Significance
Recalling information is useful for more than just accessing memories; it is also a powerful way to enhance long-term memory. Using brain imaging, we discovered that, when trying to remember, people reactivate the specific information they are searching for, along with other information from the same event. The results demonstrate that memories of the past are organized in terms of integrated events and the act of recalling even a small part of an event engages brain networks that have powerful effects on the ability to retain information from the entire event.
Keywords: retrieval, reactivation, reinstatement, memory enhancement
Abstract
Remembering is a complex process that involves recalling specific details, such as who you were with when you celebrated your last birthday, as well as contextual information, such as the place where you celebrated. It is well established that the act of remembering enhances long-term retention of the retrieved information, but the neural and cognitive mechanisms that drive memory enhancement are not yet understood. One possibility is that the process of remembering results in reactivation of the broader episodic context. Consistent with this idea, in two experiments, we found that multiple retrieval attempts enhanced long-term retention of both the retrieved object and the nontarget object that shared scene context, compared with a restudy control. Using representational similarity analysis of fMRI data in experiment 2, we found that retrieval resulted in greater neural reactivation of both the target objects and contextually linked objects compared with restudy. Furthermore, this reactivation occurred in a network of medial and lateral parietal lobe regions that have been linked to episodic recollection. The results demonstrate that retrieving a memory can enhance retention of information that is linked in the broader event context and the hippocampus and a posterior medial network of parietal cortical areas (also known as the Default Network) play complementary roles in supporting the reactivation of episodically linked information during retrieval.
Many accounts of human memory implicitly assume that memories are formed during encoding and a retrieval process is used to access the stored representation. There is substantial evidence, however, to suggest that memory retrieval can change representations of past events. For instance, during retrieval, memories can be updated with additional information (1), modified with detail lost (2), altered by misinformation (3), or strengthened for superior long-term retention. For example, recent work (4, 5) has shown that memory retrieval substantially improves long-term retention relative to reexposure to the same information, a phenomenon referred to as the testing effect. Moreover, retrieval of some information can improve long-term retention for other nontested but semantically related information (6–8), a finding that has been referred to as retrieval-induced facilitation (RIFA) (9).
Despite extensive research on the effects of retrieval of memory, little is known about the cognitive or neural mechanisms that drive these effects. There are at least two possible explanations for how retrieval enhances memory. According to one view, continuous experiences are segmented into discrete events and organized in memory (10). Retrieval of an item may be accompanied by reactivation of the spatiotemporal event in which the item was encountered (11, 12), such that the benefits of retrieval practice (Testing Effect) spill over to other items from the same context (RIFA). For example, a memory for this morning’s breakfast might include information about the cappuccino that you drank, along with your conversation with the barista; later, if you recall your morning cappuccino, you might reactivate information about the conversation, and thereby strengthen your memory for the entire event. If the testing effect and/or RIFA result from reactivation of episodic context, we might expect retrieval practice to reactivate representations in the hippocampus, which supports binding of item and context information (13, 14). Furthermore, retrieval practice might result in reactivation in lateral and medial parietal regions, which is known as the “Posterior Medial” (PM) or “Default Mode” network (15, 16).
Another nonexclusive explanation is that participants encode item-to-item associations, such that subsequent retrieval of one item directly reactivates and strengthens the semantic features of the associated item representation. For example, during breakfast, you might associate café baristas with your coffee, given their frequent cooccurrence. Later, recalling the coffee would bring to mind baristas, thereby strengthening the association between the semantic representations. If the testing effect and/or RIFA result from reactivation of semantic features of items, we might expect retrieval practice to reactivate representations in an Anterior Temporal (AT) network, including perirhinal and temporopolar regions (15), which supports learning of arbitrary associations between even unrelated items (17), particularly if they are treated as part of a unitary concept (i.e., “unitized”) (18).
The key difference between this hypothesis and the episodic reactivation hypothesis is content: Under the episodic reactivation hypothesis, RIFA is driven by reactivation of contextual information that links experiences within the same event (represented in the hippocampus and PM network), whereas, under the semantic hypothesis, RIFA is driven by reactivation of semantic features of associated items (represented in the AT network). Consistent with the latter hypothesis, previous fMRI research examining the testing effect has demonstrated increased activation in temporal lobe regions involved in semantic processing (19–21). Moreover, behavioral evidence of RIFA has only been observed in previous studies that used semantically organized materials (e.g., refs. 7 and 8). At present, there is little evidence to suggest that recovery of the spatiotemporal context of a past event (possibly supported by the hippocampus and PM network) would enhance retention of retrieved information and of contextually linked information from the same event.
To address this question, we report results from two experiments aimed at shedding light onto the cognitive and neural mechanisms by which retrieval can enhance memory for target information and for contextually related, nonretrieved information. Experiment 1 established an experimental method to investigate how retrieval of an object impacts later memory for a contextually linked object. In experiment 2, we used functional magnetic resonance imaging (fMRI) and representational similarity analysis (RSA) (22, 23), to identify reactivation of targeted and nontarget information during retrieval practice.
Results
Experiment 1.
To test whether retrieval enhances retention of contextually linked information, we developed a paradigm in which participants encoded a scene context with an object on each trial. Pairs of successive trials shared the same scene context, which served to link two objects (Fig. 1A). This method allowed us to examine whether retrieval of one object would facilitate retention of the contextually linked object.
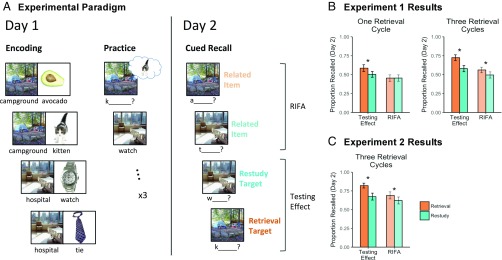
Fig. 1.
Experimental design and behavioral results for experiments 1 and 2. (A) Participants first encoded scene−object pairs, followed by retrieval or restudy of one of the two objects associated with each scene. Retrieval targets (kitten) and restudy targets (watch) were contrasted to assess the testing effect, whereas retrieval nontargets (avocado) and restudy nontargets (tie) were contrasted to assess RIFA. In experiment 1, participants performed practice for each object either one or three times. In experiment 2, practice was performed three times. (B) Recall accuracy on day 2 for experiment 1 revealed that RIFA was moderated by number of practice cycles. (C) Recall accuracy on day 2 for experiment 2 replicated the RIFA effect. Asterisk represents a significant pair-wise comparison between retrieval and restudy.
Participants were divided into two groups to understand how repeated retrieval might affect episodic memory representations. One group performed retrieval or restudy on each target object once, whereas a second group performed this practice three times. Performance during practice was designed to be at ceiling (on a scale from 1 to 4, with 1 indicating “Can picture it,” participants reported strong retrieval: 1.26 and 1.16, for one and three practice cycles, respectively).
A cued recall test, administered on day 2, revealed that retention was enhanced for tested objects in the retrieval practice condition relative to the restudy condition for both groups [one cycle, t(31) = 3.34, P = 0.002; three cycles, t(31) = 6.59, P < 0.001], replicating the testing effect in our paradigm (Fig. 1B). RIFA, however, was present only after three practice cycles [interaction between practice cycle condition (one vs. three) and practice type (retrieval vs. restudy) F(1, 62) = 4.49, P = 0.04], demonstrating that repeated retrieval plays an important role in facilitating memory for nontarget information. These findings suggest that retrieval processes might qualitatively change over the course of repeated retrieval, a possibility that we explored in depth in our second experiment.
Experiment 2.
The purpose of experiment 2 was to examine reactivation during retrieval to determine the type(s) of information that participants access during memory search. Our hypotheses centered on the hippocampus and two cortical networks, all shown to play a key role in memory encoding and retrieval. We examined the hippocampus because single-unit recording studies in rats have shown that the hippocampus “replays” recent experiences during sleep and wakeful rest (24), leading to the stabilization of new memories. Some evidence suggests that retrieval practice might be analogous to replay, as greater hippocampal activity during retrieval predicts subsequent retention of the targeted item (20, 25). However, it is unclear whether the hippocampus would be involved in reactivating the target information or the broader episode during retrieval.
We also examined two major networks that interface with the hippocampus during memory processing. The PM network—which includes the precuneus, posterior cingulate (PCC), retrosplenial cortex (RSC), angular gyrus, and parahippocampal cortex (PHC)—has been found to play a key role in recollection of event detail and processing event models (15, 26). Therefore, we predicted that the PM network might mediate RIFA by facilitating reactivation of the broader episodic representation. In contrast, the AT network—composed of the lateral orbitofrontal cortex, amygdala, ventral temporopolar cortex, and perirhinal cortex—has been implicated in the processing of unitized items and semantic information (15). If the reactivation of semantically associated objects mediates RIFA, then we might expect to see reactivation of related information in the AT network during retrieval practice.
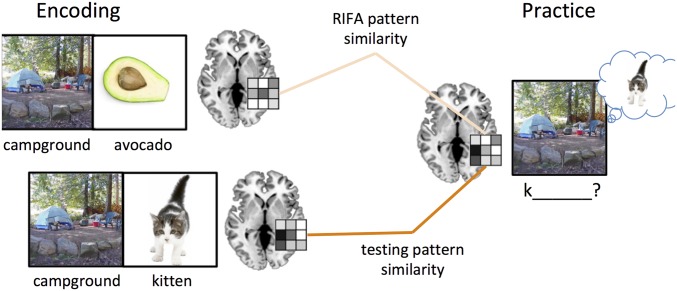
To examine reactivation in these networks in the brain, we used RSA of fMRI data, a method that allows one to examine the degree of similarity between patterns of neural activity across different trials (23). The assumption underlying this analysis is that cognitively similar events should result in similar neural pattern profiles. That is, if, during retrieval of “kitten,” participants also reactivate the contextually linked “avocado,” then pattern similarity between the encoding of “avocado” and the retrieval of “kitten” should be high (Fig. 2).
Fig. 2.
Analysis method for representation similarity analysis. To assess reactivation, patterns of activity during retrieval or restudy were compared with patterns for the encoding trial in each of our ROIs. The figure depicts an example for the retrieval practice condition; the same approach was used for the restudy condition.
Behavioral results.
This experiment involved three practice cycles for all participants. Participants reported strong retrieval of the target object (on a scale from 1 to 4, with 1 indicating “Can picture it,” participants averaged 1.11). Day 2 cued recall results replicated all of the key findings from experiment 1 (Fig. 1C): Repeated retrieval practice enhanced later recall of retrieved objects [i.e., testing effect, t(27) = 5.86, P < 0.001], and of nontarget objects that shared a scene with the retrieved objects [i.e., RIFA, t(27) = 3.56, P = 0.001], compared with corresponding objects in the restudy condition.
Functional magnetic resonance imaging results.
The main goal of our fMRI analyses was to understand the neural mechanisms that underlie the effects of retrieval practice on long-term memory. Results from several fMRI studies have shown that activity patterns evoked during encoding are reinstated when that item is recollected (27, 28). We used a similar approach in the present study by examining neural reactivation during the practice phase. To measure neural reactivation, we estimated the similarity (using Pearson’s r, with higher scores indicating more similarity) between each encoding trial and the corresponding retrieval practice or restudy trials during the practice phase, depicted in Fig. 2. Thus, we were able to assess neural reactivation separately for each retrieved item, each restudied item, and each item that was contextually linked with either a tested or a restudied item.
Differential reactivation of retrieved versus restudied items in the hippocampus and PM network.
To identify whether neural reactivation could have contributed to the testing effect, we tested for differences between the retrieval and the restudy condition. For this analysis, we focused on regions of interest (ROIs), including the hippocampus and regions in the AT and PM networks (SI Appendix, Fig. S1). If the testing effect is supported by neural reactivation of contextual representations, as suggested by the episodic context account (12), we would expect to see larger neural reactivation during retrieval practice than during restudy in the hippocampus and PM network, given their well-established role in episodic recollection. Alternatively, if the testing effect is mediated by reactivation of item-to-item semantic associations, we would expect larger neural reactivation during retrieval practice in the AT network, given extensive evidence linking these regions to semantic cognition (29, 30).
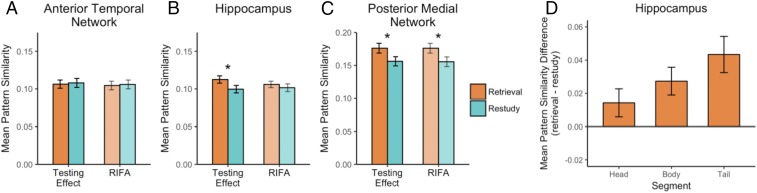
In our initial analyses, we averaged pattern similarity results across all ROIs within each network; in subsequent analyses, we compared results for each ROI in the PM network. Consistent with the episodic context account, reactivation of the target object activity pattern was more pronounced during retrieval practice than during restudy in the hippocampus, t(27) = 2.37, P = 0.03, and across the PM network, t(27) = 4.23, P < 0.001, but not the AT network [t < 1; Fig. 3 A–C; greater reactivation during retrieval vs. restudy was seen in the PM compared with AT network, F(1, 27) = 8.67, P = 0.007].
Fig. 3.
Pattern similarity between the encoding of an object and retrieval or restudy. (A–C) Pattern reactivation results during retrieval and restudy for the hippocampus and networks of interest. Asterisk represents a significant pair-wise comparison between retrieval and restudy. (D) Pattern reactivation differences for the target object scaled along the long axis of the hippocampus.
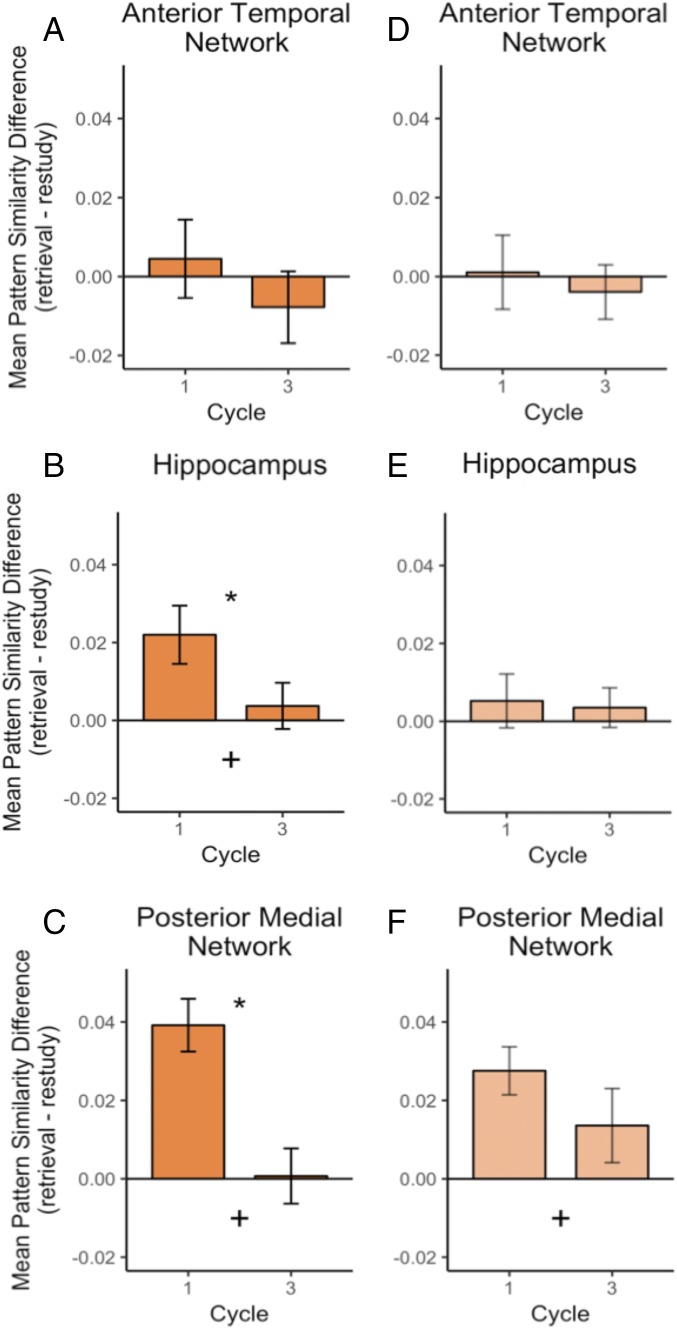
Based on results from experiment 1, which revealed that a single retrieval attempt was sufficient to elicit the testing effect, we predicted that reactivation of the target item should be more pronounced during the first retrieval attempt than the third. To test this hypothesis, we estimated the similarity between the activity pattern evoked during encoding of each item and the activity pattern evoked during the first and the third retrieval or restudy trials, separately. We focused on these two cycles to match our behavioral conditions in experiment 1. This analysis revealed a significant interaction between practice cycle (1 vs. 3) and practice type (retrieval vs. restudy) in both the hippocampus, F(1, 27) = 5.15, P = 0.03, and the PM network, F(1, 27) = 14652, P < 0.001. As shown in Fig. 4 B and C, this interaction reflected the fact that there was increased reactivation of the target item during the first cycle of retrieval, compared with restudy. However, the difference in reactivation between retrieval and restudy was absent during the third cycle. Supplementary univariate analyses revealed greater activity in the PM network and left lateral prefrontal cortex during retrieval practice compared with restudy (SI Appendix, Fig. S5 and Table S1). However, changes in mean activity across cycles could not account for the profile of pattern similarity results reported here (SI Appendix, Fig. S6). Furthermore, the pattern observed in Fig. 4C was consistent across all ROIs in the PM network (SI Appendix, Fig. S2), and none of the AT ROIs interacted with practice cycle.
Fig. 4.
Pattern similarity by cycle. (A−C) Reactivation (retrieval minus restudy) for the testing effect. (D−F) Reactivation for RIFA. Asterisk represents a significant interaction between practice type (retrieval vs. restudy) and cycle; plus sign represents a significant main effect of practice type (retrieval > restudy).
Preferential reactivation during retrieval practice differs along the long axis of the hippocampus.
Thus far, the results have indicated that, in both the hippocampus and the PM network, pattern similarity was higher between encoding and retrieval practice than between encoding and restudy. In contrast, no evidence of reactivation was observed in the AT network. Previous studies have shown that the posterior hippocampus shows preferential functional connectivity with the PM network, whereas the anterior hippocampus shows preferential connectivity with the AT network (31). Accordingly, we predicted that the difference in neural reactivation between retrieval and restudy should be larger in posterior than in anterior hippocampal regions. To test this prediction, we segmented the hippocampus into head, body, and tail to assess reactivation for the target object during cycle 1 separately for each segment. Results showed that the magnitude of the reactivation effect scaled along the long axis of the hippocampus (Fig. 3D). That is, the tail and body portions of the hippocampus showed strong testing reactivation, t(27) = 3.98, P < 0.001, t(27) = 3.26, P = 0.003, whereas the head showed no significant effects, t(27) = 1.70, P = 0.10; a comparison of the head and tail revealed a significant difference in pattern reactivation, t(27) = 2.36, P = 0.03. Thus, the hippocampal subregions that would be expected to preferentially interact with the PM network showed the most pronounced reactivation of the encoding event during retrieval practice.
Differential reactivation of contextually linked information during retrieval versus restudy in the PM network.
Having established that retrieval increased neural reactivation of the target’s encoding pattern in the hippocampus and PM network, we next examined whether differential neural reactivation could also account for RIFA. Within each ROI, we computed the similarity between the activity pattern evoked during each retrieval and restudy trial and the activity pattern evoked during encoding of the contextually linked object. Using the example depicted in Fig. 2, this would correspond to estimating pattern similarity between encoding of “avocado” and retrieval of “kitten” during the practice phase. Reactivation of the nontarget object encoding pattern was higher during retrieval practice than during restudy trials in the PM network, t(27) = 3.28, P = 0.003, but there were no significant between-condition differences in the AT network or hippocampus [ts < 1; greater reactivation during retrieval vs. restudy was seen in the PM compared with AT network, F(1, 27) = 12.02, P = 0.002]. These results are consistent with the idea that episodic reactivation—reflected by neural reactivation in the PM network—contributes to RIFA.
We further explored these effects by examining differences across practice cycles. Results from experiment 1 demonstrated that, unlike the testing effect, three retrieval attempts were necessary to obtain RIFA. To identify whether neural reactivation of contextually linked items differed across practice cycles, we separately estimated the similarity of activity patterns evoked during retrieval or restudy of a target item (separately for cycles 1 and 3) with activity patterns evoked during encoding of the contextually linked item (i.e., “RIFA pattern similiarity” in Fig. 2). As shown in Fig. 4F, analyses of these data revealed greater pattern similarity during retrieval compared with restudy, F(1, 27) = 10.77, P = 0.003. However, we found no significant interaction between practice type and practice cycle, F(1, 27) = 2.03, P = 0.17. Furthermore, the effect of practice cycle differed from that observed for the testing effect [i.e., interaction between effects in Fig. 4 C and F, F(1, 27) = 4.20, P = 0.05].
These findings, considered along with the results from analyses of reactivation of tested items, are strikingly consistent with what would be expected based on the behavioral results from experiment 1: Strong pattern reactivation for the target object occurred in the PM network on the first retrieval attempt, but it was attenuated by the third attempt; in contrast, strong pattern reactivation for the contextually linked object was sustained even after multiple retrieval attempts.
Reactivation in subnetworks of the posterior medial network.
The analyses reported above show that, on average, regions in the PM network exhibit more pronounced neural reactivation for contextually linked items during retrieval than during restudy across practice cycles 1 and 3. We next examined each ROI independently to determine whether certain ROIs were driving the effect. Interestingly, this analysis revealed two classes of ROIs. Greater pattern reactivation during retrieval of nontarget information was observed in the angular gyrus, F(1, 27) = 10.24, P = 0.003; PCC, F(1, 27) = 9.67, P = 0.004; and precuneus, F(1, 27) = 5.33, P = 0.03; and the effect did not interact with cycle in either of these regions (all Ps > 0.29). In contrast, a significant interaction was observed in PHC, F(1, 27) = 20.16, P < 0.001, and a similar, although nonsignificant, pattern was observed for RSC (SI Appendix, Fig. S3). In Fig. 4F, the pattern reactivation observed across the PM network was sustained, although somewhat decreased by cycle 3. This attenuated pattern can be explained by these two different clusters of ROIs within the network: Some regions show consistent reactivation across multiple retrieval attempts, whereas others show attenuated reactivation of the contextually linked object after the first retrieval attempt (for the same analysis on the testing effect, see SI Appendix). We speculate on the unique roles of these two subnetworks in Discussion.
Discussion
The goal of this study was to examine the effects of retrieval practice on memory for contextually linked information. Experiments 1 and 2 demonstrated that multiple retrieval attempts enhanced long-term retention of both retrieved objects and the nontarget objects that shared the same scene context. In experiment 2, we found that, relative to restudy, retrieval enhanced neural reactivation of representations of both target objects and contextually linked objects. Reactivation of the target representation occurred in the PM network as well as in the posterior hippocampal regions, which is expected to preferentially interact with the PM network. Reactivation of the contextually linked object, however, was constrained to the PM network, and it was most pronounced in a parietal subnetwork, which contributes to episodic memory retrieval. These findings demonstrate that, during recall of a particular object, reactivation of information from the broader episodic context enhances retention of both recalled and episodically linked information.
Our findings are consistent with the idea that retrieval strengthens the episodic representation associated with the targeted item (12), and they extend this account by demonstrating that retrieval practice enhances retention of episodically linked information. In other words, during retrieval, people do not just access a single object; instead, they reactivate an episode, such that the benefits of retrieval spill over to other items encountered in the same context.
Although we observed evidence for RIFA, studies of retrieval-induced forgetting (RIF) (2, 32–35) typically show that retrieval can impair recall of related, nonretrieved information. Chan (8) has determined that at least two factors affect whether retrieval will facilitate or impair memory for linked items. First, Chan found that retrieval facilitates retention of items that were well integrated with the retrieval target at the time of initial learning, whereas it inhibits retention of related items that were poorly integrated with the target. This observation fits well with the present results: A stable scene context served as the background that facilitated integration of retrieval targets and linked items. Second, Chan found that RIF tends to be observed when the final test occurs after a short delay, whereas RIFA is most apparent after a relatively long retention interval. Consistent with this finding, we observed RIFA in the present paradigm with a 1-d delay between retrieval practice and test.
One surprising finding from our research was the distinction between PHC and RSC, and the PCC, angular gyrus, and precuneus; the former grouping showed RIFA reactivation only during the first retrieval attempt, whereas the latter grouping showed sustained reactivation across three retrieval attempts. This dissociation accords with recent work that has mapped these regions onto distinct subnetworks (see yellow and dark purple networks in figure 13 of ref. 36) within the Default Network. Numerous studies have shown that activity in PHC and RSC is enhanced during processing of visual contextual information, such as processing of visual scenes or visual objects that are associated with specific contexts (37). In contrast, evidence reviewed above suggests that other parietal regions in the PM network may represent event content in a manner that generalizes across sensory modalities (38). For example, van der Linder et al. (19) found that increased angular gyrus activity accompanied memory benefits during schematic encoding. Recent work also indicates that retrieval-related activity in the parietal subnetwork is associated with increased generalization of episodic memories (39, 40).
Putting the findings together, it is possible that, during the first retrieval attempt, the hippocampus drives reactivation of sensory contextual details associated with the encoding event via reactivation in PHC and RSC (41). Although the initial retrieval attempt would be sufficient to strengthen the association between the context and the retrieval target (testing effect), it would not be sufficient to elicit a behavioral RIFA. Repeated retrieval, however, may lead to reliance on abstract event representations in the parietal subnetwork that facilitate access to other information embedded within the same event. In fact, it is interesting to consider the two subnetworks in light of the episodic context account of the testing effect (12). One core assumption of this account is that the context representation is updated each time one retrieves a target memory. Although our experiment was not optimized to test this assumption, it is possible that the medial subnetwork involving the PHC and RSC maintains a specific/sensory contextual representation that is updated with each retrieval attempt, whereas the parietal subnetwork maintains a schematic representation of the event that remains stable across retrieval attempts. As such, it is possible that activation of event representations—supported by the parietal subnetwork (38)—should facilitate semantic or conceptual associations between contextually linked items, but at the cost of sensory detail. This prediction aligns with recent neuroimaging findings from Lee et al. (39).
In recent years, research has consistently demonstrated that retrieval practice strongly enhances long-term retention, but the cognitive and neural mechanisms that drive this enhancement are not well understood. Here, we found that reactivation in parietal regions of the PM network occurs not only for the retrieval target but also for information that is linked within the same episodic context. These findings underscore the importance of spatiotemporal context in organizing information in episodic memory. In the real world, information experienced within an event is highly structured and integrated, even more so than the stimuli used in the present experiments. Accordingly, there is good reason to think that these results capture a fundamental aspect of our daily experiences: Recalling even a single detail from the past can have far-reaching effects on retention of an entire event. Our findings could therefore be used to devise ways to improve learning in educational settings and to enhance memory in patients with amnestic disorders.
Materials and Methods
Participants.
Experiment 1 included a final sample size of 64 participants (32 per group; 50 females, Mage = 20.6). Experiment 2 included a final sample size of 28 participants (13 females, Mage = 22.8).
Procedure.
An institutional review board at the University of California, Davis reviewed the experimental procedure and provided approval. Participants were provided with informed consent before the start of the experiments. On day 1, participants completed 8 (Experiment 1) or 10 (Experiment 2) blocks involving encoding and practice. During encoding, participants were shown 12 scene−object pairings along with their verbal labels (Fig. 1); two back-to-back trials shared the same scene. After viewing a pairing for 2 s, participants were prompted to indicate how well they could imagine the object in the scene, to encourage integration; they were asked to imagine both objects in the same scene. All pairings were shown twice. Following encoding, participants performed retrieval or restudy on one of the two objects paired with each of the scenes. This practice was done one or three times in experiment 1, and three times in experiment 2. For all practice trials, the scene image was shown. For retrieval trials, participants also saw a one-letter word stem (Fig. 1); after 2 s, they were to indicate how well they remembered the object, using a response scale from 1 (“Can picture it”) to 4 (“Don’t remember”). For restudy trials, participants saw the full word; after 2 s, they were told which key to press (randomly selected on each trial). Participants returned the next day to complete a cued recall test. During the test, participants were shown a scene image along with a one-letter word stem to prompt recall of the correct word.
For a description of fMRI image acquisition and preprocessing, see SI Appendix.
ROI Selection.
Anatomical ROIs were identified individually for each participant in native space. Medial temporal lobe ROIs (HC, PHC, and PRC) were manually traced using the guidelines supplied by Frankó et al. (42), and cortical ROIs were parcellated using Freesurfer (43, 44) Destrieux atlas. PM ROIs included PCC, precuneus, angular gyrus, RSC, and PHC. AT ROIs included OFC, PRC, and temporal pole.
RSA Analysis.
Similarity matrices were generated for each ROI for each participant using Pearson’s r; the similarity values of interest were extracted and averaged for each condition. For each of the networks, we assessed reactivation in each ROI independently, and then averaged across all ROIs.
Supplementary Material
Acknowledgments
We thank Alex Asera, Adira Fogel, Jacob Hansen, and Victoria Saboya for their assistance with data collection and tracing of ROIs. We also thank Lauren Farley, Boyoung Kim, Charlie Lowell, Michelle Swarovski, and Katelin Tanda for their assistance with tracing of ROIs. This work was supported by a Vannevar Bush Fellowship Office of Naval Research Grant N00014-15-1-0033 (to C.R.) and a Multi-University Research Initiative Grant Office of Naval Research Grant N00014-17-1-2961 (to C.R.) from the Office of Naval Research, and a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada (to T.R.J.). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the Office of Naval Research or the US Department of Defense.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1800006115/-/DCSupplemental.
References
- 1.Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learn Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonker TR, Seli P, MacLeod CM. Putting retrieval-induced forgetting in context: An inhibition-free, context-based account. Psychol Rev. 2013;120:852–872. doi: 10.1037/a0034246. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EF. Planting misinformation in the human mind: A 30-year investigation of the malleability of memory. Learn Mem. 2005;12:361–366. doi: 10.1101/lm.94705. [DOI] [PubMed] [Google Scholar]
- 4.Roediger HL, Karpicke JD. Test-enhanced learning: Taking memory tests improves long-term retention. Psychol Sci. 2006;17:249–255. doi: 10.1111/j.1467-9280.2006.01693.x. [DOI] [PubMed] [Google Scholar]
- 5.Karpicke JD, Roediger HL., 3rd The critical importance of retrieval for learning. Science. 2008;319:966–968. doi: 10.1126/science.1152408. [DOI] [PubMed] [Google Scholar]
- 6.Chan JCK. Long-term effects of testing on the recall of nontested materials. Memory. 2010;18:49–57. doi: 10.1080/09658210903405737. [DOI] [PubMed] [Google Scholar]
- 7.Chan JCK, McDermott KB, Roediger HL., 3rd Retrieval-induced facilitation: Initially nontested material can benefit from prior testing of related material. J Exp Psychol Gen. 2006;135:553–571. doi: 10.1037/0096-3445.135.4.553. [DOI] [PubMed] [Google Scholar]
- 8.Chan JCK. When does retrieval induce forgetting and when does it induce facilitation? Implications for retrieval inhibition, testing effect, and text processing. J Mem Lang. 2009;61:153–170. [Google Scholar]
- 9.Bäuml K-HT, Kliegl O. Retrieval-induced remembering and forgetting. In: Byrne JH, editor. Learning and Memory: A Comprehensive Reference. 2nd Ed. Psychology Press; New York: 2017. pp. 27–51. [Google Scholar]
- 10.Zacks JM, Speer NK, Swallow KM, Braver TS, Reynolds JR. Event perception: A mind-brain perspective. Psychol Bull. 2007;133:273–293. doi: 10.1037/0033-2909.133.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard MW, Kahana MJ. A distributed representation of temporal context. J Math Psychol. 2002;46:269–299. [Google Scholar]
- 12.Karpicke JD, Lehman M, Aue WR. Psychology of Learning and Motivation. Vol 61. Elsevier Science; New York: 2014. Retrieval-based learning: An episodic context account; pp. 237–284. [Google Scholar]
- 13.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 16.Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 2013;23:255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray EA, Gaffan D, Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J Neurosci. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59:554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 19.van der Linden M, Berkers RMWJ, Morris RGM, Fernández G. Angular gyrus involvement at encoding and retrieval is associated with durable but less specific memories. J Neurosci. 2017;37:9474–9485. doi: 10.1523/JNEUROSCI.3603-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu XL, Liang P, Li K, Reder LM. Uncovering the neural mechanisms underlying learning from tests. PLoS One. 2014;9:e92025. doi: 10.1371/journal.pone.0092025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson Wirebring L, et al. Lesser neural pattern similarity across repeated tests is associated with better long-term memory retention. J Neurosci. 2015;35:9595–9602. doi: 10.1523/JNEUROSCI.3550-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage. 2012;59:2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis–Connecting the branches of systems neuroscience. Front Syst Neurosci. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster DJ. Replay comes of age. Annu Rev Neurosci. 2017;40:581–602. doi: 10.1146/annurev-neuro-072116-031538. [DOI] [PubMed] [Google Scholar]
- 25.Wing EA, Marsh EJ, Cabeza R. Neural correlates of retrieval-based memory enhancement: An fMRI study of the testing effect. Neuropsychologia. 2013;51:2360–2370. doi: 10.1016/j.neuropsychologia.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilmore AW, Nelson SM, McDermott KB. The contextual association network activates more for remembered than for imagined events. Cereb Cortex. 2016;26:611–617. doi: 10.1093/cercor/bhu223. [DOI] [PubMed] [Google Scholar]
- 27.Mack ML, Preston AR. Decisions about the past are guided by reinstatement of specific memories in the hippocampus and perirhinal cortex. Neuroimage. 2016;127:144–157. doi: 10.1016/j.neuroimage.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JD, McDuff SGR, Rugg MD, Norman KA. Recollection, familiarity, and cortical reinstatement: A multivoxel pattern analysis. Neuron. 2009;63:697–708. doi: 10.1016/j.neuron.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke A, Tyler LK. Object-specific semantic coding in human perirhinal cortex. J Neurosci. 2014;34:4766–4775. doi: 10.1523/JNEUROSCI.2828-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W-C, Ranganath C, Yonelinas AP. Activity reductions in perirhinal cortex predict conceptual priming and familiarity-based recognition. Neuropsychologia. 2014;52:19–26. doi: 10.1016/j.neuropsychologia.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Anderson MC, Bjork RA, Bjork EL. Remembering can cause forgetting: Retrieval dynamics in long-term memory. J Exp Psychol Learn Mem Cogn. 1994;20:1063–1087. doi: 10.1037//0278-7393.20.5.1063. [DOI] [PubMed] [Google Scholar]
- 33.Jonker TR, Seli P, MacLeod CM. Retrieval-induced forgetting and context. Curr Dir Psychol Sci. 2015;24:273–278. [Google Scholar]
- 34.Jonker TR, Seli P, Macleod CM. Less we forget: Retrieval cues and release from retrieval-induced forgetting. Mem Cognit. 2012;40:1236–1245. doi: 10.3758/s13421-012-0224-2. [DOI] [PubMed] [Google Scholar]
- 35.Jonker TR, MacLeod CM. Retrieval-induced forgetting: Testing the competition assumption of inhibition theory. Can J Exp Psychol. 2012;66:204–211. doi: 10.1037/a0027277. [DOI] [PubMed] [Google Scholar]
- 36.Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bar M. Visual objects in context. Nat Rev Neurosci. 2004;5:617–629. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- 38.Baldassano C, et al. Discovering event structure in continuous narrative perception and memory. Neuron. 2017;95:709–721.e5. doi: 10.1016/j.neuron.2017.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee H, Samide R, Richter FR, Kuhl BA. 2017. Decomposing parietal memory reactivation to predict consequences of remembering. bioRxiv:10.1101/208678. [DOI] [PubMed]
- 40.Guerin SA, Robbins CA, Gilmore AW, Schacter DL. Interactions between visual attention and episodic retrieval: Dissociable contributions of parietal regions during gist-based false recognition. Neuron. 2012;75:1122–1134. doi: 10.1016/j.neuron.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frankó E, Insausti AM, Artacho-Pérula E, Insausti R, Chavoix C. Identification of the human medial temporal lobe regions on magnetic resonance images. Hum Brain Mapp. 2014;35:248–256. doi: 10.1002/hbm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 44.Fischl B, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.