Significance
Empirical evidence for the response of ecosystem functioning to the combined effects of warming and biodiversity loss is scarce. We show that warming and biodiversity loss interact synergistically, impairing the functioning of microbial communities. We found that as temperatures departed from ambient conditions more species were required to maintain ecosystem functioning. Our results suggest interspecific complementarity increased under thermal stress and high-diversity communities that seemed functionally redundant at ambient temperature became more functionally unique as temperatures changed. Biodiversity may therefore be even more important than previously anticipated when considering the impacts of multiple facets of environmental change.
Keywords: warming, biodiversity, ecosystem function, traits, microbial ecology
Abstract
Global warming and the loss of biodiversity through human activities (e.g., land-use change, pollution, invasive species) are two of the most profound threats to the functional integrity of the Earth’s ecosystems. These factors are, however, most frequently investigated separately, ignoring the potential for synergistic effects of biodiversity loss and environmental warming on ecosystem functioning. Here we use high-throughput experiments with microbial communities to investigate how changes in temperature affect the relationship between biodiversity and ecosystem functioning. We found that changes in temperature systematically altered the relationship between biodiversity and ecosystem functioning. As temperatures departed from ambient conditions the exponent of the diversity-functioning relationship increased, meaning that more species were required to maintain ecosystem functioning under thermal stress. This key result was driven by two processes linked to variability in the thermal tolerance curves of taxa. First, more diverse communities had a greater chance of including species with thermal traits that enabled them to maintain productivity as temperatures shifted from ambient conditions. Second, we found a pronounced increase in the contribution of complementarity to the net biodiversity effect at high and low temperatures, indicating that changes in species interactions played a critical role in mediating the impacts of temperature change on the relationship between biodiversity and ecosystem functioning. Our results highlight that if biodiversity loss occurs independently of species’ thermal tolerance traits, then the additional impacts of environmental warming will result in sharp declines in ecosystem function.
The impact of biodiversity loss on the productivity and stability of ecosystems is a major concern (1, 2). Substantial evidence exists across diverse biomes that ecosystems with higher levels of biodiversity are also more productive and stable (3, 4). Biodiversity loss driven by factors such as land-use change, nutrient pollution, and invasive species is occurring in parallel with global warming, yet our understanding of the potential for synergies between these multiple facets of environmental change on ecosystem functioning is limited (5–9).
Two key processes underlie our understanding of how biodiversity affects ecosystem functioning. “Selection effects” describe the processes whereby average levels of ecosystem functioning tend to be higher in more diverse communities because they have a greater probability of including taxa with particular traits that promote dominance in the community and contribute positively to ecosystem productivity (3, 10–12). “Complementarity effects” characterize deterministic processes like niche differentiation and facilitation that arise from species interactions and enhance resource use efficiency and productivity in more diverse communities (11–13). Ultimately both selection and complementarity effects shape ecosystem functioning through variance in phenotypic traits that determine the way organisms respond to and interact with one another and the abiotic environment. Consequently, the impacts of interactions between biodiversity loss and global warming are likely to be mediated by traits that influence the performance of species under changing thermal regimes.
Thermal tolerance curves characterize how species’ performance (fitness) responds to changes in temperature. These curves are typically unimodal and asymmetric, whereby performance declines much more rapidly after the optimum than before, and can be quantified by several key “traits” that characterize the shape of the curve (e.g., the optimal temperature) (14, 15). Theory suggests that variance in traits that determine environmental tolerance should play a key role in mediating the impacts of abiotic change on ecological dynamics and ecosystem functioning (16). If species loss occurs independently of thermal tolerance traits (as might be expected from the effects of land-use change, nutrient loading, or invasive species), then the additional impact of environmental warming could result in pronounced declines in ecosystem function, because communities with fewer species will have a lower probability of including those with thermal traits that enable them to cope with the novel temperature regime (17–19). Biodiversity loss would then interact with warming by increasing the importance of selection effects linked to thermal tolerance traits. Alternatively, if species loss is correlated with thermal performance traits—i.e., when multiple stressors drive the loss of species that are least productive in the new warmer environment—then rising temperatures may have negligible impacts on the relationship between biodiversity and ecosystem functioning. Rising temperatures are also likely to affect the nature of species interactions by changing resource requirements and/or uptake rates (20–22). Consequently, warming could also change the way in which biodiversity loss impacts ecosystem functioning by altering species interactions and complementarity effects. If warming enhances interspecific facilitation and niche partitioning, then rising temperatures would be expected to increase the strength of the diversity–functioning relationship and reduce functional redundancy. By contrast, if warming increases interspecific competition for limiting resources, then rising temperatures may increase functional redundancy and weaken the relationship between biodiversity and ecosystem functioning.
Several recent studies have shown that environmental change (e.g., drought, warming, changes in salinity) can alter the coupling between biodiversity and ecosystem functioning, with species loss resulting in more pronounced productivity declines in stressful environments (23, 24), but the processes that underpin these patterns remain unverified (19, 25, 26). Here we used a microcosm approach with microbial communities to address these key knowledge gaps. We used 24 bacterial taxa isolated from a network of geothermally warmed streams in Iceland (N64 ° 0′ 2.944″ W21 ° 11′ 17.451″) that range in temperature from 7 °C to 38 °C. We characterized the thermal tolerance traits of each taxon and placed them in randomly assembled communities of increasing diversity at eight temperatures from 10 °C to 40 °C to quantify how changes in temperature influence how biodiversity loss that is independent of species’ thermal tolerance traits and environmental warming, affect the functioning of microbial communities.
Results and Discussion
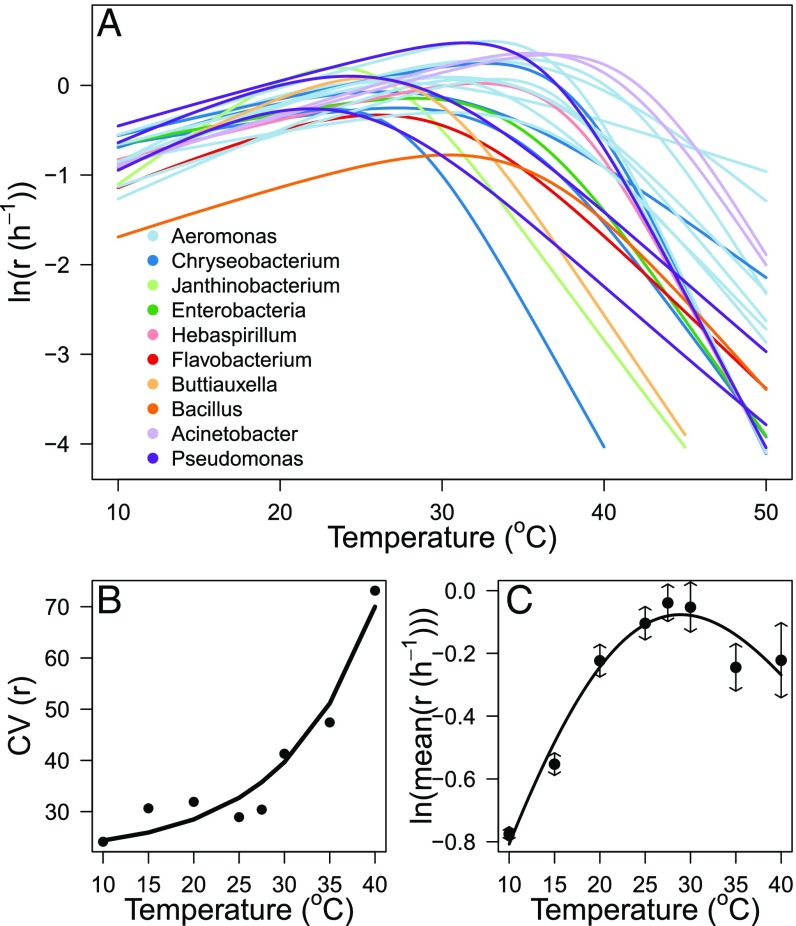
The thermal tolerance curves (characterized as the change in per capita growth rate quantified along a 10 °C to 50 °C thermal gradient) for each taxon exhibited characteristic unimodality and left skew, with performance increasing exponentially up to an optimum and then declining rapidly. However, substantial variance in the shapes and parameters of these curves was evident among the 24 taxa, with optimal temperatures () ranging from 21 °C to 37 °C (Fig. 1). Owing to the shapes of the thermal tolerance curves (unimodal and left skewed) and the marked variability in , the coefficient of variation in performance was lower at cold temperatures and increased exponentially with warming (Fig. 1B).
Fig. 1.
Thermal tolerance curves of the 24 bacterial taxa. (A) Comparison of the fitted thermal tolerance curves of population growth rate, r, for the 24 taxa quantified using the Sharpe–Schoofield equation (Materials and Methods). (B) The coefficient of variance (CV) in r among the 24 taxa at each assay temperature demonstrates an exponential increase in CV with rising temperature (y 21.98 , 0.94, 96.22, P 0.001). (C) Pooled thermal tolerance curve fitted using the Sharpe–Schoofield equation (SI Appendix, Table S1) to the mean growth rate across all 24 taxa at each assay temperature demonstrates a marked decline in average performance above 27.5 °C, which coincides with the temperature at which the CV of population growth rate rapidly increases (quasi- 0.92 of the fitted model).
This finding reflects the fact that many taxa perform poorly at temperatures that exceed the average (27 °C), while only a few perform very well. In light of this marked variation in thermal tolerance traits, in microbial communities assembled from these taxa, we expected changes in temperature to substantially alter the relationship between biodiversity and ecosystem function via temperature-driven shifts in the strength of selection and complementarity effects. Specifically, high variability in thermal tolerance traits means that when temperatures depart from ambient conditions (either via warming or cooling), levels of ecosystem functioning should change markedly when biodiversity loss is independent of species’ thermal tolerance traits—i.e., the exponent of the diversity–functioning relationship should increase because the probability of including species with thermal traits that are well suited to the new environment declines rapidly as species are lost. Furthermore, because of variability in thermal tolerance traits, changes in temperature will also have differential effects on species performance and thus alter the nature of competition (22), facilitation, and resource partitioning, likely changing the degree of interspecific complementarity.
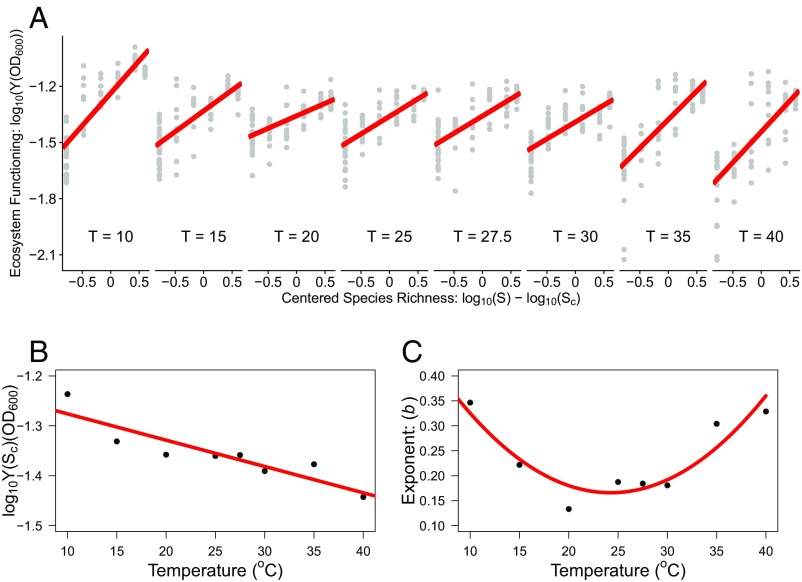
We tested these hypotheses by randomly assembling the 24 taxa in communities of increasing diversity across eight temperatures spanning 10 °C to 40 °C (the range of temperatures where all species were able to grow). We monitored the “growth” of the community by measuring the accumulation of biomass over time. In all microcosms, biomass increased exponentially and then reached a stationary phase. Ecosystem functioning was quantified as the asymptotic biomass (yield) of the community in the stationary phase, determined by fitting the logistic growth equation to the biomass time series (Materials and Methods). Yield increased with increasing species richness at all temperatures, but it did so in a decelerating manner (Fig. 2 A and B). Consequently, this relationship was well characterized by a power function (linear relationship on a log-log scale) with an exponent 1, where the exponent indicates the average effect of changes in species richness on ecosystem functioning.
Fig. 2.
Effects of temperature on the relationship between species richness and ecosystem functioning. (A) Effects of temperature on the relationship between species richness and ecosystem functioning. A power function was used to analyze the coupling between ecosystem functioning and species richness: Y(S) = b (S – ) + Y(). Ecosystem functioning was quantified as the community yield (Y) in the stationary phase of community growth, species richness (S) was centered around the mean, , so that the intercept of the linear relationship between and gives the at the average level of S, and is the exponent that captures the shape of the diversity–functioning relationship (SI Appendix, section 1.3). Analyses demonstrate major shifts in the relationship between and with temperature. (B) The intercept of the diversity–functioning relationship, (), declined with rising temperature. The red line denotes the fit of a linear model to the relationship between (Sc) and temperature ( −0.01 × −1.22, = 0.81, P 0.01). (C) Changes in the exponent reveal a U-shaped relationship with temperature, with the highest values at low and high temperatures. The red solid line represents the fit of a second-order polynomial model (y = 0.0008x2 − 0.04x + 0.63, 0.85, P 0.01).
The average yield at an intermediate level of richness (e.g., the intercept of the diversity–functioning relationship; Materials and Methods) declined exponentially with increasing temperature—e.g., on average, warmer communities supported lower levels of asymptotic biomass (Fig. 2B). This effect of temperature on the community yield is in line with predictions from metabolic scaling theory and can be explained from the exponential effects of temperature on metabolic rates (27, 28). When resource availability is fixed, finite and independent of temperature (as was the case in our experiment), higher temperatures will result in a decline in asymptotic biomass because each individual will use resources at a faster rate owing to its higher metabolic rate and thus the ecosystem can support fewer individuals (29). We also found a marked effect of temperature on the exponent of the relationship between species richness and ecosystem function (SI Appendix, Table S2). In line with our expectations, warming above and cooling below ambient conditions (e.g., 20 °C, which was the isolation and cultivation temperature of all isolates) increased the exponent (Fig. 2C), meaning that the diversity–functioning relationships became more linear and less strongly decelerating under altered thermal regimes.
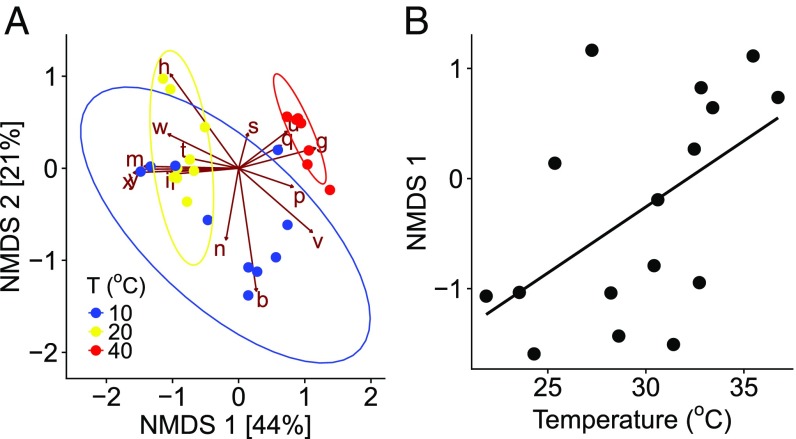
The impacts of temperature change on the exponent of the diversity–functioning relationship are particularly noteworthy because they indicate that as temperatures depart from ambient conditions, biodiversity loss has a more marked effect on ecosystem functioning. To explore the mechanisms shaping this interaction between species loss and environmental warming on ecosystem functioning, we isolated the taxa present at the end of the experiment from the high-diversity (24 species) treatments exposed to 10 °C, 20 °C, and 40 °C (i.e., the ambient and extreme ends of the temperature gradient). We found marked differences in the taxa present at the end of the experiments in the different temperature treatments. Nonmetric multidimensional scaling (NMDS) revealed a statistically significant separation in the composition and relative abundance of the taxa present at the end of the experiment among treatments (Fig. 3A; PERMANOVA; 10.00, P 0.001; see SI Appendix, Table S3 for pairwise contrasts). We also found that the species scores from the primary axis of variation in the NMDS analysis (NMDS1, which accounted for 44% of the variance in taxonomic composition) were significantly positively correlated with the thermal optima of the taxa (Fig. 3B). This result is consistent with our expectations and indicates that the presence of taxa in the different temperature treatments was associated with their thermal tolerance traits. Notably no taxa with 27 °C were present at the end of experiments in the 40 °C treatments (SI Appendix, Fig. S1). These results suggest that temperature-driven selection based on species’ thermal tolerance traits played a key role in shaping how the diversity–functioning relationship was affected by warming (3, 30).
Fig. 3.
Compositional turnover linked to variance in thermal tolerance traits. (A) Nonmetric multidimensional scaling ordination of the microbial communities in the high-diversity treatments (S 24) at high (red), low (blue), and ambient (yellow) temperatures (k 4, stress 0.01). The brown arrows and letters correspond to “species scores” and indicate the correspondence of each species with the primary axes of variation. The colored points and ellipses denote “site scores,” where each point is a replicate community and its correspondence with the axes of variation. Ellipses give the 95% CI around the centroid of each treatment—nonoverlapping ellipses suggest significant divergence in community composition between treatments (see SI Appendix, Tables S3 and S4 for results of PERMANOVA). (B) Relationship between the species scores extracted from NMDS1 and the of each species. The black line represents the fit of a linear model ( 0.28, P 0.03).
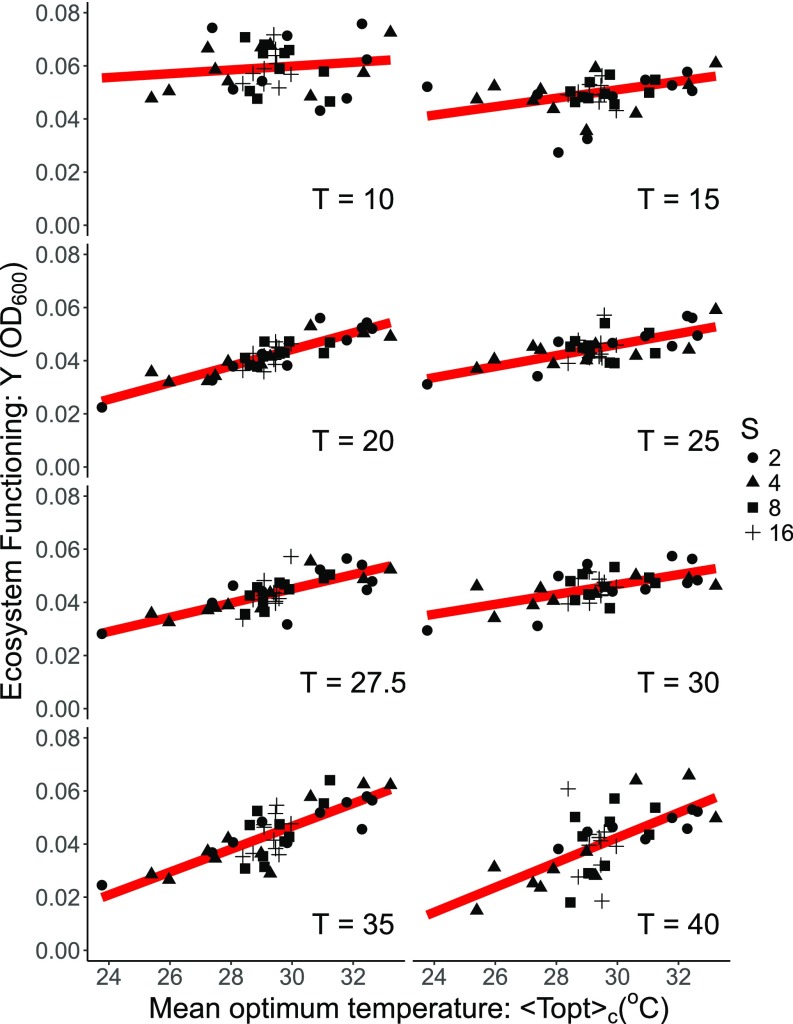
We then explored how variability in the thermal tolerance traits of the species composing each community (at each level of temperature and richness) influenced ecosystem functioning. Recent developments using trait driver theory outline how functional trait distributions change along environmental gradients and can be used to understand how ecosystem-level properties shift owing to environmental selection (on functional traits) and scaling up individual-level performance to ecosystem functioning (31). To explore the coupling between functional traits, environmental variation, and ecosystem productivity, we quantified the mean thermal optima of the taxa inoculated into each community (at each level of temperature and richness) at the start of the diversity–functioning experiment. We found that ecosystem functioning was positively correlated with and the strength of the correlation increased markedly with rising temperature across all richness levels (Fig. 4 and SI Appendix, Fig. S2). Thus, communities that comprised taxa with high were also those with the highest productivity, and this effect was most clearly manifest at warm temperatures (Fig. 4 and SI Appendix, Fig. S3). This result demonstrates that temperature-driven changes in the diversity–function relationship were mediated by variability in thermal traits (Fig. 1); high-diversity communities were more likely to include species with high , and communities with high average were generally the most productive, particularly at warm temperatures.
Fig. 4.
Linking thermal traits to the impacts of warming and species loss on ecosystem functioning. Coupling between ecosystem functioning (community yield) and the community-mean optimum temperature , derived from the species used to seed each replicate community. Analyses reveal that becomes an increasingly important predictor of ecosystem function as temperature rises, demonstrating that temperature-driven changes in the diversity–functioning relationship were mediated by variability in thermal traits. The red lines represent the fitted curves derived from the linear mixed-effect model. The different point shapes represent the level of species richness (S).
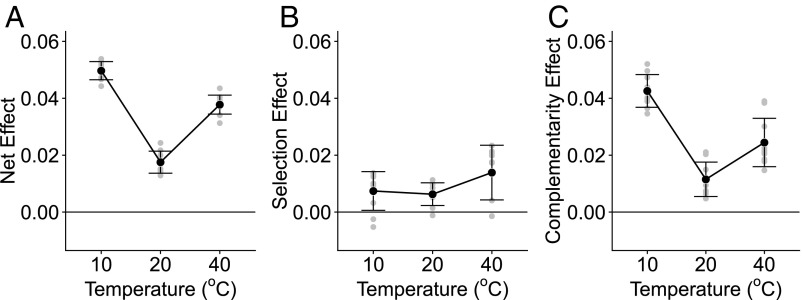
To further explore the processes that may have contributed to the strong interaction between species loss and warming on ecosystem functioning, we used the method of Isbell et al. (19) to partition the net biodiversity effect into components attributable to selection and complementarity at local (replicate communities within temperature treatments) and larger scales (total effects at the treatment level). Our results revealed a positive net biodiversity effect in all temperature treatments (Fig. 5A and SI Appendix, Table S5) and, in line with our previous analysis (Fig. 2), we found that the net biodiversity effect was higher at 10 °C and 40 °C compared with that at 20 °C (Fig. 5). We found evidence that both selection and complementarity effects contributed to the net biodiversity effect, but complementarity accounted for a much larger fraction of the net biodiversity effect across each of the temperature treatments. These patterns were consistent both at local and at larger scales (SI Appendix, Table S5). The strength of complementarity effects, however, also changed markedly with warming, increasing significantly at low and high temperatures (Fig. 5C). Indeed, the relative importance of complementarity compared with selection effects was greater at 10 °C and 40 °C (Fig. 5), indicating that as temperatures depart from ambient conditions, complementarity played an increasingly important role in mediating the effects of biodiversity of ecosystem functioning.
Fig. 5.
Partitioning the impacts of warming on ecosystem functioning into selection and complementarity effects. (A) Net effect (NE) of biodiversity on functioning at each treatment temperature. (B) Selection effect (SE) of biodiversity at each treatment temperature. (C) Complementarity effect (CE) of biodiversity at each treatment temperature. Gray circles represent the value of each replicate and the black circles and the error bars indicate the mean and the SD for each treatment. Values over the zero line indicate a positive NE, SE, or CE on ecosystem function.
Our study design precludes identification of the precise nature of the impacts of temperature change on complementarity. Without extensive further work determining the precise resource uptake characteristics of each of the species and whether they are able to grow on one another’s metabolic byproducts, we are unable to determine whether these effects are driven by changes in facilitation and cross-feeding interactions known to be prevalent in microbial communities (32) or shifts in other aspects of the functional niches (e.g., complementary resource use). Nevertheless, the strong impact of temperature on the composition of the communities, evidence that compositional turnover was linked to variability in thermal optima, and the prevalence of interspecific complementarity, suggests that variability in thermal performance played a key role in shaping the impacts of warming and species loss on ecosystem functioning. These patterns were most likely mediated by the impacts of changes in temperature on species interactions driven by differences in thermal performance among taxa (see SI Appendix, Fig. S4, where we show how complementarity effects among species pairs change along the thermal gradient).
Our experiments highlight the increasing importance of biodiversity for maintaining ecosystem functioning in the face of environmental warming. Warming fundamentally altered the relationship between biodiversity and ecosystem functioning: as temperatures shifted from ambient conditions (either via warming or cooling), diversity–functioning relationships became more linear and less saturating, indicating that functional redundancy declined and more species were required to maintain ecosystem productivity. Our results also provide clear evidence that the impacts of temperature change and biodiversity loss on ecosystem functioning were directly linked to species’ thermal traits. Temperature had a marked impact on the composition of the communities at the end of the experiment, and both the presence/absence of species and their relative abundance were linked to the optimal growth temperature (Fig. 3).
Ecosystem productivity was also positively associated with the average optimum temperature of the taxa used to seed the communities, with the strength of this coupling increasing markedly at high temperatures. This result indicates that thermal traits played a key role in mediating the combined impacts of warming and species loss on ecosystem functioning. While selection effects clearly played a role in determining how the relationship between diversity and ecosystem functioning was altered by temperature change, their impacts were secondary compared with complementarity effects. At 10 °C and 40 °C, total complementarity accounted for 86% and 63% of the net biodiversity effect, respectively (SI Appendix, Table S5). These results suggest that the impacts of temperature change and species loss on ecosystem functioning were primarily linked to temperature-driven shifts in species interactions that were in turn mediated by variability in thermal tolerance traits (SI Appendix, Fig. S4). Indeed, these findings are consistent with recent work indicating that temperature plays a fundamental role in determining the nature and outcome of species interactions in microbial communities (22).
Our results show that as temperatures depart from ambient conditions, functional redundancy rapidly declines. High biodiversity, however, facilitates greater functional complementarity among species that can maintain ecosystem productivity under varying thermal regimes. Our work therefore emphasizes that while functional redundancy may be prevalent under ambient environmental conditions (33), it is likely to rapidly decay when environmental change drives conditions outside of species’ tolerance limits. Overall, our results highlight the critical importance of biodiversity for maintaining the functioning of ecosystems, which face the double-edged sword of declining biodiversity through habitat loss (34), pollution, species invasions, and rapid changes in the abiotic environment brought about by climate change (1).
Materials and Methods
Study Site.
Biofilm samples were collected from the surface of rocks during May 2016 in Hvergerdi Valley, 45 km east of Reykjavik, Iceland. The area contains a large number of mainly groundwater-fed streams that are subjected to differential natural geothermal warming from the bedrock. The temperatures in the sampled streams ranged from 8 °C to 38 °C. Our previous work in this study site has shown that across a wide range of chemical and physical parameters (stream velocity, pH, conductivity, , , ) none correlate significantly with temperature (35). Samples were immediately frozen upon collection with 17% glycerol and transported at −20 °C for further processing in the laboratory.
Environmental Isolation of Bacterial Taxa.
Upon return to the laboratory, samples were thawed at 20 °C and were prepared by spreading 10-L serial dilutions onto R2 agar plates (Oxoid Ltd.) with sterile glass beads. Plates were incubated at 20 °C for 10 d. The resulting colonies were picked at random, placed into 200 L LB broth, and incubated for 48 h. Samples were then centrifuged, the supernatant was removed, and the pellet was resuspended in a mix of LB broth and 17% glycerol before being frozen at −80 °C. Isolates were assigned taxonomy using 16S PCR followed by Sanger sequencing within the 16S rRNA gene (SI Appendix, section 1.1). Using Mothur v.1.39.5 (36), sequences longer than 974 bp were aligned to the Silva.Bacteria.Fasta database, and taxonomy was classified using the Ribosomal Database Project (RDP) trainset 9 032012 and NCBI as a reference database (SI Appendix, Fig. S5 and Table S6). Phylogenetic trees were constructed using iToL (itol.embl.de/) (37). A total of 24 isolates from the 11 different streams were selected for the subsequent experiments.
Species-Level Thermal Tolerance Curves.
The isolates were grown in LB medium overnight immediately after coming out of the −80 °C freezer, then transferred into media made by dissolving 7.6 g of protozoan pellet in 1,000 mL of autoclaved volvic water, and then diluted by a factor of 1/10. Protozoan pellets are made from plant material and contain a diverse range of carbon sources that facilitate bacterial growth and the establishment of a diverse community (38). To characterize the thermal tolerance curves, each species was grown in Percival incubators at 10 temperatures (10 °C, 15 °C, 20 °C, 25 °C, 27.5 °C, 30 °C, 35 °C, 40 °C, 45 °C, and 50 °C) in 96-well plates containing six replicates of each isolate at each temperature. Biomass was estimated by measuring optical density at 600 nm. Growth rates were derived by fitting the logistic growth equation to the biomass time series. Thermal tolerance curves were quantified by fitting the Sharpe–Schoolfield equation (39) to the growth rate data using the methods outlined in Padfield et al. (40) (SI Appendix, section 1.2).
Biodiversity Ecosystem Functioning Experiment.
We assembled the 24 taxa into artificial communities with different levels of species richness (2, 4, 8, 16, and 24). For each richness level we built 10 different replicate communities where the species composition was determined by randomly sampling the 24 species. Each community was then grown at eight different temperatures (10 °C, 15 °C, 20 °C, 25 °C, 27.5 °C, 30 °C, 35 °C, and 40 °C). Stock cultures of all 24 taxa were first grown to stationary phase at each of the experimental temperature treatments and then diluted back to a common biomass density across all taxa and treatments. Experimental communities were then inoculated from these stocks to a standardized target biomass and microcosm volume (24 L) across all treatments, using a substitutive design. For example, at richness 2, 12 L of each species was added, while at richness 4, 6 L of each was added. A summary of the experimental design is given in SI Appendix, Table S7. We used a power function to capture the shape of the relationship between species richness and ecosystem function and quantified the effects of temperature on the exponent and intercept, using a linear mixed-effects model (SI Appendix, section 1.3). At the end of the diversity experiment we reisolated the bacterial taxa from the maximum richness treatments (S 24) that had been exposed to the ambient (20 °C) and the two extreme (10 °C and 40 °C) temperatures to explore the potential mechanisms underlying changes in the diversity–functioning relationship with temperature (SI Appendix, section 1.4).
Effects of Temperature on the Community Composition.
To determine whether the composition of the communities present at the end of the experiment differed significantly between the temperature treatments (10 °C, 20 °C, and 40 °C) we performed a NMDS ordination analysis on the relative abundance of the reisolated taxa. Analyses were performed using the R package “vegan” (41). The analysis was based on a Bray–Curtis dissimilarity matrix derived from the square-root–transformed relative abundances data. NMDS projected this matrix into a new coordinate space with a small number of dimensions (in this case, four) while preserving the original Bray–Curtis dissimilarities among samples to the extent possible. Orthogonal rotation was applied to the axes in this new coordinate space to maximize the variance in “scores” among samples along the first NMDS axis. We performed a permutational multivariate analysis of variance (PERMANOVA) to test whether there were significant differences in community composition between the different temperature treatments, using the “adonis” function from the R package vegan (SI Appendix, Table S4). We then ran separate PERMANOVA analyses for each pair of treatments (e.g., 10–20, 10–40, 20–40) to determine which pairwise treatment contrasts were significantly different (SI Appendix, Table S3). We used the Bonferroni correction to adjust the resultant P values for multiple comparisons.
Linking Thermal Traits to the Impacts of Warming and Species Loss on Ecosystem Functioning.
To explore the coupling between biodiversity, thermal tolerance traits, temperature variation, and ecosystem productivity, we quantified the community-mean thermal optima of the taxa inoculated into each community (at each level of temperature and richness) at the start of the diversity–functioning experiment. We then assessed whether was a significant predictor of ecosystem functioning after accounting for the effects of temperature and species richness using a linear mixed-effect model (SI Appendix, Table S8 and section 1.5).
Partitioning the Impacts of Biodiversity on Ecosystem Function into Selection and Complementarity Effects.
To statistically partition the net effect (NE) of biodiversity into the species-specific selection effect (SE) and the multispecies complementarity effect (CE) we followed the additive partitioning method developed by Loreau and Hector (11). We quantified these effects at both large and local scales, using the methods outlined in Isbell et al. (19). We estimated the local effects, considering each replicate as a “place” (P 10) (SI Appendix, section 1.6).
Supplementary Material
Acknowledgments
This work was supported by an European Research Council (ERC) grant awarded to G.Y.-D. (ERC StG 677278 TEMPDEP).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. MH801963 to MH801986). Files containing processed data used in the study have been deposited in Zenodo (https://doi.org/10.5281/zenodo.1418519).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805518115/-/DCSupplemental.
References
- 1.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 2.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486:105–129. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 3.Loreau M, Mouquet N, Gonzalez A. Biodiversity as spatial insurance in heterogeneous landscapes. Proc Natl Acad Sci USA. 2003;100:12765–12770. doi: 10.1073/pnas.2235465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awasthi A, Singh M, Soni SK, Singh R, Kalra A. Biodiversity acts as insurance of productivity of bacterial communities under abiotic perturbations. ISME J. 2014;8:2445–2452. doi: 10.1038/ismej.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilman D, Downing J, Wedin D. Does diversity beget stability-reply. Nature. 1994;371:114. [Google Scholar]
- 6.Bai Y, Han X, Wu J, Chen Z, Li L. Ecosystem stability and compensatory effects in the inner Mongolia grassland. Nature. 2004;431:181–184. doi: 10.1038/nature02850. [DOI] [PubMed] [Google Scholar]
- 7.Perkins DM, et al. Higher biodiversity is required to sustain multiple ecosystem processes across temperature regimes. Glob Change Biol. 2015;21:396–406. doi: 10.1111/gcb.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowles JM, Wragg PD, Wright AJ, Powers JS, Tilman D. Shifting grassland plant community structure drives positive interactive effects of warming and diversity on aboveground net primary productivity. Glob Change Biol. 2016;22:741–749. doi: 10.1111/gcb.13111. [DOI] [PubMed] [Google Scholar]
- 9.Craven D, et al. Plant diversity effects on grassland productivity are robust to both nutrient enrichment and drought. Philos Trans R Soc B Biol Sci. 2016;371:20150277. doi: 10.1098/rstb.2015.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loreau M. Biodiversity and ecosystem functioning: Recent theoretical advances. Oikos. 2000;91:3–17. [Google Scholar]
- 11.Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;413:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- 12.Cardinale BJ, et al. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci USA. 2007;104:18123–18128. doi: 10.1073/pnas.0709069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilman D, et al. Diversity and productivity in a long-term grassland experiment. Science. 2001;294:843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- 14.Angilletta M, Wilson R, Navas C, James R. Tradeoffs and the evolution of thermal reaction norms. Trends Ecol Evol. 2003;18:234–240. [Google Scholar]
- 15.Huey RB, et al. Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos Trans R Soc B Biol Sci. 2012;367:1665–1679. doi: 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norberg J, et al. Phenotypic diversity and ecosystem functioning in changing environments: A theoretical framework. Proc Natl Acad Sci USA. 2001;98:11376–11381. doi: 10.1073/pnas.171315998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leary DJ, Petchey OL. Testing a biological mechanism of the insurance hypothesis in experimental aquatic communities. J Anim Ecol. 2009;78:1143–1151. doi: 10.1111/j.1365-2656.2009.01586.x. [DOI] [PubMed] [Google Scholar]
- 18.Isbell F, et al. High plant diversity is needed to maintain ecosystem services. Nature. 2011;477:199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- 19.Isbell F, et al. Quantifying effects of biodiversity on ecosystem functioning across times and places. Ecol Lett. 2018;21:763–778. doi: 10.1111/ele.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dell AI, Pawar S, Savage VM. Systematic variation in the temperature dependence of physiological and ecological traits. Proc Natl Acad Sci USA. 2011;108:10591–10596. doi: 10.1073/pnas.1015178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dell AI, Pawar S, Savage VM. Temperature dependence of trophic interactions are driven by asymmetry of species responses and foraging strategy. J Anim Ecol. 2014;83:70–84. doi: 10.1111/1365-2656.12081. [DOI] [PubMed] [Google Scholar]
- 22.Bestion E, García-Carreras B, Schaum CE, Pawar S, Yvon-Durocher G. Metabolic traits predict the effects of warming on phytoplankton competition. Ecol Lett. 2018;21:655–664. doi: 10.1111/ele.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steudel B, et al. Biodiversity effects on ecosystem functioning change along environmental stress gradients. Ecol Lett. 2012;16:1397–1405. doi: 10.1111/j.1461-0248.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- 24.Isbell F, et al. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc Natl Acad Sci USA. 2013;110:11911–11916. doi: 10.1073/pnas.1310880110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Mazancourt C, et al. Predicting ecosystem stability from community composition and biodiversity. Ecol Lett. 2013;16:617–625. doi: 10.1111/ele.12088. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, et al. An invariability-area relationship sheds new light on the spatial scaling of ecological stability. Nat Commun. 2017;8:15211. doi: 10.1038/ncomms15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- 28.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 29.Savage VM, Gillooly JF, Brown JH, West GB, Charnov EL. Effects of body size and temperature on population growth. Am Nat. 2004;163:429–441. doi: 10.1086/381872. [DOI] [PubMed] [Google Scholar]
- 30.Hector A, et al. Plant diversity and productivity experiments in European grasslands. Science. 1999;286:1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- 31.Enquist B, et al. Scaling from traits to ecosystems: Developing a general trait driver theory via integrating trait-based and metabolic scaling theories. Adv Ecol Res. 2015;52:249–318. [Google Scholar]
- 32.Lawrence D, et al. Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 2012;10:e1001330. doi: 10.1371/journal.pbio.1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell T, Newman J, Silverman B, Turner S, Lilley A. The contribution of species richness and composition to bacterial services. Nature. 2005;436:1157–1160. doi: 10.1038/nature03891. [DOI] [PubMed] [Google Scholar]
- 34.Newbold T, et al. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520:45–50. doi: 10.1038/nature14324. [DOI] [PubMed] [Google Scholar]
- 35.Padfield D, et al. Metabolic compensation constrains the temperature dependence of gross primary production. Ecol Lett. 2017;20:1250–1260. doi: 10.1111/ele.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schloss PD, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letunic I, Bork P. Interactive tree of life (itol): An online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 38.Tan J, Pu Z, Ryberg WA, Jiang L. Species phylogenetic relatedness, priority effects, and ecosystem functioning. Ecology. 2012;93:1164–1172. doi: 10.1890/11-1557.1. [DOI] [PubMed] [Google Scholar]
- 39.Schoofield R, Sharpe P, Magnuson C. Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. J Theor Biol. 1981;88:719–731. doi: 10.1016/0022-5193(81)90246-0. [DOI] [PubMed] [Google Scholar]
- 40.Padfield D, Yvon-Durocher G, Buckling A, Jennings S, Yvon-Durocher G. Rapid evolution of metabolic traits explains thermal adaptation in phytoplankton. Ecol Lett. 2016;19:133–142. doi: 10.1111/ele.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oksanen J, et al. 2017 vegan: Community ecology package. R package version 2.4-5 (R Foundation for Statistical Computing, Vienna). Available at https://CRAN.R-project.org/package=vegan. Accessed September 20, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.