Significance
GATA-2 functions in stem and progenitor cells to control blood cell development, and its mutations cause blood diseases (immunodeficiency, myelodysplasia, and myeloid leukemia). How GATA-2 mutations cause these diseases is unclear. We innovated a genetic complementation assay to analyze functional ramifications of GATA-2 disease mutations. The activities of GATA-2 and mutants were quantified in blood progenitor cells from mice engineered to express a low level of GATA-2 due to deletion of an essential Gata2 enhancer. Unexpectedly, the mutants were not only competent to induce myeloid cells, but their activities exceeded that of GATA-2. These results transform the current paradigm that disease mutations are solely inhibitory, and ectopically low GATA-2 levels/activity constitute the disease mechanism.
Keywords: GATA-2, hematopoiesis, MDS, AML, leukemia
Abstract
By inducing the generation and function of hematopoietic stem and progenitor cells, the master regulator of hematopoiesis GATA-2 controls the production of all blood cell types. Heterozygous GATA2 mutations cause immunodeficiency, myelodysplastic syndrome, and acute myeloid leukemia. GATA2 disease mutations commonly disrupt amino acid residues that mediate DNA binding or cis-elements within a vital GATA2 intronic enhancer, suggesting a haploinsufficiency mechanism of pathogenesis. Mutations also occur in GATA2 coding regions distinct from the DNA-binding carboxyl-terminal zinc finger (C-finger), including the amino-terminal zinc finger (N-finger), and N-finger function is not established. Whether distinct mutations differentially impact GATA-2 mechanisms is unknown. Here, we demonstrate that N-finger mutations decreased GATA-2 chromatin occupancy and attenuated target gene regulation. We developed a genetic complementation assay to quantify GATA-2 function in myeloid progenitor cells from Gata2 −77 enhancer-mutant mice. GATA-2 complementation increased erythroid and myeloid differentiation. While GATA-2 disease mutants were not competent to induce erythroid differentiation of Lin−Kit+ myeloid progenitors, unexpectedly, they promoted myeloid differentiation and proliferation. As the myelopoiesis-promoting activity of GATA-2 mutants exceeded that of GATA-2, GATA2 disease mutations are not strictly inhibitory. Thus, we propose that the haploinsufficiency paradigm does not fully explain GATA-2–linked pathogenesis, and an amalgamation of qualitative and quantitative defects instigated by GATA2 mutations underlies the complex phenotypes of GATA-2–dependent pathologies.
Mechanisms underlying the heterogeneous malignancy acute myeloid leukemia (AML) are incompletely understood, and there is a vital need to develop efficacious therapies (1). Although major progress has been made in developing molecularly targeted and transplant therapies, the 5-y survival of geriatric and pediatric AML patients remains at 10–20% and 60–70%, respectively (2). Elucidating how myeloid cell genetic networks are corrupted may unveil opportunities for AML biomarker and therapeutics development. Rigorous studies have defined AML genetic and epigenetic landscapes and the vexing clonal evolution during disease progression (3–9). Germline mutations that predispose to myelodysplastic syndrome (MDS) and AML, such as those disrupting GATA-2 expression and function (10–12), have the potential to reveal clues regarding mechanisms governing disease initiation and progression.
GATA-2 is essential for multilineage hematopoiesis (13), triggers hemogenic endothelium to produce hematopoietic stem cells (HSCs) (14, 15), regulates HSC activity (16–18), and stimulates myelo-erythroid progenitor cell differentiation, proliferation, and survival (19–21). Gata2-null mice exhibit impaired multilineage hematopoiesis and die at ∼embryonic day (E) 10.5 (13). Additional instructive mouse models for analyzing GATA-2 function include the embryonic-lethal Gata2 intronic (+9.5) enhancer mutant with defective HSC genesis (18) and erythroid precursor function (19) and distal (−77) (20) enhancer mutant with defective myelo-erythroid progenitor differentiation. The results with these models, and the finding that GATA-2 overexpression in bone marrow suppresses hematopoiesis (22), indicate that GATA-2 levels/activity must be constrained within a physiological window.
In accord with critical GATA-2 functions discovered in mice, heterozygous human GATA2 mutations are pathogenic and cause immunodeficiency that often progresses to MDS and AML (23, 24). GATA2 mutations also cause other AML-linked familial diseases, and GATA2 is mutated frequently in high-risk MDS (25). GATA2 mutations often occur in the DNA binding C-finger and inhibit DNA binding (26). GATA2 +9.5 enhancer mutations decrease GATA-2 expression (18, 27). In 3q21q26 AML, the −77 enhancer is repositioned next to MECOM encoding the EVI1 oncogene (28, 29). Decreased GATA2, concomitant with elevated EVI1, underlies this malignancy. In addition, GATA-2 overexpression in AML can predict poor prognosis (30). In aggregate, mouse and human data emphasize the need to avert declines and increases in GATA-2, both being pathogenic.
GATA-2 establishes and maintains cell-type–specific genetic networks, and heterozygous GATA2 mutations that reduce GATA-2 levels/activity may differentially affect network integrity in distinct contexts (26). Inadequate or excessive target gene activity would both corrupt networks. Oncogenic Ras-dependent, multisite GATA-2 phosphorylation, coupled with GATA-2–dependent positive autoregulation of GATA2 transcription, can elevate GATA-2 levels/activity and therefore disrupt physiological GATA-2 function (31, 32). Because GATA-2 stimulates AML cell proliferation and survival in vitro (32), elevating or reducing GATA-2 may instigate or contribute to leukemogenesis.
GATA factor C-fingers mediate DNA binding (33, 34), and C-finger mutations impair GATA-2 function (23, 24). Although N-finger function remains enigmatic, N-finger mutations occur in patients with erythroleukemia (35) and AML with biallelic mutation of CEBPA (36, 37). The N-finger was reported to bind DNA with sequence-specificity in vitro (38, 39). The GATA-1 N-finger binds the critical coregulator FOG-1 (40). This interaction is mediated by GATA-1 V205 (40), and V205 mutation disrupts erythroid maturation in mice and generates familial dyserythropoietic anemia in humans (41). Although the GATA-1 and GATA-2 N-fingers are well conserved, GATA-2–expressing hematopoietic stem and progenitor cells (HSPCs) do not express FOG-1. R216 mutations in patients with X-linked gray platelet syndrome (42) attenuate GATA-1 function without influencing FOG-1 binding (43). This mutation reduces binding to sites containing single or palindromic GATA motifs. Analogous GATA-2 (R307W) and GATA-3 (R276) residues can be mutated in leukemia patients. Because the GATA-2 N-finger can be mutated in leukemia, and unlike GATA-1, FOG-1 is not expressed in the GATA-2–expressing cells, dissecting molecular consequences of these mutations has the potential to inform GATA factor mechanisms and pathologies. Herein, we analyzed the mechanistic ramifications of N-finger disease mutations in diverse systems, including a genetic complementation assay to quantify GATA-2 function in Gata2 −77 enhancer mutant primary myelo-erythroid progenitor cells. Our discovery that GATA-2 disease mutations unexpectedly enhance select GATA-2 functions in primary cells demands a reconsideration of the paradigm that inhibitory disease mutations strictly decrease GATA-2 levels/activity. These results provide a perspective into the haploinsufficiency model of GATA-2-linked pathologies.
Results
GATA-2 N-Finger Increases GATA-2 Endogenous Target Gene Chromatin Occupancy and Activation.
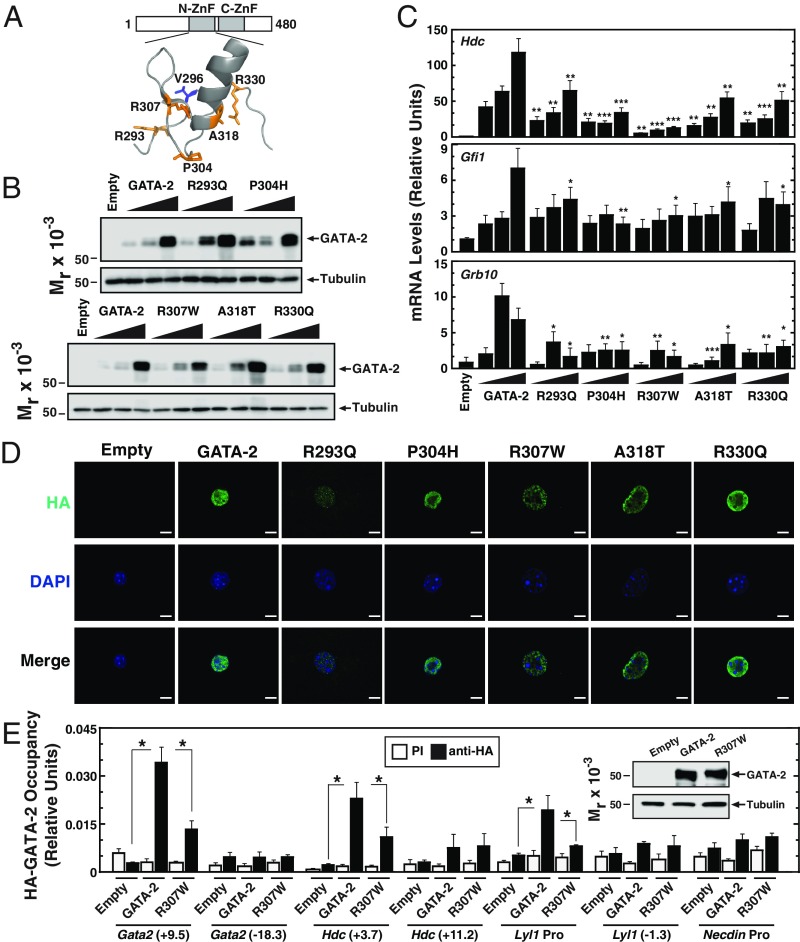
Because human disease mutations can unveil unique mechanistic insights, we analyzed the functional consequences of GATA-2 N-finger mutations (R293Q, P304H, R307W, A318T, and R330Q) (Fig. 1A) from AML patients in a mouse aortic endothelial (MAE) cell assay (31, 32) in which exogenous GATA-2 expression activates endogenous target genes. Increasing levels of murine GATA-2 (98% sequence identity to human) or mutants were expressed in MAE cells, ensuring that mutants were analyzed at levels resembling that of GATA-2 (Fig. 1B). While GATA-2 activated the target genes Hdc, Gfi1, and Grb10 (14, 19, 20, 31), N-finger leukemia mutations attenuated the transcriptional response (Fig. 1C).
Fig. 1.
GATA-2 N-finger leukemia mutations attenuate chromatin occupancy and target gene activation. (A) GATA-2 structural model based on human GATA-3 crystal structure. (B) Representative Western blot analysis with anti-HA antibody of wild-type and mutant proteins transiently expressed in MAE cells. (C) qRT-PCR analysis of mRNA levels of GATA-2 target genes in MAE cells transiently expressing HA–GATA-2 and mutant proteins (n = 6). (D) Immunofluorescence analysis with anti-HA antibody in MAE cells expressing HA–GATA-2 and mutant proteins. (Scale bars, 10 μm.) (E) Quantitative ChIP analysis of HA–GATA-2 in MAE cells transiently expressing HA–GATA-2 or HA-R307W (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001.
To elucidate the mechanism underlying the compromised activity of N-finger mutants, we tested whether the subcellular localization of the mutants resembles or differs from that of GATA-2. Immunofluorescence analysis indicated that GATA-2 and the N-finger mutants were exclusively nuclear-localized in MAE cells (Fig. 1D). Using a quantitative chromatin immunoprecipitation (ChIP) assay with anti-HA antibody, we compared GATA-2 and R307W N-finger mutant activities to occupy chromatin. R307W was analyzed, because this mutation strongly inhibited target gene induction. The R307W mutation decreased, but did not abrogate, GATA-2 occupancy at the Gata2 +9.5 enhancer (18, 44), Hdc +3.7 (31), and Lyl1 promoter (45) loci (Fig. 1E). GATA-2 did not occupy Gata2 −18.3, Hdc +11.2, Lyl1 −1.3, and Necdin promoter sites, providing evidence for specificity of chromatin occupancy. These results indicate that GATA-2 N-finger integrity promotes chromatin occupancy at target genes without grossly changing nuclear localization.
Structure-Based Design of GATA-2 Mutants to Dissect GATA-2 Leukemia Mutant Mechanisms.
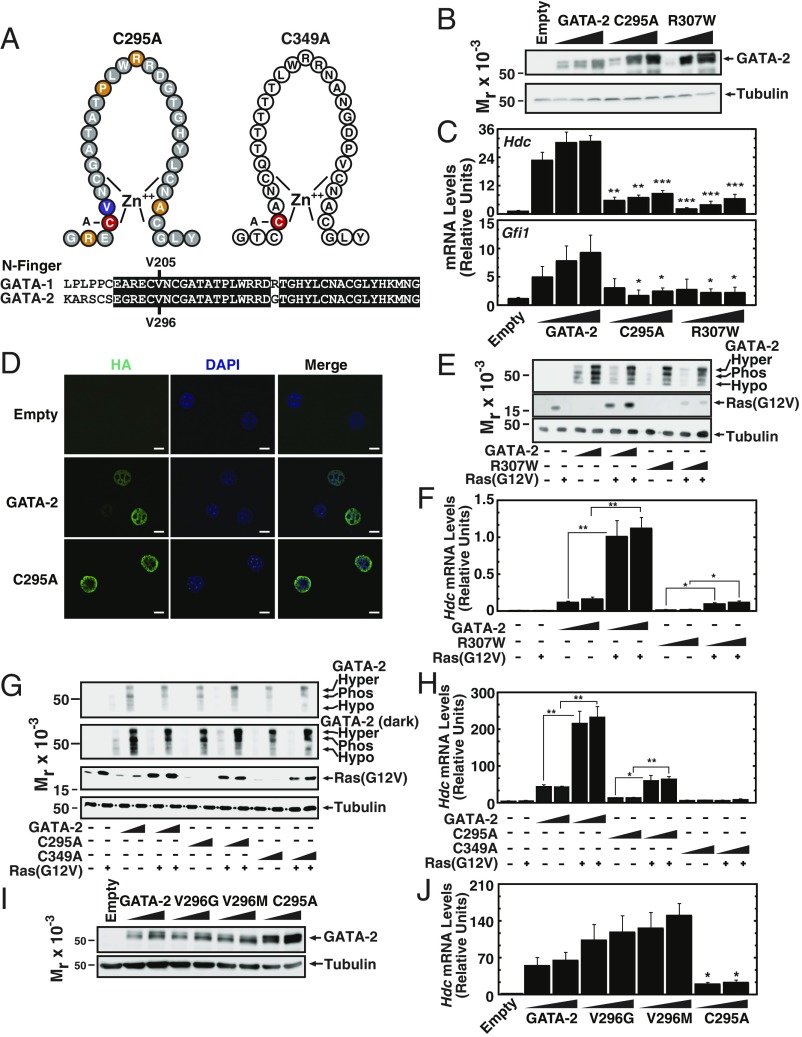
GATA factor N-fingers are highly conserved (Fig. 2A), implying important functions. To assess whether the inhibitory consequences of N-finger leukemia mutants are recapitulated by a mutation that disrupts zinc finger structure, C295A was engineered into GATA-2 (Fig. 2A). Under conditions in which GATA-2, C295A, and R307W were expressed at comparable levels (Fig. 2B), both C295A and R307W significantly decreased induction of GATA-2 target gene expression in MAE cells (Fig. 2C). Similar to the N-finger disease mutants, C295A was exclusively nuclear-localized (Fig. 2D).
Fig. 2.
Structural determinants of GATA-2 function. (A, Upper) Schematic representation of C295A and C349A mutants. (Lower) Murine GATA-1 and GATA-2 N-finger amino acid sequences. (B) Representative Western blot analysis with anti-HA antibody of MAE cells transiently expressing HA–GATA-2 or mutant proteins. (C) qRT-PCR analysis of mRNA levels of GATA-2 target genes in MAE cells transiently expressing HA–GATA-2 or mutant proteins (n = 4). (D) Immunofluorescence analysis with anti-HA antibody in MAE cells expressing HA–GATA-2 or the C295A mutant. (Scale bars, 10 μm.) (E) Representative Western blot analysis with anti-HA antibody of HA–GATA-2 and HA–R307W transiently expressed in MAE cells with or without Ras(G12V). (F) qRT-PCR analysis of mRNA levels of Hdc genes in MAE cells transiently expressing HA–GATA-2 and R307W with or without Ras(G12V) (n = 5). (G) Representative Western blot analysis with anti-HA antibody of HA–GATA-2 or mutant proteins transiently expressed in MAE cells with or without Ras(G12V). This Western blot utilized a large SDS/PAGE gel to achieve maximal isoform separation. (H) qRT-PCR analysis of Hdc mRNA levels in MAE cells transiently expressing HA–GATA-2 or mutant proteins with or without Ras(G12V) (n = 3). (I) Representative Western blot analysis with anti-HA antibody of MAE cells expressing HA–GATA-2 or mutant proteins. (J) qRT-PCR analysis of Hdc mRNA levels in MAE cells transiently expressing HA–GATA-2 or mutant proteins (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001.
Previously, we demonstrated that Ras signaling induces multisite GATA-2 phosphorylation and increases GATA-2–dependent transcriptional activation (31, 32). We tested whether N-finger leukemia mutants are competent to respond to Ras signaling. Expression of Ras(G12V) shifted GATA-2 migration to a slow-mobility species (2.2-fold increase), which we demonstrated previously to be a hyperphosphorylated isoform (Fig. 2E). Ras(G12V) also induced a mobility shift with R307W (2.4-fold increase) and increased its capacity to activate Hdc expression sevenfold, comparable to the fold-induction achieved with Ras(G12V)/GATA-2 (Fig. 2 E and F). Thus, although R307W-mediated transcriptional activation was strongly compromised, it remained competent to respond to Ras(G12V).
Because the C-finger mediates DNA binding (33), and N-finger function in vivo is unresolved, it was instructive to compare the consequences of cysteine mutations in the N- and C-fingers. We engineered C349A to disrupt C-finger structure (Fig. 2A) and analyzed its activity in MAE cells. The predominant C349A isoform exhibited a slow mobility, even without Ras(G12V) expression, and the intensity of this isoform was 2.3-fold greater than that of GATA-2 (Fig. 2G). This is consistent with our report that the T354M disease mutant linked to MDS/AML (11, 12) exhibits reduced chromatin binding and is predominantly hyperphosphorylated without Ras(G12V) (31); however, multisite phosphorylated T354M does not increase target gene expression, based on impaired chromatin binding. Ras(G12V) also induced the slow mobility C295A isoform 1.7-fold (Fig. 2G). Whereas Ras(G12V) increased GATA-2– and C295A-dependent Hdc induction fivefold, C349A was inactive in the absence or presence of Ras(G12V) (Fig. 2H), consistent with the disrupted C-finger that would not be competent for chromatin binding.
GATA-1 V205 binds FOG-1, which mediates transcriptional activation and repression of many GATA-1 target genes (40, 46, 47). FOG-1 is not expressed in GATA-2–expressing cells (e.g., MAE cells) and does not mediate GATA-2 biological activity in HSPCs. In principle, the GATA-2 residue equivalent to GATA-1 V205 might mediate transcriptional regulation through a related or novel mechanism. We tested this model by engineering mutations at GATA-2 V296, equivalent to GATA-1 V205, and analyzing its activity in MAE cells at expression levels resembling GATA-2 (Fig. 2I). V296G and V296M retained the capacity to induce Hdc (Fig. 2J). Thus, GATA-2 N-finger–dependent transcriptional activation is impaired by leukemia mutations, requires N-finger structure, and has distinct molecular determinants from the GATA-1 N-finger.
GATA-2 Genetic Complementation Assay with Gata2 −77 Enhancer Mutant Progenitor Cells: GATA-2 Leukemia Mutants Promote Myelopoiesis.
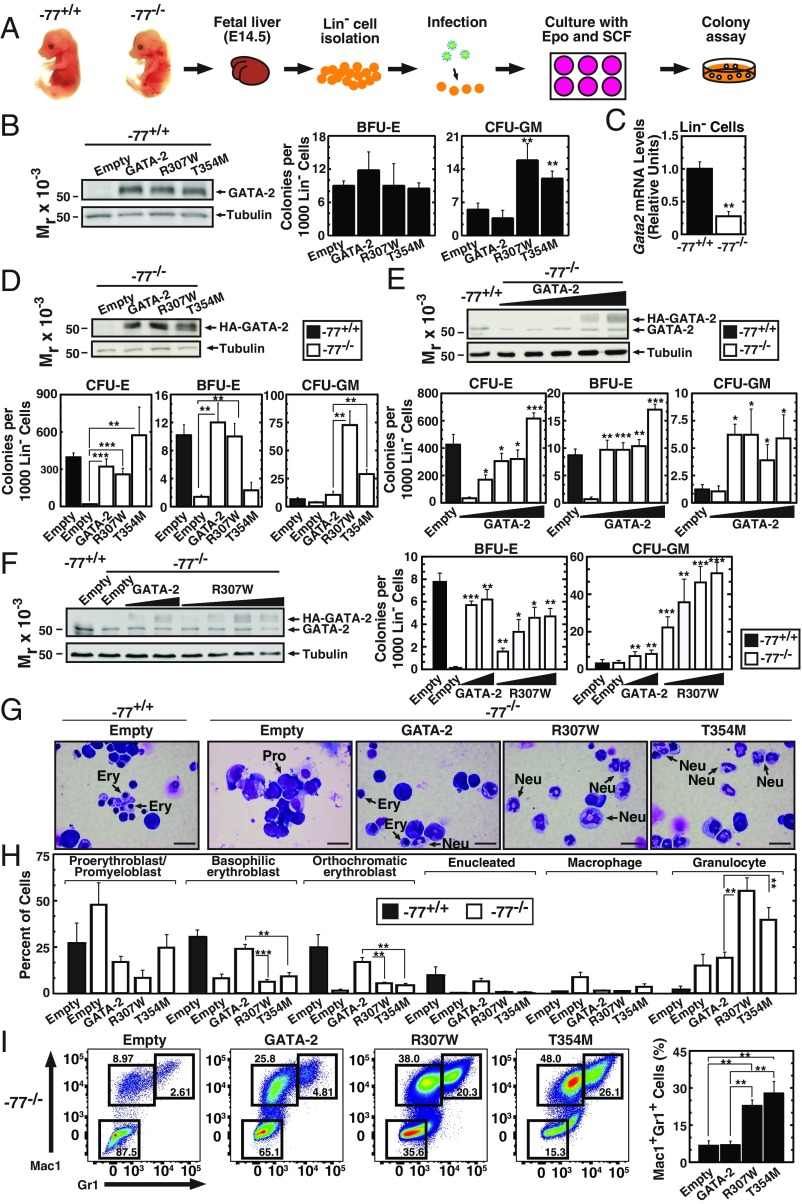
To elucidate the functional consequences of GATA-2 mutations, we compared the activity of expressed wild-type and mutant GATA-2 in primary mouse bone marrow and fetal liver HSPCs. GATA-2 or R307W expression in −77+/+ bone marrow cells decreased CFU-GM (SI Appendix, Fig. S1), consistent with the report that GATA-2 overexpression suppresses bone marrow HSPCs (22). We also expressed wild-type or mutant GATA-2 in mouse fetal liver lineage-negative (Lin−) hematopoietic precursors (Fig. 3 A and B). Although we predicted that R307W and the T354M C-finger leukemia mutant would be inactive or have attenuated activity, unexpectedly, they increased CFU-GM in −77+/+ Lin− fetal liver cells (Fig. 3B, Right).
Fig. 3.
GATA-2 leukemia mutants increase myeloid progenitor cell activity in a primary cell genetic complementation assay. (A) Schematic representation of genetic complementation assay. (B, Left) Representative Western blot analysis of −77+/+ fetal liver cells expressing HA–GATA-2 with anti-HA antibody. (Right) Quantitative analysis of CFU activity of −77+/+ fetal liver cells (n = 3). (C) qRT-PCR analysis of Gata2 mRNA levels in Lin− cells from wild-type or −77−/− fetal livers (n = 5). (D, Upper) Representative Western blot analysis with anti-HA antibody of −77−/− fetal liver cells expressing HA–GATA-2 or mutants. (Lower) Quantitative analysis of CFU activity of −77−/− fetal liver cells (n = 7). −77+/+ fetal liver cells infected with control vector were used as control. (E, Upper) Representative Western blot analysis with anti–GATA-2 antibody (recognizes both HA–GATA-2 and endogenous GATA-2) of −77−/− fetal liver cells expressing HA–GATA-2. (Lower) Quantitative analysis of CFU activity of −77−/− fetal liver cells (n = 3). −77+/+ fetal liver cells infected with control vector were used as control. (F, Left) Representative Western blot analysis with anti-GATA-2 antibody of −77−/− fetal liver cells expressing HA–GATA-2 or R307W. (Right) Quantitative analysis of CFU activity of −77−/− fetal liver cells (n = 4). −77+/+ fetal liver cells infected with control vector were used as control. Significance relative to −77−/− cells infected with empty vector was evaluated. (G) Representative image of Giemsa-stained cells from colonies. (Scale bars, 20 μm.) Ery, erythroblasts; Mac, macrophage; Neu, neutrophil; Pro, proerythroblast/promyeloblast. (H) Quantification of Giemsa stain (n = 3). (I) Flow cytometric analysis of cells isolated from colonies and stained with Gr1 and Mac1 (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001.
Because human GATA-2 disease mutations are heterozygous (23, 24), expressing GATA-2 mutants in wild-type (−77+/+) hematopoietic progenitors is not optimal for elucidating physiological or pathological mechanisms. We therefore devised a genetic complementation assay using −77−/− fetal liver myelo-erythroid progenitor cells (20), in which endogenous GATA-2 expression is reduced. It was not possible to use −77−/− bone marrow, because this homozygous mutation is embryonic-lethal (20). Genetic complementation analysis in mutant cells is a powerful strategy to dissect mechanisms, as exemplified by studies with GATA-1 (48–51). While the MAE system enables quantification of GATA-2 activity to regulate endogenous target genes (31), no GATA-2 genetic complementation systems have been described. Using Lin− myelo-erythroid progenitor cells, we compared GATA-2 and mutant activities to induce myeloid and erythroid differentiation of −77−/− cells. The −77 enhancer deletion decreased Gata2 expression by 69% (Fig. 3C).
As described previously (20), −77−/− Lin− myelo-erythroid progenitor cells have little to no capacity to generate erythroid colonies (CFU-E and BFU-E) (Fig. 3D). Wild-type progenitors formed very few myeloid colonies (CFU-GM) (Fig. 3D), which reflected the 1-d culture with erythroid factors postinfection and differed from our report in which wild-type progenitors were analyzed without culturing generate large numbers of CFU-GM (20). HA–GATA-2 expression rescued CFU-E and BFU-E, and increased CFU-GM (Fig. 3D). Comparison of GATA-2, R307W, and T354M activities revealed that R307W, but not T354M, resembled GATA-2 in rescuing BFU-E at similar expression levels (Fig. 3D). Unexpectedly, this analysis revealed that R307W and T354M increased CFU-GM 7- and 2.5-fold greater than GATA-2 (Fig. 3D). To test whether rescue involved GATA-2 expression at levels resembling endogenous GATA-2 or overexpression, we titrated the GATA-2–expressing retrovirus and analyzed HA–GATA-2 and endogenous GATA-2 expression simultaneously with anti–GATA-2 antibody. HA–GATA-2 levels that rescued CFU-E and BFU-E and elevated CFU-GM were comparable to that of endogenous GATA-2 (Fig. 3E). Furthermore, the level of R307W that strongly induced CFU-GM was comparable to that of endogenous GATA-2 (Fig. 3F). These results highlight the unique utility of the genetic complementation assay for qualitatively and quantitatively analyzing GATA-2–dependent progenitor activity. Importantly, the assay was conducted under conditions in which ectopically introduced wild-type or mutant GATA-2 was expressed at physiological GATA-2 levels.
Because R307W and T354M increased the percentage of CFU-GM among the colonies from 37% to 86% (R307W, P = 0.0014) or 92% (T354M, P = 0.0009), we analyzed colony cellularity. Granulocytes were more abundant in colonies derived from R307W- or T354M-expressing progenitors, in comparison with colonies derived from GATA-2–expressing progenitors (Fig. 3 G and H). Flow cytometric analysis revealed that R307W or T354M increased Mac1+Gr1+ myeloid cells (Fig. 3I).
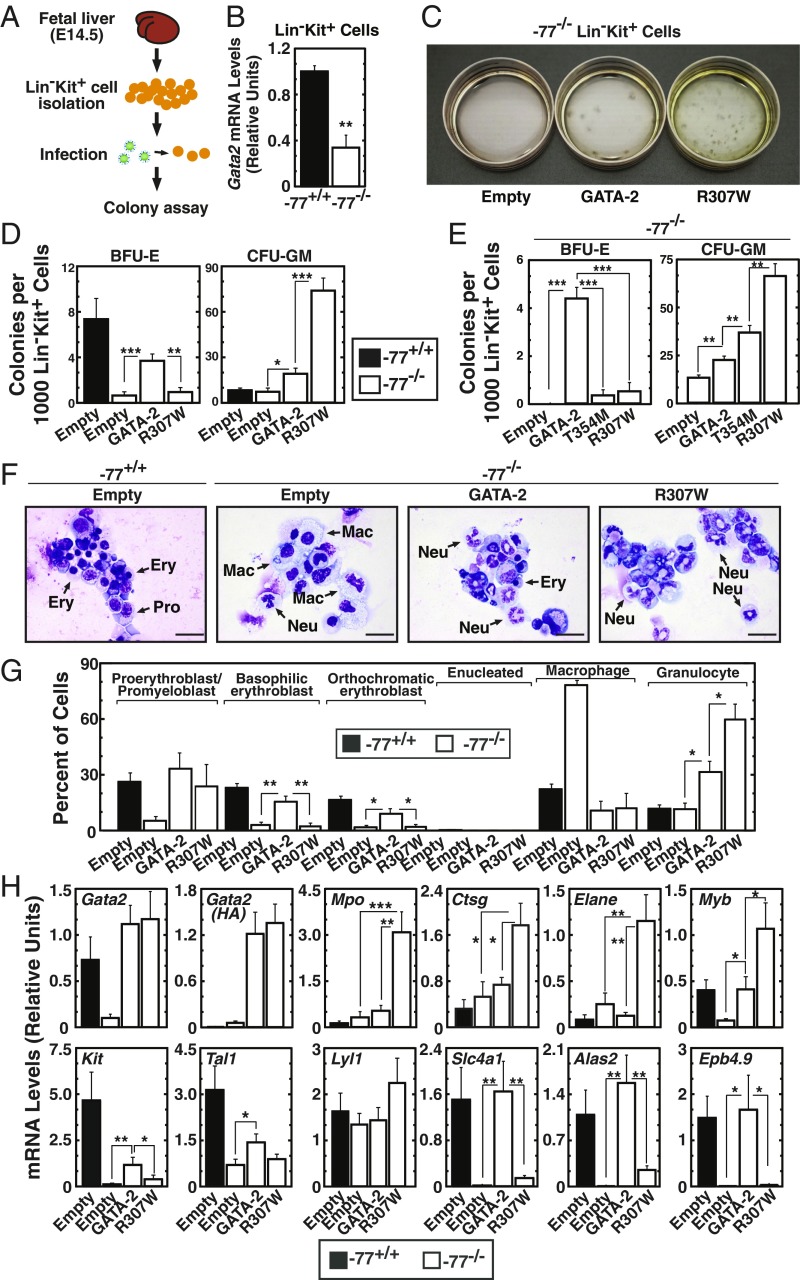
By eliminating the culture step with Epo and stem cell factor, which favors erythroid precursors at the expense of myelo-erythroid progenitors, we improved the genetic complementation assay for analyzing both erythroid and myeloid differentiation. We also utilized a more refined myelo-erythroid progenitor population, FACS-purified Lin−Kit+ myelo-erythroid progenitors, rather than the bead-sorted Lin− population. CFU activity was assayed immediately after Lin−Kit+ cell isolation to minimize the probability of cellularity transitions ex vivo (Fig. 4A). Gata2 expression was threefold lower in −77−/− progenitors in comparison with wild-type progenitors (Fig. 4B). Resembling Lin− cells, −77−/− Lin−Kit+ cells produced almost no colonies (Fig. 4C). GATA-2 expression elevated BFU-E and CFU-GM 6.4- and 2.6-fold, respectively (Fig. 4D). While R307W did not rescue BFU-E, it increased CFU-GM 3.9-fold relative to the GATA-2 control (Fig. 4D). T354M also increased CFU-GM but not BFU-E (Fig. 4E). Morphological analysis revealed only rare erythroid cells in colonies from R307W-expressing −77−/− myeloid progenitors in comparison with GATA-2–expressing progenitors (Fig. 4 F and G). Macrophages were abundant in −77−/− cells infected with empty vector, as described (20). Thus, R307W preferentially induces granulocytes. The distinct morphology of the cells derived from these −77−/− Lin−Kit+ progenitors, in comparison with the −77−/− Lin− cells (Fig. 3) reflected the assay modification in which the progenitors analyzed in Fig. 4 were immediately subjected to colony assay, rather than cultured for 1-d postinfection. Under both conditions, however, R307W induced granulocytes.
Fig. 4.
GATA-2 leukemia mutants stimulate myelopoiesis ex vivo. (A) Schematic representation of rescue assay with Lin−Kit+ cells. (B) qRT-PCR analysis of Gata2 mRNA levels in Lin−Kit+ cells from wild-type or −77−/− fetal livers (n = 3). (C) Representative image of the dishes subjected to CFU assay. (D) Quantitative analysis of CFU activity of −77−/− myeloid progenitor cells. −77+/+ fetal liver cells infected with control vector were used as control (n = 8). (E) Quantitative analysis of CFU activity of −77−/− Lin−Kit+ cells (n = 3). (F) Representative image of Giemsa-stained cells from colonies derived from Lin−Kit+ cells. (Scale bars, 20 μm.) Ery, erythroblasts; Mac, macrophage; Neu, neutrophil; Pro, proerythroblast/promyeloblast. (G) Quantification of Giemsa stain (n = 4). (H) qRT-PCR analysis of mRNA levels in cells isolated from colonies (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001.
Because R307W and T354M chromatin binding is reduced, based on ChIP analysis at select target genes, and they increased CFU-GM, we asked whether GATA-2 DNA binding activity is inconsequential for increasing CFU-GM. We tested C349A, in which the cysteine mutation destroys the structural integrity of the DNA-binding C-finger. C349A had little to no activity in the genetic complementation assay, using CFU as a read-out (SI Appendix, Fig. S2 A and B), suggesting that C-finger structure and DNA binding competence is required to induce CFU-GM. To assess whether additional GATA-2 leukemia mutants induce CFU-GM, we analyzed GATA-2 A318T, which has been detected more frequently than R307W. A318T induced CFU-GM in Lin− cells and Lin−Kit+ cells (SI Appendix, Fig. S2 C–E), and granulocytes were more abundant in colonies from −77 Lin− cells and −77 Lin−Kit+ cells (SI Appendix, Fig. S2 F and G). Thus, all GATA-2 leukemia mutants tested gain a function to increase CFU-GM, differing from GATA-2 and the inactive C349A mutant.
The gain-of-function myelopoiesis-stimulating activity of GATA-2 leukemia mutants was surprising, given the paradigm that mutations create insufficient GATA-2 activities/levels to control stem and progenitor cell transitions and function. In certain cases, cis-element mutations in MDS/AML decrease GATA-2 expression, consistent with haploinsufficiency. However, because elevated GATA-2 would deregulate target genes, which include multiple disease-linked genes (14, 19, 20, 32), and can correlate with poor prognosis of AML (30), ectopically low or high GATA-2 levels/activity will disrupt the integrity of genetic networks that ensure normal hematopoiesis.
Gene-expression analysis in R307W-expressing cells isolated from colonies revealed elevated myeloid gene (Mpo, Ctsg, and Elane) and reduced erythroid gene (Slc4a1, Epb4.9, and Alas2) expression (Fig. 4H). Genes expressed in HSPCs and erythroid cells (e.g., Myb, Kit, and Tal1) were reduced in −77−/− cells, and GATA-2 rescued expression. While R307W did not affect Tal1 expression, it was more effective than GATA-2 in elevating Myb expression (Fig. 4H). These results reinforce the conclusion from CFU analysis that the leukemia mutants are more effective than GATA-2 in inducing CFU-GM.
GATA-2 Leukemia Mutant Stimulates Myelo-Erythroid Progenitor Cell Cycle Progression.
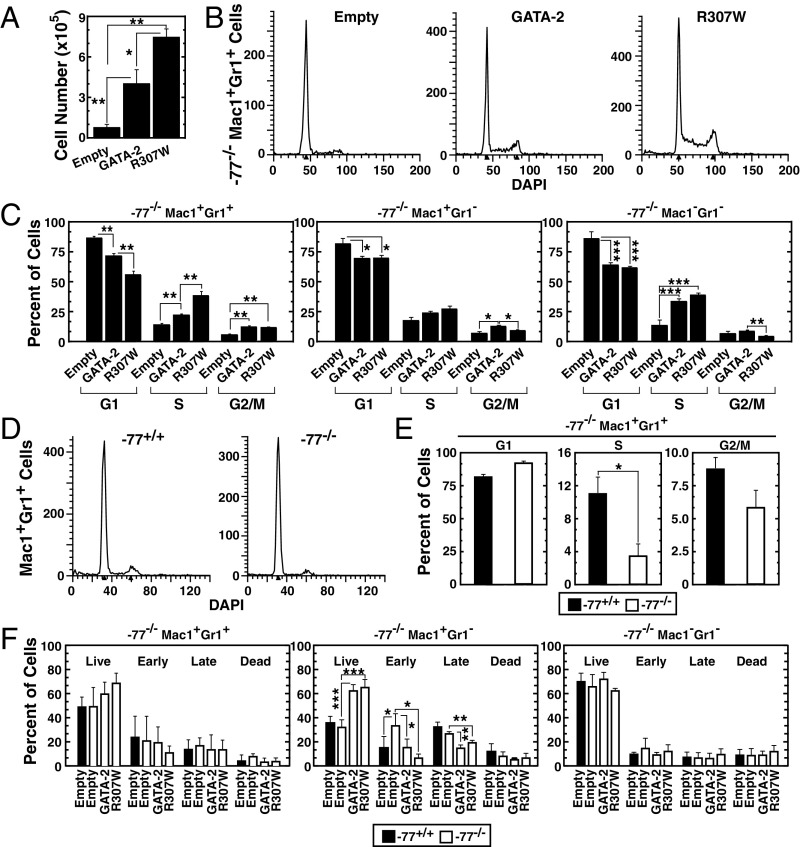
Consistent with the increased colony number, GATA-2 or R307W expression significantly increased the number of cells within colonies. R307W increased cell numbers twofold greater than GATA-2 (Fig. 5A). Because GATA-2 (21, 52) and select target genes regulate cell cycle progression, and R307W has strong activity to induce granulocytes, we compared GATA-2 and R307W activities to impact cell cycle progression in the −77−/− Lin−Kit+ cell genetic complementation assay. GATA-2 or R307W expression significantly increased S and G2/M phase cells in the Mac1+Gr1+ myeloid cell population (Fig. 5 B and C). A greater percentage of R307W-expressing cells resided in S phase in comparison with GATA-2–expressing cells. R307W was not more effective than GATA-2 in stimulating cell cycle progression of Mac1+Gr1− cells or Mac1−Gr1− cells (Fig. 5C). The Mac1+Gr1+ myeloid cell population in S phase decreased significantly in −77−/− vs. −77+/+ cells (Fig. 5 D and E). Cell survival was analyzed in the −77−/− Lin−Kit+ cell genetic complementation assay. Although altered survival was not detected in Mac1+Gr1+ cells and Mac1−Gr1− cells, GATA-2 or R307W expression significantly increased live cells in Mac1+Gr1− cells (Fig. 5F). Analysis of the erythroid cell population revealed the loss of this population in −77−/− cells. However, there was no obvious change in survival of R1 population erythroid precursor cells (SI Appendix, Fig. S3). This analysis indicates that GATA-2 stimulates cell cycle progression in −77−/− myelo-erythroid progenitor cells, and while the leukemia mutant not only retains this activity, its activity can exceed that of GATA-2.
Fig. 5.
GATA-2 leukemia mutant increases cell cycle progression in a genetic complementation assay. (A) Quantification of cell number in colonies derived from −77−/− Lin−Kit+ cells infected with empty vector, GATA-2 expression vector, or R307W expression vector. (B) Flow cytometric analysis of cell cycle in −77−/− Mac-1+Gr-1+ cells isolated from colonies. (C) Quantification of cell cycle status (n = 5). (D) Flow cytometric analysis of cell cycle in −77+/+ Mac-1+Gr-1+ cells and −77−/− Mac-1+Gr-1+ cells. (E) Quantification of cell cycle status (n = 4). (F) Quantification of early apoptotic (Annexin V+DRAQ7−), late apoptotic (Annexin V+DRAQ7+), and dead cells (Annexin V−DRAQ7+) by flow cytometry (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The paradigm for how GATA2 mutations instigate MDS/AML involves haploinsufficiency (27): inadequate GATA-2 production to fulfill its requirement to establish/maintain genetic networks that govern HSPC transitions. Heterozygous GATA2 coding region mutations inhibit DNA binding or corrupt the ORF, while +9.5 enhancer mutations reduce GATA2 expression. Both classes of mutations yield subphysiological GATA-2 levels. High-level GATA-2 expression in humans has been correlated with poor prognosis of AML (30), and GATA-2 overexpression can suppress bone marrow hematopoiesis in mice (22).
We devised a genetic complementation assay that enables quantification of GATA-2–dependent myelo-erythroid progenitor differentiation, endogenous target gene regulation, and cellular functions. We discovered that GATA-2 mutants predicted to be inactive or to have attenuated activity retain activity in primary cells. Of particular interest were their activities to induce granulocytes and stimulate cell cycle progression, which exceeded that of GATA-2. Whereas GATA-2 induced −77−/− myeloid progenitor cells to produce both erythroid and myeloid cells, R307W selectively increased granulocytes (Fig. 6). Thus, leukemia mutations corrupt, without abrogating, GATA-2 function. It is therefore instructive to consider the relationship between our findings and AML pathogenesis involving GATA2 mutations. We propose that insufficient or elevated GATA-2 levels/activity corrupt GATA-2–dependent genetic network integrity, and both GATA-2 loss-of-function and gain-of-function may constitute pathogenic mechanisms.
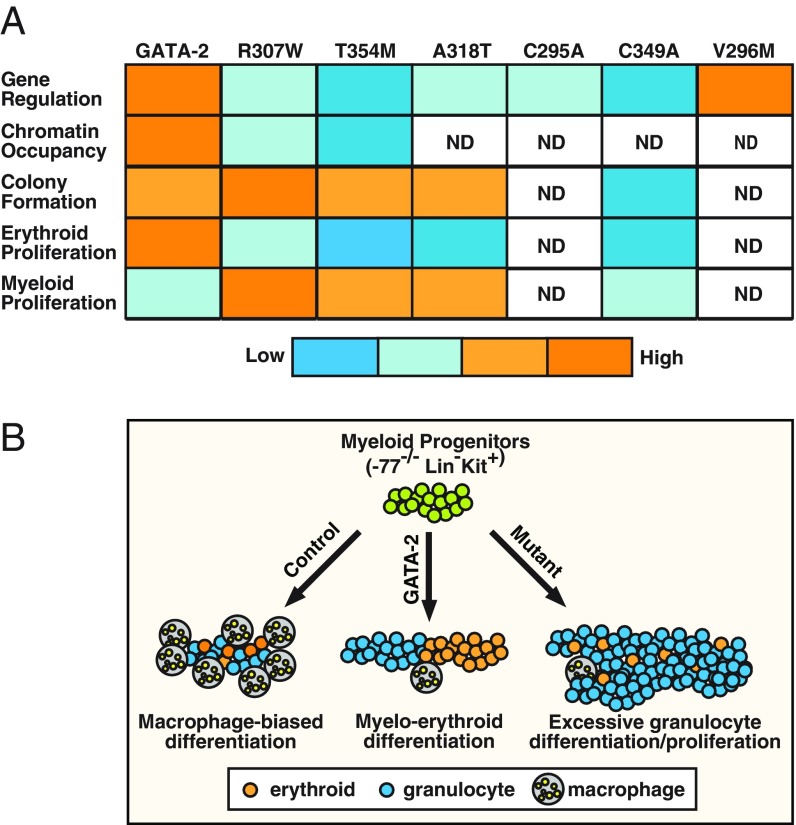
Fig. 6.
GATA-2 leukemia mutations: gain-of-function and loss-of-function consequences. (A) Molecular activities of wild-type and mutant GATA-2. DNA binding capacity of T354M was described previously (31). ND, not determined. (B) The −77−/− myeloid progenitor cells differentiate preferentially into macrophages ex vivo. While GATA-2 expression induces erythroid cells and granulocytes, GATA-2 leukemia mutants stimulate granulocyte differentiation and proliferation, and this activity can exceed that of GATA-2. Thus, GATA-2 leukemia mutants exhibit a gain-of-function activity to stimulate myelopoiesis, with a concomitant loss of activity to stimulate erythropoiesis.
Although the functional consequences of GATA-2 N-finger mutations detected in a subset of AML patients were unclear, prior studies implicated the GATA-1 N-finger in DNA binding in certain contexts (38). However, whether the N-finger is an essential determinant of chromatin occupancy in physiological contexts is uncertain. Herein, we demonstrated that N-finger leukemia mutations resembled C-finger mutations in attenuating GATA-2 chromatin binding and target gene activation. However, analysis of C295A and C349A mutations that disrupt N- and C-finger structure, respectively, indicated that N-finger mutants were more effective than C-finger mutants in activating target genes. N-finger, but not C-finger, mutants were responsive to Ras(G12V)-mediated GATA-2 activation. These results highlight functional differences in N- and C-finger mutants.
C-finger mutants have been described as germline mutations in familial MDS/AML (10–12). Somatic N-finger mutations were reported in patients with acute erythroid leukemia (35) and AML with biallelic mutation of CEBPA (53). Analyses of familial MDS/AML have identified the co-occurrence of T354M with acquired ASXL1 mutations (54, 55). Other mutations reported to co-occur with GATA2 mutations include NRAS, WT1, and DDX41, among others (56, 57). In addition, CDC25C mutations in familial platelet disorder, which predisposes to AML, can involve subsequent GATA2 somatic mutations (58). Further studies with large patient cohorts are required to rigorously analyze genotype–phenotype relationships.
The GATA-1 N-finger is an essential determinant of GATA-1 function (40). V205 mediates FOG-1 binding and FOG-1–dependent transcriptional activation and repression (40). While R216 does not impact FOG-1 binding, it contributes to the regulation of select target genes (43). Our study revealed that GATA-2 R307, the structural equivalent of GATA-1 R216, is critical for GATA-2 function, while GATA-2 V296, which is equivalent to GATA-1 V205, is dispensable for GATA-2–mediated Hdc induction. GATA-1 R216 is important for GATA-1 recognition of palindromic GATA-motifs (59), and in vivo analysis demonstrated that R216 mutation decreased occupancy at sites containing single GATA-motifs (60). Our studies revealed that GATA-2 R307W strongly increased CFU-GM. Mutations of this conserved arginine of GATA-1, GATA-2, and GATA-3 were described in hematologic malignancy and anemia patients.
How do GATA-2 leukemia mutants induce myeloid cell proliferation despite their crippled transcriptional activity? It is instructive to consider the consequences of mutating GATA-1 V205, which strongly reduces GATA-1–mediated activation and repression of target genes that require FOG-1, the majority of GATA-1 target genes. V205 mutations inhibit chromatin occupancy at select target genes and induce ectopic chromatin occupancy at sites not normally occupied by GATA-1 (47). This chromatin redistribution mechanism may have broader applicability to transcription factor mutations that influence DNA sequence-specificity or coregulator–transcription factor interactions and therefore indirectly impact chromatin occupancy. Because various factors are implicated in binding GATA factors, in principle, mutations that impact chromatin occupancy might inhibit or enhance such interactions, thereby altering networks established/maintained by interactors. In addition, DNA binding-impaired GATA factor mutants might retain the capacity to be recruited into chromatin complexes via protein–protein interactions. Regardless of these potential mechanisms, it will be instructive to consider the relationship between the unexpected myelopoiesis-inducing activity resulting from human GATA-2 disease mutations and leukemogenesis.
Materials and Methods
Cell Culture.
MAE cells (31) were maintained in medium 200 supplemented low-serum growth supplement (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific). Cells were transfected with Nucleofector II (Lonza). Fetal liver hematopoietic precursor cells were cultured in StemPro-34 (Thermo Fisher Scientific) with 1× nutrient supplement with 2 mM glutamax (Thermo Fisher Scientific), 1% penicillin/streptomycin (Thermo Fisher Scientific), 100 μM monothioglycerol (Sigma), 1 μM dexamethasone (Sigma), 0.5 U/mL of erythropoietin, and 1% conditioned medium from a Kit ligand-producing Chinese hamster ovary (CHO) cells. Cells were cultured in a humidified incubator at 37 °C and 5% carbon dioxide (61).
Plasmids.
Murine GATA-2 cDNA was cloned into pcDNA4TOFHA vector (kindly provided by Danny Reinberg, New York University, New York) and pMSCV vector (kindly provided by Mitchell Weiss, St. Jude Children’s Research Hospital, Memphis, TN). XZ-201Ras(G12V) was kindly provided by Jing Zhang, University of Wisconsin–Madison, Madison, WI.
Quantitative Real-Time RT-PCR.
Total RNA was purified with TRIzol (Thermo Fisher Scientific). cDNA was prepared by annealing 1 μg of RNA with 250 ng of a 1:5 mixture of random hexamer and oligo (dT) primers heated at 68 °C for 10 min. This was followed by incubation with Murine Moloney Leukemia Virus Reverse Transcriptase (Thermo Fisher Scientific) with 10 mM DTT, RNAsin (Promega), and 0.5 mM dNTPs at 42 °C for 1 h. This mixture was heat-inactivated at 95 °C for 5 min and diluted to a final volume of 100 μL.
Quantitative ChIP.
ChIP analysis in MAE cells was conducted as described previously (62). Samples containing 3 × 106 cells were cross-linked with 1% formaldehyde for 10 min. Lysates were immunoprecipitated with rabbit polyclonal anti-HA antibody using rabbit preimmune serum (Covance) as a control. DNA was quantitated by real-time PCR (Applied Biosystems Viia 7 instrument) with SYBR green fluorescence, and the amount of product was determined relative to a standard curve created from serial dilution of input chromatin.
Protein Analysis.
Protein samples were isolated by centrifugation of 1 × 106 cells from each condition, washing with cold PBS, and lysing in SDS sample buffer (25 mM Tris, pH 6.8, 2% β-mercaptoethanol, 3% SDS, 0.005% bromophenol blue, 5% glycerol). Samples were boiled for 10 min and stored at −80 °C. Samples were resolved by SDS/PAGE, and proteins were detected by semiquantitative Western blotting with ECL Plus (Pierce). For primary fetal liver cells, FEMTO supersignal (Pierce) was used.
Immunofluorescence.
Cells were cytospun and fixed with 3.7% paraformaldehyde in PBS for 10 min at room temperature. Slides were washed with PBS, and cells were permeabilized with 0.2% Triton X-100 for 10 min at room temperature. After washing, slides were blocked with 10% BlokHen (Aves Labs) in 0.1% Tween 20 in PBS for 1 h at 37 °C and then incubated with primary antibody (anti-HA, Covance HA11) in 2% BlokHen at 4 °C overnight. After washing, slides were incubated with secondary antibody for 1 h at 37 °C. Slides were washed and mounted using Vectashield mounting medium with DAPI (Vector Laboratories).
Colony Forming Unit Assay.
For CFU assays, dissociated Lin− cells or Lin−Kit+ cells from E14.5 fetal livers were plated in duplicate in Methocult M3434 complete media (StemCell Technologies) at 1 × 103 cells per 35-mm plate. Plates were incubated for 8 d, and colonies were identified and enumerated. For subsequent analysis, cells were isolated from the plates using PBS containing 50% calf bovine serum and centrifuged for 10 min to remove methylcellulose.
Flow Cytometry.
For Lin−Kit+ cell sorting, E14.5 fetal liver cells were dissociated and resuspended in PBS with 10% FBS and passed through 25-μm cell strainers to obtain single-cell suspensions before antibody staining. After cells were stained with FITC-conjugated CD5 (11-0051-85), CD8 (11-0081-85), CD19 (11-0193), IgM (11-5890), Il7Ra (11-1271), AA4.1 (11-5892; Thermo Fisher Scientific), B220 (103206), CD3 (100306), CD4 (100406), TER-119 (116206), and PE Cy7-conjugated c-Kit (105814; Biolegend) antibodies, Lin−Kit+ cells were collected on a FACSAria II cell sorter (BD Biosciences). For analysis of myeloid cells, cells isolated from colonies were fixed in 2% paraformaldehyde for 10 min at 37 °C. After permeabilization overnight at −20 °C in 95% methanol, cells were incubated for 1 h in HBSS/4% FBS at 4 °C. After incubation with Fc block on ice for 15 min, cells were stained with anti-mouse Mac1-APCe780 (47-0112-82; Thermo Fisher Scientific) and anti-mouse Gr1-PE-Cy7 (108416; Biolegend) at room temperature for 30 min. DAPI was added at this stage for cell cycle analysis. Cells were washed twice in PBS before analysis and analyzed on a LSR II flow cytometer (BD Biosciences). The data were analyzed using FlowJo v10.1 software (TreeStar) and ModFit LT software (Verity Software House).
Apoptosis Assay.
To quantify apoptosis after Mac1/Gr1 staining, cells were washed in Annexin V buffer (10 mM Hepes, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) and stained with Annexin V-Pacific blue (A35122; TermoFisher) and DRAQ7 (ab109292; Abcam) for 15 min in the dark at room temperature.
Statistical Analysis.
Statistical significance was determined by Student’s t-test using web-based GraphPad (https://www.graphpad.com).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK68634 and DK50107 (to E.H.B.) and K01DK113117 (to K.J.H.), and by the Carbone Cancer Center P30CA014520.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813015115/-/DCSupplemental.
References
- 1.Döhner H, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khwaja A, et al. Acute myeloid leukaemia. Nat Rev Dis Primers. 2016;2:16010. doi: 10.1038/nrdp.2016.10. [DOI] [PubMed] [Google Scholar]
- 3.Figueroa ME, et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell. 2010;17:13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih AH, et al. Mutational cooperativity linked to combinatorial epigenetic gain of function in acute myeloid leukemia. Cancer Cell. 2015;27:502–515. doi: 10.1016/j.ccell.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro AF, et al. Mutant DNMT3A: A marker of poor prognosis in acute myeloid leukemia. Blood. 2012;119:5824–5831. doi: 10.1182/blood-2011-07-367961. [DOI] [PubMed] [Google Scholar]
- 6.Welch JS, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jan M, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med. 2012;4:149ra118. doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klco JM, et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell. 2014;25:379–392. doi: 10.1016/j.ccr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uy GL, et al. Dynamic changes in the clonal structure of MDS and AML in response to epigenetic therapy. Leukemia. 2017;31:872–881. doi: 10.1038/leu.2016.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson RE, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu AP, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn CN, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai FY, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, et al. Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J Exp Med. 2013;210:2833–2842. doi: 10.1084/jem.20130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Pater E, et al. Gata2 is required for HSC generation and survival. J Exp Med. 2013;210:2843–2850. doi: 10.1084/jem.20130751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues NP, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106:477–484. doi: 10.1182/blood-2004-08-2989. [DOI] [PubMed] [Google Scholar]
- 17.Ling KW, et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KD, et al. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J Clin Invest. 2012;122:3692–3704. doi: 10.1172/JCI61623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta C, et al. Integrating enhancer mechanisms to establish a hierarchical blood development program. Cell Rep. 2017;20:2966–2979. doi: 10.1016/j.celrep.2017.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson KD, et al. Cis-regulatory mechanisms governing stem and progenitor cell transitions. Sci Adv. 2015;1:e1500503. doi: 10.1126/sciadv.1500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues NP, et al. GATA-2 regulates granulocyte-macrophage progenitor cell function. Blood. 2008;112:4862–4873. doi: 10.1182/blood-2008-01-136564. [DOI] [PubMed] [Google Scholar]
- 22.Persons DA, et al. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–499. [PubMed] [Google Scholar]
- 23.Spinner MA, et al. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickinson RE, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123:863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wlodarski MW, et al. EWOG-MDS Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127:1387–1397. doi: 10.1182/blood-2015-09-669937. [DOI] [PubMed] [Google Scholar]
- 26.Katsumura KR, Bresnick EH, Group GFM. GATA Factor Mechanisms Group The GATA factor revolution in hematology. Blood. 2017;129:2092–2102. doi: 10.1182/blood-2016-09-687871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu AP, et al. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood. 2013;121:3830–3837, S3831–S3837. doi: 10.1182/blood-2012-08-452763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki H, et al. A remote GATA2 hematopoietic enhancer drives leukemogenesis in inv(3)(q21;q26) by activating EVI1 expression. Cancer Cell. 2014;25:415–427. doi: 10.1016/j.ccr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gröschel S, et al. A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. Cell. 2014;157:369–381. doi: 10.1016/j.cell.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Vicente C, et al. Overexpression of GATA2 predicts an adverse prognosis for patients with acute myeloid leukemia and it is associated with distinct molecular abnormalities. Leukemia. 2012;26:550–554. doi: 10.1038/leu.2011.235. [DOI] [PubMed] [Google Scholar]
- 31.Katsumura KR, Yang C, Boyer ME, Li L, Bresnick EH. Molecular basis of crosstalk between oncogenic Ras and the master regulator of hematopoiesis GATA-2. EMBO Rep. 2014;15:938–947. doi: 10.15252/embr.201438808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsumura KR, Ong IM, DeVilbiss AW, Sanalkumar R, Bresnick EH. GATA factor-dependent positive-feedback circuit in acute myeloid leukemia cells. Cell Rep. 2016;16:2428–2441. doi: 10.1016/j.celrep.2016.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin DI, Orkin SH. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 1990;4:1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- 34.Omichinski JG, et al. A small single-“finger” peptide from the erythroid transcription factor GATA-1 binds specifically to DNA as a zinc or iron complex. Proc Natl Acad Sci USA. 1993;90:1676–1680. doi: 10.1073/pnas.90.5.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ping N, et al. Exome sequencing identifies highly recurrent somatic GATA2 and CEBPA mutations in acute erythroid leukemia. Leukemia. 2017;31:195–202. doi: 10.1038/leu.2016.162. [DOI] [PubMed] [Google Scholar]
- 36.Fasan A, et al. GATA2 mutations are frequent in intermediate-risk karyotype AML with biallelic CEBPA mutations and are associated with favorable prognosis. Leukemia. 2013;27:482–485. doi: 10.1038/leu.2012.174. [DOI] [PubMed] [Google Scholar]
- 37.Greif PA, et al. GATA2 zinc finger 1 mutations associated with biallelic CEBPA mutations define a unique genetic entity of acute myeloid leukemia. Blood. 2012;120:395–403. doi: 10.1182/blood-2012-01-403220. [DOI] [PubMed] [Google Scholar]
- 38.Ghirlando R, Trainor CD. Determinants of GATA-1 binding to DNA: The role of non-finger residues. J Biol Chem. 2003;278:45620–45628. doi: 10.1074/jbc.M306410200. [DOI] [PubMed] [Google Scholar]
- 39.Pedone PV, et al. The N-terminal fingers of chicken GATA-2 and GATA-3 are independent sequence-specific DNA binding domains. EMBO J. 1997;16:2874–2882. doi: 10.1093/emboj/16.10.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crispino JD, Lodish MB, MacKay JP, Orkin SH. Use of altered specificity mutants to probe a specific protein-protein interaction in differentiation: The GATA-1:FOG complex. Mol Cell. 1999;3:219–228. doi: 10.1016/s1097-2765(00)80312-3. [DOI] [PubMed] [Google Scholar]
- 41.Nichols KE, et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000;24:266–270. doi: 10.1038/73480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tubman VN, et al. X-linked gray platelet syndrome due to a GATA1 Arg216Gln mutation. Blood. 2007;109:3297–3299. doi: 10.1182/blood-2006-02-004101. [DOI] [PubMed] [Google Scholar]
- 43.Campbell AE, Wilkinson-White L, Mackay JP, Matthews JM, Blobel GA. Analysis of disease-causing GATA1 mutations in murine gene complementation systems. Blood. 2013;121:5218–5227. doi: 10.1182/blood-2013-03-488080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grass JA, et al. Distinct functions of dispersed GATA factor complexes at an endogenous gene locus. Mol Cell Biol. 2006;26:7056–7067. doi: 10.1128/MCB.01033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson KD, et al. Friend of GATA-1-independent transcriptional repression: A novel mode of GATA-1 function. Blood. 2007;109:5230–5233. doi: 10.1182/blood-2007-02-072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsang AP, et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90:109–119. doi: 10.1016/s0092-8674(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 47.Chlon TM, Doré LC, Crispino JD. Cofactor-mediated restriction of GATA-1 chromatin occupancy coordinates lineage-specific gene expression. Mol Cell. 2012;47:608–621. doi: 10.1016/j.molcel.2012.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gregory T, et al. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood. 1999;94:87–96. [PubMed] [Google Scholar]
- 49.Weiss MJ, Yu C, Orkin SH. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol Cell Biol. 1997;17:1642–1651. doi: 10.1128/mcb.17.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bresnick EH, et al. Mechanisms of erythrocyte development and regeneration: Implications for regenerative medicine and beyond. Development. 2018;145:dev151423. doi: 10.1242/dev.151423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grass JA, et al. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc Natl Acad Sci USA. 2003;100:8811–8816. doi: 10.1073/pnas.1432147100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tipping AJ, et al. High GATA-2 expression inhibits human hematopoietic stem and progenitor cell function by effects on cell cycle. Blood. 2009;113:2661–2672. doi: 10.1182/blood-2008-06-161117. [DOI] [PubMed] [Google Scholar]
- 53.Theis F, et al. Clinical impact of GATA2 mutations in acute myeloid leukemia patients harboring CEBPA mutations: A study of the AML study group. Leukemia. 2016;30:2248–2250. doi: 10.1038/leu.2016.185. [DOI] [PubMed] [Google Scholar]
- 54.Bödör C, et al. Germ-line GATA2 p.THR354MET mutation in familial myelodysplastic syndrome with acquired monosomy 7 and ASXL1 mutation demonstrating rapid onset and poor survival. Haematologica. 2012;97:890–894. doi: 10.3324/haematol.2011.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Churpek JE, et al. Genomic analysis of germ line and somatic variants in familial myelodysplasia/acute myeloid leukemia. Blood. 2015;126:2484–2490. doi: 10.1182/blood-2015-04-641100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drazer MW, et al. Prognostic tumor sequencing panels frequently identify germ line variants associated with hereditary hematopoietic malignancies. Blood Adv. 2018;2:146–150. doi: 10.1182/bloodadvances.2017013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makishima H, et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet. 2017;49:204–212. doi: 10.1038/ng.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimi A, et al. Recurrent CDC25C mutations drive malignant transformation in FPD/AML. Nat Commun. 2014;5:4770. doi: 10.1038/ncomms5770. [DOI] [PubMed] [Google Scholar]
- 59.Yu C, et al. X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction. Blood. 2002;100:2040–2045. doi: 10.1182/blood-2002-02-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hasegawa A, et al. GATA1 binding kinetics on conformation-specific binding sites elicit differential transcriptional regulation. Mol Cell Biol. 2016;36:2151–2167. doi: 10.1128/MCB.00017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McIver SC, et al. Dissecting regulatory mechanisms using mouse fetal liver-derived erythroid cells. Methods Mol Biol. 2018;1698:67–89. doi: 10.1007/978-1-4939-7428-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Im H, et al. Measurement of protein-DNA interactions in vivo by chromatin immunoprecipitation. Methods Mol Biol. 2004;284:129–146. doi: 10.1385/1-59259-816-1:129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.