Stress is among the most important risk factors for a variety of mood and anxiety disorders (1, 2), but the underlying neurobiological mechanisms remain incompletely understood, especially in humans. Previous human neuroimaging studies have identified a network of brain regions that exhibit increased activity and blood flow in response to acute stressors (3, 4) and disrupted functional coupling and structural changes after chronic stress (5–8). In PNAS, Elbau et al. (9) report an elegant series of analyses that implicate disrupted neurovascular coupling as an important potential mechanism linking these two phenomena. The authors also explore the complex interplay among the hemodynamic response, the endocrine stress response, and genetic variants related to neurovascular coupling in light of recent evidence that these factors contribute to both cognitive dysfunction (10) and major depression (11).
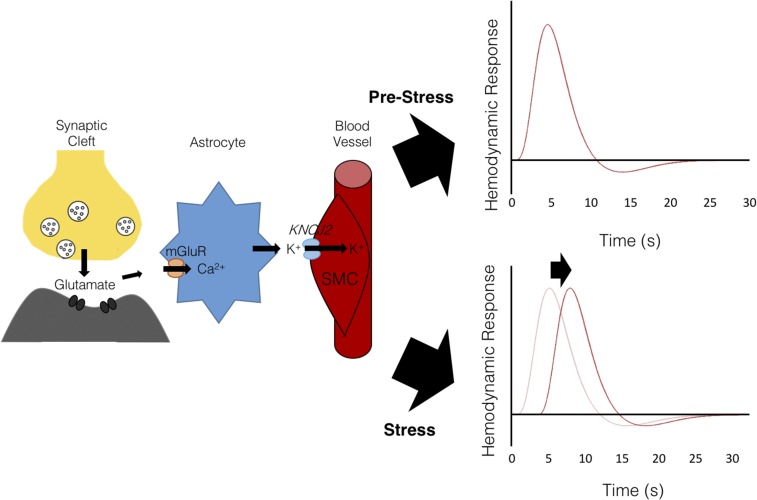
Neurons and other brain cells are highly metabolically active (12). Brain function, therefore, depends critically on neurovascular coupling, a system for regulating local cerebral blood flow to ensure an adequate supply of oxygen, glucose, and other metabolites on an “as-needed” basis in response to signals from neurons, astrocytes, and other local cell types (13, 14). Although neurovascular coupling mechanisms are not yet fully understood, one proposed mechanism is that neuronal activity increases local extracellular K+, which is taken up by nearby astrocytes. K+ is subsequently released in the perivascular space via astrocytic endfeet (15), leading to smooth muscle cell hyperpolarization via inward-rectifying K+ channels and vasodilation driven by the closure of voltage-gated Ca2+ channels (Fig. 1). Alternative molecular mediators have also been proposed, including neurotransmitters such as glutamate and nitric oxide (16) and metabolic by-products such as lactate (17). Factors that disrupt these dynamic interactions have a variety of adverse effects on brain health and have been implicated in the pathophysiology of hypertension, stroke, and Alzheimer’s disease (18, 19).
Fig. 1.
Schematic illustrating neurovascular coupling mechanisms, in which the hemodynamic response to neuronal activity is mediated in part by astrocytes, which detect glutamate released from presynaptic terminals via metabotropic glutamate receptors (mGluR). Astrocytes subsequently release potassium (K+) into the perivascular space, triggering hyperpolarization in vascular smooth muscle cells (SMC) via inward-rectifying K+ channels. The resulting vasodilation induced by this hyperpolarization is thought to underlie the hemodynamic response function. Stress increases the hemodynamic response latency.
Previous work in rodents raised the intriguing possibility that acute psychosocial stress may be sufficient to alter neurovascular coupling (20), but it has been challenging to test this directly in the human brain due to technical limitations on interrogating neurovascular coupling mechanisms noninvasively. Instead, Elbau et al. (9) were able to test for changes in neurovascular coupling indirectly, by quantifying temporal shifts in the canonical hemodynamic response function (HRF) evoked during a mental arithmetic task under stress and control conditions. In the stress condition, the task difficulty was adjusted to yield a high failure rate, and subjects received frequent performance assessments and negative verbal feedback to elicit psychosocial stress. This design allowed the authors to test for changes in the temporal dynamics of the HRF before vs. after stress, within each subject.
This approach yielded three key findings that support the idea that acute psychosocial stress alters neurovascular coupling. First, psychosocial stress rapidly increased the latency of the HRF peak in cortical and limbic regions involved in stress response regulation such that the blood oxygen level-dependent (BOLD) signal response to the mental arithmetic task was relatively sluggish and slower to respond in the acute stress condition. This suggests that neurovascular coupling mechanisms linking changes in blood flow to neuronal activity demands may be disrupted by psychosocial stress. Second, individual differences in the HRF peak latency were correlated with a genetic polymorphism influencing KCNJ2 gene expression. This finding is important because KCNJ2 encodes a protein found in smooth muscle cells that is involved in neurovascular coupling, so this result provides further evidence suggesting a specific effect of stress on neurovascular coupling, as opposed to indirect effects mediated by other changes in neuronal activity or vascular function. Third, the authors present a series of analyses specifically implicating hippocampal HRF latency in mediating adverse effects of stress on brain function: The hippocampal HRF correlated with changes in cortisol and with genetic variants involved in the transcriptional response to stress that also modulate depression risk. Critically, and most compellingly, the authors also replicated key findings in an independent sample of subjects.
These results shed light on the influence of acute psychosocial stress and genetic variants that regulate the hemodynamic response. Their findings in a healthy human sample not only support recent rodent studies illuminating the role of KCNJ2 in neurovascular coupling (20) but also studies reporting genetic variants linked to psychiatric disorders (11). Future studies will be required to understand how these acute effects on neurovascular coupling evolve over time in chronic stress paradigms and whether individuals with stress-associated psychiatric disorders exhibit similar alterations in hemodynamic responsivity. If so, there may be opportunities for intervening therapeutically to mitigate stress effects on neurovascular coupling or their downstream sequelae.
Also of clinical relevance, Elbau et al. (9) report substantial interindividual variability in the prestress hippocampal hemodynamic lag. This measure mediated the relationship between the endocrine response evoked by stress during the task and genetic variants that influence depression risk. Furthermore, prestress interindividual variability predicted the magnitude of stress-induced hemodynamic lag change such that individuals who displayed low prestress hemodynamic lag showed stress-induced increases, and vice versa. An interesting next step would be to determine whether individual differences in the hippocampal HRF lag also predict risk for developing depression, anxiety, or other psychiatric symptoms in response to stress exposure. Given that features of the hemodynamic response are heritable (7), it would also be interesting to test whether these HRF measures vary with familial risk for developing stress-related psychiatric disorders.
These findings also have important and more general implications for interpreting the results of fMRI studies involving task-evoked hemodynamic responses. They indicate that, when unaccounted for, stress effects on the HRF latency could yield apparent changes in conventional BOLD signal amplitudes that are, in fact, driven by changes in temporal dynamics. This, in turn, suggests that future studies could benefit from models that account for hemodynamic latency variability in task-based fMRI paradigms, especially those involving patients with stress-related psychiatric conditions.
Footnotes
The authors declare no conflict of interest.
See companion article on page E10206.
References
- 1.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 2.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 3.Pruessner JC, et al. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 4.Qin S, Hermans EJ, van Marle HJF, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bremner JD, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan ZY, et al. Genes influence the amplitude and timing of brain hemodynamic responses. Neuroimage. 2016;124:663–671. doi: 10.1016/j.neuroimage.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen RA, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–982, and erratum (2006) 60:1023. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Elbau IG, et al. The brain’s hemodynamic response function rapidly changes under acute psychosocial stress in association with genetic and endocrine stress response markers. Proc Natl Acad Sci USA. 2018;115:E10206–E10215. doi: 10.1073/pnas.1804340115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarantini S, et al. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arloth J, et al. Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (PGC) Genetic differences in the immediate transcriptome response to stress predict risk-related brain function and psychiatric disorders. Neuron. 2015;86:1189–1202. doi: 10.1016/j.neuron.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: Theory, procedure and normal values. J Clin Invest. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raichle ME, Gusnard DA. Appraising the brain’s energy budget. Proc Natl Acad Sci USA. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iadecola C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filosa JA, Blanco VM. Neurovascular coupling in the mammalian brain. Exp Physiol. 2007;92:641–646. doi: 10.1113/expphysiol.2006.036368. [DOI] [PubMed] [Google Scholar]
- 16.Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman RD, Li B. Neural-metabolic coupling in the central visual pathway. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150357. doi: 10.1098/rstb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 19.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 20.Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci USA. 2014;111:7462–7467. doi: 10.1073/pnas.1401811111. [DOI] [PMC free article] [PubMed] [Google Scholar]