Significance
Antibodies blocking inhibitory checkpoints on T cells have been a major advance in cancer treatment. However, agonistic antibodies have had less success due to toxicity concerns. Harnessing the knowledge that agonistic antibodies require the inhibitory Fc receptor (FcR), we engineered a CD40 antibody with improved in vivo activity. Because current models fail to recapitulate important dose-limiting toxicities in patients, we developed a mouse model carrying human CD40 and FcRs. This model mirrors human toxicities and allowed for the development of an in situ vaccination approach leading to durable tumor control. These results support the rational design of immune modulating antibodies as well as stress the importance, and possible reconsiderations needed, for optimal preclinical models allowing parallel efficacy and toxicity analyses.
Keywords: CD40, agonist antibody, immunotherapy, Fc receptor
Abstract
Immune stimulation has emerged as a promising approach to the treatment of neoplastic diseases. Currently approved therapeutics, such as anti-CTLA4 and anti-PD1, are primarily aimed at blocking inhibitory signaling by immune cells. An alternative and potentially synergistic approach would involve activation of immune pathways by agonism of stimulatory receptors, such as CD40. Agonistic antibodies, while promising in principle, have encountered significant barriers in clinical trials limited by the systemic toxicity of such approaches. Using a mouse model humanized for both Fc receptors and CD40, we previously demonstrated enhanced antitumor activity with an Fc-modified antibody. We now demonstrate that this model recapitulates the platelet and hepatic toxicities seen with anti-CD40 antibodies in patients, providing a predictive measure of the dose-limiting activity of this approach. We further show that such toxicity can be circumvented and durable systemic antitumor immunity achieved by intratumoral delivery of an Fc-engineered anti-CD40 agonistic antibody.
The CD40 pathway provides a central mechanism for the activation of B cells, dendritic cells, and macrophages and is well established as a powerful adjuvant in preclinical animal models. Despite its promise, clinical trials with agonistic, anti-CD40 antibodies have encountered dose-limiting toxicities and, as a consequence, minimal clinical responses (1). We engineered the human anti-CD40 agonist antibody CP-870,893 (2–4) with five point mutations in the Fc domain selectively increasing its binding to human FcyRIIB (referred to here as “2141-V11”), and demonstrated that it has significantly enhanced antitumor activity compared with its parental IgG2 version in several tumor models (2). Using a mouse model carrying human Fcy receptors (FcyRs) and human CD40 (hFcyR/hCD40) in place of their mouse homologs, we reported that, when given systemically, the enhanced in vivo activity of the 2141-V11 was accompanied by increasing thrombocytopenia and transaminitis (2). These same toxicities are seen with the current clinically used anti-CD40 antibodies and the primary drivers of the dose-limiting toxicities, resulting from the expression of CD40 on platelets and their activation by agonistic anti-CD40 antibodies. In preparation for clinical studies of this Fc-engineered antibody we set out to optimize a dosing and delivery regimen that would result in minimal toxicity with optimal antitumor activity.
Results
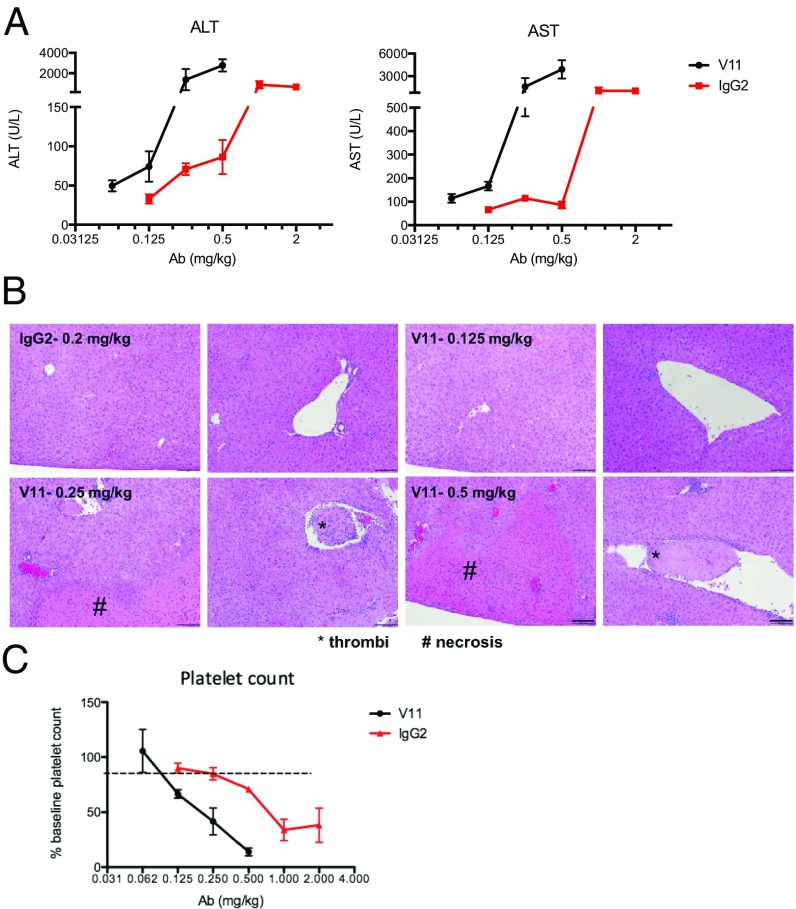
The toxicity of rat IgG2a anti-CD40 antibodies has been previously established in mouse tumor models (5), with s.c. dosing allowing activity and better tolerability (6, 7). However, this has never been evaluated using human antibodies in a system expressing both human CD40 and human FcRs. We previously generated an Fc-enhanced anti-CD40 antibody (2141-V11) with superior in vivo activity to the currently available clinical reagents (2). To test the effects of 2141-V11 on liver function tests, we treated hFcyR/hCD40 mice systemically with increasing concentrations of antibody. Interestingly, while well tolerated at low doses up to 0.1 mg/kg, higher doses of anti-CD40 agonists led to profound transaminitis and hepatotoxicity. Compared with the parental antibody, 2141-V11 led to significantly higher transaminase levels [aspartate (AST) and alanine (ALT)] at doses lower than the parent IgG2 antibody (Fig. 1A). When livers of treated mice were evaluated histologically, we found evidence of both intravascular thrombi as well as hepatocyte necrosis in mice treated with 2141-V11 at concentrations equal or above 0.25 mg/kg. No signs of hepatic toxicity were observed in histology evaluation of mice treated with 2141-V11 at 0.125 mg/kg or lower doses, and in mice treated with the parental 2141-IgG2 at 0.2 mg/kg (Fig. 1B). The liver is a primary site of immune complex clearance in normal and pathologic conditions, thus high levels of FcγRIIB on liver sinusoids (8), in addition to activation of intrasinusoidal platelets (9–11), likely both contribute to this mechanism-based toxicity. Previous studies have demonstrated that toxicity from a rat IgG2a CD40 antibody can be abrogated through pretreatment with anti–CSF1-R antibody to deplete Kupffer cells (5), suggesting that FcγRIIB-expressing Kupffer cells (12) within liver sinusoids may also play a direct role. Thus, not only does the 2141-V11 variant lead to enhanced antitumor activity, but it also worsened mechanism-based liver toxicity.
Fig. 1.
Improved FcγRIIB binding enhances in vivo toxicity of anti-CD40 antibodies. (A) Toxicity of liver transaminases in response to increasing levels of anti-CD40 antibodies. Mice were treated with increasing doses of 2141-V11 or the parental IgG2 anti-CD40 antibody and liver transaminases (AST and ALT) were measured. Data include three to five mice at each concentration. (B) Livers from mice treated with 2141-V11 show evidence of intravascular thrombi and hepatocyte necrosis. Mice treated with 2141-IgG2 showed no toxicity at 0.2 mg/kg or below. Mice treated with 2141-V11 demonstrated evidence of intravascular thrombi and hepatocyte necrosis starting at dose 0.25 mg/kg or greater. Representative images from mice treated at each concentration are shown. Intrahepatic thrombi are marked by an asterisk (*), whereas hepatocyte necrosis is marked by a pound (#) sign. Scale bars demonstrating intrahepatic necrosis and thrombi are 200 μm or 40 μm, respectively. (C) Toxicity of murine platelets in response to increasing levels of anti-CD40 antibodies. Mice were treated with increasing doses of 2141-V11 or the parental IgG2 anti-CD40 antibody and platelets were measured. Data include three to five mice at each concentration.
We next evaluated the thrombocytopenia reported in patients treated with agonistic, anti-CD40 antibodies. We found that increasing doses of 2141-V11 led to worsening thrombocytopenia (Fig. 1C) while not having any significant effect on other blood count parameters. With this information, we were able to establish an optimal dosing schedule at which this toxicity was circumvented when given systemically. Thus, unlike murine or macaque models, the hFcyR/hCD40 mouse model accurately reflected the actual dose-related toxicities observed in patient populations and provides the only predictive model for evaluating this agonistic molecule by modeling the effect of FcyRIIB enhancement under physiological conditions. The maximum tolerated dose (MTD) of the parental antibody 2141-IgG2 was found to be 0.2 mg/kg, mirroring the current clinical data (3, 4). We determined the MTD of 2141-V11 in our humanized FcyR/CD40 model and found it to be 0.1 mg/kg.
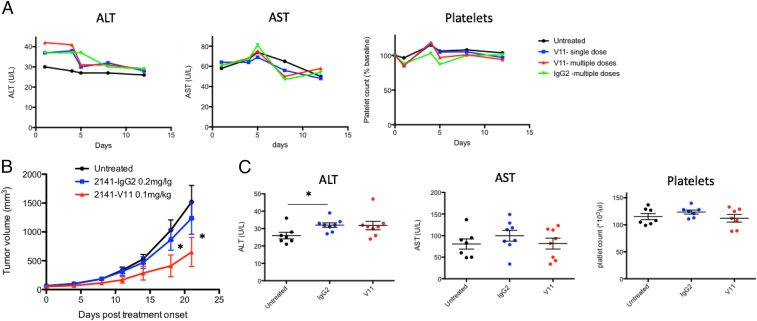
Because of the dose-limiting toxicities observed at higher systemic concentrations of agonistic anti-CD40 antibodies, we went on to confirm whether or not a multiple dose schedule at the MTD of 0.1 mg/kg would limit toxicity and allow antitumor efficacy. When 2141-V11 and 2141-IgG2 were repeatedly administered as four consecutive doses (0.1 mg/kg and 0.2 mg/kg, respectively) we did not observe any signs of toxicity (Fig. 2A). Thus, when kept at their predetermined MTD, CD40 antibodies are safe to be used in a multiple dose schedule. Next, using our humanized model, MC38 tumors were allowed to engraft for 1 wk as in prior experiments, followed by four consecutive doses of 2141-V11, starting at day 7, given every 4 d. These studies demonstrated that at their respective MTDs, 2141-V11 led to significantly better tumor control than the parental antibody with a human IgG2 Fc, 2141-IgG2 (Fig. 2B). These data also demonstrate that this approach limits toxicity as liver function tests and platelets remain similar to untreated controls at the end point of this experiment (Fig. 2C). However, as opposed to previous studies which used higher doses of the antibody (2), we did not achieve complete, durable tumor control with this approach. These results suggest that when used in patients at their respective MTDs, increased therapeutic antitumor immunity can be achieved by the Fc-engineered 2141-V11 compared with its parental IgG2 version; however, an optimal dose may never be reached for either subclass in humans when used at these doses.
Fig. 2.
Determination of the MTD in humanized mice allows antitumor activity without overt toxicity. (A) Humanized CD40/FcR mice were treated with a single (blue) or multiple dose (V11-blue, IgG2-green) regimen of the respective anti-CD40 antibody. Serum transaminases and platelets were measured over the next 2 wk and compared with untreated control mice (n = 4–5 mice per group). (B) MC38 tumors were allowed to engraft s.c. in mice for 1 wk. Mice were then treated at their respective MTDs (0.1 mg/kg for 2141-V11 and 0.2 mg/kg for 2141-IgG2) on days 7, 11, 15, and 18 (n = 7 per group). Tumor volumes were measured via caliper every 3–4 d, *P < 0.05. (C) Liver and platelet toxicity was evaluated at day 20 (n = 7 per group), *P < 0.05. There are no significant differences between groups.
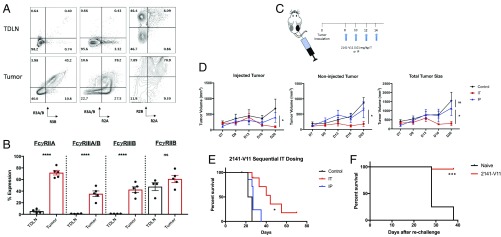
Because the goal of 2141-V11 treatment is to enhance activation of intratumoral antigen-presenting cells, leading to the stimulation of cytotoxic T cells that may then migrate to distant tumor sites (referred to as an abscopal effect), we next tested whether or not direct delivery of 2141-V11 to the tumor site maintains antitumor activity with the additional benefit of potentially reducing the toxicities limiting optimal dosing in vivo (6, 13, 14). Previous work has shown that the activity of 2141-V11 is strictly dependent on its interactions with the inhibitory Fc receptor FcyRIIB; thus, we next assessed the relative percentages of Fc receptors on leukocytes in the tumor microenvironment (TME). Although we previously demonstrated that humanized mice phenocopy the CD40 and Fc receptor expression profile similar to that found in humans (2, 15), the relative levels of human FcyRs expressed within the TME has only been recently assessed in this model (16). Because subclass activity is determined by the overall activating:inhibitory ratio of FcyRs (17), we evaluated all infiltrating leukocytes. Compared with the expression of FcyRs in the tumor draining lymph node (TDLN), we found significantly higher levels of activating FcyRIIA and FcyRIIIA, with high expression of the inhibitory FcyRIIB within both the draining lymph node and the tumor microenvironment (Fig. 3 A and B). FcyR expression within tumors was also significantly increased compared with both the spleen and peripheral blood of tumor-inoculated mice (SI Appendix, Fig. S1). As the effector function of each antibody is dependent on its collective binding to both activating (FcγRIIA and FcγRIIIA) and inhibitory (FcγRIIB) receptors (17), this result suggests that the TME and draining lymph node exhibit high levels of the inhibitory Fc receptor IIB and could limit optimal activity of other therapeutic antibodies (18–21). Because 2141-V11 has significantly enhanced binding to the inhibitory receptor FcγRIIB compared with the activating FcγRs (2), this optimizes its potential activity and further supports the design of rationale therapies based on each antibody’s mechanism of action and clinical context. Both preclinical as well as patient data continue to support a role for immune cells within the TME, as patients who present with cutaneous, lung, or visceral disease are known to have differential responses to immunotherapy (22–25).
Fig. 3.
Fc receptors are up-regulated in the tumor microenvironment. (A) Humanized CD40/FcR mice were implanted with MC38 tumor cells and allowed to engraft for 2 wk. Tumors and the TDLNs were then harvested and dissociated for analysis of infiltrating hematopoietic cells expressing different human Fc receptors. Cells were gated to exclude doublets followed by gating on live murine hematopoietic cells (live/dead aqua negative/mCD45+). Fc receptors were then evaluated on all infiltrating leukocytes. (B) Quantification of the different Fc receptors expressed on infiltrating leukocytes within either the TDLN or tumor (n = 5). Compared with the TDLN, there are significantly higher levels of all activating receptors (FcyRIIA and FcyRIIIA/B), whereas the TDLN also expresses high levels of the inhibitory receptor FcyRIIB. Data are displayed as the mean ± SEM. ****P < 0.001; ns, nonsignificant. (C) Schematic overview of bilateral tumor model and injection schedule. (D) MC38 tumors were s.c. injected and allowed to engraft into the bilateral flanks of mice until palpable. Mice were then treated at one-tenth the MTD of 2141-V11 (0.01 mg/kg) given either intratumorally or intraperitoneally on days 8, 10, 12, and 14. Tumor volumes were measured via caliper every 3–4 d (n = 5–8 mice per group). For i.p. dosing, the right tumor was measured as “injected” and the left tumor as “non-injected.” Data are displayed as the mean ± SEM. *P < 0.05; ns, nonsignificant. (E) Intratumoral 2141-V11 treatment significantly improves survival. Mice treated with either control or 2141-V11 antibody intratumoral or i.p. 2141-V11 were followed for overall survival. Intratumoral treatment with 2141-V11 (IT) led to significantly improved survival compared with mice receiving i.p. (IP) therapy, *P < 0.05. (F) Intratumoral therapy with 2141-V11 leads to long-lived protection in cured mice. Mice that were treated and cured with 2141-V11 were rechallenged with 10-fold the number of cells (10 × 106 MC38 tumor cells). Naïve, untreated mice were used as controls. Nearly all mice cured with 2141-V11 (n = 24) were protected from rechallenge with MC38 tumor cells, whereas naïve mice were not (n = 4), ***P = 0.001.
We next tested whether or not direct delivery of 2141-V11 to the tumor site could help control both local (injected) and distant (noninjected) tumor growth. Following recent work utilizing this in situ vaccination approach (6, 14), we utilized a bilateral flank MC38 tumor model which allows monitoring of tumors at both treated and untreated sites (Fig. 3C) (13). Mice were treated with four doses of intratumoral 2141-V11 at one-tenth the MTD on days 8, 10, 12, and 14, with tumor size measured over time. Compared with systemic delivery (as well as isotype control), direct administration of 2141-V11 into the tumor led to significantly lower tumor burden (Fig. 3D). Using this decreased dose also resulted in no evidence of liver or platelet toxicity. This regimen led to tumor control without evidence of toxicity, and a significant fraction of the mice displayed long-term survival (Fig. 3E). Finally, mice that cleared tumors in response to treatment with 2141-V11 were tested for protection from tumor rechallenge. Here, using a tumor dose that is 10 times higher than the initial challenge, we found that mice treated and cured with 2141-V11 were nearly all protected from rechallenge (Fig. 3F).
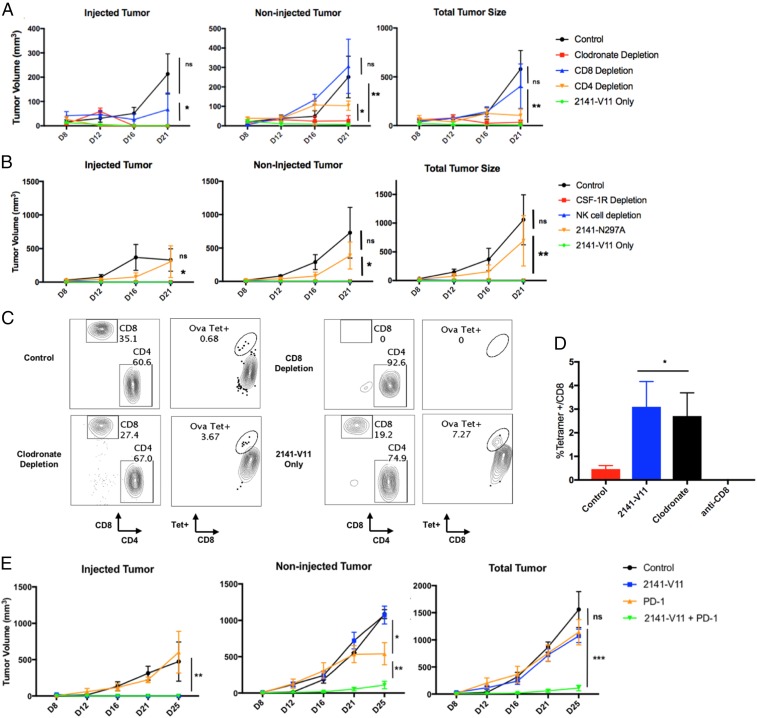
To examine the mechanism by which CD40 stimulation resulted in antitumor responses, we performed a set of depletion experiments to determine the cellular mediators required for both local and distant tumor control. Previous reports using anti-mouse CD40 antibodies have suggested a role for macrophages, and not T cells or natural killer (NK) cells, for the in vivo activity of anti-CD40 antibodies (4). To optimize the treatment in our FcγR/CD40 humanized model, we increased the intratumoral dose of 2141-V11 to what was determined to be the MTD (0.1 mg/kg) and found this led to rapid and durable tumor control of both the treated and distant sites of disease (Fig. 4A). While depletion of CD4 T cells had no effect on tumor growth at the injected site and only modest effect at a distant site of disease, depletion of CD8 T cells completely abrogated the protective effects of 2141-V11. Macrophage depletion with clodronate or anti-CSF1 antibody had no significant effect on tumor control. Additionally, depleting NK cells also had no effect on tumor growth. We also confirmed that the antitumor activity of intratumoral 2141-V11 is Fc-receptor dependent, in that tumor growth was not inhibited in mice receiving an anti-CD40 variant with an Fc unable to bind Fc receptors, N297A (2141-N297A) (Fig. 4B).
Fig. 4.
Systemic activity of intratumoral 2141-V11 is Fc-receptor dependent and requires CD8 T cells and not CD4 T cells, macrophages, or NK cells. (A) As in Fig. 3A, mice were injected with bilateral MC38 tumors and allowed to engraft until palpable. Before treatment with intratumoral 2141-V11 at 0.1 mg/kg, mice were depleted of CD8 T cells (using anti-CD8 antibody) or macrophages (using clodronate liposomes). Volume from both the injected and noninjected tumors was measured over time. Data from each treatment group (n = 4–5 per group) are shown. Data are displayed as the mean ± SEM. *P < 0.05 or **P < 0.01; ns, nonsignificant. (B) Mice were treated as above but instead before treatment with 2141-V11 received antibodies depleting NK cells or macrophages using anti–CSF1-R. One group received the non-Fc receptor binding variant, 2141-N297A, demonstrating intratumoral therapy retains its dependence on binding to the inhibitory receptor IIB. (C) Intratumoral therapy with 2141-V11 expands antigen-specific CD8 T cells. Mice were evaluated for the expansion of ovalbumin-specific T cells in response to treatment with 2141-V11. (D) The 2141-V11 therapy significantly expands OVA-specific CD8 T cells in the periphery of mice and is not affected by macrophage depletion. OVA-specific CD8 T cells are absent in mice receiving antibodies depleting CD8 T cells. Mouse peripheral blood T cells were gated on live CD3 T cells expressing CD4 or CD8. CD8 T cells were then gated for evaluation of ovalbumin-tetramer+ cells. Data are displayed as the mean ± SEM. *P < 0.05 (n = 4–5 per group). (E) Mice were injected with bilateral B16 tumors and allowed to engraft until palpable. Mice then received either control or 2141-V11 injected into the largest tumor. PD-1 therapy was given systemically (i.p.) every 2 d, starting at day 10 for four doses. Volume from both the injected and noninjected tumors was measured over time. Data from each treatment group (n = 3–5 per group) are shown. *P < 0.05, **P < 0.01, ***P < 0.001, or ns, nonsignificant.
Because CD8 T cells were shown to play a necessary role in tumor protection in our humanized model, we next evaluated whether intratumoral treatment with 2141-V11 induced expansion of T cells directed against the tumor. These T cells would thus be able to traffic through the periphery and mediate disease control at distant sites. If antigen-specific T cells indeed expand in response to 2141-V11 treatment, 2141-V11 alone may be potent enough to stimulate an antigen-specific response that can provide long-lived protection.
To address this hypothesis, we utilized a neoantigen [ovalbumin (OVA)]-expressing tumor model (MC38/OVA) to track antigen-specific T cells in response to CD40 stimulation. Following intratumoral treatment with 2141-V11, we found a statistically significant expansion of OVA-specific T cells in the periphery of treated mice. This population was lost in mice treated with CD8-depleting antibodies but not affected in mice treated with clodronate liposomes (Fig. 4 C and D). The tetramer population was also not affected by treatment with anti–CSF1-R or anti-NK cell-depleting antibodies (SI Appendix, Fig. S2). Intriguingly, we saw marked expansion of antigen-specific T cells in mice depleted of NK cells, consistent with prior findings (26). MC38 tumors are moderately immunogenic in C57BL6 mice compared with more “cold” B16 melanoma tumors, possibly given the higher somatic mutational burden in MC38 versus B16 tumors (27). Additionally, B16 melanoma tumors are traditionally resistant to single agent immunotherapeutic drugs, including PD-1 blocking antibodies and CD40 agonists (7, 21, 27, 28). We next tested the ability of 2141-V11 to control disease in this highly aggressive model. Mice treated with intratumoral 2141-V11 saw rapid and persistent control of injected tumors, while systemic single agent PD-1 blockade had no significant effect on total tumor growth. Furthermore, combination therapy with local 2141-V11 and systemic PD-1 blockade led to significantly improved control of tumors in all treated mice (Fig. 4E). Thus, intratumoral dosing of human anti-CD40 agonist antibodies can provide a potent in situ vaccination effect, expand antigen-specific CD8 T cells, and synergized with PD-1 blockade while limiting off-target toxicities (SI Appendix, Fig. S3).
Discussion
While the majority of immune therapies have focused on antagonistic antibodies targeting immune checkpoints present on T cells (e.g., PD-1 and CTLA-4), less clinical success has been demonstrated with agonistic antibodies (29). In part, this is due to toxicity concerns with agents focused on activating the immune system. It has been over a decade since the serious adverse events experienced with the agonistic anti-CD28 antibody TGN1412 (30). Although several aspects of the clinical trial design were suboptimal with TGN1412, the major toxicity of massive cytokine release syndrome was missed in both in vitro and nonhuman primate toxicology models. Further work went on to describe the discrepancy in these studies resulting from suboptimal crosslinking of human Fc receptors in vitro (31). Additionally, despite the fact that human antibodies have poor binding to macaque Fc receptors (32), nonhuman primates remain the preferred preclinical toxicology model. This contributes to underestimates of both in vivo activity and toxicity. The limitations of nonhuman primates as preclinical models for evaluating toxicity and efficacy of the Fc-engineered 2141-V11 are even more prominent, due to the selective enhancement of the V11 Fc to human, but not macaque, FcyRIIB (SI Appendix, Fig. S4).
This model also sheds light on the limitations of anti-CD40 agonistic antibodies in humans, in that when given systemically they may never reach an optimal therapeutic dose because of dose-limiting toxicities. Although accessibility (e.g., lung or liver nodules) may have limited the intratumoral approach for nonskin-based lesions in the past, the expanding access of these sites using radiographically directed therapy makes this clinically feasible. However, even if accessible tumors are controlled by intratumoral injection of 2141-V11, this may not suffice to overcome the immunosuppressive state found in many patients with metastatic disease. Additionally, the majority of patients who receive immunotherapy still do not respond, and finding novel combinations to treat these cold tumors is an active area of investigation. In mice, MC38 tumors respond to single-agent agonistic anti-CD40 antibody as well as single-agent PD-1 blockade (2, 33). B16 melanoma tumors are less immunogenic at baseline and are refractory to single-agent immunotherapy (34). At baseline, B16 tumor cells express higher levels of PD-L1 than MC38 tumors (33), suggesting their increased resistance to tumor-specific T cells which up-regulate PD-1 following antigen stimulation. Although single-agent anti-CD40 agonism with 2141-V11 could lead to enhanced activation of T cells in the local tumor microenvironment and draining lymph nodes, it remains possible that it is unable to overcome the PD-L1–induced immune suppression at distant sites of disease. Thus, blockade of PD-1 allows enhanced activity at metastatic sites of disease in the B16 model, where this is not required in MC38 tumors.
The antitumor activity of a clinically tested, agonistic anti-CD40 antibody (Pfizer CP-870,893) can be significantly enhanced by Fc engineering, by facilitating crosslinking of CD40 by Fc-mediated binding to FcyIIB (2). The results herein utilize a humanized FcγR/CD40 mouse model with predictive value that will aid in the development of these antibody-based therapeutics. Using this model, we have optimized an agonistic anti-CD40 antibody by Fc engineering and developed a treatment protocol that maximizes durable antitumor activity while minimizing toxicity. These studies provide a strong rationale for the use of Fc receptor-matched preclinical in vivo models to optimize and evaluate immunostimulatory antibodies for neoplastic diseases. They also provide further support for trials using local agonistic anti-CD40 therapy in combination with systemic PD-1 blockade.
Methods
Mice.
Humanized mice containing human Fc receptors (FcgRanull, hFcgRI+, FcgRIIaR131+, FcgRIIb+, FcgRIIIaF158+, and FcgRIIIb+) and human CD40 were generated and extensively characterized as previously described (2, 15). All mice were maintained in The Rockefeller University Comparative Bioscience Center. All experiments were performed in compliance with institutional guidelines and had been approved by The Rockefeller University Institutional Animal Care and Use Committee (IACUC).
Toxicity Analysis.
Complete blood count analysis was performed on mouse peripheral blood collected via retroorbital bleeding into heparinized tubes. Samples were analyzed using an Element HT5 hematology analyzer (Heska). Liver function tests were collected from peripheral blood isolated into BD SST Microtainer tubes. Tubes were spun down and supernatant was analyzed for AST, ALT, and alkaline phosphatase levels measured in the Laboratory of Comparative Pathology (Memorial Sloan Kettering Cancer Center) by a routine murine serum chemistry panel. Livers from treated animals were placed in formalin and following processing were stained with hematoxylin and eosin.
Tumor Challenge and Treatment.
Tumor cells were cultured in complete medium (RPMI 1640; DMEM) containing 10% FBS (HyClone), penicillin (100 units/mL), and streptomycin (100 μg/mL) (Gibco). MC38 cells (1 × 106) were implanted s.c. into bilateral flanks of 8- to 12-wk-old mice and tumor volumes were measured every 2–3 d with an electronic caliper. Volume is reported using the formula (L12 3 L2)/2, where L1 is the shortest diameter and L2 the longest diameter. Seven- to 8-d after tumor inoculation, mice were randomly assigned, based on total tumor size (day 0) and received i.p. or intratumoral injection as indicated for each experiment. Mice were followed for 24–28 d after treatment initiation or until the majority of the untreated control group had to be killed due to The Rockefeller University IACUC limitation for tumor size.
Statistical Analysis.
One-way ANOVA with Tukey’s posttest was used to compare all groups in tumor growth experiments. When two groups were compared (e.g., in experiments assessing percentages of cell types) an unpaired two-tailed t test was used. Data were analyzed with GraphPad Prism software, and P < 0.05 was considered to be statistically significant, indicated as *P < 0.05, **P < 0.01, and ***P < 0.001 in the figures. Asterisks indicate statistical comparison with the control group unless indicated otherwise on the graphs.
Supplementary Material
Acknowledgments
We thank members of the J.V.R. laboratory for their excellent technical assistance. Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health (NIH) under Awards R35CA196620 and P01CA190174. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Authors acknowledge partial support from the V Foundation for Cancer Research under Award T2017-002, from the Robertson Therapeutic Development Fund, and from The Rockefeller University. D.A.K. is supported in part by Grant UL1 TR001866 from the National Center for Advancing Translational Sciences, NIH Clinical and Translation Science Award Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810566115/-/DCSupplemental.
References
- 1.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahan R, et al. Therapeutic activity of agonistic, human anti-CD40 monoclonal antibodies requires selective FcγR engagement. Cancer Cell. 2016;29:820–831. doi: 10.1016/j.ccell.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vonderheide RH, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 4.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne KT, Leisenring NH, Bajor DL, Vonderheide RH. CSF-1R-dependent lethal hepatotoxicity when agonistic CD40 antibody is given before but not after chemotherapy. J Immunol. 2016;197:179–187. doi: 10.4049/jimmunol.1600146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJM. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17:2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 7.Sandin LC, et al. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunol Res. 2014;2:80–90. doi: 10.1158/2326-6066.CIR-13-0067. [DOI] [PubMed] [Google Scholar]
- 8.Stegner D, et al. FcγRIIB on liver sinusoidal endothelial cells is essential for antibody-induced GPVI ectodomain shedding in mice. Blood. 2016;128:862–865. doi: 10.1182/blood-2016-05-714378. [DOI] [PubMed] [Google Scholar]
- 9.Robles-Carrillo L, et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185:1577–1583. doi: 10.4049/jimmunol.0903888. [DOI] [PubMed] [Google Scholar]
- 10.Meyer T, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost. 2009;7:171–181. doi: 10.1111/j.1538-7836.2008.03212.x. [DOI] [PubMed] [Google Scholar]
- 11.Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92:1041–1048. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- 12.Ganesan LP, et al. FcγRIIb on liver sinusoidal endothelium clears small immune complexes. J Immunol. 2012;189:4981–4988. doi: 10.4049/jimmunol.1202017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marabelle A, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagiv-Barfi I, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10:eaan4488. doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc Natl Acad Sci USA. 2012;109:6181–6186. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arce Vargas F, et al. TRACERx Melanoma; TRACERx Renal; TRACERx Lung consortia Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell. 2018;33:649–663.e4. doi: 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 18.Bulliard Y, et al. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475–480. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 19.Simpson TR, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Ravetch JV. Antitumor activities of agonistic anti-TNFR antibodies require differential FcγRIIB coengagement in vivo. Proc Natl Acad Sci USA. 2013;110:19501–19506. doi: 10.1073/pnas.1319502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Ravetch JV. Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devaud C, et al. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol Ther. 2014;22:18–27. doi: 10.1038/mt.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann B, et al. Tumor location determines tissue-specific recruitment of tumor-associated macrophages and antibody-dependent immunotherapy response. Sci Immunol. 2017;2:eaah6413. doi: 10.1126/sciimmunol.aah6413. [DOI] [PubMed] [Google Scholar]
- 24.Balch CM, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang E, Rosenberg SA. Patients with melanoma metastases at cutaneous and subcutaneous sites are highly susceptible to interleukin-2-based therapy. J Immunother. 2001;24:88–90. doi: 10.1097/00002371-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Soderquest K, et al. Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J Immunol. 2011;186:3304–3308. doi: 10.4049/jimmunol.1004122. [DOI] [PubMed] [Google Scholar]
- 27.Wei SC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170:1120–1133.e17. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahan R, et al. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell. 2015;28:285–295. doi: 10.1016/j.ccell.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12:147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 31.Hussain K, et al. Upregulation of FcγRIIb on monocytes is necessary to promote the superagonist activity of TGN1412. Blood. 2015;125:102–110. doi: 10.1182/blood-2014-08-593061. [DOI] [PubMed] [Google Scholar]
- 32.Bournazos S, DiLillo DJ, Ravetch JV. The role of Fc-FcγR interactions in IgG-mediated microbial neutralization. J Exp Med. 2015;212:1361–1369. doi: 10.1084/jem.20151267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juneja VR, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214:895–904. doi: 10.1084/jem.20160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.