Significance
Adaptation to rapidly changing environments is essential for cellular fitness and is a critical component in pathogenesis. Translational control has important roles for fitness in a range of environments and requires multiple factors for efficient responses. One such factor is the translation elongation factor (EF)-P, which alleviates ribosome pausing at polyproline motifs. Our findings show that EF-P-mediated relief of ribosome queuing is integral in environmentally driven changes to translation rates. We observe that ribosome pausing leads to changes in protein yield only under rapid growth conditions, demonstrating that effects resulting from ribosome queuing correlate directly with translational demand. These results provide physiological context to previous studies establishing EF-P as a critical factor in cell growth and virulence.
Keywords: protein synthesis, cellular physiology, elongation factor P, translation elongation, adaptation
Abstract
Elongation factor P (EF-P) is a universally conserved translation factor that alleviates ribosome pausing at polyproline (PPX) motifs by facilitating peptide bond formation. In the absence of EF-P, PPX peptide bond formation can limit translation rate, leading to pleotropic phenotypes including slowed growth, increased antibiotic sensitivity, and loss of virulence. In this study, we observe that many of these phenotypes are dependent on growth rate. Limiting growth rate suppresses a variety of detrimental phenotypes associated with ribosome pausing at PPX motifs in the absence of EF-P. Polysome levels are also similar to wild-type under slow growth conditions, consistent with global changes in ribosome queuing in cells without EF-P when growth rate is decreased. Inversely, under high protein synthesis demands, we observe that Escherichia coli lacking EF-P have reduced fitness. Our data demonstrate that EF-P-mediated relief of ribosome queuing is required to maintain proteome homeostasis under conditions of high translational demands.
The average translation elongation rate in Escherichia coli is between 10 and 20 amino acids per second in rapid growth conditions (1). This rate can fluctuate, depending on environmental conditions. For example, different temperatures and nutrient conditions can increase or decrease translational speed (2). Specific transcripts are known to have programmed translation rate tuning and use ribosome pausing for regulatory and functional roles (3). Stretches of proline are also known to cause ribosome pausing (4, 5). Proline contains a unique constrained ring structure, which is important for making turns and increasing stability of proteins, but makes it a poor peptide bond donor and acceptor (6, 7). Translation elongation factor P (EF-P) alleviates ribosome pausing at polyproline (PPX; Pro-Proany amino acid) motifs by facilitating peptide bond formation between prolines (4, 5, 8). EF-P and its homologs are conserved throughout all domains of life (eIF5A and aIF5A in eukaryotes and archaea, respectively). Although all EF-P family members stimulate translation of PPX motifs, eIF5A has recently been shown to also have roles in translation termination, which is likely the cause of eIF5A essentiality in eukaryotes (9, 10).
To perform its function, EF-P enters paused ribosomes through the E-site and facilitates peptide bond formation through interactions with the P-site tRNA (11, 12). This interaction is aided by the d-arm of tRNAPro, which is shared with tRNAfMet (13). Further, EF-P requires posttranslational modification (PTM) at a conserved residue to efficiently facilitate peptide bond formation within the peptidyl transfer center of the ribosome (14–17). PTMs range from linear structures such as (R)-β-lysine and 5-aminopentanol in E. coli and Bacillus subtilis, respectively, to glycosylation with rhamnose in Pseudomonas aeruginosa. Although modification structures are diverse, the general function of the modification is to interact and stabilize proline ProtRNAPro within the peptidyl transfer center to aid peptide bond formation (12). Loss of PTM leads to comparable phenotypes to efp deletion in all organisms in which EF-P has been studied, demonstrating the importance of PTM in EF-P function.
Previous studies have shown that distal sequences, specifically the Shine-Dalgarno (SD) sequence and the start codon, influence ribosome queuing at PPX motifs. In general, in the presence of EF-P, translation initiation is the limiting factor in protein synthesis rate. When translation initiation rates are high in the absence of EF-P, pausing at PPX motifs can cause ribosome queuing and induce a translational bottleneck. When translation initiation rates are decreased by weakening the SD sequence or start codon, fewer ribosomes load on the transcript, reducing ribosome queuing at PPX motifs (18, 19). This effect can be best observed in the context of the ATP synthase proteins AtpA and AtpD. Both AtpA and AtpD have the same PPX motif (PPE) and similar immediately neighboring sequences, whereas the SD of AtpD is closer to the consensus sequence than that of AtpA. Ribosome profiling data reveal that atpD transcripts contain ribosome queuing in the absence of EF-P, whereas atpA transcripts do not, indicating that the major determinant influencing the result of EF-P dependence on PPX containing protein production is the rate of translation initiation (18).
When studying EF-P in E. coli, assays have been regularly performed in conditions in which E. coli is growing rapidly and translation is occurring at a high rate (14, 20). Recent studies have shown that translation rates in E. coli can vary depending on the growth conditions (2, 21). In this study, we determine that reducing translation rates by limiting growth rate in E. coli masks slow PPX peptide bond formation in the absence of EF-P, suggesting that high protein synthesis rates under rapid growth conditions exacerbate the effects of ribosome queuing because of slow PPX synthesis (6). These results highlight a role for EF-P in maintaining proteome homeostasis in conditions that demand rapid protein production.
Results
Net Protein Production of GFP Containing a Canonical PPX Motif Is Independent of EF-P at 25 °C.
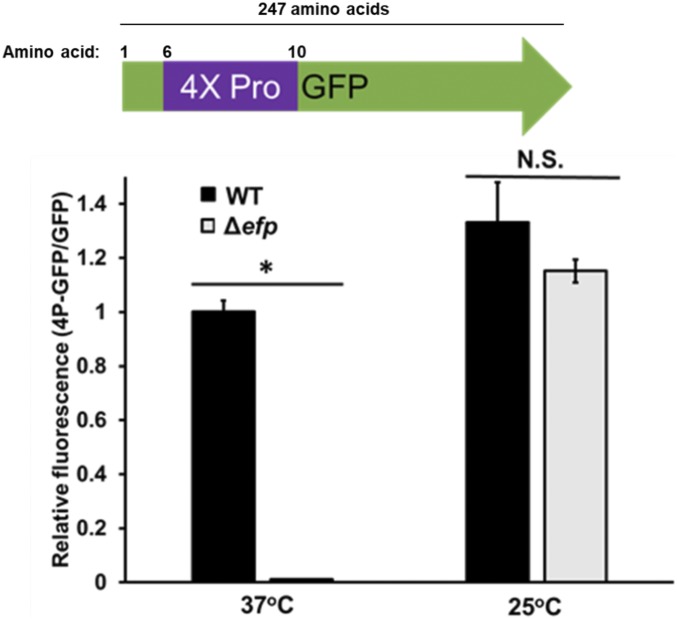
Previous studies in our laboratory demonstrated that when translation initiation rate is decreased for a specific transcript, the effect of EF-P mediated peptide bond synthesis on protein yield of a PPX-containing product is diminished (18, 19). We use growth at 25 °C, a temperature known to decrease translation initiation, in E. coli compared with 37 °C to model how global decreases in translation rate effect EF-P dependence (1, 18). There is only a slight decrease in translation elongation rate at 25 °C compared with 37 °C (21). To assess the effect of decreased growth rate on the total yield of a protein containing a PPX motif, we used a polyproline GFP reporter construct. Wild-type (WT) and Δefp E. coli transformed with pBAD30 containing either GFP or GFP with four consecutive proline residues inserted at the N terminus (4P-GFP) were grown to exponential phase (OD600 = 0.3) in Luria-Bertani broth (LB) at either 37 °C or 25 °C and washed with PBS, and GFP fluorescence was measured (19). To control for possible general translation defects between the WT and Δefp cells not directly related to polyproline peptide bond formation in the 4P-GFP construct, we compared relative levels of 4P-GFP protein production with that of GFP without a polyproline motif by dividing the emission signal of 4P-GFP by the GFP signal. If translation of GFP is equal to that of 4P-GFP, the value of 4P-GFP/GFP should be ∼1. If this number is less than 1, GFP is being made more efficiently than 4P-GFP, which is indicative of a PPX translation defect.
In agreement with previous studies, we see that 4P-GFP translation efficiency is about equal to that of GFP in WT, but is significantly reduced in the absence of EF-P at 37 °C (Fig. 1). We also confirmed that the low fluorescence levels in Δefp strains at 37 °C corresponded with low protein levels through Western blotting (SI Appendix, Fig. S1). This data show that in the absence of EF-P, addition of the 4P motif to GFP results in a greater than 95% decrease in protein yield. When this experiment is performed with cells cultured at 25 °C, we observe that insertion of a 4P motif has no change on the relative protein yield in the absence of EF-P. In our system, we observe that at 25 °C, the effect of slow PPX peptide bond formation on protein yield is relieved consistent with that seen with weakening the SD and translation start sequences (18). These data suggest that slowing translation through slow growth conditions suppresses the effect of slow peptide bond formation at PPX motifs. In the absence of EF-P, peptide bond formation at PPX motifs limits translation rate, causing ribosome queuing and decreasing protein output potential (18). When E. coli grows at 25 °C, the reduced rate of translation prevents ribosome queuing in the absence of EF-P, leaving total protein yield unaffected.
Fig. 1.
Total yield of GFP with four proline residues at the N terminus (4P-GFP) is unaffected by the absence of EF-P at 25 °C. (Top) Graphical representation of 4P-GFP construct. 4X-Pro represents four consecutive prolines at the N terminus of GFP. (Bottom) Bars indicate the average ratio of 4P-GFP to GFP fluorescence after 1 h of induction with 1 mM IPTG between three biological replicates in WT or efp::kan (Δefp) E. coli BW25113. Values normalized to OD600 at time of reading. Error bars represent the SD between ratios for each replicate. Experiment was performed at either 37 °C or 25 °C, as indicated. Significance was determined using Student’s t test (*P ≤ 0.05; N.S., P > 0.05).
Slowed Translation Rate at 25 °C Masks Global Translation Defect in Δefp Cells.
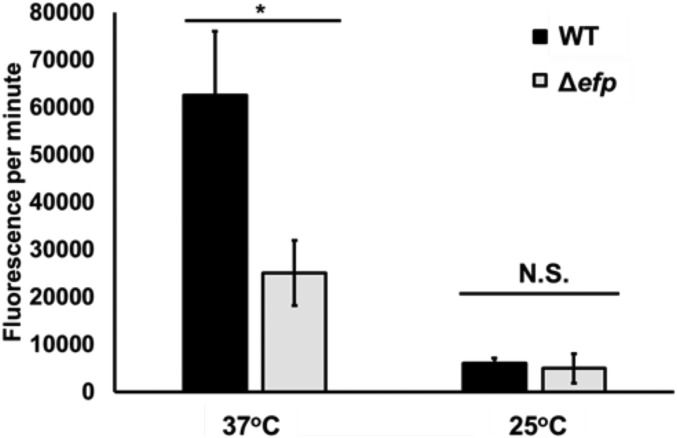
Ribosome profiling studies have shown that loss of EF-P can cause significant pausing in up to 50% of PPX motifs in E. coli, which affects cellular concentrations of PPX containing proteins (19, 22). To assess the effect of slow PPX synthesis on global protein production, we use biorthogonal noncanonical amino acid tagging (BONCAT). BONCAT uses a noncanonical amino acid, which has an available substrate to interact with a fluorescent dye in a copper-mediated alkyne-azide cycloaddition (23). This technique has been used to detect translational activity over time in both pure and mixed cultures (24). In this study, we use BONCAT to measure changes in global protein synthesis in the absence of EF-P. We labeled WT and Δefp E. coli with l-azidohomoalanine (AHA), a substrate for methionine tRNA synthetase that can be readily used in translation, at both 37 °C and 25 °C. By determining the incorporation of AHA into the proteome over time through conjugation with the dye Cy3, we can correlate an increase in fluorescence over time to protein synthesis rates.
In this assay, we observe a significantly greater AHA incorporation rate in WT compared with Δefp at 37 °C (Fig. 2 A and C), which is likely attributed to a reduced synthesis rate of PPX-containing proteins in Δefp cells that leads to a decrease in the overall rate of AHA incorporation because of the decrease in translation being inconsistent with the average abundance of PPX motifs under active translation and PPX-containing transcript abundance in exponential growth (25, 26). This effect is absent at 25 °C (Fig. 2 B and C), where general protein synthesis rates are slowed for both strains (Fig. 2 B and C). At this temperature, there is no difference in the rate of WT and Δefp incorporation rates, likely because of the rate of PPX synthesis being closer to that of the overall translation rate at 25 °C.
Fig. 2.
Cells lacking EF-P incorporate AHA into the proteome less rapidly than WT at 37 °C. Bars indicate the average increase in AHA incorporation over time over 4 or 60 min at 37 °C or 25 °C, respectively, in WT and Δefp cells. Incorporation of AHA determined by change in Cy3 fluorescence over time. Error bars represent SD between three biological replicates. Significance was determined using Student’s t test (*P ≤ 0.05; N.S., P > 0.05).
Polysome to Monosome Ratio Is Decreased in Slow-Growing Δefp Cells.
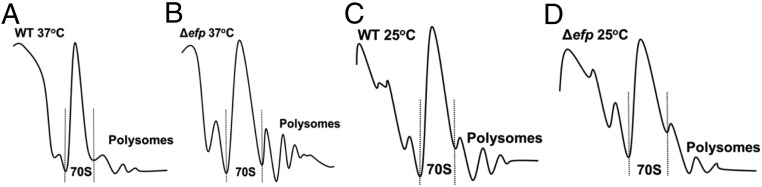
Changes in protein synthesis rates would likely also change the global translational landscape. Previous studies show that the ratio of polysomes to 70S monosomes in E. coli lacking EF-P is higher than that of WT, consistent with an increase in ribosome queuing (15). We hypothesized that decreasing translation rates using growth at 25 °C would result in more similar ribosome populations between WT and Δefp E. coli compared with that seen at 37 °C. By measuring polysome levels, we studied how changes in growth would affect the global ribosome populations in E. coli. At 37 °C, we observe an increase in the ratio of polysomes to monosomes in the Δefp cells compared with WT, a similar change to that seen in previous polysome profiling experiments (Fig. 3 and Table 1). We also report the difference in polysome-to-monosome ratios between the two strains. At 37 °C, Δefp has about a 1.8 times larger relative polysome population than WT. When grown at 25 °C, this coefficient decreases to 0.9, suggesting the global polysome landscape between WT and Δefp is similar. These data suggest that growth at 25 °C alleviates the increase in polysome population in Δefp cells likely because of changes in queuing at PPX motifs.
Fig. 3.
Polysome levels in Δefp are more similar to WT at 25 °C than at 37 °C. Images depict representative polysome profiles of WT and Δefp at 37 °C (A and B, respectively) and 25 °C (C and D, respectively). Height and width of peak represent relative abundance of ribosome species. Dotted lines separate peaks representing monosomes (70S) from the light fraction (cellular debris and subunits, left) and heavy fraction (polysomes, right).
Table 1.
Polysome ratios in Δefp are more similar to WT at 25 °C than at 37 °C
| Temperature | WT | Δefp | Δefp/WT |
| 37 °C | 0.8 ± 0.2 | 1.4 ± 0.3 | 1.8 |
| 25 °C | 1.3 ± 0.2 | 1.1 ± 0.2 | 0.9 |
Ratio of quantified polysome peaks to the 70S monosome peak. Values are reported as the average of three biological repeats ±SD.
Growth at 25 °C Alleviates EF-P Dependent Defects in Cellular Physiology.
Previous studies have shown growth defects of varying intensities by deleting efp or mutating the lysine 34, the site of modification, in multiple bacteria (16, 20, 27). We hypothesized that the global change in pausing profile resulting from slower growth at 25 °C relative to 37 °C would also alleviate previously characterized phenotypes because of loss of EF-P; specifically, whole-cell defects in doubling time and antimicrobial resistance.
Slow growth suppresses doubling-time defect in Δefp E. coli.
We quantified E. coli doubling time at 37 °C and 25 °C in LB in exponential growth phase for WT and Δefp cells (Table 2). E. coli without EF-P doubled at ∼60% the rate of those in which EF-P was present, in accordance with previous findings (20). When incubated at 25 °C, the difference in doubling times of WT and Δefp cells was not significant. We noticed that relief of this phenotype is not the result of a more rapid doubling time for Δefp E. coli; rather, the WT cells are more greatly affected by the change in temperature than those without efp (Table 2).
Table 2.
Decreased growth rate at 25 °C masks translational defects
| BW25113 | CSH142 | |||
| Growth conditions | WT | Δefp | WT | K42T |
| LB 37 °C | 30 ± 1 | 45 ± 1 | 30 ± 1 | 37 ± 1 |
| LB 25 °C | 84 ± 4 | 84 ± 6 | 77 ± 1 | 82 ± 4 |
| M9 37 °C | 77 ± 7 | 85 ± 11 | Not tested | Not tested |
Values represent the average maximum doubling time in minutes of E. coli strains BW25113 and CSH142 under various growth conditions. Values are an average of three biological replicated ±SD. M9 represents M9 minimal medium with supplemented glucose. Δefp represents E. coli BW25113 efp::kan, whereas K42T represents CSH142 with an S12 ribosomal protein mutation K42T.
We hypothesized that other defects related to translation rate would be masked in growth rate-limiting conditions. To test this, we used an E. coli strain harboring a mutation in the ribosomal protein S12 (K42T). K42T has a slowed decoding step leading to ribosome hyperaccuracy (28, 29). One cost of hyperaccuracy is that K42T has a significantly slower doubling time than its parent strain, CSH142, at 37 °C. We observe that at 25 °C, the difference in growth rate between strains was not statistically significant (Table 2). This result suggests that the effects of slow growth are global, and that various translation rate defects can be masked under growth limiting conditions.
Cells lacking EF-P are as sensitive to antibiotics as WT.
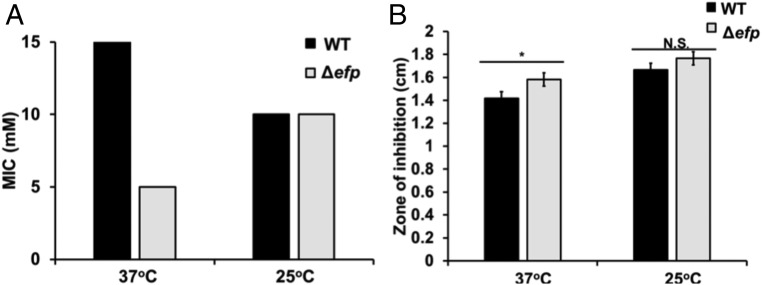
We assessed the effect of growth at 25 °C on the sensitivity to two antimicrobial agents: lauryl sulfobetaine (LSB) and polymyxin B. Both LSB and polymyxin B are chemicals known to both disrupt the E. coli membrane and have a greater effect on cells without EF-P because of the misregulation of a membrane transporter, KdgM (20). We assessed whether this phenotype is suppressed during slow growth by determining the minimal inhibitory concentration (MIC) of LSB required to inhibit E. coli growth above an OD600 of 0.05 after overnight incubation in liquid media. At 37 °C, we observe a striking difference between sensitivity to LSB between WT and Δefp E. coli, with inhibitory concentrations of 15 and 5 mM, respectively. At 25 °C, the MIC of each genotype is 10 mM (Fig. 4A). We used disk diffusion assays to test for a change in polymyxin B sensitivity at 25 °C by plating E. coli in a lawn on LB plates with a filter disk impregnated with polymyxin B overnight at either 37 °C or 25 °C. After incubation, we measured the zone of inhibition around the disk. We determined that the zones of inhibition for WT and Δefp E. coli were, on average, 142 and 158 mm, respectively. When incubated at 25 °C, the zones of inhibition increase to 167 and 177 mm in WT and Δefp cells, respectively (Fig. 4B). The difference between zones of inhibition at 37 °C between WT and Δefp cells were statistically significant at 37 °C, whereas they were not significant at 25 °C. These data are consistent with the polysome profiling data, demonstrating that the relative sensitivity to the antimicrobials LSB and polymyxin B in the Δefp cells are a product of ribosome queuing under rapid growth conditions, which was relieved at 25 °C as a result of a decrease in EF-P-dependent pausing.
Fig. 4.
Cells lacking EF-P are more sensitive to the effect of antimicrobial agents than WT at 37 °C. (A) Bars represent MIC of lauryl sulfobetaine to prevent growth over OD600 = 0.05 after 18 h growth at 37 °C or 25 °C for both WT and Δefp E. coli. Biological replicates were performed in triplicate. (B) Bars represent average zone of inhibition measured using Oxoid polymyxin B antimicrobial sensitivity disks (Thermo Fisher). Error bars represent SD between three biological replicates. Significance was determined using Student’s t test (*P ≤ 0.05; N.S., P > 0.05).
Other Growth-Limiting Conditions Relieve EF-P-Dependent Phenotypes.
Doubling-time defect in cells without EF-P is masked in nutrient-limiting conditions.
We hypothesized that the relief of EF-P-dependent phenotypes at 25 °C would be replicated using other methods of slowing E. coli growth. Previous studies have shown that under nutrient limitation, both E. coli growth and translation elongation rate are slowed (1). We first tested whether nutrient restriction would exhibit the same effects as growth at 25 °C by quantifying the doubling time of both strains in M9+Glucose minimal media at 37 °C. In this media, WT E. coli growth is slowed to a similar extent as that seen at 25 °C in LB, and the Δefp cells double nearly as rapidly as WT in minimal media (Table 2). This result suggests that it is not solely a consequence of lowered temperature that the doubling-time defect is alleviated; rather, it is an effect that can be seen in the application of different methods to slow cell growth.
WT colony morphology is similar to Δefp cells under anoxic conditions.
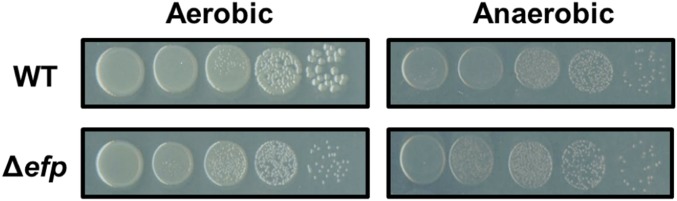
We decreased oxygen availability by growing E. coli in an anaerobic growth chamber overnight on LB plates at 37 °C and compared that with growth on plates incubated aerobically at the same temperature. We determined the effect of EF-P-dependent pausing on cell growth by comparing relative colony robustness after overnight growth (Fig. 5). Past studies have seen that E. coli without efp displays a smaller colony size than WT (30). We see a similar effect when the cells are grown aerobically, where WT colonies are larger than Δefp cells. However, when cultures are incubated in an anaerobic growth chamber, the difference in colony size is masked. As was seen with the effects of temperature and nutrient availability on Δefp-related phenotypes, WT cells seem to be more affected by the change from aerobic growth to anaerobic growth compared with those without EF-P. These data suggests that under restricted environmental conditions, EF-P is not required for optimal growth, and that under these conditions, cells without EF-P can maintain WT cell physiology.
Fig. 5.
Colony morphology of WT cells phenocopy Δefp in anaerobic conditions. WT (Top) and Δefp (Bottom) E. coli were plated on LB solid media and incubated at 37 °C either aerobically (Left) or anaerobically (Right). Cells were plated in dilution series from 10−4 to 10−8.
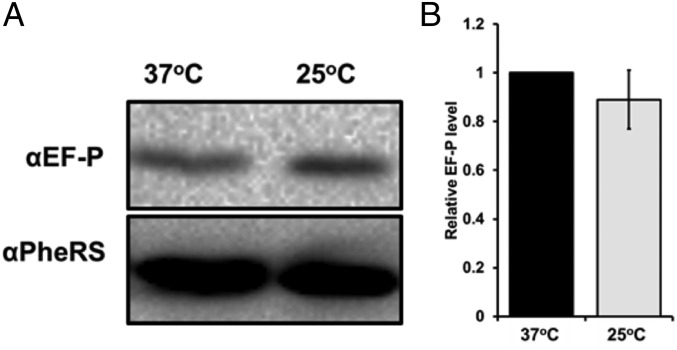
Cellular Concentrations of EF-P Are Slightly Decreased at 25 °C.
We hypothesized that a reduced role of EF-P under slow growth conditions would correlate with lower cellular EF-P protein concentrations. We extracted lysate from WT cells grown exponentially and quantified relative EF-P levels using SDS/PAGE electrophoresis and Western blot (Fig. 6). We observed that on average, EF-P levels were reduced by ∼10% at 25 °C, demonstrating a modest cellular response to a change in the role of EF-P under slow growth conditions.
Fig. 6.
EF-P levels in WT are unchanged at 25 °C. (A) Representative Western blot using αEF-P (Top) and αPheRS loading control (Bottom) antibodies. (B) Bars represent relative EF-P levels at each temperature compared with levels at 37 °C. Band intensity was quantified using ImageJ software.
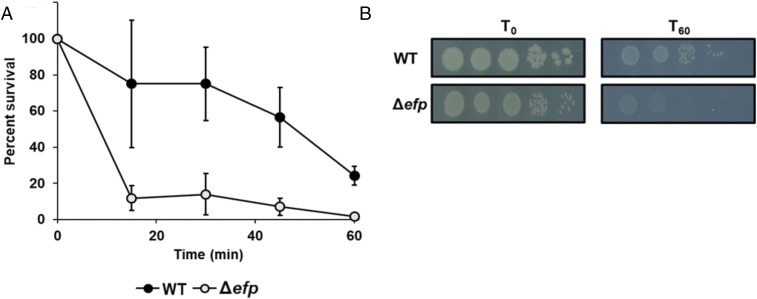
E. coli Without EF-P Is More Sensitive to Heat Stress than WT.
Given our previous results, we hypothesized that cells without EF-P would be unable to rapidly adapt to high translational demands. To test this, we use heat stress to compare the robustness of WT and Δefp cells after heat shock by placing 1 mL exponentially growing E. coli (OD600 = 0.3) in a dry bath heated to 55 °C for 1 h. We observe that WT cells are more resistant to heat stress than Δefp cells (Fig. 7). We also performed a growth curve at 42 °C, a condition in which we find that only cells without EF-P are affected (SI Appendix, Fig. S2). At this temperature, we observe an extended lag phase when Δefp cells are cultured at 42 °C, suggesting a delay in adaptation to heat stress compared with WT. These data suggest that EF-P prevents translational bottlenecks under conditions in which rapid protein production is required for cellular survival (31–33).
Fig. 7.
Cells without EF-P are more sensitive to high temperatures than WT. (A) Percentage of surviving cells over time when exposed to 55 °C dry bath. WT (closed circles) and Δefp (open circles) E. coli were plated every 15 min for 1 h while under heat stress. Survival was counted using cell counting as represented in B. Cells were plated in dilution from 10−4 to 10−8 on LB solid media.
Discussion
EF-P Allows Rapid Protein Synthesis Under High Translational Demands.
Comparison of known EF-P related phenotypes between 37 °C and 25 °C indicates that when global translation rate is decreased, many consequences of EF-P-dependent pausing are suppressed from the single transcript level to effects on whole-cell physiology. Our data demonstrate that global effects resulting from loss of EF-P are present specifically when E. coli requires rapid growth and increased translation rate. This effect is best observed when Δefp cells are assayed in conditions that require rapid protein synthesis for survival, such as under heat stress (31). Under heat stress, many genes encoding factors such as chaperones and proteases must be translated quickly for cell survival. In our heat shock assay, we see that cells without EF-P are not as fit as WT, suggesting they are unable to efficiently synthesize one or many of the protein products required for thermotolerance. These results are comparable to those observed in experiments performed to determine the role of multiple ribosomal RNA operons (rrns) in E. coli (34). In that study, E. coli with fewer rrns were unable to adapt to changing environmental conditions as rapidly as the WT, a result of having fewer available ribosomes to rapidly translate crucial adaptation proteins. Our protein synthesis rate and heat shock assays suggest a similar effect in cells lacking EF-P: Not only will PPX containing proteins be underrepresented, but pausing at PPX motifs also could sequester ribosomes away from other important transcripts. We also see the effect of translation rate on cell fitness in conditions in which all codons are translated slowly, using the K42T mutant. Only under conditions that allow rapid growth do we observe the fitness cost of hyperaccurate ribosomes, where ribosomes cannot accommodate WT protein synthesis rates and limit overall growth rate. This suggests that adaptation to dynamic translational demands is critical for cellular fitness.
Our results demonstrate an inability of Δefp E. coli to maintain cellular homeostasis during changes in its environment. For example, in a scenario in which E. coli both with and without EF-P is doubling at the same rate within a growth-limiting environment such as the gastrointestinal tract, and there is an influx of nutrients, cells without EF-P would be unable to compete with those that are able to quickly adapt to a sudden demand for rapid protein synthesis. Rapid protein synthesis in response to changing environments is also required for pathogenic organisms. One example of this is that pathogenic E. coli O157:H7 has the highest PPX content of E. coli strains, in turn increasing its reliance on EF-P to allow for responses to rapid shifts in the environment that are common in a pathogenic lifestyle (35).
EF-P Functions in Concert with Other Environmental Sensing Systems.
Recent studies have shown that protein synthesis can be regulated through temperature-sensing mRNA structural changes that can sequester or allow for recognition of specific translational signals (36, 37). Our data demonstrate that EF-P-dependent translation contributes to this model for regulation by facilitating PPX peptide bond formation, which would otherwise limit translation rate when rapid protein synthesis is required. Queuing of ribosomes on PPX-containing transcripts in the absence of EF-P during environmental shifts would decrease the concentration of available ribosomes to translate critical factors for adaptation, decreasing cell fitness. Further evidence for EF-P as a housekeeping factor is that there are no significant changes in EF-P concentrations at different temperatures, preventing delays in protein synthesis because of low levels of EF-P and allowing for immediate synthesis of PPX motifs when required.
Alternative Mechanisms for EF-P-Dependent Pausing Under Slow Growth Conditions.
Solely acting as a rapid growth factor seems to contrast with EF-P being a universally conserved translation elongation factor, even within slowly growing organisms. It is likely that although we have determined that global translation rates at PPX motifs are similar between WT and Δefp E. coli in slow growth conditions, EF-P-dependent pausing may not be completely relieved for every motif. Ribosome profiling data shows that the “X” positions in PPX motifs are crucial for determining the strength of the pause (19, 22). When translation rates are decreased, weaker pauses may be affected to an extent at which pausing is negligible in the absence of EF-P, whereas stronger pauses still influence protein yield. Curiously, we also see that in the characteristically slow-growing organism Mycobacterium tuberculosis, EF-P is thought to be an essential protein (38). This may be explained by the high density of proline in its proteome, which would be a strategy to employ EF-P-mediated translational regulation while growing slowly. This complements bioinformatic data suggesting strategic insertion of PPX motifs for regulatory function (35, 39, 40). From our findings, we conclude that EF-P may not only play a role in tuning translation rates under static conditions but also is a crucial component in cell fitness under dynamic environmental challenges.
Materials and Methods
GFP Reporter Assays.
GFP reporter assays were performed as described previously, with slight modification (16). One microliter E. coli BW25113 (WT) and E. coli BW25113 efp::kan (Δefp) were grown to OD600 = 0.3 in LB at 37 °C or 25 °C were induced with 0.02% arabinose and grown for 1 h at the same temperature. The cells were pelleted and washed once with 1× PBS. GFP signal was normalized to the OD600 at the time of sampling.
Polysome Profiling.
Polysome profiling experiments were performed as previously described (15). Briefly, E. coli cultures grown in LB at either 37 °C or 25 °C to exponential phase (OD600 = 0.6–0.8) were rapidly pelleted on ice and resuspended in lysis buffer without antibiotics. The resuspensions were lysed using freeze-thaw in liquid nitrogen. The resulting supernatant was separated in a 10–40% sucrose gradient by centrifugation at 35,000 × g for 3 h. The resulting gradient was then run through a sucrose pump, and the A260 was measured and used to determine the integral of the peaks corresponding to the 70S and polysome peaks.
BONCAT.
Determination of AHA incorporation was performed as previously described (24). E. coli BW25113 and E. coli BW25113 efp::kan were cultured at 37 °C or 25 °C in Mops media supplemented with 0.2% glucose (41). At OD600 = 0.3, 1 mM AHA was added to the media and 1 mL was taken every 1 or 15 min for samples grown at 37 °C or 25 °C, respectively. At the time of collection, cells were fixed and stored at −20 °C until all samples were processed. After addition of Cy3 dye, cells were washed in PBS and fluorescence was measured in a Horiba Fluorolog spectrofluorometer with an excitation of 554 nm and emission of 568 nm.
Growth Curves.
E. coli BW25113, E. coli BW25113 Δefp, E. coli CSH142, and E. coli CSH142 S12 K42T were grown in overnight cultures at 37 °C in LB except for the minimal media assays in which they were grown overnight in the same media in which the growth curves were conducted. Overnight cultures were diluted to OD600 = 0.05 in 5 mL fresh LB or M9 minimal media supplemented with glucose (2 g/L glucose,1 mg/L thiamine,1 mM MgSO4, and 0.1 mM CaCl2), as appropriate. The diluted cultures were then moved to the appropriate temperatures and OD600 values were taken every 30 min until the OD600 reached 2.0.
Antimicrobial Resistance Assays.
Disk diffusion.
E. coli strains grown to exponential phase in LB were spread plated on LB solid media and allowed to dry. Once dried, a single disk impregnated with polymyxin B (Oxoid) was placed in the center of the plate and incubated at 37 °C or 25 °C for 18 h.
MIC.
Overnight cultures of E. coli strains in LB were diluted 1:1,000 in fresh 2 mL LB liquid media containing the appropriate concentrations of LSB. Cultures were incubated at either 37 °C or 25 °C for 18 h while shaking. After incubation, OD600 measurements were recorded.
Anaerobic Growth.
E. coli strains were grown to exponential phase in LB at 37 °C. A dilution series was made from 10−1 to 10−5 in PBS, and 5 µL was spotted on LB agar plates. Plates were placed in an anaerobic jar with an AnaeroPack anaerobic gas generator (Thermo Fisher). This jar was incubated at 37 °C, and after 18 h, plates were imaged.
Western Blotting.
WT E. coli grown to exponential phase were lysed in lysis buffer using sonication. For detection of GFP, samples were taken every 15 min for 1 h postinduction. Protein concentrations in the clarified supernatant was determined using a NanoDrop spectrophotometer (Thermo Fisher). Thirty micrograms protein were run on a 15% polyacrylamide gel (0.1% SDS). Proteins were resolved at 180 V for 1 h and transferred to a nitrocellulose membrane. To probe for both EF-P and PheRS, the membrane was cut at 30 kD, where the bottom half was probed for EF-P or GFP and the top half was probed for PheRS using αEF-P or αGFP and αPheRS antibodies, respectively. These membranes were then probed using horseradish peroxidase-conjugated goat anti-rabbit antibodies for EF-P and donkey anti-mouse antibodies for PheRS (ProSci) and GFP (LifeTein). Blots were developed using Pierce ECL substrate (Thermo Fisher). Band intensity was quantified using ImageJ software.
Thermotolerance Assay.
E. coli strains grown to OD600 = 0.3 in 5 mL LB at 37 °C were placed in a dry bath preheated to 55 °C. Every 15 min, 100 µL was removed and put in 900 µL cold PBS. A dilution series was then made from 10−1 to 10−5, and 5 µL was spotted on LB agar plates. After 18 h incubation at 37 °C, spots containing 10–50 colonies were counted.
Supplementary Material
Acknowledgments
We thank K. Fredrick for helpful comments on the manuscript. This work was supported by National Institutes of Health training Grant T32 GM086252 (to R.T. and A.W.), Ohio State University Center for RNA Biology Fellowship (to A.W.), and National Institutes of Health Grant GM065183 (to M.I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812025115/-/DCSupplemental.
References
- 1.Dai X, et al. Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat Microbiol. 2016;2:16231. doi: 10.1038/nmicrobiol.2016.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai X, et al. Slowdown of translational elongation in Escherichia coli under hyperosmotic stress. MBio. 2018;9:e02375-17. doi: 10.1128/mBio.02375-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butkus ME, Prundeanu LB, Oliver DB. Translocon “pulling” of nascent SecM controls the duration of its translational pause and secretion-responsive secA regulation. J Bacteriol. 2003;185:6719–6722. doi: 10.1128/JB.185.22.6719-6722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doerfel LK, et al. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science. 2013;339:85–88. doi: 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- 5.Ude S, et al. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science. 2013;339:82–85. doi: 10.1126/science.1228985. [DOI] [PubMed] [Google Scholar]
- 6.Pavlov MY, et al. Slow peptide bond formation by proline and other N-alkylamino acids in translation. Proc Natl Acad Sci USA. 2009;106:50–54. doi: 10.1073/pnas.0809211106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doerfel LK, et al. Entropic contribution of elongation factor P to proline positioning at the catalytic center of the ribosome. J Am Chem Soc. 2015;137:12997–13006. doi: 10.1021/jacs.5b07427. [DOI] [PubMed] [Google Scholar]
- 8.Peil L, et al. Distinct XPPX sequence motifs induce ribosome stalling, which is rescued by the translation elongation factor EF-P. Proc Natl Acad Sci USA. 2013;110:15265–15270. doi: 10.1073/pnas.1310642110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuller AP, Wu CC, Dever TE, Buskirk AR, Green R. eIF5A functions globally in translation elongation and termination. Mol Cell. 2017;66:194–205.e5. doi: 10.1016/j.molcel.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelechano V, Alepuz P. eIF5A facilitates translation termination globally and promotes the elongation of many non polyproline-specific tripeptide sequences. Nucleic Acids Res. 2017;45:7326–7338. doi: 10.1093/nar/gkx479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaha G, Stanley RE, Steitz TA. Formation of the first peptide bond: The structure of EF-P bound to the 70S ribosome. Science. 2009;325:966–970. doi: 10.1126/science.1175800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huter P, et al. Structural basis for polyproline-mediated ribosome stalling and rescue by the translation elongation factor EF-P. Mol Cell. 2017;68:515–527.e6. doi: 10.1016/j.molcel.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Katoh T, Wohlgemuth I, Nagano M, Rodnina MV, Suga H. Essential structural elements in tRNA(Pro) for EF-P-mediated alleviation of translation stalling. Nat Commun. 2016;7:11657. doi: 10.1038/ncomms11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarre WW, et al. PoxA, yjeK, and elongation factor P coordinately modulate virulence and drug resistance in Salmonella enterica. Mol Cell. 2010;39:209–221. doi: 10.1016/j.molcel.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullwinkle TJ, et al. (R)-β-lysine-modified elongation factor P functions in translation elongation. J Biol Chem. 2013;288:4416–4423. doi: 10.1074/jbc.M112.438879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajkovic A, et al. Cyclic rhamnosylated elongation factor P establishes antibiotic resistance in Pseudomonas aeruginosa. MBio. 2015;6:e00823. doi: 10.1128/mBio.00823-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajkovic A, et al. Translation control of swarming proficiency in Bacillus subtilis by 5-amino-pentanolylated elongation factor P. J Biol Chem. 2016;291:10976–10985. doi: 10.1074/jbc.M115.712091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hersch SJ, Elgamal S, Katz A, Ibba M, Navarre WW. Translation initiation rate determines the impact of ribosome stalling on bacterial protein synthesis. J Biol Chem. 2014;289:28160–28171. doi: 10.1074/jbc.M114.593277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elgamal S, et al. EF-P dependent pauses integrate proximal and distal signals during translation. PLoS Genet. 2014;10:e1004553. doi: 10.1371/journal.pgen.1004553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou SB, et al. Loss of elongation factor P disrupts bacterial outer membrane integrity. J Bacteriol. 2012;194:413–425. doi: 10.1128/JB.05864-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu M, Dai X, Wang Y-P. Real time determination of bacterial in vivo ribosome translation elongation speed based on LacZα complementation system. Nucleic Acids Res. 2016;44:e155. doi: 10.1093/nar/gkw698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammad F, Woolstenhulme CJ, Green R, Buskirk AR. Clarifying the translational pausing landscape in bacteria by ribosome profiling. Cell Rep. 2016;14:686–694. doi: 10.1016/j.celrep.2015.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Best MD. Click chemistry and bioorthogonal reactions: Unprecedented selectivity in the labeling of biological molecules. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 24.Hatzenpichler R, et al. In situ visualization of newly synthesized proteins in environmental microbes using amino acid tagging and click chemistry. Environ Microbiol. 2014;16:2568–2590. doi: 10.1111/1462-2920.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohapatra S, Choi H, Ge X, Sanyal S, Weisshaar JC. Spatial distribution and ribosome-binding dynamics of EF-P in live Escherichia coli. MBio. 2017;8:e00300-17. doi: 10.1128/mBio.00300-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajkovic A, Ibba M. Elongation factor P and the control of translation elongation. Annu Rev Microbiol. 2017;71:117–131. doi: 10.1146/annurev-micro-090816-093629. [DOI] [PubMed] [Google Scholar]
- 27.Yanagisawa T, et al. Neisseria meningitidis translation elongation factor P and its active-site arginine residue are essential for cell viability. PLoS One. 2016;11:e0147907. doi: 10.1371/journal.pone.0147907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma D, Cukras AR, Rogers EJ, Southworth DR, Green R. Mutational analysis of S12 protein and implications for the accuracy of decoding by the ribosome. J Mol Biol. 2007;374:1065–1076. doi: 10.1016/j.jmb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Björkman J, Samuelsson P, Andersson DI, Hughes D. Novel ribosomal mutations affecting translational accuracy, antibiotic resistance and virulence of Salmonella typhimurium. Mol Microbiol. 1999;31:53–58. doi: 10.1046/j.1365-2958.1999.01142.x. [DOI] [PubMed] [Google Scholar]
- 30.Yanagisawa T, Sumida T, Ishii R, Takemoto C, Yokoyama S. A paralog of lysyl-tRNA synthetase aminoacylates a conserved lysine residue in translation elongation factor P. Nat Struct Mol Biol. 2010;17:1136–1143. doi: 10.1038/nsmb.1889. [DOI] [PubMed] [Google Scholar]
- 31.Straus D, Walter W, Gross CA. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of sigma 32. Genes Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- 32.Straus DB, Walter WA, Gross CA. The heat shock response of E. coli is regulated by changes in the concentration of σ 32. Nature. 1987;329:348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- 33.Ewalt KL, Hendrick JP, Houry WA, Hartl FU. In vivo observation of polypeptide flux through the bacterial chaperonin system. Cell. 1997;90:491–500. doi: 10.1016/s0092-8674(00)80509-7. [DOI] [PubMed] [Google Scholar]
- 34.Condon C, Liveris D, Squires C, Schwartz I, Squires CL. rRNA operon multiplicity in Escherichia coli and the physiological implications of rrn inactivation. J Bacteriol. 1995;177:4152–4156. doi: 10.1128/jb.177.14.4152-4156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi F, Motz M, Jung K, Lassak J, Frishman D. Evolutionary analysis of polyproline motifs in Escherichia coli reveals their regulatory role in translation. PLOS Comput Biol. 2018;14:e1005987. doi: 10.1371/journal.pcbi.1005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, et al. A stress response that monitors and regulates mRNA structure is central to cold shock adaptation. Mol Cell. 2018;70:274–286.e7. doi: 10.1016/j.molcel.2018.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan GJ, Burkhardt DH, Kelly JW, Powers ET. Translation efficiency is maintained at elevated temperature in Escherichia coli. J Biol Chem. 2018;293:777–793. doi: 10.1074/jbc.RA117.000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhyay S, Balaji KN. The PE and PPE proteins of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2011;91:441–447. doi: 10.1016/j.tube.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Akhter Y, Ehebauer MT, Mukhopadhyay S, Hasnain SE. The PE/PPE multigene family codes for virulence factors and is a possible source of mycobacterial antigenic variation: Perhaps more? Biochimie. 2012;94:110–116. doi: 10.1016/j.biochi.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 41.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.