Abstract
Purpose
We determine the feasibility of using a home-based tablet device to monitor retinal sensitivity (RS) in intermediate age-related macular degeneration (iAMD), the benefits of weekly reminders, and the comparison with clinic-based results.
Methods
A customized test for tablets was designed to measure RS (within central 2°) in individuals with iAMD at weekly intervals in their home, with remote data collection. Half of the participants were randomized to receive weekly test reminders. Clinic-based microperimetric macular sensitivity results were compared to tablet results. Participation rates were analyzed at 2 months.
Results
Of 38 participants (mean age, 70.3 years) with iAMD enrolled in the study, 21 (55%) were using the tablet-based test at 2 months. Common reasons for inactivity were noncompatible devices (41.1%) or other technology access issues (35.3%). Participants with weekly reminders completed tests more regularly (6.6 ± 3.9 vs. 8.7 ± 4.1 days, P = 0.01), but weekly reminders showed no effect on participation rates (P = 0.69). Mean RS from the tablet device (25.03 ± 2.41 dB) was not significantly different from the clinic-based microperimetry performance (25.21 ± 2.20 dB; P = 0.58).
Conclusions
Regular monitoring of retinal function on a tablet device in a home setting in individuals with iAMD is feasible with results comparable to those of clinic-based microperimetry. Weekly reminders resulted in more frequent testing. Seamless ability to access technology will be important for higher participation rates.
Translational Relevance
The use of home-monitoring on a tablet-device is promising, but adequate support for an older cohort to take up technology is required if such a tool is to be useful for long-term home monitoring.
Keywords: iPad, tablet, visual function, microperimetry, age-related macular degeneration, home monitoring
Introduction
The widespread use of intravitreal injections of antivascular endothelial growth factor (anti-VEGF) for neovascular age-related macular degeneration (nAMD) has transformed the hitherto bleak outlook for this devastating complication of AMD, with significant reductions in the rates of legal blindness being reported.1,2 However, the positive outcomes of anti-VEGF treatment on visual acuity (VA) are not uniform. We, and others have identified that a delay to treatment, with resultant poor presenting vision, is a major factor in determining the final VA after treatment.3,4 In results from one large series of anti-VEGF treatments in the United Kingdom, it was clear that while those presenting with poor vision, gained vision, they remained with relatively poor vision and never achieved the same vision as those who presented with good vision before treatment.5 A recent special communication reiterated the large amount of data that now support the need for earlier intervention for better vision outcomes.6
Therefore, it is clear that a major impact on saving vision could be made by gaining access to “at risk” individuals earlier, when neovascular complications occur, so that treatment could commence before irreversible loss of vision occurs. However, the onset of nAMD usually occurs without warning and often remains undetected; thus, leading to delayed presentation and irreversible damage to the retina before commencing treatment, limiting the ability of the anti-VEGF therapy to restore vision.
The Amsler grid is the current standard of care for individual home monitoring in those identified at risk of nAMD. Despite being used for over 50 years, it is well known that the Amsler grid detection rate is low. One study showed the detection of visual abnormalities to be less than 30% in patients who subsequently required treatment for nAMD.7 A need exists for a totally different, modern approach in the design of a monitoring tool that addresses all issues related to delayed presentation.8–10
Home monitoring devices already are available and approved by the United States Food and Drug Administration (FDA), such as the ForeseeHome instrument and the MyVisionTrack (mVT) application, but both work on shape discrimination hyperacuity (SDH). In a trial, the ForeseeHome instrument reported detecting nAMD with less loss of VA when compared to standard clinical care.11 Unfortunately, 20% of the cohort did not continue using the device, during the average participation of 1.4 years.11 The positive results of the trial, however, led to home monitoring for AMD, using the ForeseeHome device, being covered by Medicare in the USA. The mVT smart phone application was the first FDA-approved application for tracking changes to visual function before VA loss in diabetes mellitus and AMD, and requires a monthly subscription.12 A pilot study for mVT in AMD, performed in clinic and remotely, was encouraging in showing that elderly individuals (64% >75 years old) were compliant over a 16-week period to regularly test their eyes using a portable handheld device.13 As an alternative to evaluating hyperacuity, our aim was to explore home monitoring, testing retinal sensitivity using a portable, tablet-based application, with an easily scalable monitoring and surveillance system, the result of which could be validated with a sensitivity measure on clinic-based microperimetry.14,15
Central retinal sensitivity in mesopic conditions using microperimetry has been shown to be a sensitive measure of AMD-related functional deficit within the macula.16 Formal microperimetry testing however requires clinic-based, expensive and relatively immobile devices that require trained staff to instruct, help, and monitor the patient while they perform the test. We developed a tablet-based application with a purpose-designed central retinal sensitivity test using the open access PsyPad platform.17,18 In the clinical setting, we found a good correlation between macular sensitivity measured on formal microperimetry and that recorded using the tablet application.18 We also reported that among a large group of people with intermediate AMD with a mean age of 70.5 years, 91.4% used one or more personal electronic devices. Of participants aged 50 to 69 years, 92% were willing to use a tablet device to monitor their vision, compared to 78% of those aged 70 years and older.19
Thus, we determined the initial feasibility of using our tablet-based application in a home setting in a cohort of people with intermediate AMD (iAMD), at high risk for nAMD. We recorded retinal sensitivity in the home, compared the results to those obtained in the clinic with microperimetry, and assessed the benefit of using text message (SMS) reminders to participants' phones to influence the regularity of testing as it has been reported to have significant improvement in prevention modification practices in various clinical settings, such as self-examination breast screenings,20 use of peak flow monitoring in asthma,21 and glucose monitoring in diabetes.22
Methods
This prospective study involved consecutive participants with iAMD who were currently involved in natural history AMD research studies where microperimetry was performed. It was approved by the human ethics committee of the Royal Victorian Eye and Ear Hospital (RVEEH) and was conducted in adherence with the Declaration of Helsinki. Written informed consent was obtained from all participants following an explanation of all test procedures.
Participants
Participants aged 50 years and over, with bilateral drusen >125 μm (a subset of iAMD,23 taking part in natural history studies, with best corrected VA (BCVA) of >60 letters read (>20/60) or better were approached for participation in this substudy. To be eligible, participants required ready access at home to an iPad 2 tablet device or later (Apple, Inc., Cupertino, CA) as these were the only tablets able to run the application, and an internet connection. Exclusion criteria were late AMD in either eye; other ocular diseases that could have influenced retinal sensitivity, such as glaucoma, significant cataracts, corneal pathology, diabetes, uncontrolled hypertension, amblyopia, neurological or systemic disease affecting vision; or any medication known to affect retinal function. Participants also were excluded if they had any physical and/or mental impairment preventing them from participating in this study or an inability to sign a consent form.
Procedure
Consecutive participants taking part in a natural history study were asked to participate in the tablet monitoring study and their AMD status was confirmed during the consultation with multimodal imaging. Participants underwent formal ophthalmic examination, including BCVA tested using a standardized refraction protocol monocularly using an Early Treatment of Diabetic Retinopathy Study (ETDRS) refraction chart at a testing distance of 4 m. Central retinal sensitivity at recruitment into this substudy was performed using the macular assessment integrity analyzer (MAIA; CenterVue, Padova, Italy) microperimetry before any imaging or examination was performed. Retinal imaging including color fundus photography with a C46-45NM non-mydriatic retinal camera (Canon, Inc. Tokyo, Japan) and spectral-domain optical coherence tomography (SD-OCT) scans using a Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany) was performed.
During the clinical visit, eligible participants were educated to use the customized retinal sensitivity application (described below). They were advised to test their eyes using the application while wearing their reading glasses in the same darkened area and were prompted to test one eye at a time while maintaining a distance of 50 cm from the screen to their eyes (a string was given to participants to assist them with this procedure). A follow-up call was conducted with the aim to ensure that participants were able to use their personal iPad, log on to the PsyPad platform, and access the customized test while at their home. Initial PsyPad tests completed in the clinic before commencing home monitoring were not used in this study.
After completion of the study, participants who did not remain actively engaged in home monitoring at the 2-month time period were contacted by phone by the coordinator to ask their reasons for ceasing the home monitoring test to gain a better understanding of reasons for noncompliance.
Measurements of Retinal Sensitivity Using the PsyPad App
Using the open access application platform PsyPad,17 our group developed a test to measure the central retinal sensitivity to luminance increment. The PsyPad platform required at least an iPad 2 and operating system of iOS 8 (Apple, Inc.). Participants downloaded the PsyPad application from the Apple App Store onto their personal iPad device with the guidance of research staff either in the clinic or over the phone.
The PsyPad platform17 and previous testing algorithms have been described in full previously.18 However in brief, the PsyPad platform allows a library of images (customized Portable Network Graphics [PNG] files) to be displayed at specified timing and with standard algorithms (in our case, a 4-2 staircase with two reversals over 12 locations in the central 2° of fixation). Central retinal sensitivity, in decibels, was defined as mean threshold estimate across the stimuli. Test results were automatically uploaded to a central server when the iPad was next connected to the internet. This allowed for remote analyses of retinal sensitivity and rates of use for each participant.
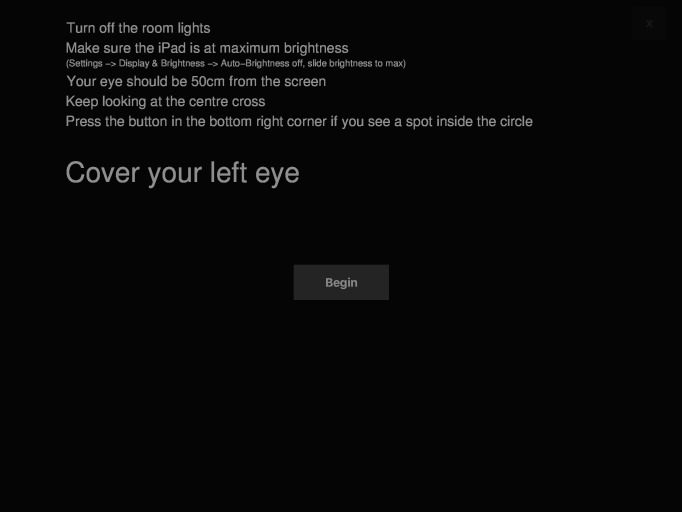
Compared to the predecessor study,18 we changed our test to start with an instruction screen (Fig. 1a) and increased the area of retinal sensitivity assessed from 1° to 2° across the central macula (based on a 50 cm viewing distance). Previously, only five points were used to assess retinal sensitivity (one point at 0° and four points at 1°) and utilized a red fixation ring with a radius of 3°. We modified the test to include a white central fixation cross at 0° and fixation ring with a radius of 3° (Fig. 1b). The number of retinal sensitivity assessment points was increased to 12 (four points at 1° and eight points at 2°). We previously utilized the PR-650 Spectra-Scan Colorimeter (Photo Research, Inc., Chatsworth, CA) to assess the luminance of the iPad. Our results showed the test images consisted of a uniform black background (luminance of 1.27 cd/m2) and white circular stimuli (Goldmann Size III, or 0.43° based on a 50 cm viewing distance) at specific luminance levels across a 31 dB dynamic range and in 1 dB increments (this corresponded to minimum and maximum stimuli luminance of 1.52 and 317.50 cd/m2, respectively).
Figure 1a.
Instruction screen for the test on the PsyPad platform. The example given is before the test for the left eye. Once the left eye is completed, a similar screen appears before commencement of testing the right eye.
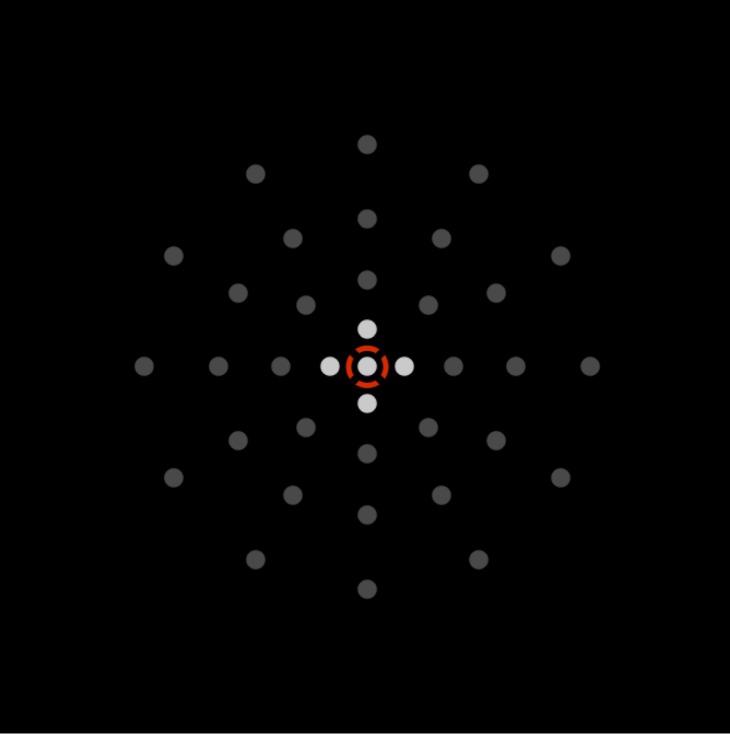
Figure 1b.
Illustration of the customized test designed on the PsyPad platform to measure central retinal sensitivity (within the central 2° radius) and the location of the 12 test stimuli are shown. The participant focuses on the central fixation target with an outer ring (white ring, 3° in radius). Participants responded to seeing a stimulus at any of the 12 circular locations by pressing the gray square at the bottom corner.
SMS Reminder and Randomization
An aim of the study was to analyze how compliant and consistent participants were in using the test at least weekly and whether an SMS reminder increased the frequency of the testing and reduced dropout rate with the testing procedure. Therefore, participants were randomized into one of two groups; either to receive weekly SMS messages that prompted the participant to perform their iPad test, or to receive no reminders. Randomization of participants occurred at the initial visit in clinic. The message received in the reminder group was as follows - “PsyPad Reminder from Centre for Eye Research Australia: Please complete your weekly PsyPad test. Call (contact name and phone number inserted) if you have any problems or changes to your vision.”
Formal Microperimetry Assessment
Participants had their retinal sensitivity measured in clinic with the MAIA microperimetry at the time they were set up for the home monitoring study. MAIA measures individual's retinal sensitivity across a dynamic range of 36 dB (luminance from 318–1.37 cd/m2) at points 0°, 1°, 2.3°, 4°, and 6° (in radius) over the central macula. The MAIA microperimeter performs fundus tracking with a line scanning laser ophthalmoscope at a rate of 25 frames per second. A red circle fixation target was used with a diameter of 1°, while a 4-2-staircase threshold strategy with a Goldman III sized stimuli on a 1.27 cd/m2 background was performed. As our test on the tablet was designed to measure only retinal sensitivity at 1° and 2°, only the central retinal sensitivity recorded from the MAIA was averaged for this study; 12 points at 1° and 2.3° from the fovea (Fig. 2). The reliability of the test was determined as the percentage of false-positive responses (to suprathreshold stimuli at the optic nerve head, that was manually placed before the beginning of the threshold measurements). Any test with a >25% false-positive rate was excluded from the study. All participants underwent two full examinations of both eyes. The first test of both eyes was deemed a practice and, therefore, only the second tests were used for comparison with the results obtained on the PsyPad platform.
Figure 2.
Customized grid on microperimetry with the fixation target represented by the red ring. The central retinal sensitivity defined as the average of the 12 points (lighter gray points) at 1 and 2.3 radius was used in this study to compare to the PsyPad sensitivity.
Statistical Analysis
To report on the feasibility of the application over the initial 2-month period, participants were divided into those who were still actively using the test at the 2-month mark and those inactive participants who had not performed the test for at least 30 days.
We assessed the frequency of performing the test, 2 months after initial randomization. Welch Two Sample t-tests were used to examine differences in examination duration, testing frequency, and age across two groups. Participation rates and sex were compared using Pearson's χ2 test.
For comparison of mean retinal sensitivity using formal microperimetry (MAIA) and the first home test performed on the PsyPad platform, a paired t-test was used to assess statistical difference and the 95% limits of agreement were calculated using a method described by Bland and Altman.24 All data processing and statistical analyses were performed using the open source statistical software R.25
Results
A total of 38 consecutive iAMD participants (average age, 70.3 ± 7.5 years; range, 56–83 years) were initially enrolled into the study. By the time all had been enrolled for 2 months, 21 individuals had ongoing participation with the application (10 [47.6%] were in the SMS reminder group, with 11 [52.2%] not receiving reminders). Of the 17 inactive participants, 7 (41.2%) were in the SMS reminder group and 10 (58.8%) had no reminders; the proportion of participants in each group was not significantly different between those who were ongoing or inactive after two months (Fisher's exact P = 0.752).
Characteristics of Test Parameters on PsyPad
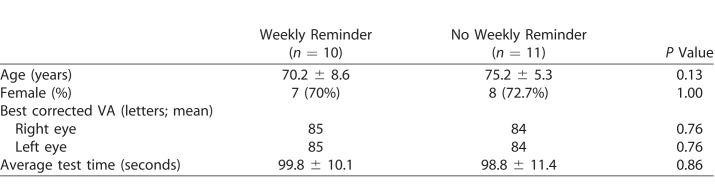
A statistically significant difference in age was evident between those still participating (72.8 ± 7.3 years) and those inactive (67.2 ± 6.6 years) at 2 months (t[35.62] = 2.48, P = 0.02). Participation rates were similar for sex (P = 0.69) and study group (SMS reminder vs. no reminder; P = 0.69) at 2 months. A summary of participants' demographic and characteristics of test parameters is shown in Table 1 for individuals who successfully continued with home testing for 2 months.
Table 1.
Participants who Completed 2 Months of Testing (60 days)
The test duration per eye across both groups (reminder and nonreminder) was 99.3 ± 10.8 seconds (mean ± standard deviation), with no significant difference between groups (t[983.11] = 1.52, P = 0.13). Participants in the reminder group more frequently performed their tests compared to the no-reminder group during the initial first 2 months (P = 0.01); however, there was no significant difference between the total number of tests completed within 2 months when comparing those who did and did not have reminders (13.34 vs. 13.01 tests, respectively, P = 0.87).
We looked further at the 17 inactive participants to determine reasons for their inactivity. Seven of the 17 participants (41.1%) were unable to download and activate the application on their home iPad. This was either due to the iPad having an operating system that would not support the application, or a screen that lacked the appropriate resolution. Another six (35.3%) inactive participants started using the application, but for technology reasons, had to cease, such as an upgrade being required in the operating system but not implemented, or the participant forgot how to log on and use the application. Three of 17 (17.6%) participants were unable to continue because of personal reasons due to either being medically unwell or a family member being unwell. One person (5.9%) felt that the test was ‘not useful' and ‘too easy.' There was no significant difference in baseline BCVA between those who were ongoing or inactive after 2 months (P = 0.93).
Agreement Between PsyPad at Home and Microperimetry
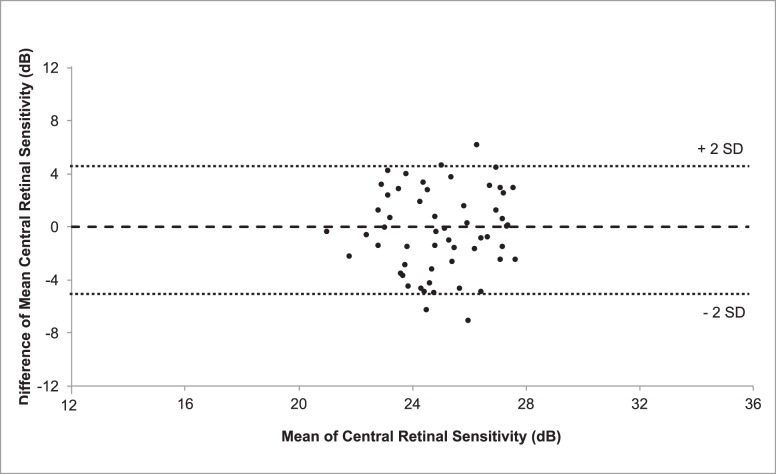
The results of the first tablet examination for each eye, performed at home, after practice in the clinic, were compared to those of the microperimetry test performed on randomization in the clinic. A paired t-test showed that mean retinal sensitivity (± standard error of mean) was not statistically different between the PsyPad (25.03 ± 2.41 dB) and microperimetry central sensitivity (25.21 ± 2.20 dB; P = 0.58). The 95% limits of agreement between the two modalities were between −5.39 and 4.87 dB. Figure 3 illustrates the Bland-Altman plot to demonstrate the agreement between the two methods.
Figure 3.
Bland-Altman plot of mean central retinal sensitivity measurements obtained using PsyPad and microperimetry, illustrating the agreement between the two measures (the difference was obtained by subtracting the central retinal sensitivity results of microperimetry from PsyPad). Horizontal dashed lines represent the upper limits of 95% of the mean (+2 SD), mean and lower limits of 95% of the mean (−2 SD) from top to bottom respectively, and the solid line represents zero.
Discussion
Our pilot study for monitoring AMD, using retinal sensitivity on a portable tablet “in the home” device shows that the concept is feasible and offers some promising results, in agreement with formal, clinic-based microperimetry and the improved frequency of testing in those with reminders. The high level of inactivity was disappointing; however, over three-quarters of these participants had issues with their personal iPad not being able to support the application or having other issues with their ability to continue to access the application to keep using it, rather than the participants losing interest in the task. Indeed, only one participant ceased activity due to their not perceiving the usefulness of the tool. Ideally these compatibility issues and ease of access to the technology would be addressed to improve ongoing activity in any further testing. Going forward in larger trials, it will be important to have a well-resourced help desk facility and actively re-engage with participants not returning any tests.
Our study showed that at least half of elderly individuals (average age, 70 years) with iAMD were active and compliant, over 2 months, using tablet technology to regularly test themselves in their home environment. Weekly reminders resulted in an improvement in the time between tests by an average of 2.3 days in the first 2 months. This highlights the important role of prompts to ensure improved compliance with the recommended testing frequency of at least once a week. Although this difference was statistically significant, the actual difference of 2.3 days for testing frequency is likely to have a minimal impact on the early detection of nAMD. However, this study established the use of such a strategy that warrants evaluation in a longer-term study, since its effect could have a greater impact on long-term adherence. Age was the only characteristic identified that was different between the participation rates. Those who stopped performing the test were on average younger than those who continued performing the test at 2 months (P = 0.02). One reason for this counterintuitive result may be that older individuals would be more likely to be retired and, thus, have more time to invest in their own health and in the study.
The study extended our previous work, which compared retinal sensitivity achieved with formal clinic-based microperimetry conducted by trained staff, with results obtained on the tablet device in the same controlled clinical environment and supervised by the same trained staff.18,26 While the laboratory-based microperimetry method was unchanged, we increased the complexity of the tablet-based task by increasing the test of five points in the central 1° of the macula to 12 points across the central 2° of the macula and by asking the participants to perform the testing unsupervised at home.
The study found good agreement between retinal sensitivity measured on the tablet device in the home and that using formal clinic-based, supervised microperimetry. We note that thresholds gathered with an iPad-based test across a wider field of view in people with glaucoma, also show values similar to those with automated perimeters.27 Part of the success of the agreement is attributed to the quality of tablet screen technology. The iPad screen used in the study is able to provide a dynamic range of 31 dB for the visual stimuli. Other than the MAIA microperimeter used in the study (dynamic range, 36 dB), most commercially available microperimeters can only produce a dynamic range of 20 dB. The agreement when comparing tablet and controlled clinical results also has been demonstrated previously with contrast sensitivity.28,29
Our study for monitoring mean central retinal sensitivity in participants with iAMD showed promising initial results, but has limitations. We did not have the capability to enforce testing parameters or check whether individuals were adhering to them. For example, our test had no means of checking testing distance, ambient lighting, screen brightness, gaze direction, whether spectacle corrections were being worn (if required) or which eye was being used. We relied on educating participants to maintain a consistent testing environment, with instructions reiterated before each assessment (Fig. 1a) and provided a string to guide them as to the correct viewing distance of the iPad. Another limitation to our study is a potential for selection bias of a population that is perhaps more tech savvy as our cohort was selected based upon the requirement for a smartphone for SMS reminders, an iPad tablet for test application, and internet access at home.
The weekly reminder system worked effectively in improving testing regularity; however, it did not result in better 2-month participation rates. The high percentage of inactivity at 2 months is disappointing, but was more a reflection of a participant having technology that was not as up to date as perhaps younger participants might have and as a result could not support our application. However, poor continued engagement is reported by other home monitoring devices. The ForseeHome device recorded a 20% noncompliance rate at 1.4 years11 and mVT had a 64% compliance rate in those over 75 years old at 16 weeks.13
A number of strategies could be considered to improve long-term activity. Additional incentives, prior training, or more frequent reminders based upon monitored compliance of participants may be simple examples that could potentially improve participation rates. We can learn from other examples where techniques adopted from the gaming industry have shown that combining player involvement, immediate performance feedback and social connectivity via competition, improves motivation and increase adherence to the task, such as engagement in health-related activities.30–32 We aim to take a similar approach to improve long-term compliance for self-monitoring vision, by implementing features, such as offering immediate feedback on the frequency of testing with appropriate encouragement (e.g., positive reinforcement).
Future developments in software design, longer study period, and robust testing of tablet-based home monitoring retinal functional tests will be required to determine whether such tests can identify cases with retinal sensitivity changes corresponding with the earliest indication of nAMD with the aim of more rapid access to treatment before permanent loss of vision is incurred.
Conclusion
This pilot study found that elderly individuals would engage in an activity to regularly test their visual function using the PsyPad platform on an iPad in a home setting. Participants completed home testing more regularly with weekly SMS reminders than those without. The sensitivity results obtained in the home mirrored those obtained in formal clinic-based perimetry. Issues and solutions related to initially accessing and continuing to access an application from personal electronic equipment is important and must be considered as part of any future trial design. The study highlights the potential feasibility for using home-based digital methods with remote surveillance for monitoring visual function in people at high risk of vision loss from AMD and the benefit of reminders in increasing testing frequency. This opens up enormous opportunities to optimize “in the home” monitoring of the community at risk of vision loss, for whom long-term treatment outcomes are significantly better the sooner interventions are started.
Acknowledgments
Supported by The National Health and Medical Research Council), Principal Research Fellowship (GNT1103013) and Early Career Fellowship (#1104985, ZW). The Centre for Eye Research Australia (CERA) receives Operational Infrastructure Support from the Victorian Government.
Disclosure: M. Adams, None; C.Y.D. Ho, None; E. Baglin, None; P. Sharanan, None; Z. Wu, None, D. J. Lawson, None, C.D. Luu, None, A. Turpin, None, A.M. McKendrick, None, R.H. Guymer, Advisory Board member for Novartis, Bayer, Roche/Genentech (C)
References
- 1.Block S, Larsen M, Munch I. Incidence of legal blindness from age-related macular degeneration in denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153:209–213. doi: 10.1016/j.ajo.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Bressler N, Doan Q, Dolan C, Ferreira A, Obsorne A. Estimated cases of blindness and visual impairment from neovascular age-related macular degeneration avoided in Australia by ranibizumab treatment. PLoS One. 2014;9:e101072. doi: 10.1371/journal.pone.0101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim J, Wickremasinghe S, Xie J, Chauhan D, Baird P, Robman L. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am J Ophthalmol. 2012;153:678–686. doi: 10.1016/j.ajo.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldstein S, Simader C, Staurenghi G, Chong N, Mitchell P, Jaffe G. Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age-related macular degeneration in the VIEW trials. Ophthalmology. 2016;12:1521–1529. doi: 10.1016/j.ophtha.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Tufail A, Xing W, Johnston R, Akerele T, McKibbin M, Downey L, et al. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121:1092–1101. doi: 10.1016/j.ophtha.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Ho A, Albini T, Brown D, Boyer D, Regillo C, Heier J. The potential importance of detection of neovascular age-related macular degeneration when visual acuity is relatively good. JAMA Ophthalmol. 2017;135:268–273. doi: 10.1001/jamaophthalmol.2016.5314. [DOI] [PubMed] [Google Scholar]
- 7.Zaidi F, Cheong-Leen R, Gair E, Weir R, Sharkawi E, Lee N. The Amsler chart is of doubtful value in retinal screening for early laser therapy of subretinal membranes. The West London Survey. Eye (Lond) 2004;18:503–5038. doi: 10.1038/sj.eye.6700708. [DOI] [PubMed] [Google Scholar]
- 8.Bittner A, Torr-Brown S, Arnold E, Nwankwo A, Beaton P, Rampat R. Improved adherence to vision self-monitoring with the vision and memory stimulating (vms) journal for non-neovascular age-related macular degeneration during a randomized controlled trial. Clin Exp Ophthalmol. 2014;5:320. doi: 10.4172/2155-9570.1000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervantes-Castaneda R, Banin E, Hemo I, Shpigel M, Averbukh E, Chowers I. Lack of benefit of early awareness to age-related macular degeneration. Eye. 2007;22:777–781. doi: 10.1038/sj.eye.6702691. [DOI] [PubMed] [Google Scholar]
- 10.Crossland M, Rubin G. The Amsler chart: absence of evidence is not evidence of absence. Br J Ophthalmol. 2007;91:391–393. doi: 10.1136/bjo.2006.095315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chew E, Clemons T, Bressler S, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121:535–544. doi: 10.1016/j.ophtha.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micheletti J, Hendrick A, Khan F, Ziemer D, Pasquel F. Current and next generation portable screening devices for diabetic retinopathy. J Diabetes Sci Technol. 2016;10:295–300. doi: 10.1177/1932296816629158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser P, Wang Y, He Y, Weisberger A, Wolf S, Smith C. Feasibility of a novel remote daily monitoring system for age-related macular degeneration using mobile handheld devices: results of a pilot study. Retina. 2013;33:1863–1870. doi: 10.1097/IAE.0b013e3182899258. [DOI] [PubMed] [Google Scholar]
- 14.Aslan T, Murray I, Lai M, Linton E, Tahir H, Parry N. An assessment of a modern touch-screen tablet computer with reference to core physical characteristics necessary for clinical vision testing. J R Soc Interface. 2013;10:20130239. doi: 10.1098/rsif.2013.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahir H, Murray I, Parry N, Aslam T. Optimisation and assessment of three modern touch screen tablet computers for clinical vision testing. PLoS One. 2014;9:e95074. doi: 10.1371/journal.pone.0095074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunness J, Rubin G, Broman A, Applegate C, Bressler N, Hawkins B. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1480–1488. doi: 10.1016/j.ophtha.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turpin A, Lawson DJ, McKendrick AM. PsyPad: a platform for visual psychophysics on the iPad. J Vis. 2014;14:16. doi: 10.1167/14.3.16. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Guymer R, Jung C, Goh J, Ayton L, Luu C. Measurement of retinal sensitivity on tablet devices in age-related macular degeneration. Transl Vis Sci Technol. 2015;4:13. doi: 10.1167/tvst.4.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razavi H, Baglin E, Sharangan P, et al. Gaming to improve vision: 21st century self-monitoring for patients with age-related macular degeneration. Clin Exp Ophthalmol. 2018;46:480–484. doi: 10.1111/ceo.13097. [DOI] [PubMed] [Google Scholar]
- 20.Khokhar A. Short text messages (SMS) as a reminder system for makingworking women from Delhi Breast Aware. Asian Pac J Cancer Prev. 2009;10:319–322. [PubMed] [Google Scholar]
- 21.Ostojic V, Cvoriscec B, Ostojic S, Reznikoff D, Stipic-Markovic A, Tudjman Z. Improving asthma control through telemedicine: a study of short-message service. Telemed J E Health. 2005;11:28–35. doi: 10.1089/tmj.2005.11.28. [DOI] [PubMed] [Google Scholar]
- 22.Hanauer D, Wentzell K, Laffel N, Laffel L. Computerized Automated Reminder Diabetes System (CARDS): e-mail and SMS cell phone text messaging reminders to support diabetes management. Diabetes Technol Ther. 2009;11:99–106. doi: 10.1089/dia.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferris F, Wilkinson C, Bird A. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;129:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bland J, Altman D. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–582. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- 25.Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austra: R Foundation for Statistical Computing;; 2015. [Google Scholar]
- 26.Ho C, Wu Z, Turpin A, et al. A tablet-based retinal function test in neovascular age-related macular degeneration eyes and at-risk fellow eye. Trans Vis Sci Tech. 2018;7:2. doi: 10.1167/tvst.7.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong Y, He M, Crowston J, Vingrys A. A comparison of perimetric results from a tablet perimeter and Humphrey field analyzer in glaucoma patients. Transl Vis Sci Technol. 2016;5:2. doi: 10.1167/tvst.5.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorr M, Lesmes L, Lu Z, Bex P. Rapid and reliable assessment of the contrast sensitivity function on an iPad. Invest Ophthalmol Vis Sci. 2013;54:7266–7273. doi: 10.1167/iovs.13-11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kollbaum P, Jansen M, Kollbaum E, Bullimore M. Validation of an iPad test of letter contrast sensitivity. Optom Vis Sci. 2014;91:291–296. doi: 10.1097/OPX.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 30.Baranowski T, Buday R, Thompson D, Baranowski J. Playing for real: video games and stories for health-related behavior change. Am J Prevent Med. 2008;34:74–82. doi: 10.1016/j.amepre.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett A, Cerin E, Baranowski T. Active video games for youth: a systematic review. J Phys Act Health. 2011;8:724–737. doi: 10.1123/jpah.8.5.724. [DOI] [PubMed] [Google Scholar]
- 32.Gamberini L, Alcaniz M, Barresi G, Fabregat M, Prontu L, Sergalia B. Playing for a real bonus: videogames to empower elderly people. J CyberTher Rehab. 2008;1:37–47. [Google Scholar]