Abstract
Many infectious diseases have emerged or reemerged in Africa in the 21st century. Some of them are associated with newly discovered microorganisms such as Rickettsia felis and Tropheryma whipplei; others are known, historical diseases such as plague and cholera. In addition are diseases related to previously known microorganisms which recently have been involved for the first time in massive outbreaks with worldwide impacts (such as Ebola virus, Zika virus and Chikungunya virus). Research on emerging infectious diseases needs to be identified as a priority.
Keywords: Africa, cholera, emerging infectious diseases, fever, plague
At the beginning of the 21st century, infectious diseases remain responsible for about one quarter of deaths worldwide, causing at least 10 million deaths per year, mainly in the tropical countries [1], [2]. Emerging infectious diseases are a high burden on public health but have also an impact on global economies [3]. Their origin is generally connected to social and economic conditions as well as environmental and ecologic factors [3]. A substantial risk of wildlife zoonotic and vector-borne emerging infectious diseases exists mainly at lower-latitude developing countries such as tropical Africa [3]. Overall, 60% of emerging infectious diseases are zoonoses, of which 72% are from wildlife [3].
Emerging infectious diseases are mainly those that have recently appeared in a population or have already existed but are rapidly increasing in incidence or geographic range [4]. Although much depends on the type of emerging infection, the development of modern rapid, sensitive and accurate methods of microorganism detection has played an important role in the diagnosis and identification of emerging infectious diseases, such as for the molecular detection of Rickettsia felis and Tropheryma whipplei.
Several factors of emergence have been identified: microbial adaptation and change, human susceptibility to infection, climate and weather, changing ecosystems, human demographics and behavior, economic development and land use, international travel and commerce, technology and industry, breakdown of public health measures, poverty and social inequality, war and famine, lack of political will and intent to harm [5]. Unfortunately, most of these factors are overrepresented in many countries of Africa. Thus, many emerging diseases are reported from or originated from Africa. Among the most important are HIV infection and malaria, which emerged from wild monkeys [6]. Finally, improved disease assessment through improved public health surveillance could also contribute to the apparent onset and reappearance of some diseases.
Here we briefly describe new diseases and emerging or reemerging known diseases that have been involved in outbreaks since the beginning of the 21st century in Africa. Although most are zoonotic, some of them, such as T. whipplei infections, cholera and measles, do not have a zoonotic component.
Africa can be seen as distinct from the rest of the world, and even from other parts of the developing world which are also affected by the emergence and reemergence of diseases. Most dangerous infectious agents that kill humans originate in Africa. In addition, the difficulties with morbidity surveillance in Africa are well known. In the context of the multiple epidemics that have been recognized over the past decade, numerous assessments of surveillance systems have revealed problems essentially across the board [7]. Poverty and poor healthcare exacerbate health problems. African countries rank among the lowest in per-capita spending on health and the availability of physicians. According to the Worldwatch Institute (http://www.worldwatch.org/), in 2001 an average of $36 per person was spent on healthcare in Africa. The spending amounted only $6 in Niger, $7 in Sierra Leone and $15 in Nigeria compared to $4800 in the United States. Moreover, 32% of the population is undernourished; five of the six worst countries for mortality of children under 5 years are in Africa, with an under-5 mortality rate above 100 deaths per 1000 live births, according to the World Health Organization. Half of all deaths in Africa are caused by infectious diseases, compared to only 2% in Europe. This means that Africa should be the number one priority in the world community's struggle against infectious diseases. Surveillance of emerging infectious diseases is vital for the early identification of public health threats.
Rickettsia felis was reported for the first time in the United States and was officially described in 2002. Before 2010, the bacterium was mainly found in arthropods, especially in cat fleas, Ctenocephalides felis, with a worldwide distribution. It was also considered as a rare cause of rickettsial spotted fever transmitted by fleas. Only about 70 described human cases of infection due to R. felis have been reported. In 2010, two studies conducted in Senegal and Kenya by two independent teams showed a prevalence of about 4% of R. felis DNA in blood samples of febrile individuals without malaria (Table 1) [8], [9]. A more consistent study, involving 2024 febrile and 391 afebrile subjects, confirmed the role of R. felis in fever in Senegal [10]. R. felis was also detected in eschar samples (5/68, 7.4%) and skin swabs (3/60, 5%) from healthy Senegalese villagers [11]. The presence of R. felis was also observed in faecal samples from Senegalese villagers (8/451) [12]. In Gabon, R. felis was detected in blood samples of febrile patients, reaching a prevalence of 39.7% in the rural area of Fougamou [13]. The bacterium was also detected in the rural area of Lastourville (11.2%) and, less frequently, in the city areas of Koulamoutou (2.1%) and Franceville (1.3%) [3], [14]. Overall, R. felis is commonly detected in febrile patients in sub-Saharan Africa. R. felis is also usually detected in cat fleas, although not in Senegal [15]. Anopheles gambiae mosquitoes are suspected to transmit the bacterium; this was strongly supported by a recent experimental model [16], [17]. R. felis was also detected in a nonhaematophagous ubiquitous household pest, the booklouse Liposcelis bostrychophila, which also may play a role in the R. felis transmission [18]. However, an experimental model remained to be developed to better determine its role. Moreover, the omnipresence of L. bostrychophila in households and its 100% level of R. felis infection may explain the occasional detection of R. felis on healthy skin [11]. Other rickettsial diseases also may be considered to be neglected and emerging diseases in Africa [19]: African tick-bite fever is more frequently being diagnosed among travellers returning from Africa [20].
Table 1.
Emerging and reemerging bacterial diseases in Africa in 21st century
| Bacteria | Implications for Africa | Targets for prevention | References |
|---|---|---|---|
| Rickettsia felis |
|
Vector-control measures | [8], [9], [10], [13] |
| Tropheryma whipplei |
|
Sanitation facilities | [22], [26], [56] |
| Yersinia pestis (plague) |
|
Reduce risk of wildlife-to-human transmission | [29], http://www.who.int/csr/don/27-november-2017-plague-madagascar/en/ |
| Vibrio cholera (cholera) |
|
Appropriate water and sanitation facilities Oral cholera vaccination (transient protection about 3–5 years) To be alert during conflicts or natural disasters |
[32], [33], http://www.who.int/csr/don/archive/disease/cholera/en/ |
DRC, Democratic Republic of the Congo.
Tropheryma whipplei is the agent of Whipple disease, a chronic disease mainly observed in white men about 50 years old. The disease was first described in 1907, but the bacterium was cultured only in 2000 [21], [22]. The high prevalence of asymptomatic carriage of T. whipplei in feces and saliva was reported in 2008 in Senegal [23] and in 2016 in Ghana [24]. T. whipplei was also detected in the blood of febrile Senegalese patients without malaria, with a prevalence of 6.4% (Table 1) [21]. No correlation was reported between the presence of T. whipplei in stool and saliva and bacteraemia, but the presence of one significant clinical sign, cough, was observed. T. whipplei was also found in important quantities in bronchoalveolar lavage samples in Malawi [25]. In the Sine-Saloum area in Senegal in August 2010, T. whipplei was the main detected cause of fever (58.5% in febrile patients in the village of Dielmo and 69% in Ndiop) [26]. Most of the cases were grouped in the same households. In addition, the same new T. whipplei genotype was detected among patients. Overall, the data show that T. whipplei is a cause of epidemic acute fever in Senegal. The examples of R. felis and T. whipplei support the notion that malaria in people with undifferentiated fever has long been overdiagnosed. They also show that diagnostic tests are essential to improve care, and that targeted surveys have a role to play in providing insight into the causes of the fever in order to suggest the tests to be performed.
Plague has a remarkable place in history. Yersinia pestis has been involved in several pandemics. It is present as a permanent focus in many countries in Africa and is still involved in epidemics (Table 1) [27]. Among recent examples, an epidemic of bubonic plague emerged in Oran (Algeria) in June 2003 [28]. The last human cases of plague had occurred in this city in 1946 and in this country in 1950. More recently, in the central highlands of Madagascar, a man visiting Ankazobe district was bitten by a flea in August 2017 [29]. Within a week, he developed malaria-like symptoms. While travelling to the eastern coast, he took a public taxi to reach the capital, where he died. A week later, an epidemic was officially confirmed. The infection of more than 2348 confirmed, probable and suspected cases was reported in November 2017. Although the disease can be treated with antibiotics if detected early, it is worth noting that more than 200 people have died [29]. Overall, the fact that there was a plague outbreak in Madagascar is not astonishing. Indeed, there is an ongoing epizootic that leads to an annual emergence of human infections [29]. This emergence is attributed to seasonal ecologic conditions that cause a decrease in rat populations and then lead fleas to feed directly on humans. The surprising features of this 2017 epidemic are that it appeared earlier than normal; it was predominantly a pneumonic form; and it hit the capital [29]. It is not just bad luck that this plague outbreak emerged in a country with an extremely fragile health system [29]. Indeed, poor access to healthcare, the lack of rapid diagnostic tests in many health facilities, the missing link between communities and the frontline health system and national health authorities as well as weakness of supply chains and infrastructure throughout the country are all factors that favour the spread of the bacterium, resulting in a high mortality rate from a treatable disease [29].
Cholera, caused by the bacterium Vibrio cholerae, is reemerging in Africa as a result of limited access to drinking water and conflicts. Overpopulation, poverty, lack of hygiene, poor sanitation facilities, contamination of food and lack of safe water are the main risk factors for cholera [30], [31]. All these elements are also among the consequences of war and civil strife. In 2006, cholera was reported in 33 African countries; 88% of reports came from conflict-affected countries [31]. Cholera epidemics occur almost every year in Africa (Table 1). The most severe outbreak of cholera was observed in Zimbabwe, with 98 585 reported cases including more than 4000 deaths from 2008 to 2009 [32], [33].
Although mortality from measles has declined in sub-Saharan Africa, it remains a major public health problem in numerous countries like the Democratic Republic of the Congo (DRC), with 294 455 cases including 5045 deaths reported between 2010 and 2013 [33], [34], [35]. A total of 186 178 patients (63%) were younger than 5 years old. After the first mass vaccination campaigns, weekly reported cases decreased by 21.5%. However, results of postvaccination campaign coverage surveys indicated suboptimal (less than 95%) vaccination coverage among children surveyed.
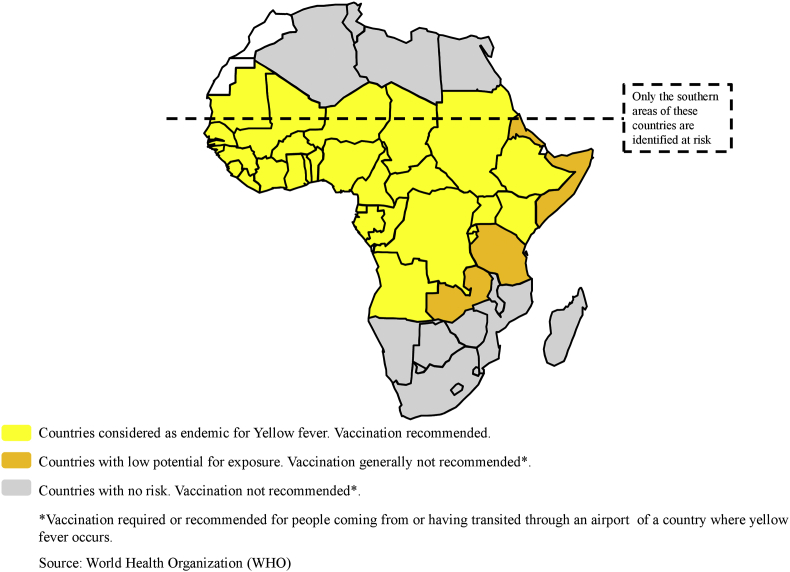
Yellow fever virus is a mosquito-borne Flavivirus (family Flaviviridae) causing infection in humans, with symptoms ranging from mild nonspecific illness to severe disease with jaundice, haemorrhage and death [36]. A single-dose vaccine has available since the 1940s. It has allowed virus transmission to be controlled and substantially reduced. However, the sylvatic cycle of the virus prevents its eradication. In this sylvatic cycle, nonhuman primates act as primary hosts, and Aedes aegypti mosquitoes are responsible for transmission to people. Lack or intermittence of vaccination campaigns, mainly in low-income and/or politically unstable countries, is another factor that can explain the failure to eradicate yellow fever in Africa (Fig. 1) [37]. Since 2000, numerous outbreaks of yellow fever have been observed in West Africa, the Horn of Africa and east-central Africa (Table 2) [36]. Since 2008, an increased number of cases have been also reported in Central African countries, raising the question of whether these reports were related to better surveillance or a real increase in disease [36]. A large epidemic of yellow fever was reported in Angola and the DRC from 2015 to 2016, with 7334 suspected cases [33], [38]. Finally, regardless of the cause of the uptick in reports (surveillance improvements or epidemic), the root cause remains the same: a lack of sustained vaccination in the human population.
Fig. 1.
Yellow fever risks in Africa and vaccination recommendations.
Table 2.
Emerging and reemerging viral diseases in Africa in 21st century
| Viral diseases | Implications for Africa: Main outbreaks since 2000 | Targets for prevention | References |
|---|---|---|---|
| Measles |
|
Sustained vaccination in human population | http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/Country_slides_measles.pdf?ua=1 |
| Yellow fever virus |
|
Sustained vaccination in human population | [33], [36], [38], http://www.who.int/csr/don/archive/disease/yellow_fever/en/ |
| Monkeypox |
|
Reduce risk of wildlife-to-human transmission | [42], [43] |
| Ebola |
|
Reduce risk of wildlife-to-human transmission | [44], [45], [46], http://www.who.int/csr/don/archive/country/cod/en/ |
| Rift Valley fever |
|
Sustained vaccination in animals; vector-control measures | [47], http://www.who.int/news-room/fact-sheets/detail/rift-valley-fever |
| Zika virus |
|
Vector-control measures | [48] |
| Chikungunya virus |
|
Vector-control measures | [50] |
DRC, Democratic Republic of the Congo.
Monkeypox is a zoonotic disease caused by Orthopoxvirus, a virus very close to that causing smallpox in humans. This virus is endemic to central and western African countries. It was first identified in 1958 as a pathogen of Macaca cynomolgus (then in use as laboratory animals) and then in 1970 was described as a human pathogen in DRC [39]. The virus is capable of infecting humans, other primates and rodents. Most of the reported cases were related to animal-to-human transmission and were associated with the handling and eating of infected animals, but several cases of human-to-human transmission occurred [40]. Since 2000, human monkeypox cases have been reported in the Central African Republic, Republic of the Congo, DRC, South Sudan, Nigeria, Liberia and Sierra Leone [41], [42]. In 2017, Nigeria experienced the largest documented outbreak around 40 years after the last confirmed cases of monkeypox [43].
Ebola fever due to Ebola virus (Filoviridae) has caused severe human epidemics identified since 1976. The transmission from animal reservoirs to humans likely happens via direct contact with ill, dead or killed wild animals mainly collected as bushmeat. The virus then spreads in the human population through human-to-human transmission. Since 2001, the disease reemerged in the forest zone between Gabon and Republic of the Congo, in Uganda and the DRC [44], [45]. The world's largest ever outbreak of Ebola fever probably started in December 2013 in rural Guinea after the infection of the suspected index case, a 2-year-old child [46]. The subsequent epidemic crossed into Sierra Leone and Liberia [33]. Case numbers escalated quickly [33], [46]. Overall, 881 healthcare workers were also infected, and 513 of them died (https://www.cdc.gov/vhf/ebola/pdf/impact-ebola-healthcare.pdf). In central Africa, the outbreaks were rural epidemics that occurred in remote locations and involved relatively small case numbers, thus allowing targeted intervention in the affected villages, whereas the West African epidemic was an urban epidemic that occurred in a highly mobile population, leading to rapid spread of the virus. In DRC, new outbreaks still occur regularly (Table 2), such as the two declared in 2018.
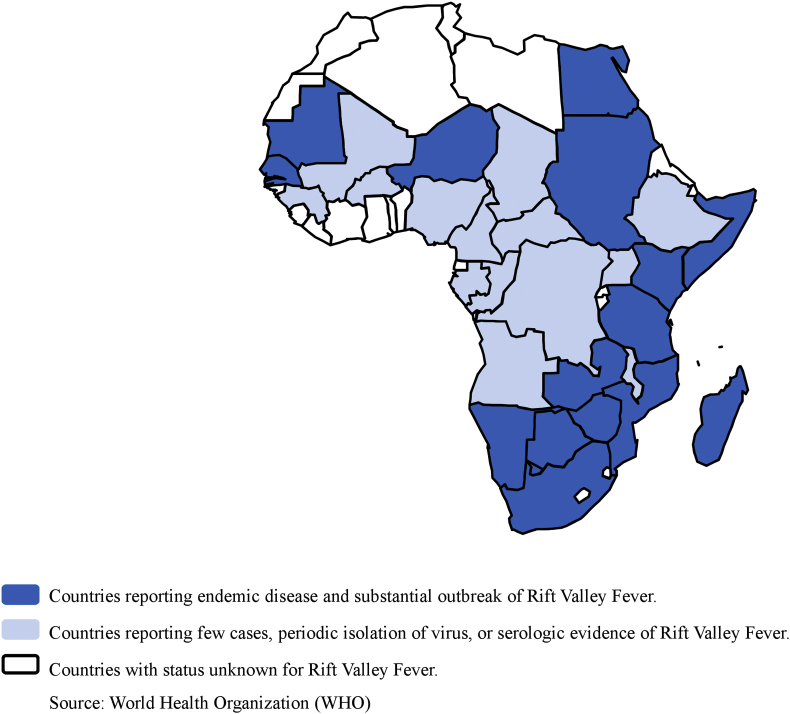
Rift Valley fever is an acute fever most commonly observed in domesticated animals (such as goats, sheep, cattle, buffalo and camels) caused by a virus, a member of the genus Phlebovirus (family Bunyaviridae), with the ability to infect and cause illness in humans. The virus was first reported in livestock by veterinary officers in Kenya's Rift Valley in the early 1910s. Rift Valley fever is endemic in several African countries (Fig. 2). Human outbreaks also regularly occur in Africa (Table 2). The two largest outbreaks were those observed in Sudan, with a suspicion of 75 000 human cases and a total of 747 confirmed human cases including 230 deaths, and in Kenya, with a total of 684 cases including 234 deaths (http://www.who.int/news-room/fact-sheets/detail/rift-valley-fever) [47].
Fig. 2.
Rift Valley Fever risks in Africa.
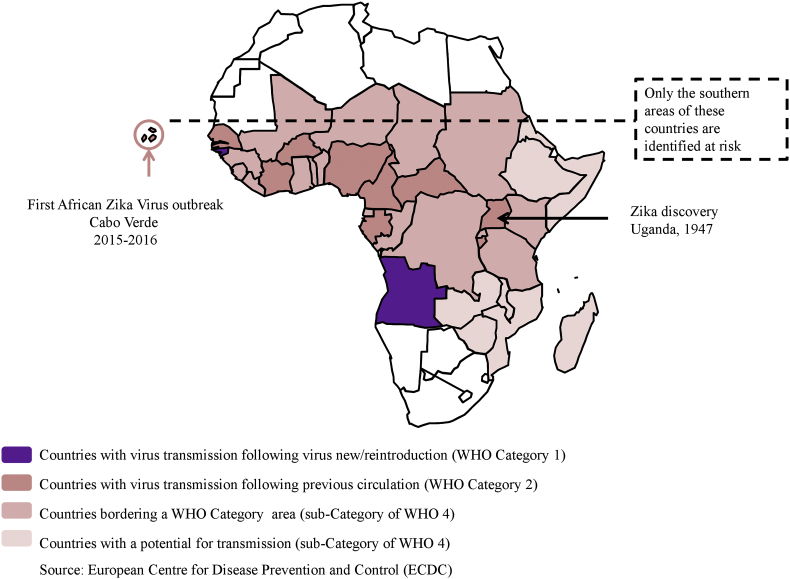
Zika virus is another Flavivirus (family Flaviridae) transmitted by Aedes mosquitoes. The virus was first detected in April 1947 in a febrile sentinel rhesus macaque monkey in the Zika forest on the shores of Lake Victoria (Uganda) [48]. The first human case was diagnosed in 1962–1963 in Uganda. Overall, data currently support a silent transmission of Zika virus among humans, mosquitoes and animals throughout tropical Africa for more than 70 years without reports of epidemic [48]. In 2007, the first epidemic occurred in the Pacific (Yap Island, Micronesia). The second epidemic affected Polynesia in 2013. The first outbreak of Zika virus in Africa was detected in 2015 in Cabo Verde (Fig. 3). Zika virus exhibited two unique features among the arboviruses: sexual transmission and congenital central nervous system malformations [48]. Sexual transmission was first suspected in an American citizen returning from Senegal and then was confirmed after its emergence in the Americas [48]. The link between Zika virus and severe malformations of the central nervous system (particularly microcephaly) has been demonstrated by many studies in Brazil and has been reported retrospectively in French Polynesia [48]. Genetic differences between African and Asian/American strains of Zika virus and appearance of the ‘pathogenic clone’ may explain the emergence of Zika virus infections in South America and Asia [48], [49].
Fig. 3.
Zika virus risks in Africa.
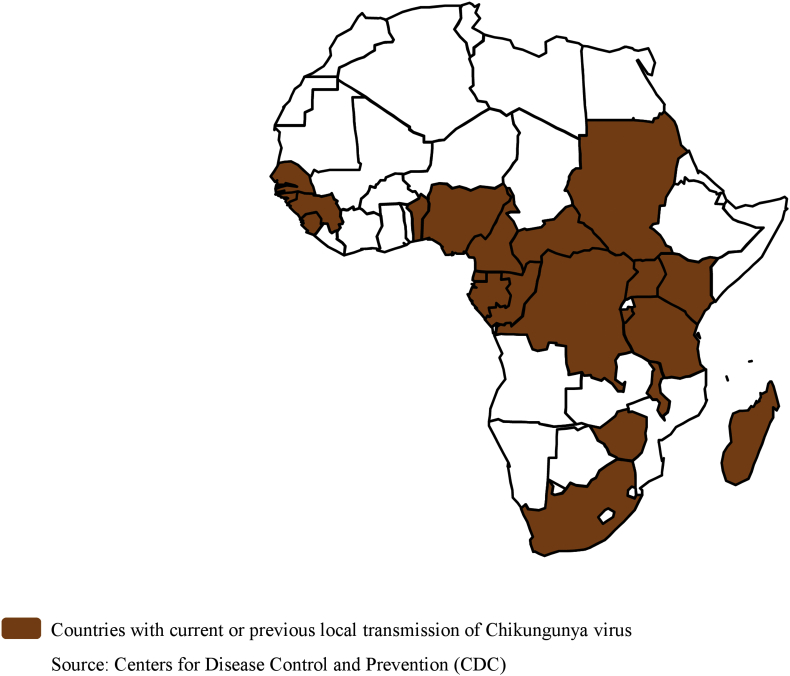
Chikungunya virus, an Alphavirus (family Togaviridae), first reported in Tanzania in 1952, is transmitted by Aedes mosquitoes [50], [51]. The virus is associated with fever with rash and arthralgias [52]. Severe joint pains can persist for a long time. From 1960 to 1990, epidemics were recorded in the DRC, Central African Republic, Malawi, Uganda, Burundi, Angola, Guinea, South Africa and Nigeria. In 2004, a large epidemic involving almost half a million cases was reported in Kenya [50]. Then this epidemic reached the southwestern Indian Ocean region, India and Southeast Asia [50], [51]. From 2004 to 2007, several outbreaks were reported in Guinea, Tanzania, Sudan, Gabon and Cameroon. Two outbreaks were also reported on La Réunion Island in 2009 and 2010 [50]. In 2011, an epidemic hit the DRC [50]. The circulation of chikungunya was also reported in Senegal in the area of Kédougou in 2015 (Fig. 4).
Fig. 4.
Chikungunya virus risks in Africa.
Many other diseases deserve to be mentioned here. Among the most remarkable is the emergence (since 2001–2002) of recombinant poliovirus strains between vaccine strains and circulating enterovirus strains. These strains derived from oral vaccination rendering poliomyelitis eradication strategies more complex [53]. Tick-borne relapsing fever, although it always existed in Western and Eastern Africa, seems to be reemerging, although this is likely the result of the growing availability of diagnostic methods in rural Africa. Indeed, relapsing fever may be the second leading cause, after malaria, of acute febrile illnesses in rural West African countries such as Senegal [54].
In conclusion, increasing efforts targeting infectious disease studies in Africa have identified new infectious agents. It concerns not only bacterial and viral diseases but also protozoal and helminthic ones. For instance, a new genetic variant of Mansonella associated with fever in children was recently reported from Gabon [55]. It is evident that Africa is characterized by the greatest infectious disease burden as well as by the weakest public health infrastructure in the world; further, efforts to establish public health infrastructures that are actually effective may take a period of years, even decades. Emerging infectious diseases should be identified as priority diseases. The challenge will be to combine surveillance and epidemic preparedness and response activities for these priority diseases. Evidently this task is quite difficult because the infrastructure and level of support for surveillance, research and training on emerging infectious diseases in Africa are limited. Laboratory-based surveillance and targeted research surveys to identify common sources of infection in different community types would allow a unified approach to target this enormous challenge. We are persuaded that the most important step towards the elimination of existing burden of infectious diseases in Africa is a massive increase in the number of qualified personnel, including both physicians and scientists.
Conflict of interest
This study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency under the program « Investissements d’avenir », reference ANR-10-IAHU-03, the Région Provence Alpes Côte d’Azur and European funding FEDER PRIMI.
References
- 1.Global Health Estimates 2016 . World Health Organization; Geneva: 2018. Deaths by cause, age, sex, by country and by region, 2000–2016. [Google Scholar]
- 2.Dye C. After 2015: infectious diseases in a new era of health and development. Philos Trans R Soc Lond B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morse S.S. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute of Medicine . National Academies Press; Washington, DC: 2003. Microbial threats to health: emergence, detection, and response. [PubMed] [Google Scholar]
- 6.Calvignac-Spencer S., Leendertz S.A., Gillespie T.R., Leendertz F.H. Wild great apes as sentinels and sources of infectious disease. Clin Microbiol Infect. 2012;18:521–527. doi: 10.1111/j.1469-0691.2012.03816.x. [DOI] [PubMed] [Google Scholar]
- 7.Perkins B.A. In: Integrated disease surveillance and epidemics: preparedness and response in Africa in emerging infectious diseases from the global to the local perspective: a summary of a workshop of the forum on emerging infections. Davis J.R., Lederberg J., editors. National Academies Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- 8.Socolovschi C., Mediannikov O., Sokhna C., Tall A., Bassene H., Trape J.F. Rickettsia felis, a common cause of uneruptive fever in rural Senegal. Emerg Infect Dis. 2010;16:1140–1142. doi: 10.3201/eid1607.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards A.L., Jiang J., Omulo S., Dare R., Abdirahman K., Ali A. Human infection with Rickettsia felis, Kenya. Emerg Infect Dis. 2010;16:1081–1086. doi: 10.3201/eid1607.091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mediannikov O., Socolovschi C., Edouard S., Fenollar F., Mouffok N., Bassene H. Common epidemiology of Rickettsia felis infection and malaria, Africa. Emerg Infect Dis. 2013;19:1775–1783. doi: 10.3201/eid1911.130361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mediannikov O., Socolovschi C., Million M., Sokhna C., Bassene H., Diatta G. Molecular identification of pathogenic bacteria in eschars from acute febrile patients, Senegal. Am J Trop Med Hyg. 2014;91:1015–1019. doi: 10.4269/ajtmh.13-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keita A.K., Fenollar F., Socolovschi C., Ratmanov P., Bassene H., Sokhna C. The detection of vector-borne-disease–related DNA in human stool paves the way to large epidemiological studies. Eur J Epidemiol. 2015;30:1021–1026. doi: 10.1007/s10654-015-0022-9. [DOI] [PubMed] [Google Scholar]
- 13.Mourembou G., Lekana-Douki J.B., Mediannikov O., Nzondo S.M., Kouna L.C., Essone J.C. Possible fole of Rickettsia felis in acute febrile illness among children in Gabon. Emerg Infect Dis. 2015;21:1808–1815. doi: 10.3201/eid2110.141825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mourembou G., Fenollar F., Socolovschi C., Lemamy G.J., Nzoughe H., Kouna L.C. Molecular detection of fastidious and common bacteria as well as Plasmodium spp. in febrile and afebrile children in Franceville, Gabon. Am J Trop Med Hyg. 2015;92:926–932. doi: 10.4269/ajtmh.14-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roucher C., Mediannikov O., Diatta G., Trape J.F., Raoult D. A new Rickettsia species found in fleas collected from human dwellings and from domestic cats and dogs in Senegal. Vector Borne Zoonotic Dis. 2012;12:360–365. doi: 10.1089/vbz.2011.0734. [DOI] [PubMed] [Google Scholar]
- 16.Dieme C., Bechah Y., Socolovschi C., Audoly G., Berenger J.M., Faye O. Transmission potential of Rickettsia felis infection by Anopheles gambiae mosquitoes. Proc Natl Acad Sci U S A. 2015;112:8088–8093. doi: 10.1073/pnas.1413835112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parola P., Mediannikov O., Dieme C., Raoult D. Reply to Slesak et al. So much about Rickettsia felis infection to be discovered. Proc Natl Acad Sci U S A. 2015;112:e6595–e6596. doi: 10.1073/pnas.1517919112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelakis E., Mediannikov O., Parola P., Raoult D. Rickettsia felis: the complex journey of an emergent human pathogen. Trends Parasitol. 2016;32:554–564. doi: 10.1016/j.pt.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Mediannikov O., Diatta G., Fenollar F., Sokhna C., Trape J.F., Raoult D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl Trop Dis. 2010;4:e821. doi: 10.1371/journal.pntd.0000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensenius M., Parola P., Raoult D. Threats to international travellers posed by tick-borne diseases. Travel Med Infect Dis. 2006;4:4–13. doi: 10.1016/j.tmaid.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Raoult D., Birg M., La Scola B., Fournier P., Enea M., Lepidi H. Cultivation of the bacillus of Whipple’s disease. N Engl J Med. 2000;342:620–625. doi: 10.1056/NEJM200003023420903. [DOI] [PubMed] [Google Scholar]
- 22.La Scola B., Fenollar F., Fournier P.E., Altwegg M., Mallet M.N., Raoult D. Description of Tropheryma whipplei gen. nov, sp. nov, the Whipple’s disease bacillus. Int J Syst Evol Microbiol. 2001;51:1471–1479. doi: 10.1099/00207713-51-4-1471. [DOI] [PubMed] [Google Scholar]
- 23.Fenollar F., Trape J.F., Bassene H., Sokhna C., Raoult D. Tropheryma whipplei in fecal samples from children, Senegal. Emerg Infect Dis. 2009;15:922–924. doi: 10.3201/eid1506.090182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinnemeier C.D., Klupp E.M., Krumkamp R., Rolling T., Fischer N., Owusu-Dabo E. Tropheryma whipplei in children with diarrhoea in rural Ghana. Clin Microbiol Infect. 2016;22 doi: 10.1016/j.cmi.2015.09.022. 65–3. [DOI] [PubMed] [Google Scholar]
- 25.Rylance J., Kankwatira A., Nelson D.E., Toh E., Day R.B., Lin H. Household air pollution and the lung microbiome of healthy adults in Malawi: a cross-sectional study. BMC Microbiol. 2016;16:182. doi: 10.1186/s12866-016-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassene H., Mediannikov O., Socolovschi C., Ratmanov P., Keita A.K., Sokhna C. Tropheryma whipplei as a cause of epidemic fever, Senegal, 2010–2012. Emerg Infect Dis. 2016;22:1229–1334. doi: 10.3201/eid2207.150441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gracio A.J.D.S., Gracio M.A.A. Plague: a millenary infectious disease reemerging in the 21st century. Biomed Res Int. 2017;2017 doi: 10.1155/2017/5696542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitam I., Baziz B., Rolain J.M., Belkaid M., Raoult D. Zoonotic focus of plague, Algeria. Emerg Infect Dis. 2006;12:1975–1977. doi: 10.3201/eid1212.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonds M.H., Ouenzar M.A., Garchitorena A., Cordier L.F., McCarty M.G., Rich M.L. Madagascar can build stronger health systems to fight plague and prevent the next epidemic. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lessler J., Moore S.M., Luquero F.J., McKay H.S., Grais R., Henkens M. Mapping the burden of cholera in sub-Saharan Africa and implications for control: an analysis of data across geographical scales. Lancet. 2018;391:1908–1915. doi: 10.1016/S0140-6736(17)33050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okonko I.O., Donbraye E., Babalola E.T., Mejeha O.K., Fadeyi A., Udeze A.O. Conflict and the spread of emerging infectious diseases: where do we go from here? Afr J Microbiol Res. 2009;3:1015–1028. [Google Scholar]
- 32.Mukandavire Z., Liao S., Wang J., Gaff H., Smith D.L., Morris J.G., Jr. Estimating the reproductive numbers for the 2008–2009 cholera outbreaks in Zimbabwe. Proc Natl Acad Sci U S A. 2011;108:8767–8772. doi: 10.1073/pnas.1019712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sands P., Mundaca-Shah C., Dzau V.J. The neglected dimension of global security—a framework for countering infectious-disease crises. N Engl J Med. 2016;374:1281–1287. doi: 10.1056/NEJMsr1600236. [DOI] [PubMed] [Google Scholar]
- 34.Maurice J. Measles outbreak in DR Congo an ‘epidemic emergency’. Lancet. 2015;386:943. doi: 10.1016/S0140-6736(15)00115-4. [DOI] [PubMed] [Google Scholar]
- 35.Ashbaugh H.R., Hoff N.A., Doshi R.H., Alfonso V.H., Gadoth A., Mukadi P. Predictors of measles vaccination coverage among children 6–59 months of age in the Democratic Republic of the Congo. Vaccine. 2018;36:587–593. doi: 10.1016/j.vaccine.2017.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monath T.P., Vasconcelos P.F. Yellow fever. J Clin Virol. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 37.Markoff L. Yellow fever outbreak in Sudan. N Engl J Med. 2013;368:689–691. doi: 10.1056/NEJMp1300772. [DOI] [PubMed] [Google Scholar]
- 38.Kraemer M.U.G., Faria N.R., Reiner R.C., Jr., Golding N., Nikolay B., Stasse S. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–16: a modelling study. Lancet Infect Dis. 2017;17:330–338. doi: 10.1016/S1473-3099(16)30513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morand A., Delaigue S., Morand J.J. Review of poxvirus: emergence of monkeypox. Med Sante Trop. 2017;27:29–39. doi: 10.1684/mst.2017.0653. [DOI] [PubMed] [Google Scholar]
- 40.Learned L.A., Reynolds M.G., Wassa D.W., Li Y., Olson V.A., Karem K. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73:428–434. [PubMed] [Google Scholar]
- 41.Durski K.N., McCollum A.M., Nakazawa Y., Petersen B.W., Reynolds M.G., Briand S. Emergence of monkeypox—west and central Africa, 1970–2017. MMWR Morb Mortal Wkly Rep. 2018;67:306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalthan E., Dondo-Fongbia J.P., Yambele S., Dieu-Creer L.R., Zepio R., Pamatika C.M. Twelve cases of monkeypox virus outbreak in Bangassou District (Central African Republic) in December 2015. Bull Soc Pathol Exot. 2016;109:358–363. doi: 10.1007/s13149-016-0516-z. [DOI] [PubMed] [Google Scholar]
- 43.Yinka-Ogunleye A., Aruna O., Ogoina D., Aworabhi N., Eteng W., Badaru S. Reemergence of human monkeypox in Nigeria, 2017. Emerg Infect Dis. 2018;24:1149–1151. doi: 10.3201/eid2406.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rouquet P., Froment J.M., Bermejo M., Kilbourn A., Karesh W., Reed P. Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003. Emerg Infect Dis. 2005;11:283–290. doi: 10.3201/eid1102.040533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebola Outbreak Epidemiology Team Outbreak of Ebola virus disease in the Democratic Republic of the Congo, April–May, 2018: an epidemiological study. Lancet. 2018;392:213–221. doi: 10.1016/S0140-6736(18)31387-4. [DOI] [PubMed] [Google Scholar]
- 46.Rojek A., Horby P., Dunning J. Insights from clinical research completed during the West Africa Ebola virus disease epidemic. Lancet Infect Dis. 2017;17:e280–e292. doi: 10.1016/S1473-3099(17)30234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan O.A., Ahlm C., Sang R., Evander M. The 2007 Rift Valley fever outbreak in Sudan. PLoS Negl Trop Dis. 2011;5:e1229. doi: 10.1371/journal.pntd.0001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gubler D.J., Vasilakis N., Musso D. History and emergence of Zika virus. J Infect Dis. 2017;216(Suppl. 10):S860–S867. doi: 10.1093/infdis/jix451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freire C.C.M., Palmisano G., Braconi C.T., Cugola F.R., Russo F.B., Beltrao-Braga P.C. NS1 codon usage adaptation to humans in pandemic Zika virus. Mem Inst Oswaldo Cruz. 2018;113 doi: 10.1590/0074-02760170385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahid B., Ali A., Rafique S., Idrees M. Global expansion of chikungunya virus: mapping the 64-year history. Int J Infect Dis. 2017;58:69–76. doi: 10.1016/j.ijid.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Zeller H., Van B.W., Sudre B. Chikungunya: its history in Africa and Asia and its spread to new regions in 2013–2014. J Infect Dis. 2016;214(Suppl. 5):S436–S440. doi: 10.1093/infdis/jiw391. [DOI] [PubMed] [Google Scholar]
- 52.Simon F., Parola P., Grandadam M., Fourcade S., Oliver M., Brouqui P. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore) 2007;86:123–137. doi: 10.1097/MD/0b013e31806010a5. [DOI] [PubMed] [Google Scholar]
- 53.Rousset D., Rakoto-Andrianarivelo M., Razafindratsimandresy R., Randriamanalina B., Guillot S., Balanant J. Recombinant vaccine-derived poliovirus in Madagascar. Emerg Infect Dis. 2003;9:885–887. doi: 10.3201/eid0907.020692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mediannikov O., Socolovschi C., Bassene H., Diatta G., Ratmanov P., Fenollar F. Borrelia crocidurae infection in acutely febrile patients, Senegal. Emerg Infect Dis. 2014;20:1335–1358. doi: 10.3201/eid2008.130550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mourembou G., Fenollar F., Lekana-Douki J.B., Ndjoyi M.A., Maghendji N.S., Matsiegui P.B. Mansonella, including a potential new species, as common parasites in children in Gabon. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mourembou G., Nzondo S.M., Ndjoyi-Mbiguino A., Lekana-Douki J.B., Kouna L.C., Matsiegui P.B. Co-circulation of Plasmodium and bacterial DNAs in blood of febrile and afebrile children from urban and rural areas in Gabon. Am J Trop Med Hyg. 2016;95:123–132. doi: 10.4269/ajtmh.15-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]