Abstract
Infective endocarditis is a severe disease with high mortality. Despite a global trend towards an increase in staphylococcal aetiologies, in older patients and a decrease in viridans streptococci, we have observed in recent studies great epidemiologic disparities between countries. In order to evaluate these differences among Mediterranean countries, we performed a PubMed search of infective endocarditis case series for each country. Data were available for 13 of the 18 Mediterranean countries. Despite great differences in diagnostic strategies, we could classify countries into three groups. In northern countries, patients are older (>50 years old), have a high rate of prosthetic valves or cardiac electronic implantable devices and the main causative agent is Staphylococcus aureus. In southern countries, patients are younger (<40 years old), rheumatic heart disease remains a major risk factor (45–93%), viridans streptococci are the main pathogens, zoonotic and arthropod-borne agents are frequent and blood culture–negative endocarditis remains highly prevalent. Eastern Mediterranean countries exhibit an intermediate situation: patients are 45 to 60 years old, the incidence of rheumatic heart disease ranges from 8% to 66%, viridans streptococci play a predominant role and zoonotic and arthropod-borne diseases, in particular brucellosis, are identified in up to 12% of cases.
Keywords: Diagnosis, endocarditis, epidemiology, Mediterranean Sea, zoonoses
Introduction
Infective endocarditis (IE) is a life-threatening disease most often caused by bacteria. Its epidemiology varies greatly according to geographic area [1]. Over the past decades, the eradication of acute rheumatic fever in high-income countries has led to a progressive replacement of IE related to prior rheumatic disease in younger patients by cases related to degenerative valvulopathies, prosthetic valves and cardiac implantable electronic devices (CIED) in older patients [2]. Nosocomial and non–nosocomial-acquired cases have risen, as has the proportion of IE caused by staphylococci. In low-income countries, in contrast, rheumatic disease remains the main risk factor [3] and streptococci the most frequent causative agents. In addition, the rate of blood culture–negative endocarditis (BCNE) is elevated. This latter characteristic may be explained by the early use of antibiotics before blood culture collection and the elevated incidence of zoonotic diseases, including brucellosis, bartonelloses and Q fever. However, the aetiologies identified strongly depend on the diagnostic methods and strategies used. The more standardized and diversified the tests, the lower the rate of IE without any identified aetiologic diagnosis [4].
To date, no study has focused on the aetiologies of IE in the Mediterranean Basin despite the heavy travel of the population from country to country. Our objective was therefore to describe the profiles of microbial aetiologies of IE in Mediterranean countries.
Methods
We searched the international literature for series of patients, by country, diagnosed between 1990 and 2018, using the MeSH terms ‘endocarditis’ and ‘Albania,’ ‘Algeria,’ ‘Bosnia,’ ‘Croatia,’ ‘Egypt,’ ‘France,’ ‘Greece,’ ‘Israel,’ ‘Italy,’ ‘Lebanon,’ ‘Libya,’ ‘Malta,’ ‘Montenegro,’ ‘Morocco,’ ‘Slovenia,’ ‘Spain,’ ‘Tunisia’ or ‘Turkey.’ We excluded series of ten patients or fewer, articles that did not describe the identified aetiologic agents or articles that were focused on specific causative agents.
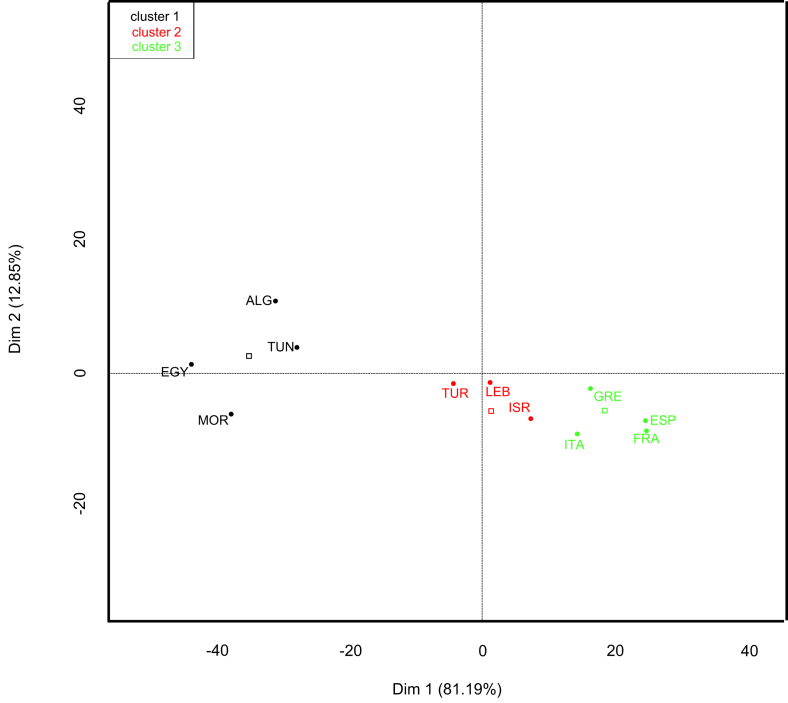
In order to compare the epidemiologic characteristics of IE among Mediterranean countries, we performed a principal component analysis with the open-source statistical software R [5] and the FactoMineR package [6] using the following characteristics: patient age, ratio of rheumatic heart disease (RHD) and percentages of Staphylococcus aureus, coagulase-negative staphylococci, viridans streptococci, Streptococcus gallolyticus, Enterococcus spp., Gram-negative bacteria, zoonotic and arthropod-borne bacteria, fungi and other microorganisms.
Results
Our literature search retrieved 6, 3, 5, 35, 38, 1452, 203, 339, 831, 35, 0, 9, 1, 23, 24, 816, 37 and 491 articles on endocarditis published between 1990 and 2018 for Albania, Algeria, Bosnia, Croatia, Egypt, France, Greece, Israel, Italy, Lebanon, Libya, Malta, Montenegro, Morocco, Slovenia, Spain, Tunisia and Turkey, respectively. Of these, on the basis of our selection criteria, we only retained 0, 1, 0, 2, 1, 8, 2, 4, 6, 2, 0, 0, 0, 3, 1, 6, 5 and 11 articles, respectively. We acknowledge the fact that our study suffers from several limitations, notably that the numbers of patients are highly variable according to studies and countries (18 to 2756), that recruitment and diagnostic methods are heterogeneous among studies and that the time frames covered by the selected articles may vary depending on countries, from 1974 to 2014 in Turkey to 2010 only in Egypt.
Albania
No data were available on IE in Albania, except a few cases reports of S. aureus [7], [8] and Streptococcus bovis [9] endocarditis.
Algeria
In Algeria, few studies on endocarditis have been published. The most comprehensive is a study by Benslimani et al. [10] of 110 cases diagnosed in 108 patients and in seven referral hospitals in Algiers, Algeria's capital, from 2000 to 2003. In addition to a young mean age (Supplementary Data), poor living conditions and overcrowding were frequent (61%). Blood cultures were sterile in 62 cases (56.4%). Zoonotic and arthropod-borne agents were identified in 18 cases (16.4%). In particular, Bartonella quintana, the agent of trench fever, caused 12.7% of cases, representing a high risk for patients living in poor conditions in this country. In addition, Q fever, although an active agent of IE in Algeria, is rarely diagnosed as a result of the lack of appropriate tools.
Bosnia
In Bosnia, two IE case reports have been published, including one caused by Brucella melitensis [11] and one caused by Streptococcus pneumoniae in a 12-year-old child [12]. In addition, although no case of endocarditis has been reported in the scientific literature, Q fever is common in Bosnia, with 373 cases diagnosed between 1998 and 2003 [13].
Croatia
In Croatia, two recent IE series totaling 324 patients have been published [14], [15]. The mean patient age ranged 54.5 to 63 years old. Although the causative agents are not fully detailed, S. aureus is the main pathogen (35%), followed by viridans streptococci (20.1%) and enterococci (14.6%). Q fever was identified in one patient in one series, but no information was provided regarding other zoonotic and arthropod-borne microorganisms.
Egypt
In a 2010 series of 132 patients with IE in Egypt, the patients were young (mean age, 31 years; Supplementary Data) and had high rates of RHD, prosthetic valves and negative blood cultures (25.0%, 30.3% and 69.7%, respectively) [16]. The main identified microorganisms were S. aureus and viridans streptococci (10.6% of patients each), but zoonotic and arthropod-borne agents, including Brucella spp. and B. quintana, infected 3.7% of patients (Supplementary Data). Moreover, Q fever, although not detected in this series, is endemic in Egypt and may thus also cause cases of BCNE [17]. The addition of serologic and PCR assays enabled a reduction of patients without an identified causative agent from 69.7% using blood culture to 49.2% [16].
France
In France, several studies have described large series of IE cases (Supplementary Data). In an attempt to evaluate the impact of the discontinuation of systematic antibioprophylaxis in case of dental treatments, Duval et al. [18] compared three cohorts of IE cases diagnosed in 1991, 1999 and 2008. They observed that this drastic prophylaxis change did not result in an increase in streptococcal IE. Rather, the authors observed an increased frequency of staphylococcal IE, caused by both S. aureus and coagulase-negative staphylococci. Such a trend may be explained in part by the increasing number of prosthetic valve and CIED infections (Supplementary Data). Hoen et al. [19] compared a cohort of 390 patients with IE diagnosed in 1999 with a previous series from 1991. They observed a reduced incidence of IE caused by oral streptococci but an increased role of group D streptococci and staphylococci, as well as an increased rate of early valve surgery [19]. We acknowledge the fact that the latter two studies may partially be redundant, as some patients were included in both for the year 1999 [18], [19]. This was taken into account in the Supplementary Data to avoid a biased representation of the French epidemiology. Mourvillier et al. [20] and Sonneville et al. [21] studied 426 patients with severe IE requiring admission to the intensive care unit. They reported that 26.3% of cases were healthcare associated, with a predominant role of staphylococci, in particular S. aureus (48.3% of patients), which was an independent factor of poor prognosis. The overall mortality rate was high (49.0%) and was higher in patients with prosthetic valve endocarditis (58.8%). Demonchy et al. [22] observed a high rate of IE complicating prosthetic valves or implantable cardiac electronic devices (ICEDs) (42.4%) and a predominance of staphylococcal infections (31.8%). By studying 197 IE cases occurring in patients with ICEDs, Deharo et al. [23] observed that blood cultures were negative in 60.4% of cases and that staphylococci were by far the most common agents (71.6%). However, globally, the rate of BCNE in France ranged from 6.0% to 9.0% of cases; zoonotic and arthropod-borne agents accounted for 0.8% to 1.5% of IE cases [19], [20], [21], [22]. Finally, by focusing on patients with BCNE, we observed that Bartonella spp., Coxiella burnetii and Tropheryma whipplei were responsible for 25.5% of cases and that 1.8% of patients had noninfective endocarditis [24].
Greece
In Greece, two studies dating to the early 2000s described a total of 296 patients [25], [26]. The mean age of patients ranged from 54.4 to 63.3 years. RHD was identified in up to 14.3% of patients [26], but the prevalence of prosthetic valve endocarditis was also elevated (21.5–30.7%). Oral streptococci and S. aureus exhibited similar incidences (18.8–20.5% and 17.4–21.8%, respectively), but a discrepancy was observed between both series regarding the incidence of Enterococcus spp. IE (2.9% and 37.6%, respectively). Zoonotic diseases (brucellosis and Q fever) accounted for 2.8% of cases in one series [25].
Israel
In Israel, three IE series were published that spanned the 1980s and early 1990s [27], the 1990s [28] and the 2000s [29]. The patients' mean age increased over time, from 50 years old in the earliest period [27] to 65 years old in the latest [29]. This higher mean age is similar to those observed in European countries [27], [28], [29]. The rate of RHD decreased from 37% in the 1980s to 8% in the 2000s (Supplementary Data). Inversely, the rate of prosthetic valve endocarditis increased from 27% to 39%, as did the death rate, from 15% to 33%. The microbiologic spectrum of IE agents in Israel has also evolved, with an increasing prevalence of staphylococci, notably S. aureus (up to 31% in the most recent study) and enterococci, but with a reduction of streptococci (Supplementary Data). Few cases of brucellosis were diagnosed [27], [28] before the 2000s. Q fever represented 3% to 4% of IE cases in the 1990s and 2000s. In a retrospective study from 1983 to 1992, Siegman-Igra et al. [30] reported 35 cases of C. burnetii endocarditis, thus representing an annual incidence of 0.75 cases per million inhabitants. In addition, Wiener-Well et al. [31] observed that Q fever may cause low-grade infection and should be part of the systematic screening of BCNE in Israel. No case of Bartonella endocarditis was detected, but no specific tools were used to detect these bacteria.
Italy
In Italy, the incidence rate of IE is 4.6/100 000 person-years, with a higher rate in people older than 65 years (11.7/100 000 person-years) [32]. Several studies demonstrated temporal changes in IE epidemiology, similar to those observed in other European countries [32], [33], [34], [35], [36], [37]: an increase in patient age (57 to 69 years old, depending on the series), rate of prosthetic heart valve and comorbidity factors such as diabetes mellitus, chronic renal failure and cancer [33]. In the largest series, Leone et al. [35] reported 1082 IE cases. Prosthetic valve endocarditis and ICED infections represented 25.6% and 4.8% of cases, respectively. The main IE agent was S. aureus (21.9%), followed by viridans streptococci (17.7%), enterococci (13.3%), bovis-group streptococci (11.0%) and coagulase-negative staphylococci (10.9%). Similar distributions of causative microorganisms were reported in other Italian studies [32], [33], [34], [36], [37]. Other characteristics of IE in Italy include an elevated ratio of healthcare-associated cases (12.4–64.6% of cases) and a low rate of zoonotic and arthropod-borne microorganisms, notably C. burnetii and Bartonella spp. [37], although specific diagnostic tools for these agents are not routinely used in published studies [34], [35], [36] and their incidence might thus be underestimated. Regarding IE-associated in-hospital mortality, Cresti et al. [32] observed an increase from 1998 to 2014, with being elderly associated with a worse prognosis.
Lebanon
In Lebanon, RHD has remained stable over time (15–16%), but the patient age has increased, as have the ratios of IE on prosthetic valve and ICEDs, many of which were nosocomial (Supplementary Data). Although the prevalence of streptococcal infections has decreased, they remain predominant, but infections caused by coagulase-negative staphylococci and enterococci are more frequent [38], [39]. Zoonoses and arthropod-borne infections are not detected in published series, but Q fever is present in Lebanon and should thus not be overlooked in patients with BCNE [40].
Libya
No data were available on IE in Libya.
Malta
No data were available on IE in Malta.
Montenegro
No data were available on IE in Montenegro.
Morocco
In Morocco, patients with IE were young (22–27.5 years old) [41], [42], had a history of RHD in 63% to 77.8% but few valvular prostheses (3.8–5.5% of patients). BCNE represented two thirds of all cases of IE. Viridans streptococci were the most prevalent causative agents (15.9–27.8%). Although present, zoonotic and arthropod-borne infections were underdiagnosed because of the lack of appropriate diagnostic tools [43].
Slovenia
In Slovenia, a single series of patients with IE has been published [44]. However, this study focused on patients who had undergone valvular surgery and were tested by 16S ribosomal RNA PCR from valvular biopsy samples. In 21 patients, a microorganism was identified, including S. aureus in seven, followed by coagulase-negative staphylococci and viridans streptococci in four each and enterococci in three [44]. In addition, a few case reports of IE caused by Abiotrophia defectiva [45], Bartonella henselae [46] and Candida spp. [47] have been reported.
Spain
Several large IE studies have been published in Spain [48], [49], [50], [51], [52], including a temporal trend study over a 12-year period [50]. In this study of 16 867 IE episodes from 2003 to 2014, Olmos et al. observed an increase of IE incidence from 2.72/100 000 in 2003 to 3.49/100 000 person-years in 2014. This increase in incidence paralleled that of patient age, which ranged from 66.4 to 69 years old in the most recent series [51], [52], the prevalence of prosthetic valves (37.4%) and CIEDs (1.1%), and the proportion of patients who underwent surgery (18.3–26.5%). In contrast, and similar to what is observed in other European countries, rheumatoid heart disease (8.9%) and intravenous drug use (2.6%) decreased considerably [50]. Depending on the study, about one third of IEs were healthcare associated [51], [52]. In addition, the in-hospital mortality rate did not significantly change, ranging 20% to 29%, depending on the series. Regarding the aetiologic diagnosis, the incidence of viridans streptococci decreased (24.0–12.3%), that of S. aureus remained stable (∼20%) but those of enterococci (10.3–16.7%) and coagulase-negative staphylococci (8.0–14.4%) increased (13.1%). Regarding zoonotic and arthropod-borne causative agents, when searched, Q fever is regularly detected (1–4%), whereas Brucella spp. (0.05%) appear to be uncommon IE agents in Spain. As for Bartonella spp., they may be underdiagnosed in Spain because specific diagnostic methods are not routinely available in all diagnostic laboratories.
Tunisia
In Tunisia, patients with IE exhibited an age range of 29.9 to 34.2 years [53], [54], [55], [56], [57]. Similar to other southern Mediterranean countries, a history of RHD was frequently reported by patients (44.8–93.0%), the rate of BCNE was elevated (49.2–69.0%) and the main identified causative agents were viridans streptococci (10.7–23.8% of patients) [53], [54], [56], [57]. Zoonotic and arthropod-borne agents were frequent (up to 8.9% of cases), but Znazen et al. [58] demonstrated the major role of Bartonella spp., in particular B. quintana, and proposed that Bartonella serology should be systematic in case of BCNE in this country.
Turkey
In Turkey, endocarditis appears to be a frequent disease. In an article summarizing the data of 857 Turkish patients with fever of unexplained origin from 13 articles, IE represented 4.5% of all cases, 5.4% of patients with an identified aetiology and 9.6% of all infectious aetiologies [59]. Despite the improvement of medical technology in this country, acute rheumatic fever remains a serious public health threat and the primary cause of valvular heart disease [60]. An analysis of the Turkish registry of heart valve disease demonstrated that 46% of patients had a history of RHD, with a clear causative link between the disease and mitral stenosis or multiple valve disease [60]. Since 2001, 11 series of patients with endocarditis in various areas of Turkey have been published (Supplementary Data), for a total of 1433 patients diagnosed and treated in 40 hospitals in Turkey [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71]. These studies span a period from 1974 to 2014, so the epidemiologic data should be analysed with caution. However, 1215 of the 1433 patients were diagnosed since 1997. Endocarditis mostly occurred in male subjects (male/female ratio, 1.38) with a mean age of 44 years old (Supplementary Data). Rheumatic valvular disease was highly prevalent (37.6%), including in the most recent studies. The death rate was 25.8% (range, 15.0–36.4%). Blood cultures were negative in 36.8% of patients (range, 16.0–57.1%). Staphylococcus aureus was the main agent of endocarditis (29.1%), followed by viridans streptococci (20.5%), coagulase-negative staphylococci (17.0%) and Enterococcus spp. (11.1%). A striking characteristic of IE in Turkey was the high prevalence of Brucella endocarditis (7.5%; range, 0–27.7), as previously reported [72], [73]. In addition, it should be noted that other zoonotic agents such as Bartonella spp. and C. burnetii might be present in Turkey but may be underdiagnosed because they are not systematically searched for. In the 11 reviewed studies, Bartonella and Q fever serology were not used in the diagnostic panel for endocarditis, but two cases of Bartonella endocarditis were diagnosed by culture, and Q fever endocarditis has been reported [74], thus suggesting that these agents should be included in the diagnostic screening of IE.
Principal component analysis
Principal component analysis distributed Mediterranean countries within three groups: Algeria, Egypt, Morocco and Tunisia; Israel, Lebanon and Turkey; and France, Greece, Italy and Spain. Croatia and Slovenia were not included in the analysis because the IE series available lacked several of the criteria used in the analysis or included only patients who had undergone valvular surgery, respectively.
Discussion
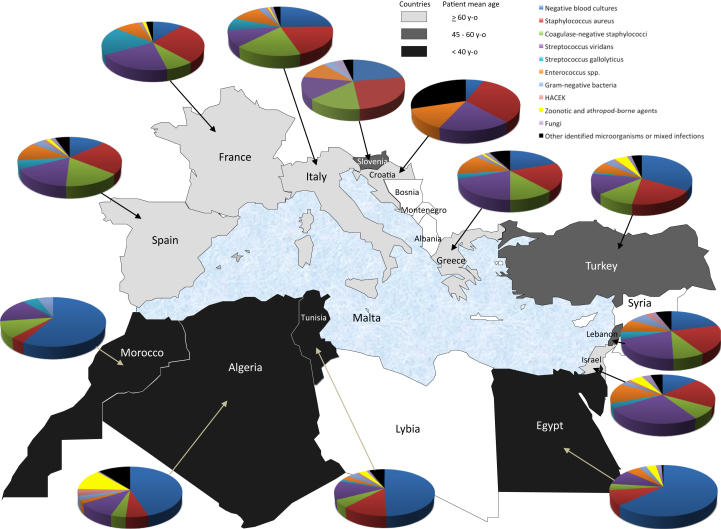
Around the Mediterranean Sea, the epidemiology of IE varies according to country (Fig. 1, Fig. 2). In southern countries (Morocco, Algeria, Tunisia, Egypt), RHD remains a major risk factor (25–93% of patients) and explains the young age of patients (22–40 years old). In contrast, the ratio of patients with prosthetic valves ranges from 3.8% to 30.3%, and CIEDs are rare. BCNE remains highly prevalent (49–69% of cases). When identified, causative agents are dominated by viridans streptococci (up to 28%), followed by S. aureus (up to 22%). Zoonotic and arthropod-borne agents are frequent (up to 16.4% of patients) and are dominated by Bartonella spp.
Fig. 1.
Map of Mediterranean area showing distribution of aetiologic agents and age range of patients with infective endocarditis according to country.
Fig. 2.
Principal component analysis (factor map) comparing Mediterranean countries using following infective endocarditis characteristics: patient age, ratio of rheumatic heart disease and percentages of Staphylococcus aureus, coagulase-negative staphylococci, viridans streptococci, Streptococcus gallolyticus, Enterococcus spp., nonfermentative Gram-negative bacteria, Enterobacteriaceae, HACEK (Haemophilus, Aggregatibacter (previously Actinobacillus), Cardiobacterium, Eikenella, Kingella), zoonotic and arthropod-borne bacteria, fungi and other microorganisms. ALG, Algeria; EGY, Egypt; FRA, France; GRE, Greece; ISR, Israel; ITA, Italy; MOR, Morocco; SPA, Spain; TUN, Tunisia; TUR, Turkey.
In northern countries (Croatia, France, Greece, Italy, Spain), RHD is no longer a risk factor of cardiovascular diseases (<5% of patients). The ratio of patients with prosthetic valves and/or CIED is higher than in southern countries and ranges from 21.9% to 33.7%. The mean age of patients with IE ranges from 51 to 69.5 years. BCNE represented 5.5% to 29% of cases. The main causative agents are S. aureus (8.0–26% of patients), followed by viridans streptococci (2.8–24.4%), coagulase-negative staphylococci (0.8–20.0%) and enterococci (3.0–16.7%). Zoonotic and arthropod-borne agents are rare (0.7–8.3%) and are dominated by Bartonella spp. in France and Italy and by Q fever in Spain. Brucella IE is rare in Spain and Italy and is not detected in France.
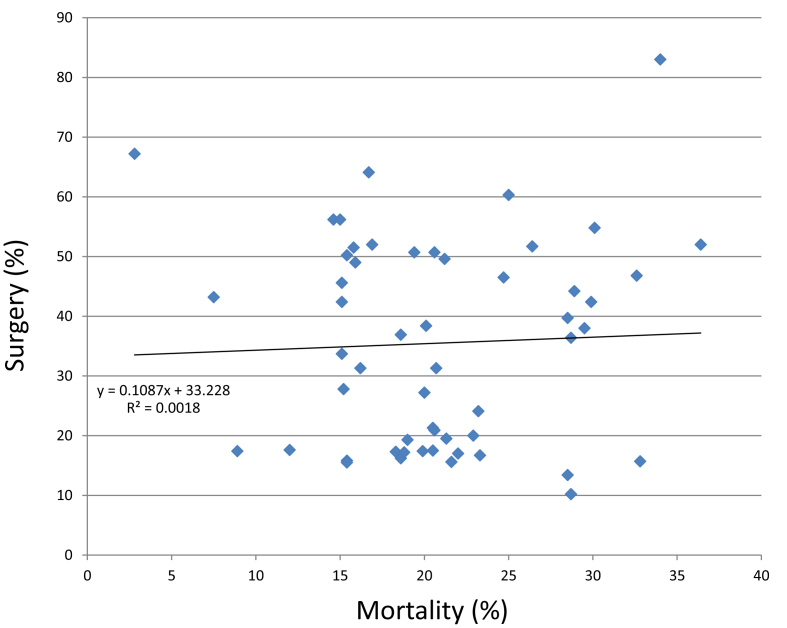
In eastern countries (Israel, Lebanon, Turkey), distinct situations are observed, as follows. In Lebanon and Turkey, the age of patients (46.6–53.3 years old) is younger than in European countries but older than in southern countries, and the prevalence of RHD ranges from to 15.1% to 66.2%. Zoonotic and arthropod-borne diseases, especially brucellosis, represent up to 12.5% of cases in Turkey, but they are not part of the systematic screening of IE in Lebanon, although present. In Israel, patients are older (50–66 years old), but up to 36.6% of patients may have a history of RHD, and viridans streptococci are still more frequent than S. aureus. Surgery rates varied greatly from one country to another: 10.2% to 15.8% in Morocco to 13.4% to 75.6% in Turkey (Supplementary Data). In addition, no correlation (R2 = 0.0018) could be found between surgery and in-hospital mortality rates (2.8% to 34%) among studied series or Mediterranean countries (Fig. 3; Supplementary Data).
Fig. 3.
Surgery vs. mortality rates in published series of infective endocarditis in Mediterranean countries. Series for which either surgery and/or mortality rates were not available and those that included only patients requiring admission to intensive care unit were not included. Shown are tendency curve, its equation and correlation coefficient.
Conclusion
Despite a global trend towards an increase in patient age and staphylococcal aetiologies in endocarditis, great epidemiologic disparities remain among Mediterranean countries. In addition, the diagnostic methods, notably the use of specific serologic assays for the detection of zoonotic and arthropod-borne agents, are heterogenous, despite the need for specific antibiotic therapies for diseases such as brucellosis, Q fever or Whipple endocarditis.
Acknowledgements
The study was funded by the Fondation Méditerranée Infection and the Agence National de recherché under reference Investissements d’Avenir 10-IHAU-03.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.nmni.2018.05.004.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Brouqui P., Raoult D. New insight into the diagnosis of fastidious bacterial endocarditis. FEMS Immunol Med Microbiol. 2006;47:1–13. doi: 10.1111/j.1574-695X.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A., Baumgartner H., Bax J., Butchart E., Dion R., Filippatos G. Guidelines on the management of valvular heart disease: the task force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 3.Zuhlke L., Karthikeyan G., Engel M.E., Rangarajan S., Mackie P., Cupido-Katya M.B. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: two-year follow-up of the global rheumatic heart disease registry (the REMEDY Study) Circulation. 2016;134:1456–1466. doi: 10.1161/CIRCULATIONAHA.116.024769. [DOI] [PubMed] [Google Scholar]
- 4.Fournier P.E., Thuny F., Richet H., Lepidi H., Casalta J.P., Arzouni J.P. Comprehensive diagnostic strategy for blood culture–negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis. 2010;51:131–140. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]
- 5.R: a language and environment for statistical computing [computer program] R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- 6.Le S., Josse J., Husson R. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008;25:1–18. [Google Scholar]
- 7.Prifti E., Ademaj F., Baboci A., Demiraj A. Acquired Gerbode defect following endocarditis of the tricuspid valve: a case report and literature review. J Cardiothorac Surg. 2015;10:115. doi: 10.1186/s13019-015-0320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xhabija N., Prifti E., Allajbeu I., Sula F. Gerbode defect following endocarditis and misinterpreted as severe pulmonary arterial hypertension. Cardiovasc Ultrasound. 2010;8:44. doi: 10.1186/1476-7120-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasmi G., Andoni R., Mano V., Kraja D., Muco E., Kasmi I. Streptococcus bovis isolated in haemoculture a signal of malignant lesion of the colon. Clin Lab. 2011;57:1007–1009. [PubMed] [Google Scholar]
- 10.Benslimani A., Fenollar F., Lepidi H., Raoult D. Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis. 2005;11:216–224. doi: 10.3201/eid1102.040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehanic S., Mulabdic V., Baljic R., Hadzovic-Cengic M., Pinjo F., Hadziosmanovic V. Brucella endocarditis in prosthetic valves. Mater Sociomed. 2012;24(Suppl. 1):11–12. doi: 10.5455/msm.2012.24.s11-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesihovic-Dinarevic S., Halimic M., Begic Z., Kadic A., Kacila M., Omerbasic E. Mitral valve replacement in a patient with infective endocarditis and aneurysm of the cerebral artery: a case report. Acta Med Acad. 2014;43:165–169. doi: 10.5644/ama2006-124.116. [DOI] [PubMed] [Google Scholar]
- 13.Puvacic S., Dizdarevic Z., Zvizdic S., Tandir S., Alikovic I., Celiks S. Epidemiological investigations transmission of Q fever among humans in Bosnia and Herzegovina. Med Arch. 2005;59:118–120. [PubMed] [Google Scholar]
- 14.Krajinovic V., Ivancic S., Gezman P., Barsic B. Association between cardiac surgery and mortality among patients with infective endocarditis complicated by sepsis and septic shock. Shock. 2018;49:536–542. doi: 10.1097/SHK.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 15.Lepur D., Barsic B. Incidence of neurological complications in patients with native-valve infective endocarditis and cerebral microembolism: an open cohort study. Scand J Infect Dis. 2009;41:708–713. doi: 10.1080/00365540903146995. [DOI] [PubMed] [Google Scholar]
- 16.El-Kholy A.A., El-Rachidi N.G., El-Enany M.G., AbdulRahman E.M., Mohamed R.M., Rizk H.H. Impact of serology and molecular methods on improving the microbiologic diagnosis of infective endocarditis in Egypt. Infection. 2015;43:523–529. doi: 10.1007/s15010-015-0761-2. [DOI] [PubMed] [Google Scholar]
- 17.Vanderburg S., Rubach M.P., Halliday J.E., Cleaveland S., Reddy E.A., Crump J.A. Epidemiology of Coxiella burnetii infection in Africa: a OneHealth systematic review. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duval X., Delahaye F., Alla F., Tattevin P., Obadia J.F., Le Moing V. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol. 2012;59:1968–1976. doi: 10.1016/j.jacc.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Hoen B., Alla F., Selton-Suty C., Beguinot I., Bouvet A., Briancon S. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA. 2002;288:75–81. doi: 10.1001/jama.288.1.75. [DOI] [PubMed] [Google Scholar]
- 20.Mourvillier B., Trouillet J.L., Timsit J.F., Baudot J., Chastre J., Regnier B. Infective endocarditis in the intensive care unit: clinical spectrum and prognostic factors in 228 consecutive patients. Intensive Care Med. 2004;30:2046–2052. doi: 10.1007/s00134-004-2436-9. [DOI] [PubMed] [Google Scholar]
- 21.Sonneville R., Mirabel M., Hajage D., Tubach F., Vignon P., Perez P. Neurologic complications and outcomes of infective endocarditis in critically ill patients: the ENDOcardite en REAnimation prospective multicenter study. Crit Care Med. 2011;39:1474–1481. doi: 10.1097/CCM.0b013e3182120b41. [DOI] [PubMed] [Google Scholar]
- 22.Demonchy E., Dellamonica P., Roger P.M., Bernard E., Cua E., Pulcini C. Audit of antibiotic therapy used in 66 cases of endocarditis. Med Mal Infect. 2011;41:602–607. doi: 10.1016/j.medmal.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Deharo J.C., Quatre A., Mancini J., Khairy P., Le D.Y., Casalta J.P. Long-term outcomes following infection of cardiac implantable electronic devices: a prospective matched cohort study. Heart. 2012;98:724–731. doi: 10.1136/heartjnl-2012-301627. [DOI] [PubMed] [Google Scholar]
- 24.Fournier P.E., Gouriet F., Casalta J.P., Lepidi H., Chaudet H., Thuny F. Blood culture–negative endocarditis: improving the diagnostic yield using new diagnostic tools. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loupa C., Mavroidi N., Boutsikakis I., Paniara O., Deligarou O., Manoli H. Infective endocarditis in Greece: a changing profile. Epidemiological, microbiological and therapeutic data. Clin Microbiol Infect. 2004;10:556–561. doi: 10.1111/j.1469-0691.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- 26.Giannitsioti E., Skiadas I., Antoniadou A., Tsiodras S., Kanavos K., Triantafyllidi H. Nosocomial vs. community-acquired infective endocarditis in Greece: changing epidemiological profile and mortality risk. Clin Microbiol Infect. 2007;13:763–769. doi: 10.1111/j.1469-0691.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 27.Borer A., Riesenberg K., Uriel N., Gilad J., Porath A., Weber G. Infective endocarditis in a tertiary-care hospital in southern Israel. Public Health Rev. 1998;26:317–330. [PubMed] [Google Scholar]
- 28.Fefer P., Raveh D., Rudensky B., Schlesinger Y., Yinnon A.M. Changing epidemiology of infective endocarditis: a retrospective survey of 108 cases, 1990–1999. Eur J Clin Microbiol Infect Dis. 2002;21:432–437. doi: 10.1007/s10096-002-0740-2. [DOI] [PubMed] [Google Scholar]
- 29.Korem M., Israel S., Gilon D., Cahan A., Moses A.E., Block C. Epidemiology of infective endocarditis in a tertiary-center in Jerusalem: a 3-year prospective survey. Eur J Intern Med. 2014;25:550–555. doi: 10.1016/j.ejim.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Siegman-Igra Y., Kaufman O., Keysary A., Rzotkiewicz S., Shalit I. Q fever endocarditis in Israel and a worldwide review. Scand J Infect Dis. 1997;29:41–49. doi: 10.3109/00365549709008663. [DOI] [PubMed] [Google Scholar]
- 31.Wiener-Well Y., Fink D., Schlesinger Y., Raveh D., Rudensky B., Yinnon A.M. Q fever endocarditis; not always expected. Clin Microbiol Infect. 2010;16:359–362. doi: 10.1111/j.1469-0691.2009.02805.x. [DOI] [PubMed] [Google Scholar]
- 32.Cresti A., Chiavarelli M., Scalese M., Nencioni C., Valentini S., Guerrini F. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther. 2017;7:27–35. doi: 10.21037/cdt.2016.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassetti M., Venturini S., Crapis M., Ansaldi F., Orsi A., Della M.A. Infective endocarditis in elderly: an Italian prospective multi-center observational study. Int J Cardiol. 2014;177:636–638. doi: 10.1016/j.ijcard.2014.09.184. [DOI] [PubMed] [Google Scholar]
- 34.Ferraris L., Milazzo L., Ricaboni D., Mazzali C., Orlando G., Rizzardini G. Profile of infective endocarditis observed from 2003–2010 in a single center in Italy. BMC Infect Dis. 2013;13:545. doi: 10.1186/1471-2334-13-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leone S., Ravasio V., Durante-Mangoni E., Crapis M., Carosi G., Scotton P.G. Epidemiology, characteristics, and outcome of infective endocarditis in Italy: the Italian Study on Endocarditis. Infection. 2012;40:527–535. doi: 10.1007/s15010-012-0285-y. [DOI] [PubMed] [Google Scholar]
- 36.Cecchi E., Ciccone G., Chirillo F., Imazio M., Cecconi M., Del P.S. Mortality and timing of surgery in the left-sided infective endocarditis: an Italian multicentre study. Interact Cardiovasc Thorac Surg. 2018;26:602–609. doi: 10.1093/icvts/ivx394. [DOI] [PubMed] [Google Scholar]
- 37.Cecchi E., Chirillo F., Castiglione A., Faggiano P., Cecconi M., Moreo A. Clinical epidemiology in Italian Registry of Infective Endocarditis (RIEI): focus on age, intravascular devices and enterococci. Int J Cardiol. 2015;190:151–156. doi: 10.1016/j.ijcard.2015.04.123. [DOI] [PubMed] [Google Scholar]
- 38.El-Chakhtoura N., Yasmin M., Kanj S.S., Baban T., Sfeir J., Kanafani Z.A. A 27-year experience with infective endocarditis in Lebanon. J Infect Public Health. 2017;10:734–739. doi: 10.1016/j.jiph.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Kanafani Z.A., Mahfouz T.H., Kanj S.S. Infective endocarditis at a tertiary care centre in Lebanon: predominance of streptococcal infection. J Infect. 2002;45:152–159. doi: 10.1016/s0163-4453(02)91041-8. [DOI] [PubMed] [Google Scholar]
- 40.Dabaja M.F., Greco G., Villari S., Bayan A., Vesco G., Gargano V. The first serological study of Q fever in humans in Lebanon. Vector Borne Zoonotic Dis. 2018;18:138–143. doi: 10.1089/vbz.2016.2102. [DOI] [PubMed] [Google Scholar]
- 41.Zarzur J., Amri R., Cherradi R., Housni A., Balafrej K., Arharbi M. Vascular complications of infective endocarditis: a retrospective analysis of 18 cases. J Mal Vasc. 2002;27:82–87. [PubMed] [Google Scholar]
- 42.Bennis A., Zahraoui M., Azzouzi L., Soulami S., Mehadji B.A., Tahiri A. Bacterial endocarditis in Morocco. Ann Cardiol Angeiol (Paris) 1995;44:339–344. [PubMed] [Google Scholar]
- 43.Boudebouch N., Sarih M., Chakib A., Fadili S., Boumzebra D., Zouizra Z. Blood culture–negative endocarditis, Morocco. Emerg Infect Dis. 2017;23:1908–1909. doi: 10.3201/eid2311.161066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller P.M., Lejko Z.T., Klokocovnik T., Ruzic S.E., Cerar T. Broad-range 16S rDNA PCR on heart valves in infective endocarditis. J Heart Valve Dis. 2016;25:221–226. [PubMed] [Google Scholar]
- 45.Lainscak M., Lejko-Zupanc T., Strumbelj I., Gasparac I., Mueller-Premru M., Pirs M. Infective endocarditis due to Abiotrophia defectiva: a report of two cases. J Heart Valve Dis. 2005;14:33–36. [PubMed] [Google Scholar]
- 46.Lejko-Zupanc T., Slemenik-Pusnik C., Kozelj M., Klokocovnik T., Avsic-Zupanc T., Dolenc-Strazar Z. Native valve endocarditis due to Bartonella henselae in an immunocompetent man. Wien Klin Wochenschr. 2008;120:246–249. doi: 10.1007/s00508-008-0951-3. [DOI] [PubMed] [Google Scholar]
- 47.Lejko-Zupanc T., Logar M. Candida endocarditis: a review of twelve episodes in eleven patients. J Heart Valve Dis. 2017;26:98–102. [PubMed] [Google Scholar]
- 48.Galvez-Acebal J., Rodriguez-Bano J., Martinez-Marcos F.J., Reguera J.M., Plata A., Ruiz J. Prognostic factors in left-sided endocarditis: results from the Andalusian multicenter cohort. BMC Infect Dis. 2010;22(10):17. doi: 10.1186/1471-2334-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruiz-Morales J., Ivanova-Georgieva R., Fernandez-Hidalgo N., Garcia-Cabrera E., Miro J.M., Munoz P. Left-sided infective endocarditis in patients with liver cirrhosis. J Infect. 2015;71:627–641. doi: 10.1016/j.jinf.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Olmos C., Vilacosta I., Fernandez-Perez C., Bernal J.L., Ferrera C., Garcia-Arribas D. The evolving nature of infective endocarditis in Spain: a population-based study (2003 to 2014) J Am Coll Cardiol. 2017;5(70):2795–2804. doi: 10.1016/j.jacc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Munoz P., Kestler M., de A.A., Miro J.M., Bermejo J., Rodriguez-Abella H. Current epidemiology and outcome of infective endocarditis: a multicenter, prospective, cohort study. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Hidalgo N., Almirante B., Tornos P., Gonzalez-Alujas M.T., Planes A.M., Galinanes M. Immediate and long-term outcome of left-sided infective endocarditis. A 12-year prospective study from a contemporary cohort in a referral hospital. Clin Microbiol Infect. 2012;18:E522–E530. doi: 10.1111/1469-0691.12033. [DOI] [PubMed] [Google Scholar]
- 53.Ben Hamda K., Betbout F., Gamra H., Maatouk F., Dridi Z., Zidi M.A. Pronostic de l’endocardite infectieuse. Tunis Med. 2002;80:739–750. [PubMed] [Google Scholar]
- 54.Fradi I., Drissa M.A., Cheour M., Ben I.M., Drissa H. Retrospective study on 100 cases of infective endocarditis, Rabta University Hospital, Tunis, from 1980 to 2004. Arch Mal Coeur Vaiss. 2005;98:966–971. [PubMed] [Google Scholar]
- 55.Letaief A., Boughzala E., Kaabia N., Ernez S., Abid F., Ben C.T. Epidemiology of infective endocarditis in Tunisia: a 10-year multicenter retrospective study. Int J Infect Dis. 2007;11:430–433. doi: 10.1016/j.ijid.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Trabelsi I., Rekik S., Znazen A., Maaloul I., Abid D., Maalej A. Native valve infective endocarditis in a tertiary care center in a developing country (Tunisia) Am J Cardiol. 2008;102:1247–1251. doi: 10.1016/j.amjcard.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 57.Omezzine-Letaief A., Alaoui F.Z., Bahri F., Mahdhaoui A., Boughzela E., Jemni L. [Infectious endocarditis with negative blood cultures] Arch Mal Coeur Vaiss. 2004;97:120–124. [PubMed] [Google Scholar]
- 58.Znazen A., Rolain J.M., Hammami N., Kammoun S., Hammami A., Raoult D. High prevalence of Bartonella quintana endocarditis in Sfax, Tunisia. Am J Trop Med Hyg. 2005;72:503–507. [PubMed] [Google Scholar]
- 59.Sipahi O.R., Senol S., Arsu G., Pullukcu H., Tasbakan M., Yamazhan T. Pooled analysis of 857 published adult fever of unknown origin cases in Turkey between 1990–2006. Med Sci Monit. 2007;13:CR318–CR322. [PubMed] [Google Scholar]
- 60.Demirbag R., Sade L.E., Aydin M., Bozkurt A., Acarturk E. The Turkish registry of heart valve disease. Turk Kardiyol Dern Ars. 2013;41:1–10. doi: 10.5543/tkda.2013.71430. [DOI] [PubMed] [Google Scholar]
- 61.Heper G., Yorukoglu Y. Clinical, bacteriologic and echocardiographic evaluation of infective endocarditis in Ankara, Turkey. Angiology. 2002;53:191–197. doi: 10.1177/000331970205300210. [DOI] [PubMed] [Google Scholar]
- 62.Sucu M., Davutoglu V., Ozer O., Aksoy M. Epidemiological, clinical and microbiological profile of infective endocarditis in a tertiary hospital in the south-east Anatolia region. Turk Kardiyol Dern Ars. 2010;38:107–111. [PubMed] [Google Scholar]
- 63.Cancan G.N., Vardar I., Demirdal T., Gursul E., Ural S., Yesil M. Clinical and microbiological findings of infective endocarditis. J Infect Dev Ctries. 2016;10:478–487. doi: 10.3855/jidc.7516. [DOI] [PubMed] [Google Scholar]
- 64.Simsek-Yavuz S., Sensoy A., Kasikcioglu H., Ceken S., Deniz D., Yavuz A. Infective endocarditis in Turkey: aetiology, clinical features, and analysis of risk factors for mortality in 325 cases. Int J Infect Dis. 2015;30:106–114. doi: 10.1016/j.ijid.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Agca F.V., Demircan N., Peker T., Ari H., Karaagac K., Ozluk O.A. Infective endocarditis: a tertiary referral centre experience from Turkey. Int J Clin Exp Med. 2015;8:13962–13968. [PMC free article] [PubMed] [Google Scholar]
- 66.Ozveren O., Ozturk M.A., Sengul C., Bakal R.B., Akgun T., Izgi C. Infective endocarditis and complications; a single center experience. Turk Kardiyol Dern Ars. 2014;42:629–634. doi: 10.5543/tkda.2014.80708. [DOI] [PubMed] [Google Scholar]
- 67.Elbey M.A., Akdag S., Kalkan M.E., Kaya M.G., Sayin M.R., Karapinar H. A multicenter study on experience of 13 tertiary hospitals in Turkey in patients with infective endocarditis. Anadolu Kardiyol Derg. 2013;13:523–527. doi: 10.5152/akd.2013.172. [DOI] [PubMed] [Google Scholar]
- 68.Kucukates E., Gultekin N., Bagdatli Y. Cases of active infective endocarditis in a university hospital during a 10-year period. J Pak Med Assoc. 2013;63:1163–1167. [PubMed] [Google Scholar]
- 69.Tugcu A., Yildirimturk O., Baytaroglu C., Kurtoglu H., Kose O., Sener M. Clinical spectrum, presentation, and risk factors for mortality in infective endocarditis: a review of 68 cases at a tertiary care center in Turkey. Turk Kardiyol Dern Ars. 2009;37:9–18. [PubMed] [Google Scholar]
- 70.Leblebicioglu H., Yilmaz H., Tasova Y., Alp E., Saba R., Caylan R. Characteristics and analysis of risk factors for mortality in infective endocarditis. Eur J Epidemiol. 2006;21:25–31. doi: 10.1007/s10654-005-4724-2. [DOI] [PubMed] [Google Scholar]
- 71.Cetinkaya Y., Akova M., Akalin H.E., Ascioglu S., Hayran M., Uzuns O. A retrospective review of 228 episodes of infective endocarditis where rheumatic valvular disease is still common. Int J Antimicrob Agents. 2001;18:1–7. doi: 10.1016/s0924-8579(01)00344-2. [DOI] [PubMed] [Google Scholar]
- 72.Inan M.B., Eyileten Z.B., Ozcinar E., Yazicioglu L., Sirlak M., Eryilmaz S. Native valve Brucella endocarditis. Clin Cardiol. 2010;33:E20–E26. doi: 10.1002/clc.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cay S., Cagirci G., Maden O., Balbay Y., Aydogdu S. Brucella endocarditis—a registry study. Kardiol Pol. 2009;67:274–280. [PubMed] [Google Scholar]
- 74.Simsek-Yavuz S., Ozbek E., Basaran S., Celebi B., Yilmaz E., Basaran M. The first case of chronic Q fever endocarditis and aortitis from Turkey: a 5-year infection before diagnosis with drain in sternum. Anatol J Cardiol. 2016;16:814–816. doi: 10.14744/AnatolJCardiol.2016.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.