Abstract
Human mansonellosis is caused by M. perstans, M. ozzardi and M. streptocerca, the three main filarial species in the genus Mansonella. Despite accumulating evidence of a high prevalence in endemic areas, there is currently no filariasis control programme targeting mansonellosis. The health-related impact on people living with these filariae remains unknown, and evidences regarding treatment strategies are scarce. Like other neglected diseases, it mainly affects poor populations living in tropical and subtropical climates. Mansonellosis can be considered one of the most neglected tropical infectious diseases. The objective of this literature review was to draw attention to the gap of knowledge regarding Mansonella spp. taxonomy, the transmission of these arthropod-borne filariasis and the health outcomes of people living with mansonellosis.
Keywords: Filariasis, Mansonella perstans, Mansonella ozzardii, Mansonella streptocerca, neglected tropical diseases
Introduction
Mansonella Faust, 1929, is a large genus of nematodes of Onchocercidae family. The type species is Mansonella ozzardi [1]; another major human parasite, Mansonella perstans, was originally reported by P. Manson in 1891 as ‘Filaria sanguinis hominis minor’ [2]. After several modifications of the genus name from Filaria to Dipetalonema, Tetrapetalonema and Acanthocheilonema, both nematodes are now attributed to Mansonella. To date, this genus includes 29 valid species that are distributed worldwide, except in Australia [1].
Burden of mansonellosis
M. perstans is considered to be the most frequent filariasis in Africa (Fig. 1). It is distributed mostly in wet subtropical and tropical areas of Africa, from Senegal (Fig. 2) to Zimbabwe. It has also been recently reported in South America, in sympatry with M. ozzardi [3]. Many publications refer to mansonellosis as one of the most common human helminthiases in endemic areas. Indeed, a prevalence of up to 92% among microscopically assessed microfilaraemia has been reported historically in endemic regions of Cameroon [4]. More recent real-time quantitative PCR (qPCR)-based prevalence data from Senegal found an overall prevalence of 14.5%, which could extend up to 40% to 50% in some villages [5]. Similarly, the qPCR-based prevalence was 32% in 2247 participants studied in Ghana [6] and 76% in 1085 participants in Cameroon [7]. Simonsen et al. [8] estimated in a review that more than 100 million people are infected in Africa, and that ∼600 million people live in 33 countries at high risk for M. perstans infection in Africa.
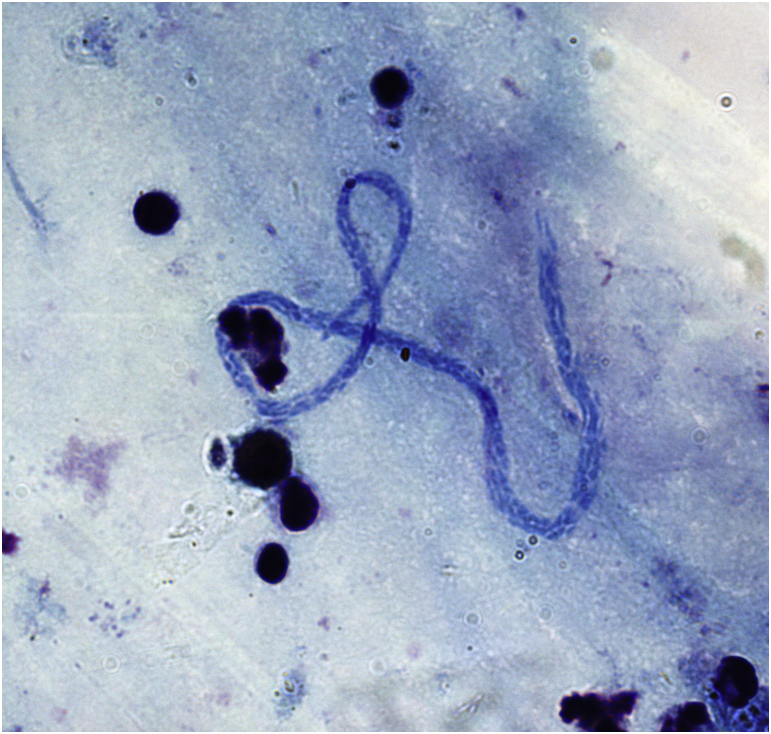
Fig. 1.
Mansonella perstans microfilaria in thick blood film collected from a Senegalese patient (Giemsa straining; original magnification, ×1000).
Fig. 2.
Village in Oriental Senegal in which half of inhabitants are living with mansonellosis.
M. ozzardi is evenly distributed in tropical regions of North and South America, from Mexico to Argentina [9], [10]. A recent study reported a prevalence of ∼35% in Brazilian Amazon autochthons [11], but prevalence may reach 61% in endemic regions [9].
Recently a novel genetic variant referred to as Mansonella sp. ‘Deux’ has been described in blood samples of febrile children in Gabon [5]. Unfortunately, it is still difficult to say exactly whether Mansonella sp. ‘Deux’ corresponds to M. rodhaini or any other ape-associated Mansonella from Central Africa (M. gorillae, M. lopeensis, M. leopoldi, M. vanhoofi) because nucleotide sequences for these species are not available.
Biology of Mansonella spp.
Adult Mansonella worms are relatively small filarial nematodes, with a length ranging from 2 cm (M. streptocerca) to 8 cm (M. perstans and M. ozzardi) [1], [9]. As for other Onchocercidae, the life cycle includes the development in both an insect vector and a vertebrate (mammal) host. In mammalian hosts, adult Mansonella worms are located in connective tissues (fascia, subcutaneous, etc.) or natural (peritoneal, pleural or pericardial) cavities. Viviparous female worms release sharp-tailed unsheathed microfilariae that reach the bloodstream. Microfilariae of M. streptocerca have a unique tropism to the skin, similar to Onchocerca volvulus. In contrast to Wuchereria bancrofti or Loa loa, the microfilariae of the three Mansonella species have no circadian periodicity in the peripheral blood. Table 1 summarizes the biologic characteristics of these three filariasis.
Table 1.
Key features of human mansonellosis
| Characteristic | Mansonella streptocerca | Mansonella ozzardi | Mansonella perstans |
|---|---|---|---|
| Areas of endemicity | Western and Central Africa | Central and South America, Caribbean | Sub-Saharan and North Africa, South America |
| Arthropod vectors | Culicoides spp. | Culicoides spp.; Simulium amazonicum | Culicoides spp. |
| Filariae localization | Dermis of the trunk and upper shoulder girdle | Lymphatic vessels, pericardium, pleura and peritoneum | Pericardium, pleura and peritoneum; mesentery, and retroperitoneum |
| Microfilariae localization | Skin | Blood and skin | Blood |
Haematophagous arthropods acquire microfilaria during their blood meal. Culicoides spp. (biting midges) are considered to be effective vectors for all species of Mansonella [4]; Simulium (blackflies) have also been reported to transmit M. ozzardi [10]. Once ingested by a female arthropod, microfilariae penetrate the insect's gut and go through three maturation stages (L1 to L3) in the thoracic muscles over 6 to 12 days. The infective third-stage larvae (L3) migrate to the arthropod proboscis, where they can infect a human when the arthropod takes a blood meal. Body heat activates the larvae; it prompts them to leave the vector, actively penetrate the bite wound and mature into adult worms [4].
Mansonella hosts
Mansonella hosts include several species of primates, carnivores, ungulates, rodents and tupaids in tropical Africa, South America and Asia. Three Mansonella species often infect humans: M. perstans, M. ozzardi and M. streptocerca. Whereas M. perstans has never been reported to infect nonhuman primates, M. streptocerca has been found in primates [1]. Only humans appear to be naturally infected with M. ozzardi [14], [15]; African patas monkeys (Erythrocebus patas), but not chimpanzees, rhesus, capuchin or squirrel monkeys, are susceptible to experimental infection with this nematode [12]. Chimpanzees in Central Africa are the reservoir of Mansonella rodhaini, which has been identified in skin biopsy samples of several villagers in Gabon [13].
Clinical features
In-depth clinical studies aiming to describe the symptoms associated with mansonellosis are lacking. Most microfilaremic individuals are reported to be asymptomatic; eosinophilia is a common feature. Nonspecific symptoms, including pruritus, urticaria, arthralgia, abdominal pain and fatigue, have been described. In M. perstans, symptoms of infection are probably related to the migration of the worms, and include transient subcutaneous swellings (similar to the Calabar swellings caused by L. loa), pericarditis, pleuritis and inflammatory granulomatous nodules surrounding dead adult worms [8]. Although M. ozzardi infection is usually described as relatively harmless, it has been associated with keratitis [23]. The symptoms associated with M. streptocerca infection are similar to onchocerciasis, with the following characteristics: papular dermatitis and pruritus predominates on the upper parts of the body, and the absence of nodules.
Parasitologic diagnosis
The morphologic identification of microfilariae in Giemsa-stained blood smears is based on size, presence or absence of a sheath, tail shape and arrangement of terminal nuclei in the tail. Other features are the presence or not of microfilariae in the bloodstream and its periodicity. All Mansonella microfilariae are unsheathed, and those circulating in blood are aperiodic [11].
Microfilariae of Mansonella perstans circulate in the blood. They are 190 to 200 μm long, and nuclei extend to the tip of the tail, which is blunt. M. perstans microfilariae are distinguishable from other sympatric microfilariae, including those from L. loa or W. bancrofti, which are longer and sheathed, and in whose terminal nuclei are bigger than the others.
Microfilariae of Mansonella ozzardi circulate in the blood and can occasionally be found in the skin. They are 160 to 205 μm long, unsheathed and have no nuclei in the tip of the tail, which is bent in a small hooklike shape. In the blood, they can be easily distinguished from W. bancrofti microfilariae, which are longer and sheathed. In the skin, they resemble O. volvulus microfilariae, which are slightly longer (300 μm) and have a flexed tail.
Microfilariae of Mansonella streptocerca are found in skin and not in blood. They are 180 to 240 μm long and unsheathed, and the nuclei extend to the end of the hooklike tail. They are smaller and thinner than O. volvulus microfilariae, which have no nuclei in the tip of the tail.
Treatment
Anthelminthic drugs have a limited efficacy against M. perstans. However, one study showed a remarkable efficacy of a prolonged (6 weeks) doxycycline treatment targeting a Wolbachia endosymbiont [16]. Diethylcarbamazine treatment is effective against both microfilariae and adults of M. streptocerca; however, it has been commonly associated with significant adverse drug reactions. Although ivermectin reduces M. streptocerca microfilaria loads, its clinical effects have not been documented [24]. Neither the presence of Wolbachia endosymbionts nor the use of doxycycline against M. streptocerca has been documented to date. A recent phase 3 clinical trial in the Brazilian Amazon autochthones showed high efficacy of ivermectin in the reduction of M. ozzardi infection [18]. In contrast, single-dose ivermectin treatment was relatively ineffective in studies conducted in Northern Argentina [10], and diethylcarbamazine was also demonstrated to be ineffective [25].
Despite accumulating evidence of a high prevalence of human infections, no current large-scale filariasis control programme is targeting mansonellosis. Mansonellosis is not listed among the neglected diseases of the World Health Organization, and no control strategy has been defined against this human filariasis. The major reasons for this lack of concern regarding Mansonella infections are that they prevail in poor, rural populations, they have not been associated with distinct clinical features and there is currently no drug that could be used in community-directed treatment against mansonellosis. Further, and more generally, published evidence to guide a treatment strategy against these filariases are scarce. Mansonellosis can thus be regarded as a neglected tropical infectious disease.
Knowledge gaps in the biology of Mansonella spp.
Few studies aim to investigate vector–Mansonella relationships. The range and transmission competency of the distinct possible Mansonella spp. vectors have not been identified. The results of the few entomologic studies that have been performed in mansonellosis-endemic areas report either a very low (0.8%) or zero prevalence of Mansonella-infected wild-caught Culicoides [5], [19]. These findings are inconsistent with the high prevalence of Mansonella microfilaraemia in humans, thus suggesting that an arthropod other than Culicoides—possibly mosquitos or other sympatric haematophagous arthropods—are vectors.
Another issue is the controversial symbiosis of the obligate intracellular bacteria of the genera Wolbachia and Mansonella. The only evidence of the presence of Wolbachia in Mansonella ozzardi was published in 2001 [20]. The first attempt to detect Wolbachia in M. perstans failed [21]. Nevertheless, the presence of the bacterium was evidenced later [17], [22], though not in all samples, thus either indicating that Wolbachia are facultative M. perstans endosymbionts or that there were some technical difficulties. Although the efficacy of doxycycline against M. perstans microfilaraemia in a clinical trial [16] promoted the hypothesis of obligatory endosymbiosis, further studies are needed to better understand the Wolbachia–Mansonella association.
Perspectives
Summarizing the issues regarding mansonellosis, it is clear that (a) the data on morbidity, mortality and clinical picture of mansonellosis remain scarce, (b) the limited data on vector transmission hinder the development of effective control strategies, and (c) effective treatment is lacking. All these issues reflect governments', stakeholders', interest groups' and other decision makers' disregard of mansonellosis.
Mansonellosis is a genuinely neglected disease that calls out for attention. A PubMed query using the term ‘Mansonella’ retrieved an average of 7.12 publications per year for the last 50 years (1968–2017). Equally, a Nucleotide database query (https://www.ncbi.nlm.nih.gov/nuccore/?term=mansonella) using the term ‘Mansonella’ retrieved only 207 items; in comparison, a query using the term ‘Staphylococcus’ retrieved 1 973 105 items. These two examples dramatically emphasize the scarce knowledge and limited scientific literature available despite the hundreds of millions of people living with mansonellosis [8].
Acknowledgement
The authors thank O. Cusack for English-language editorial work. This work was supported by the French Government under the “Investissements d'avenir” (Investments for the Future) program managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research), (reference: Méditerranée Infection 10-IAHU-03).
Conflict of interest
None declared.
References
- 1.Bain O., Mutafchiev Y., Junker K., Guerrero R., Martin C., Lefoulon E. Review of the genus Mansonella Faust, 1929 sensu lato (Nematoda: Onchocercidae), with descriptions of a new subgenus and a new subspecies. Zootaxa. 2015;3918:151–193. doi: 10.11646/zootaxa.3918.2.1. [DOI] [PubMed] [Google Scholar]
- 2.Manson P. The filaria sanguinis hominis major and minor, two new species of haematozoa. Lancet. 1891;137:4–8. [Google Scholar]
- 3.Tavares da Silva L.B., Crainey J.L., Ribeiro da Silva T.R., Suwa U.F., Vicente A.C., Fernandes de M.J. Molecular verification of new world Mansonella perstans parasitemias. Emerg Infect Dis. 2017;23:545–547. doi: 10.3201/eid2303.161159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyce Sharp N.A. Filaria perstans; its development in Culicoides austeni. Trans R Soc Trop Med Hyg. 1928;21:371–396. [Google Scholar]
- 5.Bassene H., Sambou M., Fenollar F., Clarke S., Djiba S., Mourembou G. High prevalence of Mansonella perstans filariasis in rural Senegal. Am J Trop Med Hyg. 2015;93:601–606. doi: 10.4269/ajtmh.15-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debrah L.B., Nausch N., Opoku V.S., Owusu W., Mubarik Y., Berko D.A. Epidemiology of Mansonella perstans in the middle belt of ghana. Parasit Vectors. 2017;10:15. doi: 10.1186/s13071-016-1960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drame P.M., Montavon C., Pion S.D., Kubofcik J., Fay M.P., Nutman T.B. Molecular epidemiology of blood-borne human parasites in a Loa loa–, Mansonella perstans–, and Plasmodium falciparum-endemic region of cameroon. Am J Trop Med Hyg. 2016;94:1301–1308. doi: 10.4269/ajtmh.15-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonsen P.E., Onapa A.W., Asio S.M. Mansonella perstans filariasis in Africa. Acta Trop. 2011;120(Suppl. 1):S109–S120. doi: 10.1016/j.actatropica.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Downes B.L., Jacobsen K.H. A systematic review of the epidemiology of mansonelliasis. Afr J Infect Dis. 2010;4:7–14. doi: 10.4314/ajid.v4i1.55085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shelley A.J., Coscaron S. Simuliid blackflies (Diptera: Simuliidae) and ceratopogonid midges (Diptera: Ceratopogonidae) as vectors of Mansonella ozzardi (Nematoda: Onchocercidae) in northern Argentina. Mem Inst Oswaldo Cruz. 2001;96:451–458. doi: 10.1590/s0074-02762001000400003. [DOI] [PubMed] [Google Scholar]
- 11.Ta-Tang T.H., Crainey J.L., Post R.J., Luz S.L., Rubio J.M. Mansonellosis: current perspectives. Res Rep Trop Med. 2018;9:9–24. doi: 10.2147/RRTM.S125750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima N.F., Veggiani Aybar C.A., Dantur Juri M.J., Ferreira M.U. Mansonella ozzardi: a neglected new world filarial nematode. Pathog Glob Health. 2016;110:97–107. doi: 10.1080/20477724.2016.1190544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richard-Lenoble D., Kombila M., Bain O., Chandenier J., Mariotte O. Filariasis in Gabon: human infections with Microfilaria rodhaini. Am J Trop Med Hyg. 1988;39:91–92. doi: 10.4269/ajtmh.1988.39.91. [DOI] [PubMed] [Google Scholar]
- 14.Orihel T.C., Lowrie R.C., Jr., Eberhard M.L., Raccurt C., Kozek W.J., Tidwell M.A. Susceptibility of laboratory primates to infection with Mansonella ozzardi from man. Am J Trop Med Hyg. 1981;30:790–794. doi: 10.4269/ajtmh.1981.30.790. [DOI] [PubMed] [Google Scholar]
- 15.Orihel T.C., Eberhard M.L. Mansonella ozzardi: a redescription with comments on its taxonomic relationships. Am J Trop Med Hyg. 1982;31:1142–1147. doi: 10.4269/ajtmh.1982.31.1142. [DOI] [PubMed] [Google Scholar]
- 16.Coulibaly Y.I., Dembele B., Diallo A.A., Lipner E.M., Doumbia S.S., Coulibaly S.Y. A randomized trial of doxycycline for Mansonella perstans infection. N Engl J Med. 2009;361:1448–1458. doi: 10.1056/NEJMoa0900863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gehringer C., Kreidenweiss A., Flamen A., Antony J.S., Grobusch M.P., Belard S. Molecular evidence of Wolbachia endosymbiosis in Mansonella perstans in Gabon, Central Africa. J Infect Dis. 2014;210:1633–1638. doi: 10.1093/infdis/jiu320. [DOI] [PubMed] [Google Scholar]
- 18.de Almeida B.S., de Almeida Aranha Camargo J.S., Fontes G., Pereira A.R., Medeiros J.F., de Oliveira Laudisse M.C. Phase III clinical trial to evaluate ivermectin in the reduction of Mansonella ozzardi infection in the Brazilian Amazon. Am J Trop Med Hyg. 2018;98:786–790. doi: 10.4269/ajtmh.17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noireau F., Itoua A., Carme B. Epidemiology of Mansonella perstans filariasis in the forest region of south Congo. Ann Trop Med Parasitol. 1990;84:251–254. doi: 10.1080/00034983.1990.11812464. [DOI] [PubMed] [Google Scholar]
- 20.Casiraghi M., Favia G., Cancrini G., Bartoloni A., Bandi C. Molecular identification of Wolbachia from the filarial nematode Mansonella ozzardi. Parasitol Res. 2001;87:417–420. doi: 10.1007/s004360000368. [DOI] [PubMed] [Google Scholar]
- 21.Grobusch M.P., Kombila M., Autenrieth I., Mehlhorn H., Kremsner P.G. No evidence of Wolbachia endosymbiosis with Loa loa and Mansonella perstans. Parasitol Res. 2003;90:405–408. doi: 10.1007/s00436-003-0872-z. [DOI] [PubMed] [Google Scholar]
- 22.Keiser P.B., Coulibaly Y., Kubofcik J., Diallo A.A., Klion A.D., Traore S.F. Molecular identification of Wolbachia from the filarial nematode Mansonella perstans. Mol Biochem Parasitol. 2008;160:123–128. doi: 10.1016/j.molbiopara.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer P., Tukesiga E., Büttner D.W. Long-term suppression of Mansonella streptocerca microfilariae after treatment with ivermectin. J Infect Dis. 1999;180(4):1403. doi: 10.1086/315014. [DOI] [PubMed] [Google Scholar]
- 24.Fischer P., Bamuhiiga J., Büttner D.W. Occurrence and diagnosis of Mansonella streptocerca in Uganda. Acta Trop. 1997;63:43. doi: 10.1016/s0001-706x(96)00607-9. [DOI] [PubMed] [Google Scholar]
- 25.Bartholomew C.F., Nathan M.B., Tikasingh E.S. The failure of diethylcarbamazine in the treatment of Mansonella ozzardi infections. Trans R Soc Trop Med Hyg. 1978;72:423–424. doi: 10.1016/0035-9203(78)90141-4. [DOI] [PubMed] [Google Scholar]