Abstract
Most infectious diseases are unequally distributed between male and female subjects. This sex dimorphism is confirmed by epidemiologic studies which suggest an increased number of male septic patients, while, due to the class age of septic patients, an overrepresentation of female patients would be expected. Lifestyle, recreational activities, professional exposition and access to care are plausible reasons for this dimorphism. However, biological differences should be carefully considered, particularly the weight of X-linked variability and the role of sex hormones. Animal models clearly show that clinical response to infection is more exuberant in males than in females. This is partly explained by an attenuation of the inflammatory response by female sex hormones. However, the translation from experimental studies to the bedside remains challenging as a result of confounding factors like age, hormone changes and response to treatment.
Keywords: Bacteria, estradiol, gender, infection, sex
Introduction
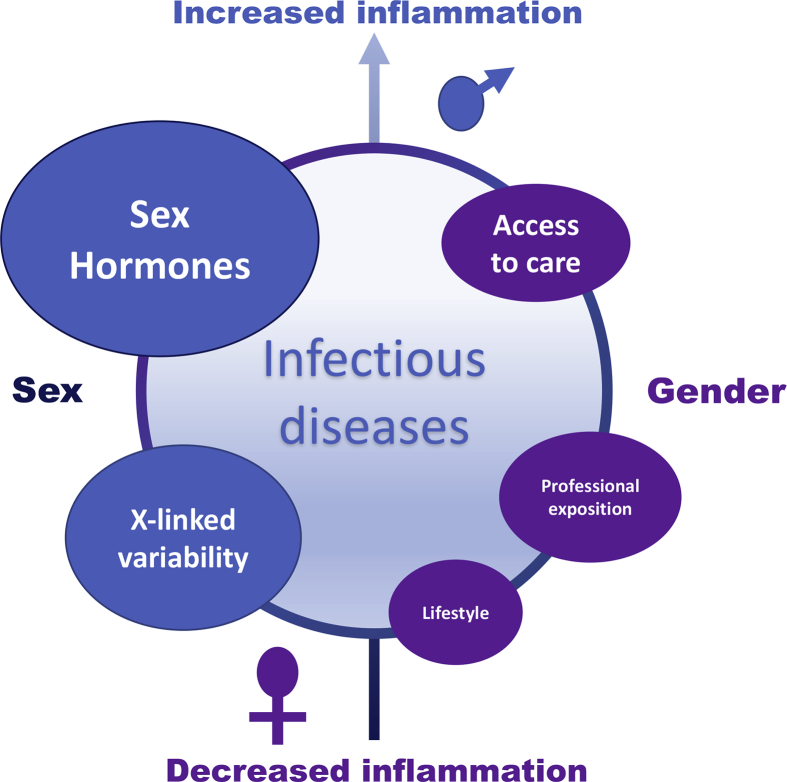
Most diseases are unequally balanced between male and female subjects. In infectious diseases, female subjects seem less prone to develop infection than male subjects, although this fluctuates. Lifestyle, professional exposition, recreational activities and access to healthcare are plausible reasons to explain this dimorphism. On the other hand, X-linked variability and sex hormones may also participate to the sex dimorphism (Fig. 1). Differences due to environmental factors are grouped under the designation ‘gender,’ while ‘sex’ refers to biological dimorphism.
Fig. 1.
Sex, gender and infection: weight of players.
Here we report findings about epidemiology, gender effect and sex differences reported in bacterial infectious diseases.
Epidemiology
Epidemiologic studies have demonstrated that male subjects are more prone to develop most infectious diseases than female subjects. In a meta-analysis, Campanelli et al. [1] assessed 498 146 patients from 12 multicentre randomized clinical trials and 22 original articles published in the three highest-ranked journals focusing on septic shock and severe sepsis. They found that the majority of septic patients were men, confirming the findings of largest cohort studies [2], [3]. Men represent from 54% to 61% of septic patients, whereas the sex ratio of the class age >65 is around 0.7 [1]. Hence, a majority of women would be expected. In parallel, among women referred to intensive care unit, 7.6% developed severe sepsis/septic shock, while 10.4% of all men experienced severe sepsis or septic shock [4].
This dimorphism is reported in most infectious diseases, including Pseudomonas aeruginosa bacteraemia [5], Q fever [6], [7], Legionnaires' disease [8] and tuberculosis [9]. Among patients with Q fever, men are symptomatic more often than women, with a male:female ratio of 2.5 [6]. From 2011 to 2015, the European Legionnaires' Disease Surveillance Network showed that infection due to Legionella pneumophila occurred in male subjects in for 71% of cases [8]. Tuberculosis is a worldwide disease with increasing incidence in low- and middle-income countries. A meta-analysis including 53 publications showed that the overall random effects weighted prevalence per 100 000 individuals was about two times higher in men than in women in bacteriologically positive tuberculosis and smear-positive tuberculosis [9].
In addition, the site of infection, which affects outcome, differs between men and women [3]. Urinary tract infections are more common in women, whereas endocarditis and mediastinitis occur more frequently in men [10]. One should note that urinary tract infections are associated with the lowest mortality rate among the different types of infection [3]. Taken together, the incidence of infections is marked by an overrepresentation of men and a different distribution infections sites between men and women.
Gender effect
Professional activities, recreational activities and lifestyle affect exposure to pathogens. Serology can be used as a surrogate for a group's exposure to a specific pathogen. In the south of France, Q fever incidence is higher in men than in women. Because Coxiella burnetii is found in hunting areas—with hunting being a recreational activity traditionally performed by men—one may hypothesize that men were more exposed than women to C. burnetii. Yet the antibodies directed against C. burnetii in men and women were equally distributed, suggesting a similar level of exposure [6], [11].
More importantly, access to care in septic patients differs between men and women. Regarding gender equity in healthcare, Diaz-Granados et al. [12] reported inequities in all countries, regardless of income level. In a study measuring the delay of antibiotic administration, the mean time to antibiotics in women was longer than in men [13]. In contrast, in patients with tuberculosis living in low- and middle-income countries, there is strong evidence that men are disadvantaged in seeking and/or accessing tuberculosis care in many settings [9]. A French study which measured the level of care given by nurses to men and women in an intensive care unit did not report significant differences [14]. Thus, access to care remains disparate among men and women, depending on culture, country and infection type.
Biological mechanisms
As reported elsewhere [15], sex-related differences in infection may be related to the large quantity of X-linked polymorphic immunocompetent genes, differences in X chromosome regulation and inheritance patterns between the sexes and the presence of X-linked cellular mosaicism, which is unique to females. This is illustrated by the impact of the abundance of immune-related genes on the X chromosome on susceptibility to tuberculosis. The X-linked TLR8 polymorphisms have been linked with susceptibility to tuberculosis in boys [16]. Sperry et al. [17] described an X chromosome–linked interleukin 1 receptor–associated kinase (IRAK-1) polymorphism as possibly responsible for sex differences in trauma patients. They identified an IRAK-1 variant in a cohort of patients which was associated with increased rates of organ failure and death. This variant was found in 88% of male and 67% of female subjects; female subjects who were heterozygous for the variant had worse outcome.
Sex hormones
Sex hormones play a role in dimorphic clinical expression of acute infectious diseases. Hence, clinical presentation is often attenuated in women, partly explaining the differences in reported incidences of infectious diseases. Chen et al. [18] exposed male or female C57BL/6 mice to various infectious insults and then evaluated their cardiac function using echocardiography. Cardiac dysfunction in immune sepsis that was reported in both sexes of mice was significantly less pronounced in female than in male animals. In rats subjected to cecal ligation puncture, we first confirmed that septic cardiac dysfunction was markedly increased in male compared to female animals. β-Blockers were then administered to improve stroke volume and cardiac relaxation. The response to β-blockers improved the cardiac function in male animals, while it was associated with deleterious effects in female animals [19]. Although the host response was mediated by β-blockers, this is likely related to sex-dependent expression of adrenergic receptors. Experimental data revealed a reduced β-adrenergic responsiveness of female hearts [20], [21]. In an experimental model conducted in healthy volunteers subjected to endotoxin administration, females developed a more pronounced proinflammatory response associated with less attenuation of norepinephrine sensitivity than men [22]. Nevertheless, a sexual dimorphism of the cardiovascular response between males and females is still reported.
In animal models of sepsis, it has been found that sex hormones modulate severity of septic-induced damage. Hence, castration of females worsens the lesions, and addition of oestradiol induces the reverse. In a model of trauma-haemorrhage, castration of females was associated with an increased mortality [23], suggesting a protective role of sex hormones. The administration of dehydroepiandrosterone, an intermediate in sex hormone synthesis, to pro-oestrus females after trauma-haemorrhage enhances immunoresponse [24]. Both testosterone [25] and oestradiol [26] have been shown to reduce the production of proinflammatory mediators. In a cohort of intensive care unit patients, Tsang et al. [27] showed that bioavailable oestradiol levels were elevated in those patients, particularly nonsurvivors, and were independently associated with mortality. Because most intensive care unit patients are immunosuppressed, one can hypothesize the existence of an association between immune and hormone responses during sepsis [28].
Sex hormones also play a role in the susceptibility to intracellular pathogens. In a mouse model of C. burnetii infection, we found an increased number of tissue granulomas and bacterial burden in male compared to female animals [29]. After ovariectomy, the number of granuloma and bacteria increased in female animals; this increase was corrected by the addition of exogenous oestradiol [29].
Using a transcriptomic approach, we showed that 86% of genes were differentially expressed in males and females infected with C. burnetii and more than 60% of gene modulation was due to sex hormones [30]. In males, there was an enrichment with gene categories related to inflammation and antibacterial immunoresponse, suggesting an enhanced susceptibility of males to C. burnetii infection that is related to inappropriate immunoresponse. In females, we found that the circadian rhythm pathway was altered after C. burnetii infection, suggesting no previous mechanism for the role of oestradiol.
Oestradiol and progesterone produced during menstrual cycle influence the susceptibility to sexually transmitted infections via their actions on the female reproductive tract [31]. Elderly women are particularly prone to recurrent urinary tract infections, but oestrogen supplementation after menopause protects against infections, likely by boosting innate immunity [32].
Challenge of translating animal models into clinical findings
A discrepancy was often reported between animal and human studies, restricting the development of research programmes in this field. Firstly, this is a complex issue with a large number of players, which evolve with time and disease progression. Disease evolves over a long duration, affecting hormone production in septic patients [33]. Secondly, age is a critical variable in sepsis [33] and in chronic infections such as Q fever [6]. In an experimental model of C. burnetii infection, we showed that age affects host response to C. burnetii infection, with a repressed immunoresponse in mature mice [34]. In clinical studies, results should be analysed according to patient age and hormone status. Thirdly, metabolisms may differ in animals and humans. We previously found that C. burnetii infection affected circadian rhythm through an increased expression of the Per2 gene in females [30]. A converse result was found in humans, with an overexpression in men [35]. This contradiction is due to an inversion of circadian rhythm between mice and humans in relation with the timing of activity [36]. Fourthly, for safety reasons, most clinical trials exclude women who may become pregnant. Hence, new treatments are never tested in this specific population, which makes the risk–benefit balance speculative.
Conclusion
Sexual dimorphism can be regarded as the first step of personalized medicine [37]. Recourse to hormone treatments probably remains speculative at this stage. However, the use of immune treatments or steroids should probably be assessed differently in male and female subjects. Studies must consider sex as a critical factor for subgroup analysis.
Conflict of interest
None declared.
References
- 1.Campanelli F., Landoni G., Cabrini L., Zangrillo A. Gender differences in septic intensive care unit patients. Minerva Anestesiol. 2018;84:504–508. doi: 10.23736/S0375-9393.17.12187-5. [DOI] [PubMed] [Google Scholar]
- 2.Rhee C., Dantes R., Epstein L., Murphy D.J., Seymour C.W., Iwashyna T.J. Incidence and trends of sepsis in US hospitals using clinical vs. claims data, 2009–2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent J.L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 4.Wichmann M.W., Inthorn D., Andress H.J., Schildberg F.W. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26:167–172. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hasan M.N., Wilson J.W., Lahr B.D., Eckel-Passow J.E., Baddour L.M. Incidence of Pseudomonas aeruginosa bacteremia: a population-based study. Am J Med. 2008;121:702–708. doi: 10.1016/j.amjmed.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tissot Dupont H., Raoult D., Brouqui P., Janbon F., Peyramond D., Weiller P.J. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am J Med. 1992;93:427–434. doi: 10.1016/0002-9343(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 7.Eldin C., Mélenotte C., Mediannikov O., Ghigo E., Million M., Edouard S. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017;30:115–190. doi: 10.1128/CMR.00045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beauté J. European Legionnaires’ disease surveillance network. Legionnaires’ disease in Europe, 2011 to 2015. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.27.30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton K.C., MacPherson P., Houben R.M., White R.G., Corbett E.L. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Vught L.A., Scicluna B.P., Wiewel M.A., Hoogendijk A.J., Klein Klouwenberg P.M.C., Ong D.S.Y. Association of gender with outcome and host response in critically ill sepsis patients. Crit Care Med. 2017;45:1854–1862. doi: 10.1097/CCM.0000000000002649. [DOI] [PubMed] [Google Scholar]
- 11.Brandwagt D.A., Herremans T., Schneeberger P.M., Hackert V.H., Hoebe C.J., Paget J. Waning population immunity prior to a large Q fever epidemic in the south of The Netherlands. Epidemiol Infect. 2016;144:2866–2872. doi: 10.1017/S0950268816000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Granados N., McDermott S., Wang F., Posada-Villa J., Saavedra J., Rondon M.B. Monitoring gender equity in mental health in a low-, middle-, and high-income country in the Americas. Psychiatr Serv. 2011;62:516–524. doi: 10.1176/ps.62.5.pss6205_0516. [DOI] [PubMed] [Google Scholar]
- 13.Madsen T.E., Napoli A.M. The DISPARITY-II study: delays to antibiotic administration in women with severe sepsis or septic shock. Acad Emerg Med. 2014;21:1499–1502. doi: 10.1111/acem.12546. [DOI] [PubMed] [Google Scholar]
- 14.Adrie C., Azoulay E., Francais A., Clec’h C., Darques L., Schwebel C. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 15.Spolarics Z., Peña G., Qin Y., Donnelly R.J., Livingston D.H. Inherent X-linked genetic variability and cellular mosaicism unique to females contribute to sex-related differences in the innate immune response. Front Immunol. 2017;8:1455. doi: 10.3389/fimmu.2017.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nhamoyebonde S., Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. 2014;209(Suppl. 3):S100–S106. doi: 10.1093/infdis/jiu147. [DOI] [PubMed] [Google Scholar]
- 17.Sperry J.L., Zolin S., Zuckerbraun B.S., Vodovotz Y., Namas R., Neal M.D. X chromosome–linked IRAK-1 polymorphism is a strong predictor of multiple organ failure and mortality postinjury. Ann Surg. 2014;260:698–705. doi: 10.1097/SLA.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J., Chiazza F., Collino M., Patel N.S., Coldewey S.M., Thiemermann C. Gender dimorphism of the cardiac dysfunction in murine sepsis: signaling mechanisms and age-dependency. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathieu C., Desrois M., Kober F., Lalevée N., Lan C., Fourny N. Sex-mediated response to the beta-blocker landiolol in sepsis: an experimental, randomized study. Crit Care Med. 2018 Apr 7 doi: 10.1097/CCM.0000000000003146. [DOI] [PubMed] [Google Scholar]
- 20.McIntosh V.J., Chandrasekera P.C., Lasley R.D. Sex differences and the effects of ovariectomy on the β-adrenergic contractile response. Am J Physiol Heart Circ Physiol. 2011;301:H1127–H1134. doi: 10.1152/ajpheart.00711.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeker G.S., Hood A.R., Katra R.P., Poelzing S., Pogwizd S.M. Sex differences in β-adrenergic responsiveness of action potentials and intracellular calcium handling in isolated rabbit hearts. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Eijk L.T., Dorresteijn M.J., Smits P., van der Hoeven J.G., Netea M.G., Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 23.Knöferl M.W., Angele M.K., Diodato M.D., Schwacha M.G., Ayala A., Cioffi W.G. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235:105–112. doi: 10.1097/00000658-200201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knoferl M.W., Angele M.K., Catania R.A., Diodato M.D., Bland K.I., Chaudry I.H. Immunomodulatory effects of dehydroepiandrosterone in proestrus female mice after trauma-hemorrhage. J Appl Physiol. 2003;95:529–535. doi: 10.1152/japplphysiol.01201.2002. [DOI] [PubMed] [Google Scholar]
- 25.Torres M.B., Trentzsch H., Stewart D., Mooney M.L., Fuentes J.M., Saad D.F. Protection from lethal endotoxic shock after testosterone depletion is linked to chromosome X. Shock. 2005;24:318–323. doi: 10.1097/01.shk.0000177639.22863.99. [DOI] [PubMed] [Google Scholar]
- 26.Rettew J.A., Huet Y.M., Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150:3877–3884. doi: 10.1210/en.2009-0098. [DOI] [PubMed] [Google Scholar]
- 27.Tsang G., Insel M.B., Weis J.M., Morgan M.A., Gough M.S., Frasier L.M. Bioavailable estradiol concentrations are elevated and predict mortality in septic patients: a prospective cohort study. Crit Care. 2016;20:335. doi: 10.1186/s13054-016-1525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boomer J.S., Green J.M., Hotchkiss R.S. The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence. 2014;5:45–56. doi: 10.4161/viru.26516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leone M., Honstettre A., Lepidi H., Capo C., Bayard F., Raoult D. Effect of sex on Coxiella burnetii infection: protective role of 17beta-estradiol. J Infect Dis. 2004;189:339–345. doi: 10.1086/380798. [DOI] [PubMed] [Google Scholar]
- 30.Textoris J., Ban L.H., Capo C., Raoult D., Leone M., Mege J.L. Sex-related differences in gene expression following Coxiella burnetii infection in mice: potential role of circadian rhythm. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wira C.R., Rodriguez-Garcia M., Patel M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015;15:217–230. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham S.N., Miao Y. The nature of immune responses to urinary tract infections. Nat Rev Immunol. 2015 Oct;15:655–663. doi: 10.1038/nri3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angstwurm M.W., Gaertner R., Schopohl J. Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit Care Med. 2005;33:2786–2793. doi: 10.1097/01.ccm.0000190242.24410.17. [DOI] [PubMed] [Google Scholar]
- 34.Leone M., Bechah Y., Meghari S., Lepidi H., Capo C., Raoult D. Coxiella burnetii infection in C57BL/6 mice aged 1 or 14 months. FEMS Immunol Med Microbiol. 2007;50:396–400. doi: 10.1111/j.1574-695X.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 35.Mehraj V., Textoris J., Capo C., Raoult D., Leone M., Mege J.L. Overexpression of the Per2 gene in male patients with acute Q fever. J Infect Dis. 2012;206:1768–1770. doi: 10.1093/infdis/jis600. [DOI] [PubMed] [Google Scholar]
- 36.Méndez-Ferrer S. Human and mouse leukocytes: different clockwork. Blood. 2017;130:1960–1961. doi: 10.1182/blood-2017-09-805374. [DOI] [PubMed] [Google Scholar]
- 37.Mathieu C., Leone M. Gender and sepsis: first step of personalized medicine? Minerva Anestesiol. 2018;84:434–436. doi: 10.23736/S0375-9393.18.12625-3. [DOI] [PubMed] [Google Scholar]