Abstract
Arthropod vectors can transmit pathogenic microorganisms from one vertebrate to another during their blood meal. Although some vector-borne diseases have been eradicated in the Mediterranean area, such as malaria and dengue, recent endemic microorganisms (Toscana virus, Rickettsia spp.) remain neglected even though they cause many more cases. New diagnostic tools and innovative tools for the identification and characterization of vector species and microorganisms have been developed at IHU Méditerranée Infection, either internally or through collaborative and integrated projects. We have detected Rickettsia slovaca as a human pathogen and have described the disease; we have shown that Rickettsia felis can be transmitted by Anopheles mosquitoes; we have emphasized the increasing importance of bedbug (Cimex lectularius) as a potential vector of Bartonella quintana; and we have described the Toscana virus, a major agent of meningitis and meningoencephalitis which was disseminated in North Africa and Central and Eastern Europe, where it frequently cocirculates with a large number of newly described phleboviruses transmitted by sand flies.
Keywords: Arbovirus, arthropods, fleas, lice, phlebovirus, sand fly, ticks, Toscana virus, virus
Introduction
Some arthropods can actively transmit pathogenic microorganisms (virus, bacteria, parasites) from one vertebrate to another during their blood meal; such arthropods are known as vectors. Mosquitoes and ticks are the main vectors of human infectious diseases. In the Mediterranean area, major mosquito-borne diseases such as malaria and dengue have been considered to be eradicated for decades. However, Lyme disease, the well-known human tick-borne diseases, has not be described in the Mediterranean area because the major vector, Ixodes ricinus, is not prevalent in places with a Mediterranean climate, even if the exact limits of the geographic distribution of the tick vector and the disease are not precisely known [1]. Among vector-borne viral diseases, the scientific and medical literature emphasize epidemic diseases. Despite this, these diseases usually have a lesser long-term medical impact on exposed populations than endemic diseases. As a consequence, endemic diseases are commonly neglected compared to the epidemic ones, which benefit from public interest and media attention. Newspaper focus has been recently directed towards chikungunya virus, Zika virus and dengue virus, even in geographic areas where these viruses have played a negligible role in public health in Europe [2], [3]. In contrast, Crimean-Congo hemorrhagic fever, Rift Valley fever and Toscana virus (TOSV) infections have not been popularized. For instance, TOSV, which is the most prevalent arthropod-borne virus in Europe and in the Mediterranean area, is totally unknown—not only by the public but also by physicians [4].
West Nile virus (WNV) holds a peculiar situation. Undoubtedly, WNV is an emerging virus in the New World, which it stunningly entered in the fall of 1999 before spreading over North America [5]. In the Old World, it is endemic in Africa. The first noticeable outbreak in Europe was described in 1996 in Romania. Since then, WNV circulation has clearly increased in Europe over the last two decades with, special emphasis on Italy and Greece; however, other western European countries, such as Portugal, Spain and France, have not reported the same trend [6].
At the University Hospital Institute Méditerranée Infection in Marseille, France, vector-borne diseases represent a major axis of research and surveillance [7]. We have been involved in the surveillance of imported cases of major tropical vector-borne diseases (e.g. malaria) and arboviral infections (e.g. dengue, chikungunya and Zika), and more specifically in the surveillance of autochthonous transmission and even local outbreaks after importation [8]. This has been illustrated by several sentinel reports of autochthonous malaria cases and a local chikungunya outbreak [9], [10]. However, our main contribution in the field of vector-borne diseases in the Mediterranean area concerns neglected vector-borne bacterial diseases and phleboviruses.
As a reference centre for rickettsiology, we have been involved in major contributions to the knowledge on rickettsioses in the Mediterranean area through clinical, microbiologic and entomologic studies [11]. Indeed, although tick-borne rickettsioses, caused by obligate intracellular bacteria belonging to the spotted fever group (SFG) of the genus Rickettsia, are among the oldest known vector-borne diseases, the scope and importance of the recognized tick-associated rickettsial pathogens have dramatically increased, making this complex of diseases a paradigm of emerging diseases in the Mediterranean area. Until 1990, only Mediterranean spotted fever, caused by Rickettsia conorii conorii, had been described in this area. In the past decades, we added to the knowledge of clinical, epidemiologic and entomologic aspects of Mediterranean spotted fever in France and Algeria, including atypical and serious life-threatening presentations or the explanation of the peak of Mediterranean spotted fever during warmer months related to a warming-mediated increase in the aggressiveness of Rhipicephalus sanguineus ticks to bite humans [12], [13], [14].
With the use of arthropods as specimen to study the repertoire of tick-associated rickettsia and the development and expansion of molecular tools such as PCR and sequencing on arthropods, we identified several new pathogens in ticks collected from North Africa, and we therefore complemented the knowledge already collected on arthropod-borne pathogens circulating in the Mediterranean area, particularly in France, Italy and North Africa. We detected Rickettsia slovaca, the causative agent of tick-borne lymphadenopathy, also known as SENLAT syndrome (scalp eschar and neck lymphadenopathy after a tick bite), and contributed to the description of the clinical presentation of the disease. We also detected in ticks and/or patients several other emerging agents of SFG rickettsioses, including R. aeschlimanni, R. massiliae, R. monacensis, R. helvetica, R. sibirica mongolotimonae, R. conorii israelensis, R. conorii caspia, R. conorii indica and many other SFG rickettsia of unknown pathogenicity [15]. New approaches in the diagnosis have also been reported, such as swabbing of eschars to obtain material to be tested by PCR [11], [16] or the use of molecular tools and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry as a new tool to identify the tick that had bitten the patient as well as potential pathogens in ticks [17], [18]. In addition to tick-borne rickettsia and rickettsioses studies, we detected one of the causative agents of Lyme disease, Borrelia garinii, in Ixodes ricinus ticks collected in Algeria, where the epidemiology of the disease is not known [19].
When studying fleas, we expanded the knowledge of R. felis, an emerging agent of rickettsioses, and also detected Rickettsia typhi, the agent of murine typhus in several areas. Several cases of these infections were diagnosed [16], [20]. Experimental models highlighted the role of fleas in the transmission of Bartonella spp., and interestingly we have also shown that R. felis might also be transmitted by mosquitoes [21]. Finally, we have long been involved in surveying and managing infections in the homeless population, particularly louse-borne diseases such as trench fever. We have continued to study other louse-associated bacteria [22]. We have investigated strategies to eradicate lice in the homeless through study of the resistance of lice to pyrethrinoids and clinical trials on infested clothes [23]. More recently, and in the context of increasingly recognized infestations of human houses by bedbugs (Cimex lectularius), we have begun to study the potential vectorial capacity of these arthropods. We have shown their potential to transmit Bartonella quintana, the agent of trench fever, which is known to be transmitted by body lice [24]. A relationship has been established between three research structures of southeastern France: IRD-Montpellier, IHU-Marseille and CHU-Nice. It allowed us to evaluate the presence of this insect at the national level [25], to evaluate its genetics [26] and to look for the presence of Wolbachia bacteria in its gut [27].
Toscana virus
Discovered in 1971 in sand flies (Phlebotomus perniciosus, Phlebotomus perfiliewi) collected in the Tuscany region of Central Italy, TOSV was an orphan virus for 14 years before it was first recognized as the causative agent of neuroinvasive infections in southwestern Europe [28], [29]. Soon after the first case report–based evidence that TOSV was causing meningitis and encephalitis, several large studies were conducted in central Italy on adults and children with central nervous system infection [30], [31], [32], [33], [34], [35].
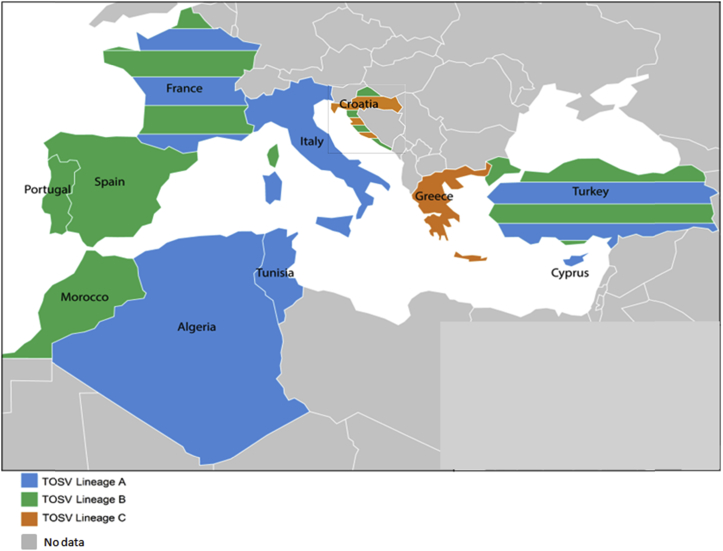
Researchers and physicians in Marseille became interested in this virus in 2003, when two patients without a relevant travel history were diagnosed with TOSV infection. One patient presented with aseptic meningitis, the other with febrile illness only [36]. Investigations conducted in the Marseille region and in coastal southeastern France indicated that (1) TOSV strains could be detected and isolated in sand flies present in the region [37], (b) autochthonous human TOSV cases may be caused by strains belonging to two different lineages (A and B), demonstrating that both types of strains cocirculate in southeastern France [37] (Fig. 1) and (c) other sand fly–borne phleboviruses genetically and antigenically related to but clearly distinct from TOSV are transmitted by sand flies belonging to the same species, such as Massilia virus [38].
Fig. 1.
Countries where Toscana virus is present.
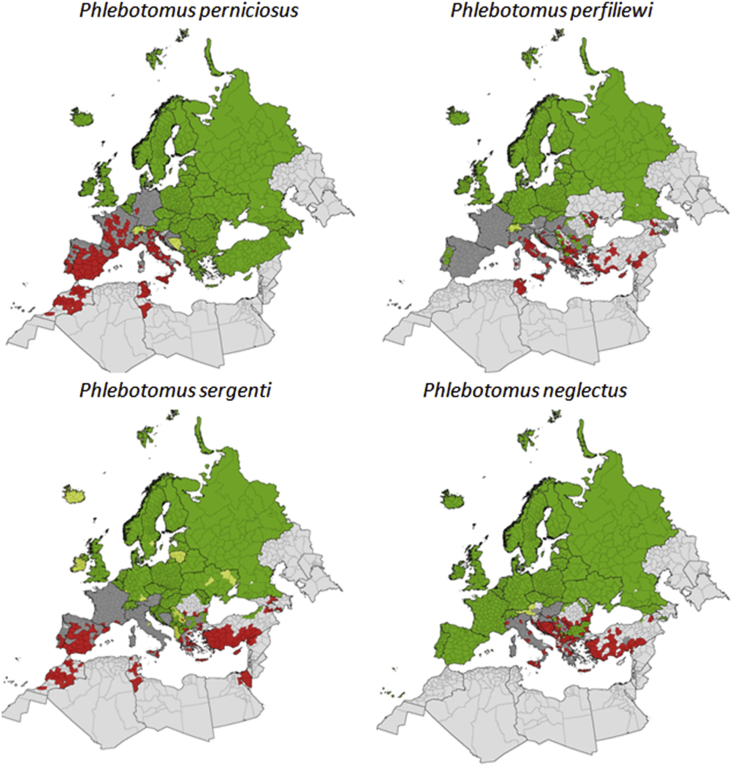
Studies conducted by our research group outside of France in collaboration with local researchers have demonstrated that TOSV is present in North Africa with undisputable evidence in Algeria [39], Tunisia [40] and Morocco [41]; and that TOSV can be transmitted by sand flies belonging to species distinct from P. perniciosus and P. perfiliewi, such as P. neglectus in the Balkans [42], P. sergenti in Morocco [41] and Sergentomyia minuta in France [43] (Fig. 2).
Fig. 2.
Current known distribution of sand fly species transmitting Toscana virus.
We demonstrated that seroprevalence studies must be based on techniques with high specificity to provide undisputable rates of TOSV antibodies and to avoid overestimation due to techniques prone to cross-reactions, such as ELISA or immunofluorescence. From these studies, it is clear that exposure of human populations to TOSV is much higher in northern Africa (Algeria 30%, northern Tunisia 42%) than in Southern Europe, where rates are consistently lower, at 10% to 15% in high exposure areas, and commonly <5%. [39], [40].
We also discovered several new viruses belonging to the Phlebovirus genus and transmitted by phlebotomines, such as Punique virus and Medjerda Valley virus in Tunisia [44], [45], Massilia virus in France [38], Adana, Toros and Zerdali viruses in Turkey [46], [47] and Dashli virus in Iran [48].
Together with veterinarians, we have demonstrated that the sand fly fever Sicilian virus circulates at a high rate in various regions of the Mediterranean Basin, such as Greece and Cyprus [49] and Portugal [50].
Molecular diagnostic tools for TOSV
Because one of the major options to improve the awareness about TOSV is to disseminate molecular diagnosis to clinical microbiology laboratories, we developed a real-time reverse transcriptase PCR assay [51]. At the beginning of the 21st century, TOSV was the most prominent cause of aseptic meningitis during summertime in central Italy—far ahead of enteroviruses and other causes such as WNV, herpes simplex viruses 1/2, varicella zoster virus and lymphocytic choriomeningitis virus. At this time there were no data to assess the medical impact of TOSV in other areas such as central south Europe (Croatia, Greece), Eastern Europe (Turkey) or North Africa (Morocco, Algeria, Tunisia). Evidence that TOSV is also circulating in those countries at rates that are at least equivalent (although frequently much higher) were provided after 2006 [39], [41], [42], [52], [53], [54], [55], [56], [57] (Fig. 1). Clinical data indicate that TOSV can cause meningitis, meningoencephalitis or encephalitis. Clinically, there is no marker to distinguish TOSV from other causes. Laboratory-based diagnosis using direct or indirect methods are therefore the only possibility to identify TOSV. Interestingly, encephalitis is frequently seen in central nervous system infections caused by TOSV, although the outcome is favourable [58], [59], [60], [61]. Detection of TOSV RNA using real-time molecular techniques is now the reference standard.
In the Mediterranean area, an estimated 250 million people are exposed to TOSV, with seroprevalence rates higher than 40% in certain regions (Fig. 1). In France, >8 million people are at risk of TOSV infection. TOSV is the most prevalent arthropod-borne virus in Europe. However, there are only 295 references using the keyword ‘Toscana virus’ in the National Center for Biotechnology Information PubMed database for a virus that was discovered almost 50 years ago. In comparison, for the same period of time (1971–2018), West Nile, Zika and chikungunya viruses have about 6500, 3360 and 3000 references, respectively. This is undisputable evidence that TOSV remains a neglected pathogen. Researchers in Marseille, working in research groups that have joined the IHU Méditerranée Infection, have produced 66 articles in the field; they now hold the leading position according to the Web of Science for studies related to viruses transmitted by sand flies in the Mediterranean Basin.
Conflict of interest
None declared.
References
- 1.Chomel B. Lyme disease. Rev Sci Tech. 2015;34:569–576. doi: 10.20506/rst.34.2.2380. [DOI] [PubMed] [Google Scholar]
- 2.Paixão E.S., Teixeira M.G., Rodrigues L.C. Zika, chikungunya and dengue: the causes and threats of new and re-emerging arboviral diseases. BMJ Glob Health. 2018;3(Suppl. 1) doi: 10.1136/bmjgh-2017-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver S.C., Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372:1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 4.Charrel R.N., Gallian P., Navarro-Mari J.M., Nicoletti L., Papa A., Sánchez-Seco M.P. Emergence of Toscana virus in Europe. Emerg Infect Dis. 2005;11:1657–1663. doi: 10.3201/eid1111.050869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David S., Abraham A.M. Epidemiological and clinical aspects on West Nile virus, a globally emerging pathogen. Infect Dis (Lond) 2016;48:571–586. doi: 10.3109/23744235.2016.1164890. [DOI] [PubMed] [Google Scholar]
- 6.Rizzoli A., Jimenez-Clavero M.A., Barzon L., Cordioli P., Figuerola J., Koraka P. The challenge of West Nile virus in Europe: knowledge gaps and research priorities. Euro Surveill. 2015;20:21135. doi: 10.2807/1560-7917.es2015.20.20.21135. [DOI] [PubMed] [Google Scholar]
- 7.Laroche M., Bérenger J.M., Delaunay P., Charrel R., Pradines B., Berger F. Medical entomology: a reemerging field of research to better understand vector-borne infectious diseases. Clin Infect Dis. 2017;65(Suppl. 1):S30–S38. doi: 10.1093/cid/cix463. [DOI] [PubMed] [Google Scholar]
- 8.Vega-Rúa A., Lourenço-de-Oliveira R., Mousson L., Vazeille M., Fuchs S., Yébakima A. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calba C., Guerbois-Galla M., Franke F., Jeannin C., Auzet-Caillaud M., Grard G. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Euro Surveill. 2017;22(39) doi: 10.2807/1560-7917.ES.2017.22.39.17-00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florescu S.A., Popescu C.P., Calistru P., Ceausu E., Nica M., Toderan A. Plasmodium vivax malaria in a Romanian traveller returning from Greece, August 2011. Euro Surveill. 2011;16:19954. [PubMed] [Google Scholar]
- 11.Parola P., Paddock C.D., Socolovschi C., Labruna M.B., Mediannikov O., Kernif T. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parola P., Socolovschi C., Jeanjean L., Bitam I., Fournier P.E., Sotto A. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl Trop Dis. 2008;2:e338. doi: 10.1371/journal.pntd.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouffok N., Parola P., Lepidi H., Raoult D. Mediterranean spotted fever in Algeria—new trends. Int J Infect Dis. 2009;13:227–235. doi: 10.1016/j.ijid.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Socolovschi C., Gaudart J., Bitam I., Huynh T.P., Raoult D., Parola P. Why are there so few Rickettsia conorii conorii–infected Rhipicephalus sanguineus ticks in the wild? PLoS Negl Trop Dis. 2012;6:e1697. doi: 10.1371/journal.pntd.0001697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kernif T., Socolovschi C., Bitam I., Raoult D., Parola P. Vector-borne rickettsioses in North Africa. Infect Dis Clin North Am. 2012;26:455–478. doi: 10.1016/j.idc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Delord M., Socolovschi C., Parola P. Rickettsioses and Q fever in travelers (2004–2013) Travel Med Infect Dis. 2014;12:443–458. doi: 10.1016/j.tmaid.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Yssouf A., Almeras L., Raoult D., Parola P. Emerging tools for identification of arthropod vectors. Future Microbiol. 2016;11:549–566. doi: 10.2217/fmb.16.5. [DOI] [PubMed] [Google Scholar]
- 18.Aubry C., Socolovschi C., Raoult D., Parola P. Bacterial agents in 248 ticks removed from people from 2002 to 2013. Ticks Tick Borne Dis. 2016;7:475–481. doi: 10.1016/j.ttbdis.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Benredjem W., Leulmi H., Bitam I., Raoult D., Parola P. Borrelia garinii and Rickettsia monacensis in Ixodes ricinus ticks, Algeria. Emerg Infect Dis. 2014;20:1776–1777. doi: 10.3201/eid2010.140265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelakis E., Mediannikov O., Parola P., Raoult D. Rickettsia felis: the complex journey of an emergent human pathogen. Trends Parasitol. 2016;32:554–564. doi: 10.1016/j.pt.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Dieme C., Bechah Y., Socolovschi C., Audoly G., Berenger J.M., Faye O. Transmission potential of Rickettsia felis infection by Anopheles gambiae mosquitoes. Proc Natl Acad Sci USA. 2015;112:8088–8093. doi: 10.1073/pnas.1413835112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mana N., Louni M., Parola P., Bitam I. Human head lice and pubic lice reveal the presence of several Acinetobacter species in Algiers, Algeria. Comp Immunol Microbiol Infect Dis. 2017;53:33–39. doi: 10.1016/j.cimid.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Benkouiten S., Drali R., Badiaga S., Veracx A., Giorgi R., Raoult D. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273–279. doi: 10.1001/jamadermatol.2013.6398. [DOI] [PubMed] [Google Scholar]
- 24.Leulmi H., Bitam I., Berenger J.M., Lepidi H., Rolain J.M., Almeras L. Competence of Cimex lectularius bed bugs for the transmission of Bartonella quintana, the agent of trench fever. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jourdain F., Delaunay P., Bérenger J.M., Perrin Y., Robert V. The common bed bug (Cimex lectularius) in metropolitan France. Survey on the attitudes and practices of private- and public-sector professionals. Parasite. 2016;23:38. doi: 10.1051/parasite/2016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akhoundi M., Kengne P., Cannet A., Brengues C., Berenger J.M., Izri A. Spatial genetic structure and restricted gene flow in bed bugs (Cimex lectularius) populations in France. Infect Genet Evol. 2015;34:236–243. doi: 10.1016/j.meegid.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Akhoundi M., Cannet A., Loubatier C., Berenger J.M., Izri A., Marty P. Molecular characterization of Wolbachia infection in bed bugs (Cimex lectularius) collected from several localities in France. Parasite. 2016;23:31. doi: 10.1051/parasite/2016031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrnst A., Peters C.J., Niklasson B., Svedmyr A., Holmgren B. Neurovirulent Toscana virus (a sandfly fever virus) in Swedish man after visit to Portugal. Lancet. 1985;1(8439):1212–1213. doi: 10.1016/s0140-6736(85)92886-7. [DOI] [PubMed] [Google Scholar]
- 29.Calisher C.H., Weinberg A.N., Muth D.J., Lazuick J.S. Toscana virus infection in United States citizen returning from Italy. Lancet. 1987;1(8525):165–166. doi: 10.1016/s0140-6736(87)92005-8. [DOI] [PubMed] [Google Scholar]
- 30.Braito A., Ciufolini M.G., Pippi L., Corbisiero R., Fiorentini C., Gistri A. Phlebotomus-transmitted Toscana virus infections of the central nervous system: a seven-year experience in Tuscany. Scand J Infect Dis. 1998;30:505–508. doi: 10.1080/00365549850161539. [DOI] [PubMed] [Google Scholar]
- 31.Braito A., Corbisiero R., Corradini S., Fiorentini C., Ciufolini M.G. Toscana virus infections of the central nervous system in children: a report of 14 cases. J Pediatr. 1998;132:144–148. doi: 10.1016/s0022-3476(98)70500-1. [DOI] [PubMed] [Google Scholar]
- 32.Braito A., Corbisiero R., Corradini S., Marchi B., Sancasciani N., Fiorentini C. Evidence of Toscana virus infections without central nervous system involvement: a serological study. Eur J Epidemiol. 1997;13:761–764. doi: 10.1023/a:1007422103992. [DOI] [PubMed] [Google Scholar]
- 33.Nicoletti L., Verani P., Caciolli S., Ciufolini M.G., Renzi A., Bartolozzi D. Central nervous system involvement during infection by phlebovirus Toscana of residents in natural foci in central Italy (1977–1988) Am J Trop Med Hyg. 1991;45:429–434. doi: 10.4269/ajtmh.1991.45.429. [DOI] [PubMed] [Google Scholar]
- 34.Valassina M., Meacci F., Valensin P.E., Cusi M.G. Detection of neurotropic viruses circulating in Tuscany: the incisive role of Toscana virus. J Med Virol. 2000;60:86–90. doi: 10.1002/(sici)1096-9071(200001)60:1<86::aid-jmv14>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Valassina M., Cuppone A.M., Bianchi S., Santini L., Cusi M.G. Evidence of Toscana virus variants circulating in Tuscany, Italy, during the summers of 1995 to 1997. J Clin Microbiol. 1998;36:2103–2104. doi: 10.1128/jcm.36.7.2103-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemmersbach-Miller M., Parola P., Charrel R.N., Paul Durand J., Brouqui P. Sandfly fever due to Toscana virus: an emerging infection in southern France. Eur J Intern Med. 2004;15:316–317. doi: 10.1016/j.ejim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Charrel R.N., Izri A., Temmam S., Delaunay P., Toga I., Dumon H. Cocirculation of 2 genotypes of Toscana virus, southeastern France. Emerg Infect Dis. 2007;13:465–468. doi: 10.3201/eid1303.061086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charrel R.N., Moureau G., Temmam S., Izri A., Marty P., Parola P. Massilia virus, a novel Phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519–530. doi: 10.1089/vbz.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alkan C., Allal-Ikhlef A.B., Alwassouf S., Baklouti A., Piorkowski G., de Lamballerie X. Virus isolation, genetic characterization and seroprevalence of Toscana virus in Algeria. Clin Microbiol Infect. 2015;21:1040. doi: 10.1016/j.cmi.2015.07.012. e1–9. [DOI] [PubMed] [Google Scholar]
- 40.Sakhria S., Bichaud L., Mensi M., Salez N., Dachraoui K., Thirion L. Co-circulation of Toscana virus and Punique virus in northern Tunisia: a microneutralisation-based seroprevalence study. PLoS Negl Trop Dis. 2013;7:e2429. doi: 10.1371/journal.pntd.0002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Es-Sette N., Ajaoud M., Bichaud L., Hamdi S., Mellouki F., Charrel R.N. Phlebotomus sergenti, a common vector of Leishmania tropica and Toscana virus in Morocco. J Vector Borne Dis. 2014;51:86–90. [PubMed] [Google Scholar]
- 42.Ayhan N., Alten B., Ivovic V., Martinkovic F., Kasap O.E., Ozbel Y. Cocirculation of two lineages of Toscana virus in Croatia. Front Public Health. 2017;5:336. doi: 10.3389/fpubh.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charrel R.N., Izri A., Temmam S., de Lamballerie X., Parola P. Toscana virus RNA in Sergentomyia minuta flies. Emerg Infect Dis. 2006;12:1299–1300. doi: 10.3201/eid1208.060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhioua E., Moureau G., Chelbi I., Ninove L., Bichaud L., Derbali M. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91(Pt 5):1275–1283. doi: 10.1099/vir.0.019240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bichaud L., Dachraoui K., Alwassouf S., Alkan C., Mensi M., Piorkowski G. Isolation, full genomic characterization and neutralization-based human seroprevalence of Medjerda Valley virus, a novel sandfly-borne phlebovirus belonging to the Salehabad virus complex in northern Tunisia. J Gen Virol. 2016;97:602–610. doi: 10.1099/jgv.0.000389. [DOI] [PubMed] [Google Scholar]
- 46.Alkan C., Erisoz Kasap O., Alten B., de Lamballerie X., Charrel R.N. Sandfly-borne phlebovirus isolations from Turkey: new insight into the sandfly fever Sicilian and sandfly fever Naples species. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alkan C., Alwassouf S., Piorkowski G., Bichaud L., Tezcan S., Dincer E. Isolation, genetic characterization, and seroprevalence of Adana virus, a novel phlebovirus belonging to the Salehabad virus complex, in Turkey. J Virol. 2015;89:4080–4091. doi: 10.1128/JVI.03027-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alkan C., Moin Vaziri V., Ayhan N., Badakhshan M., Bichaud L., Rahbarian N. Isolation and sequencing of Dashli virus, a novel Sicilian-like virus in sandflies from Iran; genetic and phylogenetic evidence for the creation of one novel species within the Phlebovirus genus in the Phenuiviridae family. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alwassouf S., Christodoulou V., Bichaud L., Ntais P., Mazeris A., Antoniou M. Seroprevalence of sandfly-borne phleboviruses belonging to three serocomplexes (sandfly fever Naples, sandfly fever Sicilian and Salehabad) in dogs from Greece and Cyprus using neutralization test. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alwassouf S., Maia C., Ayhan N., Coimbra M., Cristovao J.M., Richet H. Neutralization-based seroprevalence of Toscana virus and sandfly fever Sicilian virus in dogs and cats from Portugal. J Gen Virol. 2016;97:2816–2823. doi: 10.1099/jgv.0.000592. [DOI] [PubMed] [Google Scholar]
- 51.Brisbarre N., Plumet S., Cotteaux-Lautard C., Emonet S.F., Pages F., Leparc-Goffart I. A rapid and specific real time RT-PCR assay for diagnosis of Toscana virus infection. J Clin Virol. 2015;66:107–111. doi: 10.1016/j.jcv.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Ergünay K., Saygan M.B., Aydoğan S., Lo M.M., Weidmann M., Dilcher M. Sandfly fever virus activity in central/northern Anatolia, Turkey: first report of Toscana virus infections. Clin Microbiol Infect. 2011;17:575–581. doi: 10.1111/j.1469-0691.2010.03346.x. [DOI] [PubMed] [Google Scholar]
- 53.Bahri O., Fazaa O., Ben Alaya-Bouafif N., Bouloy M., Triki H., Bouattour A. Role of Toscana virus in meningo-encephalitis in Tunisia. Pathol Biol (Paris) 2011;59:e125–e127. doi: 10.1016/j.patbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Santos L., Simões J., Costa R., Martins S., Lecour H. Toscana virus meningitis in Portugal, 2002–2005. Euro Surveill. 2007;12:E3–E4. doi: 10.2807/esm.12.06.00715-en. [DOI] [PubMed] [Google Scholar]
- 55.Punda-Polić V., Mohar B., Duh D., Bradarić N., Korva M., Fajs L. Evidence of an autochthonous Toscana virus strain in Croatia. J Clin Virol. 2012;55:4–7. doi: 10.1016/j.jcv.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Es-Sette N., Nourlil J., Hamdi S., Mellouki F., Lemrani M. First detection of Toscana virus RNA from sand flies in the genus Phlebotomus (Diptera: Phlebotomidae) naturally infected in Morocco. J Med Entomol. 2012;49:1507–1509. doi: 10.1603/me12042. [DOI] [PubMed] [Google Scholar]
- 57.Papa A., Paraforou T., Papakonstantinou I., Pagdatoglou K., Kontana A., Koukoubani T. Severe encephalitis caused by Toscana virus, Greece. Emerg Infect Dis. 2014;20:1417–1419. doi: 10.3201/eid2008.140248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dupouey J., Bichaud L., Ninove L., Zandotti C., Thirion-Perrier L., de Lamballerie X. Toscana virus infections: a case series from France. J Infect. 2014;68:290–295. doi: 10.1016/j.jinf.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Jaijakul S., Arias C.A., Hossain M., Arduino R.C., Wootton S.H., Hasbun R. Toscana meningoencephalitis: a comparison to other viral central nervous system infections. J Clin Virol. 2012;55:204–208. doi: 10.1016/j.jcv.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pierro A., Ficarelli S., Ayhan N., Morini S., Raumer L., Bartoletti M. Characterization of antibody response in neuroinvasive infection caused by Toscana virus. Clin Microbiol Infect. 2017;23:868–873. doi: 10.1016/j.cmi.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 61.Vocale C., Bartoletti M., Rossini G., Macini P., Pascucci M.G., Mori F., Tampieri A. Toscana virus infections in northern Italy: laboratory and clinical evaluation. Vector Borne Zoonotic Dis. 2012;12:526–529. doi: 10.1089/vbz.2011.0781. [DOI] [PubMed] [Google Scholar]