Abstract
Recent interest in reversal of the hypnotic effects of anesthesia has mainly focused on overcoming a surge in GABA-mediated inhibitory signaling through activation of subcortical arousal circuits or antagonizing GABA receptors. Here we examine the reversal of anesthesia produced from non-GABA agents ketamine/xylazine and the effects of antagonists of adrenoreceptors. These antagonists vary in selectivity and produce temporally unique waking behavior post-anesthesia. We compared two antagonists with differential selectivity for α1- vs. α2-receptors, yohimbine (YOH, 1:40 selectivity) and atipamezole (ATI, 1:8500). Adult mice received intraperitoneal injections of either YOH (4.3 mg/kg), ATI (0.4 mg/kg), or saline after achieving sustained loss of righting following injection of ketamine/xylazine (ketamine: 65.0 mg/kg; xylazine: 9.9 mg/kg). Behaviors indicative of the post-anesthesia, re-animation sequence were carefully monitored and the timing of each behavior relative to anesthesia induction was compared. Both YOH and ATI hastened behaviors indicative of emergence, but ATI was faster than YOH to produce certain behaviors, including whisker movement (YOH: 21.9±1.5 min, ATI: 17.5±0.5 min, p = 0.004) and return of righting reflex (RORR) (YOH: 40.6±8.8 min, ATI: 26.0±1.2 min, p<0.001). Interestingly, although YOH administration hastened early behavioral markers of emergence relative to saline (whisking), the completion of the emergence sequence (time from first marker to appearance of RORR) was delayed with YOH. We attribute this effect to antagonism of α1 receptors by yohimbine. Also notable was the failure of either antagonist to hasten the re-establishment of coordinated motor behavior (e.g., attempts to remove adhesive tape on the forepaw placed during anesthesia) relative to the end of emergence (RORR). In total, our work suggests that in addition to pharmacokinetic effects, re-establishment of normal waking behaviors after anesthesia involves neuronal circuits dependent on time and/or activity.
Introduction
Reconstruction of consciousness has been studied in the context of anesthesia [1–3] and has been likened to waking from sleep [4]. Despite the active pharmacological reversal of some aspects of anesthesia such as neuromuscular blockade and opioid induced respiratory depression, the recovery of consciousness following clinical anesthesia has traditionally been considered a passive process. Recently, stimulation of arousal pathways [5] and antagonism of inhibitory signaling [6] have been investigated as potential strategies for hastening the arrival of consciousness after isoflurane anesthesia. This work has been extended to reversal of other GABA-ergic anesthetic agents such as propofol [7]. In contrast, the reversal of non-GABA agents (i.e., ketamine, xylazine, dexmedetomidine) has received much less attention, perhaps because their use as sole agents for maintenance of anesthesia is less common in human clinical practice [8–10].
Despite their lack of study, non-GABA agents remain in common use. Ketamine (K) is one of the most popular non-GABA veterinary anesthetic agents. It has been in use for over 50 years, yet there is still much to learn about its pharmacodynamic effects. Although glutamate receptors are known targets of its neurophysiologic effects (NMDA antagonism, AMPA agonism) other receptors involved in neuronal excitability have demonstrated bioactivity.
Ketamine is most often administered in combination with other anesthetics, such as xylazine, which is thought to counteract some of ketamine’s sympathomimetic effects. The intraperitoneal or intramuscular injection of xylazine in combination with ketamine is a common anesthesia technique used for procedures performed in the laboratory on mice, rats, and other animals [11–13]. Xylazine (X) specifically agonizes the α2-adrenoceptor [13]. Its action at the α2-receptor in the brain stem produces sedation through increased noradrenergic release throughout the cortex [14].
While ketamine/xylazine (K/X) is an effective combination for veterinary anesthesia, it can produce side effects such as acute hyperglycemia [15] and corneal lesions [16] if administered without a reversal agent. Currently, no pharmacologic reversal of ketamine anesthesia exists. However, in veterinary practice, the sedative actions of α2-agonists can be pharmacologically reversed with α2-antagonists, such as yohimbine and atipamezole [13]. Yohimbine (YOH) is an indole alkaloid derived from the bark of the Pausinystaliayohimbe tree. It has highest affinity for the α2-receptor, but also antagonizes the α1-receptor, as well as some serotonin and dopamine receptors. It is considered a selective α2-antagonist, with a 40:1 α2:α1 selectivity ratio. Atipamezole (ATI) is also an α2-antagonist, with a higher α2:α1 selectivity ratio 8500:1 [17]. ATI is also more potent than YOH. Ten times the amount of YOH is needed to block central α2-receptors to the same level that ATI does [18, 19].
The combination of an anesthetic cocktail and a reversal agent (of varying selectivity) can have complex influences on behavior while the animal emerges from anesthesia. A more efficient hastening of emergence with ATI compared to YOH has been suggested in the context of ketamine/xylazine anesthesia [20], but an examination of the behaviors during emergence and recovery from anesthesia with these reversal agents has yet to be studied.
Studying anesthesia produced by ketamine and α2-agonists has clinical relevance. Ketamine has recently experienced a renaissance in clinical usage [9] as some benefits have been demonstrated in the treatment of depression [21], attenuation of postoperative delirium [22], and reduction of opioid administration for analgesia [23]. Although xylazine is not approved for use in humans, other selective α2-agonists, like dexmedetomidine and clonidine, are used for blood pressure control, sedation, and as adjuncts to general anesthesia. Dexmedetomidine in combination with ketamine has been used as a preferred technique intending to minimize post-anesthesia confusion in humans [24, 25].
Here we investigated an animal model of recovery from anesthesia in the absence of GABA-ergic anesthetic drugs. We measured the appearance of behavioral markers of emergence and describe their canonical sequence of arrival following K/X anesthesia in the presence and absence of reversal by α2-antagonists.
Methods
Animals
Both male (n = 24, and female (n = 6) adult (approximately 20–30 grams) C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were used. Animals were experimentally naïve and were used for only one trial each. Mice were housed under a 12:12 light:dark cycle and given standard mouse chow ad libitum. All procedures were approved by the Atlanta Veterans Administration Institutional Animal Care and Use Committee.
Emergence procedure, anesthesia challenge, and early behavioral markers
Fig 1 depicts the experimental protocol. Animals were first weighed, and then placed in an open top, clear observation box for acclimation. Before induction of anesthesia, each mouse received training on a “sticky dot” test (see below). Following the training trials, mice were given an intraperitoneal injection of K/X cocktail (65 mg/kg of ketamine [Ketaved, Vedco Inc., St. Joseph, MO], 9.9 mg/kg xylazine [Anased, Akorn, Decatur, IL]), and the timer was started. Animals were considered “anesthetized” when they failed to right themselves (by placing all four paws on the surface of the chamber) after being gently placed on their back. This time was noted as the appearance of loss of righting reflex (LORR). To investigate post-anesthesia behaviors in the absence of pharmacological manipulation of alpha receptors, six mice were administered intraperitoneal ketamine (65 mg/kg) and then immediately placed into 4% isoflurane for 60 seconds which resulted in until LORR (K/I regimen). Immediately after LORR, each mouse was placed on a heating pad beneath a heat lamp adjusted to maintain body temperature between 39.4–40.0 degrees Celsius. While anesthetized, adhesive tape (“sticky dot”) was applied to the right forepaw. At exactly 15 minutes after the injection of anesthesia/sedation each mouse was given either YOH [Yobine, Akorn Inc, Decatur, IL; 4.3 mg/kg], ATI [Antisedan, Orion Corporation, Espoo, Finland; 0.4 mg/kg], ATI and prazosin (ATI+PRA) [Prazosin HCl, Sigma-Aldrich, St. Louis, MO; 2.0 mg/kg], or saline (SAL) [Hospira Inc., Lake Forest, IL; 0.1mL 0.9% solution. Consequently, the groups are depicted SAL, YOH, ATI, ATI+PRA for all groups with ketamine/xylazine and K/I-SAL for the ketamine/isoflurane group in the reminder of the manuscript. The time of the following behavioral markers was taken at their first occurrence: whisker movement (any movement of whiskers), forelimb movement (any movement of either forelimb), and respiration change (either a change in rate or change in breathing depth, judged by the size of the chest excursion with each breath). Next, the time for each mouse to regain its righting reflex (RORR) was recorded. As rodents do not sleep on their backs, it is common to use RORR as an arbitrary marker for cessation of the anesthetized state (end-emergence), and so it is used here to delineate the emergence and recovery periods. Upon righting, an attempt to return the mouse to its back was performed in each mouse to ensure the righting reflex was robust. Following the RORR, each mouse was placed into a clear-walled open top box to enable observation of the recovery period behaviors.
Fig 1. Waking behavior observation protocol.
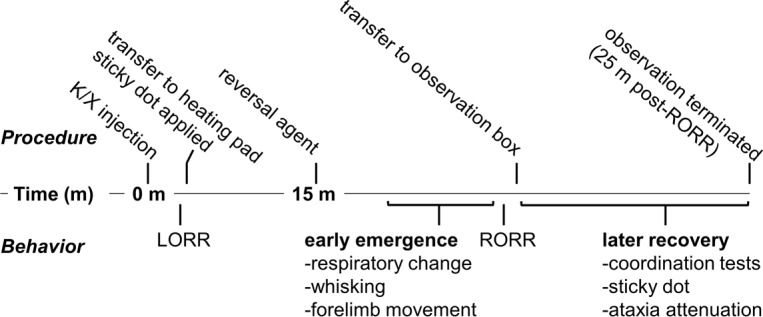
Timeline describing observation protocol. Procedures listed on top, with behaviors listed below. K/X: ketamine/xylazine, LORR: loss of righting reflex, RORR: return of righting reflex.
Late behavioral markers and early recovery from anesthesia
The sticky dot test is a complex measure of perception and motor coordination previously used to evaluate animals after ischemic stroke [26]. Briefly, the animals receive a 2.5 x 0.5 cm adhesive tape folded over their forepaw; then the time to investigation of the tape (paw shaking or any purposeful movement towards the tape involving the nose, mouth, or alternate forepaw) is recorded. Prior to the anesthesia challenge, all mice received three trials of the sticky dot test. For our experiments we defined recovery period as the time between RORR and the appearance of the final marker of our observed behavioral sequence (sticky dot notice). If the animal did not attempt to remove the tape within 25 minutes after RORR, the trial ended. Ataxia was assessed at five-minute intervals after RORR by testing for splaying of the legs. This was accomplished by lifting the mouse by the tail, suspending both hind limbs and observing the hind limb reflexes after subsequent dropping of the hindquarters. Other ataxic features were recorded if present: ambulation with only the forelimbs, or otherwise uncoordinated movement. Coordinated movement was defined as diagonal cross-matched ambulation, in which the right forelimb movement was followed by movement of the left hind limb. Latency to return of diagonal cross-matched ambulation was recorded. After 25 minutes post-RORR, observation was terminated, and the mouse was sacrificed by cervical dislocation.
Statistics
We applied non-parametric testing for evaluating our data set, because of our modest sample size. We used the Kruskal-Wallis test to evaluate possible differences between all groups and the Mann-Whitney U test as post-hoc test. We did not correct for multiple comparisons in order to prevent increase of false negatives [27]. But therefore, additionally to the hypothesis-based tests, we calculated the area under the receiver operating curve (AUC) together with 10000-fold bootstrapped 95% confidence intervals as effect size. As a rough estimate, according to the traditional point system, an effect can be classified as: excellent (very strong) AUC = 0.9–1; good AUC = 0.8–0.9; fair AUC = 0.7–0.8; poor AUC = 0.6–0.7; or fail: AUC = 0.5–0.6. We used MATLAB R2017 (The Mathworks, Natick, MA) for our statistical tests and the MATLAB-based MES toolbox [28] to calculate AUC and 95% CI. We present our data as raw data together with the mean and the median.
Results
Baseline testing and induction of anesthesia
During baseline experiments of the sticky dot test all mice noticed and removed the adhesive tape in less than 2 minutes by the third trial. No animal noticed or removed the tape before RORR.
All 24 mice that received the K/X dose described in the methods experienced LORR in less than 5 minutes.
Emergence from ketamine/xylazine anesthesia is hastened with the administration of α2-antagonists
Among the early behaviors observed before RORR, change in respiration rate, whisker movement, and forelimb movement were recorded. Fig 2 contains the detailed information. ATI and YOH produced signs of waking earlier than SAL in all three behaviors.
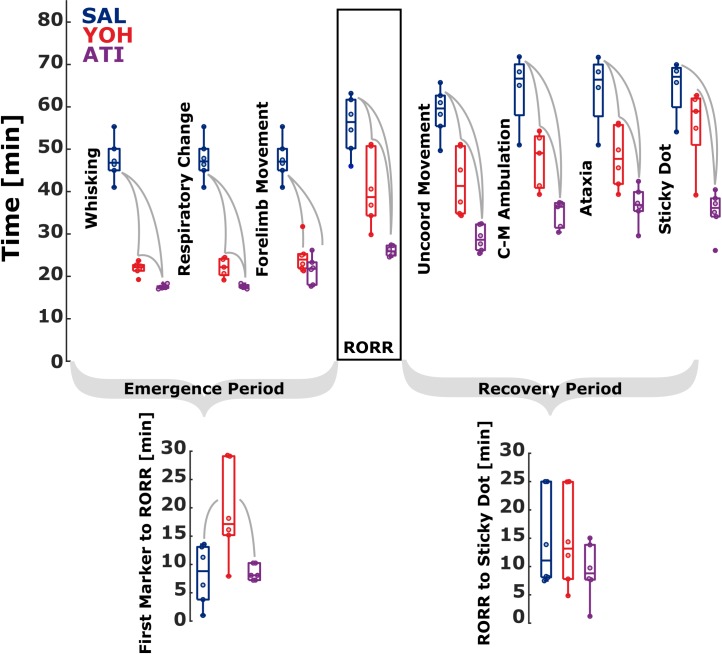
Fig 2. Reversal agents exert specific influence on the time to achieve different behavioral markers characteristic of emergence and recovery from ketamine / xylazine anesthesia in mice.
The timing of waking behaviors and the duration of the emergence and recovery processes vary after animals were administered saline (SAL), yohimbine (YOH), or atipamezole (ATI) 15 minutes after anesthesia induction. Early emergence: Both YOH and ATI reduce the time required to exhibit the first behavioral signs of emergence from ketamine/xylazine anesthesia. Time for first incidence of individual waking behaviors is plotted, including whisker movement, respiratory change, and forelimb movement. Return of righting reflex (RORR) and Emergence Period: Both α2 antagonist treated groups ATI and YOH recover righting reflex faster, but YOH has increased delay between early markers (first marker to RORR) and RORR. Later Recovery and Recovery Period: Appearance of motoric behaviors during recovery show ATI elicits these behaviors faster than both YOH and SAL. There was no significant difference in the duration from RORR to Sticky Dot notice between the groups. Time measurements are from ketamine/xylazine injection. The gray lines between boxes indicate a statistically significant difference between the groups.
The time to whisker movement was different among the groups (p = 0.0005, χ2 = 15.16). Compared to SAL (n = 6) treated animals, YOH (n = 6, p = 0.0022), and ATI (n = 6 p = 0.0022), showed faster recovery to whisker movement. AUC indicated perfect separation (very strong effect) between the groups, i.e., AUC = 1. We observed the same result when comparing the ATI with the YOH group (p = 0.0022; AUC = 1, very strong effect). Time required for anesthetized mice to exhibit a change in respiration was also different among groups (p = 0.0013, χ2 = 13.35) depending on reversal agent. We derived very similar statistical results for time to respiration as for time to whisker movement. The SAL (n = 6) group took significantly longer to express respiration signs when compared to YOH (n = 5, p = 0.0043) and when compared to ATI (n = 5, p = 0.0043). When compared to YOH, ATI animals reached this behavioral milestone significantly earlier as well (p = 0.0079). AUC also showed perfect separation (AUC = 1) between all groups. We observed very similar results for the time to forelimb movement as well. Overall, ATI and YOH have similar profiles in the sequence of early markers of emergence. The first section of Table 1 depicts the median latency to these early markers of emergence and Fig 2 graphically represents the time required to achieve these behavioral events for each animal.
Table 1. Durations from ketamine/xylazine injection until the behavioral marker as well as between defined markers.
| Time in minutes (median and 1st and 4th quartile) | |||||
|---|---|---|---|---|---|
| SAL | YOH | ATI | χ2 | p* | |
| Time to Marker | |||||
| Emergence Period | |||||
| Whisker Movement | 46.8 [41.0–55.3] | 22.2 [19.3–23.7] | 17.4 [17.0–18.3] | 15.16 | p<0.001 |
| Respiration | 47.1 [41.0–55.3] | 22.2 [19.2–24.5] | 17.5 [17.0–18.3] | 13.35 | p = 0.001 |
| Forelimb Movement | 47.1 [41.0–55.3] | 24.0 [21.3–31.8] | 21.8 [17.7–26.2] | 11.94 | p = 0.003 |
| RORR | 56.4 [46.0–63.2] | 38.7 [29.8–51.2] | 25.9 [24.6–27.4] | 13.66 | p = 0.001 |
| Recovery Period | |||||
| Uncoordinated Movement | 59.6 [49.7–65.7] | 41.3 [34.3–51.2] | 28.6 [25.4–32.4] | 14.36 | p = 0.001 |
| Cross-Matched Ambulation | 66.7 [51.0–71.8] | 49.1 [39.3–54.3] | 36.4 [30.4–37.4] | 10.65 | p = 0.001 |
| Ataxia | 66.4 [51.0–71.7] | 47.7 [39.3–56.2] | 36.8 [29.6–42.4] | 10.88 | p = 0.004 |
| Sticky Dot Notice | 67.1 [54.1–70.0] | 59.0 [39.2–62.7] | 36.1 [26.1–40.4] | 10.32 | p = 0.006 |
| Sticky Dot Notice maxed* | 69.2 [54.1–88.2] | 57.0 [39.2–62.7] | 36.1 [26.1–40.4] | 12.88 | p = 0.002 |
| Time between Markers | |||||
| 1st behavior and RORR | 8.8 [1.0–13.6] | 17.1 [7.9–29.3] | 8.1 [7.2–10.3] | 7.4 | p = 0.025 |
| RORR to C-M Ambulation | 10.1 [5.0–10.5] | 4.6 [1.5–8.5] | 10.0 [5.0–10.0] | 6.76 | p = 0.034 |
| RORR to Ataxia | 10.0 [5.0–10.0] | 5.0 [5.0–20.0] | 10.0 [5.0–15.0] | 3.49 | p = 0.175 |
| RORR to Sticky Dot | 11.1 [7.5–25] | 13.2 [4.9–25.0] | 8.8 [1.2–15.0] | 1.28 | p = 0.527 |
SAL: saline; YOH: yohimbine; ATI: atipamezole; RORR: return of righting reflex; C-M: cross-matched
* p-values (χ2-values) were calculated using the Kruskal-Wallis test.
Yohimbine increases the time to completion of the emergence sequence
The timing of RORR was significantly different among treatments (p = 0.0011; χ2 = 13.66) as shown in Fig 2. The SAL group (n = 6) took longer to right themselves after ketamine/xylazine anesthesia compared to YOH (n = 6) with p = 0.0260 and AUC = 0.89 [0.67 1] (strong effect) and ATI (n = 6) with p = 0.0022 and AUC = 1 (very strong effect). ATI animals also exhibited RORR faster than YOH animals (p = 0.0022 and AUC = 1; very strong effect). Interestingly, the time delay between the first exhibited behavior and RORR showed significant difference among groups (p = 0.0248; χ2 = 7.40), but with a different pattern. YOH showed a significantly longer delay in completion of the emergence sequence, compared to SAL with p = 0.0152 and AUC = 0.92 [0.67 1] and ATI with p = 0.0260 and AUC = 0.89 [0.58 1]. There was no significant difference between SAL and ATI (p = 1; AUC = 0.5 [0.17 0.83]). This suggests that although YOH hastened the start of emergence behaviors, completion of the entire sequence of emergence behaviors was lengthened by administration of YOH (Fig 2). Table 1 presents the latency to RORR specific for each reversal agent.
Atipamezole and yohimbine hasten recovery from ketamine/xylazine anesthesia
The recovery of locomotor activity in an uncoordinated fashion (uncoordinated movement) was significantly different among treatment groups (p = 0.0008, χ2 = 14.36). YOH mice exhibited uncoordinated movement faster (n = 6), than SAL mice (n = 6), with p = 0.0087 and AUC = 0.94 [0.78 1] (very strong effect), while ATI mice showed uncoordinated movement earlier (n = 6), than both SAL (p = 0.0022; AUC = 1 (very strong effect)) and YOH (p = 0.0022; AUC = 1 (very strong effect); Fig 2). Latency to the first notice of the sticky dot was different between groups (p = 0.0057, χ2 = 10.32). ATI mice were faster (n = 6), to identify the sticky dot compared to both SAL (n = 4), and YOH (n = 6); (respectively: p = 0.0095; p = 0.0087 and AUC = 1; AUC = 0.97 [0.8 1], very strong effects). YOH mice were statistically indistinguishable from SAL mice in noticing the sticky dot (p = 0.19, AUC = 0.8 [0.4 1]; strong, but not significant effect); Fig 2). Two SAL-treated mice failed to show diagonally cross-matched ambulation, notice the sticky dot, or show ataxia attenuation within 25 minutes after RORR and one YOH-treated mouse did not notice the sticky dot within 25 minutes after RORR. These three animals were removed from the analysis in Fig 2 (see also S1 Fig). But for complete presentation of the results without removed animals, we set the times of appearance of behavioral markers these animals to the maximum RORR+25 min. The results were similar to the reduced data set. The time to sticky dot notice was significantly different among the groups (p = 0.0016, χ2 = 12.88). See Table 1 for median times for each group and behavior. The pairwise comparison led to p = 0.0411, AUC = 0.86 [0.58 1] for SAL vs. YOH indicating a strong effect, to p = 0.0022, AUC = 1 for SAL vs. ATI and to p = 0.0043, AUC = 0.97 [0.83 1] for YOH vs. ATI. The recovery of more coordinated (diagonally cross-matched ambulation) locomotor efforts was different between groups (p = 0.0005, χ2 = 10.65). YOH mice (n = 5) showed a trend towards faster diagonally cross-matched ambulation than SAL controls (n = 4) with p = 0.0635 and AUC = 0.9 [0.6 1] (very strong effect), while ATI mice (n = 6) were faster than SAL (p = 0.0159, AUC = 1, very strong effect) and YOH mice (p = 0.0080, AUC = 1; very strong effect; Fig 2). The time delay to ataxia attenuation was similarly dependent on treatment (p = 0.0043, χ2 = 10.88). YOH mice exhibited ataxia attenuation earlier (n = 6) than SAL mice (n = 4) with p = 0.0381 and AUC = 0.92 [0.67 1] (very strong effect), while ATI mice (n = 6) were faster to show ataxia attenuation compared to both SAL p = 0.0095 and AUC = 1 (very strong effect) and YOH (p = 0.0152, AUC = 0.92 [0.70 1]; very strong effect; Fig 2). Table 1 contains the detailed times to the respective marker.
Emergence and recovery behaviors are influenced by activity at α1-receptors
Three animals (2, SAL and 1, YOH) did not regard the sticky dot before the experiment timed out (25 minutes after RORR) so a maximum of 25 minutes was assigned as their recovery period. We did not find a significant difference in the Recovery Period, i.e., from RORR to sticky dot notice between the groups (p = 0.5273, χ2 = 1.28). Fig 2 graphs the latencies. The pairwise post hoc comparisons did not reveal any significant changes between the groups, neither did the AUC analysis reveal any trends. As an additional piece of information, the latencies between RORR to uncoordinated movement, diagonally cross matched ambulation and ataxia attenuation can be found in S1 Fig and Table 1. The observed difference between ATI and YOH during the Emergence Period (Fig 2) and the observation that no animal timed out during recovery for ATI, but one did for YOH (Fig 2) prompted us to further examine the role of α1-receptors in these post-anesthesia behaviors.
Prazosin, a selective α1 inverse agonist, does not hasten emergence from ketamine/xylazine anesthesia in the presence of atipamezole
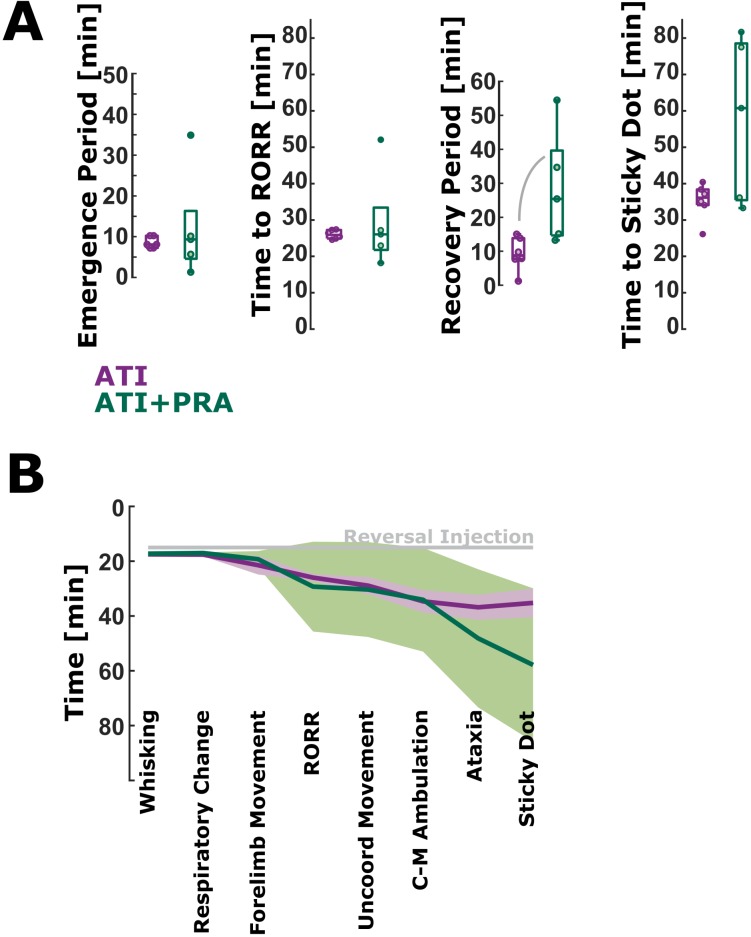
Co-administration of prazosin (2.0 mg/kg) with atipamezole does not hasten RORR but prolongs sticky dot removal in the recovery from ketamine/xylazine anesthesia. Time to RORR for ATI+PRA was not significantly different from the ATI group (p = 0.7922; AUC = 0.57 [0.20 0.93]; Fig 3A). The time to sticky dot notice for ATI+PRA was also not significantly different from the ATI group (p = 0.2468; AUC = 0.73 [0.33 1] (fair effect); Fig 3A). However, the AUC>0.7 may indicate a trend towards a longer time to sticky dot notice with ATI+PRA. The time from the first marker of emergence to RORR was not significantly different between the ATI+PRA and the ATI group (AUC = 0.53 [0.13 0.90]; Fig 3A). The recovery period, i.e., the time from RORR to sticky dot notice, was significantly slower (p = 0.0173; AUC = 0.93 [0.73 1]; very strong effect) compared to the ATI group (Fig 3A). Table 2 contains the detailed times. Fig 3B presents the course of the emergence and recovery for ATI and ATI+PRA.
Fig 3. Latency to return of righting reflex (RORR) and sticky dot notice and the emergence and recovery period duration for the atipamezole (ATI) and ATI+PRA (prazosin) group.
A) There were no significant differences between the ATI and ATI+PRA group in the latency to RORR and sticky dot notice. There was no difference in the duration of the emergence period as well. The recovery period was significantly shorter for ATI than for ATI+PRA. The solid, gray lines indicate significant differences between groups. B) Time course of the durations to the different emergence and recovery markers. The solid lines present the mean and the 95% confidence interval on the mean. The gray line indicates time of reversal injection, 15 minutes post-ketamine/xylazine injection.
Table 2. Durations from ketamine/xylazine injection until the behavioral marker as well as between defined markers.
| Time in minutes (median and 1st and 4th quartile) | ||||
|---|---|---|---|---|
| ATI | ATI+PRA | AUC | p* | |
| Time to Marker | ||||
| RORR | 25.9 [24.6–27.4] | 26.1 [18.2 52.1] | 0.57 [0.20 0.93] | p = 0.7922 |
| Sticky Dot Notice | 36.1 [26.1–40.4] | 60.8 [33.3 81.7] | 0.73 [0.33 1] | p = 0.2468 |
| Time between Markers | ||||
| 1st behavior and RORR | 8.1 [7.2–10.3] | 9.3 [1.3 34.9] | 0.53 [0.13 0.90] | p = 0.8918 |
| RORR to Sticky Dot | 8.8 [1.2–15.0] | 25.4 [13.2 54.5] | 0.93 [0.73 1] | p = 0.0173 |
ATI: atipamezole; PRA: prazosin; RORR: return of righting reflex
* p-values were calculated using the Mann-Whitney U test; AUC: area under the receiver operating curve; AUC was used as effect size
Through a disruption of normal α1-receptor activity, the recovery from ketamine/xylazine in the ATI+PRA group is lengthened. In similar fashion, the emergence period (whisking to RORR) is increased for animals given YOH for reversal (Fig 3A). This highlights the importance of alpha receptor pharmacology in emergence and recovery from this ketamine/xylazine regimen.
Lengthy recovery is associated with effects on α1 receptors
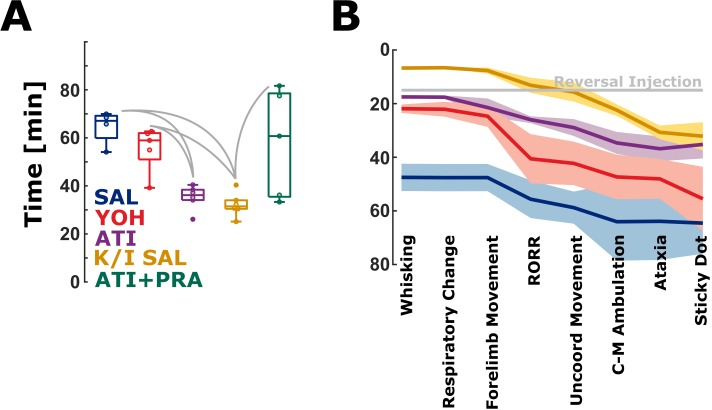
These mice were given a brief exposure to isoflurane to induce LORR and an equivalent amount of ketamine to the K/X regimen. Fig 4A compares the latency to noticing the sticky dot for the ketamine/isoflurane regimen with sham (saline) reversal (K/I-SAL, 31.5 [25.1 40.4] min). The Kruskal-Wallis test indicated a significant difference among groups (p = 0.0052, χ2: 14.79). Time to sticky dot notice for KET was significantly shorter when compared to SAL (p = 0.010, AUC = 1, very strong effect), YOH (p = 0.009, AUC = 0.97 [0.80 1], very strong effect), and ATI+PRA (p = 0.0303, AUC = 0.9 [0.67 1], strong to very strong effect). There was no significant difference when compared to the ATI group (p = 0.180, AUC = 0.75 [0.42 1], fair effect). This is the group that was given only α2-selective agonists and antagonists and like the K/I-SAL regimen no α1-antagonism. Fig 4B is a summary of all the emergence and recovery observations for all 5 regimens. Qualitatively, the appearance of emergence and recovery behaviors indicative of a return to neurocognitive baseline varied in time but the order of these behaviors was largely unaltered across groups. To complete the picture, S2 Fig presents a model of the pharmacodynamic effects and the latencies of emergence and recovery period for all groups.
Fig 4. Timing of waking behavior incidence varies with reversing agent, while order is generally maintained.
A) Animals that did not receive alpha receptor agonists or antagonists (ketamine+isoflurane+saline) notice the sticky dot earlier than animals from the saline (SAL) and yohimbine (YOH) group The Kruskal-Wallis test indicated a significant difference among groups (p = 0.0052, χ2: 14.79). The significant differences between the groups exclusive K/I-SAL (K/I: ketamine/isoflurane) are reported in the results section. Time to sticky dot notice for K/I-SAL was significantly shorter when compared to SAL, YOH. There was no significant difference when compared to the atipamezole (ATI) group The solid, gray lines indicate significant differences between groups. B) All measured waking behavior hallmarks compared between the groups. The gray line indicates time of reversal injection, 15 minutes post-ketamine/xylazine injection. The solid lines present the mean and the 95% confidence interval on the mean.
Discussion
In this study, behavioral milestones indicative of the approach to normal neurocognitive function after anesthesia with ketamine/xylazine were observed following injection of reversal agents. As predicted, both YOH and ATI effectively shortened the time required to reach these milestones during emergence from anesthesia compared to saline.
The behavioral profile between YOH and ATI mice during emergence and recovery from anesthesia was not identical, in agreement with previous studies [20]. Differential pharmacology between YOH and ATI offers some possible explanations for this. First, there are slight differences between YOH and ATI affinity for α2-receptor subtypes. ATI has an equal affinity for α2a, α2b, α2c, and α2d, while YOH has similar affinity for all the α2-subtypes except for α2d, for which it has a lower affinity. Xylazine indiscriminately targets all of the α2-subtypes [29]. Due to YOH’s lower affinity for the α2d-subtype, it is possible that some α2-receptors could still be agonized by xylazine resulting in a prolonged emergence as compared to ATI.
While both drugs predominately target α2-receptors, YOH interacts with other systems. Unlike ATI, which has a negligible affinity for serotonin (5-HT) [17, 18], β1/β2-adrenergic, muscarinic, dopamine2, tryptamine, GABA, opiate or benzodiazepine receptors [17], YOH is less discriminatory. High doses of YOH (>1 mg/kg; the current study used 4.3 mg/kg) have been shown to have 5-HT1A agonistic properties, which lead to decreases in heart rate, blood pressure, activity level, and body temperature [30]. Similarly, in doses approximating those used in the current study, YOH has been shown to decrease ambulation, an effect not seen with more selective α2-antagonists [19]. During the post-RORR period in the observation box, YOH and ATI animals exhibited qualitatively different levels of exploratory behavior.
In an attempt to characterize non-α2-interactions, the current study dosed the reversal agents in order to equalize α2blockade between the two reversal agents. Pertovaara et al. found that, in order to block α2 receptors to the same level that ATI does, about ten times the amount of YOH needs to be administered [18, 19].We dosed 10.8 times more YOH compared to ATI. This provides some evidence that non-α2-interactions in YOH are slowing down the emergence process, while possibly leading to hypoactivity during recovery.
Because YOH has an α2:α1 selectivity ratio over 200 times smaller than ATI [17], α1 antagonism could be implicated in YOH mice. Antagonism of α1-receptors is mechanistically involved in the sedating effects of some anti-psychotics (quetiapine, risperidone) [31] as well as anti-hypertensives. Some of the behavioral effects of co-administering the selective α1-inverse agonist prazosin [32] with atipamezole mimicked the yohimbine reversal, specifically the time to notice of sticky dot, the final behavioral marker we observed. This suggests that the slowing of recovery after ketamine/xylazine anesthesia after yohimbine reversal (relative to ATI) could be mediated by antagonism of α1-receptors and likely not subsequent hypoactivity due to the effect of yohimbine on 5-HT, or other receptors. Our experiments with ketamine in the absence of xylazine or any other adrenoreceptor manipulation further support the notion that α1-receptor antagonism can delay complex behaviors indicative of the restoration of normal behaviors post-anesthesia (notice of sticky dot). In situ hybridization experiments reveal that neurons in layers II-V of most areas of the cerebral cortex, the lateral amygdala, hippocampus, and reticular thalamus all have high density of α1-receptors [33]. Distribution of normal signaling of these regions may contribute to failure to achieve a normal recovery sequence efficiently.
While ATI mice were more active than YOH mice, they showed a profound lack of coordination once righted. This ataxia did not completely resolve during the 25-minute observation. α2-adrenoceptors are known to be present in the cerebellum [34]. Further studies will be necessary to determine if this prolonged ataxia is related to the hastening of emergence, causing enhanced locomotion before coordination is re-established, or a lingering effect of atipamezole on cerebellar function.
A limitation of our study is the failure to pharmacologically antagonize the effects of ketamine. Although ketamine is known to inhibit NMDA receptors, it is pharmacologically promiscuous and its exact mechanism for producing surgical anesthesia is unknown. Unlike xylazine, dexmedetomidine, opioids, and benzodiazepines, no pharmacologic agent specifically reverses all of the pharmacodynamic effects of ketamine. Some evidence suggests that ketamine minimally interacts with adrenoceptors [35], but these interactions have yet to be thoroughly examined. While it is not possible from this data to distinguish the effects of residual ketamine from lingering effects of the antagonists, our observations of mild hyperactive ataxic behavior in ATI animals are similar to the clinical situation often described for recovery from ketamine anesthesia characterized by excitation and features of emergence delirium [36]. This supports the notion that ATI treated animals may be exhibiting behavior typical of ketamine after effectively eliminating xylazine’s pharmacodynamic effects (S2 Fig). Interestingly, ataxia appeared to be attenuated after approximately the same delay (in reference to RORR as opposed to anesthesia injection) in all groups. In parallel, if measured in reference to RORR, other measures of coordination recovery (uncoordinated movement, diagonally cross-matched ambulation) and higher perception and motor processing (sticky dot notice) had the same delay across groups. It appears that, although ATI produces a more efficient emergence from ketamine/xylazine anesthesia, it does not improve late recovery compared to YOH or even no reversal agent at all (Fig 4B). It is possible that the differences in waking behaviors between ATI and YOH are arising from a differential clearance in ketamine and xylazine, rather than off-target interactions. It would be excellent to characterize these effects further with additional experiments examining the potential for a dose-effect of YOH and/or ATI. Based on our findings we conclude that proper reconstruction of network activity, requiring specific activation of networks involving adrenoreceptors, underlies restoration of coordinated movement, as opposed to this being solely a consequence of ketamine pharmacokinetics. Future studies involving careful blood sampling over time would be necessary to determine which has the greatest influence.
Although both YOH and ATI produced waking behaviors before saline, ATI was slightly quicker to elicit several markers. These differences are likely attributable to the differential affinity between the two drugs for α2-subtypes, as well as α1-interactions. Because the effects of adrenoceptor antagonism on behavior are dose-dependent [19, 30], further experiments are needed to compare these results to lower doses of these drugs. During recovery from anesthesia it is difficult to determine if the animal is attempting to explore their environment versus exhibiting an escape response, however motoric behaviors can still be observed and measured. Quantification of arousal, exploratory behavior, and balance should be done given the observations made during the current experiment. In total, our results highlight that an efficient emergence is not necessarily a preferred trajectory for the immediate post-anesthesia recovery. In addition to pharmacokinetic effects, the re-establishment of normal behaviors after anesthesia likely involves neuronal circuits dependent on time and/or activity.
Supporting information
Latency from RORR to A) uncoordinated movement, B) cross-matched ambulation, and C) ataxia. There was no significant difference between these groups except for B) Mice from the YOH group had a significantly shorter time span from RORR to cross-matched ambulation. The solid lines between the boxes indicate significance.
(TIF)
The upper graph is an idealized model that schematically depicts the expected pharmacodynamic effects (estimated overall effect of the drugs on the animal) of anesthetic agents over time (x-axis on the same scale as lower graph). Ketamine = yellow line, xylazine = green, xylazine with reversal agent = light green line. Mean latency for emergence period (whisker movement to RORR, solid lines) and recovery period (RORR to sticky dot notice, dashed lines) is plotted on the lower graph. SAL = blue, YOH = red, ATI = purple, K/I-SAL = yellow, ATI+PRA = green. Mean values are plotted. Vertical dashed gray line represents time of reversal agent injection.
(TIF)
Data Availability
All relevant data are available from the Figshare repository through the following link: https://figshare.com/s/4f268a9c44e24d126574.
Funding Statement
Dr. García’s research efforts are supported in part by a Career Development Award #BX00167 (PI: PS García, MD, PhD) from the United States Department of Veteran Affairs (https://www.research.va.gov/funding/), Biomedical Laboratory Research and Development Service (https://www.research.va.gov/services/blrd/) and the James S. McDonnell Foundation grant #220023046 (PI: PS García, MD, PhD) (https://www.jsmf.org/apply/). Dr. Pardue’s research efforts are supported by a Research Scientist Award C9257-5 from the Department of Veterans Affairs, Rehabilitation Research and Development Service (http://www.rehab.research.va.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mashour GA, Alkire MT. Evolution of consciousness: phylogeny, ontogeny, and emergence from general anesthesia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110 Suppl 2:10357–64. 10.1073/pnas.1301188110 ; PubMed Central PMCID: PMC3690605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hight DF, Dadok VM, Szeri AJ, Garcia PS, Voss L, Sleigh JW. Emergence from general anesthesia and the sleep-manifold. Front Syst Neurosci. 2014;8:146 10.3389/fnsys.2014.00146 ; PubMed Central PMCID: PMCPMC4131673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Fong R, Mason P, Fox AP, Xie Z. Caffeine accelerates recovery from general anesthesia. Journal of neurophysiology. 2014;111(6):1331–40. 10.1152/jn.00792.2013 ; PubMed Central PMCID: PMC3949308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chander D, Garcia PS, MacColl JN, Illing S, Sleigh JW. Electroencephalographic variation during end maintenance and emergence from surgical anesthesia. PLoS One. 2014;9(9):e106291 10.1371/journal.pone.0106291 ; PubMed Central PMCID: PMC4180055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013;118(1):30–9. 10.1097/ALN.0b013e318278c896 ; PubMed Central PMCID: PMCPMC3527840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safavynia SA, Keating G, Speigel I, Fidler JA, Kreuzer M, Rye DB, et al. Effects of gamma-Aminobutyric Acid Type A Receptor Modulation by Flumazenil on Emergence from General Anesthesia. Anesthesiology. 2016;125(1):147–58. 10.1097/ALN.0000000000001134 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012;116(5):998–1005. 10.1097/ALN.0b013e3182518bfc ; PubMed Central PMCID: PMCPMC3339625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Vlisides PE. Ketamine: 50 Years of Modulating the Mind. Front Hum Neurosci. 2016;10:612 10.3389/fnhum.2016.00612 ; PubMed Central PMCID: PMCPMC5126726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia P, Sleigh J. Ketamine: A Drug at War with Itself. Anesthesiology. 2017;126(3):371–2. 10.1097/ALN.0000000000001513 . [DOI] [PubMed] [Google Scholar]
- 10.Makary L, Vornik V, Finn R, Lenkovsky F, McClelland AL, Thurmon J, et al. Prolonged recovery associated with dexmedetomidine when used as a sole sedative agent in office-based oral and maxillofacial surgery procedures. J Oral Maxillofac Surg. 2010;68(2):386–91. 10.1016/j.joms.2009.09.107 . [DOI] [PubMed] [Google Scholar]
- 11.Komulainen AO Merle. Antagonism of ketamine-xylazine anesthesia in rats by administration of yohimbine, tolazoline, or 4-aminopyridine. Am J Vet Res. 1991;52(4):585–7. [PubMed] [Google Scholar]
- 12.Maze M, Tranquilli W. Alpha-2 adrenoceptor agonists: defining the role in clinical anesthesia. Anesthesiology. 1991;74(3):581–605. . [PubMed] [Google Scholar]
- 13.Greene ST, J C. Xylazine—a review of its pharmacology and use in veterinary medicine. Journal of Veterinary Pharmacology and Therapeutics 1988;4(11). [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto H, Fukuda S, Minakawa Y, Sawamura S. Clonidine induces sedation through acting on the perifornical area and the locus coeruleus in rats. J Neurosurg Anesthesiol. 2013;25(4):399–407. 10.1097/ANA.0b013e3182978ff0 . [DOI] [PubMed] [Google Scholar]
- 15.Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med (Maywood). 2005;230(10):777–84. . [DOI] [PubMed] [Google Scholar]
- 16.Turner PV, Albassam MA. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp Med. 2005;55(2):175–82. . [PubMed] [Google Scholar]
- 17.Virtanen RS, J M; Saano V Highly selective and specific antagonism of central and peripheral alpha 2-adrenoceptors by atipamezole. Arch Int Pharmacodyn Ther 1989. [PubMed] [Google Scholar]
- 18.Pertovaara A, Haapalinna A, Sirvio J, Virtanen R. Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist. CNS Drug Rev. 2005;11(3):273–88. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haapalinna A, Viitamaa T, MacDonald E, Savola JM, Tuomisto L, Virtanen R, et al. Evaluation of the effects of a specific alpha 2-adrenoceptor antagonist, atipamezole, on alpha 1- and alpha 2-adrenoceptor subtype binding, brain neurochemistry and behaviour in comparison with yohimbine. Naunyn Schmiedebergs Arch Pharmacol. 1997;356(5):570–82. . [DOI] [PubMed] [Google Scholar]
- 20.Janssen CF, Maiello P, Wright MJ Jr., Kracinovsky KB, Newsome JT. Comparison of Atipamezole with Yohimbine for Antagonism of Xylazine in Mice Anesthetized with Ketamine and Xylazine. J Am Assoc Lab Anim Sci. 2017;56(2):142–7. . [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, et al. Ketamine for treatment-resistant unipolar depression: current evidence. CNS drugs. 2012;26(3):189–204. 10.2165/11599770-000000000-00000 ; PubMed Central PMCID: PMC3677048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Byrne AJ, Hudetz AG, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. Journal of cardiothoracic and vascular anesthesia. 2009;23(5):651–7. 10.1053/j.jvca.2008.12.021 . [DOI] [PubMed] [Google Scholar]
- 23.Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesthesia and analgesia. 2004;99(2):482–95, table of contents. 10.1213/01.ANE.0000118109.12855.07 . [DOI] [PubMed] [Google Scholar]
- 24.Levanen J, Makela ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82(5):1117–25. . [DOI] [PubMed] [Google Scholar]
- 25.Hadi SM, Saleh AJ, Tang YZ, Daoud A, Mei X, Ouyang W. The effect of KETODEX on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane based-anesthesia. Int J Pediatr Otorhinolaryngol. 2015;79(5):671–6. 10.1016/j.ijporl.2015.02.012 . [DOI] [PubMed] [Google Scholar]
- 26.Balkaya M, Krober JM, Rex A, Endres M. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab. 2013;33(3):330–8. 10.1038/jcbfm.2012.185 ; PubMed Central PMCID: PMCPMC3587814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald JH. Handbook of biological statistics: Sparky House Publishing; Baltimore, MD; 2009. [Google Scholar]
- 28.Hentschke. Measures of Effect Size Toolbox. In: mes.m, editor. MATLAB2011.
- 29.Schwartz DD, Clark TP. Affinity of detomidine, medetomidine and xylazine for alpha-2 adrenergic receptor subtypes. J Vet Pharmacol Ther. 1998;21(2):107–11. . [DOI] [PubMed] [Google Scholar]
- 30.Zaretsky DV, Zaretskaia MV, DiMicco JA, Rusyniak DE. Yohimbine is a 5-HT1A agonist in rats in doses exceeding 1 mg/kg. Neuroscience letters. 2015;606:215–9. 10.1016/j.neulet.2015.09.008 ; PubMed Central PMCID: PMC4726473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanibuchi Y, Fujita Y, Kohno M, Ishima T, Takatsu Y, Iyo M, et al. Effects of quetiapine on phencyclidine-induced cognitive deficits in mice: a possible role of alpha1-adrenoceptors. Eur Neuropsychopharmacol. 2009;19(12):861–7. 10.1016/j.euroneuro.2009.07.005 . [DOI] [PubMed] [Google Scholar]
- 32.Melchiorre C, Bolognesi ML, Budriesi R, Chiarini A, Giardina D, Minarini A, et al. Search for selective antagonists at alpha 1-adrenoreceptors: neutral or negative antagonism? Farmaco. 1998;53(4):278–86. . [DOI] [PubMed] [Google Scholar]
- 33.Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14(7):4252–68. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strazielle C, Lalonde R, Hebert C, Reader TA. Regional brain distribution of noradrenaline uptake sites, and of alpha1-alpha2- and beta-adrenergic receptors in PCD mutant mice: a quantitative autoradiographic study. Neuroscience. 1999;94(1):287–304. . [DOI] [PubMed] [Google Scholar]
- 35.Scholz J, Tonner PH, Krause T, Paris A, Steinfath M, Wappler F, et al. [Interactions of intravenous anesthetics with cerebral alpha-2-adrenoceptors]. Anasthesiol Intensivmed Notfallmed Schmerzther. 1999;34(10):642–7. 10.1055/s-1999-219 . [DOI] [PubMed] [Google Scholar]
- 36.Dundee JW, Knox JW, Black GW, Moore J, Pandit SK, Bovill J, et al. Ketamine as an induction agent in anaesthetics. Lancet. 1970;1(7661):1370–1. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Latency from RORR to A) uncoordinated movement, B) cross-matched ambulation, and C) ataxia. There was no significant difference between these groups except for B) Mice from the YOH group had a significantly shorter time span from RORR to cross-matched ambulation. The solid lines between the boxes indicate significance.
(TIF)
The upper graph is an idealized model that schematically depicts the expected pharmacodynamic effects (estimated overall effect of the drugs on the animal) of anesthetic agents over time (x-axis on the same scale as lower graph). Ketamine = yellow line, xylazine = green, xylazine with reversal agent = light green line. Mean latency for emergence period (whisker movement to RORR, solid lines) and recovery period (RORR to sticky dot notice, dashed lines) is plotted on the lower graph. SAL = blue, YOH = red, ATI = purple, K/I-SAL = yellow, ATI+PRA = green. Mean values are plotted. Vertical dashed gray line represents time of reversal agent injection.
(TIF)
Data Availability Statement
All relevant data are available from the Figshare repository through the following link: https://figshare.com/s/4f268a9c44e24d126574.