Abstract
Dogs diagnosed with appendicular osteosarcoma typically succumb to metastatic disease within a year of diagnosis. The current standard of care for curative intent, amputation followed by adjuvant chemotherapy, increases survival time but chemoresistance is a major contributor to mortality. Unfortunately, the mechanisms driving the progression of metastatic disease and the development of chemoresistance are unknown. One theory is that autophagy may contribute to chemoresistance by providing neoplastic cells with a mechanism to survive chemotherapy treatment. Our objective was to evaluate the effect of combining an autophagy inhibitor with a standard chemotherapeutic drug on response to chemotherapy in canine appendicular osteosarcoma cells. We hypothesized that combining the autophagy inhibitor spautin-1 with doxorubicin treatment would enhance chemoresponsiveness. Using commercial (D17) and primary cell lines derived from 1° and 2° sites of osteosarcoma, we showed that this combination treatment enhances cell killing and inhibits colony formation. Our findings support the theory that autophagy contributes to chemoresistance in canine appendicular osteosarcoma and indicate that adding an autophagy inhibitor to the standard of care has the potential to improve outcome.
Introduction
Despite being the most common and aggressive bone neoplasm of dogs, the treatment used for canine appendicular osteosarcoma has been largely unchanged for decades [1]. The addition of adjuvant chemotherapy post-amputation was investigated in the late 1980s [2–6], was further evaluated and made common practice in the 1990s [3,7–10], and remains the standard of care for curative intent today. Unfortunately, even with aggressive chemotherapy post-amputation, most dogs succumb to metastatic disease less than a year after diagnosis [11].
Multiple attempts have been made to extend survival time by altering the current standard of care for curative intent, as well as to improve the efficacy of treatment against metastatic disease, but canine osteosarcoma is highly chemoresistant. Alternating doses of the most commonly used chemotherapeutics, doxorubicin and carboplatin, does not improve survival time, but may reduce adverse effects [12–18]. The use of these two chemotherapeutics has also been compared retrospectively with no significant difference in outcome [19]. New or alternative therapeutic agents, including other platinum compounds, different classes of chemotherapeutics, bisphosphonates and other palliative therapies, liposome-encapsulated drugs, matrix metalloproteinase inhibitors, mTOR inhibitors, tyrosine kinase inhibitors, human cytotoxic T-cells, immunotherapies, drugs that target multi-drug resistance, and even personalized strategies have not proved superior to the current regimen [1,20–32]. There is however, a new vaccine with promising phase I results [33]. Neoadjuvant chemotherapy is a component of the standard of care for the treatment of human conventional osteosarcoma, the human equivalent of canine appendicular osteosarcoma. However, there is currently no evidence that neoadjuvant treatment improves outcome in dogs with appendicular osteosarcoma [34]. Additional in vivo studies in dogs have investigated the treatment of metastatic disease after chemotherapy fails, but to date no significant improvements in survival time have been gained [18,24,35,36]. Chemoresistance is therefore a major hurdle in the management of canine appendicular osteosarcoma, since metastatic disease develops despite aggressive chemotherapy. The search continues for treatments that can prolong life and prevent progression in dogs diagnosed with appendicular osteosarcoma.
Autophagy is a self-digestion and recycling mechanism used by cells to survive harsh environmental conditions. Autophagy and/or autophagy dysregulation are suspected to play various, sometimes conflicting roles in the development and progression of neoplasia [37–39]. One hypothesis is that neoplastic cells use autophagy to survive treatment with chemotherapeutic agents. Blocking autophagy under these circumstances could trigger apoptosis, enhancing the effects of the chemotherapeutic agent [40].
Spautin-1 (specific and potent autophagy inhibitor-1) is a small molecule that inhibits autophagy by enhancing degradation of beclin-1, a protein required for the initiation of autophagy [41]. Since its discovery in 2011, multiple in vitro studies confirm that this drug can inhibit autophagy, induce cancer cell death, and enhance the effectiveness of radiation therapy and chemotherapy in multiple human cancer cell lines, including a human osteosarcoma cell line [40,42–47]. The chemotherapeutic agent doxorubicin induces autophagy in human osteosarcoma cell lines [48]. We hypothesized that inhibition of autophagy would render canine osteosarcoma cells more sensitive to chemotherapy. Our objective was to evaluate the effect of pre-treatment with the autophagy inhibitor spautin-1 on the response of canine osteosarcoma cells to doxorubicin.
Materials and methods
Cell lines and cell maintenance
Both primary and commercial cells lines were used. The primary cell lines were derived in-house from 1° and 2° tumours removed from dogs with appendicular osteosarcoma. For most experiments, there were 6 canine cell lines used, 2 of each representing: non-cancer cells [MDCK (Madin Darby Canine Kidney) and OVC-cMES-103], cells derived from 1° osteosarcoma (OVC-cOSA-75 and OVC-cOSA-106), and cells derived from 2° osteosarcoma (D17 and OVC-cOSA-31). The commercial cells lines, D17 and MDCK, were obtained from the American Type Culture Collection (ATCC; S1 Table). The primary cell lines were derived in our laboratory either by the current author (CS), OVC-cMES-103 and OVC-cOSA-106, or by previous members of the laboratory. All cells were maintained at 37.0°C, 5% CO2 (Thermo Fisher Scientific, HERAcell 150i CO2 Incubator) in 10 cm plates (Corning, 430167) with Dulbecco′s Modified Eagle′s Medium (HyClone DMEM High Glucose, SH30022.01) with L-glutamine (Corning, 25-005-Cl) and 10% fetal bovine serum (FBS; Gibco, 12483–020), hereafter called standard media.
Derivation of primary cell lines
All animal experiments were performed according to Canadian Council of Animal Care guidelines. This study was approved by the Animal Care Committee of the University of Guelph and consent to collect tissue samples was obtained from the dogs’ owners. For derivation of cell lines OVC-cMES-103 and OVC-cOSA-106, tissue samples were collected from 2 dogs (see S1 Table for animal details) that underwent amputation at the Ontario Veterinary College (OVC) Health Sciences Centre (HSC) as a component of the standard of care for appendicular osteosarcoma. Sterile dissection instruments were used to extract tissue. Samples were dipped in 70% ethanol, rinsed with phosphate buffered saline (PBS), and then submerged in sterile PBS or standard media for 1–2 hours at 4°C in 50 mL tubes (Fisher Scientific, 06-443-18).
Sterile procedures were used to transfer the tissue samples into 10 cm culture plates. Tissues were manually minced with a scalpel blade into 1–2 mm pieces and covered with 10 mL of standard media plus 10 μg/mL gentamicin (Sigma, G1272-100ML). These plates were then transferred into the incubator for a minimum of 21 days at 37.0°C, 5% CO2. Once the cells reached confluency, they were rinsed with 2 mL of PBS, trypsinized with 1 mL of 0.05% Trypsin with 0.53 mM EDTA (Corning, 25-052-Cl), resuspended in media, and split into multiple 10 cm plates. Cells were continually passaged as plates reached confluency and gentamicin was eventually eliminated from the media, prior to freezing or experimental use.
Cells from various passages were frozen and stored in liquid nitrogen for future use. First, they were resuspended in standard media plus an additional 10% FBS and 10% DMSO (dimethyl sulfoxide; Fisher Scientific, BP231-100). Resuspended cells were aliquoted into 1.2 mL cryogenic vials (Corning, 430487). Prior to being transferred into liquid nitrogen, vials were immersed in isopropyl alcohol and placed in a -80°C freezer for 24 hours. Individual vials of cells were thawed as necessary. The other primary cell lines, OVC-cOSA-75 and OVC-cOSA-31, were previously derived in-house using similar methods.
Colony formation in soft agar
For the base layer, 1.5 mL of molten 0.8% agarose (UltraPure LMP Agarose; Invitrogen, 16520–100) was added to each well of a 6-well plate (Costar, 3506) and was left to solidify at room temperature for at least 1 hour. Confluent cells were trypsinized and resuspended in standard media. Resuspended cells were mixed 1:1 with 0.4% Trypan Blue (Gibco, 15250–06) and live cells were counted using the TC20 Automated Cell Counter (BioRad, Serial No. 508BR02851). Cells were resuspended in 0.4% agarose (5000 cells/1.5 mL) at 37.0°C and 1.5 mL/well was added on top of the base layer. The plates were left to solidify in the biological safety cabinet for 1 hour prior to being moved to the incubator. An entire 6-well plate was used for each cell line. After 28 days, colonies were stained and fixed with 0.05% crystal violet (Fisher Scientific, C581-25) containing 3% formalin (Surgipath 10% neutral buffered formalin; Leica, 3800780) for 15 minutes. Plates were then washed with PBS and after drying, the number of colonies per well was quantified using an Olympus SZX7 microscope. Cell lines that did not form colonies in soft agar were designated as non-cancer cells.
Protein collection and western blot
Cells were maintained in standard media at 37.0°C, 5% CO2. For each cell line, the seeding density necessary to reach 90–100% confluency at 72 hours was determined for 6-well plates under untreated conditions. For each experimental replicate for each cell line, cells for all treatment conditions (see Treatment subsection) were plated in duplicate wells. In half of these wells, 6 hours prior to the endpoint, 75 μM per well of hydroxychloroquine sulfate (HCQ; Sigma, H0915-5MG Lot# 046M4758V) was added to inhibit lysosome fusion with the autophagosome, thus preventing degradation of the proteins required to evaluate autophagic carrier flux via western blot analysis. HCQ treated proteins were used for the LC3 and P62 western blots. Optimization experiments where protein was collected for LC3 and P62 western blots in the absence of HCQ were also performed.
At the endpoint, 72 hours after seeding, the media was aspirated and cells were rinsed with cold PBS, then the plates were transferred onto ice. Either 60 or 120 μL/well, depending on cell viability, of RIPA buffer (Sigma Life Science, R0278-50ML) was applied to the cells for 1 minute, then cells were harvested using a cell scraper (25 cm Sterile Disposable Cell Scraper; Fisher Scientific, 08-100-241). Cell lysate from each well was transferred into separate 1.5 mL tubes (Fisher Scientific, 05-408-129) and was quickly vortexed. Lysate remained on ice for 30 minutes, being vortexed every 2–3 minutes. The samples were centrifuged at 16,000 x g for 30 minutes at 4°C before removing the supernatant and transferring it to new 1.5 mL tubes. If not being used immediately, proteins were stored at -80°C. Protein concentration was determined using a Bradford assay (Bio-rad Protein Assay Dye Reagent Concentrate, 500–0006).
Individual proteins were combined 1:1 with 2x reducing buffer [5 mL Laemmli Sample Buffer (BioRad, 161–0737) and 270 mg Dithiothreitol (BioRad, 161–0611)] in preparation for loading, then maintained at 95°C for 5 minutes in a heating block. Experimental protein samples (20 μg) and PiNK Plus Prestained Protein Ladder (GeneDireX Inc., PM0005) were loaded into separate wells of 12% TGX Stain-Free FastCast Acrylamide gels (BioRad, 1610185). Gels were submerged in 1x Tris-Glycine SDS running buffer (diluted from 10x; BioRad, 1610732) and proteins were separated by molecular weight at 200 V for up to 45 minutes. Proteins were transferred from the gel to a Trans-Blot Turbo Mini-size PVDF membrane (BioRad, 1704272) using the Trans-Blot Turbo Transfer System (BioRad, 1704150) at 25 V for 3 minutes. The membrane was then washed twice while being gently agitated in tris-buffered saline (TBS) containing 0.5% Tween 20 (MP Biomedicals, 194841; TBST). The membrane was blocked with 5% skim milk in TBST for 1 hour at room temperature with motion. Primary antibody was diluted (Table 1) with 5% skim milk in TBST and incubated for 14–16 hours at 4°C on a rocker. The membrane was then washed twice with TBST. Secondary antibody diluted with 5% skim milk in TBST (Table 1) was applied to the membrane for 1 hour at room temperature with motion, prior to being washed twice with TBST and once with TBS. Clarity Western ECL Substrate (BioRad, 1705060), 10 mL/membrane, was applied for 5 minutes before the chemiluminescence signal was detected by the ChemiDoc XRS (BioRad, 1708265).
Table 1. Antibodies used for western blot analysis.
| Primary Antibody | Secondary Antibody | |||||||
|---|---|---|---|---|---|---|---|---|
| Protein Target | Species | Company | Product #, Lot# | Dilution | Species | Company | Product #, Lot# | Dilution |
| Beclin-1 | Mouse | LSBio | LS-C172820, 66461 | 1:2500 | Goat | Thermo Fisher Scientific | G21040 | 1:2000 |
| P62 | Rabbit | MBL | PM045, 018 | 1:10000 | Goat | Thermo Fisher Scientific | G-21234, 1419716 | 1:10000 |
| LC3 | Rabbit | Cell Signaling | PM036, 029 | 1:10000 | Goat | Thermo Fisher Scientific | G-21234, 1419716 | 1:10000 |
| pS6RP | Rabbit | Cell Signaling | 4857, 2 | 1:10000 | Goat | Thermo Fisher Scientific | G-21234, 1419716 | 1:5000 |
| Beta actin | Goat | Santa Cruz Biotechnology | Sc-1616, F1213 | 1:10000 | Rabbit | Thermo Fisher Scientific | 611620, 1305431A | 1:2500 |
P62, sequestosome 1; LC3, microtubule-associated protein light chain 3; pS6RP, phospho-S6 ribosomal protein
Cell viability assays
For each cell line, the seeding density determined for the 6-well plates (described previously in the Protein Collection and Western Blot subsection), was adjusted to the surface area of a 96-well plate (Corning Costar, 3997). Cells were exposed to various conditions (see Treatment subsection) and 6 hours prior to treatment endpoint 11 μL of resazurin (R&D Systems, AR002) was added to each well. Fluorescence was measured at 525/590 nm with a BioTek Synergy HT plate reader using Gen5 version 1.11 software (BioTek) and values were normalized to the mean of the cell-free media-only wells. The values of the non-treated control wells were assigned 100% viability and cell viability for the other conditions was calculated accordingly. For each experiment, 6 plates were used with 36 wells per condition and the experiment was performed twice for each cell line (n = 72 wells for each condition of each cell line). Repetitions were combined for statistical analysis.
Clonogenic assays
Confluent cells were trypsinized and resuspended in standard media to create a single cell suspension. The cell density of the solution was measured using the TC20 Automated Cell Counter. The cell suspension was further diluted with standard media to reach the appropriate seeding density. For each cell line, the seeding density necessary for non-treated cells to reach approximately 50–100 colonies per well in 7–9 days was established; to meet the definition of a colony, a minimum of 50 cells was required. After seeding, the cells were left to attach for 24 hours prior to being treated with various conditions (see Treatment subsection). Under all conditions, 72 hours after seeding, treated media was removed and cells were covered with fresh media before being returned to the incubator. At the endpoint, 7–9 days post-seeding, cells were rinsed twice with PBS then 1 mL of 0.01% crystal violet in 10% formalin was applied to the cells for 15 minutes. The fixative was removed, and cells were rinsed at least twice with PBS, and if necessary submerged in water until excess stain was removed. The number of colonies per well was counted and a minimum of 20 colonies per well was required. Each treatment condition was represented by 3–6 wells. Plating efficiency (PE) was calculated based on the colonies formed in the non-treated wells and was subsequently used to calculate the surviving fraction (SF) for the treated wells. For each cell line, the experiment was performed twice (n = 6–12 wells for each condition of each cell line).
Treatment conditions
The drugs used for the various treatments were spautin-1 (Sigma, SML0440) and a chemotherapeutic agent (doxorubicin hydrochloride; Pfizer DIN 02410397). Doxorubicin IC50s were determined for cell viability (96-well plates, 18 wells/dose), using R statistical analysis software [49], and for colony formation (6-well plates, 2–4 wells/dose). Similarly, determination of the spautin-1 IC50 was attempted for each cell line for the cell viability experiments. For the IC50s calculated using R statistical analysis software, the “ic50” package [50] was used, and the goal was to attain a coefficient of variation of < 0.05 and a maximum standard deviation of ≤ 0.2 [51].
Cells were exposed to both single-agent and combination treatments using both high and low doses of each drug. For the various conditions, cells were exposed to each individual drug for 24 hours at a time. Pre-treatment with spautin-1, followed by doxorubicin defines the combination treatment. All drugs were diluted to the appropriate concentration in standard media.
Cells used for viability assays and western blot were seeded in their respective 96- and 6-well plates simultaneously from the same single cell suspension (i.e. experiments were run in parallel). At 24 hours post-seeding, spautin-1 was applied to the appropriate wells and fresh media was applied to the cells not receiving pre-treatment. At 48 hours post-seeding, the treated and non-treated media was removed from all wells and either doxorubicin or fresh media was added to the wells depending on the condition. At 68 hours post-seeding, 11 μL of resazurin was added to each well of the 96-well plates for the cell viability assays and 75 μM of HCQ was added to half of the wells for western blot. At 72 hours, fluorescence was determined for the viability assays and protein was collected for western blot analysis.
Statistical analysis
R statistical analysis software was used to perform all analyses [49]. The Shapiro-Wilk test was used to test for normality. For normally distributed data, the Bartlett test was used to test the assumption of homogeneity of variance, while for non-normal data the “car” package was used to perform the Levene test [52].
For normal/non-normal data where groups had equal variances, one-way ANOVA followed by Tukey’s Honestly Significant Difference test was performed using the “multcomp” package [53]. For normal/non-normal data where groups had unequal variances, Welch’s ANOVA followed by the Games-Howell posthoc test was performed using the “onewaytests” and “userfriendlyscience” packages respectively [54,55]. When p < 0.05, differences were considered significant.
Results
Derivation, seeding densities, and IC50s
All osteosarcoma cell lines, both commercial (D17) and those derived in our lab, were adherent in culture, had a spindle-cell morphology, and formed colonies in soft agar, suggesting a neoplastic origin. OVC-cMES-103 cells also had a spindle-cell morphology but did not form colonies and are therefore presumed to have a non-cancerous mesenchymal origin. The 72 hour seeding densities for each cell line in 96- and 6-well plates, used for cell viability assays and protein collection, are provided in S2 Table. The seeding densities used for the clonogenic assays varied by treatment and are also provided in S2 Table. Non-cancer cell line OVC-cMES-103 did not form colonies within 8 days.
Cells varied in their susceptibility to doxorubicin, with the non-cancer cell lines being least susceptible, and the cell lines derived from 2° osteosarcoma being most susceptible (S1 Fig and S2 Table). The standard deviation was ≤ 0.02 for all cell lines and the coefficient of variations was < 0.05 for all cell lines except MDCK. For the cell viability assays, doxorubicin IC50 was designated as the high dose and for the low dose, the IC50 was divided by 20. Only one dose, the IC50, was used in the colony formation assays (S2 Fig and S2 Table).
During spautin-1 IC50 determination, drug precipitation occurred at concentrations > 100 μM. Most cell lines were minimally affected by spautin-1, and IC50 determination was only possible for a single cell line (2° osteosarcoma, D17; S3 Fig), but was not considered reliable because the IC50 occurred at a dose > 100 μM. Subsequently, 100 μM was designated as the high dose of spautin-1 for all cell lines and experiments. In the most severely affected cell line, D17, 5 μM was the lowest dose where an effect on cell viability was seen (S3 Fig), and therefore 5 μM was selected as the low dose of spautin-1 for all cell lines and experiments.
Cell viability assays
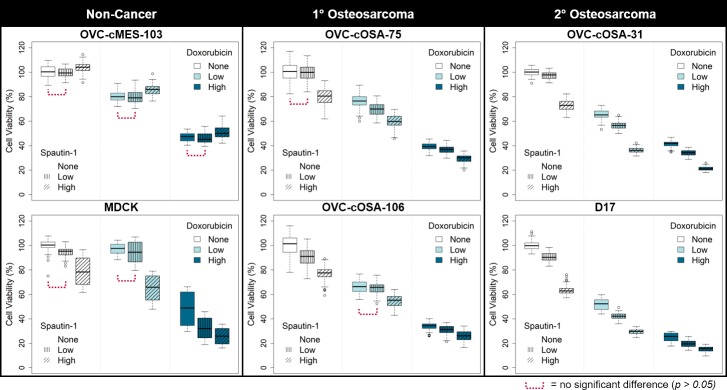
For all cell lines the treatment groups had non-equal variances, thus requiring Welch’s ANOVA. The cell viability results are graphically depicted in Fig 1. For all cell lines except MDCK, the difference between replicate treatments varied by ≤ 7% (Fig 1). For MDCK, the difference in the effect caused by the same treatment between replicate wells varied by up to 28% (Fig 1).
Fig 1. Cell viability of canine cells treated with the autophagy inhibitor spautin-1, doxorubicin, or a combination, with spautin-1 applied pre-chemotherapy.
Box plots representing cell viability measured using a resazurin assay for canine cell lines treated with spautin-1 (Low = 5 μM, High = 100 μM) or doxorubicin (Low = IC50/20, High = IC50) for 24 hours each as single-agent or combination treatments, using both low and high doses. Using Welch’s ANOVA followed by the Games-Howell test, differences between all treatments were statistically significant (p < 0.05), except where indicated (n = 72 wells per box).
Single-agent treatment
Both low and high doses of doxorubicin consistently reduced cell viability in all cell lines by up to 52% and 38–78%, respectively (p < 0.05, Fig 1). Low dose spautin-1 reduced cell viability in all cell lines by up to 11% (Fig 1); in both non-cancer cell lines, this was not significantly different from no treatment, but it was statistically significant in 3 of 4 cell lines derived from osteosarcoma (p < 0.05 for OVC-cOSA-106, OVC-cOSA-31, and D17). High dose spautin-1 significantly reduced cell viability in all cell lines, except OVC-cMES-103, by 12–38% (p < 0.05); in OVC-cMES-103, a non-cancer mesenchymal cell line, high dose spautin-1 significantly increased viability by 3–5% (p < 0.05; Fig 1). Cell lines derived from 2° osteosarcoma were most sensitive to high dose spautin-1, where it decreased cell viability by 27–38% (p < 0.05; Fig 1).
Combination treatment
Low dose spautin-1 applied prior to chemotherapy reduced cell viability compared to low and high dose doxorubicin alone by up to 12% (p < 0.05 for OVC-cOSA-75, OVC-cOSA-31, and D17) and by up to 8% (p < 0.05 for all but OVC-cMES-103), respectively (Fig 1). Pre-chemotherapy high dose spautin-1 reduced cell viability compared to low and high dose doxorubicin alone by an additional 10–30% and 6–20%, respectively (p < 0.05; Fig 1). In the cell lines derived from osteosarcoma, the effect of the pre-treatment became less evident with increasing doxorubicin (Fig 1). The combination effect was never more than the sum of the effects caused by each drug given alone.
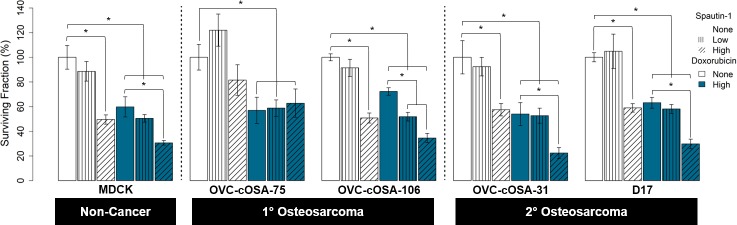
Clonogenic assays
For all cell lines except D17, the data was normally distributed. All cell lines except MDCK met the homogeneity of variance assumption. The standard error within treatment groups across all cell lines ranged from 0.96–6.94% and was typically highest for low dose spautin-1 alone.
Single-agent treatment
Doxorubicin significantly reduced colony formation for all cell lines by 27–52% (p < 0.05; Fig 2). Low dose spautin-1 was not significantly different from no treatment in any cell line, but either mildly reduced colony formation (4–18%) or enhanced it by 2–30% (Fig 2). High dose spautin-1 significantly and consistently reduced colony formation by 13–53% (p < 0.05) in all cell lines except OVC-cOSA-75; this reduction was greater than that of doxorubicin alone for 2 of 4 osteosarcoma cell lines (Fig 2).
Fig 2. Colony formation in canine cells treated with the autophagy inhibitor spautin-1, doxorubicin, or a combination, with spautin-1 applied pre-chemotherapy.
Bar graphs representing the mean surviving fraction of colonies determined using a clonogenic assay for canine cell lines treated with spautin-1 or doxorubicin for 24 hours each as single-agent or combination treatments, using both low (5 μM) and high (100 μM) doses of spautin-1. One-way ANOVA followed by Tukey’s Honestly Significant Difference test used for statistical analysis (* = p < 0.05, n = 6–12 wells per bar). Error bars represent standard error.
Combination treatment
For all cell lines, except OVC-cOSA-75, pre-treatment with low or high dose spautin-1 further reduced colony formation compared to doxorubicin alone (Fig 2). The difference compared to doxorubicin alone when low dose spautin-1 pre-treatment was added was statistically significant only for OVC-cOSA-106 (p < 0.05), while the addition of high dose spautin-1 pre-treatment compared to doxorubicin alone was significant in all cell lines (p < 0.05), except OVC-cOSA-75. For OVC-cOSA-106, the effect of pre-treatment with low dose spautin-1 combined with doxorubicin was more than the sum of the effects of the individual drugs given alone (Fig 2).
Western blot
All western blots are displayed in Fig 3. In general, the intensity of the beta actin bands was reduced with increasing doxorubicin, despite equal loading of total protein (Fig 3). For MDCK, most bands representing cells treated with high dose doxorubicin were not detectable (Fig 3). Therefore, the bands from MDCK cells treated with high dose doxorubicin were not examined for trends. Unlike the others, protein levels in OVC-cMES-103 and OVC-cOSA-31 were stable, even after high dose doxorubicin treatment, based on the loading control. For the autophagy related proteins in most osteosarcoma cell lines treated with high dose doxorubicin, band intensity was reduced, and sometimes lost, beyond what would be expected based on the beta actin bands.
Fig 3. Expression of autophagy related proteins in canine cells treated with the autophagy inhibitor spautin-1, doxorubicin, or a combination, with spautin-1 applied pre-chemotherapy.
Western blot expression of beclin-1, P62, pS6RP (phospho-S6 ribosomal protein), and LC3 (microtubule‑associated protein light chain 3) in canine cell lines treated with spautin-1 (Low = 5 μM, High = 100 μM) or doxorubicin (Low = IC50/20, High = IC50) for 24 hours each as single-agent or combination treatments, using both low and high doses. Beta actin was used as a loading control.
Single-agent treatment
Chemotherapy is known to induce autophagy [56], thus beclin-1 would be expected to increase in response to treatment with increasing concentration of chemotherapy. This was observed in OVC-cMES-103 cells (non-cancer mesenchymal cells) but was not seen in any in cell lines derived from osteosarcoma; instead, beclin-1 expression typically decreased with increasing doxorubicin (lanes 1 vs 4 vs 7; Fig 3). As a beclin-1 inhibitor, spautin-1 is expected to reduce beclin-1 expression because it enhances its degradation. This expected trend of decreasing beclin-1 expression was observed in the 2° osteosarcoma cell lines, but spautin-1 had no effect on beclin-1 expression in the non-cancer and 1° osteosarcoma cell lines (1 vs 2 vs 3; Fig 3).
In most cell lines, autophagic carrier flux was induced by low dose doxorubicin, based on an increase in LC3II expression, as expected (lanes 1 vs 4; Fig 3), while there was no change for OVC-cOSA-106 and OVC-cMES-103. In OVC-cMES-103, a non-cancer cell line, and the 2° osteosarcoma cell lines, LC3II expression was either maintained or further intensified with high dose doxorubicin, as expected (lanes 4 vs 7; Fig 3). However, this was not the case for our 1° osteosarcoma cell lines, which showed a marked reduction in LC3II expression after treatment with high dose doxorubicin (lanes 1 vs 4 vs 7; Fig 3). Also, unexpectedly, in all cell lines, LC3II expression either did not change or actually increased with increasing spautin-1 (lanes 1 vs 2 vs 3; Fig 3); as an autophagy inhibitor, spautin-1 is expected to reduce LC3II expression. Optimization experiments in the absence of HCQ with D17 cells indicate that both spautin-1 and doxorubicin alter complete autophagic flux, and not just autophagic carrier flux (S4 Fig).
P62 is depleted during autophagy. P62 band intensity typically decreased with increasing doxorubicin (lanes 1 vs 4 vs 7; Fig 3); this occurred in the presence and absence of HCQ (lanes 1 vs 4 and 7 vs 10; S4 Fig), indicating increased autophagic flux. In the cell lines derived from osteosarcoma, P62 expression tended to remain fairly constant or to subtly increase with increasing spautin-1 (lanes 1 vs 2 vs 3; Fig 3), as expected. Unexpectedly, treatment with increasing spautin-1 concentration in the non-cancer cell lines resulted in decreasing P62 intensity.
In the non-cancer cell lines, pS6RP expression increased with increasing doxorubicin, while the opposite was observed in all osteosarcoma lines (lanes 1 vs 4 vs 7 if applicable; Fig 3). pS6RP expression generally increased with increasing spautin-1 (lanes 1 vs 2 vs 3; Fig 3), which is a logical pattern if autophagy is being inhibited.
Combination treatment
When considering the effects of combination treatment, one must keep in mind that in most cell lines high dose doxorubicin diminished the expression of the loading control. This decreased expression was observed despite both the presence of live cells reflected in the cell viability results during IC50 determination (S1 Fig) and equal protein loading. The most common observation across all cell lines was that the effect of increasing spautin-1 on autophagy related protein expression was either greatly reduced or completely lost in the presence of high dose doxorubicin (lanes 1 vs 2 vs 3, 4 vs 5 vs 6, and 7 vs 8 vs 9); this was true for P62 expression in all osteosarcoma cell lines (Fig 3). Occasionally, the expression trend seen with increasing spautin-1 in the absence of doxorubicin (lanes 1 vs 2 vs 3) and in combination with low dose doxorubicin (lanes 4 vs 5 vs 6) was exactly the opposite to the trend seen with increasing spautin-1 in combination with high dose doxorubicin (lanes 7 vs 8 vs 9); this was true for LC3II in D17, beclin-1 in OVC-cOSA-106, and pS6RP in OVC-cOSA-31 (Fig 3).
Discussion
Our investigation evaluated the effect of pre-treatment with the autophagy inhibitor spautin-1 on the response of canine osteosarcoma cells to doxorubicin with respect to cell viability, colony formation, and expression of autophagy related proteins. We hypothesized that doxorubicin would induce autophagy in canine osteosarcoma cells and that the concomitant autophagy inhibition induced by spautin-1 would render these cells more sensitive to chemotherapy. Consistent with our hypothesis, spautin-1 treatment prior to doxorubicin treatment resulted in a reduction in both cell survival and colony formation compared to doxorubicin alone in the D17 commercial cell line and all of our primary cell lines derived from canine osteosarcoma. The effect on cell viability was never more than the sum of the effects of the individual drugs given alone, but for colony formation in a single cell line derived from 1° osteosarcoma, the effect was greater than this sum. When used in combination, spautin-1’s ability to enhance cell killing diminished with increasing concentration of doxorubicin, but the overall trend of enhanced killing was consistent.
A study using triple negative breast cancer cells found that 10 μM of spautin-1 for 24 hours (our low dose was 5 μM for 24 hours) had minimal effect on colony formation, but when used in combination with a chemotherapy drug, paclitaxel, it significantly reduced colony formation, even in paclitaxel-resistant cells [42]. In our canine osteosarcoma cells, the reduction in colony formation induced by high dose spautin-1 alone was equivalent to, or even greater than, that of doxorubicin alone (Fig 2). These findings are significant to canine osteosarcoma because the disseminated neoplastic cells that remain after amputation and survive chemotherapy to form new colonies are responsible for metastatic disease, which is the most common cause of mortality. A new therapy that can limit colony formation at a level that is similar or superior to the current standard of care has great potential to improve the overall outcome for canine osteosarcoma and may have potential applications to human osteosarcoma treatment as well.
Osteosarcoma is the most common 1° bone malignancy in dogs and humans, and progression to metastatic disease and chemoresistance represent the most important treatment hurdles in both species [40,57,58]. Horie et al. induced autophagy in a human osteosarcoma cell line using rapamycin, an mTOR inhibitor, while using spautin-1 to prevent the cancer cells from using autophagy as a self-defence mechanism [40]. Spautin-1 alone (100 μM, the same as our high dose) had minimal effect on cell proliferation in vitro but significantly reduced cell proliferation in combination with rapamycin, when compared to rapamycin alone [40]. In the same study, a similar effect was seen in vivo with shrinkage of xenograft tumours in mice; however, the difference in tumour size when spautin-1 was added as a combination treatment was not significantly different from that of rapamycin alone [40]. These findings are in line with our cell viability results using spautin-1 as a pre-treatment, but our results also indicate that a high dose of spautin-1 was effective on its own. During the in vivo portion of Horie et al.’s study, no adverse effects were observed in the mice after receiving 40 mg/kg of spautin-1 intraperitoneally, 5 times per week for a period of 4 weeks [40]. A different study stated that spautin-1 induced cardiotoxicity in mice, but there was no data provided to support this statement [43]. In a phase I/II clinical trial for a different autophagy inhibitor, HCQ, used in combination with reduced-dose doxorubicin, 30 dogs with lymphoma had a 93.3% overall response rate, and HCQ-related adverse effects were limited to mild lethargy and gastrointestinal upset [59]. Further in vivo studies are required to determine whether spautin-1 is suitable for clinical trials in canine patients with various cancer types.
One study recently showed that a Heat shock protein 90 (Hsp90) inhibitor induced autophagy in 2 metastatic canine osteosarcoma cell lines, one being D17 [60]. Interestingly, the 2 cells lines differed with respect to drug-induced apoptosis, with one cell line exhibiting a significant dose- and time-dependent increase in apoptosis and the other, D17, only minimal apoptosis [60]. The authors suggested that the autophagy induced by the Hsp90 inhibitor was not adequate to enhance apoptosis in the D17 cells [60]. These findings provide evidence that canine osteosarcoma cells perform autophagy and highlight the necessity of using multiple cell lines, based on the observed cell line dependent relationship between autophagy and apoptosis [60].
There were some unexpected expression patterns for several autophagy related proteins between both treatment conditions and cell lines. Doxorubicin consistently reduced cell viability and colony formation in all osteosarcoma cell lines at both doses, as expected. We also anticipated that doxorubicin would increase autophagic activity, and this would be reflected by increased LC3II expression in western blot analysis. This increase was observed in most cell lines after treatment with low dose doxorubicin (lanes 1 vs 4) and in most cell lines with high dose doxorubicin (lanes 4 vs 7), except for the cell lines derived from 1° osteosarcoma (Fig 3). These chemotherapy-naïve cells may be less adept at utilizing autophagy during chemotherapy treatment, compared to the cell lines derived from 2° osteosarcoma which may have previously survived treatment with chemotherapy. Our primary cell line derived from a 2° osteosarcoma had previously been treated with both carboplatin and doxorubicin (S1 Table), but the chemotherapy exposure status of the tumour from which the D17 cell line was generated is unknown.
Protein expression patterns also differed between the non-cancer and the cancer cell lines for pS6RP and beclin-1. The measurement of pS6RP, although it is not a direct substrate of mTOR, can be used to assess mTOR activity [61]. mTOR supresses autophagy, and phosphorylation of S6RP occurs downstream of mTOR activation [61]; therefore, phosphorylation of S6RP is indicative of autophagy suppression and one would expect a decrease in pS6RP expression after doxorubicin treatment. In our study, pS6RP expression increased with increasing doxorubicin concentration in the non-cancer cells but decreased in response to increasing doxorubicin in the osteosarcoma cell lines. This likely reflects the difference in the proliferative rate of normal cells versus neoplastic cells, as doxorubicin targets actively dividing cells. As beclin-1 is required for induction of autophagy, one might expect that treatment with a chemotherapeutic agent would result in increased beclin-1 expression, as was observed in our non-cancer cell lines. However, the opposite effect was observed in the cell lines derived from osteosarcoma, suggesting that autophagy pathways might be altered in these neoplastic cells. Defective autophagy has been implicated in the acceleration of tumour development in some forms of neoplasia [62]. Seeing differences in the expression of autophagy related proteins in our non-cancer versus cancer cells may indicate that abnormal autophagy is involved in the initiation or progression of canine osteosarcoma.
Treating both normal and neoplastic cells with spautin-1 inhibits autophagy, as is indicated by decreased LC3II expression [41,43,45–47,63–73] and increased P62 expression [45,64,66,72] assessed by western blot. In our canine cell lines, we observed the opposite for LC3II; we saw either no change, or increased expression with increasing spautin-1 concentration (Fig 3). For P62, our cancer cell lines produced the expected expression pattern in response to increasing spautin-1; however, the same pattern was not observed in the non-cancer cell lines. Others have similarly reported unexpected protein expression patterns in response to spautin-1 treatment, including a lack of effect on LC3II or P62 [74] and decreased P62 [70]. Expression of both LC3II and P62 are context and cell type specific, they both contribute to cellular processes beyond autophagy, and changes in their expression are not always correlated [61].
Spautin-1, an autophagy inhibitor that increases beclin-1 degradation, typically results in decreased beclin-1 expression using western blot [45,46,66–68,71,75]. Although we observed this pattern in our cell lines derived from 2° osteosarcoma, which were the cells most sensitive to spautin-1 in our study, no effect on beclin-1 was observed in the non-cancer and 1° osteosarcoma cell lines (lanes 1 vs 2 vs 3; Fig 3). Similar to our findings, other investigators did not observe a decrease in beclin-1 expression after spautin-1 treatment [47,76]. These results may indicate that dysregulation of autophagy is relevant to canine appendicular osteosarcoma and that different portions of the pathway may be affected in different cell types (i.e. non-cancerous vs neoplastic and 1° vs 2° osteosarcoma). It is widely accepted that autophagy can enhance both the survival and death of neoplastic cells depending the type of cancer, its genetic profile, and microenvironmental conditions [77]. Therefore, interpreting autophagy related protein expression is not always straightforward, and further investigation into the different patterns between cell types may help elucidate the role of autophagy in pathogenesis and progression of osteosarcoma.
Autophagy related protein expression did not always follow the expected pattern for a given treatment; however, when considering the changes in protein expression, the effects caused by chemotherapy alone must also be considered. High dose doxorubicin diminished the expression of all proteins, including the loading control, most prominently in MDCK cells. This was observed despite both the presence of live cells reflected by the cell viability assays (S1 Fig) and equal protein loading. Some of the inconsistent results observed for high dose doxorubicin may indicate that the cells were unable to perform normal autophagy. Additionally, to assess autophagic carrier flux by western blot, the use of a lysosomal inhibitor is recommended to block degradation of LC3. Using these inhibitors for extended periods of time can lead to nonspecific effects, and maximum exposure time should be under 12 hours [78]. We applied HCQ 6 hours prior to protein collection, which was 18 hours after spautin-1 was removed from the cells. Perhaps the autophagy inhibition induced by spautin-1 was not accurately captured due to the timing of our protein collection, but it did occur and caused significant effects in the cell viability and colony formation assays. Autophagy inhibition could therefore have been responsible for the enhanced chemoresponsiveness seen in our canine osteosarcoma cells.
A definitive explanation of how autophagy contributes to chemoresistance in canine osteosarcoma was not uncovered, but we did observe differences in the expression of autophagy related proteins in response to different treatment conditions, as well as when comparing non-cancer cells to cancer cells and cells derived from 1° osteosarcoma to those derived from 2°. Our results may indicate that cells that have already survived chemotherapy treatment may be better equipped to use autophagy to enhance survival if re-exposed to that drug. Determining which differential response patterns reflect intact versus defective or abnormal autophagy in these canine cell lines will require further investigation. We showed that an autophagy inhibitor, spautin-1, effectively kills canine osteosarcoma cells and reduces their ability to form colonies in vitro; and as a pre-treatment, it enhances the effect of doxorubicin in most canine osteosarcoma cell lines. To improve the outcome for dogs diagnosed with appendicular osteosarcoma, new ways to prevent, delay, and/or treat metastatic disease are necessary. Clinically, the addition of spautin-1 to the standard of care could potentially enhance the killing of neoplastic cells and reduce the ability of metastatic osteosarcoma cells to form colonies post-amputation.
Supporting information
Cell viability measured using a resazurin assay. The solid vertical line denotes the IC50 value, and the dashed vertical lines denote 95% confidence interval (CI) bounds. Error bars denote standard deviation (SD) [CV, coefficient of variation].
(TIFF)
Surviving fraction measured using a colony formation assay.
(TIFF)
Cell viability measured using a resazurin assay. The solid vertical line denotes the IC50 value, and the dashed vertical lines denote 95% confidence interval (CI) bounds. Error bars denote standard deviation (SD) [CV, coefficient of variation].
(TIFF)
Western blot expression of P62 and LC3 (microtubule‑associated protein light chain 3) in metastatic canine osteosarcoma cells treated with spautin-1 (Low = 15 μM, High = 120 μM) or doxorubicin (IC50) for 24 hours each as a single-agent, or both drugs in combination. Beta actin was used as a loading control. Both in the presence and absence of the lysosomal inhibitor HCQ, LC3II expression is reduced with increasing spautin-1 (1 vs 2 vs 3 and 7 vs 8 vs 9), indicative of autophagy inhibition. Both in the presence and absence of HCQ, doxorubicin increases LC3II expression (1 vs 4 and 7 vs 10), indicative of autophagy induction.
(TIF)
(PDF)
(PDF)
Acknowledgments
The authors thank Mary Ellen Clark for her expertise and support in the laboratory. They also thank Jeremy Wong and Dr. Christopher Pinelli who provided training for certain experimental techniques and advice on experimental design. and Kadi White for her assistance with the optimization of several experimental protocols.
Data Availability
Data are available from the University of Guelph Research Data Repository (https://dataverse.scholarsportal.info/dataverse/ugrdr). DOI for the data set is: https://doi.org/10.5683/SP2/ZT4AZV.
Funding Statement
This work was funded by a grant to GAW by the Pet Trust (https://ovc.uoguelph.ca/pettrust). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Marley K, Helfand SC, Simpson J, Mata JE, Tracewell WG, Brownlee L, et al. Pharmacokinetic study and evaluation of the safety of taurolidine for dogs with osteosarcoma. J Exp Clin Cancer Res. 2013;32: 74 10.1186/1756-9966-32-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauldin GN, Matus RE, Withrow SJ, Patnaik AK. Canine osteosarcoma chemotherapy using doxorubicin and cisplatin. J Vet. 1988; 177–180. [DOI] [PubMed] [Google Scholar]
- 3.Straw RC, Withrow SJ, Richter SL, Powers BE, Klein MK, Postorino NC, et al. Amputation and cisplatin for treatment of canine osteosarcoma. J Vet Intern Med. 1991;5: 205–210. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro W, Fossum TW, Kitchell BE, Couto CG, Theilen GH. Use of cisplatin for treatment of appendicular osteosarcoma in dogs. J Am Vet Med Assoc. 1988;192: 507–11. [PubMed] [Google Scholar]
- 5.Kraegel S a, Madewell BR, Simonson E, Gregory CR. Osteogenic sarcoma and cisplatin chemotherapy in dogs: 16 cases (1986–1989). J Am Vet Med Assoc. 1991;199: 1057–1059. [PubMed] [Google Scholar]
- 6.Thompson JP, Fugent MJ. Evaluation of survival times after limb amputation, with and without subsequent administration of cisplatin, for treatment of appendicular osteosarcoma in dogs: 30 cases (1979–1990). J Am Vet Med Assoc. 1992;200: 531–533. [PubMed] [Google Scholar]
- 7.Berg J, Weinstein MJ, Springfield DS, Rand WM. Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. J Am Vet Med Assoc. 1995;206: 1555–1560. [PubMed] [Google Scholar]
- 8.Berg J. Canine osteosarcoma: amputation and chemotherapy. Vet Clin North Am Small Anim Pract. 1996;26: 111–121. 10.1016/S0195-5616(96)50010-0 [DOI] [PubMed] [Google Scholar]
- 9.Berg J, Gebhardt MC, Rand WM. Effect of timing of postoperative chemotherapy on survival of dogs with osteosarcoma. Cancer. 1997;79: 1343–50. [DOI] [PubMed] [Google Scholar]
- 10.Bergman PJ, MacEwen EG, Kurzman ID, Henry CJ, Hammer AS, Knapp DW, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J Vet Intern Med. 1996;10: 76–81. 10.1111/j.1939-1676.1996.tb02031.x [DOI] [PubMed] [Google Scholar]
- 11.Schmidt AF, Nielen M, Klungel OH, Hoes a. W, de Boer A, Groenwold RHH, et al. Prognostic factors of early metastasis and mortality in dogs with appendicular osteosarcoma after receiving surgery: an individual patient data meta-analysis. Prev Vet Med. 2013;112: 414–422. 10.1016/j.prevetmed.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 12.Skorupski K a., Uhl JM, Szivek A, Allstadt Frazier SD, Rebhun RB, Rodriguez CO. Carboplatin versus alternating carboplatin and doxorubicin for the adjuvant treatment of canine appendicular osteosarcoma: a randomized, phase III trial. Vet Comp Oncol. 2016;14: 81–87. 10.1111/vco.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent MS, Strom A, London CA, Seguin B. Alternating carboplatin and doxorubicin as adjunctive chemotherapy to amputation or limb-sparing surgery in the treatment of appendicular osteosarcoma in dogs. J Vet Intern Med. 2004;18: 540–544. 10.1111/j.1939-1676.2011.0697.x [DOI] [PubMed] [Google Scholar]
- 14.Bacon NJ, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Use of alternating administration of carboplatin and doxorubicin in dogs with microscopic metastases after amputation for appendicular osteosarcoma: 50 cases (1999–2006). J Am Vet Med Assoc. 2008;232: 1504–1510. 10.2460/javma.232.10.1504 [DOI] [PubMed] [Google Scholar]
- 15.Lane a. E, Black ML, Wyatt KM. Toxicity and efficacy of a novel doxorubicin and carboplatin chemotherapy protocol for the treatment of canine appendicular osteosarcoma following limb amputation. Aust Vet J. 2012;90: 69–74. 10.1111/j.1751-0813.2011.00878.x [DOI] [PubMed] [Google Scholar]
- 16.Frimberger AE, Chan CM, Moore AS. Canine osteosarcoma treated by post-amputation sequential accelerated doxorubicin and carboplatin chemotherapy: 38 cases. J Am Anim Hosp Assoc. 2016;52: 149–156. 10.5326/JAAHA-MS-6315 [DOI] [PubMed] [Google Scholar]
- 17.Bailey D, Erb H, Williams L, Ruslander D, Hauck M. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med. 2003;17: 199–205. 10.1111/j.1939-1676.2003.tb02434.x [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Matsuyama A, Mutsaers AJ, Woods JP. Retrospective evaluation of toceranib (Palladia) treatment for canine metastatic appendicular osteosarcoma. Can Vet J. 2017;58: 1059–1064. [PMC free article] [PubMed] [Google Scholar]
- 19.Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. 2014;28: 554–563. 10.1111/jvim.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon M, Mathie T, Stingle N, Romansik E, Vail D, London C. Adjuvant carboplatin and gemcitabine combination chemotherapy postamputation in canine appendicular osteosarcoma. J Vet Intern Med. 2011;25: 511–7. 10.1111/j.1939-1676.2011.0697.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirpensteijn J, Teske E, Kik M, Klenner T, Rutteman GR. Lobaplatin as an adjuvant chemotherapy to surgery in canine appendicular osteosarcoma: a phase II evaluation. Anticancer Res. 2002;22: 2765–70. [PubMed] [Google Scholar]
- 22.Hahn K a, Legendre a M, Schuller HM. Amputation and dexniguldipine as treatment for canine appendicular osteosarcoma. J Cancer Res Clin Oncol. 1997;123: 34–38. 10.1007/BF01212612 [DOI] [PubMed] [Google Scholar]
- 23.Alvarez FJ, Kisseberth W, Hosoya K, Lara-Garcia A, Kosarek C, Murahari S, et al. Postoperative adjuvant combination therapy with doxorubicin and noncytotoxic suramin in dogs with appendicular steosarcoma. J Am Anim Hosp Assoc. 2013;50: 12–18. 10.5326/JAAHA-MS-5958 [DOI] [PubMed] [Google Scholar]
- 24.Laver T, London CA, Vail DM, Biller BJ, Coy J, Thamm DH. Prospective evaluation of toceranib phosphate in metastatic canine osteosarcoma. Vet Comp Oncol. 2017;2: 1–7. 10.1111/vco.12328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurzman ID, MacEwen EG, Rosenthal RC, Fox LE, Keller ET, Helfand SC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. 1995;1: 1595–601. [PubMed] [Google Scholar]
- 26.Moore AS, Dernell WS, Ogilvie GK, Kristal O, Elmslie R, Kitchell B, et al. Doxorubicin and BAY 12–9566 for the treatment of osteosarcoma in dogs: a randomized, double-blind, placebo-controlled study. J Vet Intern Med. 2007;21: 783–790. 10.1111/j.1939-1676.2007.tb03022.x [DOI] [PubMed] [Google Scholar]
- 27.Kozicki AR, Robat C, Chun R, Kurzman ID. Adjuvant therapy with carboplatin and pamidronate for canine appendicular osteosarcoma. Vet Comp Oncol. 2015;13: 229–36. 10.1111/vco.12040 [DOI] [PubMed] [Google Scholar]
- 28.Davis LE, Hofmann NE, Li G, Huang ET, Loriaux MM, Bracha S, et al. A case study of personalized therapy for osteosarcoma. Pediatr Blood Cancer. 2013;60: 1313–9. 10.1002/pbc.24512 [DOI] [PubMed] [Google Scholar]
- 29.Chun R, Garrett LD, Henry C, Wall M, Smith A, Azene NM. Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. J Am Anim Hosp Assoc. 2005;41: 382–387. 10.5326/0410382 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Visonneau S, Cesano A, Jeglum KA, Santoli D. Adjuvant treatment of canine osteosarcoma with the human cytotoxic T-cell line TALL-104. Clin Cancer Res. 1999;5: 1868–75. [PubMed] [Google Scholar]
- 31.London CA, Gardner HL, Mathie T, Stingle N, Portela R, Pennell ML, et al. Impact of toceranib/piroxicam/cyclophosphamide maintenance therapy on outcome of dogs with appendicular osteosarcoma following amputation and carboplatin chemotherapy: a multi-institutional study. PLoS One. 2015;10: 1–17. 10.1371/journal.pone.0124889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paoloni MC, Mazcko C, Fox E, Fan T, Lana S, Kisseberth W, et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS One. 2010;5: e11013 10.1371/journal.pone.0011013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, et al. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res. 2016;22: 4380–4390. 10.1158/1078-0432.CCR-16-0088 [DOI] [PubMed] [Google Scholar]
- 34.Phillips B, Powers BE, Dernell WS, Straw RC, Khanna C, Hogge GS, et al. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J Am Anim Hosp Assoc. 2009;45: 33–38. 10.5326/0450033 [DOI] [PubMed] [Google Scholar]
- 35.London C, Mathie T, Stingle N, Clifford C, Haney S, Klein MK, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia) in solid tumours. Vet Comp Oncol. 2012;10: 194–205. 10.1111/j.1476-5829.2011.00275.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogilvie GK, Straw RC, Jameson VJ, Walters LM, Lafferty MH, Powers BE, et al. Evaluation of single-agent chemotherapy for treatment of clinically evident osteosarcoma metastases in dogs: 45 cases (1987–1991). J Am Vet Med Assoc. 1993;202: 304–6. [PubMed] [Google Scholar]
- 37.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15: 5308–16. 10.1158/1078-0432.CCR-07-5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi KS. Autophagy and cancer. Exp Mol Med. 2012;44: 109–120. 10.3858/emm.2012.44.2.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7: 961–967. 10.1038/nrc2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horie R, Nakamura O, Yamagami Y, Mori M, Nishimura H, Fukuoka N, et al. Apoptosis and antitumor effects induced by the combination of an mTOR inhibitor and an autophagy inhibitor in human osteosarcoma MG63 cells. Int J Oncol. 2015; 37–44. 10.3892/ijo.2015.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147: 223–34. 10.1016/j.cell.2011.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen J, Yeo S, Wang C, Chen S, Sun S, Haas MA, et al. Autophagy inhibition re-sensitizes pulse stimulation-selected paclitaxel-resistant triple negative breast cancer cells to chemotherapy-induced apoptosis. Breast Cancer Res Treat. 2015;149: 619–629. 10.1007/s10549-015-3283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vakifahmetoglu-Norberg H, Kim M, Xia H-G, Iwanicki MP, Ofengeim D, Coloff JL, et al. Chaperone-mediated autophagy degrades mutant p53. Genes Dev. 2013;27: 1718–30. 10.1101/gad.220897.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei Y, Kansy BA, Li J, Cong L, Liu Y, Trivedi S, et al. EGFR-targeted mAb therapy modulates autophagy in head and neck squamous cell carcinoma through NLRX1-TUFM protein complex. Oncogene. 2016;35: 4698–707. 10.1038/onc.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JM, Tougeron D, Huang S, Okamoto K, Sinicrope FA. Beclin 1 and UVRAG confer protection from radiation-induced DNA damage and maintain centrosome stability in colorectal cancer cells. PLoS One. 2014;9: 1–11. 10.1371/journal.pone.0100819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shao S, Li S, Qin Y, Wang X, Yang Y, Bai H, et al. Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia. Int J Oncol. 2014; 1661–1668. 10.3892/ijo.2014.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Correa RJM, Valdes YR, Peart TM, Fazio EN, Bertrand M, McGee J, et al. Combination of AKT inhibition with autophagy blockade effectively reduces ascites-derived ovarian cancer cell viability. Carcinogenesis. 2014;35: 1951–1961. 10.1093/carcin/bgu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao D, Yuan H, Yi F, Meng C, Zhu Q. Autophagy prevents doxorubicin-induced apoptosis in osteosarcoma. Mol Med Rep. 2014;9: 1975–1981. 10.3892/mmr.2014.2055 [DOI] [PubMed] [Google Scholar]
- 49.R Core Team. R: A Language and Environment for Statistcal Computing Vienna, Austria: R Foundation for Statistical Computing; 2016. p. https://www.r-project.org/. [Google Scholar]
- 50.Frommolt P. ic50: standardized high-throughput evaluation of cell-based compound screens. 2010. [DOI] [PMC free article] [PubMed]
- 51.Frommolt P, Thomas RK. Standardized high-throughput evaluation of cell-based compound screens. BMC Bioinformatics. 2008;9: 475 10.1186/1471-2105-9-475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fox J, Weisberg S. An {R} Companion to Applied Regression Second. Thousand Oaks, CA: Sage; 2011. [Google Scholar]
- 53.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical J. 2008;50: 346–363. [DOI] [PubMed] [Google Scholar]
- 54.Peters G. userfriendlyscience: quantitative analysis made accesible. 2017. doi:10.17605/OSF.IO/TXEQU
- 55.Dag O, Dolgun A, Konar NM. onewaytests: an R package for one-way tests in independent groups designs. 2017.
- 56.Kimmelman AC. The dynamic nature of autophagy in cancer. Genes Dev. 2011;25: 1999–2010. 10.1101/gad.17558811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez CO. Using canine osteosarcoma as a model to assess efficacy of novel therapies: can old dogs teach us new tricks? Adv Exp Med Biol. 2014;804: 237–56. 10.1007/978-3-319-04843-7_13 [DOI] [PubMed] [Google Scholar]
- 58.Botter SM, Neri D, Fuchs B. Recent advances in osteosarcoma. Curr Opin Pharmacol. 2014;16: 15–23. 10.1016/j.coph.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 59.Barnard RA, Wittenburg LA, Amaravadi RK, Gustafson DL, Thorburn A, Thamm DH. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy. 2014;10: 1415–1425. 10.4161/auto.29165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massimini M, Palmieri C, De Maria R, Romanucci M, Malatesta D, De Martinis M, et al. 17-AAG and apoptosis, autophagy, and mitophagy in canine osteosarcoma cell lines. Vet Pathol. 2017;54: 405–412. 10.1177/0300985816681409 [DOI] [PubMed] [Google Scholar]
- 61.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S, Akemat ZS. Guidelines for use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12: 1–222. 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou S, Zhao L, Kuang M, Zhang B, Liang Z, Yi T, et al. Autophagy in tumorigenesis and cancer therapy: Dr. Jekyll or Mr. Hyde? Cancer Lett. 2012;323: 115–127. 10.1016/j.canlet.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 63.Vlahava V-M, Eliopoulos AG, Sourvinos G. CD40 ligand exhibits a direct antiviral effect on Herpes Simplex Virus type-1 infection via a PI3K-dependent, autophagy-independent mechanism. Cell Signal. 2015;27: 1253–63. 10.1016/j.cellsig.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 64.Chen S, Zhou L, Zhang Y, Leng Y, Pei X-Y, Lin H, et al. Targeting SQSTM1/p62 induces cargo loading failure and converts autophagy to apoptosis via NBK/Bik. Mol Cell Biol. 2014;34: 3435–3449. 10.1128/MCB.01383-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solano JD, González-Sánchez I, Cerbón MA, Guzmán Á, Martínez-Urbina MA, Vilchis-Reyes MA, et al. The products of the reaction between 6-amine-1,3-dimethyl uracil and bis-chalcones induce cytotoxicity with massive vacuolation in HeLa cervical cancer cell line. Eur J Med Chem. 2013;60: 350–9. 10.1016/j.ejmech.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 66.Lee TG, Jeong EH, Kim SY, Kim HR, Kim CH. The combination of irreversible EGFR TKIs and SAHA induces apoptosis and autophagy-mediated cell death to overcome acquired resistance in EGFR T790M-mutated lung cancer. Int J Cancer. 2015;136: 2717–2729. 10.1002/ijc.29320 [DOI] [PubMed] [Google Scholar]
- 67.Lin Han ZX. Upregulation of autophagy by angiotensin II triggers phenotypic switching of aortic vascular smooth muscle cells. J Clin Exp Cardiolog. 2014;5: 308 10.4172/2155-9880.1000308 [DOI] [Google Scholar]
- 68.Liu J, Zhang Y, Liu A, Wang J, Li L, Chen X, et al. Distinct dasatinib-induced mechanisms of apoptotic response and exosome release in imatinib-resistant human chronic myeloid leukemia cells. Int J Mol Sci. 2016;17: 531 10.3390/ijms17040531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu X-C, Wu Y-F, Zhou J-S, Chen H-P, Wang Y, Li Z-Y, et al. Autophagy inhibitors suppress environmental particulate matter-induced airway inflammation. Toxicol Lett. 2017;280: 206–212. 10.1016/j.toxlet.2017.08.081 [DOI] [PubMed] [Google Scholar]
- 70.Xiao J, Feng X, Huang X-Y, Huang Z, Huang Y, Li C, et al. Spautin-1 ameliorates acute pancreatitis via inhibiting impaired autophagy and alleviating calcium overload. Mol Med. 2016;22: 643–652. 10.2119/molmed.2016.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferraresi A, Phadngam S, Morani F, Galetto A, Alabiso O, Chiorino G, et al. Resveratrol inhibits IL-6-induced ovarian cancer cell migration through epigenetic up-regulation of autophagy. Mol Carcinog. 2017;56: 1164–1181. 10.1002/mc.22582 [DOI] [PubMed] [Google Scholar]
- 72.Qiang L, Zhao B, Shah P, Sample A, Yang S, He Y-Y. Autophagy positively regulates DNA damage recognition by nucleotide excision repair. Autophagy. 2016;12: 357–68. 10.1080/15548627.2015.1110667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parks A, Charest-Morin X, Boivin-Welch M, Bouthillier J, Marceau F. Autophagic flux inhibition and lysosomogenesis ensuing cellular capture and retention of the cationic drug quinacrine in murine models. PeerJ. 2015;3: e1314 10.7717/peerj.1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Llewellyn KJ, Nalbandian A, Weiss LN, Chang I, Yu H, Khatib B, et al. Myogenic differentiation of VCP disease- induced pluripotent stem cells: a novel platform for drug discovery. 2017; 1–19. 10.1371/journal.pone.0176919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han L, Schubert C, Köhler J, Schemionek M, Isfort S, Brümmendorf TH, et al. Calreticulin-mutant proteins induce megakaryocytic signaling to transform hematopoietic cells and undergo accelerated degradation and Golgi-mediated secretion. J Hematol Oncol. 2016;9: 45 10.1186/s13045-016-0275-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salabei J, Cummins T, Singh M, Jones S, Bhatnagar A, Hill B. PDGF-mediated autophagy regulates vascular smooth muscle cell phenotype and resistance to oxidative stress. Biochem J. 2014;451: 375–388. 10.1042/BJ20121344.PDGF-mediated [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanahan D, Weinberg R a. Hallmarks of cancer: the next generation. Cell. 2011;144: 646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 78.Chittaranjan S, Bortnik S, Gorski SM. Monitoring autophagic flux by using lysosomal inhibitors and western blotting of endogenous MAP1LC3B. Cold Spring Harb Protoc. 2015;2015: 743–50. 10.1101/pdb.prot086256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell viability measured using a resazurin assay. The solid vertical line denotes the IC50 value, and the dashed vertical lines denote 95% confidence interval (CI) bounds. Error bars denote standard deviation (SD) [CV, coefficient of variation].
(TIFF)
Surviving fraction measured using a colony formation assay.
(TIFF)
Cell viability measured using a resazurin assay. The solid vertical line denotes the IC50 value, and the dashed vertical lines denote 95% confidence interval (CI) bounds. Error bars denote standard deviation (SD) [CV, coefficient of variation].
(TIFF)
Western blot expression of P62 and LC3 (microtubule‑associated protein light chain 3) in metastatic canine osteosarcoma cells treated with spautin-1 (Low = 15 μM, High = 120 μM) or doxorubicin (IC50) for 24 hours each as a single-agent, or both drugs in combination. Beta actin was used as a loading control. Both in the presence and absence of the lysosomal inhibitor HCQ, LC3II expression is reduced with increasing spautin-1 (1 vs 2 vs 3 and 7 vs 8 vs 9), indicative of autophagy inhibition. Both in the presence and absence of HCQ, doxorubicin increases LC3II expression (1 vs 4 and 7 vs 10), indicative of autophagy induction.
(TIF)
(PDF)
(PDF)
Data Availability Statement
Data are available from the University of Guelph Research Data Repository (https://dataverse.scholarsportal.info/dataverse/ugrdr). DOI for the data set is: https://doi.org/10.5683/SP2/ZT4AZV.