Abstract
The phytotoxic potential of the legume shrubs Ulex europaeus L. (gorse) and Cytisus scoparius (L.) Link. (Scotch broom) is studied in this work for the first time. On the basis of their richness in active principles, the previous evidence of biological activity, and the abundance of biomass in their native range and invaded areas, a question arose: can U. europaeus and C. scoparius be considered as potential sources of natural herbicides for sustainable agriculture? By means of volatile bioassays, the flowering fresh plant material of both shrub species was shown to produce and emit volatile organic compounds (VOCs) able to inhibit the germination and/or early growth of two agricultural weeds: Amaranthus retroflexus and Digitaria sanguinalis. Novel complete VOCs profiles from the volatile extracts of the shrub species were obtained by GC and GC/MS. A total of 20 compounds were identified from U. europaeus flowering biomass, theaspirane and eugenol, among others, being described in gorse for the first instance. The chemical profile of C. scoparius yielded 28 compounds and was rich in oxygenated monoterpenes such as terpinen-4-ol, verbenol, α-terpineol, and verbenone, which were also identified in this species for the first time. Using dose-response bioassays with pure compounds, these VOCs were argued to be involved in the phytotoxicity observed for the plant materials, even at very low concentrations. The phytotoxic effects were predominantly irreversible, particularly for D. sanguinalis, since the seeds exposed to the VOCs produced damaged seedlings, were unable to recover germination capacity after removing the phytotoxin or, when recovered, produced unviable seedlings. Our results extend the interest of the abundant U. europaeus and C. scoparius for the obtention of natural products with bioherbicide potential, or to be used as allelopathic biomass in the development of new sustainable agricultural practices.
Introduction
Legume species have major relevance in agriculture and agroforestry worldwide. They are vital components in ecosystems for their role as atmospheric nitrogen fixers through their association with Rhizobium bacteria. From the Fabaceae family, the perennial shrubs Ulex europaeus L. (gorse) and Cytisus scoparius (L.) Link. (Scotch broom) are native to the Atlantic region. Gorse is native to the western coast of continental Europe and the British Isles, whereas Scotch broom is widely distributed all across Europe. The Atlantic shrubland is dominant in the native range [1–3]. Outside their natural distribution range, U. europaeus and C. scoparius are considered highly invasive weeds [4–6]. In fact, gorse is considered one of the 100 worst invasive species in the world [7].
The invasiveness of these species is closely linked to their ability to sprout and regenerate rapidly, the difficulty for their eradication, a high seed production, and their fire-resistance [8, 9]. However, other factors underlying the competitive ability of plants are also key mechanisms to establish successfully, including interference against other species. One of these mechanisms modeled by evolution is allelopathy [10, 11], which has gained significance in recent years for the explanation of plant invasion success. The phenomenon of allelopathy refers to ‘any direct or indirect effect of one plant on other plants through the release of bioactive compounds (named allelochemicals) by volatilization, leaching, exudation from roots or decomposition of plant residues’ [10].
Both gorse and Scotch broom have been appraised from ancient times for their high content in bioactive compounds. Several species of the genus Cytisus have been used in folk medicine ([12, 13] and references therein). In particular, C. scoparius extracts based on organic solvents have shown antifungal [12], antimicrobial [14] and antioxidant activities, e.g., [12, 15]. Also, U. europaeus has been studied for its antioxidant [16] and antifungal [17, 18] properties. However, can we consider these species to be allelopathic? Some recent evidence supports it. Grove et al. [19] argued allelopathy as the possible mechanism of C. scoparius for competing intensively with native vegetation, thus reducing recruitment of seedlings and growth of understory species in open forest areas. Also, López-Nogueira et al. [20] suggested that the legume species from the Atlantic shrubland are highly competitive also in the native range, being able to stop the spread of invasive tree species through allelopathy. The emission of volatile organic compounds (VOCs) from U. europaeus in the different phenological stages throughout the year has been studied by some authors [16, 21, 22] thus demonstrating the continuous release of potentially bioactive compounds from this species. VOCs are in fact allelochemicals produced and emitted by plants that play important roles in biotic interactions such as pollinator attraction or plant defense [23–25]. However, the volatile profiles of gorse and Scotch broom are as yet poorly known.
Allelopathy also plays essential roles in agroecosystems influencing weed growth and crop yield [26, 27]. For this reason, allelopathy and the allelochemicals involved are receiving notable attention as possible sustainable alternatives to the use of synthetic herbicides [28, 29]. Especially, VOCs have excited the greatest interest as natural herbicides [30–32]. They have been described as potent inhibitors of seed germination and growth of several plant species, e.g., [33–35]. However, they continue to be a largely untapped source of active compounds for potential use in agricultural fields. Interestingly, very recent approaches have proposed the use of allelopathic biomass from forest residues or invasive tree species as bioherbicide green manures [36, 37]. Allelopathic biomass incorporated into the soil can release genuine ‘cocktails’ of allelochemicals that could control the germination and growth of different weed species.
Then, considering previous evidence of biological activity, the richness in active principles, and the abundance of shrub biomass in our agroecosystems, a question arises: can U. europaeus, and C. scoparius be considered as potential sources of natural herbicides for sustainable agriculture? As far as we know, the potential phytotoxic effects of gorse and Scotch broom on agricultural weeds have not yet been studied.
To answer this question, the following objectives were proposed: (i) to assess the in vitro phytotoxic potential of volatiles emitted by flowering biomass of each shrub species, on the germination and early growth of two agricultural weed species; (ii) by GC and GC/MS, to determine the chemical profile of the volatile extracts of flowers and flowering branches of both shrub species; (iii) to identify which VOCs are potentially involved in the phytotoxicity observed, by means of dose-response bioassays of isolated compounds on the germination and early growth of the two agricultural weeds; and (iv) to test the reversibility of the effects of the most phytotoxic VOCs.
Materials and methods
Plant material
Flowering branches of U. europaeus and C. scoparius were collected over April and May 2014 at different locations, the first species in Cabo Home (Galicia, NW Spain, 42°16'08.9" N 8°51'38.0" W), and the latter in the vicinity of the University of Vigo (Galicia, NW Spain, 42°09'56.0" N 8°41'04.7" W). No specific permission was required for these locations and plant species, since both gorse and Scotch broom are regularly thinned to clean paths and viewpoints, and they quickly regenerate. Fresh plant material was taken immediately to the laboratory for further processing.
Once in the laboratory, the pool of plant material for each species was separated into two portions: one for in vitro volatile bioassays, and the other one for the extraction and chemical analysis of VOCs. All the volatile bioassays and analyses were carried out for flowering branches and also for flowers alone.
Naturally emitted volatile bioassays
To assess the in vitro phytotoxicity of VOCs emitted by U. europaeus, and C. scoparius, a battery of bioassays was performed after Barney et al. [38]. Plant material was wrapped in a sterile cotton gauze swab (1 mm mesh size) and introduced into a 1-L hermetic glass chamber, hanging from the top for preventing physical contact between seeds of the target species and the donor plant material, but allowing VOCs to flow inside the chamber atmosphere (S1 Fig). Treatments consisted of fresh plant material equivalent to 2 g of dry weight of green flowering branches (flowers, leaves and shoots, and thorns in the case of U. europaeus) or flowers alone [i.e., 6.0 and 7.85 or 6.45 and 12.7 g fw, of flowering green branches and flowers of U. europaeus or C scoparius, respectively]. Control treatment consisted of cotton gauze swab containing pieces of drinking straws at the same volume as fresh plant material.
Amaranthus retroflexus L. (redroot pigweed) and Digitaria sanguinalis (L.) Scop. (large crabgrass) from Herbiseed (Twyford, England, UK) were used as representative dicotyledon and monocotyledon highly competitive weed species [36]. Amaranthus retroflexus seeds were previously synchronized by soaking in distilled water for 15 days at 4°C and then air dried, whereas D. sanguinalis seeds were placed under light for 56 days at 4°C.
For germination bioassays, twenty-five seeds per chamber were placed on a filter paper layer wetted with 4 ml of distilled water; then, the chamber was hermetically closed and incubated at 27°C in the dark. This way, the seeds were continuously exposed to the VOCs emitted by the plant material over the time assayed. The number of germinated seeds (rupture of seed coats and the emergence of radicle ≥1 mm [39]) was counted every 12 h for A. retroflexus, and every 24 h for D. sanguinalis, until no further germination events were observed in the control. The total percentage of germinated seeds (Gt) and the coefficient of the rate of germination (CRG) were calculated after Chiapusio et al. [40] and De Bertoldi et al. [41]. For early growth bioassays, fifteen pre-germinated seeds (root length between 1 and 3 mm [39]) were used under the same conditions as for germination bioassays. Root and shoot lengths of the pre-germinated seeds were measured after 48 h, and a morpho-anatomical description of the seedlings was made by using a Nikon SMZ 1500 electronic magnifier (Nikon, Melville, NY, USA). Then, root and shoot biomass per chamber were obtained by drying the fresh material at 70°C for 72 h. For each treatment and target species, four replicates were carried out.
Extraction and chemical analyses of VOCs
Volatile extracts from flowering branches or flowers of U. europaeus or C. scoparius were obtained by continuous water distillation/solvent extraction for four h, using a Likens-Nickerson type apparatus [42] and n-pentane as solvent [43].
Gas chromatography and gas chromatography/mass spectrometry analyses
Analytical GC was carried out in a Hewlett-Packard 6890 (Agilent Technologies, Palo Alto, CA, USA) gas chromatograph with an HP GC ChemStation Rev. A.05.04 data handling system equipped with a single injector and two flame ionization detection (FID) system. A GraphPad divider (Agilent Technologies, part no. 5021–7148) was used for simultaneous sampling to two Supelco (Supelco, Bellefonte, PA, USA) fused silica capillary columns with different stationary phases: SPB-1 (polydimethylsiloxane 30 m × 0.20 mm i.d., film thickness 0.20 μm), and SupelcoWax-10 (polyethylene glycol 30 m × 0.20 mm i.d., film thickness 0.20 μm). Oven temperature program: 70–220°C (3°C min-1), 220°C (15 min); injector temperature: 250°C; carrier gas: helium, adjusted to a linear velocity of 30 cm s-1; splitting ratio 1:40; detectors temperature: 250°C. GC/MS was carried out in a Hewlett-Packard 6890 gas chromatograph fitted with an HP1 fused silica column (polydimethylsiloxane 30 m × 0.25 mm i.d., film thickness 0.25 μm), interfaced with a Hewlett-Packard mass selective detector 5973 (Agilent Technologies) operated by HP Enhanced ChemStation software version A.03.00.GC, with parameters as described above; interface temperature: 250°C; MS source temperature: 230°C; MS quadrupole temperature: 150°C; ionization energy: 70 eV; ionization current: 60 μA; scan range: 35–350 units; scans s-1: 4.51.
Identification of VOCs from flowering branches and flowers of gorse and Scotch broom
Compounds were identified by their GC retention indices on both SPB-1 and SupelcoWax-10 columns and from their mass spectra. Retention indices, calculated by linear interpolation relative to retention times of C8–C23 of n-alkanes, were compared with those of reference samples included in our laboratory database (C.E.F. / Faculty of Pharmacy, University of Coimbra). Acquired mass spectra were compared with reference spectra from the laboratory database, Wiley / NIST library [44] and literature data [45, 46]. Relative amounts of individual components were calculated based on GC raw data areas without FID response factor correction.
Phytotoxicity dose-response bioassays of some identified isolated VOCs
Twelve volatile compounds present in the chemical profiles of the flowering branches and the flowers of U. europaeus and C. scoparius were selected by (i) previous evidence of phytotoxicity in the literature, (ii) the commercial availability, and (iii) their abundance in the analyzed plant material. Four oxygenated monoterpenes (linalool, terpinen-4-ol, α-terpineol and verbenone), one benzenoid compound (eugenol), one oxygenated norisoprenoid (theaspirane) and six aliphatic compounds (n-nonadecene, n-eicosane, n-heneicosane, n-docosane, n-tricosane and n-tetracosane) were tested on the germination and early growth of A. retroflexus, and D. sanguinalis.
Chemicals were purchased from Sigma–Aldrich Chemical (St. Louis, MO, USA) and used without further purification. For dose-response bioassays, a filter paper strip was fixed to the top lid of the plate, and the corresponding quantity of compound was added with a micropipette; in this way, the compound was only in aerial contact with the target seeds [34]. Each compound was tested at 0, 0.5, 1, 1.5 and 2 μl corresponding to concentrations of 0, 6.25, 12.5, 18.75 and 25 ppm in the sealed Petri dish atmosphere, respectively. The solid aliphatic compounds were dissolved in pentane for their use; dose calculations were done basing on their density values, ranging between 0.778 and 0.797g·ml-1, and an average density of 0.79 g·ml-1 was assumed. For these aliphatic compounds, the corresponding volume of pentane was used for the control treatment. Pentane was let to evaporate before closing the Petri dish.
Germination and early growth bioassays were performed in Petri dishes (9 cm diameter) sealed with Parafilm under the same conditions described for the in vitro volatile bioassays. For each compound, concentration and target species, four replicates were carried out.
Reversibility bioassays of the phytotoxic isolated VOCs
The viability of the non-germinated seeds resulting from the phytotoxicity dose-response bioassays was tested. Those compounds that inhibited the germination of at least ten target seeds per replicate were selected. Non-germinated seeds were incubated in 6-well dishes, at a rate of 10 seeds per well placed on a filter paper layer wetted with 750 μl of distilled water. Ten non-pretreated seeds for each species were used as control treatment. Seeds were incubated under the same conditions described for the dose-response bioassays. The total percentage of germinated seeds (Gt) was calculated after 20 days, and the morpho-anatomical description of the obtained seedlings was made as described above.
Statistical analyses
Replicated experiments were conducted in a completely randomized design. Data were expressed as percentages relative to the control. After testing for normality by Kolmogorov-Smirnov test and for homogeneity of variances by Levene’s test, data were analyzed by one-way ANOVA (P ≤ 0.05) and LSD test (P = 0.05) for post hoc multiple comparisons of means. In the case of heteroscedasticity, the variance was analyzed by Kruskal-Wallis H test and Tamhane´s T2 for post hoc multiple comparisons. For the dose-response bioassays of identified isolated VOCs, two-way ANOVA was previously used to test the effects of the independent variables (compound and concentration) and their interactions (compound x concentration) on each measured parameter. Dose-response curves were modeled by regression analysis with mathematical models, and the most appropriate model was selected for each case after the adjusted coefficient of determination (r2adj). IC50 and IC80 values (concentrations required to obtain 50 and 80% inhibition, respectively) were calculated from the generated dose-response curves, because of their usefulness to compare phytotoxicity among compounds [47]. Statistical analyses were performed using the SPSS v.19 (IBM SPSS Inc., Chicago, IL, USA) software for Windows.
Results
Naturally emitted volatile bioassays
The flowering branches and the flowers of both shrub species produced conspicuous phytotoxic effects on the target weed species.
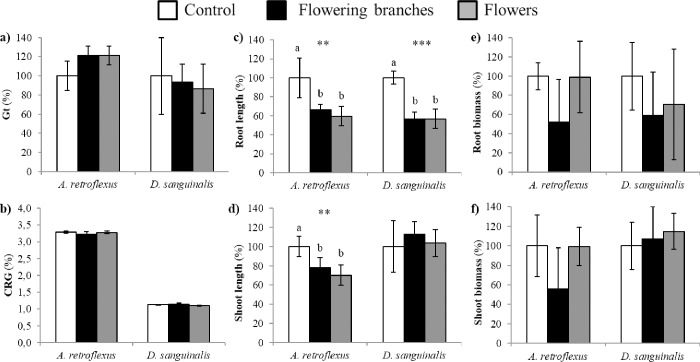
The phytotoxic effects of volatiles naturally emitted from U. europaeus fresh material are represented in Fig 1. From the analysis of variance, root and shoot lengths of A. retroflexus were very significantly affected (P ≤ 0.01) by the VOCs emitted from the flowering branches and the flowers of U. europaeus, thus achieving inhibitions up to ca. 40% (Fig 1C) and ca. 30% (Fig 1D) of control, respectively. In the case of D. sanguinalis, only root length was affected, with reductions about 45% of control (Fig 1C). No significant effects were observed on the total germination, CRG, or root and shoot biomass.
Fig 1. Effects of Ulex europaeus volatiles on the germination, growth, and biomass of two agricultural weed species (Amaranthus retroflexus and Digitaria sanguinalis).
a) Total germination (Gt) b) CRG index c) root length d) shoot length e) root biomass and f) shoot biomass after the exposure to VOCs released from flowering branches and flowers of U. europaeus. Mean values are represented as percentages relative to the control. Error bars represent standard deviation (SD). For each target weed species, asterisks denote statistically significant effects of treatments at *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 (ANOVA or Kruskal-Wallis H test). Mean values labeled with distinct letters are significantly different at P ≤ 0.05 (post-hoc LSD or Tamhane`s T2 test).
Volatiles emitted from the fresh flowers of C. scoparius (Fig 2) significantly reduced A. retroflexus root length to 41% (P ≤ 0.01, Fig 2C), whereas shoot length was inhibited 50% by both flowering branches and flowers (P ≤ 0.001, Fig 2D); nonetheless, germination was not significantly affected. Otherwise, total germination of D. sanguinalis was reduced by 50% with respect to control (P ≤ 0.05, Fig 2A). D. sanguinalis also suffered inhibitions of root growth (P ≤ 0.001, Fig 2C) and biomass (P ≤ 0.01, Fig 2E) higher than 40% by both treatments, whereas shoot length was only significantly reduced to 32% by the flowers (P ≤ 0.05, Fig 2D). No significant effects were observed on the CRG.
Fig 2. Effects of Cytisus scoparius volatiles on the germination, growth, and biomass of two agricultural weed species (Amaranthus retroflexus and Digitaria sanguinalis).
a) Total germination (Gt) b) CRG index c) root length d) shoot length e) root biomass and f) shoot biomass after the exposure to VOCs released from flowering branches and flowers of U. europaeus. Mean values are represented as percentages relative to the control. Error bars represent standard deviation (SD). For each target weed species, asterisks denote statistically significant effects of treatments at *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 (ANOVA or Kruskal-Wallis H test). Mean values labeled with distinct letters are significantly different at P ≤ 0.05 (post-hoc LSD or Tamhane`s T2 test).
Identification of VOCs from flowering branches and flowers of gorse and Scotch broom
The extraction of volatile compounds from fresh plant material of the shrub species rendered a yellowish liquid with fresh, light liquorice odour, obtained at a mean yield of 0.06% (w/w, on a fresh mass basis) with a mean density of 0.85 g mL-1. Table 1 summarizes the results obtained from the GC and GC/MS analyses of the volatile extracts (from flowering branches or flowers) of U. europaeus and C. scoparius.
Table 1. Volatile organic compounds identified by GC and GC/MS from the volatile extracts of flowering branches and flowers of Ulex europaeus and Cytisus scoparius.
Data are expressed as percentages of the total yield of the extract.
| Ulex europaeus | Cytisus scoparius | |||||
|---|---|---|---|---|---|---|
| IRa | IRb | Compound | Flowering branches | Flowers | Flowering branches | Flowers |
| Monoterpene hydrocarbons | ||||||
| 929 | 1030 | α-pinene | 0.23 | |||
| 1010 | 1187 | α-terpinene | 0.57 | 0.62 | ||
| 1046 | 1249 | γ-terpinene | 1.04 | 1.17 | ||
| Oxygenated monoterpenes | ||||||
| 1148 | 1496 | isomenthone | 0.32 | |||
| 1082 | 1543 | linalool | 3.08 | |||
| 1158 | 1597 | terpinen-4-ol | 2.66 | 4.30 | ||
| 1122 | 1648 | verbenol | 1.58 | 1.50 | ||
| 1169 | 1692 | α-terpineol | 1.23 | |||
| 1177 | 1698 | verbenone | 2.07 | 4.09 | ||
| 1142 | 1723 | p-menth-1,5-diene-8-ol | 0.58 | |||
| Sesquiterpene hydrocarbons | ||||||
| 1510 | 1763 | β-sesquiphellandrene | 0.94 | |||
| Oxygenated sesquiterpenes | ||||||
| 1545 | 2039 | E-nerolidol | 0.45 | 0.70 | ||
| 1828 | 2126 | 6,10,14-trimethylpentadecanone | 0.52 | 0.74 | 0.86 | |
| Oxygenated diterpenes | ||||||
| 2096 | n.d. | phytol | 1.85 | 0.92 | 1.90 | |
| Oxygenated norisoprenoids | ||||||
| 1298 | 1487 | theaspirane A | 0.24 | |||
| 1315 | 1522 | theaspirane B | 0.30 | |||
| Benzenoid compounds | ||||||
| 1339 | 2159 | eugenol | 0.21 | |||
| n.d. | 2190 | 4-vinyl-2-methoxyphenol | 0.22 | |||
| Aliphatic compounds | ||||||
| 1084 | 1393 | nonanal | 0.29 | |||
| 959 | 1442 | 1-octen-3-ol | 3.37 | 0.91 | 8.34 | |
| n.d. | 1707 | 8-heptadecene | 0.79 | |||
| 1900 | 1900 | n-nonadecane | 1.50 | 1.04 | ||
| 2000 | 2000 | n-eicosane | 1.69 | 1.47 | ||
| 1697 | 2022 | 2-pentadecanone | 1.18 | |||
| 2100 | 2100 | n-heneicosane | 2.46 | 3.06 | 14.75 | 14.28 |
| 2200 | 2200 | n-docosane | 0.58 | 1.28 | 3.43 | 2.90 |
| n.d. | n.d. | 2-heptadecanone | 0.76 | |||
| 2300 | 2300 | n-tricosane | 21.29 | 30.01 | 23.02 | 29.56 |
| 2400 | 2400 | n-tetracosane | 2.50 | 3.99 | 1.54 | |
| n.d. | n.d. | lauric acid | 1.57 | 1.10 | 1.66 | |
| 2500 | 2500 | n-pentacosane | 12.80 | 6.03 | 8.71 | |
| 2600 | 2600 | n-hexacosane | 16.81 | 8.50 | 8.26 | |
| n.d. | n.d. | myristic acid | 3.62 | 8.82 | 1.49 | 0.83 |
| 2700 | 2700 | n-heptacosane | 11.37 | 9.47 | 7.94 | |
| n.d. | n.d. | palmitic acid | 13.25 | 12.59 | 5.99 | 2.84 |
| Grouped components (%) | ||||||
| Monoterpene hydrocarbons | 1.61 | 2.02 | ||||
| Oxygenated monoterpenes | 0.32 | 10.62 | 10.47 | |||
| Sesquiterpene hydrocarbons | 0.94 | |||||
| Oxygenated sesquiterpenes | 0.97 | 1.44 | 0.86 | |||
| Oxygenated diterpenes | 1.85 | 0.92 | 1.90 | |||
| Oxygenated norisoprenoids | 0.54 | |||||
| Benzenoid compounds | 0.43 | |||||
| Aliphatic compounds | 89.91 | 61.76 | 85.87 | 82.1 | ||
| Total identified | 94.02 | 64.12 | 100 | 96.39 | ||
IRa retention index on SPB-1 column
IRb retention index on a Supelcowax-10 column.
A total of 20 compounds representing 94.02% of the flowering branches volatile extract, and 11 compounds representing 64.12% of the flowers extract were identified in U. europaeus. Both extracts were qualitatively and quantitatively dominated by aliphatic compounds, n-tricosane being the most abundant. No monoterpene and sesquiterpene hydrocarbons were found, but some oxygenated terpenes of different complexity (e.g., isomenthone, E-nerolidol, phytol), oxygenated norisoprenoids (theaspirane), and benzenoids (eugenol, 4-vinyl-2-methoxyphenol) were identified.
The volatile extracts from the flowering branches and flowers of C. scoparius revealed the presence of 20 and 23 compounds constituting 100% and 96.39% of the total identified compounds, respectively (Table 1). These volatile extracts were also abundant in aliphatic compounds, thus representing more than 80% of the total composition, n-tricosane being the most abundant in both extracts (23.02% for the flowering branches and 29.56% for the flowers). The volatile extracts of Scotch broom also contained oxygenated monoterpenes representing ca. 10% of the total. Linalool, terpinen-4-ol, verbenol, α-terpineol, and verbenone were found in the flowering branches, linalool being the most abundant (3.08%) followed by terpinen-4-ol (2.66%) and verbenone (2.07%). From the flowers volatile extract, terpinen-4-ol, verbenol, verbenone, and p-menth-1,5-diene-8-ol were identified, with terpinen-4-ol (4.3%) and verbenone (4.09%) as majority compounds. Other identified compounds were β-sesquiphellandrene, 6,10,14-trimethylpentadecanone, phytol, and the monoterpene hydrocarbons α-pinene, α-terpinene, and γ-terpinene.
Phytotoxicity dose-response bioassays of identified isolated VOCs
From the twelve compounds primarily selected to be tested for their phytotoxic potential on the germination of the target weed species, the analysis of variance did not yield statistically significant effects of the aliphatic compounds at their different concentrations including 0 ppm, or any significant interaction between compound and concentration (S1 and S2 Tables). Concomitantly, no visual phytotoxic effects were observed on the roots and shoots of the germinated seeds. Then, aliphatic compounds were discarded for further analysis.
All the non-aliphatic compounds assayed produced conspicuous phytotoxic effects on the target weed species. For the two target species and for all compounds, the treated pre-germinated seeds produced seedlings with curved-yellowish roots, necrotic root tips, malformed calyptra, and/or abnormal shoot growth.
The two-way ANOVA of the effects of the oxygenated monoterpenes (linalool, terpinen-4-ol, α-terpineol, and verbenone), the benzenoid eugenol, and the oxygenated norisoprenoid theaspirane, on the germination, root and shoot length and biomass of A. retroflexus and D. sanguinalis is shown in Table 2. The statistical analyses yielded general highly significant differences (P ≤ 0.001) among compounds and their concentrations and, with the exception A. retroflexus root biomass and D. sanguinalis shoot biomass, significant inter-subject effects (compound × concentration) were also observed.
Table 2. P-values obtained for the two-way ANOVA of the effects of six VOCs: Linalool, terpinen-4-ol, α-terpineol, verbenone, eugenol and theaspirane, the concentration assayed, and their interactions, on the germination and early growth of two agricultural weed species.
| Compound | Concentration | Compound × Concentration | ||
|---|---|---|---|---|
| Amaranthus retroflexus | Germination | 0.000 | 0.000 | 0.000 |
| CRG index | 0.000 | 0.000 | 0.000 | |
| Root length | 0.000 | 0.000 | 0.000 | |
| Shoot length | 0.000 | 0.000 | 0.000 | |
| Root biomass | 0.000 | 0.000 | 0.372 | |
| Shoot biomass | 0.000 | 0.000 | 0.013 | |
| Digitaria sanguinalis | Germination | 0.000 | 0.000 | 0.000 |
| CRG index | 0.000 | 0.000 | 0.000 | |
| Root length | 0.000 | 0.000 | 0.000 | |
| Shoot length | 0.000 | 0.000 | 0.000 | |
| Root biomass | 0.000 | 0.000 | 0.000 | |
| Shoot biomass | 0.000 | 0.000 | 0.180 |
Effects of the independent variables are considered significant at P ≤ 0.05, very significant at P ≤ 0.01, highly significant at P ≤ 0.001, and not significant at P > 0.05.
The best-fit equation based on the value of the adjusted coefficient of determination (r2adj), and the IC50 and IC80 values (in ppm) obtained from the dose-response curves (S2 and S3 Figs) are shown in Tables 3 and 4. The obtained r2adj values, generally above 0.850, indicated the adequacy of the models to describe the tendency of variables and the response to different concentrations of the tested compounds, with IC50 and IC80 values mainly in the range of the concentrations assayed.
Table 3. Regression analyses of the dose-response effects of six VOCs on the germination and early growth of Amaranthus retroflexus.
| Regression equation | r2adj | IC 50 (ppm) | IC 80 (ppm) | ||
|---|---|---|---|---|---|
| Linalool | Germination | y = -3.662x + 102.0 | 0.915 | 14.20 | 22.39 |
| Root length | y = 0.091x2–5.685x + 102.7 | 0.980 | 11.32 | 23.06 | |
| Shoot length | y = 0.138x2–6.808x + 102.3 | 0.987 | 9.52 | 21.19 | |
| Root biomass | y = 0.092x2–5.313x + 99.09 | 0.993 | 11.55 | o.r. | |
| Shoot biomass | y = 0.123x2–6.176x + 99.82 | 0.956 | 10.10 | o.r. | |
| Terpinen-4-ol | Germination | y = -2.231x + 104.0 | 0.908 | 24.20 | 37.65 |
| Root length | y = -2.408x + 108.0 | 0.687 | 24.09 | 36.54 | |
| Shoot length | y = -1.496x + 93.84 | 0.680 | 29.30 | 49.36 | |
| Root biomass | y = 0.039x2–3.601x + 106.9 | 0.804 | 20.24 | o.r. | |
| Shoot biomass | y = -1.642x + 91.55 | 0.683 | 25.30 | 43.57 | |
| α-Terpineol | Germination | y = -4.354x + 102.4 | 0.924 | 12.03 | 18.92 |
| Root length | y = -3.450x + 105.2 | 0.832 | 16.00 | 24.69 | |
| Shoot length | y = 0.092x2–5.836x + 105.2 | 0.928 | 11.57 | 22.78 | |
| Root biomass | y = -3.438x + 99.54 | 0.901 | 14.40 | 23.13 | |
| Shoot biomass | y = 0.105x2–6.195x + 103.7 | 0.961 | 10.56 | 20.95 | |
| Verbenone | Germination | y = 0.358x2–12.08x + 89.69 | 0.837 | 3.69 | 7.39 |
| Root length | y = 0.123x2–6.308x + 100.2 | 0.999 | 9.85 | 23.30 | |
| Shoot length | y = 0.166x2–7.479x + 98.88 | 0.996 | 7.93 | 16.84 | |
| Root biomass | y = -3.808x + 98.06 | 0.972 | 12.62 | 20.50 | |
| Shoot biomass | y = 0.158x2–7.321x + 97.43 | 0.984 | 7.79 | 16.34 | |
| Eugenol | Germination | y = -0.074x2–1.262x + 106.4 | 0.787 | 20.37 | 26.69 |
| Root length | y = 0.108x2–5.281x + 99.46 | 0.991 | 12.63 | o.r. | |
| Shoot length | y = 0.207x2–7.976x + 96.09 | 0.956 | 7.08 | 17.37 | |
| Root biomass | y = -0.022x2–1.725x + 98.26 | 0.972 | 21.87 | 32.17 | |
| Shoot biomass | y = 0.185x2–7.515x + 96.42 | 0.952 | 7.60 | o.r. | |
| Theaspirane | Germination | y = -0.064x2 + 1.956x + 103.9 | 0.336 | 48.08 | 54.58 |
| Root length | y = -0.015x2–1.077x + 105.1 | 0.617 | 34.57 | 47.54 | |
| Shoot length | y = 0.018x2–2.221x + 103.5 | 0.825 | o.r. | o.r. | |
| Root biomass | y = 0.028x2–2.530x + 105.7 | 0.795 | 32.82 | o.r. | |
| Shoot biomass | y = 0.027x2–3.039x + 107.5 | 0.703 | 24.07 | o.r. |
r2 adj = adjusted coefficient of determination
IC50 = concentration that inhibits or reduces germination and growth at 50% of control
IC80 = concentration that inhibits or reduces germination and growth at 80% of control
o.r. = out of range
IC values calculated from equation overcoming the maximum assayed concentration are expressed in italics
Table 4. Regression analyses of the dose-response effects of six VOCs on the germination and early growth of Digitaria sanguinalis.
| Regression equation | r2adj | IC 50 (ppm) | IC 80 (ppm) | ||
|---|---|---|---|---|---|
| Linalool | Germination | y = -3.294x + 106.8 | 0.888 | 17.25 | 26.35 |
| Root length | y = 0.122x2–6.633x + 100.3 | 0.999 | 9.11 | 18.20 | |
| Shoot length | y = 0.210x2–8.791x + 95.02 | 0.952 | 5.97 | 11.94 | |
| Root biomass | y = -3.495x + 94.49 | 0.965 | 12.73 | 21.31 | |
| Shoot biomass | y = 0.194x2–8.331x + 93.95 | 0.925 | 6.16 | 12.54 | |
| Terpinen-4-ol | Germination | y = 0.086x2–3.748x + 98.71 | 0.796 | o.r. | o.r. |
| Root length | y = -3.088x + 104.5 | 0.935 | 17.65 | 27.36 | |
| Shoot length | y = -3.424x + 95.40 | 0.935 | 13.26 | 22.02 | |
| Root biomass | y = -0.090x2–0.788x + 105.7 | 0.884 | 20.88 | 26.79 | |
| Shoot biomass | y = -3.264x + 92.69 | 0.928 | 13.08 | 22.27 | |
| α-Terpineol | Germination | y = 0.150x2–7.436x + 101.6 | 0.987 | 8.34 | 16.40 |
| Root length | y = 0.128x2–5.713x + 102.2 | 0.931 | 12.82 | o.r. | |
| Shoot length | y = 0.098x2–5.948x + 100.3 | 0.996 | 10.16 | 20.27 | |
| Root biomass | y = 0.110x2–5.613x + 103.4 | 0.951 | 12.65 | o.r. | |
| Shoot biomass | y = 0.093x2–5.769x + 100.6 | 0.996 | 10.57 | 21.25 | |
| Verbenone | Germination | y = -2.126x + 92.09 | 0.715 | 19.80 | 33.90 |
| Root length | y = 0.157x2–7.079x + 100.7 | 0.976 | 8.93 | o.r. | |
| Shoot length | y = 0.196x2–8.447x + 97.98 | 0.977 | 6.73 | 13.39 | |
| Root biomass | y = 0.104x2–5.830x + 103.3 | 0.957 | 11.50 | o.r. | |
| Shoot biomass | y = 0.185x2–8.192x + 96.93 | 0.968 | 6.76 | 13.52 | |
| Eugenol | Germination | y = -0.101x2–0.705x + 99.09 | 0.993 | 18.83 | 24.71 |
| Root length | y = 0.239x2–8.824x + 93.25 | 0.887 | 5.82 | 12.60 | |
| Shoot length | y = 0.295x2–10.62x + 91.89 | 0.877 | 4.51 | 9.04 | |
| Root biomass | y = 0.212x2–8.186x + 97.39 | 0.979 | 7.09 | 16.53 | |
| Shoot biomass | y = 0.277x2–10.38x + 94.46 | 0.944 | 4.93 | 9.67 | |
| Theaspirane | Germination | y = 0.007x2–0.754x + 97.93 | 0.660 | o.r. | o.r. |
| Root length | y = -0.123x2 + 3.640x + 102.4 | 0.668 | 40.19 | 44.61 | |
| Shoot length | y = 0.123x2–5.415x + 97.92 | 0.977 | 12.27 | o.r. | |
| Root biomass | y = -0.073x2 + 2.129x + 105.3 | 0.101 | 45.73 | 51.75 | |
| Shoot biomass | y = 0.116x2–5.231x + 98.71 | 0.980 | 13.14 | o.r. |
r2 adj = adjusted coefficient of determination
IC50 = concentration that inhibits or reduces germination and growth at 50% of control
IC80 = concentration that inhibits or reduces germination and growth at 80% of control
o.r. = out of range
IC values calculated from equation overcoming the maximum assayed concentration are expressed in italics
In the case of A. retroflexus (Table 3), all the assayed compounds except theaspirane caused at least 50% inhibition of germination in the range of concentrations assayed, with verbenone achieving the lowest IC50 and IC80 values of 3.69 and 7.39 ppm, respectively. This compound, together with linalool and α-terpineol, also caused intense growth reductions below 25 ppm, the shoots being more sensitive than the roots.
The germination of D. sanguinalis was inhibited 50% by linalool, α-terpineol, verbenone, and eugenol (Table 4). The monoterpene α-terpineol was the most phytotoxic to D. sanguinalis, being able to inhibit germination 50% and 80% at 8.34 and 16.40 ppm in the Petri dish atmosphere, respectively. Eugenol, followed by linalool and verbenone were the most effective compounds in reducing seedling growth and biomass.
Reversibility bioassays of the phytotoxic isolated VOCs
From the results obtained in the previous dose-response bioassays, linalool, terpinen-4-ol, α-terpineol, verbenone, and eugenol were assayed for reversibility at those concentrations that achieved a minimum of ten non-germinated seeds per replicate. The results of total germination of these pre-treated seeds incubated for 20 days in distilled water are represented in Table 5. Germination values are expressed as percentages relative to the control (non-pretreated seeds).
Table 5. Reversibility of the phytotoxic effects on the germination of two agricultural weed species pre-treated with different VOCs and then transferred to water.
Data are expressed as percentages relative to the control ± SD.
| Compound | Pre-treated concentration (ppm) |
Germination (% ± S.D.) | |
|---|---|---|---|
| Amaranthus retroflexus | Digitaria sanguinalis | ||
| Linalool |
6.25 | # | # |
| 12.5 | # | # | |
| 18.75 | 60.0 ± 14.1 | 2.6 ± 5.3 | |
| 25 | 67.5 ± 20.6 | 7.9 ± 10.1 | |
| Terpinen-4-ol | 6.25 | # | # |
| 12.5 | # | # | |
| 18.75 | 60.0 ± 8.2 | 5.3 ± 10.5 | |
| 25 | 45.0 ± 36.9 | 13.2 ± 13.2 | |
| α-Terpineol | 6.25 | # | 26.3 ± 13.6 |
| 12.5 | 60.0 ± 24.5 | 18.4 ± 17.9 | |
| 18.75 | 57.5 ± 20.6 | 7.9 ± 10.1 | |
| 25 | 62.5 ± 12.6 | 10.5 ± 14.9 | |
| Verbenone | 6.25 | 52.0 ± 25.0 | # |
| 12.5 | 42.5 ± 25.0 | 42.1 ± 14.9 | |
| 18.75 | 50.0 ± 16.3 | 13.2 ± 5.3 | |
| 25 | 40.0 ± 8.2 | 10.5 ± 8.6 | |
| Eugenol | 6.25 | # | # |
| 12.5 | # | # | |
| 18.75 | 78.9 ± 6.1 | 10.5 ± 8.6 | |
| 25 | 97.4 ± 10.1 | 5.3 ± 6.1 | |
# Concentrations for which the minimum number of ten non-germinated seeds for the reversibility bioassay was not achieved
In the case of A. retroflexus, only the seeds pre-treated with verbenone or terpinen-4-ol at 25 ppm showed reversibility values below 50% after being transferred to distilled water, the effects of verbenone being the most persistent.
In contrast, the phytotoxic effects of the isolated VOCs were in general highly persistent on D. sanguinalis seeds. For all the VOCs, the germination percentages of D. sanguinalis seeds pre-treated with 18.75 and 25 ppm scored the lowest values of the bioassay, ranging between 2.6 and 13.2%.
For both target species and all compounds, the seeds that recovered the germination capacity produced seedlings with curved-yellowish roots, necrotic root tips, malformed calyptra, and/or prostrated shoots.
Discussion
Following the methodology of Barney et al. [38], other authors have demonstrated the phytotoxic properties of different plant species (e.g., Artemisia vulgaris, Calamintha nepeta, Acacia longifolia) through the natural emission of volatile organic compounds in a closed atmosphere, e.g., [38, 48, 49]. However, the bioherbicide potential of the VOCs emitted by the aerial biomass of U. europaeus and C. scoparius is reported here for the first time. From our results, the flowering fresh plant material of both species can produce and emit volatile compounds able to inhibit at different extend the germination and/or early growth of two weed species. Both flowering branches and flowers of U. europaeus resulted phytotoxic to A. retroflexus and D. sanguinalis root growth and also reduced A. retroflexus shoot growth. Moreover, C. scoparius was able to inhibit significantly D. sanguinalis germination, and early growth of both target weeds, also exerting significant reduction of D. sanguinalis root biomass. The equivalent biomass quantity on a dry mass basis, composed of only flowers or flowering green branches, was able to produce similar phytotoxic effects, thus pointing out the potential of both species to produce and emit bioactive VOCs from the total aerial biomass. These results extend the interest of both shrub species as sources of natural products with bioherbicide potential, or to be used as bioherbicide green manures, as has been proposed for other allelopathic biomass-rich invasive species [36, 37].
The volatile profiles obtained by GC and GC/MS from both species and plant parts (flowering branches and flowers) gave us the qualitative and quantitative composition of VOCs that could be potentially released from them. Of course, the presence of a given compound in the volatile extract does not guarantee its natural release but reflects the potential for VOCs synthesis. In this sense, Cao et al. [21] and Boissard et al. [22] measured the emission fluxes of VOCs from U. europaeus as well as their seasonal variations. They found that branches from U. europaeus from England were large emitters of isoprene and the monoterpenes trans-ocimene and α-pinene, mainly over the hottest months from June to September, besides others such as camphene, sabinene, β-pinene, myrcene, limonene, and γ-terpinene to a lesser extent. In contrast, from our chemical profile of the volatile extract of U. europaeus from NW Spain gathered over spring blossom (April and May), just one monoterpene, the limonene derivative isomenthone, was detected. Otherwise, other not previously reported compounds were identified, i.e., two sesquiterpenes (E-nerolidol and 6, 10, 14-trimethylpentadecanone), one diterpene (phytol), two norisoprenoids (theaspirane A and B), and two benzenoids (eugenol and 4-vinyl-2-methoxyphenol). However, 89.91 and 61.12 percent of the volatile extract of flowering branches and flowers, respectively, was composed of aliphatic compounds. Of course, the environmental conditions such as different climate, geographical location, soil type, seasonality, phenological stage, and stress can underlie the different chemical compositions found in U. europaeus. It may also be possible that part of the VOCs from U. europaeus is largely emitted by the living plant, but otherwise completely or partially lost during harvesting, transportation, or processing. Moreover, they could be present but undetected, since 36% of the components were not identified in the flower volatile extract.
Because of their role in pollinator attraction, flowers are prone to be especially rich in allelochemicals. Cao et al. [21] found more than two-fold VOCs emission from flowering branches of U. europaeus than for branches without flowers, whereas López-Hortas et al. [16] found that the phenolic and volatile contents of flowers were higher than those in any other part of the plant. In our case, phytol, E-nerolidol, and 6, 10, 14-trimethylpentadecanone, where the main terpenoid constituents of the volatile extract of U. europaeus flowers. As this species cannot set seeds in the absence of active pollination [50], it is possible that some of those three terpenoids (i.e., E-nerolidol [51]) together with the other abundant aliphatic compounds found in the flowers extract, may play a role in pollinators attraction in U. europaeus, as has been discussed for the essence of flowers of other species [52] including Leguminous [53]. Nonetheless, we identified other VOCs in the extract of flowering branches of U. europaeus which were not present in the flower extract, i.e., isomenthone, theaspirane, eugenol, 4-vinyl-2-methoxyphenol, and certain aliphatic compounds. These compounds produced by vegetative parts (shoots, foliage or leaf spines) have been argued to play a bioactive role in plant defense [54, 55]. If naturally emitted, these VOCs should also be responsible for the phytotoxic effects on weeds observed in the volatile bioassays with flowering branches, since some of them have reputed bioherbicide effects (e.g., eugenol, [56, 57]) or have been isolated from phytotoxic essential oils [48].
The richness of active principles of C. scoparius and other species of the genus Cytisus is well known, and their wide traditional pharmacological uses ‘support the further application and exploitation for new drug development’ [12]. However, differently from other groups of natural compounds (i.e., alkaloids, phenolic acids, flavones, flavonols, and isoflavones), only one previous report dealt with the analysis of the volatile profile of C. scoparius flowers [58]. Kurihana and Kikuchi [58] detected a discrete number of VOCs by GC/MS in the essential oil of fresh flowers of C. scoparius from Japan. Some of them, as 1-octen-3-ol, palmitic, lauric and myristic acids, and linalool, also occurred in our chemical profile; others like isovaleranol, guaiacol, benzoic acid, cresols, and eugenol were not detected in our volatile extract. In our case, the flowering branches and flowers of C. scoparius from NW Spain yielded a high percentage of aliphatic compounds (>82%) and were rich in monoterpenes, oxygenated monoterpenes being quite abundant (>10%). Here, the monoterpene hydrocarbons α-pinene, α-terpinene, γ-terpinene, and the oxygenated monoterpenes terpinen-4-ol, verbenol, α-terpineol, verbenone, and p-menth-1,5-diene-8-ol were identified for the first time in C. scoparius.
Whereas U. europaeus presents some plasticity to regenerate vegetatively [6, 50], C. scoparius lacks the capacity for vegetative reproduction. So, since Scotch broom is an obligate outcrossing species [59], the investment of secondary metabolites to ensure pollination should be higher, even more diverse to attract a wider diversity of pollinators [55] over a single and shorter flowering period. In fact, some of the terpenoids identified in the floral extract have been reported to be involved in pollinators’ attraction ([51, 52], and references therein), including verbenone [60]. These compounds can be responsible for the significant to highly significant phytotoxic effects described for the volatile bioassays with C. scoparius flowering branches and flowers, as linalool, terpinen-4-ol or α-terpineol have well known phytotoxic activity, e.g., [34, 61]. Also, verbenone, a semiochemical with reputed pest deterrent properties because of its ecological role as anti-aggregation pheromone (see [62] as a review), has very recently been explored for its phytotoxicity [63]. Verbenone is synthetically obtained from the abundantly available pinenes in pine oleoresins, and then commercially used in tree protection [64]. This oxygenated monoterpene is also a particularly attractive starting material for the synthesis of the antitumoral diterpene Taxol (Bristol-Myers Squibb Company, Princeton, NJ). Verbenone is quite abundant in flavor complexes of edible aromatic species such as strawberry, raspberry, dill, rosmarinus, and spearmint ([64] and references therein). The natural occurrence of verbenone in the abundant biomass of C. scoparius, described here for the first time, extends the interest of this species for the exploitation of its natural active principles.
From the twelve compounds selected to be tested isolatedly for their phytotoxicity on the germination of the target agricultural weed species A. retroflexus and D. sanguinalis, the aliphatic compounds n-nonadecene, n-eicosane, n-heneicosane, n-docosane, n-tricosane and n-tetracosane were innocuous to A. retroflexus and D. sanguinalis germination and early growth at any concentration assayed. So, despite the high percentages of the aliphatic compounds in the volatile extracts of both shrub species, they seemed to have a limited contribution to the phytotoxicity observed in the volatile assays. The low phytotoxicity of the natural aliphatic compounds if compared with the aromatic ones is well known [65], as well as the stronger phytotoxic properties of terpenes compared with other VOC classes [66].
From the results obtained from the dose-response in vitro bioassays, the other six minority compounds (linalool, terpinen-4-ol, α-terpineol, verbenone, eugenol, and theaspirane) showed different degrees of phytotoxicity on the germination and early growth of both target species, even at very low concentrations.
Monoterpenes are argued to be involved in allelopathic processes as effective inhibitors of seed germination and seedling growth [67, 68], oxygenated monoterpenes being more bioactive than non-oxygenated [34, 69]. As a background of our work, the presence of high concentrations of linalool and α-terpineol in the Petri dish atmosphere was shown to prevent the germination and growth of both A. retroflexus and D. sanguinalis [70]. Kordali et al. [69] reported that linalool, α-terpineol, and terpinen-4-ol at 100 ppm completely inhibited seed germination of A. retroflexus. Also, eugenol sprayed at 1.5% (v/v) on A. retroflexus seedling caused loss of membrane integrity and inhibited growth severely [71]. Lower concentrations of linalool, terpinen-4-ol, and eugenol were shown to inhibit the germination of the model species Lactuca sativa [34] or the early growth of Cassia occidentalis and Bidens pilosa [57]. From the results obtained from our dose-response bioassays, verbenone, linalool, and α-terpineol seemed to be responsible for the phytotoxicity observed in C. scoparius, terpinen-4-ol showing more moderate phytotoxic effects on both A. retroflexus and D. sanguinalis, with higher IC values. Following the trend observed in the volatile bioassay with plant material, shoot growth was generally more sensitive than root elongation to the effects of the isolated VOCs, with lower IC50 and IC80 values for shoot length and biomass.
Noteworthy, verbenone was able to inhibit 80% germination of A. retroflexus and 50% root and shoot length of D. sanguinalis at less than 9 ppm in the Petri dish atmosphere. As far as we know, only two recent studies dealt with the phytotoxicity of verbenone added to the culture solution [63, 72]. These studies showed that verbenone applied at 100 to 150 μg mL-1 exerted extremely weak inhibition of the seedling growth of Echinochloa crus-galli and Brassica campestris [63], or just a delay on L. sativa germination [72]. We explain such discrepancy of results by the relative insolubility of VOCs in water [73], which could have prevented verbenone to reaching the target seeds when added to an aqueous culture solution, whereas being highly phytotoxic when volatilized in the Petri dish atmosphere.
The richness and relative abundance of oxygenated monoterpenes in the volatile profile of C. scoparius may underlie the higher phytotoxic effects observed of its flowering biomass if compared with U. europaeus, being able to inhibit the root and shoot growth of both weed species. Note that linalool, which could control by 50% the germination and early growth of both dicotyledon and monocotyledon agricultural weed species at very few ppm, was detected only in the volatile extract of the flowering branches, but not in the flowers alone, as was the case for α-terpineol. In the same direction, the benzenoid eugenol, which was extremely effective in controlling A. retroflexus and D. sanguinalis shoot and root growth- with the lowest IC80 values-, was only found in the flowering branches of U. europaeus, but not in the flowers extract, just like the oxygenated norisoprenoid theaspirane, which was moderately phytotoxic to D. sanguinalis shoot growth. These findings extend the interest of Scotch broom and gorse as natural sources of phytotoxins also outside their flowering periods, and also to be used both together as bioherbicide biomass under a practical point of view.
The study of the reversibility of the phytotoxic effects of linalool, terpinen-4-ol, α-terpineol, verbenone, and eugenol also threw quite exciting results. The toxic effects were highly persistent on D. sanguinalis, since the seeds that had been exposed to 18.75 to 25 ppm of any of the five bioassayed VOCs were not able to recover more than 13.5% mean germination. Noteworthy, those seeds that reversed the germination inhibition produced damaged seedlings with similar symptoms than those exposed to the phytotoxic VOCs during germination and early growth but additionally showed prostrated shoots applied to the wet filter paper. So that, even when the weed seeds subjected to inhibitory concentrations could recover germination capacity in different degree, we can consider the effects on the embryo as permanent or at least persistent [74, 75], because of producing unviable seedlings even after removing the phytotoxin.
Finally, reconsidering the naturally emitted VOCs bioassays, we must bear in mind that the volatile extracts of the shrub species rendered a mean yield of 0.06% (w/w, on a fresh mass basis) with a mean density of 0.85 g mL-1. That little yield, if related to the fresh flowering biomass used in the volatile assay, corresponds to ca. 4.2 to ca. 4.5 μL of volatile extract in each of the 1 L chambers for U. europaeus and C. scoparius, respectively. So, as an example, the conspicuous phytotoxic effects on the weeds germination and seedling growth produced by the flowering branches of C. scoparius in the volatile assay were caused by, at most, the joint action of only ca. 0.14, 0.12, 0.06, and 0.09 ppm of linalool, terpinen-4-ol, α-terpineol, and verbenone, respectively, among other VOCs. Obviously, the real concentration of VOCs emitted by the plant material into the test chamber must be even lower. Considering the dose-response curves of each bioassayed VOC and the obtained IC50 values, the phytotoxicity observed in the volatile bioassays had to be due not to one VOC in particular, but to the combined action of minority components [38, 76], or to the interactions among some of them and other non-studied compounds present in the volatile extract. In fact, the synergistic phytotoxic action of the open-chain alcohol linalool and the monocyclic one terpinen-4-ol have been described in the literature [34, 77]. Also, we do not discard possible synergies or antagonisms with the abundant aliphatic compounds, if emitted by the plant material, and other phytotoxic VOCs taking part in the profile, interactions which are worth studying. Approaches that use headspace trapping techniques to collect and concentrate the volatiles released into the airspace, combined with GC/MS, would through light on which VOCs are responsible for the phytotoxicity observed in these naturally emitted volatile bioassays, as well as their real concentrations in the controlled atmosphere.
Conclusions
The bioherbicide potential of the legume shrub species Ulex europaeus and Cytisus scoparius is reported in this work for the first time. The flowering fresh plant material of both species can produce and emit volatile compounds able to inhibit at different extend the germination and/or early growth of two agricultural weeds: Amaranthus retroflexus and Digitaria sanguinalis. Complete VOCs profiles from volatile extracts of U. europaeus and C. scoparius were obtained by GC and GC/MS. From these novel profiles, both species receive re-energized attention as sources of bioactive compounds. Particularly, C. scoparius revealed as rich in oxygenated monoterpenes as terpinen-4-ol, α-terpineol, and verbenone, as well as the previously reported linalool. Theaspirane and eugenol, among others, are also described for the first instance in U. europaeus.
Using dose-response bioassays with pure compounds, these VOCs were argued to be involved in the phytotoxicity observed for the plant materials, even at very low concentrations. The seeds of the agricultural weeds exposed to these VOCs produced damaged seedlings, were unable to recover germination capacity after removing the phytotoxin or, when recovered, produced unviable seedlings.
Our results extend the interest of the abundant U. europaeus and C. scoparius for the obtention of natural products with bioherbicide potential, or to be used as allelopathic biomass in the development of new sustainable agricultural practices. Further studies are required to evaluate the herbicide effectiveness under realistic field approaches, in which soil could compromise the persistence of phytotoxicity, as well as the putative side-effects on crops.
Supporting information
Schematic representation of the experiments carried on seeds and seedlings of the weed species Amaranthus retroflexus and Digitaria sanguinalis.
(TIF)
Mean values are represented as percentages relative to the control. Error bars represent standard deviation (SD). Mean values labelled with distinct letters are significant different at P ≤ 0.05 (ANOVA or Kruskal-Wallis H test).
(TIF)
Mean values are represented as percentages relative to the control. Error bars represent standard deviation (SD). Mean values labelled with distinct letters are significant different at P ≤ 0.05 (ANOVA or Kruskal-Wallis H test).
(TIF)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Spanish Ministry of Economy and Competitiveness (http://www.mineco.gob.es/; BIOINPUT (CGL2016-78660-R)) (NP). This research has been developed in the frame of the ‘Agri-Food Research and Transfer Centre of the Water Campus (CITACA) at the University of Vigo (Spain), which is economically supported by the Galician Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Basanta M, Diaz Vizcaino E, Casal M, Morey M. Diversity measurements in shrubland communities of Galicia (NW Spain). Plant Ecol. 1989; 82: 105–112. [Google Scholar]
- 2.Núñez-Regueira L, Proupín-Castiñeiras J, Rodrıguez-Anón JA. Energy evaluation of forest residues originated from shrub species in Galicia. Bioresour. Technol. 2004; 91: 215–221. [DOI] [PubMed] [Google Scholar]
- 3.Haveman R, De Ronde I, Schaminée JH. Retamoid scrubs of the Cytisetea scopario-striati in the Netherlands. Tuexenia. 2017; 37: 143–161. [Google Scholar]
- 4.Peterson DJ, Prasad R. The biology of Canadian weeds. 109. Cytisus scoparius (L.) Link. Can J Plant Sci. 1998; 78: 497–504. [Google Scholar]
- 5.Richardson RG, Hill RL. The biology of Australian weeds. 34. Ulex europaeus L. Plant Prot Q. 1998; 13: 46–58. [Google Scholar]
- 6.Clements DR, Peterson DJ, Prasad R. The biology of Canadian weeds. 112. Ulex europaeus L. Can J Plant Sci. 2001; 81: 325–337. [Google Scholar]
- 7.Global Invasive Species Database (GISD) [Internet] Auckland (NZ): 100 of the World’s Worst Invasive Alien Species. c2013 - [cited 2017 Dec 8]. Available from: http://www.iucngisd.org/gisd/100_worst.php.
- 8.Fogarty G, Facelli JM. Growth and competition of Cytisus scoparius, an invasive shrub, and Australian native shrubs. Plant Ecol. 1999; 144: 27–35. [Google Scholar]
- 9.Rees M, Hill RL. Large-scale disturbances, biological control and the dynamics of gorse populations. J Appl Ecol. 2001; 38: 364–377. [Google Scholar]
- 10.Rice EL. Allelopathy 2nd ed Orlando: Academic Press; 1984. 189 p. [Google Scholar]
- 11.Pedrol N, González L, Reigosa MJ. Allelopathy and abiotic stress In: Reigosa MJ, Pedrol N, González L, editors. Allelopathy: a physiological process with ecological implications. The Netherlands: Springer; 2006. pp. 171–209. [Google Scholar]
- 12.Sundararajan R, Koduru R. Cytisus scoparius: A review of ethnomedical, phytochemical and pharmacological information. Indo Am J Pharm Res. 2014; 4: 2151–2169. [Google Scholar]
- 13.Larit F, Nael MA, Benyahia S, Radwan MM, León F, Jasicka-Misiak I, et al. Secondary metabolites from the aerial parts of Cytisus villosus Pourr. Phytochem Lett. 2018; 24: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lores M, Pájaro M, Álvarez-Casas M, Domínguez J, García-Jares C. Use of ethyl lactate to extract bioactive compounds from Cytisus scoparius: Comparison of pressurized liquid extraction and medium scale ambient temperature systems. Talanta. 2015; 140: 134–142. 10.1016/j.talanta.2015.03.034 [DOI] [PubMed] [Google Scholar]
- 15.Pinela J, Barros L, Carvalho AM, Ferreira IC. Influence of the drying method in the antioxidant potential and chemical composition of four shrubby flowering plants from the tribe Genisteae (Fabaceae). Food Chem Toxicol. 2011; 49: 2983–2989. 10.1016/j.fct.2011.07.054 [DOI] [PubMed] [Google Scholar]
- 16.López-Hortas L, Conde E, Falqué E, Domínguez H. Flowers of Ulex europaeus L.–Comparing two extraction techniques (MHG and distillation). C R Chim. 2016; 19: 718–725. [Google Scholar]
- 17.Máximo P, Lourenço A, Feio SS, Roseiro JC. Flavonoids from Ulex airensis and Ulex europaeus ssp. europaeus. J Nat Prod. 2002; 65: 175–178. [DOI] [PubMed] [Google Scholar]
- 18.Spínola V, Llorent-Martínez EJ, Gouveia-Figueira S, Castilho PC. Ulex europaeus: from noxious weed to source of valuable isoflavones and flavanones. Ind Crops Prod. 2016; 90: 9–27. [Google Scholar]
- 19.Grove S, Haubensak KA, Parker IM. Direct and indirect effects of allelopathy in the soil legacy of an exotic plant invasion. Plant Ecol. 2012; 213:1869–1882. [Google Scholar]
- 20.López-Nogueira A, Pardo-Muras M, Pedrol N. Can the Atlantic Shrubland compromise the invasive success of Eucalyptus globules Labill? In: EWRS and AFPP (French Plant Protection Association), editors. Proceedings of the 17th European Weed Research Society Symposium, Weed management in changing environments; 2015 Jun 23–26; Montpellier. France: Numeric Print Services; 2015. pp. 163.
- 21.Cao XL, Boissard C, Juan AJ, Hewitt CN, Gallagher M. Biogenic emissions of volatile organic compounds from gorse (Ulex europaeus): Diurnal emission fluxes at Kelling Heath, England. J Geophys Res Atmos. 1997; 102: 18903–18915. [Google Scholar]
- 22.Boissard C, Cao XL, Juan CY, Hewitt CN, Gallagher M. Seasonal variations in VOC emission rates from gorse (Ulex europaeus). Atmos Environ. 2001; 35: 917–927. [Google Scholar]
- 23.Griffiths DW, Robertson GW, Shepherd T, Ramsay G. Epicuticular waxes and volatiles from faba bean (Vicia faba) flowers. Phytochemistry. 1999; 52: 607–612. [Google Scholar]
- 24.Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002; 5: 237–243. [DOI] [PubMed] [Google Scholar]
- 25.Marin-Loaiza JC, Cespedes CL. Volatile compounds from plants. Origin, emission, effects, analysis and agro applications. Rev Fitotec Mex. 2007; 30: 327–351. [Google Scholar]
- 26.De Albuquerque MB, Dos Santos RC, Lima LM, De Albuquerque Melo Filho P, Nogueira RJMC, Da Câmara CAG, et al. Allelopathy, an alternative tool to improve cropping systems. A review. Agron Sustain Dev. 2011; 31: 379–395. [Google Scholar]
- 27.Tesio F, Ferrero A. Allelopathy, a chance for sustainable weed management. Int J Sust Dev World. 2010; 17: 377–389. [Google Scholar]
- 28.Bhowmik P, Inderjit C. Challenges and opportunities in implementing allelopathy for natural weed management. Crop Protect. 2003; 22: 661–671. [Google Scholar]
- 29.Shah AN, Iqbal J, Ullah A, Yang G, Yousaf M, Fahad S, et al. Allelopathic potential of oil seed crops in production of crops: a review. Environ Sci Pollut Res Int. 2016; 23: 14854–14867. 10.1007/s11356-016-6969-6 [DOI] [PubMed] [Google Scholar]
- 30.Macías FA, Molinillo JM, Varela RM, Galindo JC. Allelopathy—a natural alternative for weed control. Pest Manag Sci. 2007; 63: 327–348. 10.1002/ps.1342 [DOI] [PubMed] [Google Scholar]
- 31.Verdeguer M, Blázquez MA, Boira H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem Syst Ecol. 2009; 37: 362–369. [Google Scholar]
- 32.Benvenuti S, Cioni PL, Flamini G, Pardossi A. Weeds for weed control: Asteraceae essential oils as natural herbicides. Weed Res. 2017; 57: 342–353. [Google Scholar]
- 33.Weston LA, Duke SO. Weed and crop allelopathy. Crit Rev Plant Sci. 2003; 22: 367–389. [Google Scholar]
- 34.Vokou D, Douvli P, Blionis GJ, Halley JM. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J Chem Ecol. 2003; 29: 2281–2301. [DOI] [PubMed] [Google Scholar]
- 35.De Martino L, Mancini E, Almeida LFRD, Feo VD. The antigerminative activity of twenty-seven monoterpenes. Molecules. 2010; 15: 6630–6637. 10.3390/molecules15096630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puig CG, Álvarez-Iglesias L, Reigosa MJ, Pedrol N. Eucalyptus globulus leaves incorporated as green manure for weed control in maize. Weed Sci. 2013; 61: 154–161. [Google Scholar]
- 37.Souza-Alonso P, Puig CG, Pedrol N, Freitas H, Rodríguez-Echeverría S, Lorenzo P. Exploring the use of residues from the invasive Acacia sp. for weed control. Renew Agr Food Syst. 2018; 1–12. [Google Scholar]
- 38.Barney JN, Hay AG, Weston LA. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J Chem Ecol. 2005; 31: 247–265. [DOI] [PubMed] [Google Scholar]
- 39.Mayer AM, Poljakoff-Mayber A. The Germination of Seeds 1st ed Oxford: Pergamon Press; 1963. pp. 244. [Google Scholar]
- 40.Chiapusio G, Sanchez AM, Reigosa MJ, Gonzalez L, Pellissier F. Do germination indices adequately reflect allelochemical effects on the germination process? J Chem Ecol. 1997; 23: 2445–2453. [Google Scholar]
- 41.De Bertoldi C, De Leo M, Braca A, Ercoli L. Bioassay-guided isolation of allelochemicals from Avena sativa L.: allelopathic potential of flavone C-glycosides. Chemoecology. 2009; 19: 169–176. [Google Scholar]
- 42.Likens ST, Nickerson GB. Detection of certain hop oil constituents in brewing products. J Am Soc Brew Chem. 1964; 22: 5–13. [Google Scholar]
- 43.Marsili R. Flavor, fragrance, and odor analysis 1st ed New York: CRC Press; 2001. [Google Scholar]
- 44.McLafferty H. Wiley registry of mass spectral data 9th/NIST 08. Mass spectral libraly; 2009. [Google Scholar]
- 45.Adams RP. Identification of essential oils components by gas chromatography / mass spectrometry 4th ed Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- 46.Joulain D, König WA. The atlas of spectral data of sesquiterpene hydrocarbons Hamburg: EB-Verlag; 1998. [Google Scholar]
- 47.Dayan FE, Romagni JG, Duke SO. Investigating the mode of action of natural phytotoxins. J Chem Ecol. 2000; 26: 2079–2094. [Google Scholar]
- 48.Araniti F, Lupini A, Sorgonà A, Statti GA, Abenavoli MR. Phytotoxic activity of foliar volatiles and essential oils of Calamintha nepeta (L.) Savi. Nat Prod Res. 2013; 27: 1651–1656. 10.1080/14786419.2012.746337 [DOI] [PubMed] [Google Scholar]
- 49.Souza-Alonso P, González L, López-Nogueira A, Cavaleiro C, Pedrol N. Volatile organic compounds of Acacia longifolia and their effects on germination and early growth of species from invaded habitats. Chem Ecol. 2018; 34: 126–145. [Google Scholar]
- 50.Atlan A, Schermann-Legionnet A, Udo N, Tarayre M. Self-incompatibility in Ulex europaeus: variations in native and invaded regions. Int J Plant Sci. 2015; 176: 515–524. [Google Scholar]
- 51.Lemaitre AB, Pinto CF, Niemeyer HM. Generalized pollination system: Are floral traits adapted to different pollinators?. Arthropod Plant Interact. 2014; 8: 261–272. [Google Scholar]
- 52.Maffei ME. Sites of synthesis, biochemistry and functional role of plant volatiles. S. African J Bot. 2010; 76: 612–631. [Google Scholar]
- 53.Marinho CR, Souza CD, Barros TC, Teixeira SP. Scent glands in legume flowers. Plant Biol. 2014; 16: 215–226. 10.1111/plb.12000 [DOI] [PubMed] [Google Scholar]
- 54.Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002; 5: 237–243. [DOI] [PubMed] [Google Scholar]
- 55.Baldwin IT. Plant volatiles. Curr Biol. 2010; 20: R392–R397. 10.1016/j.cub.2010.02.052 [DOI] [PubMed] [Google Scholar]
- 56.Singh HP, Batish DR, Kaur S, Ramezani H, Kohli RK. Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Ann Appl Biol. 2002; 141: 111–116. [Google Scholar]
- 57.Vaid S, Batish DR, Singh HP, Kohli RK. Phytotoxic effect of eugenol towards two weedy species. The Bioscan. 2010; 5: 339–341. [Google Scholar]
- 58.Kurhara T, Kikuchi M. Studies on the constituents of flowers. XIII. on the components of the flower of Cytisus scoparius Link”, Yakugaku Zasshi. 1980; 100: 1054–1057. [Google Scholar]
- 59.Simpson SR, Gross CL, Silberbauer LX. Broom and honeybees in Australia: an alien liaison. Plant Biol. 2005; 7: 541–548. 10.1055/s-2005-865855 [DOI] [PubMed] [Google Scholar]
- 60.Abdullah ZS, Ficken KJ, Greenfield BP, Butt TM. Innate responses to putative ancestral hosts: is the attraction of Western flower thrips to pine pollen a result of relict olfactory receptors?. J Chem Ecol. 2014; 40: 534–540. 10.1007/s10886-014-0450-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angelini LG, Carpanese G, Cioni PL, Morelli I, Macchia M, Flamini G. Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. J Agric Food Chem. 2003; 51: 6158–6164. 10.1021/jf0210728 [DOI] [PubMed] [Google Scholar]
- 62.Seybold SJ, Bentz BJ, Fettig CJ, Lundquist JE, Progar RA, Gillette NE. Management of Western North American Bark Beetles with Semiochemicals. Annu Rev Entomol. 2018; 63:407–432. 10.1146/annurev-ento-020117-043339 [DOI] [PubMed] [Google Scholar]
- 63.Hu Q, Lin GS, Duan WG, Huang M, Lei FH. Synthesis and Biological Activity of Novel (Z)-and (E)-Verbenone Oxime Esters. Molecules. 2017; 22: 1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodrigues‐Corrêa KCDS, Lima JC, Fett‐Neto AG. Pine oleoresin: tapping green chemicals, biofuels, food protection, and carbon sequestration from multipurpose trees. Food Energy Secur. 2012; 1: 81–93. [Google Scholar]
- 65.Barbosa LCDA, de Alvarenga ES, Demuner AJ, Figueiredo R, da Silva AA. Synthesis of new aliphatic and aromatic phytotoxic derivatives of 2α, 4α‐dimethyl‐8‐oxabicyclo [3.2.1] oct‐6‐en‐3‐one. Pest Manag Sci. 2003; 59: 1043–1051. 10.1002/ps.717 [DOI] [PubMed] [Google Scholar]
- 66.Kordali S, Cakir A, Akcin TA, Mete E, Akcin A, Aydin T, Kilic H. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan. (Asteraceae). Ind Crops Prod. 2009; 29: 562–570. [Google Scholar]
- 67.Macías FA, Molinillo JM, Varela RM, Galindo JC. Allelopathy—a natural alternative for weed control. Pest Manag Sci. 2007; 63: 327–348. 10.1002/ps.1342 [DOI] [PubMed] [Google Scholar]
- 68.Ishii-Iwamoto EL, Marusa Pergo Coelho E, Reis B, Sebastiao Moscheta I, Moacir Bonato C. Effects of monoterpenes on physiological processes during seed germination and seedling growth. Curr Bioact Compd. 2012; 8: 50–64. [Google Scholar]
- 69.Kordali S, Caki A, Sutay S. Inhibitory effects of monoterpenes on seed germination and seedling growth. Z Naturforsch C. 2007; 62: 207–214. [DOI] [PubMed] [Google Scholar]
- 70.Bainard LD, Isman MB, Upadhyaya MK. Phytotoxicity of clove oil and its primary constituent eugenol and the role of leaf epicuticular wax in the susceptibility to these essential oils. Weed Sci. 2006; 54: 833–837. [Google Scholar]
- 71.Vaughn SF, Spencer GF. Volatile monoterpenes as potential parent structures for new herbicides. Weed Sci. 1993; 41: 114–119. [Google Scholar]
- 72.Ortiz de Elguea-Culebras G, Sánchez-Vioque R, Berruga MI, Herraiz-Peñalver D, Santana-Méridas O. Antifeedant effects of common terpenes from Mediterranean aromatic plants on Leptinotarsa decemlineata. J Soil Sci Plant Nutr. 2017; 17: 475–485. [Google Scholar]
- 73.Weidenhamer JD, Macias FA, Fischer NH, Williamson GB. Just how insoluble are monoterpenes? J Chem Ecol. 1993; 19: 1799–1807. 10.1007/BF00982309 [DOI] [PubMed] [Google Scholar]
- 74.Dudai N, Poljakoff-Maybe A, Mayer AM, Putievsky E, Lerner HR. Essential oils as allelochemicals and their potential use as bioherbicides. J Chem Ecol. 1999; 25: 1079–1089. [Google Scholar]
- 75.Sobrero MC, Ronco A. Ensayo de toxicidad aguda con semillas de lechuga (Lactuca sativa L.) In: Castillo MG, editor. Ensayos toxicológicos y métodos de evaluación de calidad de aguas. Canadá: IDRC; 2004. pp. 71–79. [Google Scholar]
- 76.Koroch AR, Juliani HR. Bioactivity of essential oils and their components In: Berger RG, editor. Flavours and fragrances: chemistry, bioprocessing and sustainability. Heidelberg: Springer; 2007. pp. 87–115. [Google Scholar]
- 77.Vasilakoglou I, Dhima K, Paschalidis K, Ritzoulis C. Herbicidal potential on Lolium rigidum of nineteen major essential oil components and their synergy. J Essent Oil Res. 2013; 25: 1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of the experiments carried on seeds and seedlings of the weed species Amaranthus retroflexus and Digitaria sanguinalis.
(TIF)
Mean values are represented as percentages relative to the control. Error bars represent standard deviation (SD). Mean values labelled with distinct letters are significant different at P ≤ 0.05 (ANOVA or Kruskal-Wallis H test).
(TIF)
Mean values are represented as percentages relative to the control. Error bars represent standard deviation (SD). Mean values labelled with distinct letters are significant different at P ≤ 0.05 (ANOVA or Kruskal-Wallis H test).
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.