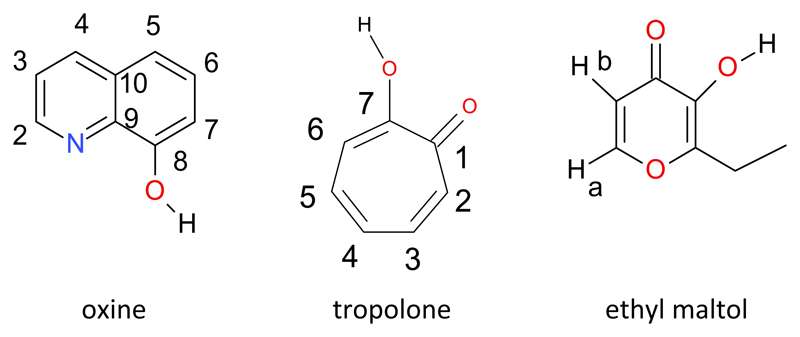
Abstract
The increasing availability of the long half-life positron emitter Zr-89 (half life 78.4 h) suggests that it is a strong candidate for cell labelling and hence cell tracking using positron emission tomography. The aim was to produce a range of neutral ZrL4 lipophilic complexes for cell labelling which could be prepared under radiopharmaceutical conditions. This was achieved when the ligand was oxine, tropolone or ethyl maltol. The complexes can be prepared in high yield from zirconium(IV) precursors in hydrochloric or oxalic acid solution. The oxinate and tropolonate complexes were the most amenable to chromatographic characterisation, and HPLC and ITLC protocols have been established to monitor their radiochemical purity. The radiochemical synthesis and quality control of 89Zr(oxinate)4 is reported as well as preliminary cell labelling data for the oxinate, tropolonate and ethyl maltolate complexes which indicates that 89Zr(oxinate)4 is the most promising candidate for further evaluation.
Keywords: Zirconium, oxine, tropolone, ethyl maltol, zirconium-89, PET
Introduction
The increasing availability of the positron emitter zirconium-89 combined with its half life of 78.4 h suggests that it is a strong candidate for cell labelling and hence cell tracking in vivo using positron emission tomography (PET), affording the opportunity to exploit the advantages of PET over conventional gamma camera imaging of cells labelled with the gamma emitter indium-111. Although the favoured oxidation state of zirconium is 4+ (compared to 3+ for indium) there are parallels between the two metals in their reactivity and preferred ligand types. This raises the possibility that the mechanism exploited to label cells with In-111 (i.e. use of metastable chelates that enter cells by virtue of their lipophilicity followed by dissociation within cells leading to intracellular trapping of the radiometal1–4) might be exploited in the case of zirconium-89. Tetravalent zirconium is known to form complexes with monobasic bidentate ligands such as oxinate,5 tropolonate6 and hydroxamates7 of the stoichiometry ZrL4, bearing analogy to InL3.8,9
Here we describe methodologies for the synthesis and characterisation of Zr(IV) complexes containing oxine, tropolone and ethyl maltol ligands. Conditions compatible with the radiopharmacy, where zirconium-89 is typically available in hydrochloric or oxalic acid solution, were investigated. The characterisation of these compounds underpins understanding of their utility in radiolabelled form for cell labelling, and provides chromatographic standards for identification and quality control of the radioactive species.
The synthesis of Zr(oxinate)4, by reacting ZrCl4 with oxine either in THF or in the absence of solvent was first reported in 196810 and the X-ray crystal structure of Zr(oxinate)4.(PhMe)3 was reported in 1974.11 There has been renewed interest in Zr(oxinate)4 in recent years because of its potential applications in organic light emitting diodes (OLEDs) and two new crystalline forms of Zr(oxinate)4 have since been reported.5 The molecular structures are very similar in the various crystal structures: the metal centre is eight coordinated in approximately dodecahedral geometry by four chelating N,O pairs from four oxinate ligands. Tropolonate zirconium complexes have also been known for over fifty years and the crystal structure of Zr(tropolonate)4.(CHCI3)2.25 was published in 1978 displaying again a neutral eight coordinate complex of approximately dodecahedral geometry.12 ZrCl2(ethyl maltolate)2 has been shown to be a catalyst for ethylene polymerisation,13 but we can find no previous reports of Zr(ethyl maltolate)4.
Results and Discussion
Synthesis and Characterisation
Zr(oxinate)4 was initially prepared in 71% yield by the reaction of ZrCl4 with 4 molar equivalents of oxine and an excess of piperidine followed by Soxhlet extraction with 1, 4-dioxane, in a similar manner to the reported synthesis of beta-Zr(oxinate)4 (Method 1).5 NMR spectroscopic data and elemental analysis were in accord with the formation of the desired compound and previous reports (see experimental).5 IR and Raman spectra were assigned by comparison with Ga(oxinate)3.14 The characteristic ν(OH) stretching band, located at 3176 cm-1 in the IR spectrum of oxine disappears in the complex due to deprotonation of the ligand and the band assigned to the υ(C-O) vibration moves to lower energy in the complex (from 1093 to 1054 cm-1 in the IR and from 1101 to 1054 cm-1 in the Raman spectrum).
The UV-visible spectrum (see Table 1) of Zr(oxinate)4 in acetonitrile has an absorption at 380 nm (previously reported in toluene at 387 nm)5 assigned to a π→π* transition in the ligand by comparison with related complexes.15 The molar absorptivity is approximately four times that observed for oxine in accord with the chromophore being ligand-based rather than involving the zirconium.
Table 1. UV-visible spectroscopic data for ligands and complexes.
| Solvent | λmax /nm (ε/dm3 mol-1 cm-1) | |
| Oxine | MeCN | 240 (35000 ± 2000), 308 (2570 ± 10) |
| Zr(oxinate)4 | MeCN | 250 (47000 ± 500), 380 (9430 ± 30) |
| Tropolone | MeOH | 307 (sh), 320 (6500 ± 600), 354 (5900 ± 200), 370 (4700 ± 100) |
| Zr(tropolonate)4 | MeOH | 333 (63000 ± 5000), 369 (33000 ± 1000), 380 (sh), 390 (sh) |
| Ethyl maltol | MeOH | 268 (10200 ± 300) |
| Zr(ethyl maltolate)4 | MeOH | 224 (64000 ± 5000), 312 (21000 ± 1000) |
In order to prepare Zr(oxinate)4 from zirconium (IV) chloride in 1M HCl the acidic solution was first neutralised with NaOH followed by addition of aqueous oxine resulting in the precipitation of Zr(oxinate)4 after reduction of the solvent volume (Method 2). Spectroscopic data confirmed that the complex isolated was the desired compound. Attempts to prepare Zr(oxinate)4 from a solution of zirconium (IV) chloride in 1M HCl failed unless the solution was first neutralised. A similar approach was taken to the synthesis from K4[Zr(C2O4)4] in 1M oxalic acid, the neutralisation being effected with Na2CO3 (Method 3). Importantly these results demonstrate the formation of Zr(oxinate)4 in the presence of a high excess of chloride or oxalate, as would be encountered in a radiopharmaceutical synthesis.
Zr(tropolonate)4 was prepared by adapting the published method of synthesis of zirconium tetrakis isopropyltropolone (Method 4).6 ZrCl4 and 4 molar equivalents of tropolone were reacted in chloroform and the product isolated by evaporation of the chloroform. 1H and 13C NMR spectra were in accord with the proposed product showing ligand resonances perturbed from their positions in tropolone. 1H NMR spectroscopy and elemental analysis data suggest inclusion of CHCl3. NMR integration indicates approximately 0.85 CHCl3 while elemental analysis fits best for 0.55 CHCl3. The previously reported crystal structure contained 2.25 CHCl3.12 IR and Raman spectra were assigned by comparison with bistropolonato vanadium, cobalt, copper and zinc complexes,16 and tristropolonato lanthanum, neodymium, samarium and ytterbium complexes.17
Electronic absorptions for free tropolone in methanol were in accord with those previously observed in DMSO.16 The absorptions are due to π→π* transitions and analogous transitions are observed in Zr(tropolonate)4 with increased extinction coefficients attributed to the presence of four tropolone rings with enhancement by the contribution of a charge transfer transition.16 Zr(tropolonate)4 was also successfully prepared from of ZrCl4 in 1M HCl or K4[Zr(C2O4)4] in 1M oxalic acid if the acidic solutions were first neutralised (Method 5). Zr(ethyl maltolate)4 was prepared in 91% yield by the reaction of 4 equivalents of ethyl maltol with ZrCl4 in tetrahydrofuran (method 6). Elemental analysis data were in accord with the desired complex (see experimental). As expected the 1H NMR spectrum shows no resonance due to free ligand OH in the region of 8.8 ppm and the shift of the Ha and Hb protons to lower frequencies in relation to the ligand demonstrates the loss of aromaticity due to the donation of electron density to the metal. IR and Raman spectra were assigned by comparison with bis(maltolato) vanadium18 and zinc19 and tris(maltolato) gallium20 complexes. An electronic absorption for ethyl maltol in methanol was recorded as 268 nm having been observed previously in toluene at 286 nm and interpreted as a π→π* transitions of the β unsaturated enone.13 In Zr(ethyl maltolate)4 this band disappears due to the loss of conjugation of the enone, demonstrating the complexation by the carbonyl group. Absorption bands appear in the UV spectrum at 224 and 312 nm. The latter may be attributed to a ligand-to-metal charge transfer from a π ligand orbital to an empty zirconium d orbital. Zr(ethyl maltolate)4 was also successfully prepared from K4[Zr(C2O4)4] in 1M oxalic acid if the acidic solution was first neutralised (Method 7).
High Performance Liquid Chromatography
The HPLC of oxinate complexes has previously been reported when oxine was used as a pre-column chelating agent for reverse-phase HPLC multi-element (Zn, Al, Co, Cr, Cu, Ga, In and Fe) determinations.21 For ions other than the kinetically inert Co(III) and Cr(III), excess ligand was required in the mobile phase to observe good chromatographic properties, presumably due to ready ligand dissociation occurring during passage through the column. A large range of columns and solvents were evaluated in order to arrive at conditions suitable for using these compounds as standards for chromatographic identification of their radioactive analogues.
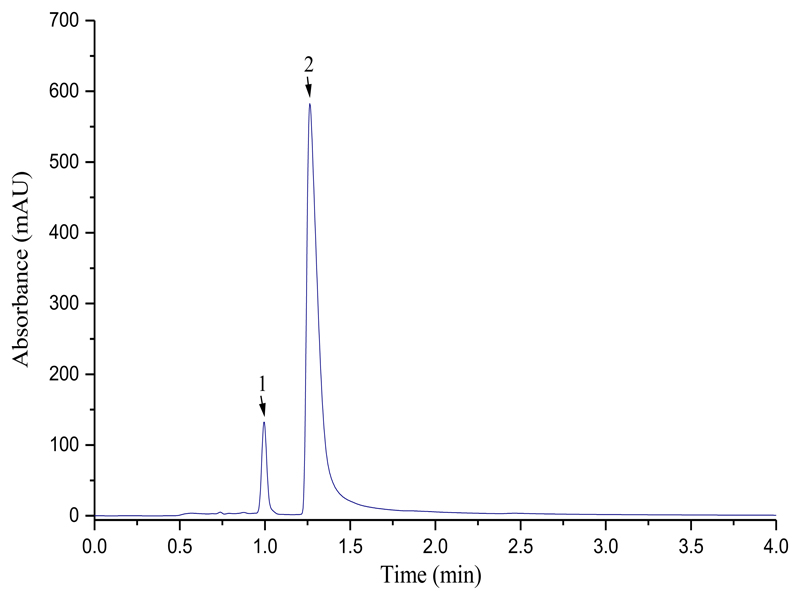
For Zr(oxinate)4 using a XBD-C8 eclipse column very poor results were obtained without excess ligand in the mobile phase. However addition of ca. 1000 ppm ligand to the mobile phase gives chromatograms (MeCN:H2O 75:25, 1.0 mL min-1, 380 nm) with a retention time of 2.96 min. With a Altima C18 5 μm column (MeOH:H2O 50:50, 0.1% formic acid, 2.0 mL min-1), monitored at 245 nm to detect ligand and complex simultaneously, separation was obtained with retention times of oxine and Zr(oxinate)4 being 1.27 and 1.00 min respectively without the use of excess ligand in the mobile phase (figure 1).
Figure 1.
HPLC of a 1:1 mixture of Zr(oxinate)4 (1) and oxine (2). Altima C18 5 μm column (MeOH:H2O 50:50,0.1% formic acid, 2.0 mL min-1, 245 nm)
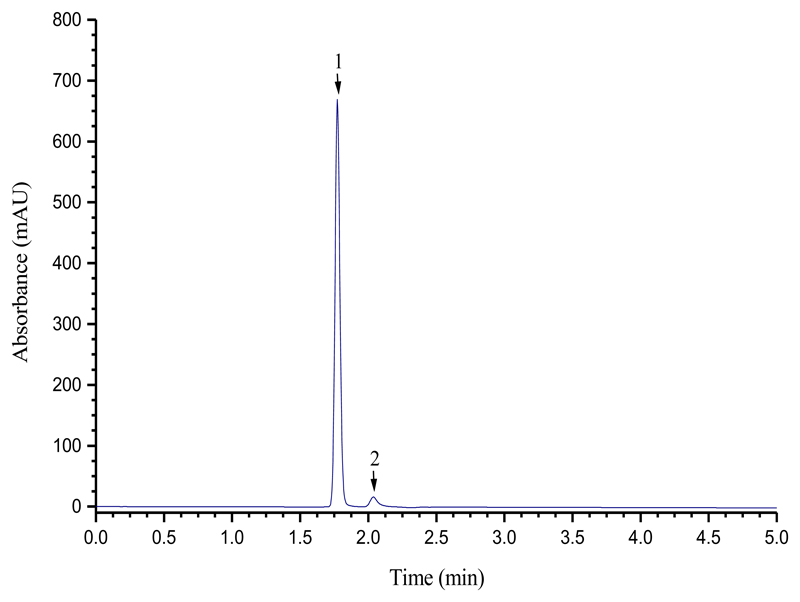
Chromatograms of Zr(tropolonate)4 (figure 2) were obtained using a Luna 3 silica column (MeCN:H2O 90:10, 1.0 mL min-1, 333 nm). A retention time of 1.78 min was observed, clearly resolvable from the free ligand at 2.04 min.
Figure 2.
HPLC of a 1:1 mixture of Zr(tropolonate)4 (1) and tropolone (2). Luna 3 silica column (MeCN:H2O 90:10, 1.0 mL min-1, 333 nm)
Numerous conditions were attempted to produce chromatograms demonstrating separation of Zr(ethyl maltolate)4 / ethyl maltol without success. In all cases the chromatograms were indistinguishable from those of the free ligand.
Instant Thin Layer Chromatography (ITLC)
Instant thin layer chromatography (ITLC) can provide a quick and accurate test for radiochemical purity and hence its use was explored for the zirconium complexes (see Table 2). The oxine and tropolone ligands and their zirconium complexes were visualised by UV light, and ZrCl4 by staining with silver nitrate, however it was not possible to find a method of visualising K4[Zr(C2O4)4]. The Rf value could only be determined by radio-ITLC of K4[89Zr(C2O4)4].
Table 2. ITLC Results.
| Compound | Solvent | Visualisation | Rf Value |
|---|---|---|---|
| ZrCl4 | 20 mM sodium citrate | AgNO3 | 1.0 |
| K4[Zr(C2O4)4] | 20 mM sodium citrate | Zr-89 | 0.9 |
| K4[Zr(C2O4)4] | Ethyl acetate | Zr-89 | 0.0 |
| Oxine | 20 mM sodium citrate | UV 254 nm | 0.8 |
| Zr(oxinate)4 | 20 mM sodium citrate | UV 254 nm | 0.0 |
| Oxine | Ethyl acetate | UV 254 nm | 0.2 |
| Zr(oxinate)4 | Ethyl acetate | UV 254 nm/ Zr-89 | 0.9 |
| Tropolone | 20 mM sodium citrate | UV 254 nm | 1.0 |
| Zr(tropolonate)4 | 20 mM sodium citrate | UV 254 nm | 0.0 |
Oxine and Zr(oxinate)4 were applied to the ITLC sheets as 1 mg/mL solutions in acetonitrile. Eluting with 20 mM sodium citrate solution provided clearly distinguishable chromatographic behaviour with the ligand displaying a spot at Rf 0.8 with some streaking, the complex remaining at the origin (Rf = 0) compared with the precursors ZrCl4 moving with the solvent front and K4[Zr(C2O4)4] having a Rf of 0.9. Alternatively eluting with ethyl acetate results in Rf for Zr(oxinate)4 of 0.9 and K4[Zr(C2O4)4] zero.
Similar results were obtained eluting with 20 mM sodium citrate solution when tropolone and Zr(tropolonate)4 were applied to the ITLC sheets as 1 mg/mL solutions in methanol. Non-radioactive visualisation methods could not be established for ethyl maltol and Zr(ethyl maltolate)4. Performing the synthesis of 89Zr(oxinate)4 from 89Zr in 0.1 M oxalic acid by adapting method 3 (see experimental) resulted in a yield of ca. 60% in the chloroform phase and a radiochemical purity of 99% as determined by ITLC with ethyl acetate, exhibiting a Rf of 0.9 in agreement with that of the spectroscopically and analytically characterised sample of Zr(oxinate)4. This ITLC method was therefore adopted as the main quality control of the 89Zr(oxinate)4 complex performed before each biological evaluation, as reported elsewhere.22, 23
Cell Labelling
The uptake of 89Zr(oxinate)4 in breast cancer cell line MDA-MB-231 was found to be ca. 16% after 1 min rising to ca. 20% after 1h, in contrast to 89Zr(oxalate)4 which rose to ca. 5% after 1 h. Retention of 89Zr(oxinate)4 was high with 82% retention after 24 h. Similar results were obtained with mouse macrophage J447 with ca. 5% uptake of 89Zr(oxalate)4 and ca. 22% uptake of 89Zr(oxinate)4 after 1 h. Approximately 91% of 89Zr(oxinate)4 was retained after 24 h. Uptake of 89Zr(tropolonate)4 in this cell line was found to be ca. 22% after 1 h, with ca. 49% being retained after 24 h. Uptake of 89Zr(ethyl maltolate)4 by colon cancer cells HTC-116 was found to be ca. 43% after 1 h, with ca. 26% retention after 24 h. These cell labelling experiments were conducted with around one million cells per sample, a much smaller number than typically used with clinical samples, and therefore the % labelling yields reported here are expected to translate into much higher yields when used in clinical samples. These preliminary experiments indicate that 89Zr(oxinate)4 is a promising candidate for cell tracking and detailed evaluation is currently ongoing.
Conclusions
The aim was to produce a range of neutral ZrL4 complexes for cell labelling which could be prepared under radiopharmaceutical conditions. This was achieved using oxinate, tropolonate or ethyl maltolate as uninegative bidentate ligands to prepare neutral eight coordinate Zr(IV) complexes. The complexes can be prepared in high yield from zirconium precursors supplied in high concentrations of hydrochloric or oxalic acid following neutralisation. The oxine and tropolone complexes were the most amenable to chromatographic characterisation with both HPLC and ITLC conditions established to monitor their purity. 89Zr(oxinate)4 complex is able to radiolabel cells with good yields and good retention over 24 h; it is currently undergoing detailed evaluation for cell labelling and in vivo tracking by PET imaging.
Experimental
NMR analysis was undertaken using a JEOL NMR ECS-400 and JEOL Delta v5.02 software. FTIR spectra were obtained using a Thermo Scientific Nicolet 380 FT-IR spectrometer with a Smart Orbit high performance diamond single bounce ATR accessory. CHN analyses were obtained at the University of Kent utilising an A EMA Syst 1106 elemental analyser and at the Science Centre, London Metropolitan University utilising a Carlo Erba Flash 2000 elemental analyser. Raman spectra were obtained using a Horiba LabRAM-HR Raman spectrometer utilising a laser operating at 784 nm. A ×50 objective lens was used giving a beam diameter of approximately 2 µm on the sample. The spectrometer was calibrated against the silicon line at 520.6 cm-1. UV-visible spectra were obtained with a Shimadzu UV-1800 spectrometer.
HPLC was performed using a Dionex Ultimate 3000 system with data processing using Chromeleon 7. For ITLC a 20 mM citrate elutant solution (pH 4.0) was made by the addition of 0.262 g of citric acid and 0.193 g of tri sodium citrate to 100 mL of millipore water. ITLC-SG plates were obtained from Varian. K4[Zr(C2O4)4] was prepared by a literature method.24
Synthesis of Zr(oxinate)4
Method 1
A solution of oxine (5.00 g; 34.5 mmol) in ethanol (35 mL) was added dropwise to a solution of zirconium (IV) chloride (2.01 g; 8.63 mmol) in ethanol (25 mL) which had been cooled to 10°C with an ice-water bath. The resulting mixture was stirred for 20 min at room temperature (20°C). The solution was then heated to 50°C in a water bath and piperidine (10 mL; 100 mmol) was added dropwise resulting in the formation of a yellow precipitate. This suspension was then refluxed for 1 h and allowed to cool to room temperature. The precipitate was collected by filtration and washed with ethanol (15 mL), tetrahydrofuran (15 mL) and diethyl ether (15 mL) before drying at 70°C for 1 h affording a crude yield 5.06 g (89%). To further purify the compound Soxhlet extraction was undertaken with 1, 4-dioxane for 8 h. Concentration of the extract resulted in a yellow precipitate which was collected with a Büchner funnel and washed with ethanol (15 mL). The product was then dried for 2 h in an oven at 50°C and stored in a glass air tight screw top bottle. Yield 4.01 g (71%). Found C 64.1, H 3.8, N 8.6%; C36H24N4O4Zr requires 64.8, H 3.6, N 8.7%.
δH(400 MHz, d6-DMSO), 8.60 (1 H, b s, 2-H), 8.15 (1 H, d, J3,4 7.3, 4-H), 7.33-7.26 (2 H, m, 5-H and 6-H), 7.02 (1 H, d, J6,7 8.2, 7-H), 6.69 (1 H, d, J3,4 7.3, 4-H)
δc(100 MHz, d6-DMSO) 162.84 (C8), 145.88 (C2), 141.75 (C9), 138.39 (C4), 129.92 (C10), 129.63 (C6), 122.19 (C3), 114.17 (C5), 112.39 (C7)
IR υmax (cm−1): 3045 w (υCH), 1604 m (υC=N), 1573 s (υC=C), 1495 vs, 1462 vs, 1423 m, 1378 vs, 1318 vs (υC-C), 1274 s, 1227 m, 1173 w, 1107 vs (δCH in plane), 1054 m (υC-O), 1030 m, 910 w, 822 s, 806 m (δCH out of plane), 785 s, 737 vs (ring breathing), 643 m, 616 s, 560 w, 514 vs (ring deformations).
Raman υmax (cm−1): 1606 w (υC=N), 1589 m, 1578 m 1572 sh (υC=C), 1498 m, 1463 w, 1421 m, 1388 vs, 1378 s, 1327 w (υC-C), 1284 m, 1209 w, 1175 w, 1143 w, 1137 w, 1112 w (δCH in plane), 1054 w (υC-O), 1032 w, 907 m, 824 w, 805 m (δCH out of plane), 754 m, 736 w (ring breathing), 650 w, 617 m, 558 w, 524 vs, 500 sh, 492 m, 469 w, 449 w (ring deformations).
Method 2
Zirconium(IV) tetrachloride (1.01 g; 4.31 mmol) was dissolved in 1M HCl (20 mL). The pH was then raised to 7.60 by addition of 1 M NaOH (28 mL). A solution of oxine (2.50 g; 17.3 mmol) in reverse osmosis water (150 mL) was then added drop wise. The solution was brought to the boil and reduced in volume by 90%. After cooling to room temperature the resulting precipitate was then collected by the use of a Büchner funnel and washed with ethanol (15 mL), tetrahydrofuran (15 mL) and diethyl ether (15 mL) and dried in an electric oven at a 100 °C for 10 minutes. Yield 2.55 g (91%).
Method 3
K4[Zr(C2O4)4] (2.00 g; 2.89 mmol) was dissolved in 1 M oxalic acid (20 mL) and then neutralised with 1 M sodium carbonate (Na2CO3) (19 mL) before diluting to 50 mL with water. A solution of oxine (2.10 g; 14.5 mmol) in chloroform (50 mL) was then added drop wise with stirring. The mixture was stirred for 20 minutes at room temperature and then the pale yellow chloroform layer separated. The product was isolated by evaporation at 60 °C. Yield 1.70 g (88%).
Synthesis of Zr(tropolonate)4
Method 4
A solution of tropolone (3.80 g; 31.0 mmol) in chloroform (75 mL) was added drop wise to a solution of zirconium (IV) chloride (1.78 g; 7.64 mmol) in chloroform (75 mL). The solution was then stirred for 20 min during which time it became pale yellow in colour. The solution was then heated in a water bath at between 50°C and 55°C for 2 h. The volume of the solution was reduced to ~40 mL during this period. The remaining chloroform was removed by use of a rotary evaporator. The compound was then dried at 40°C for 2 h in an electric oven. Yield 4.18 g (83%)
Found C 53.33, H 3.51, N 0.0%; C28H20O8Zr.0.55CHCl3 requires C 53.47, H 3.23, N 0.0%
δH(400 MHz, d6-DMSO), 8.27 (0.125H, s, CHCl3), 7.59 (2H, dd, J 9.6, 11.0, H3 & 5), 7.19 (2 H, d, J2,3 J5,6 11.0, H2 & 6), 7.13 (1 H, t, J 9.6, H4)
δc(100 MHz, d6-DMSO), 181.72 (C1 &C7), 140.31 (C2 & C6), 128.28 (C4), 125.05 (C3 & C5)
IR υmax (cm−1): 3013 m (υCH), 1590 vs (υC=C and υC=O), 1516 vs (υC=O and υC=C), 1418 sh, 1408 vs, (υC=O, υC-C and δCH), 1349 vs (υC=C), 1223 vs (υC-O), 1079 m (υC-C and δCH), 968 m, 934 m, 919 m (υC-C), 875 s, 728 vs (υC-C and δCH), 712 vs (υCH), 661 m (υC-C), 586 m, 530 vs (υZr-O)
Raman υmax (cm−1): 1593 vs (υC=C and υC=O), 1533 w, 1521 w (υC=O and υC=C), 1471 m (δCH), 1422 m, 1408 m (υC=O, υC-C and δCH), 1390 w, 1350 w (υC=C), 1227 m (υC-O), 1082 m (υC-C and δCH), 975 s (υC-C), 879 m (υC-C and δCH), 722 vs (υCH), 662 w (υC-C), 575m, 538 m (υZr-O)
Method 5
Tropolone (3.60 g; 29.8 mmol) was dissolved in chloroform (50 mL). K4[Zr(C2O4)4] (5.00g; 7.25 mmol) was dissolved in 1 M oxalic acid (20 mL) and then neutralised with 1 M sodium carbonate (19 mL) before diluting to 50 mL with water. The solution of tropolone was added to the solution of K4[Zr(C2O4)4] drop wise with stirring. The mixture was stirred for 20 mins before separating the pale yellow chloroform phase. The product was isolated by evaporation at 60°C. Yield 4.64 g (97%).
Synthesis of Zr(ethyl maltolate)4
Method 6
A solution of ethyl maltol (4.00 g; 28.6 mmol) in tetrahydrofuran (100 mL) was added drop wise to solution of zirconium (IV) chloride (1.66 g; 7.12 mmol) in tetrahydrofuran (50 mL) and then stirred for 2 h. The solution was then heated in a water bath at between 50°C and 55°C for 3 h during which time the volume was reduced to ~30 mL. Hexane (50mL) was added to the solution and a pale yellow precipitate was formed. The resulting precipitate was collected by the use of a Büchner funnel. The product was washed with hexane (4 x 20 mL) and dried at 55°C for 3 h in an electric oven. Yield 4.12 g (91%).
Found C 51.8, H 4.3, N 0%; C28H28O12Zr requires 51.9, H 4.4, N 0%
δH (400 MHz, d6-DMSO), 8.24 (1H, d, Ja,b 5.2, Ha), 6.64 (1 H, d, Ja,b 4.8, Hb), 2.50 (2 H, q, J 7.3, CH2), 0.91 (3 H, t, J 7.6, CH3)
δc(100 MHz, d6-DMSO), 180.3 (CO), 155.97 (Ca), 154.36 (C2), 153.79 (C3), 109.94 (Cb), 21.46 (CH2), 11.42 (CH3)
IR υmax (cm−1): 3065 w, 2986 m, 2943 w, 2885 w, 1628 sh (υC=O), 1606 sh (υC=O), 1556 vs (υC=C), 1519 s, 1507 sh, 1473 vs, 1456 sh, 1433 sh (υC-C), 1393 m, 1368 m, 1331 s, 1262 vs, 1235 s, 1187 vs, 1102 w
Raman υmax (cm−1): 1599 vs (υC=O), 1582 sh (υC=O), 1518 s, 1476 m, 1428 w(υC-C), 1327 w, 1264 w, 1048 s, 938 w, 845 m, 769 w, 725 s, 621 w, 603 m, 546 m, 539 m, 525 m, 511 m
Method 7
Ethyl maltol (4.20 g; 30.0 mmol) was dissolved in chloroform (50 mL). K4[Zr(C2O4)4] (5.00 g; 7.25 mmol) was dissolved in oxalic acid (20 mL) and then neutralised with 1 M Na2CO3 before diluting to 50 mL with water. The solution of ethyl maltol was added to the solution of K4[Zr(C2O4)4] drop wise whilst stirring and then stirred for a further 20 mins. The chloroform layer became off white in colour as the Zr(ethyl maltolate)4 was extracted into the chloroform phase which was separated and heated on an electric hotplate at 60°C until the product had precipitated and the remaining chloroform evaporated. Yield 4.22 g (90%).
Synthesis of 89Zr(oxinate)4
89Zr was supplied as Zr4+ (20-90 MBq, measured with a Capintec CRC-25 dose calibrator) in 0.1 M oxalic acid (Perkin-Elmer, Seer Green, UK), brought to pH 7 with 1M Na2CO3 and diluted to 500 μL with distilled water. Oxine in chloroform (500 μL, 1 mg/mL) was added and the vessel was vortexed (1000 RPM) for 15 mins to facilitate phase transfer. The two phases were then allowed to separate and the aqueous phase was transferred into a separate vessel. The chloroform extract was evaporated at 60 °C and the residue containing 89Zr(oxinate)4 was dissolved in 10-20 μL of DMSO and diluted to 1-3 mL with phosphate buffered saline (PBS). Samples were analysed on silica gel impregnated ITLC strips (ITLC-SG; Agilent, UK) with ethyl acetate mobile phase. 8-9 cm long strips were developed in 50 mL centrifuge tubes. Chromatograms were evaluated on a LabLogic MINI-SCAN radio TLC linear scanner connected to a LabLogic B-FC-3200 NaI detector for gamma photon detection. 89Zr(tropolonate)4 and 89Zr(ethyl maltolate)4 were prepared similarly.
Cell Cultures
HCT116: colon cancer, J774: mouse macrophage and MDA-MB-231 breast cancer cell lines were cultured as adhesion cells at 37°C with CO2 at 5 % under a humidified atmosphere.
Uptake Experiments
89Zr(oxinate)4, 89Zr(tropolonate)4 and 89Zr(ethyl maltolate)4 were diluted to an activity of 0.05 MBq in 50 µL of a serum free medium. This was then added to glass test tubes containing the cell line under investigation (~1 x 106 cells) in 500 µL of a serum free medium. The percentage of uptake was determined in triplicate at 1, 15, 30, 45 and 60 minutes after adding the tracer to the cell line under investigation. Percentage uptake was determined at each of the time points by centrifuging the sample for 5 minutes at 2500 RPM. 450 µL of supernatant was collected using a micro pipette and placed into a clean glass vial. The supernatant (S) and cell pellet (C) were placed separately into a gamma counter to calculate the percentage of radioactivity in the labelled cells using the equation; (C / (C + S)) x 100. Controls were the uptake of neutralised 89Zr(oxalate)4 and the determination of the degree of binding of the tracers to the glass test tubes.
Efflux Experiments
Following the method reported above the 89Zr tracers were allowed 30 minutes for uptake to occur in the respective cell lines. Efflux was measured at time points of 1, 2, 3, 20 and 24 hrs depending on the tracer. Samples were centrifuged at ~ 2000 RPM for 5 mins. 450 µL of supernatant was then removed from the glass reaction vial. Cell pellets were washed with phosphate buffered saline (PBS) 500 µL x 2 to remove any excess tracer. Fresh media (with serum, 500 µL) was added to each reaction vial. Samples were incubated at 37°C until the required time point. At the required time point the sample was centrifuged at 3000 RPM for 5 mins to obtain a cell pellet. Supernatant (~400-500 µL) from each reaction vial was placed into a new vial and matched to its cell pellet and both were analysed for radioactivity utilising a gamma counter. Percentage retention of the tracers in the labelled cells was calculated by the comparison of the total activity in the cells and the supernatant.
Supplementary Material
Acknowledgements
This research was supported by the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC under grant number WT088641/Z/09/Z, and the Kings College London and UCL Comprehensive Cancer Imaging Centre funded by the CRUK and EPSRC in association with the MRC and DoH (England), and by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. LM was supported by a grant from Leukaemia and Lymphoma Research. PC was supported by a PhD scholarship funded by Ramathibodi Hospital, Faculty of Medicine, Mahidol University, Thailand.
Footnotes
Footnote
Electronic supplementary information (ESI) available: Contains NMR, IR, Raman and UV-visible spectra.
References
- 1.Intenzo CM, Desai AG, Thakur ML, Park CH. Journal of Nuclear Medicine. 1987;28:438–441. [PubMed] [Google Scholar]
- 2.Puncher MRB, Blower PJ. Journal of Nuclear Medicine. 1995;36:499–505. [PubMed] [Google Scholar]
- 3.Yoon J, Park B, Shim W, Shin JY, Lee G, Ahn YH. Nucl Med Biol. 2010;37:381–388. doi: 10.1016/j.nucmedbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Kotze HF, Heyns AD, Lotter MG, Pieters H, Roodt JP, Sweetlove MA, Badenhorst PN. Journal of Nuclear Medicine. 1991;32:62–66. [PubMed] [Google Scholar]
- 5.Kathirgamanathan P, Surendrakumar S, Antipan-Lara J, Ravichandran S, Reddy VR, Ganeshamurugan S, Kumaraverl M, Arkley V, Blake AJ, Bailey D. Journal of Materials Chemistry. 2011;21:1762–1771. doi: 10.1039/c0jm02644a. [DOI] [Google Scholar]
- 6.Muetterties EL, Alegranti CW. J Am Chem Soc. 1969;91:4420–4425. doi: 10.1021/ja01044a016. [DOI] [Google Scholar]
- 7.Guerard F, Lee Y, Tripier R, Szajek LP, Deschamps JR, Brechbiel MW. Chemical Communications. 2013;49:1002–1004. doi: 10.1039/c2cc37549d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nepveu F, Jasanada F, Walz L. Inorg Chim Acta. 1993;211:141–147. doi: 10.1016/S0020-1693(00)85593-0. [DOI] [Google Scholar]
- 9.Abrahams I, Choi N, Henrick K, Joyce H, Matthews R, McPartlin M, Brady F, Waters S. Polyhedron. 1994;13:513–516. doi: 10.1016/S0277-5387(00)81668-X. [DOI] [Google Scholar]
- 10.Frazer MJ, Rimmer B. J Chem Soc A. 1968:2273–2275. doi: 10.1039/J19680002273. [DOI] [Google Scholar]
- 11.Lewis D, Fay R. Journal of the Chemical Society-Chemical Communications. 1974:1046–1047. doi: 10.1039/c39740001046. [DOI] [Google Scholar]
- 12.Davis A, Einstein F. Acta Crystallographica Section B-Structural Science. 1978;34:2110–2115. doi: 10.1107/S0567740878007530. [DOI] [Google Scholar]
- 13.Fim FDC, Machado T, De Sa DS, Livotto PR, Da Rocha ZN, Basso NRDS, Galland GB. Journal of Polymer Science Part A-Polymer Chemistry. 2008;46:3830–3841. doi: 10.1002/pola.22734. [DOI] [Google Scholar]
- 14.Wagner CC, Gonzalez-Baro AC, Baran EJ. Spectrochimica Acta Part A-Molecular and Biomolecular Spectroscopy. 2011;79:1762–1765. doi: 10.1016/j.saa.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 15.Monzon LMA, Burke F, Coey JMD. Journal of Physical Chemistry C. 2011;115:9182–9192. doi: 10.1021/jp201019c. [DOI] [Google Scholar]
- 16.Parajon-Costa BS, Baran EJ, Romero J, Saez-Puche R, Arrambide G, Gambino D. Journal of Coordination Chemistry. 2011;64:57–70. doi: 10.1080/00958972.2010.531131. [DOI] [Google Scholar]
- 17.Jianlin Y, Yaxian Y, Renao G. Spectrochimica acta.Part A, Molecular and biomolecular spectroscopy. 2006;64:1072–6. doi: 10.1016/j.saa.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Parajon-Costa BS, Baran EJ. Spectrochimica Acta Part A-Molecular and Biomolecular Spectroscopy. 2011;78:133–135. doi: 10.1016/j.saa.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Parajon-Costa BS, Baran EJ. Spectrochimica Acta Part A-Molecular and Biomolecular Spectroscopy. 2013;113:337–339. doi: 10.1016/j.saa.2013.04.119. [DOI] [PubMed] [Google Scholar]
- 20.Wagner CC, Parajon-Costa BS, Baran EJ. Latin American Journal of Pharmacy. 2011;30:1454–1456. [Google Scholar]
- 21.Baiocchi C, Saini G, Bertolo P, Cartoni G, Pettiti G. Analyst. 1988;113:805–807. doi: 10.1039/an9881300805. [DOI] [Google Scholar]
- 22.Meszaros LK, Charoenphun P, Chuamsaamarkkee K, Ballinger JR, Mullen GED, Ferris TJ, Went MJ, Blower PJ. 89Zr-Oxine Complex: a Long-Lived Radiolabel for Cell Tracking Using PET. World Molecular Imaging Conference; 2013. [Accessed 26 June 2014]. http://www.wmis.org/abstracts/2013/data/index.htm. [Google Scholar]
- 23.Ferris TJ, Charoenphun P, Went MJ, Blower PJ. Nucl Med Commun. 2013;34:362. [Google Scholar]
- 24.Johnson FA, Larsen EM, Rollinson CL, Lindsay J. In: Inorganic Syntheses. Holtzlaw Henry F., Jr, editor. John Wiley & Sons; Hoboken, NJ, USA: 1966. pp. 40–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.