Abstract
Radioisotopes of elements from all parts of the periodic table find both clinical and research applications in radionuclide molecular imaging and therapy (nuclear medicine). This article provides an overview of these applications in relation to both the radiological properties of the radionuclides and the chemical properties of the elements, indicating past successes, current applications and future opportunities and challenges for inorganic chemistry.
Introduction
Since the first experimental use of the beta-emitting cyclotron-produced radionuclide phoshorus-32 for treatment of patients with haematological disease in the 1930s, the use of radioisotopes in medicine has expanded into a mainstream clinical speciality incorporating both diagnostic imaging (exploiting the tissue penetration of gamma rays derived from nuclear decay or positron annihilation) and targeted therapy (exploiting the cellular toxicity of non-penetrating alpha and beta particles and secondary electrons). Via landmark steps including clinical use of iodine-131 for therapy and diagnosis of thyroid disease, and key technologies such as the Anger gamma camera and, later, single-photon emission tomography (SPET), the technetium-99m generator, positron emission tomography (PET) and medical cyclotrons, radioisotope imaging has evolved into today’s set of cutting-edge technologies with applications and instrumentation dedicated not only to clinical medicine but to basic biomedical and preclinical research. Over this time the range of available radioisotopes, produced by cyclotrons, nuclear reactors and generators, has grown and now represents all areas of the periodic table with applications that have evolved to make best use of both the radiological (half-life, specific activity and of course emission type – alpha, beta, gamma, positron, Auger electron, and even associated Cerenkov light emission) and chemical properties (biological behaviour and chemical suitability for incorporation into complex targeting molecules and bioconjugates) of the radioelement for the biomedical purpose at hand. This article presents a survey of the variety of these properties and their uses, linking properties to applications and briefly reporting on current developments and future challenges for inorganic chemists in this field, using the Periodic Table (Fig. 1) – the “nuclear chocolate box”, from which selections can be made to suit a variety of different needs - as a basis of classification. In this way it is hoped that the article will provide a map for navigation of radionuclide imaging and therapy specifically for inorganic chemists. It should indicate of the role of the inorganic chemist, who must partner with biologists, clinicians, physicists, pharmacologists, radiochemists and other specialists to move forward in this highly multidisciplinary field. The material is arranged roughly by periodic group, with some flexibility in this approach to allow for parallels in applications to be grouped together where this is appropriate; lanthanides and actinides are accorded the status of “honorary groups” rather than periods for this purpose. Only elements that have radioisotopes with a significant current or promising clinical or research application are included; some groups offer greater riches in this respect than others. Elements of particularly current topical interest are considered in somewhat more detail.
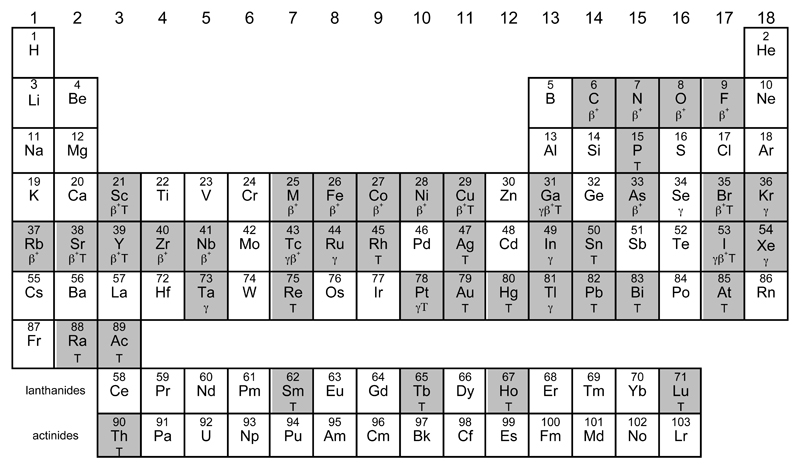
Figure 1.
Periodic table highlighting (shaded) elements with radionuclides with uses or identified potential uses in molecular imaging or targeted radionuclide therapy. Main uses are identified by symbols: γ (gamma camera imaging/SPET), β+ (positron emission tomography) and T (therapy, using beta-, alpha, or Auger-emitting radioisotopes). Some unshaded elements also have indirect importance e.g. as parent radionuclides for directly-used radionuclides, or as cyclotron target materials, or chemical binding sites for biomolecule labelling, hence the “usefulness” is somewhat arbitrary.
Group 1
In group 1, only rubidium offers an imaging radioisotope with a significant biomedical application – the short half-life positron emitter 82Rb (β+, 95%, half-life 75 s). As a group 1 metal whose chemical speciation under biological conditions is almost completely restricted to the hydrated cation, its potential for incorporation into complex targeting molecules is negligible. Nevertheless, its periodic position underlies its application; Rb+ is a potassium mimic and its ionic radius, despite being larger than that of K+, does not prevent it serving as a substrate, in place of K+, for the Na+/K+-ATPase, a ubiquitous antiporter in cell plasma membranes that pumps potassium ions into cells and sodium ions out, in the ratio 2:3, driven by and coupled to the hydrolysis of ATP. This pump is most abundant and active in the myocardium, resulting in 82Rb+ that has been delivered to the heart via blood becoming trapped rapidly in the myocardium, provided blood flow is adequate to deliver it and the tissue is sufficiently metabolically active and energetic to drive the pump. Images of the distribution in the myocardium, obtained by PET, provide a map of its perfusion, giving valuable information about the patient’s condition for the cardiologist.1 An example is shown in Fig. 2.
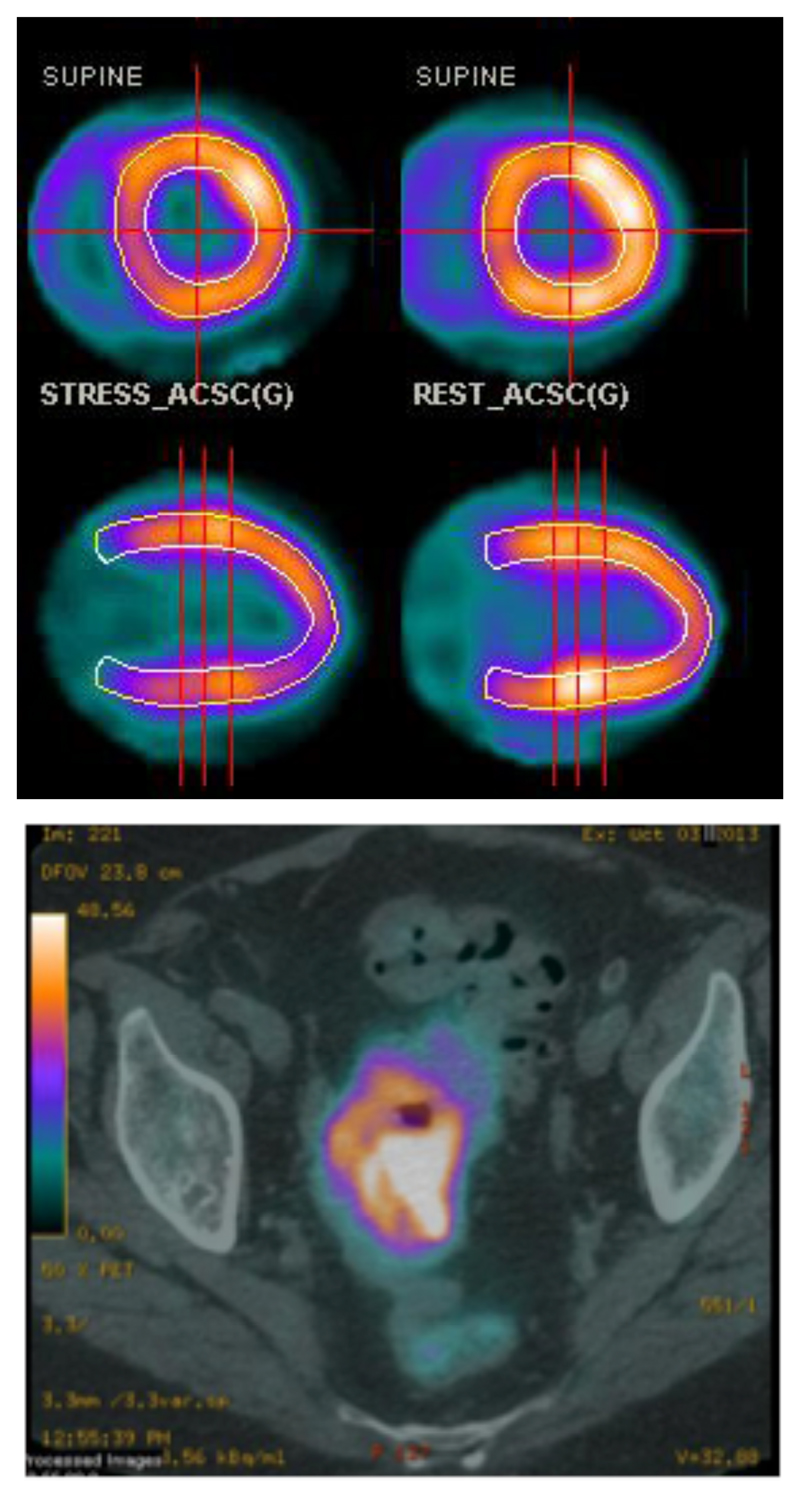
Figure 2.
Top: Example of myocardial perfusion PET imaging using 82Rb+ (transverse (top) and sagittal (bottom) sections); bottom: PET-CT scan (transverse section, pelvic region) of a patient with colorectal cancer after injection of 82Rb+. (Images courtesy of Prof A. M. Groves, University College London Hospitals)
The excellent utility of this radioisotope for this purpose arises from its high-abundance positron emission, and its availability from a convenient generator in which the parent isotope is strontium-82 (half-life 25 days), adsorbed on a solid phase such as stannic oxide, decays to 82Rb which is eluted with physiological saline. Because of the 75 s half-life, there is not time for manipulation with syringes or procedures for radiolabelling or sterilisation, and the generator is “plumbed” directly into the vein of the subject via an infusion set. The short half-life allows repeated administration and imaging after a short interval, while keeping the radiation dose to the patient low. Although costly, this is now a commonplace procedure especially in the US.
Myocardial perfusion scanning using the 82Rb-generator relies on the same biochemical principle as the older (>30 years) use of monovalent thallium-201 (gamma-emitter), which is also a potassium mimic, for obtaining similar clinical diagnostic information with a gamma camera (see Group 13). 201Tl+ scanning has also been applied in the past to tumour imaging, relying on the same biochemical pathway, suggesting that application of 82Rb could be extended in the same way. Accordingly, a recent evaluation in patients with colorectal and lung cancer confirmed tumour avidity of the tracer and the ability to map tumour perfusion (Fig. 2),2 opening up a new clinical field of application for this radionuclide.
Group 2
Like the group 1 elements, the group 2 metals are not amenable to kinetically stable incorporation into molecules and bioconjugates, and their radioisotopes find their application mainly through mimicry of calcium. Thus the beta emitter strontium-89 (half-life 53 days) has been used for many years as a palliative therapy for painful bone metastases in cancer, especially prostate cancer, by exploiting toxicity of the beta emissions, most likely towards inflammatory cells in these lesions. After intravenous administration of 89SrCl2, the strontium is transported in way that, to an extent, mimics calcium and it accumulates in sites of active bone mineral deposition (rather than mineral lysis, as found in for example multiple myeloma bone lesions) found in such metastases, leading to higher radiation doses in these tumours than in normal tissues.3 89Sr has no positron or gamma emissions and so is not amenable to high quality quantitative imaging, but the positron emitter 83Sr (β+, 24%, half-life 33 h) which is produced by proton irradiation of 85Rb in a cyclotron,4 or the gamma emitter 85Sr (γ, 100%, half-life 65 days) can in principle be used to determine its quantitative biodistribution.
The other group 2 radionuclide of major importance emerging recently is radium-223 (α, 94%, half life 11.4 days), an alpha emitter, which also mimics calcium in accumulating selectively in sites of bone mineralisation (Fig. 3). The recent success of clinical trials of 223RaCl2 in patients with metastatic prostate cancer represents the first major application of alpha emitting isotopes in humans. It is a significant step forward in therapeutic nuclear medicine opening the door to wider use of alpha-emitter therapy in other cancers.
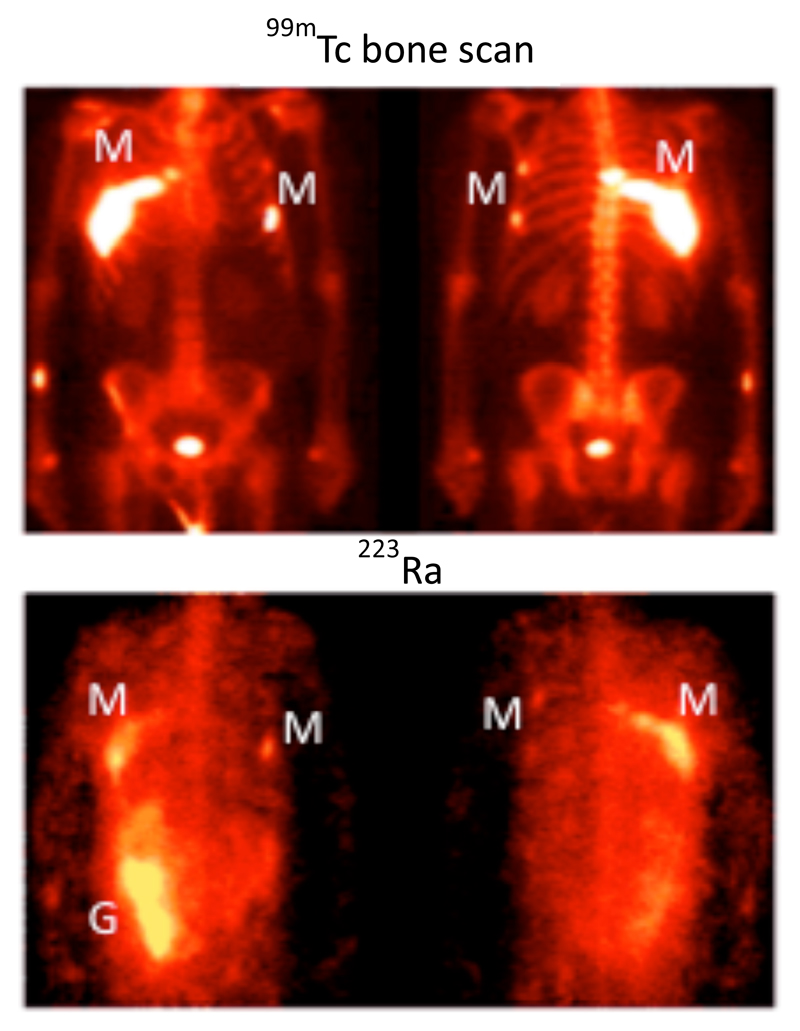
Figure 3.
Biodistribution of 223Ra in a patient with metastatic prostate cancer after i.v. administration of 223RaCl2.5 The anterior (left) and posterior (right) views show specific uptake in the sites of bone metastases indicated by the 99mTc bone scan (M), and activity in the gut (G) indicating radioactivity that has undergone hepatobiliary excretion. The gamma camera image was possible by virtue of a low abundance gamma emission from 223Ra accompanying the alpha decay. (Adapted with permission from ref. 5).
Applications of these therapeutic isotopes in other cancers will require their incorporation into more complex targeting molecules, which is extremely challenging for the group 2 metals as they do not readily form kinetically stable chelates; indeed, the development of novel chemistry to incorporate these metals into bioconjugates with a degree of kinetic stability might be considered a challenge to inorganic chemists. The insoluble nature of many of their salts, including minerals such as hydroxyapatite, may offer the possibility of incorporating them into inorganic nanoparticles. These could then be targeted by derivatising their surface using conjugates of targeting biomolecules with phosphonates and bisphosphonates, which form stable links with the surface of such nanoparticulate materials.6
Group 3
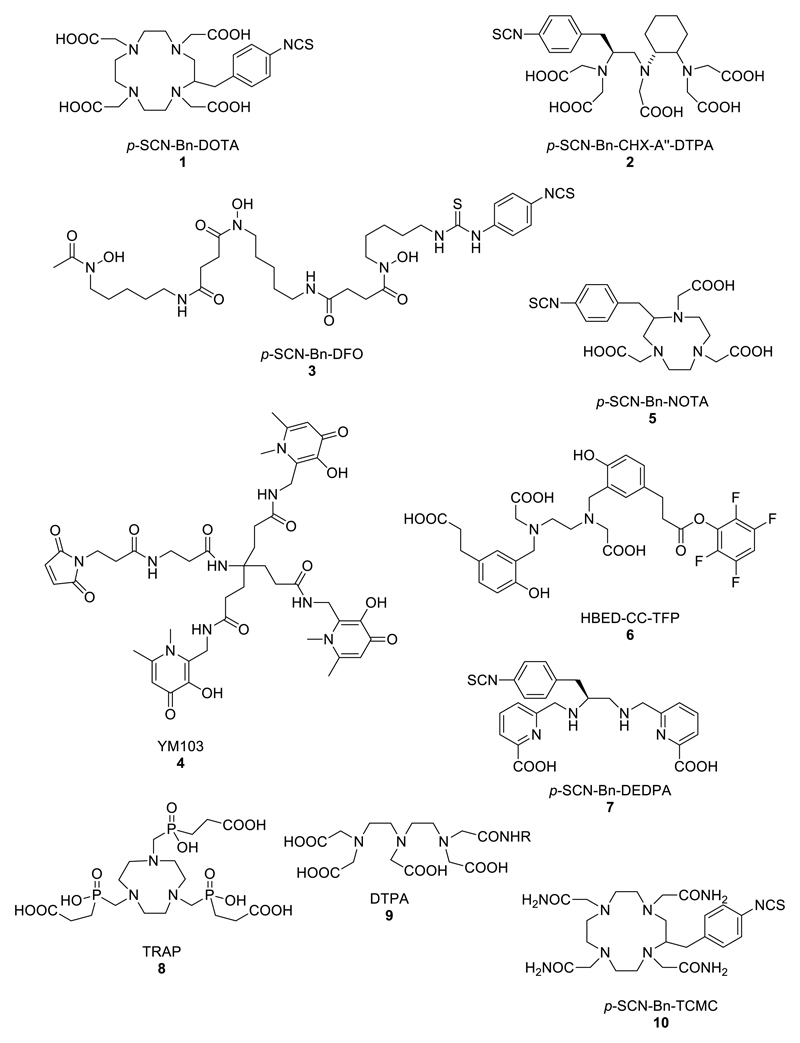
While the basis of the uses of the group 1 and 2 elements is restricted to mimicry of the biological behaviour of their biologically important congeners (namely potassium and calcium), from group 3 onwards there is opportunity to incorporate the radioelement into more complex molecules and bioconjugates for targeted molecular imaging, by chelation or covalent bond formation. From a historical perspective the Group 3 elements are not an ideal starting point since the groundwork for their applications was laid in past decades using related trivalent metals from other parts of the periodic table, especially Group 13 and lanthanides (vide infra). The trivalent, “hard” metal ions from these groups find their main applications as radiolabels for biomolecules, incorporated with use of bifunctional chelators (Fig. 4) which share, to an extent, common design features but with subtle variations to suit individual metals. These are discussed in more detail in their respective sections and have recently been much more comprehensively reviewed elsewhere.7
Figure 4.
Structures of bifunctional chelators used for radioactive di-, tri- and tetravalent metal ions
Group 3 is represented by scandium-44 (β+, 94%, half-life 3.97 h), yttrium-90 (β-, 100%, half-life 64 h) and yttrium-86 (β+, 33%, half life 14.7 h). 44Sc is a recent innovation,8 available through development of the 44Ti/44Sc generator and offering the possibility, after further development, of a PET radionuclide chemically similar to gallium-68 (vide infra) but for applications requiring a somewhat longer half-life (3.97 h c.f. 68 min). The 44Ti parent has a long half-life of 60 years, which raises problems with radioactive waste management. There is potential for a “theranostic” (PET imaging/radionuclide therapy) pair by using it alongside the potential therapeutic radionuclide 47Sc (β-, 100%, half-life 3.35 days).9 DOTA-based chelators (1, Fig. 4) have been employed to link it to targeting biomolecules.
90Y is a pure high-energy (2.28 MeV) beta-emitting radionuclide that has growing applications in radionuclide therapy when coupled to small tumour-targeting peptides and monoclonal antibodies. Its beta emissions have a relatively longer range - about a centimetre in tissue - compared to other therapeutic beta emitters such as 131I and 177Lu. Most prominent currently among the targeting molecules used are the somatostatin analogues, used for radionuclide therapy of neuroendocrine and other tumours that express somatostatin receptors,10 and the antibody-based treatment Zevalin for lymphoma.11 The yttrium is coupled to the targeting peptide or antibody by means of a bifunctional chelator with amine and carboxylate donors, such as the macrocyclic DOTA and non-cyclic CHX-A″-diethylenetriaminepentaacetic acid (2), Fig. 4 (vide infra). Since 90Y has no positron or gamma emissions, quantification of its biodistribution by imaging to assess radiation doses is difficult, and analogues labelled with the gamma-emitter indium-111 have been used as surrogates for this purpose. Inorganic chemists will recognise that this poses uncertainties because the coordination chemistry of indium and yttrium is subtly different and can (and does) lead to differences in biodistribution and clearance. Therefore to obtain definitive and quantitative biodistribution information to underpin radionuclide therapy, the positron emitting isotope 86Y, which can be produced on many biomedical cyclotrons, can be used.12
Group 4
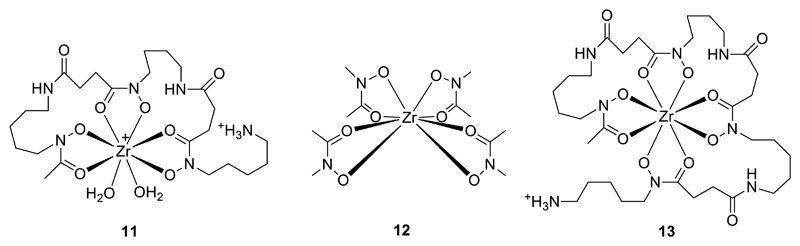
Zirconium-89 (β+, 23%, half-life 78.4 h) is a rising star in Group 4, emerging in response to the need for a longer half-life positron emitter to match the growth in availability of PET scanners in the last decade. 89Zr is quickly becoming the PET replacement for the gamma-emitter indium-111 (see Group 13), which, with its half-life of 67.3 h, has been the radioisotope of choice since the 1980’s for labelling molecules with slow pharmacokinetics, i.e. mainly monoclonal antibodies. IgG antibodies clear very slowly from blood and continue to accumulate in tumour targets and improve target-to-background ratios over a period of several days. Consequently a relatively long half-life is required, and imaging is typically carried out 24-72 h after injection of the tracer. The improvements in image sensitivity and quantification offered by PET compared to SPET have created a desire for a radionuclide with similar chemistry (i.e. amenability to kinetically stable chelation) and radiological half-life. Despite some disadvantages (low positron yield, abundant high-energy gamma emissions, both of which lead to concerns about high patient radiation dose), 89Zr fits this need. This presents a new challenge for inorganic chemists: new chelators are needed that impart both kinetic stability and efficient, mild labelling that overcomes the tendency towards hydrolysis in higher pH conditions, while fundamental studies of the coordination preferences of Zr4+ are less complete than is the case for some other metals described in this article. A number of bifunctional chelators conventionally used with the trivalent metals (see Group 13; Fig. 5), such as DOTA and DTPA derivatives were evaluated without great success but, somewhat fortuitously, the iron chelator desferrioxamine-B (DFO, 3, Fig. 4) proved to combine the most efficient labelling with the best long-term in vivo stability, despite the general acceptance of DFO as a ligand for hard trivalent metals rather than tetravalent ones such as Zr4+, and despite the fact that it cannot satisfy the evident preference shown by zirconium for coordination numbers greater than 6 (Fig. 5).13 Zr4+ readily transchelates from the oxalate complex [Zr(oxalate)4]4- (the form in which is it most often purified from the yttrium target after proton irradiation in the cyclotron) to form the DFO complex under mild conditions. Antibodies labelled with 89Zr through conjugation with DFO allow imaging for several days, in both experimental animals and humans, with very little loss of 89Zr (which is manifested in uptake in the bones and joints, as exemplified in Fig. 6). This has made possible the use of 89Zr-PET to evaluate the biodistribution in humans of several candidate antibody therapeutics,13–16 and to demonstrate the advantages of 89Zr-PET over SPET (even with the optimal SPET isotope 99mTc), for example in sentinel lymph node imaging in humans.17 Efforts are in progress to develop ligands with superior Zr4+ chelation properties compared to DFO, including the tripodal tris(hydroxypyridinone) hexadentate chelator YM10318 (4, Fig. 4) developed for use with gallium-68.19 A promising strategy is represented by octadentate designs based on a tetrapodal array of four bidentate oxygen donor ligands.20, 21 The structure of the Zr4+ tetrakis(N-methylacetohydroxamate) complex, which has a 4:1 ligand:metal stoichiometry forming a mononuclear eight-coordinate structure22 (Fig. 5), suggests that an assembly of four such bidentate ligands is the ideal way to meet the coordination preferences of zirconium. It has been most conveniently and elegantly implemented by linearly extending the DFO ligand to include a fourth hydroxamate group, producing an octadentate ligand 13 that on (very preliminary) evaluation appears to have the expected advantages over DFO itself23 (Fig. 5). This is a highly topical endeavour in radiometal chemistry; nevertheless, it should be recognised that, meanwhile, DFO itself is an extremely effective chelator (Fig. 6), with only slight translocation of radioactivity from labelled antibody to bone allowing high-quality PET imaging to be continued clinically.
Figure 5.
Left: Modelled coordination of Zr4+ by DFO (11);20 Centre: Schematic structure of ZrL4 (HL = N-methylacetohydroxamic acid) (12), serving as a template for the design of new octadentate chelators for Zr+ (note that disorder in the C and N orientations of the hydroxamate rings precludes determination of their preferred relative orientation and suggests that any such preferences are weak);22 Right: Schematic structure of Zr4+ complex 13 of an octadentate DFO homologue.23
Figure 6.
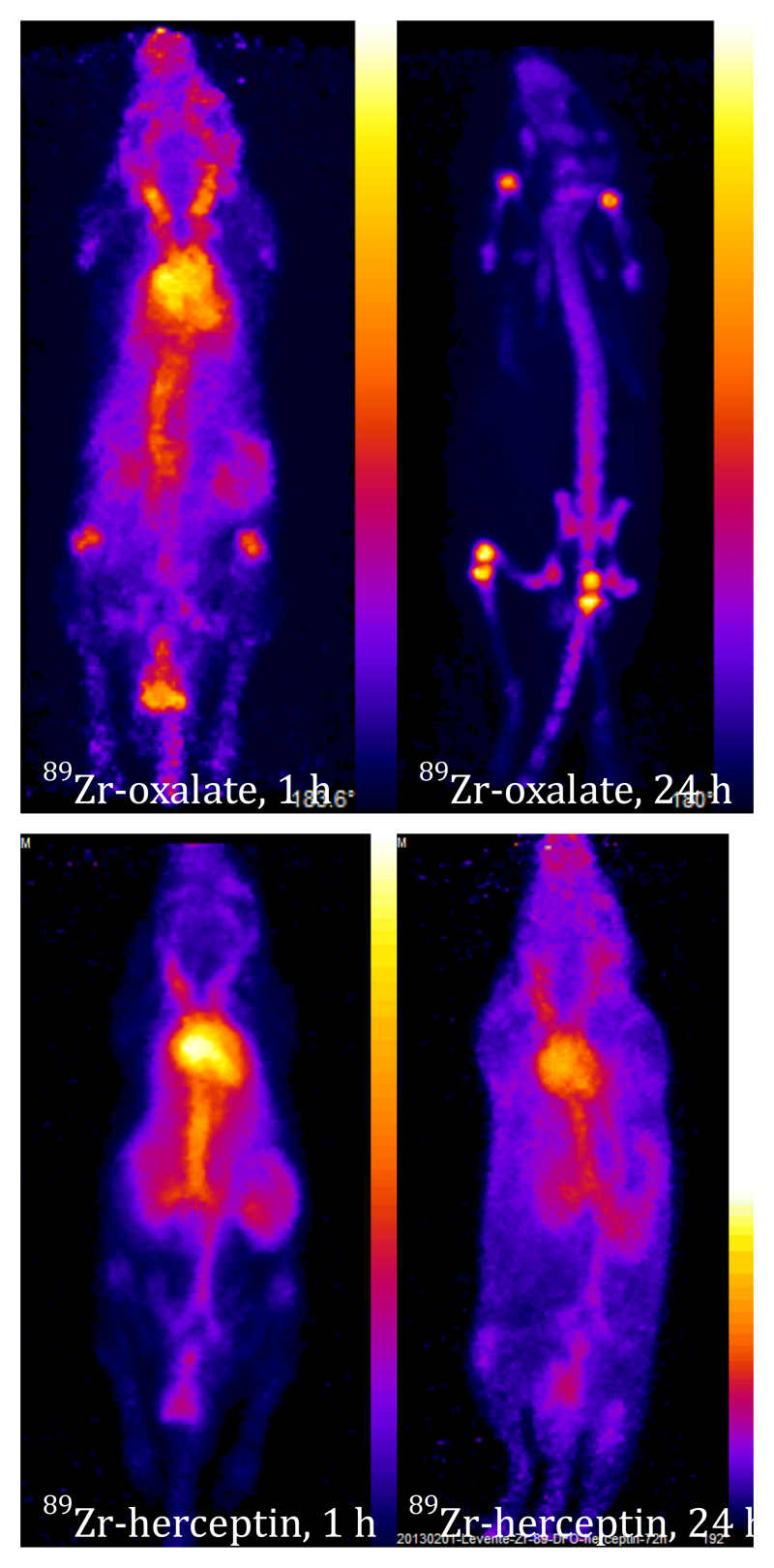
PET images showing biodistribution of 89Zr-oxalate complex (top) and 89Zr-labelled trastuzumab-DFO conjugate (bottom) at 1 h (left) and 4 h (right) post-injection in mice. Oxalate complex is gradually cleared from blood to bone and to some extent excreted through kidney, while the labelled antibody remains largely in the blood pool until at least 24 h, with only slight uptake in bone by 24 h.
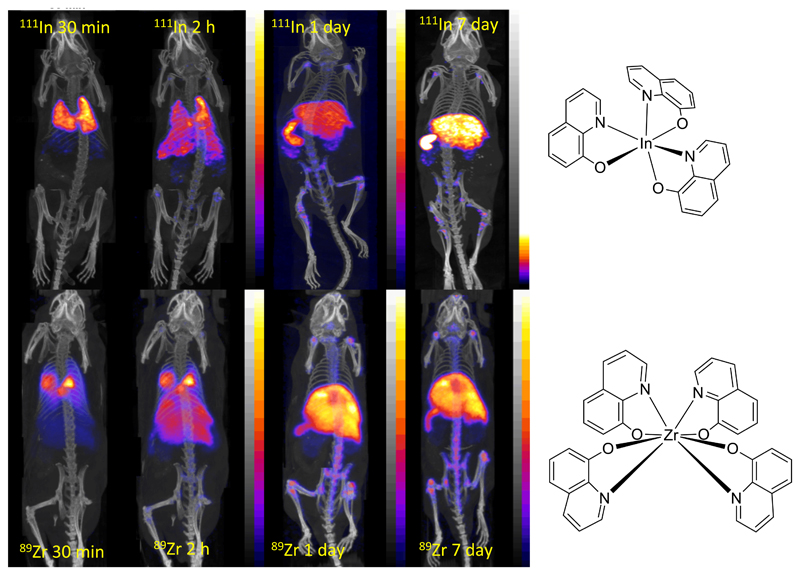
Another application, of growing importance, that calls for a long half-life positron emitter is radiolabelling of living cells for tracking their migration through the body over a period of several days. This has been a major role for the gamma-emitter 111In (see Group 13) for more than three decades, but the advantages of PET over SPET stimulated a search for positron-emitting analogues. Despite the difference in preferred oxidations state, there are parallels in chemical behaviour that provide a rationale for design of such tracers. Thus, by analogy with the metastable lipophilic complex [InL3] (L = bidentate uninegative ligand such as oxinate and tropolonate, Fig. 7), which non-specifically diffuses into cells and dissociates allowing the released In3+ to bind to intracellular macromolecules, Zr4+ forms metastable lipophilic complexes [ZrL4] which behave similarly. The 89Zr oxinate complex has shown great promise in labelling a range of cell types, giving, in addition to the imaging advantages of 89Zr over 111In, equivalent cell labelling yields, better cellular retention of radioactivity and lower toxicity in vivo than 111In. Cancer cell migration has been followed for up to 14 days in a mouse model of multiple myeloma using this tracer (Fig. 7).24
Figure 7.
PET/CT scans comparing cell tracking with 111In(oxinate)3 (top) and 89Zr(oxinate)4 (bottom) labelled myeloma cells in mice. Cells initially accumulate in lungs, followed by migration to spleen, liver and bone marrow. Less uptake in kidney is observed for 89Zr, suggesting greater stability and cell survival.24
Group 5
Very recently niobium-90 (β+, 53%, half-life 14.6 h) has emerged as a promising radionuclide for positron emission tomography (PET). To date, although it can be attached to monoclonal antibodies using the bifunctional chelator DFO, limitations in the separation chemistry have impeded attempts at exploring the radiochemistry and imaging potential.25 Tantalum-178 (γ, half–life 9.3 min) is generated and concentrated on site as needed from W–178 (half–life 21.7 days) using a semi–automated generator system to maximise consistency.26 Its chemistry has not been developed for specific targeting applications.
Group 6
Chromium-51 (γ, 9.9%, half-life 27.8 days) emits gamma rays of too high energy, and has too long a half life, for imaging applications but is used in nuclear medicine for non-imaging tracer applications. Molybdenum-99 and tungsten-188 are indirectly important as parent isotopes for technetium-99m and rhenium-188 (see Group 7).
Group 7
Manganese-52m (β+, 98.3%, half-life 21.1 min) can be produced from the 52Fe/52mMn generator, and in unchelated form (most likely Mn2+) is rapidly taken up in the myocardium and is a potential myocardial perfusion agent.27 However, it has not been adopted in clinical use.
Technetium-99m (γ, 90%, half-life 6.02 h) has for several decades been the radioisotope used in the largest number of clinical radionuclide scans, and the invention of the 99mTc generator, enabling daily availability of radiopharmaceuticals in all major hospitals, was one of the key steps in the evolution of nuclear medicine as a routine service. The generator overcomes the problem of worldwide distribution of the short half-life (6 h) radionuclide, by enabling it to be shipped in the form of its parent radionuclide molybdenum-99 (half-life 60 h), as molybdate bound to an alumina stationary phase. Upon decay of 99Mo, [99mTc]-pertechnetate is formed and can be eluted from the stationary phase with physiological saline. The chemistry of technetium is so diverse, and its importance to nuclear medicine so great (because of the generator availability, because the single gamma photopeak at 141 KeV offers a close to ideal balance of attenuation and gamma camera sensitivity and resolution, and because many complexes can be synthesised by a simple one-step “kit” process) that it would warrant a Dalton Discussion to itself. Therefore, after a brief historical summary, this section will focus on the most current and topical developments.
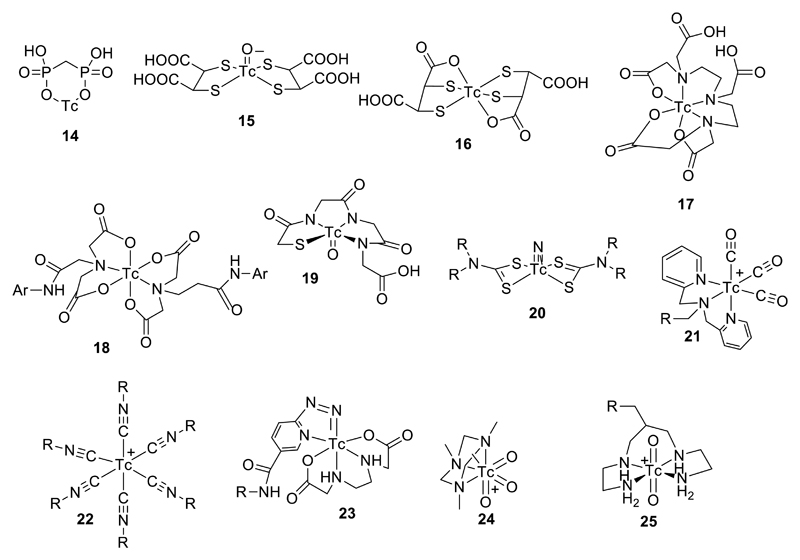
Early implementation of 99mTc radiopharmaceuticals (1970s) was based on empirical biological evaluation of a variety of 99mTc chelates, with ligands such as dithiolates, bisphosphonates and amino- and hydroxyl-carboxylates, without guidance from the detailed knowledge of technetium coordination chemistry that is available today. This led to the first generation of 99mTc radiopharmaceuticals, many of which are still in routine use today (e.g. 99mTc-MDP (14), 99mTc-DMSA (15, 16), 99mTc-DTPA (17), 99mTc-HIDA (18), Fig. 8) for imaging bone disease, kidney perfusion, kidney function and liver function, respectively) despite lack of full understanding of their structure and mechanism. In the 1980s, a more design-and-mechanism based approach began to emerge, informed by a growing knowledge of the coordination and oxidation state preferences of technetium. This led to a range of technetium “cores” – stable metal complex units that could be modified to control pharmacokinetic and targeting properties, without loss of control of the technetium coordination sphere. This was the basis for the second generation of 99mTc tracers which include well-defined compounds such as lipophilic cations for myocardial perfusion imaging (99mTc-sestamibi, 99mTc-tetrofosmin)28 and uncharged metastable lipophilic complexes capable of crossing the blood-brain barrier and cell-labelling (99mTc-HMPAO,29, 30 99mTc-NOET31). In these, the biological behaviour is a consequence of the physicochemical properties of the complex such as charge, protic equilibria, lipophilicity, size, kinetic stability and reactivity, and possible redox reactivity. The development of a set of technetium cores32 including TcO3+ (e.g. 19),33, 34 TcN2+ (e.g. 20),35 Tc(CO)33+ (e.g. 21),36, 37 Tc(CNR)6+ (e.g. 22),38 Tc(hynic)3+ (e.g. 23),39 etc., see Fig. 9) with well-defined coordination chemistry also laid the foundation for the incorporation of 99mTc into bioconjugates such as peptides and antibodies, and conjugates with small molecules and nanoparticles. Most current work is based on these established cores – there is rather little recent literature reporting new cores; one interesting recent example is the Tc(VII) core TcO3+ (e.g. 24), to which the 3+1 cycloaddition of alkenes offers a potential new route to bioconjugation of 99mTc40, 41 (Fig. 8).
Figure 8.
A selection of established 99mTc radiopharmaceuticals and core structures (if known): TcMDP (14), Tc(V)DMSA (15), TcDMSA (16), TcDTPA (17), TcHIDA (18) TcMAG3 (19), Tc(V)-nitridobis(dithiocarbamate) (20), Tc(I)-tricarbonyl (21), Tc(I)-hexakis(isonitrile) (22), Tc(V)-hynic (23), Tc(VII)-trioxo (24), and Tc(V)-dioxo (25). Structures of 15, 19, 20, 21, 22, 24 and 25 are known and well defined; the others are not clearly defined.
Figure 9.
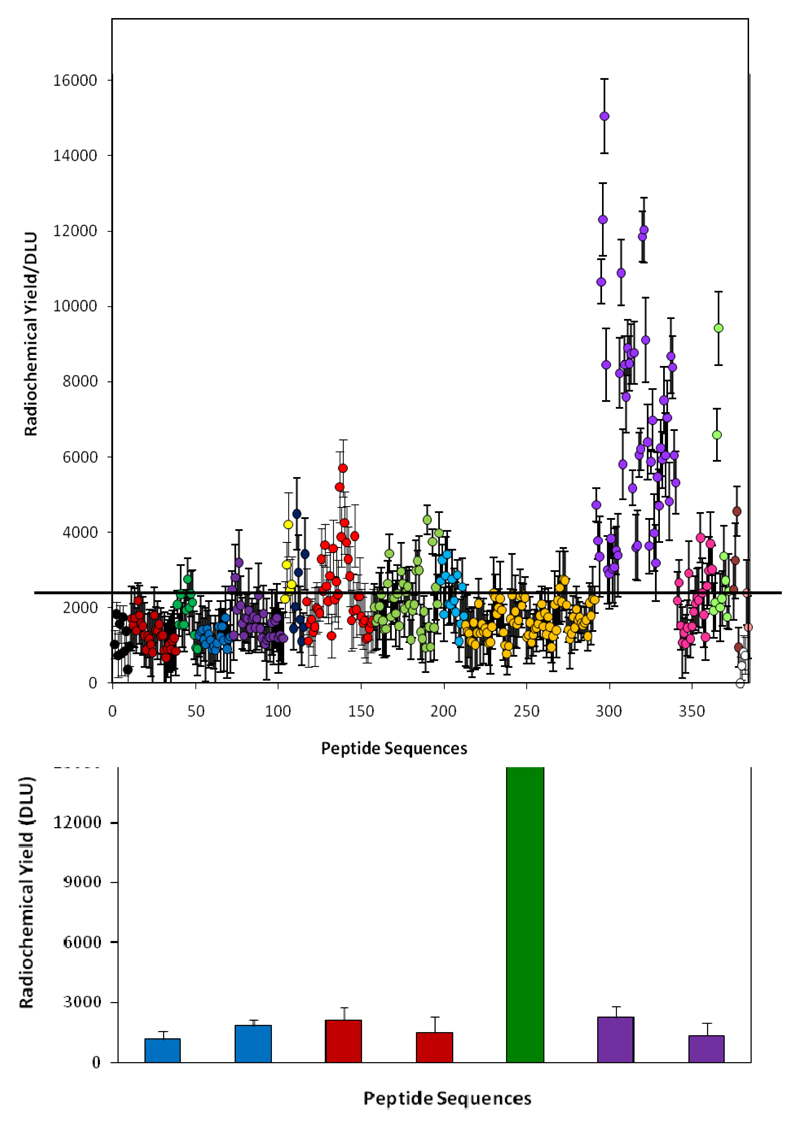
Optimisation of His-tag sequences for 99mTc tricarbonyl labelling from high throughput screening. Top: relative labelling efficiency of a range of peptides. Purple symbols represent His-tags with adjacent arginine or lysine residues (i.e. positively charged sequences). Other colours represent uncharged and negatively charged sequences. Bottom: Comparison of labelling efficiency of optimal binding sequence selected from the screening (green)53 with established his tags sequences.48–50
Despite the historical importance of 99mTc to nuclear medicine, the nuclear medicine community is approaching something of a crossroads in the development of technetium chemistry, and decisions will have to be made about how to, and indeed whether to, make its future secure. The world’s supply of the parent isotope 99Mo emanates from a small number of aging nuclear reactors, some of which have recently closed temporarily for planned refurbishment or unplanned repairs, during which 99mTc was in short supply and nuclear medicine departments resorted to alternatives (such as [201Tl]-TlCl for myocardial perfusion imaging (see Group 13) and [18F]-fluoride for bone metastases. This situation will inevitably repeat itself and the future unlimited availability of 99mTc cannot be assured without new investment – either in new reactors to produce more 99Mo, or in development of alternative methods (e.g. cyclotron methods) to produce 99Mo, or cyclotron routes to produce 99mTc directly.42 Given that the overriding advantage of 99mTc as the most widely used radioisotope in nuclear medicine is the 99Mo/99mTc generator, cyclotron production of 99mTc has limited appeal because of distribution problems. At a time when PET is rapidly growing and the gallium-68 generator is set to make PET more widely accessible (see Group 13), perhaps even (to stretch a point) allowing 68Ga PET to supplant 99mTc SPET as the workhorse of nuclear medicine, there are some hard decisions to be made in the near future.
Despite the present uncertainty, there is some innovation in 99mTc chemistry currently taking place. The main current challenges being addressed are improving the efficiency of bioconjugate labelling and the quality (homogeneity, specific activity, in vivo stability, target affinity) of the labelled product, and producing radiotracers for combined modality imaging (e.g. SPET/MRI, SPET/optical) for use with the emerging generation of combined modality scanners. One of the key attributes of 99mTc that has led to its predominance is the ability to synthesise a range of complexes in a one-pot, one-step process whereby [99mTc]-pertechnetate in saline (generator eluate) is simply added to a “kit” vial containing the necessary reducing agent, ligand and other constituents required to produce the desired radiopharmaceutical without need for purification or other subsequent processing. This simplicity means that many sterile 99mTc radiopharmaceuticals can be produced daily with minimal technologist input or costly equipment. While this is well-established for the “first generation” radiopharmaceuticals referred to above, the development of targeted 99mTc bioconjugates has not yet reached this level of simplicity. Until it does, these “third generation” 99mTc-labelled biomolecular imaging agents will not be routinely available to maximally benefit patients. The current most popular cores for bioconjugate labelling include Tc(hynic)3+, TcO3+, and Tc(CO)3+. All have limitations at present. Hynic is a bifunctional chelator that provides reasonably stable links to biomolecules, very efficiently at very low concentration, via one-step labelling, with high specific activity, at room temperature. However, while its coordination chemistry is better understood than when first introduced,39, 43 it is subject to exchange of co-ligands giving rise to mixtures of subtly different bioconjugates and potential co-ligand exchange in vivo. The ideal accompanying ligand to remedy these limitations (replacing tricine, ethylenediamine diacetic acid, water-soluble phosphines etc.) has yet to be introduced. The TcO3+ core can be linked to biomolecules by a variety of nitrogen- and sulfur-donor polydentate ligands such as mercaptoacetyl triglycine (MAG3, 19),44 1,4,8,11-tetraaminododecane (25),45, 46 dimercaptosuccinic acid (15)47 etc. (Fig. 8). These are highly stable but none have been developed into a simple, mild one-step labelling process. The Tc(I) tricarbonyl core Tc(CO)3+ has been the subject of much attention and application, for labelling a range of small molecules, peptides and especially for radiolabelling proteins incorporating a histidine tag (a sequence of histidines first developed as a motif to assist protein purification during biological production). The labelling procedure requires first the synthesis of the putative intermediate [99mTc(CO)3(H2O)3]+,37 which is then incubated with the His-tagged protein producing a highly stable conjugate in which the technetium is coordinated by at least two imidazole groups of histidine residues. Recently, attempts have been made to place this bioconjugation step on a more efficient footing, by modifying the design of the His-tag, either to improve the biodistribution properties of the labelled protein48, 49 or to improve the labelling efficiency50–53 so that milder conditions can be employed and lower protein quantities can be labelled without need for the purification step (usually size-exclusion chromatography) which is usually a part of the process (Fig. 9). Incorporation of positively charged residues, and/or methionine or cysteine, adjacent to the His-tag sequence resulted in almost an order of magnitude increase in labelling efficiency when conjugating in phosphate buffers, for reasons that are yet to be elucidated.53 This shows that there are ways in which the properties and general utility of biomolecule labelling with 99mTc can be improved, and the further development of such methods to achieve a truly single-step, kit-based radiolabelling method for biomolecules is still a challenge for chemists.
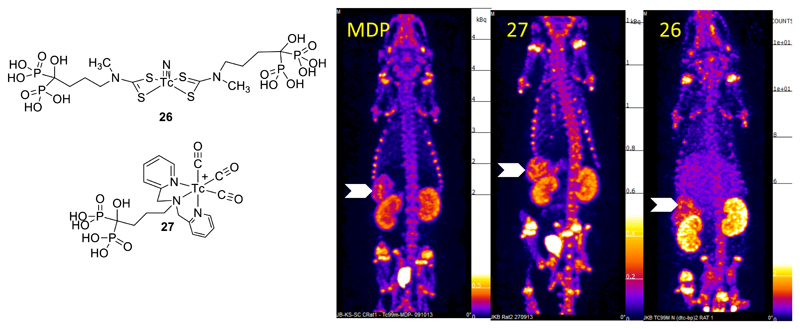
The Tc(V) nitride core,35 with the nitride ligand derived from hydrazine derivatives such as succinic dihydrazide, has recently been the subject of increased interest as a centre to which small molecules can be attached by means of dithiocarbamate ligands, a wide range of which can be easily synthesised by treatment of a primary or, preferably, secondary amine-containing molecule with carbon disulfide. This was first applied in the myocardial imaging agent 99mTc-NOET,54, 55 and more recently extended to other derivatives such as antibiotics, bisphosphonates and other drug-like molecules.56 In some circumstances the presence of two dithiocarbamate ligands offers, in theory, the opportunity to improve target affinity. An attempt to exploit this to develop improved agents for imaging bone disease and small soft-tissue calcifications produced a bis(bisphosphonate) derivative (Fig. 10) with two pendant bisphosphonate groups) which shows higher in vivo stability than 99mTc-MDP and is able to image vascular calcification in small animal models. However, the extent to which it can improve on molecules containing just one pendant bisphosphonate group (such as the tricarbonyl derivative, Fig. 11), or even 99mTc-MDP, is as yet unproven. New synthetic precursors from which the nitride-ligand may derived have been developed recently, for both technetium and rhenium.57
Figure 10.
SPET images (maximum intensity projection) of vascular calcification in a mouse model 30 min after injection with [99mTc]-MDP, [99mTc]-27 and [99mTc]-26. Arrows indicate uptake in calcified mesenteric arteries. Uptake in bone and clearance via kidneys and bladder is also evident.
Figure 11.
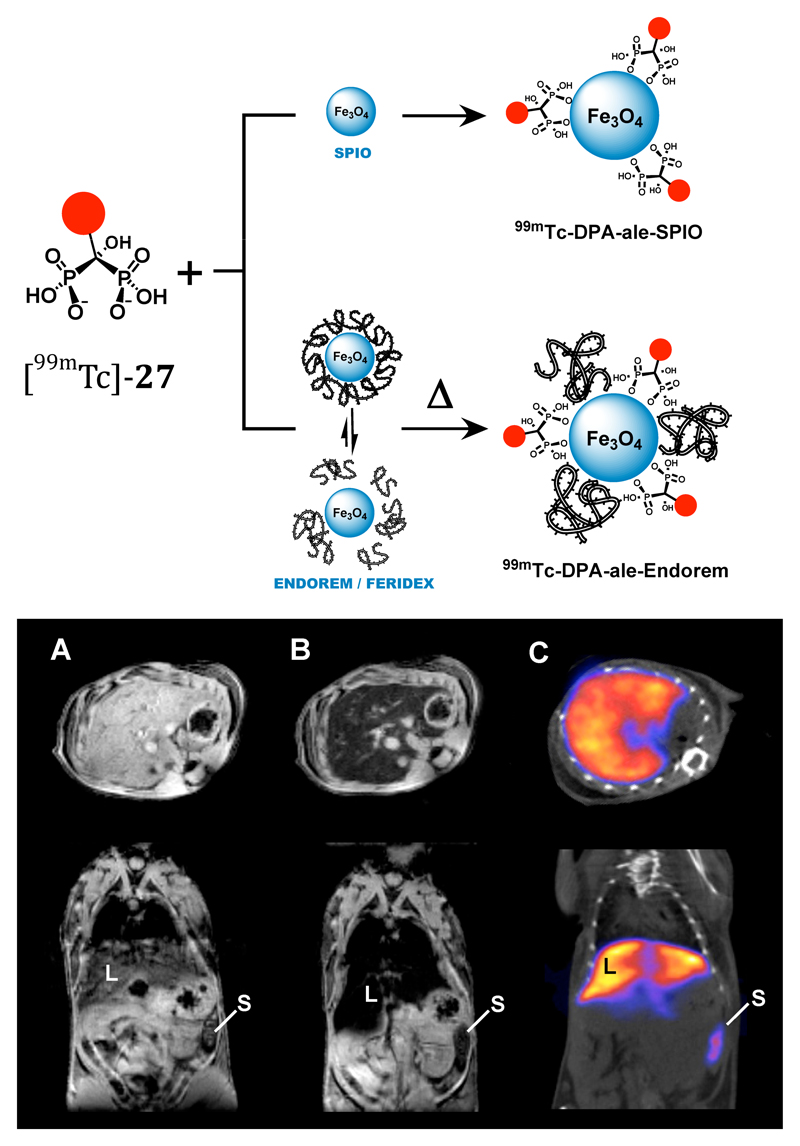
Dual-modality (MRI/SPET) contrast agent combining iron oxide nanoparticles with 99mTc radiolabel linked to the inorganic iron oxide surface via bisphosphonate groups. Top: assembly of composite particle; bottom: Mouse images, transverse sections through liver. A: MR image pre-injection of contrast agent; B: MR image post-injection of contrast agent showing darkening of liver due to accumulation of iron oxide nanoparticles; C: SPET/CT image showing co-localisation of 99mTc with iron oxide contrast in liver (L) and spleen (S).
The arrival of combined modality imaging incorporating radionuclide and magnetic resonance contrast, to exploit simultaneously the advantages of each, has created interest in developing contrast agents that support both modalities in a single species. An attractive clinical use is sentinel lymph node imaging, where surgeons value both pre-surgical imaging (SPET to identify a location and MRI to provide soft tissue contrast and fine structural detail) to identify lymph nodes draining tumours prior to surgery, and fluorescent contrast as a guide during surgery (Fig. 11). Several versions of iron oxide nanoparticles derivatised with radionuclides for such purposes have been reported, including materials with 99mTc linked to the nanoparticle using the tricarbonyl core directly58, 59 or via a bisphosphonate linker60 (Fig. 11); the latter provides greatly improved stability compared to the conventional carboxylate linker used to anchor various modifiers to the surface of iron oxides.
Beta-emitting isotopes rhenium-186 (β-, 92.5%, half-life 90 h) and rhenium-188 (β-, 100%, half-life 17 h) have been of interest to researchers for many years as therapeutic analogues of 99mTc radiopharmaceuticals. However, while other beta emitters such as yttrium-90 and lutetium-177 have become established in clinical use, so far rhenium isotopes have not made their presence felt widely in the clinic despite the convenience of the 188W/188Re generator, which is chemically analogous to the 99mTc generator. The chemical analogy between rhenium and technetium, which is often the basis of a diagnostic/therapeutic “matched pair” plan, must be implemented with caution61 since, when the ligand and core choice is not optimal to create thermodynamically and kinetically stable complexes with strongly preferred geometry and oxidation state, the structures, reactivity and biological behaviour may differ. The bisphosphonate complexes of technetium and rhenium, for example, behave very differently.62 On the other hand the tricarbonyl complexes, M(V) nitride-dithiocarbamate complexes and M(V) oxo-complexes (including those of MAG3, DMSA63 and tetraamines), are closely similar in structure and reactivity when M = Tc or Re, although they may require different synthesis conditions. The tricarbonyl core, in particular provides a highly stable and reliable means of synthesising structurally analogous protein and peptide conjugates64 via the His-tag coordination chemistry described above for 99mTc, and indeed the improvements in labelling efficiency shown in Fig. 9 apply equally to 188Re. There is thus every prospect that high quality 188Re radiopharmaceuticals could be developed for routine therapeutic use to exploit the convenience and potential cost-effectiveness of the 188Re-generator, if an appropriate business model can be identified.
Group 8
Iron-52 is a positron emitter (β+, 55.5%, half-life 8.28 h) but has no advantages for general clinical use and is of interest mainly as a tracer for investigating iron metabolism and distribution in health and disease in vivo.65, 66 Even this application is complicated by the fact that its decay leads to manganese-52m, which is also a positron emitter (β+, 98.3%, half-life 21.1 min) so it is not possible to know that PET images reflect iron distribution rather than the redistribution of the decay product 52mMn. This decay scheme can however be used as the basis of a generator system for producing the short half-life 52mMn27 (see Group 7).
Ruthenium-97 has attractive gamma emission properties (γ, 85%, half-life 2.9 days) that would make it suitable as a radiolabel for biomolecules with slow pharmacokinetics (i.e. applications similar to indium-111, see Group 13) but its production demands very high energy proton bombardment, limiting its availability and preventing general clinical development.67
Group 9
Cobalt-55 (β+, 76%, half-life 17.5 h), as unchelated Co2+, is believed to mimic transport of calcium ions and has found applications in imaging tissue damage especially in brain, e.g. in stroke and vascular dementia.68 Its long half-life allows time for radiosynthesis to incorporate the isotope into structurally diverse targeting molecules, but this has been barely exploited.69 Efforts to label cells for in vivo cell tracking have been largely unsuccessful.70
Rhodium-105 (β-, 100%, half-life 1.47 days) is a beta-emitter with potential applications in radionuclide therapy. Various bifunctional chelator systems based on combinations of soft donor groups (amine, thioether and phosphine) have been evaluated with some promise but have not yet translated into therapeutic evaluation.71–73
Group 10
Nickel-57 (β+, 43.4%, half-life 35.6 h) has been briefly explored as a PET imaging radiolabel but its use has been limited to labelling of metal chelating drugs (doxorubicin)74 and use in studying the toxicology of nickel itself.75
Platinum-195m (γ, half-life 4 days) is a reactor-produced radionuclide that emits low-abundance gamma photons. It has been used primarily for studying the biodistribution and pharmacokinetics of platinum anticancer drugs,59 and also experimentally as a radionuclide therapy by virtue of its Auger electron emissions.76
Group 11
Copper radioisotopes have made a major impact in the last two decades due to a combination of a versatile range of radionuclides for both imaging and therapy (Table 1), and chemical properties that are amenable to various imaging applications.77, 78 These properties include the kinetic stability of well-designed macrocyclic chelates,79, 80 suitable for labelling peptides and antibodies; well-defined redox properties, especially bioreduction from Cu(II) to Cu(I), that lead to applications in blood flow and hypoxia imaging;81, 82 and studies of the innate biological handling of copper and abnormalities in these processes associated with disease.83 A range of isotopes for PET imaging is available, with half-lives ranging from 9 minutes (62Cu, generator-produced) to 12.7 h (64Cu, cyclotron-produced). In addition, 64Cu (which is a beta- and Auger electron-emitter as well as a positron emitter) and 67Cu (which combines beta and imageable gamma emissions) are attractive as therapeutic radionuclides. Despite early promise in clinical trials with 67Cu-labelled antibodies, therapeutic application of 67Cu has not lived up to expectations because of lack of a reliable and cost-effective production route, which remains unresolved.
Table 1. Copper radioisotopes available for PET and radionuclide therapy. EC = electron capture.
| Isotope | β %, Eave | β+, %, Eave | other | t1/2 |
|---|---|---|---|---|
| Cu-60 | 93%, 0.87 MeV | EC | 24 min | |
| Cu-61 | 62%, 0.53 MeV | EC | 3.33 h | |
| Cu-62 | 98%, 1.32 MeV | EC | 9.75 min | |
| Cu-64 | 39%, 0.19 MeV | 18%, 0.28 MeV | EC | 12.7 h |
| Cu-67 | 100%, 0.12 MeV | γ, 93 KeV, 52% | 62 h | |
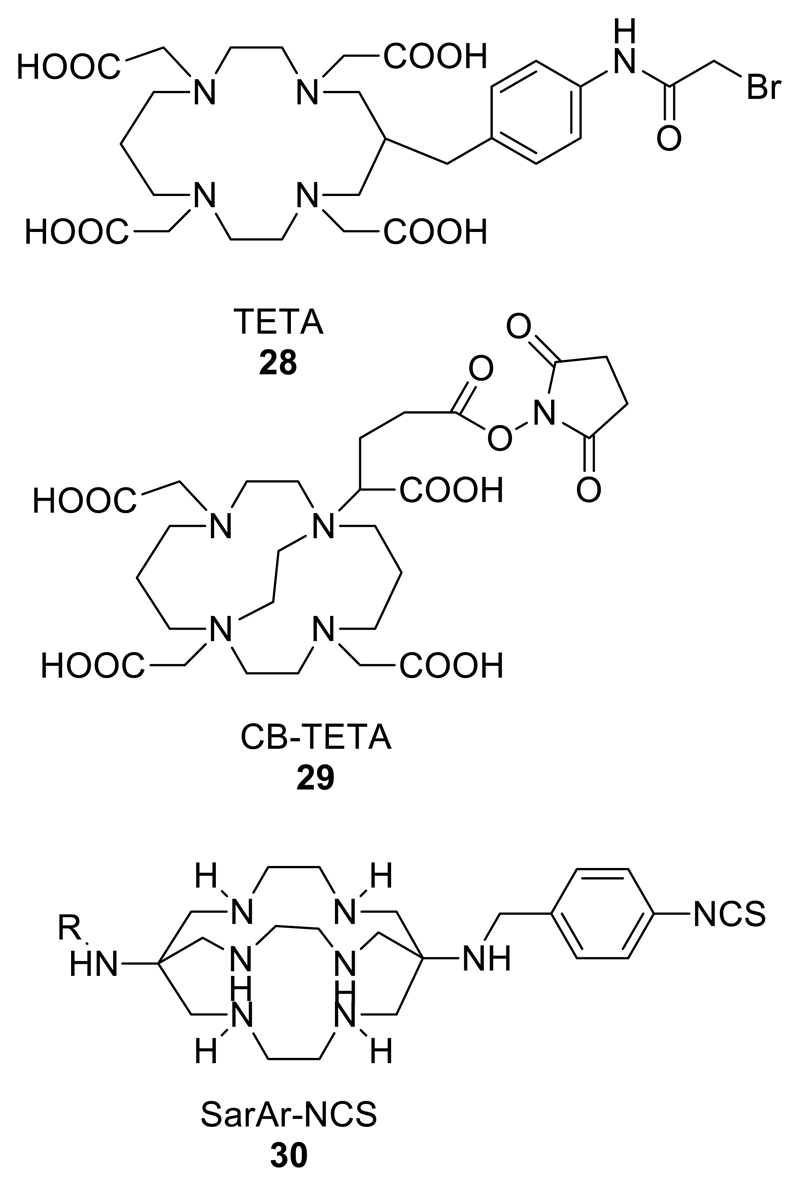
Because of the attractive applications of the longer half-life isotopes 64Cu (PET imaging and radionuclide therapy) and 67Cu (radionuclide therapy and gamma imaging), many studies over the years have been devoted to identifying the best chelators for copper; some of the more significant ones are shown in Fig. 4; some more specific to copper are shown in Fig. 12. Early studies favoured macrocyclic ligands TETA (Fig. 12) and cyclam,77 but with more experience DOTA, and most recently NOTA (5, Fig. 4) and the sarcophagine bicyclic structures84–86 (Fig. 12) have emerged as the leading chelators combining both high in vivo kinetic stability and high labelling efficiency at low chelator-antibody conjugate concentration under mild conditions.87 Non-macrocyclic ligands are consistently inferior, and cross-bridged cyclams (Fig. 12) other than the sarcophagines, designed to impart kinetic stability by virtue of the greater rigidity imparted by the cross-bridge, are still being evaluated.88, 89 In general, cross-bridging leads to greater kinetic stability, but also to a higher activation energy barrier to complex formation, leading to slower labelling and a requirement for harsher conditions. The sarcophagine ligands appear to represent an appropriate balance between these two effects. Despite the effective labelling and good stability in blood shown by antibodies labelled with 64Cu via NOTA and DOTA derivatives, a comparison of peptides labelled with 64Cu, 68Ga and 111In via these ligands suggested that dissociation of copper from the chelator, once the conjugate enters cells, leads to translocation of 64Cu to liver and hence to gut. This does not occur for the 111In and 68Ga labelled conjugates, indicating that these ligands remain far from optimal for tracking biomolecules with 64Cu.90 The design of bifunctional chelators for copper remains a highly active area.
Figure 12.
Selection of chelators for radioisotopes of Cu2+; others of significance include 1 and 5 from Fig. 4.
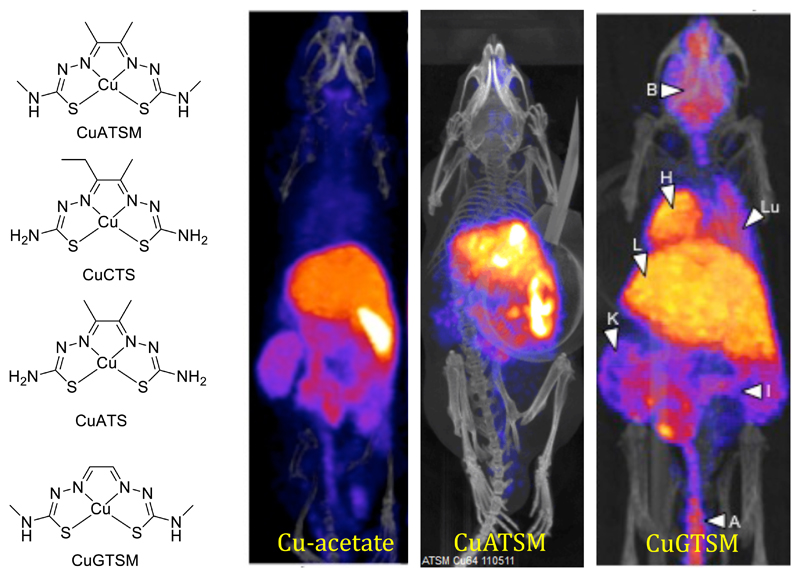
The redox properties of copper can be exploited in several different ways and for different purposes in diagnostic imaging. The common putative mechanistic theme in all these applications is that lipophilic Cu(II) complexes of bis(thiosemicarbazone) ligands (Fig. 13) diffuse into cells non-specifically, whereupon they are exposed to intracellular reducing agents causing reduction to Cu(I). The complexes may then dissociate either slowly (in the case of ATSM and related complexes with two backbone alkyl groups) or quickly (PTSM, GTSM and other complexes with <2 backbone alkyl groups). Once liberated from the ligand, the copper becomes subject to the cellular processes involved in tight regulation of intracellular copper, leading to incorporation into proteins or efflux from the cell, depending on the cell type. The slowly-dissociating complexes can be re-oxidised by molecular oxygen in the cell, whereupon they may escape by diffusing out, as the concentration gradient that drove them in is reversed by clearance from blood and excretion. This is the hypothetical basis of selectivity for hypoxic cells in CuATSM and related complexes,91–94 although other factors also appear to control the cellular uptake and in vivo biodistribution of copper administered in the form of CuATSM.95–98 The rapidly-dissociating complexes, on the other hand, show no selectivity and deposit copper in cells and tissues in proportion to their delivery via blood, hence they are effectively blood flow imaging agents.
Figure 13.
Structures of copper bis(thiosemicarbazone) complexes and PET imaging with 64Cu and its bis(thiosemicarbazone) complexes in mice 30 min post injection. Cu-acetate is rapidly taken up in liver and excreted into the gut; CuATSM (hypoxia-selective tracer) behaves in a superficially similar way; CuGTSM (non-hypoxia-selective tracer) likewise shows liver and gut activity but also shows high uptake in normal brain and myocardium.
This non-specific trapping has been put to use in cell labelling with 64CuPTSM99, 100 and 64CuGTSM, both of which show extremely efficient uptake and initial trapping in cells.101 Lipophilic 64Cu(dithiocarbamate) complexes behave similarly.101 However, cell tracking over the period of days allowed by the half-life of 64Cu is not effective because the copper-regulating processes cause efflux of copper over a period of several hours.101 To an extent this problem might be overcome by prior treatment of cells with chelating agents that will sequester 64Cu released inside the cell, preventing their transport out by specific cellular transport mechanisms.102 This approach deserves further evaluation, since cell tracking with 64Cu is an attractive application because of the high-resolution PET imaging achievable with 64Cu.
Non-specific trapping of CuPTSM and particularly CuGTSM is also being used as a basis for imaging biological transport processes for copper. Thus, 64CuGTSM can efficiently deliver 64Cu across the blood brain barrier and release it into cells (in contrast to, for example, CuATS which does not cross the blood brain barrier103). The resulting intracellular deposition of copper is sufficient to monitor the fate of the copper as it is exported from cells and redistributed; ionic 64Cu (e.g. Cu(acetate)2), on the other hand, does not enter cells and tissues in sufficient quantitities for this to be possible, and uptake in tissues reflects entry of copper into cells via specific transport systems. Thus, both delivery/influx and efflux mechanisms can be imaged separately. This can be applied to imaging changes (and early indications indicate that there are indeed imageable changes) in copper homeostasis and transport associated with disease, including Alzheimer’s disease104, 105 and other dementias, Menkes’ disease, and Wilson’s disease,83 and their treatment with copper-chelating drugs. Recent observations of uptake of 64Cu administered as ionic copper in tumours, including changes in uptake due to hypoxia, indicate that there are some interesting applications of tracking copper metabolism in tumours as well.95, 106 The full potential for imaging changes in copper metabolism to diagnose disease is only just beginning to dawn, and this represents a big opportunity and challenge for metals in imaging over the next few years.
Hypoxia imaging with CuATSM is an important application, as hypoxia is a prognostic factor for response of tumours to radiotherapy and there are many other clinical indications for hypoxia measurement. It has been a subject of controversy recently because of observations that ionic 64Cu shows a biodistribution that is similar to that of 64CuATSM, including in some tumours studied.95 This is compounded by the likely mechanism, which entails dissociation and hence rapid release of free copper, resulting in potential supposition that 64Cu distribution after administration of 64CuATSM reflects distribution of ionic copper rather than hypoxia, and that in some tumours uptake of ionic copper may be related to hypoxia as well. These questions certainly complicate the interpretation of the 64CuATSM scans. However, Cu bis(thiosemicarbazone) complexes do not behave like unchelated copper in the early phase of biodistribution; CuATSM is hypoxia-selective, CuPTSM is (almost) not;92, 107 CuGTSM is taken up in normal myocardium, CuATSM is not;105 CuATSM and CuGTSM cross the blood brain barrier, CuATS and ionic copper do not.103 These differences are evident from Fig. 13. The most likely underlying mechanism is that all the complexes are, to a large extent, quickly metabolised in liver, releasing copper, part of which is excreted into the gut via the bile, and part of which is recirculated as copper transport proteins, leading to delayed uptake in tissues by specific copper transport mechanisms, which may themselves be disturbed in tumours and in hypoxic tissues. Since the clearance of CuATSM from non-hypoxic tissues is relatively quick, imaging can be performed early (e.g. 20 min), as in most studies in Japan (where the short lived isotope 62Cu is most often used). New, less lipophilic analogues of CuATSM (e.g. CuATS and CuCTS, Fig. 13) show promise as potential superior replacements for CuATSM; they clear more quickly from normoxic tissue and reach higher hypoxic:normoxic tissue ratios in isolated rat hearts in a shorter time.107 In vivo evaluation of these compounds is anticipated with interest.
Much of the clinical work with CuATSM, especially in Japan, has been done with 62Cu. A 62Cu generator is not commercially available in Europe and very little work with 62Cu has been reported outside Japan.108 For clinical trials its short half-life (9 min) has the advantage of offering the opportunity to perform repeat scans with and without an intervention (e.g. oxygen breathing), with the patient in the same position on the scanner. For such purposes a 62Zn/62Cu generator has been implemented at King’s College London by means of a collaboration in which natural copper is irradiated using the cyclotron at the University of Birmingham, and the resulting 62Zn is processed to fabricate the generator at King’s. Fast chemistry for synthesis of 62CuATSM (to GMP standards) and other bis(thiosemicarbazone) complexes has been developed by implementing the solid phase transmetallation method reported by Dilworth et al.109 whereby the ligand, in the form of a zinc complex, is linked to a solid phase via a pyridine ligand, which binds an axial site of zinc. Passage of 62Cu solution over this stationary phase allows transmetallation to form the copper complex, releasing the ligand from the column and providing a product very quickly and with very high specific activity. This opens the door to several interesting clinical trials with very low radiation doses to patients.
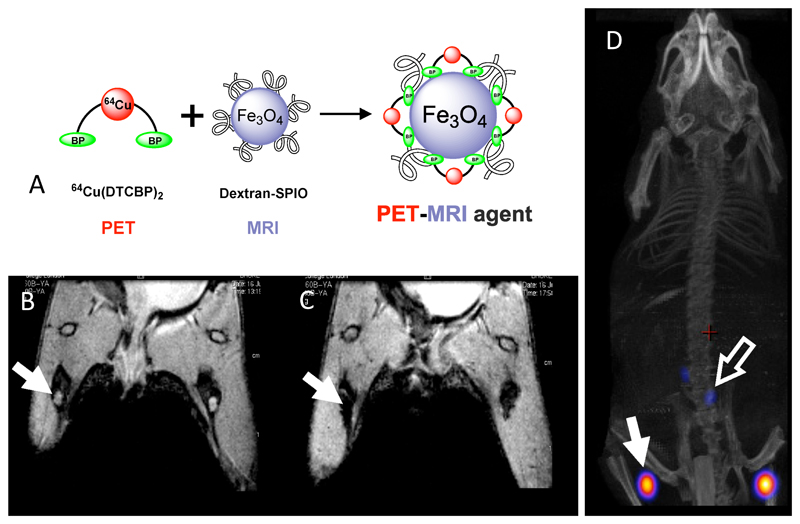
Because of its half-life, availability and excellent PET imaging properties, 64Cu has been a radioisotope of choice in radiolabelling nanoparticulate materials110–112 to assess drug delivery and combine imaging modalities. An example of the latter is radiolabelling of magnetic iron oxide nanoparticulate contrast agents with 64Cu using the bis(dithiocarbamate-bisphosphonate) complex shown in Fig. 14, in which the bisphosphonate groups anchor the 64Cu to the inorganic particle surface, allowing the potential of the new generation of PET/MRI scanners to be applied, for example, to sentinel lymph node localisation.113
Figure 14.
Dual modality (PET/MR) imaging of sentinel lymph nodes in mice. A: assembly of 64Cu-labelled iron oxide/dextran nanoparticles using a dithiocarbamate-bisphosphonate ligand (as used in technetium complex 26, see Fig. 10). B: MR scan (coronal section) of hind limbs and lower abdomen, pre-injection of contrast agent. Lymph nodes are visible as bright areas (arrow). C: MR scan post-injection of contrast agent, showing darkening of lymph nodes due to uptake of MR contrast. D: PET/CT scan showing radioactivity co-localised with magnetic particles (arrows). The radioactivity remains bound to the magnetic particles in vivo.
Other Group 11 metallic isotopes of interest to molecular targeting include 111Ag114 and 198Au115 (beta-emitters with potential, if not yet actual, application in targeted radionuclide therapy).
Group 12
62Zn is important as the parent isotope in the 62Zn/62Cu generator (see Group 11). 197Hg-chlormerodrin116 (γ, 100%, half-life 64.14 h) was used in the early days of nuclear medicine as a renal perfusion agent before replacement with 99mTc tracers. 197Hg can be coupled to biomolecules using a bifunctional chelator incorporating the soft macrocyclic ligand 1,4,7-trithiacyclonane.117
Group 13
Group 13 is a feast for the radiometal molecular imager. Gallium, indium and thallium have radionuclides of great historic and current importance, and even boron and aluminium, while offering no useful radionuclides, have come to prominence very recently because of their role as inorganic binding sites for the important radionuclide fluorine-18, a role which will be discussed under Group 17.
Gallium-67 (γ, 86%, half-life 3.26 days) has been important in nuclear medicine for decades. Among the various applications and complexes explored are two main clinical roles: imaging of lymphoma and other tumours,118, 119 and imaging of infection.120 Uptake in lymphoma is believed to be mediated by transferrin receptors, taking advantage of the serum speciation of gallium when administered as the citrate complex, which involves a significant fraction of the radionuclide binding to the iron-binding sites of the iron transport protein transferrin (reliable experimental evidence for this mechanism, however, is hard to pin down in the literature).121 The clearance of radioactivity from blood is very slow, resulting in delays of several days before useful imaging can be performed, making the long half-life of 67Ga essential to this application. The mechanism by which 67Ga (again administered as citrate complex) is accumulated in sites of infection is unclear and may be related either to targeting of the infiltrating immune cells, or targeting the microorganisms themselves, by binding to secreted polysaccharides or by transchelation to bacterial siderophores followed by siderophore-mediated uptake into the bacterial (or fungal) cells. Thus, these applications might rely fundamentally on the biological mimicry of iron by gallium.
A newly-emerging potential role for 67Ga arises from the Auger electron emissions that accompany its nuclear decay, offering possible application as a therapeutic radionuclide. These electrons are higher in number and energy than some other Auger electron emitters, leading to substantial local radiation dose to the individual cells in which the 67Ga is located. The higher energy means that the radionuclide may not need to be located within the cell nucleus in order to have DNA damaging effects. Early indications supported the idea that 67Ga may be more cytotoxic than 111In which has previously been evaluated as a radionuclide therapy agent in clinical trials.122–127 The radiobiological effects of intracellular 67Ga on cell survival warrant further study, in light of recent improvements in chelation and delivery of gallium isotopes.
68Ga (β+, 88.9%, half-life 68 min) is set to make an impact on PET imaging comparable to that made by 99mTc half a century ago. It is a generator produced positron-emitting isotope with a half-life of 68 min – long enough for reasonably quick radiolabelling chemistry and for biodistribution of most small molecules, peptides etc., but short enough to keep radiation doses reasonably low. Despite commercial generators with marketing authorisation not having been available, many centres, especially in Europe, have been using 68Ga clinically, mainly in the context of somatostatin receptor-targeting peptides such as DOTATATE, DOTATOC and analogues,128, 129 to which the radiometal is attached via a DOTA chelator (Fig. 4). Many of the technical issues that have delayed the regulatory approval of the generator have now been overcome and in 2014, the first marketing authorisation for a 68Ge/68Ga generator was granted by the EMEA and clinical use of 68Ga is likely to spread and increase greatly, creating a second revolution in clinical PET (the first being the spread of cyclotrons and PET scanners driven by the enormous clinical utility of 18F-fluorodeoxyglucose) by making it available in centres that do not have a cyclotron nearby. One might provoke a lively discussion by observing that this development coincides with the current (and probably future) era of shortages of 99mTc, and could be a factor that informs decisions about the future of 99mTc as the workhorse of nuclear medicine (see Group 7). The arrival of the generator has sparked a proliferation of new 68Ga imaging agents, particularly biomolecules such as peptides and small proteins, and conjugates of small molecules such as bisphosphonates130, 131 and folic acid,132, 133 to make economic use of the generator to bring its benefits to the maximum number of patients world wide. The development of new agents, and in particular their widespread application, requires radiolabelling chemistry that can be performed quickly, with the minimum of manipulation and the minimum of costly equipment, on the hospital premises. That is, labelling methodology akin to the 99mTc labelling kits that made radiopharmaceuticals available at low cost, that can be performed in facilities little more complex than conventional hospital radiopharmacies rather than PET radiochemistry centres with cyclotrons and arrays of robotic synthesisers and hot cells.
Despite having become the standard bifunctional chelator used in the first wave of 68Ga-labelled peptides, DOTA (Fig. 4) was not designed specifically for Ga3+ chelation and is not ideal because labelling it requires relatively harsh conditions – heat (e.g. 90°C), time (30 min) and low pH, all of which bring inconvenience and undesirable complexity and may be incompatible with the biomolecule concerned. The last few years has seen something of a “beauty pageant” of new bifunctional chelators for 68Ga, aiming to improve the labelling conditions and efficiency while maintaining adequate in vivo stability. A selection is shown in Fig. 4; a more comprehensive selection is discussed in an excellent recent review of metal chelators for radionuclides.7, 134 Many of these ligands, such as HBED (6, Fig. 4), DEDPA (7) and TRAP (8), significantly improve the labelling conditions compared to DOTA, and have good biostability and other potential advantages. Indeed HBED is the basis of very promising 68Ga-bioconjugates (e.g. the PSMA ligand for imaging prostate cancer) now being used in the clinic.135 For rapid labelling under the mildest conditions at the lowest concentrations, the acyclic tripodal tris(hydroxypyridinone) ligand CP256, and its bifunctional conjugate YM103 (4, Fig. 4), appear to be the optimal choice, based on direct side-by-side comparison with some of the other ligands. Its design was based on the premise that rapid, mild and efficient labelling overrides in vivo stability as an important feature, because of the short half-life. The immediate future promises rapid development in this field and a small number of purpose-designed gallium chelators will soon emerge as a basis for commercial development of kit-based 68Ga labelled radiopharmaceuticals.
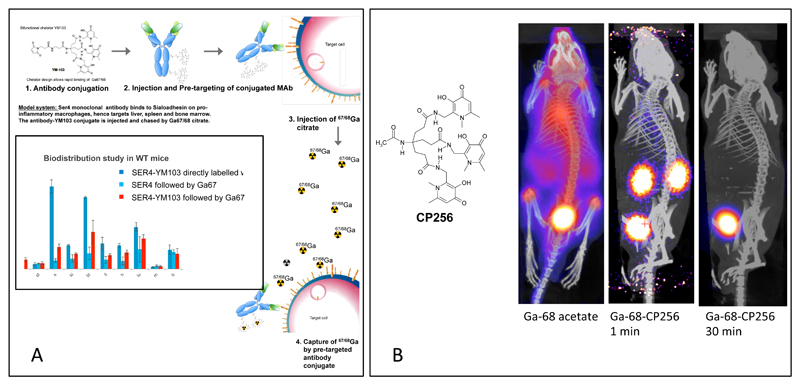
A surprising property of YM103 is its ability to transchelate 68Ga bound to transferrin in serum. This has been demonstrated in vitro by incubating in serum.19 The extent to which other chelators can do this has not been established but it raises the intriguing possibility of radiolabelling in vivo. This has been demonstrated by injecting 68Ga acetate into mice, observing the typical slow clearance from blood to bone, and following with an injection of CP256 whereupon the circulating 68Ga is immediately cleared from the circulation and excreted via the kidney (Fig. 15). Such behaviour, on a longer timescale, has been demonstrated previously for DFO using 67Ga.136, 137 This in vivo transchelation is being explored as a basis for pretargeting with antibodies: injection of an antibody-YM103 conjugate, followed after a suitable period to allow targeting by an injection of 68Ga-acetate. The pre-injected antibody perturbed the biodistribution of the 68Ga, increasing its uptake in the tissues targeted by he antibody. This observation indicates potential for a new form of pre-targeting based on in vivo metal transchelation,138 allowing use of short half-life radioisotopes such as 68Ga even with targeting biomolecules that have very slow pharmacokinetics.
Figure 15.
A. Concept of pre-targeting by in vivo transchelation of 68Ga, which is feasible with YM103 (4 in Fig. 4) because of its ability to transchelate Ga3+ from transferrin in plasma and in vivo. Inset: Uptake of 68Ga in most organs (including spleen which is the location of the macrophages expressing sialoadhesin, the SER4 antibody target) when given as a chase after administration of antibody-YM103 conjugate, is intermediate between that of the pre-labelled antibody and the distribution of 68Ga given as a chase after administration of unconjugated antibody. The exception is bone, which is the target of unchelated 68Ga (see part B, left image). This suggests that 68Ga is able to find and bind to the pre-targeted antibody-YM103 conjugate in vivo. B: Demonstration that the tris(hydroxypyridinone) chelator CP256 (non-derivatised form of YM103) can bind to 68Ga in vivo and excrete it rapidly via kidney. Left: Biodistribution of 68Ga administered in unchelated form; centre and right: biodistribution of 68Ga-CP256 complex 1 min and 30 min post-injection respectively, showing rapid renal excretion in progress and completed. Similar images are obtained if the 68Ga is administered first, followed by the CP256 ligand.
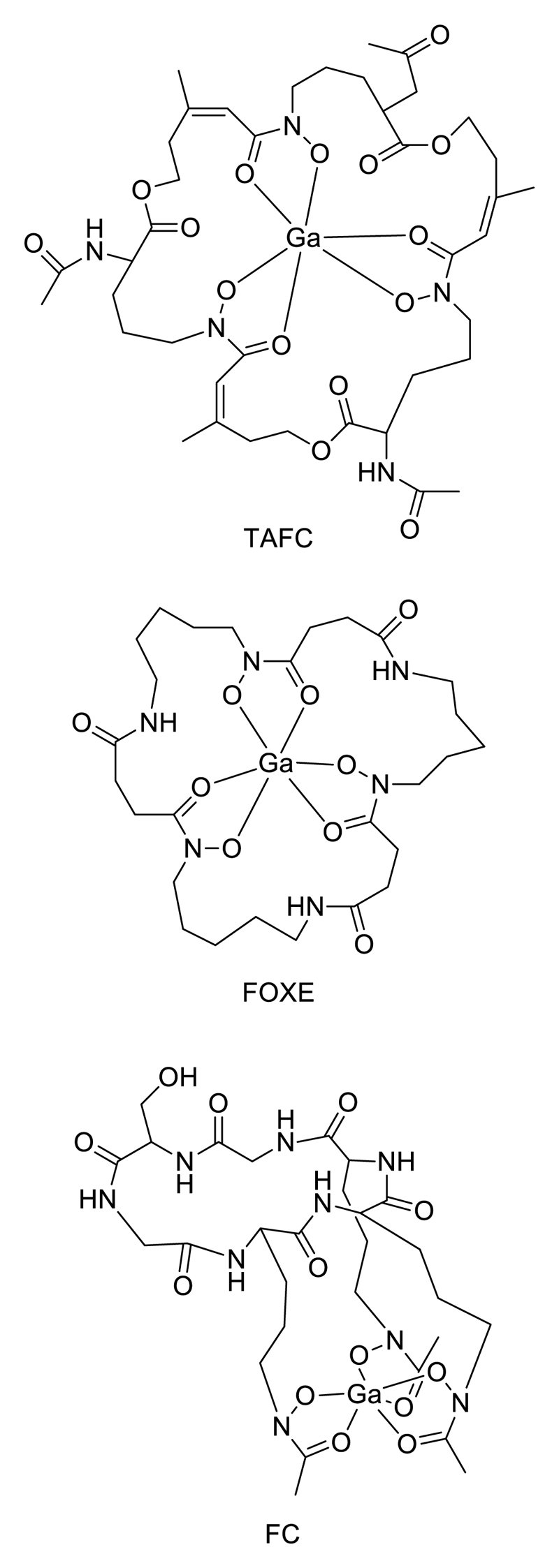
The similarity in ligand donor and geometric preferences between Ga(III) and Fe(III) underlies a new application of molecular imaging that is specific to gallium: species-specific imaging of microbial infection (bacterial or fungal) using 68Ga-labelled siderophores. This exploits the microorganisms’ highly developed systems for acquiring iron from their environment, in which they secrete low molecular weight chelators (siderophores) which bind Fe(III) with extremely high affinity, and then take up the complexes via specific transporters. By labelling these molecules with 68Ga in place of iron, the structural analogy of the complex is adequate for the Ga complex to be taken up by the siderophore receptor, allowing this molecular mechanism to be used for specific imaging of the presence of the living microorganisms in vivo. This has been applied to detect Aspergillus fumigatus infection using its specific sidrerophores desferri-triacetylfusarinine C (TAFC) and desferri-ferricrocin (FC) (Fig. 16), and similarly other microbe/siderophore combinations, in animal models.139–142 This represents a highly promising new area for development.
Figure 16.
Siderophores labelled with 68Ga for targeting microorganisms: TAFC = triacetylfusarinine; FOXE = ferrixoamine E; FC = desferriferricrocin.
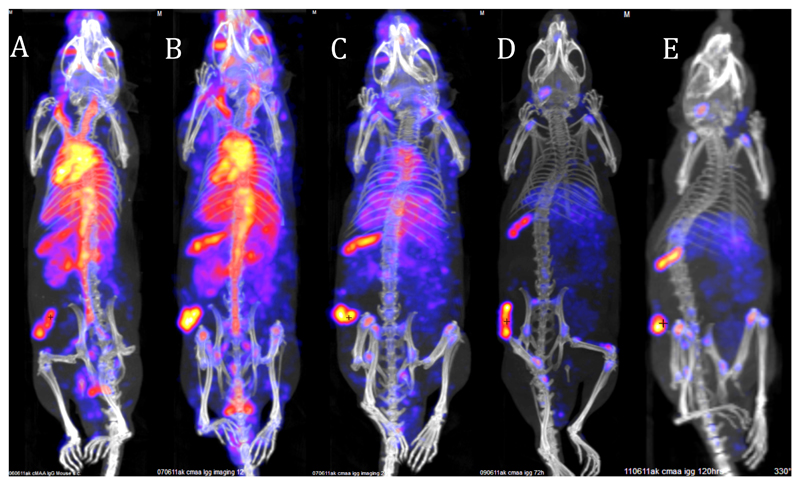
Indium-111 (γ, 100%, half-life 2.8 days) was the first radionuclide to be commercially and clinically established in routine practice in gamma imaging applications that necessitate a long half-life: labelling monoclonal antibodies and labelling cells. Its commercial development in the 1980’s coincided with the emergence of monoclonal antibody technology, which underpinned hopes of “magic bullet” drugs and diagnostics. It forms reasonably stable chelates with various polyaminocarboxylates and unlike copper it does not appear to be essential to use a macocyclic chelator for 111In. Even the prototype DTPA (9, Fig. 4), coupled to antibodies by reaction of its cyclic anhydride with amine side chains of lysine residues, provided excellent results in the early days of antibody imaging, and was the chelator employed in the first commercially available peptide-based imaging agent Octreoscan.143 DTPA has since been supplanted by related ligands (Fig. 4) offering greater kinetic inertness through incorporation of cross-bridging, backbone alkylation and cyclisation, e.g. DOTA144 or CHX-A”-DTPA145. 111In remains the most important gamma-emitting radiolabel for antibodies (see for example Fig. 17).
Figure 17.
Typical tumour SPET/CT imaging with whole IgG monoclonal antibody in mouse bearing an experimental melanoma on the flank, illustrating the need for long half-life radionuclide (in this case 111In, half-life 2.8 days). CSPG4 IgG antibody, which binds to melanoma associated antigen, was labelled with 111In using the bifunctional chelator CHX-A”-DTPA. Images show predominantly blood pool at 4 h (A) and 24 h (B) with increasing tumour- and spleen-to-blood ratio 48 h and optimal images are not seen until 48-120 h.
Cell labelling with 111In, developed to allow clinical imaging and localisation of infection and inflammation sites, was first developed in the late 1970s and entered widespread clinical use in the 1980s. The design principle was the opposite of that required in antibody labelling – rather than kinetic stability the aim was to form biologically unstable lipophilic complexes able to diffuse into cells and dissociate when exposed to the intracellular milieu. This was achieved with a number of uninegative bidentate ligands such as oxinate and tropolonate, forming InL3 complexes (Fig. 7) and these complexes remain in demand for imaging inflammation and infection.146 The rise of cellular therapies (transplants, stem cells etc.) has created new applications for cell tracking and 111In is likely to continue to fill this need for some time to come.
Thallium-201 (γ, 10%, half-life 73 h), in the form of thallium(I) chloride, was the first routinely used myocardial perfusion imaging agent. Since it became established in clinical use it has become recognised that the 201Tl+ cation, like rubidium discussed earlier, is a potassium mimic and a substrate of the sodium/potassium ATPase and so, like 82Rb+, is efficiently taken up in well-perfused myocardium.147 Despite its low γ-yield (which is compensated by a higher abundance of X-ray emissions) it remained the regular clinical myocardial imaging agent until supplanted by the lipophilic cationic technetium complexes, and made something of a come-back during recent 99mTc shortages. Its cationic charge was, misguidedly but ultimately successfully, the design template148 for these hugely commercially successful new 99mTc myocardial agents developed in the 1980s; these are in fact not potassium mimics but are trapped in mitochondria by virtue of their cationic charge, and access them by virtue of their lipophilicity.149 201Tl has been (and is in many centres) also used to detect tumours, being taken up presumably by the same mechanism.
Group 14
Group 14 offers carbon-11 (β+, 99.6%, half-life 20 min). This radioisotope makes possible the labelling of organic compounds without structural modification and provides a role for synthetic organic chemists in molecular imaging, which is outside the scope of this Perspective. In this field there is no escaping the dependence on a local cyclotron, automated multi-step synthesis and purification and so the challenges are rather different to those of inorganic radiochemistry. Other contributions from Group 14 include tin-117m (γ, 86%, and conversion electron emitter, half-life 13.6 days) which, as its Sn(IV) DTPA complex, shows exquisite selectivity for bone metastases, where its very short range electron emissions keep bone normal marrow irradiation to a minimum.150 Despite this advantage it has not become established as a widely-used bone therapy agent, possibly because of limitations on the scale of production. Lead-212 (β-, 100%, half-life 10.6 h) is of interest as an “in vivo generator” of the alpha-emitter bismuth-212. The in vivo generator principle poses the additional challenge that the energetic decay process of the parent isotope (recoil energy and shower of conversion or Auger electrons) can induce dissociation, and the chelating system must be able to resist this or allow rapid reassociation with the daughter nuclide. Macrocyclic polyaminocarboxlyate chelators, particularly DOTA (Fig. 4) show efficacy as bifunctional chelators of Pb2+ but it has been shown that replacing the carboxylate groups with primary amides, giving an octadentate ligand TCMC (10, Fig. 4) which coordinates through the four amines and four carbonyl oxygens of the amide groups, is advantageous and in use in a clinical trial of 212Pb-labelled antibody.151–153
Group 15
Nitrogen-13 (β+, 99.8%, half life 10 min) is a very short-lived positron-emitting radionuclide most readily produced from cyclotrons in the form of ammonia or, less commonly, nitrate/nitrite. Its only established clinical application is use of 13NH3 as a myocardial perfusion PET imaging agent. The ubiquitous appearance of nitrogen in biomolecules, metabolites and drugs creates attractive applications based on incorporating 13N into more complex molecules, but this has been to all intents and purposes unrealised because of the perception that the half-life is too short for the relatively complex chemistry to be practicable. However, very recently the feasibility has been demonstrated to utilise 13NH3 as a precursors for more complex molecules,154, 155 opening a potential new field of organic PET radiochemistry. Inorganic applications might also be envisaged utilising the ability of ammonia to bind to metals.
Phosphorus-32 (β-, 100%, half-life 14 days), a high-energy beta-emitter, is the oldest radionuclide used in nuclear medicine, having been evaluated in the 1930s in the form of phosphate for treatment of haematological disorders. It is still used for treatment of polycythemia vera. It has also been widely used for palliative therapy of bone metastases, since it targets areas of bone mineral deposition, but has fallen out of favour because of high bone marrow dose associated with uptake in rapidly dividing cells in bone marrow. Its complete lack of photoemission precludes reliable imaging. Lack of imageable emissions has not prevented widespread therapeutic use of other beta-emitters such as 90Y, however, and it is surprising that 32P has barely been used therapeutically in any chemically targeted form other than phosphate and polyphosphates (except radiolabelled colloids for topical application in tumours and arthritis). Given its relatively low cost and an amenable half-life, it is possible to imagine that useful agents could be developed by broadening the chemistry, perhaps to incorporate 32P into some of the many bisphosphonate drugs that are now widely used in treatment of bone diseases. The very few attempts to develop more complex targeted agents containing 32P include labelling of ATP156 and labelling of targeting proteins.157 33P (β-, 100%, half-life 25.3 days), a medium-energy beta-emitter, has been suggested as an alternative to 32P as a therapeutic radionuclide with lower energy beta emissions,158 but no biological or clinical evaluations have been reported to date.
Arsenic has several positron emitting isotopes with potential for PET imaging including 71As (β+, 30%, half-life 64 h), 72As (β+, 88%, half-life 26 h), and 74As (β+, 29%, half-life 17.8 days), and beta-emitting isotopes that in principle could be used for radionuclide therapy, including 77As (β-, 100%, half-life 38.8 h) and 76As (β-, 100%, half-life 26.3 h). The half-lives of all the positron emitters are compatible with use with antibody targeting. 72As can be produced from a generator (parent isotope 72Se)159 by eluting it in the form of arsenic triiodide AsI3, which can be covalently attached to antibodies modified to create thiol side chains, by elimination of HI with formation of arsenic-sulfur bonds.160 Although radionuclide imaging with 74As dates back to the 1950s,161 it has been explored surprisingly little to date.
Bismuth-212 (α, 36%, β-, 64%, half-life 60.6 min) and bismuth-213 (complex decay chain involving alpha and beta emissions, half-life 46 min) are alpha emitters. Their short half-lives are less than ideal for targeted radionuclide therapy but still, some targeting molecules may have sufficiently rapid target accumulation in vivo that they could be used. 213Bi is eluted from the 225Ac/213Bi generator and is readily chelated by DOTA derivatives for attachment to antibodies; applications are envisaged in diffuse cancers in which the radiopharmaceutical can easily access individual cancer cells (e.g. leukaemias),162 or possibly using a pre-targeting approach using 213Bi-conjugated biotin.163 212Bi will most likely find application in the form of the in vivo generator whereby 212Pb attached to a targeting molecule (see Group 14) decays in vivo to 212Bi, which, if the chelate withstands the decay process, remains in situ long enough to deliver its alpha particles to the target tissue.151–153
Group 16
The half-life of oxygen-15 (β+, 100%, half-life 2 min), which can be produced in cyclotrons in the form of H2O (used for blood flow quantification) or O2, is so short that even die-hards who consider 15N and 62Cu amenable to incorporation into targeting molecules draw the line at labelling complex molecules with it. Nevertheless CO, butanol and 2-deoxy-D-glucose have been labelled for potential in vivo applications.164
Group 17
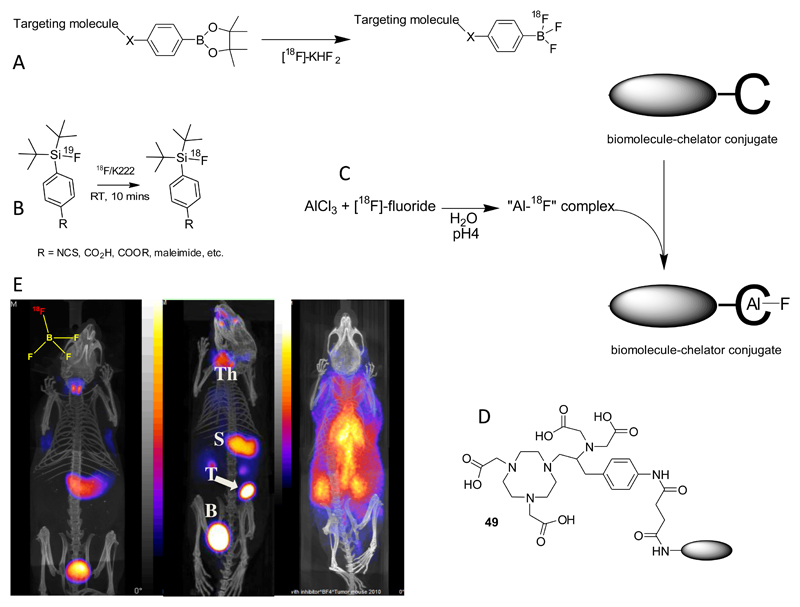
Fluorine-18 (β+, 96.7%, half-life 20 min) is currently by far the most important radionuclide in PET, and is used most often in the form of [18F]-2-fluoro-2-deoxyglucose. By far the major route to incorporating it into targeting molecules is formation of carbon-fluorine bonds, which falls outside the scope of this inorganic Perspective. However, interest in the inorganic chemistry of fluorine, using elements other than carbon as a binding site for 18F, is growing. Since it has been reviewed recently165 it will be introduced here very briefly. Boron, aluminium and silicon, all of which have very high bond energy in binding with fluorine, have been the most studied. 18F-labelled aryl trifluoroborate (ArBF3-) and alkylfluorosilanes166, 167 have been used as prosthetic groups for labelling biomolecules, and the 18F-labelled tetrafluoroborate ion is, like iodide (vide infra) and pertechnetate, a substrate of the sodium/iodide symporter and is under development as a PET imaging agent for thyroid disease, with the potential to improve on the sensitivity of gamma camera and SPET imaging with [99mTc]-pertechnetate (Fig. 18).168, 169 Coordinatively unsaturated aluminium complexes based on bifunctional NOTA derivatives can be linked to biomolecules and will complex fluoride ions to produce stable radiopharmaceuticals. This is an exciting new area which has recently been reviewed.170, 171 With further development to introduce aluminium chelates that will bind fluoride under milder conditions and reach higher specific activity, it may have the potential to bring simple, one-step kit-like labelling chemistry to 18F labelling of biomolecules.
Figure 18.
A. Nucleophilic radiolabelling of boronic ester prosthetic group; B: Isotopic exchange labelling of fluorosilane prosthetic group; C: Use of coordinatively unsaturated aluminium complex as a binding site for fluoride ions in biomolecule labelling; D: currently favoured ligand for chelation of Al3+ and labelling with [18F]-fluoride; E: PET-CT imaging of sodium/iodide symporter (NIS) activity mouse using NIS substrate [18F}-BF4-, 30 min post-injection. Left: normal mouse showing thyroid and stomach uptake and excretion into bladder; Centre: mouse with implanted NIS-expressing breast tumour showing tumour (T) uptake as well as thyroid (Th) and stomach (S) uptake; right: normal mouse pre-injected with perchlorate to block NIS activity, showing radioactivity largely confined to blood pool.
Bromine has positron emitting isotopes 75Br (half-life 96.7 min) and 76Br (half-life 16.2 h) that can usefully be incorporated into organic molecules, usually by formation of aromatic C-Br bonds using oxidative electrophilic bromination. Much of this chemistry is analogous to iodination chemistry referred to below. Despite the suitable emission properties of the isotopes they have not found wide use. The production and utility of these isotopes have been reviewed recently.172
Iodine isotopes are extremely versatile, both from a radiological and chemical perspective. Iodine-131 (β-, 91%, γ, 82%, half-life 8.1 days) is of great historical significance in nuclear medicine and was among the earliest isotopes used clinically, originally used primarily as a radionuclide therapy in thyroid disease and later, with the advent of imaging, as an imaging agent for “thyroid function”. Its half-life is suitable for therapy in this context but its imaging characteristics are not ideal and better images, with smaller radiation doses, were later accessible with the introduction of the gamma-emitter 123I (γ, 83%, half-life 13 h). In the age of PET, iodine is represented by 124I (β+, 23%, half-life 4.2 days) which, despite less than ideal imaging properties and an unnecessarily long half-life, advances the sensitivity of imaging a step further compared to the gamma-emitting isotopes. As well as imaging “thyroid function” (later transforming into “molecular imaging” of sodium/iodide symporter activity once that transporter had been identified, cloned and characterised in the 1990s, nicely illustrating a semantic distinction between functional imaging and molecular imaging as different interpretations of the same imaging process in different eras), it is readily incorporated into organic and biological molecules, usually by formation of aromatic carbon-iodine bonds. In the case of proteins and peptides this is most often by electrophilic substitution in the activated aromatic groups of tyrosine side chains. The chemistry of incorporation of radioiodine into organic and biological molecules has been well-reviewed elsewhere.173–175
Astatine-211 (α, 100%, half-life 7 h) is one of the prominent alpha-emitting radionuclides being evaluated for radionuclide therapy. It holds a fascination for many inorganic chemists, because, having no stable elements, its chemistry has barely been explored. Most of the chemistry for incorporating it into targeting molecules is based on an assumed analogy to iodine, with a tendency towards preferring higher oxidation states by comparison. Hence it is usually incorporated with reasonable stability into aromatic groups by C-At bond formation using electrophilic substitution.173 However, it has recently been the subject of interest from an inorganic perspective, on the one hand being treated as a metal to see whether chelating agents could bind it satisfactorily in its higher oxidation states, and on the other by exploring the coordination of astatide ions to the softer transition metals. These approaches are at the very early stages of evaluation and have not yet shown signs of replacing conventional organic astatine chemistry as the basis of radiolabelling.176–180
Group 18
Being “noble” the group 18 elements cannot be incorporated into targeting molecules but are useful in the form of the elemental gas for lung ventilation studies and perfusion of tissues, especially brain and liver. Gamma emitting members of the family are krypton-81m (γ, 68%, half-life 13 s)181 and xenon-133 (γ, 37%, β-, 100%, half-life 5.24 days).29 The gaseous state is exploited in the design of the krypton-81m generator, from which the gas is eluted from a solid support with air directly into the inhaled airstream.
Lanthanides
The coordination preferences of the lanthanides bear similarity to the group 3 and group 13 metals, and all form 3+ cations that can be chelated with oxygen/nitrogen donor ligands, with maximum kinetic stability obtained using macrocyclic ligands, especially DOTA. Although all can be effectively bound by the same chelator, there is a contraction in ionic radius towards the right of the period (the “lanthanide contraction”), which affects the coordination number, potentially leading to variations in biological behaviour when bioconjugates with the same chelator are labelled with different metals. Most important are lutetium-177 (β-, 100%, γ, 14%, half-life 6.65 days),10 samarium-153 (β-, 100%, γ, 29.8%, half-life 46.27 h),182 holmium-166 (β-, 100%, half-life 1.12 days),183 and terbium-149 (α, 16.7%, β+, 83.3%, half-life 4.12 h),184 all of which are therapeutic isotopes and some of which can be imaged with a gamma or PET camera. The overlap in chemistry between the lanthanide elements and the group 3 and 13 metals is useful as a basis for multimodality imaging (aspects of which are discussed elsewhere in this issue), exploiting the luminescent properties of the elements for optical imaging, the high relaxivity of gadolinium complexes for magnetic resonance imaging, and the radionuclide properties of the lanthanides and chemically similar elements like 68Ga and 111In which can share the same chelators.
Actinides
Actinium-225 (α, complex decay chain, half-life 10.0 days) decays via a sequence of alpha-emissions and so imparts an extraordinarily high local radiation dose per atom, which can be exploited for targeted radionuclide therapy. Despite the large ionic radius, the well-established macrocyclic chelator DOTA is a satisfactory means for coupling Ac3+ to targeting molecules such as antibodies, and the DOTA complex is indeed more kinetically stable than complexes of related macrocyclic ligands of higher denticity.185 Thorium-227 (α, complex decay chain, half-life 18.5 days) also decays via a sequence of alpha-emissions, and is also adequately bound to antibodies by DOTA-based bifunctional chelators.186 These alpha-decay series elements are currently involved in a number of early-phase clinical trials with commercial sponsorship; it is a rapidly developing field, invigorated by the success of larger trials with 223Ra (see Group 2) in palliative treatment of bone metastases.
Conclusion
Nuclear medicine and molecular imaging has provided a gratifying mix of inorganic chemistry challenges, multidisciplinary collaboration, and tangible benefits to patients, health services and business, making it a stimulating and hugely varied area in which to spend a career in inorganic chemistry and providing opportunities to apply chemistry not only from all areas of the periodic table, but different types of materials and molecules including organic molecules, metal chelates, proteins, peptides and nanoparticles. There have been significant developments recently in many of these areas, raising prospects for translation of new radionuclides and molecular imaging mechanisms, which in turn creates new inorganic chemical challenges. It will be interesting for inorganic chemists not only to observe the outcomes of their past developments for clinical applications and commercial products, but to drive them by providing new chemistry to improve the quality and accessibility of molecular imaging and radionuclide therapy for patients. Old challenges remain and new ones emerge across the periodic table. New non-cardiac applications should emerge for the 82Rb generator. The 68Ga generator is becoming established clinically as a source of positron emitting radiopharmaceuticals, with the possibility to revolutionise PET by making it available to centres without cyclotrons and complex radiochemistry facilities. To achieve this requires a shift in emphasis in chelator design from long-term kinetic stability towards rapid complexation under mild conditions and at very low concentration. New generator-produced medium-half-life positron emitting radionuclides such as 44Sc and 90Nb are emerging and may offer similar possibilities. All these isotopes require new chemistry for effective, and above all, simple incorporation into targeting molecules. Longer half life positron emitters such as 89Zr isotopes are already making an impact in imaging with antibodies in humans, and this impact may now spread to cell tracking. The growing availability and versatility of very short half-life (<20 min) positron emitters (13N, 62Cu, 82Rb, 15O, 52mMn) presents a new opportunity to perform multiple scans in individual patients to explore multiple facets of tissue physiology and gene expression in a single scanning session, without unduly high radiation doses; realisation of this possibility also creates needs and opportunities for innovative fast chemistry. The emergence of generator-produced positron emitters, and the closure of reactors producing 99Mo, threatens the position of 99mTc at the centre of radionuclide imaging, but a renaissance of 99mTc may follow if the production infrastructure becomes more secure. This will highlight a need for new technetium chemistry, particularly for convenient kit-based incorporation into biomolecules, which despite many years of research remains far from optimal and 99mTc has surrendered a lead to 68Ga in this respect. Copper radioisotopes continue to command very wide interest and there is a growing recognition of their potential in the study of copper biochemistry in health and disease, creating a major new field of activity in PET. The use of metals such as aluminium as binding sites for fluoride shows promise for easy incorporation of 18F into biomolecules but this has some way to go to achieve its potential; this approach could be extended to other metals and radiolabels. Finally, the clinical success with 223Ra radionuclide therapy may stimulate a resurgence of interest in radionuclide therapy, especially with alpha-emitting isotopes, e.g. of Bi, Ac, Th, Pb, At and Tb, whose coordination chemistry has yet to be optimised for the purpose, and of 223Ra which requires completely new thinking if it is to be incorporated into biological targeting molecules. There is something for everyone.
Acknowledgment
I am grateful to many colleagues for the opportunity to include recent data and for reading and offering helpful advice on the manuscript. This research was supported by the Centre of Excellence in Medical Engineering Centre funded by the Wellcome Trust and EPSRC under grant number WT088641/Z/09/Z, and the King’s College London and UCL Comprehensive Cancer Imaging Centre funded by CRUK and EPSRC in association with the MRC and DoH (England), and by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London. PET and SPECT scanning equipment was funded by an equipment grant from the Wellcome Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Notes and references
- 1.Ghotbi AA, Kjaer A, Hasbak P. Clin Physiol Functional Imaging. 2014;34:163. doi: 10.1111/cpf.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickson JC, Endozo R, Erlandsson K, Wan S, Neriman D, Blower PJ, Groves AM. J Nucl Med. 2014 in press. [Google Scholar]
- 3.Lin A, Ray ME. Cancer Metastasis Rev. 2006;25:669. doi: 10.1007/s10555-006-9025-z. [DOI] [PubMed] [Google Scholar]
- 4.Simpson WJ, Orange RP. Can Med Assoc J. 1965;93:1237. [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson S, Larsen RH, Fosså SD, Balteskard L, Borch KW, Westlin JE, Salberg G, Bruland OS. Clin Cancer Res. 2005;11:4451. doi: 10.1158/1078-0432.CCR-04-2244. [DOI] [PubMed] [Google Scholar]
- 6.Jauregui-Osoro M, Williamson PA, Glaria A, Sunassee K, Charoenphun P, Green MA, Mullen GED, Blower PJ. Dalton Trans. 2011;40:6226. doi: 10.1039/c0dt01618g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price EW, Orvig C. Chem Soc Rev. 2014;43:260. doi: 10.1039/c3cs60304k. [DOI] [PubMed] [Google Scholar]
- 8.Koumarianou E, Loktionova NS, Fellner M, Roesch F, Thews O, Pawlak D, Archimandritis SC, Mikolajczak R. Appl Radiat Isot. 2012;70:2669. doi: 10.1016/j.apradiso.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Majkowska-Pilip A, Bilewicz A. J Inorg Biochem. 2011;105:313. doi: 10.1016/j.jinorgbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Romer A, Seiler D, Marincek N, Brunner P, Koller MT, Ng QKT, Maecke HR, Mueller-Brand J, Rochlitz C, Briel M, Schindler C, et al. Eur J Nucl Med Mol Imaging. 2014;41:214. doi: 10.1007/s00259-013-2559-8. [DOI] [PubMed] [Google Scholar]
- 11.Emmanouilides C. Cancer Management Res. 2009;1:131. doi: 10.2147/cmr.s6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walrand S, Jamar F, Mathieu I, Camps J, Lonneux M, Sibomana M, Labar D, Michel C, Pauwels S. Eur J Nucl Med Mol Imaging. 2003;30:354. doi: 10.1007/s00259-002-1068-y. [DOI] [PubMed] [Google Scholar]
- 13.Holland JP, Vasdev N. Dalton Trans. 2014;43:9872. doi: 10.1039/c4dt00733f. [DOI] [PubMed] [Google Scholar]
- 14.Rice SL, Roney CA, Daumar P, Lewis JS. Semin Nucl Med. 2011;41:265. doi: 10.1053/j.semnuclmed.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vugts DJ, Visser GWM, van Dongen GAMS. Current Topics Med Chem. 2013;13:446. doi: 10.2174/1568026611313040005. [DOI] [PubMed] [Google Scholar]
- 16.Deri MA, Zeglis BM, Francesconi LC, Lewis JS. Nucl Med Biol. 2013;40:3. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heuveling DA, van Schie A, Vugts DJ, Hendrikse NH, Yaqub M, Hoekstra OS, Karagozoglu KH, Leemans CR, van Dongen GAMS, de Bree R. J Nucl Med. 2013;54:585. doi: 10.2967/jnumed.112.115188. [DOI] [PubMed] [Google Scholar]
- 18.Ma MT, Meszaros L, Berry DJ, Cooper M, Clark SJ, Ballinger JR, Blower PJ, Paterson BM, Ma Y, Hider RC. J Biol Inorg Chem. 2014;19:S649. [Google Scholar]
- 19.Berry DJ, Ma Y, Ballinger JR, Tavare R, Koers A, Sunassee K, Zhou T, Nawaz S, Mullen GED, Hider RC, Blower PJ. Chem Commun. 2011;47:7068. doi: 10.1039/c1cc12123e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deri MA, Ponnala S, Zeglis BM, Pohl G, Dannenberg JJ, Lewis JS, Francesconi LC. J Med Chem. 2014;57:4849. doi: 10.1021/jm500389b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerard F, Lee Y-S, Brechbiel MW. Chem Eur J. 2014;20:5584. doi: 10.1002/chem.201304115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerard F, Lee Y-S, Tripier R, Szajek LP, Deschamps JR, Brechbiel MW. Chem Commun. 2013;49:1002. doi: 10.1039/c2cc37549d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patra M, Bauman A, Mari C, Fischer CA, Blacque O, Häussinger D, Gasser G, Mindt TL. Chem Commun. 2014;50:11523. doi: 10.1039/c4cc05558f. [DOI] [PubMed] [Google Scholar]
- 24.Charoenphun P, Meszaros LK, Chuamsaamarkkee, Sharif-Paghaleh E, Ballinger JR, Ferris TJ, Went MJ, Mullen GED, Blower PJ. Eur J Nucl Med MoImaging. 2014;41 doi: 10.1007/s00259-014-2945-x. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radchenko V, Hauser H, Eisenhut M, Vugts DJ, van Dongen GAMS, Roesch F. Radiochim Acta. 2012;100:857. [Google Scholar]
- 26.Lacy JL, Layne WW, Guidry GW, Verani MS, Roberts R. J Nucl Med. 1991;32:2158. [PubMed] [Google Scholar]
- 27.Buck A, Nguyen N, Burger C, Ziegler S, Frey L, Weigand G, Erhardt W, SenekowitschSchmidtke R, Pellikka R, Blauenstein P, Locher JT, et al. Eur J Nucl Med. 1996;23:1619. doi: 10.1007/BF01249625. [DOI] [PubMed] [Google Scholar]
- 28.Jain D. Semin Nucl Med. 1999;29:221. doi: 10.1016/s0001-2998(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 29.Saha GB, Macintyre WJ, Go RT. Semin Nucl Med. 1994;24:324. doi: 10.1016/s0001-2998(05)80022-4. [DOI] [PubMed] [Google Scholar]
- 30.Peters AM. Semin Nucl Med. 1994;24:110. doi: 10.1016/s0001-2998(05)80226-0. [DOI] [PubMed] [Google Scholar]
- 31.Vanzetto G, Fagret D, Ghezzi C. J Nucl Cardiol. 2004;11:647. doi: 10.1016/j.nuclcard.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Tisato F, Porchia M, Bolzati C, Refosco F, Vittadini A. Coord Chem Rev. 2006;250:2034. [Google Scholar]
- 33.Banerjee S, Pillai MRA, Ramamoorthy N. Semin Nucl Med. 2001;31:260. doi: 10.1053/snuc.2001.26205. [DOI] [PubMed] [Google Scholar]
- 34.Johannsen B, Spies H. Technetium and Rhenium. 1996;176:77. doi: 10.1016/0969-8051(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 35.Boschi A, Duatti A, Uccelli L. Development of technetium-99m and rhenium-188 radiopharmaceuticals containing a terminal metal-nitrido multiple bond for diagnosis and therapy. In: Krause W, editor. Contrast Agents III: Radiopharmaceuticals - from Diagnostics to Therapeutics. Springer; Berlin/Heidelberg: 2005. (Topics in Current Chemistry series no. 252). [Google Scholar]
- 36.Coogan MP, Doyle RP, Valliant JF, Babich JW, Zubieta J. J Labelled Compd Radiopharm. 2014;57:255. doi: 10.1002/jlcr.3164. [DOI] [PubMed] [Google Scholar]
- 37.Kluba CA, Mindt TL. Molecules. 2013;18:3206. doi: 10.3390/molecules18033206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herman LW, Sharma V, Kronauge JF, Barbarics E, Herman LA, Piwnicaworms D. J Med Chem. 1995;38:2955. doi: 10.1021/jm00015a018. [DOI] [PubMed] [Google Scholar]
- 39.Meszaros LK, Dose A, Biagini SCG, Blower PJ. Inorg Chim Acta. 2010;363:1059. [Google Scholar]
- 40.Braband H, Benz M, Tooyama Y, Alberto R. Chem Commun. 2014;50:4126. doi: 10.1039/c4cc00718b. [DOI] [PubMed] [Google Scholar]
- 41.Wuillemin MA, Stuber WT, Fox T, Reber MJ, Bruehwiler D, Alberto R, Braband H. Dalton Trans. 2014;43:4260. doi: 10.1039/c3dt53019a. [DOI] [PubMed] [Google Scholar]
- 42.van Noorden R. Nature. 2013;504:202. doi: 10.1038/504202a. [DOI] [PubMed] [Google Scholar]
- 43.King RC, Surfraz MB-U, Biagini SCG, Blower PJ, Mather SJ. Dalton Trans. 2007:4998. doi: 10.1039/b705111e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Liu X, Hnatowich DJ. Nature Protocols. 2007;2:972. doi: 10.1038/nprot.2007.144. [DOI] [PubMed] [Google Scholar]
- 45.Huber GJ, Alberto RA, Blauenstein P, Anderegg G. J Chem Soc Chem Commun. 1989:879. [Google Scholar]
- 46.Abiraj K, Mansi R, Tamma M-L, Forrer F, Cescato R, Reubi JC, Akyel KG, Maecke HR. Chem Eur J. 2010;16:2115. doi: 10.1002/chem.200902011. [DOI] [PubMed] [Google Scholar]
- 47.Choudhry U, Greenland WEP, Goddard WA, Maclennan TAJ, Teat SJ, Blower PJ. Dalton Trans. 2003:311. [Google Scholar]
- 48.Hofstrom C, Altai M, Honarvar H, Strand J, Malmberg J, Hosseinimehr SJ, Orlova A, Graslund T, Tolmachev V. J Med Chem. 2013;56:4966. doi: 10.1021/jm400218y. [DOI] [PubMed] [Google Scholar]
- 49.Tolmachev V, Hofstrom C, Malmberg J, Ahlgren S, Hosseinimehr SJ, Sandstrom M, Abrahmsen L, Orlova A, Graslund T. Bioconjugate Chem. 2010;21:2013. doi: 10.1021/bc1002357. [DOI] [PubMed] [Google Scholar]
- 50.Badar A, Williams J, de Rosales RT, Tavare R, Kampmeier F, Blower PJ, Mullen GE. Eur J Nucl Med Mol Imaging Res. 2014;4:14. doi: 10.1186/2191-219X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tavare R, De Rosales RTM, Blower PJ, Mullen GED. Bioconjugate Chem. 2009;20:2071. doi: 10.1021/bc900160j. [DOI] [PubMed] [Google Scholar]
- 52.Tavare R, Williams J, Howland K, Blower PJ, Mullen GED. J Inorg Biochem. 2012;114:24. doi: 10.1016/j.jinorgbio.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Williams JD, Cooper M, Kampmeier F, Tavare R, Mullen GE, Blower P. Eur J Nucl Med Mol Imaging. 2012;39:S216. [Google Scholar]
- 54.Pasqualini R, Duatti A. J Chem Soc Chem Commun. 1992:1354. [Google Scholar]
- 55.Pasqualini R, Duatti A, Bellande E, Comazzi V, Brucato V, Hoffschir D, Fagret D, Comet M. J Nucl Med. 1994;35:334. [PubMed] [Google Scholar]
- 56.Berry DJ, de Rosales RTM, Charoenphun P, Blower PJ. Mini-Rev Med Chem. 2012;12:1174. doi: 10.2174/138955712802762112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carta D, Jentschel C, Thieme S, Salvarese N, Morellato N, Refosco F, Ruzza P, Bergmann R, Pietzsch H-J, Bolzati C. Nucl Med Biol. 2014;41:570. doi: 10.1016/j.nucmedbio.2014.04.126. [DOI] [PubMed] [Google Scholar]
- 58.Gomez Blanco N, Jauregui-Osoro M, Cobaleda-Siles M, Maldonado CR, Henriksen-Lacey M, Padro D, Clark S, Mareque-Rivas JC. Chem Commun. 2012;48:4211. doi: 10.1039/c2cc31045g. [DOI] [PubMed] [Google Scholar]
- 59.Maldonado CR, Gomez-Blanco N, Jauregui-Osoro M, Brunton VG, Yate L, Mareque-Rivas JC. Chem Commun. 2013;49:3985. doi: 10.1039/c3cc39104c. [DOI] [PubMed] [Google Scholar]
- 60.Torres Martin de Rosales R, Tavare R, Glaria A, Varma G, Protti A, Blower PJ. Bioconjugate Chem. 2011;22:455. doi: 10.1021/bc100483k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres Martin de Rosales R, Blower PJ. The role of 99mTc in the development of rhenium radiopharmaceuticals. In: Duatti A, editor. Technetium radiopharmaceuticals: status and prospective. IAEA; Venna: 2008. pp. 250–278. [Google Scholar]
- 62.Torres Martin de Rosales R, Finucane C, Mather SJ, Blower PJ. Chem Commun. 2009:4847. doi: 10.1039/b908652h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blower PJ, Kettle AG, O'Doherty MJ, Coakley AJ, Knapp FF. Eur J Nucl Med. 2000;27:1405. doi: 10.1007/s002590000307. [DOI] [PubMed] [Google Scholar]
- 64.Mueller C, Schubiger PA, Schibli R. Nucl Med Biol. 2007;34:595. doi: 10.1016/j.nucmedbio.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 65.Bruehlmeier M, Leenders KL, Vontobel P, Calonder C, Antonini A, Weindl A. J Nucl Med. 2000;41:781. [PubMed] [Google Scholar]
- 66.Beshara S, Sorensen J, Lubberink M, Tolmachev V, Langstrom B, Antoni G, Danielson BG, Lundqvist H. Brit J Haematol. 2003;120:853. doi: 10.1046/j.1365-2141.2003.03590.x. [DOI] [PubMed] [Google Scholar]
- 67.Srivastava SC, Richards P, Som P, Meinken G, Atkins HL, Sewatkar A, Ku TH. Ruthenium-97-labeled compounds - a new class of radiopharmaceuticals. In: Horst W, et al., editors. Frontiers in Nuclear Medicine. Spinger-Verlag; Berlin Heidelberg: 1980. [Google Scholar]
- 68.De Reuck J, Vonck K, Santens P, Boon P, De Bleecker J, Strijckmans K, Lemahieu I. J Neurolog Sci. 2000;181:13. doi: 10.1016/s0022-510x(00)00382-8. [DOI] [PubMed] [Google Scholar]
- 69.Ferreira CL, Lapi S, Steele J, Green DE, Ruth TJ, Adam MJ, Orvig C. Appl Radiat Isot. 2007;65:1303. doi: 10.1016/j.apradiso.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Schmaljohann J, Karanikas G, Sinzinger H. J Labelled Compd Radiopharm. 2001;44:395. [Google Scholar]
- 71.Akgun Z, Engelbrecht H, Cutler CS, Barnes CL, Jurisson SS, Lever SZ. J Labelled Compd Radiopharm. 2005;48:S299. [Google Scholar]
- 72.Ballard BD, Kannan R, Katti KV, Hoffman T, Engelbrecht HP, Cutler CS, Jurisson SS. J Labelled Compd Radiopharm. 2005;48:S19. [Google Scholar]
- 73.Goswami N, Higginbotham C, Volkert W, Alberto R, Nef W, Jurisson S. Nucl Med Biol. 1999;26:951. doi: 10.1016/s0969-8051(99)00070-0. [DOI] [PubMed] [Google Scholar]
- 74.Zweit J, Carnochan P, Goodall R, Ott R. J Nucl Biol Med. 1994;38:18. [PubMed] [Google Scholar]
- 75.Nielsen GD, Andersen O, Jensen M. Fund Appl Toxicol. 1993;21:236. doi: 10.1006/faat.1993.1094. [DOI] [PubMed] [Google Scholar]
- 76.Packard AB, Spaeth K, Kronauge JF, Mirzadeh S. J Labelled Compd Radiopharm. 1997;40:354. [Google Scholar]
- 77.Blower PJ, Lewis JS, Zweit J. Nucl Med Biol. 1996;23:957. doi: 10.1016/s0969-8051(96)00130-8. [DOI] [PubMed] [Google Scholar]
- 78.Shokeen M, Anderson CJ. Acc Chem Res. 2009;42:832. doi: 10.1021/ar800255q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson CJ, Ferdani R. Cancer Biother Radiopharm. 2009;24:379. doi: 10.1089/cbr.2009.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai Z, Anderson CJ. J Labelled Compd Radiopharm. 2014;57:224. doi: 10.1002/jlcr.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holland JP, Lewis JS, Dehdashti F. Quart J Nucl Med Mol Imaging. 2009;53:193. [PMC free article] [PubMed] [Google Scholar]
- 82.Vavere AL, Lewis JS. Dalton Trans. 2007:4893. doi: 10.1039/b705989b. [DOI] [PubMed] [Google Scholar]
- 83.Hueting R. J Labelled Compd Radiopharm. 2014;57:231. doi: 10.1002/jlcr.3155. [DOI] [PubMed] [Google Scholar]
- 84.Ma MT, Karas JA, White JM, Scanlon D, Donnelly PS. Chem Commun. 2009:3237. doi: 10.1039/b903426a. [DOI] [PubMed] [Google Scholar]
- 85.Di Bartolo N, Sargeson AM, Smith SV. Org Biomol Chem. 2006;4:3350. doi: 10.1039/b605615f. [DOI] [PubMed] [Google Scholar]
- 86.Ma MT, Cooper MS, Paul RL, Shaw KP, Karas JA, Scanlon D, White JM, Blower PJ, Donnelly PS. Inorg Chem. 2011;50:6701. doi: 10.1021/ic200681s. [DOI] [PubMed] [Google Scholar]
- 87.Cooper MS, Ma MT, Sunassee K, Shaw KP, Williams JD, Paul RL, Donnelly PS, Blower PJ. Bioconjugate Chem. 2012;23:1029. doi: 10.1021/bc300037w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silversides JD, Smith R, Archibald SJ. Dalton Trans. 2011;40:6289. doi: 10.1039/c0dt01395a. [DOI] [PubMed] [Google Scholar]
- 89.Lima LMP, Halime Z, Marion R, Camus N, Delgado R, Platas-Iglesias C, Tripier R. Inorg Chem. 2014;53:5269. doi: 10.1021/ic500491c. [DOI] [PubMed] [Google Scholar]
- 90.Roosenburg S, Sosabowski JK, Joosten L, Cooper MS, Kolenc-Peit PK, Foster J, Burnet J, Oyen WJG, Blower PJ, Mather SJ, Boerman OC, et al. Mol Pharm. doi: 10.1021/mp500283k. in press. [DOI] [PubMed] [Google Scholar]
- 91.Dearling JLJ, Lewis JS, McCarthy DW, Welch MJ, Blower PJ. Chem Commun. 1998:2531. [Google Scholar]
- 92.Dearling JLJ, Lewis JS, Muller GED, Welch MJ, Blower PJ. J Biol Inorg Chem. 2002;7:249. doi: 10.1007/s007750100291. [DOI] [PubMed] [Google Scholar]
- 93.Maurer RI, Blower PJ, Dilworth JR, Reynolds CA, Zheng YF, Mullen GED. J Med Chem. 2002;45:1420. doi: 10.1021/jm0104217. [DOI] [PubMed] [Google Scholar]
- 94.Mullen GE, Zheng Y, Reynolds CA, Blower PJ, Dilworth JR. J Nucl Med. 2000;41:123P. [Google Scholar]
- 95.Hueting R, Kersemans V, Cornelissen B, Tredwell M, Hussien K, Christlieb M, Gee AD, Passchier J, Smart SC, Dilworth JR, Gouverneur V, et al. J Nucl Med. 2014;55:128. doi: 10.2967/jnumed.113.119917. [DOI] [PubMed] [Google Scholar]
- 96.Burgman P, O'Donoghue JA, Lewis JS, Welch MJ, Humm JL, Ling CC. Nucl Med Biol. 2005;32:623. doi: 10.1016/j.nucmedbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Donnelly PS, Liddell JR, Lim S, Paterson BM, Cater MA, Savva MS, Mot AI, James JL, Trounce IA, White AR, Crouch PJ. Proc Nat Acad Sci USA. 2012;109:47. doi: 10.1073/pnas.1116227108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Price KA, Crouch PJ, Volitakis I, Paterson BM, Lim S, Donnelly PS, White AR. Inorg Chem. 2011;50:9594. doi: 10.1021/ic201334q. [DOI] [PubMed] [Google Scholar]
- 99.Griessinger CM, Kehlbach R, Bukala D, Wiehr S, Bantleon R, Cay F, Schmid A, Braumueller H, Fehrenbacher B, Schaller M, Eichner M, et al. J Nucl Med. 2014;55:301. doi: 10.2967/jnumed.113.126318. [DOI] [PubMed] [Google Scholar]
- 100.Huang J, Lee CCI, Sutcliffe JL, Cherry SR, Tarantal AF. Mol Imaging. 2008;7:1. [PubMed] [Google Scholar]
- 101.Charoenphun P, Paul R, Weeks A, Berry D, Shaw K, Mullen G, Ballinger J, Blower PJ. Eur J Nucl Med Mol Imaging. 2011;38:S294. [Google Scholar]
- 102.Bhargava KK, Gupta RK, Nichols KJ, Palestro CJ. Nucl Med Biol. 2009;36:545. doi: 10.1016/j.nucmedbio.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dearling JLJ, Mullen GED, Lewis JS, Welch MJ, Blower PJ. J Labelled Compd Radiopharm. 1999;42:S276. [Google Scholar]
- 104.Fodero-Tavoletti MT, Villemagne VL, Paterson BM, White AR, Li Q-X, Camakaris J, O'Keefe GJ, Cappai R, Barnham KJ, Donnelly PS. J Alzheimers Disease. 2010;20:49. doi: 10.3233/JAD-2010-1359. [DOI] [PubMed] [Google Scholar]
- 105.Bagunya-Torres J, Andreozzi E, Blower PJ. submitted.
- 106.Qin C, Liu H, Chen K, Hu X, Ma X, Lan X, Zhang Y, Cheng Z. J Nucl Med. 2014;55:812. doi: 10.2967/jnumed.113.133850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Handley MG, Medina RA, Mariotti E, Kenny GD, Shaw KP, Yan R, Eykyn TR, Blower PJ, Southworth R. J Nucl Med. 2014;55:488. doi: 10.2967/jnumed.113.129015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ng Y, Lacy JL, Fletcher JW, Green MA. Appl Radiat Isot. 2014;91:38. doi: 10.1016/j.apradiso.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Betts HM, Barnard PJ, Bayly SR, Dilworth JR, Gee AD, Holland JP. Angew Chem Int Ed. 2008;47:8416. doi: 10.1002/anie.200801936. [DOI] [PubMed] [Google Scholar]
- 110.Sun X, Huang X, Guo J, Zhu W, Ding Y, Niu G, Wang A, Kiesewetter DO, Wang ZL, Sun S, Chen X. J Amer Chem Soc. 2014;136:1706. doi: 10.1021/ja410438n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wong RM, Gilbert DA, Liu K, Louie AY. ACS Nano. 2012;6:3461. doi: 10.1021/nn300494k. [DOI] [PubMed] [Google Scholar]
- 112.Zeng D, Lee NS, Liu Y, Zhou D, Dence CS, Wooley KL, Katzenellenbogen JA, Welch MJ. ACS Nano. 2012;6:5209. doi: 10.1021/nn300974s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Torres Martin de Rosales R, Tavare R, Paul RL, Jauregui-Osoro M, Protti A, Glaria A, Varma G, Szanda I, Blower PJ. Angew Chem Int Ed. 2011;50:5509. doi: 10.1002/anie.201007894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alberto R, Blauenstein P, Novakhofer I, Smith A, Schubiger PA. Appl Radiat Isot. 1992;43:869. [Google Scholar]
- 115.Berning DE, Katti KV, Volkert WA, Higginbotham CJ, Ketring AR. Nucl Med Biol. 1998;25:577. doi: 10.1016/s0969-8051(98)00023-7. [DOI] [PubMed] [Google Scholar]
- 116.Gadbois WF, Corriere JN. J Urol. 1974;112:420. doi: 10.1016/s0022-5347(17)59749-9. [DOI] [PubMed] [Google Scholar]
- 117.Blower PJ, Smith RJ, Jolley C. Nucl Med Commun. 1992;13:231. [Google Scholar]
- 118.Draisma A, Maffioli L, Gasparini M, Savelli G, Pauwels E, Bombardieri E. Tumori. 1998;84:434. doi: 10.1177/030089169808400402. [DOI] [PubMed] [Google Scholar]
- 119.Even-Sapir E, Israel O. Eur J Nucl Med Mol Imaging. 2003;30:S65. doi: 10.1007/s00259-003-1164-7. [DOI] [PubMed] [Google Scholar]
- 120.Goldsmith SJ, Vallabhajosula S. Semin Nucl Med. 2009;39:2. doi: 10.1053/j.semnuclmed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 121.Weiner RE. Nucl Med Biol. 1996;23:745. doi: 10.1016/0969-8051(96)00119-9. [DOI] [PubMed] [Google Scholar]
- 122.Jonkhoff AR, Huijgens PC, Versteegh RT, Vandieren EB, Ossenkoppele GJ, Martens HJM, Teule GJJ. Brit J Cancer. 1993;67:693. doi: 10.1038/bjc.1993.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jonkhoff AR, Huijgens PC, Versteegh RT, Vanlingen A, Ossenkoppele GJ, Drager AM, Teule GJJ. Leukemia Res. 1995;19:169. doi: 10.1016/0145-2126(94)00130-3. [DOI] [PubMed] [Google Scholar]
- 124.Jonkhoff AR, Plaizier M, Ossenkoppele GJ, Teule GJJ, Huijgens PC. Brit J Cancer. 1995;72:1541. doi: 10.1038/bjc.1995.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Michel RB, Brechbiel MW, Mattes MJ. J Nucl Med. 2003;44:632. [PubMed] [Google Scholar]
- 126.Ochakovskaya R, Osorio L, Goldenberg DM, Mattes MJ. Clin Cancer Res. 2001;7:1505. [PubMed] [Google Scholar]
- 127.Othman MF, Cooper M, Blower PJ, Lewington V. Eur J Nucl Med Mol Imaging. 2014 in press. [Google Scholar]
- 128.Banerjee SR, Pomper MG. Appl Radiat Isotopes. 2013;76:2. doi: 10.1016/j.apradiso.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Velikyan I. Theranostics. 2014;4:47. doi: 10.7150/thno.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fellner M, Baum RP, Kubicek V, Hermann P, Lukes I, Prasad V, Roesch F. Eur J Nucl Med Mol Imaging. 2010;37:834. doi: 10.1007/s00259-009-1355-y. [DOI] [PubMed] [Google Scholar]
- 131.Meckel M, Fellner M, Thieme N, Bergmann R, Kubicek V, Roesch F. Nucl Med Biol. 2013;40:823. doi: 10.1016/j.nucmedbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 132.Fani M, Tamma M-L, Nicolas GP, Lasri E, Medina C, Raynal I, Port M, Weber WA, Maecke HR. Mol Pharm. 2012;9:1136. doi: 10.1021/mp200418f. [DOI] [PubMed] [Google Scholar]
- 133.Mathias CJ, Lewis MR, Reichert DE, Laforest R, Sharp TL, Lewis JS, Yang ZF, Waters DJ, Snyder PW, Low PS, Welch MJ, et al. Nucl Med Biol. 2003;30:725. doi: 10.1016/s0969-8051(03)00080-5. [DOI] [PubMed] [Google Scholar]
- 134.Burke BP, Clemente GS, Archibald SJ. J Labelled Compd Radiopharm. 2014;57:239. doi: 10.1002/jlcr.3146. [DOI] [PubMed] [Google Scholar]
- 135.Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, Holland-Letz T, Hadaschik BA, Giesel FL, Debus J, Haberkorn U. Eur J Nucl Med Mol Imaging. 2014;41:11. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 136.Hoffer PB, Samuel A, Bushberg JT, Thakur M. Radiol. 1979;131:775. doi: 10.1148/131.3.775. [DOI] [PubMed] [Google Scholar]
- 137.Hoffer PB, Samuel A, Bushberg JT, Thakur M. J Nucl Med. 1979;20:248. [PubMed] [Google Scholar]
- 138.Blower PJ, Cooper MS, Nawaz S, O'Neill A, Koers A, Sunassee K, Berry DJ, Mullen GED, Ballinger JR. Eur J Nucl Med Mol Imaging. 2012;39:S262. [Google Scholar]
- 139.Petrik M, Franssen GM, Haas H, Laverman P, Hoertnagl C, Schrettl M, Helbok A, Lass-Floerl C, Decristoforo C. Eur J Nucl Med Mol Imaging. 2012;39:1175. doi: 10.1007/s00259-012-2110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Petrik M, Haas H, Dobrozemsky G, Lass-Floerl C, Helbok A, Blatzer M, Dietrich H, Decristoforo C. J Nucl Med. 2010;51:639. doi: 10.2967/jnumed.109.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Petrik M, Haas H, Laverman P, Schrettl M, Franssen GM, Blatzer M, Decristoforo C. Mol Imaging Biol. 2014;16:102. doi: 10.1007/s11307-013-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Petrik M, Haas H, Schrettl M, Helbok A, Blatzer M, Decristoforo C. Nucl Med Biol. 2012;39:361. doi: 10.1016/j.nucmedbio.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bomanji JB, Papathanasiou ND. Eur J Nucl Med Mol Imaging. 2012;39:113. doi: 10.1007/s00259-011-2013-8. [DOI] [PubMed] [Google Scholar]
- 144.Virgolini I, Britton K, Buscombe J, Moncayo R, Paganelli G, Riva P. Semin Nucl Med. 2002;32:148. doi: 10.1053/snuc.2002.31565. [DOI] [PubMed] [Google Scholar]
- 145.Malmberg J, Tolmachev V, Orlova A. Exp Ther Med. 2011;2:523. doi: 10.3892/etm.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peters AM. Quart J Nucl Med Mol Imaging. 2005;49:304. [PubMed] [Google Scholar]
- 147.Pagnanelli RA, Basso DA. J Nucl Med Technol. 2010;38:1. doi: 10.2967/jnmt.109.068593. [DOI] [PubMed] [Google Scholar]
- 148.Deutsch E, Bushong W, Glavan KA, Elder RC, Sodd VJ, Scholz KL, Fortman DL, Lukes SJ. Science. 1981;214:85. doi: 10.1126/science.6897930. [DOI] [PubMed] [Google Scholar]
- 149.Beller GA, Watson DD. Semin Nucl Med. 1991;21:173. doi: 10.1016/s0001-2998(05)80038-8. [DOI] [PubMed] [Google Scholar]
- 150.Bishayee A, Rao DV, Srivastava SC, Bouchet LG, Bolch WE, Howell RW. J Nucl Med. 2000;41:2043. [PubMed] [Google Scholar]
- 151.Baidoo KE, Milenic DE, Brechbiel MW. Nucl Med Biol. 2013;40:592. doi: 10.1016/j.nucmedbio.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim Y-S, Brechbiel MW. Tumor Biol. 2012;33:573. doi: 10.1007/s13277-011-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yong K, Brechbiel MW. Dalton Trans. 2011;40:6068. doi: 10.1039/c0dt01387k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gomez-Vallejo V, Gaja V, Gona KB, Llop J. J Labelled Compd Radiopharm. 2014;57:244. doi: 10.1002/jlcr.3163. [DOI] [PubMed] [Google Scholar]
- 155.Blower JE, Cousin SF, Gee AD. submitted.
- 156.Cheng Y, Senthamizhchelvan S, Agarwal R, Green GM, Mease RC, Sgouros G, Huso DL, Pomper MG, Meltzer SJ, Abraham JM. Cell Cycle. 2012;11:1878. doi: 10.4161/cc.19955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Patrick MR, Chester KA, Pietersz GA. Cancer Immunol Immunother. 1998;46:229. doi: 10.1007/s002620050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Goddu SM, Bishayee A, Bouchet LG, Bolch WE, Rao DV, Howell RW. J Nucl Med. 2000;41:941. [PubMed] [Google Scholar]
- 159.Jennewein M, Qaim SM, Kulkarni RV, Mason RP, Hermanne A, Rosch F. Radiochim Acta. 2005;93:579. [Google Scholar]
- 160.Jennewein M, Lewis MA, Zhao D, Tsyganov E, Slavine N, He J, Watkins L, Kodibagkar VD, O'Kelly S, Kulkarni P, Antich PP, et al. Clin Cancer Res. 2008;14:1377. doi: 10.1158/1078-0432.CCR-07-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sweet WH, Brownell GL. J Amer Med Assoc. 1955;157:1183. doi: 10.1001/jama.1955.02950310009002. [DOI] [PubMed] [Google Scholar]
- 162.Rosenblat TL, McDevitt MR, Mulford DA, Pandit-Taskar N, Divgi CR, Panageas KS, Heaney ML, Chanel S, Morgenstern A, Sgouros G, Larson SM, et al. Clin Cancer Res. 2010;16:5303. doi: 10.1158/1078-0432.CCR-10-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pagel JM, Kenoyer AL, Back T, Hamlin DK, Wilbur DS, Fisher DR, Park SI, Frayo S, Axtman A, Orgun N, Orozco J, et al. Blood. 2011;118:703. doi: 10.1182/blood-2011-04-347039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Miller PW, Long NJ, Vilar R, Gee AD. Angew Chem Int Ed. 2008;47:8998. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 165.Smith GE, Sladen HL, Biagini SCG, Blower PJ. Dalton Trans. 2011;40:6196. doi: 10.1039/c0dt01594f. [DOI] [PubMed] [Google Scholar]
- 166.Kostikov AP, Chin J, Orchowski K, Niedermoser S, Kovacevic MM, Aliaga A, Jurkschat K, Waengler B, Waengler C, Wester H-J, Schirrmacher R. Bioconjugate Chem. 2012;23:106. doi: 10.1021/bc200525x. [DOI] [PubMed] [Google Scholar]
- 167.Waengler C, Niedermoser S, Chin J, Orchowski K, Schirrmacher E, Jurkschat K, Iovkova-Berends L, Kostikov AP, Schirrmacher R, Waengler B. Nature Protocols. 2012;7:1946. doi: 10.1038/nprot.2012.109. [DOI] [PubMed] [Google Scholar]
- 168.Jauregui-Osoro M, Sunassee K, Weeks AJ, Berry DJ, Paul RL, Cleij M, Banga JP, O'Doherty MJ, Marsden PK, Clarke SEM, Ballinger JR, et al. Eur J Nucl Med Mol Imaging. 2010;37:2108. doi: 10.1007/s00259-010-1523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weeks AJ, Jauregui-Osoro M, Cleij M, Blower JE, Ballinger JR, Blower PJ. Nucl Med Commun. 2011;32:98. doi: 10.1097/MNM.0b013e3283419540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Laverman P, McBride WJ, Sharkey RM, Goldenberg DM, Boerman OC. J Labelled Compd Radiopharm. 2014;57:219. doi: 10.1002/jlcr.3161. [DOI] [PubMed] [Google Scholar]
- 171.McBride WJ, Sharkey RM, Goldenberg DM. Eur J Nucl Med Mol Imaging Res. 2013;3:36. doi: 10.1007/s00259-013-2434-7. [DOI] [PubMed] [Google Scholar]
- 172.Tolmachev V. Curr Radiopharm. 2011;4:76. doi: 10.2174/1874471011104020076. [DOI] [PubMed] [Google Scholar]
- 173.Adam MJ, Wilbur DS. Chem Soc Rev. 2005;34:153. doi: 10.1039/b313872k. [DOI] [PubMed] [Google Scholar]
- 174.Seevers RH, Counsell RE. Chem Rev. 1982;82:575. [Google Scholar]
- 175.Wilbur DS. Bioconjugate Chem. 1992;3:433. doi: 10.1021/bc00018a001. [DOI] [PubMed] [Google Scholar]
- 176.Champion J, Alliot C, Huclier S, Deniaud D, Asfari Z, Montavon G. Inorg Chim Acta. 2009;362:2654. [Google Scholar]
- 177.Guerard F, Rajerison H, Faivre-Chauvet A, Barbet J, Meyer GJ, Haddad F, Da Silva I, Gestin JF. Eur J Nucl Med Mol Imaging. 2010;37:S361. [Google Scholar]
- 178.Pruszynski M, Bilewicz A, Zalutsky MR. Bioconjugate Chem. 2008;19:958. doi: 10.1021/bc700413r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rajerison H, Guerard F, Mougin-Degraef M, Bourgeois M, Da Silva I, Cherel M, Barbet J, Faivre-Chauvet A, Gestin J-F. Nucl Med Biol. 2014;41(Suppl):e23. doi: 10.1016/j.nucmedbio.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 180.Wilbur DS, Chyan M-K, Hamlin DK, Perry MA. Bioconjugate Chem. 2009;20:591. doi: 10.1021/bc800515d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lambrecht RM, Tomiyoshi K, Sekine T. Radiochim Acta. 1997;77:103. [Google Scholar]
- 182.Anderson P, Nunez R. Expert Rev Anticancer Ther. 2007;7:1517. doi: 10.1586/14737140.7.11.1517. [DOI] [PubMed] [Google Scholar]
- 183.Thompson S, Ballard B, Jiang Z, Revskaya E, Sisay N, Miller WH, Cutler CS, Dadachova E, Francesconi LC. Nucl Med Biol. 2014;41:276. doi: 10.1016/j.nucmedbio.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Beyer GJ, Miederer M, Vranjes-duric S, Comor JJ, Kunzi G, Hartley O, Senekowitsch-Schmidtke R, Soloviev D, Buchegger F. Eur J Nucl Med Mol Imaging. 2004;31:547. doi: 10.1007/s00259-003-1413-9. [DOI] [PubMed] [Google Scholar]
- 185.Miederer M, Scheinberg DA, McDevitt MR. Adv Drug Deliv Rev. 2008;60:1371. doi: 10.1016/j.addr.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Abbas N, Bruland OS, Brevik EM, Dahle J. Nucl Med Commun. 2012;33:838. doi: 10.1097/MNM.0b013e328354df7c. [DOI] [PubMed] [Google Scholar]