Abstract
Background
Men who have sex with men (MSM) and transgender women in Peru are at high risk for acquiring syphilis and HIV infection. The World Health Organization highly recommends screening for HIV and syphilis to reduce morbidity and mortality associated with untreated infections. We aimed to identify factors associated with dual testing preferences for HIV and syphilis infection among MSM and transgender women in Lima, Peru.
Methods
We used conjoint analysis, an innovative method for systematically estimating consumer preferences. We created eight hypothetical test profiles varying across six dichotomous attributes: cost (free vs. $4), potential for false positive syphilis result (no false positive vs. some risk of false positive), time-to-result (20 minutes vs. 1 week), blood draw method (finger prick vs. venipuncture), test type (rapid vs. laboratory), and number of draws (1 vs. 2). We fit a conjoint analysis model for each participant using a simple main effects ANOVA. Attribute importance values were calculated using percentages from relative ranges in the attribute’s utility values. Results were summarized across participants and averages were reported.
Results
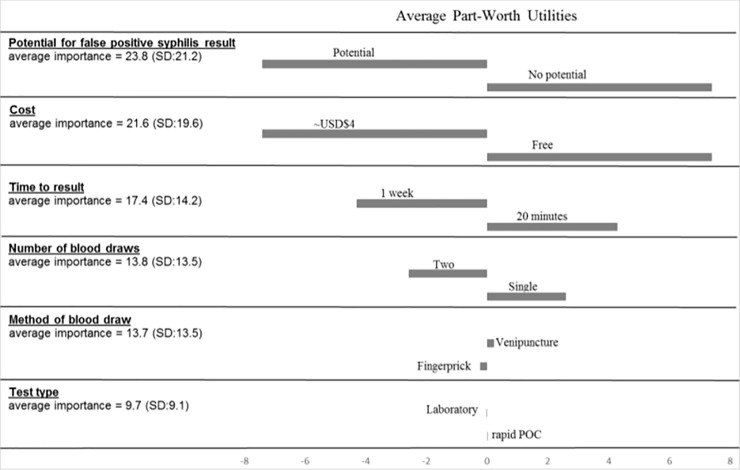
We recruited 415 MSM/transgender women over 18 years of age from two STD clinics in Lima, Peru. No potential for syphilis false positive result (no false positive vs. some potential for false positive) had the largest average impact on willingness to use the test and on average accounted for 23.8% of test type preference, followed by cost (free vs. ~USD$4; 21.6%), time to results (20 minutes vs. 1 week; 17.4%), number of blood draws (1 draw vs. 2 draws; 13.8%), method of blood draw (fingerprick vs. venipuncture; 13.7%), and test type (rapid POC vs. laboratory; 9.7%).
Conclusion
MSM/transgender women in Peru prioritized accuracy, cost, timeliness and number of blood draws for HIV and syphilis testing. Implementing a low cost, accurate, rapid and dual testing strategy for HIV and syphilis could improve screening uptake and accessibility of testing to accelerate time to treatment.
Background
The World Health Organization highly recommends screening for human immunodeficiency virus (HIV) and syphilis to reduce morbidity and mortality associated with untreated infections [1, 2]. In South America, HIV and syphilis is endemic among men who have sex with men (MSM) and transgender women [3–5]. In a study conducted in 2013 to 2014 among MSM and transgender women in Lima, Peru, our group observed the prevalence of HIV infection to be 30.1% among MSM and 33.7% among transgender women and the prevalence of recent syphilis infection to be 16.8% among MSM and 6.7% among transgender women [6]. In addition, MSM and transgender women may be averse to health care engagement and those who access care are often lost to follow up [7–11]. Among MSM and transgender women attending sexually transmitted infection (STI) clinics in Lima, Peru, 41% reported never being tested for HIV prior to their current visit [12].
Implementing a preferred and acceptable dual testing strategy for HIV and syphilis could improve screening uptake, accessibility, retention, and buy-in from patient. For example, the introduction of point-of-care (POC) rapid tests, rapid tests that can be used in settings outside of the laboratory, improved overall rates of syphilis screening and treatment among pregnant women in low-resource countries, including Peru [13–15]. However, many types of diagnostics tools are available for syphilis screening and preferred testing modalities for MSM and transgender women have not been determined in South America [16–18]. These testing tools include rapid tests for HIV and syphilis as well as laboratory based tests.
We aimed to determine which attributes of diagnostic tests are preferred among MSM and transgender women in Lima, Peru.
Methods
Participants and study setting
MSM and transgender women aged 18 years and over seeking testing or care in one of two STI clinics in Lima, Peru were recruited to participate in a study to assess HIV and syphilis dual testing acceptability and preferences. The two clinical sites were the Alberto Barton Clinic, a public STI Health Center located in Callao, the main port of Peru that regularly provides services to female and male sex workers, MSM and transgender women; and the Epicentro Clinic, a gay men’s community health center in southern Lima provides health services to MSM and transgender women. All interviews were conducted individually by trained counselors in the two clinics in-person in a private room and responses were collected electronically. Interviews took approximately 15 minutes and participants were reimbursed approximately USD$4 for their time.
Data collection and procedures
Conjoint analysis is a method for systematically estimating consumer preferences [19], and has been applied in health care research [20, 21]. Therefore, we used conjoint analysis to determine which attributes of diagnostic tests are preferred among MSM and transgender women in Lima, Peru.
We created eight hypothetical test profiles, which varied across six dichotomous testing attributes to yield 64 possible test profiles (26 = 64). Given that the number of possible test profiles is too large to ask participants to rate every scenario, we used a fractional factorial design to reduce the number of hypothetical test profiles to eight to measure the main effect of each attribute [22, 23]. That design with eight hypothetical test profiles is 100% efficient, balanced and orthogonal [23]. The testing attributes were: test type (rapid POC vs. laboratory), cost (free vs. approximately four US dollars in the local currency (Peruvian Nuevos Soles S/.12)) (the price of 1 liter of milk at this time was about S/.3.85–4.16 [24]), potential for false positive syphilis result (no risk of false positive vs. some risk of false positive), time-to-result (20 minutes vs. 1 week), blood draw method (finger prick vs. venipuncture), and number of blood draws (1 vs. 2). We included potential for false positive result because often screening tests that are used for syphilis that detect treponemal antibody, this is an antibody that is produced in response to syphilis infection, however this antibody can be present for life even following curative syphilis treatment. Therefore, if a person has had syphilis in the past, they may still test positive for syphilis using a treponemal test. The testing attributes were created using characteristics of existing HIV and syphilis testing strategies [25–27].
Each participant was presented with the eight different testing scenarios, one at a time, described on laminated cards. The cards were presented in random order and were not marked with any schema that might suggest a sequence or preference rating. Participants were asked to rate the eight testing scenarios in terms of how likely they would be to ever test for HIV and syphilis given the test attributes on that test profile card. Participants’ ratings of each hypothetical test profile were recorded using a five-point Likert preference scale: highly likely, somewhat likely, neutral, somewhat unlikely, and highly unlikely.
Data analysis
The overall goal of conjoint analysis is to understand preferences between product (in this case, a test for HIV and syphilis) attributes and the impact of individual attributes on consumers’ willingness to use a product. Because it may not be possible for a test for HIV and syphilis to be a combination of every desirable attribute, trade-offs must be made to select those attributes that are most highly valued. For analysis, we converted the Likert preference scale values to a 100-point numeric scale using multiplication; higher scores suggested increased preference. We fit a conjoint analysis model for each participant using a simple main effects ANOVA with attributes as independent variables [28], participant ratings as the dependent variable to give part-worth utility values (represented by the β terms from the ANOVA). Part-worth utilities mean values were calculated from each individuals’ subjective preference or the utility they associated with each level (in this study we had two levels) of each attribute. The part-worth utility values measure how much attribute and level influenced the participants’ willingness to use a test. Participants who gave the same Likert scale response for every hypothetical test profile or whose adjusted R square were less than 0.3 were excluded from data analysis. Attribute importance values were calculated using percentages from relative ranges in the attribute’s utility values. Results were summarized across participants and averages were reported. Data were analyzed using SAS v9.4 (Cary, NC, USA).
Ethical approval
All participants gave written informed consent for participation. Ethical approval and oversight was provided by the Institutional Review Board at the Universidad Peruana Cayetano Heredia. The approval was filed under the reference number SIDISI 59996.
Results
We recruited 415 MSM and transgender women, with a mean age of 30.1 years (standard deviation: 9.0). Of the 415 participants, 310 (74.7%) were men and 105 (25.3%) were transgender women.
Of the 415 participants, 40 gave the same Likert scale response for all eight hypothetical test profiles and thus were excluded from the conjoint analysis. In addition, 28 who had an adjusted R square less than 0.3 were excluded from analysis, leaving 347 evaluable results. Average preference scores of the eight hypothetical testing scenarios ranged from 44.4 (SD = 32.0) to 79.2 (SD = 28.2). Results of the metric conjoint analysis model of participant willingness to use a hypothetical HIV and syphilis test are displayed in Fig 1. For the sample population, no potential for syphilis false positive result (no false positive vs. some potential for false positive) had the largest average impact on willingness to use the test and on average accounted for 23.8% of test type preference, followed by cost (free vs. ~USD$4; 21.6%), time to results (20 minutes vs. 1 week; 17.4%), number of blood draws (1 draw vs. 2 draws; 13.8%), method of blood draw (fingerprick vs. venipuncture; 13.7%), and test type (rapid POC vs. laboratory; 9.7%). We stratified the conjoint analysis by gender identity (men vs. transgender women), however we did not observe any significant differences in testing preferences between groups.
Fig 1. Average importance and average part-worth utilities for attributes of human immunodeficency virus and syphilis testing from conjoint analysis results among men who have sex with men and transgender women in Lima, Peru.
*Standard deviation, SD; United States Dollar, USD; Point of Care, POC. Positive values suggest participant relative preference while negative values suggest lack of preference. Part-worth utility values measure how much attribute and level influenced the participants’ willingness to test. Attributes with the largest part-worth utility range are considered the most important in predicting willingness to test. The attribute importance values were calculated using the percentages from relative ranges in the attribute’s utility values.
Discussion
We conducted a study among a key group of MSM and transgender women to identify HIV and syphilis testing preferences using metric conjoint analysis. HIV and syphilis testing preferences among those key populations in Peru prioritized accuracy, cost, timeliness, and number of blood draws. While it is well described that MSM and transgender women have difference risk contexts, their preferences regarding HIV and syphilis testing preferences are not dissimilar in our setting. However, given their different risk contexts, it is likely that testing programs will require different strategies to target those groups and further research may be needed to determine those optimal testing programs.
Lee et al. used conjoint analysis to address the willingness to test for HIV among MSM in Los Angeles based on varying factors that found similar results to our study [21]. Populations in both studies prioritized cost and timeliness of results. Among our participants, the method of specimen collection (venipuncture vs. fingerprick) would not affect their likelihood of testing for HIV and syphilis. Additionally, the participants did not prioritize the testing modality, laboratory versus rapid POC. This is unsurprising because this attribute does not provide further information about how the test will be administered and results generated. In addition, participants identified preferred attributes of a POC test that have low potential for a false positive syphilis result, however rapid tests for syphilis usually detect treponemal antibody, thus they cannot distinguish between past and present infection. Rapid tests that include a non-treponemal result, a second test that is often used for confirmation, may have additional utility in this population [29, 30].
There are some potential limitations to this study. The study sample may not be generalizable to all MSM and transgender women in Lima, Peru. The study sample selection also could have impacted the findings in that the participants were already seeking HIV and syphilis testing, therefore attributes of the test would not necessarily have as large of an impact for them compared with those not seeking testing. This may have led to our findings that some attributes of tests were similarly important when deciding whether to test for HIV and syphilis. In addition, further research is needed to determine if availability of preferred tests leads to improved test uptake.
Given that MSM and transgender women are often difficult to access populations that are often lost to follow up, it is important for policy makers and health care workers to understand these groups’ preferences if there is the potential to increase testing and retention in care [7–11]. We found that MSM and transgender women prefer tests that have lower costs, improved accuracy, timeliness, and lower number of blood draws. Policy makers should ensure access to accurate HIV and syphilis tests at a low cost. Test manufacturers should work to create tests that reduce numbers of blood draws and provide accurate results in a timely manner. Health care workers should prioritize optimal clinic management to reduce that chance of having to redraw blood from patients and to provide testing results in a timely manner.
Supporting information
(XLS)
Acknowledgments
We would like to thank the study sites, Barton Clinic and Epicentro clinic in Lima, Peru, as well as the study program staff and research participants.
Data Availability
All data are contained in the paper and in the supplementary file S1 Dataset. That is the minimal dataset used to draw the conclusions of our manuscript. Interested researchers will be able to replicate our study's findings using this file.
Funding Statement
This study was funded by the NIH funded though NIH/NIAID R-01 study #1R01AI099727-01. CCB’s time was supported but NIH/NIAID through grant numbers T32AI007384 and K01AI136725. NK is supported by the Fogarty International Center of the National Institutes of Health (NIH) under award #D43TW009343 and the University of California Global Health Institute (UCGHI). Dr. Lee’s time on this manuscript was supported by the National Institute of Mental Health (5K01MH085503 & P30MH058107), UCLA AIDS Institute, UCLA Center for AIDS Research (AI28697), and the UCLA Clinical and Translational Science Institute, NCRR and NCATS (UL1TR000124). Segundo R Leon was also supported by NIH research training grant #R25TW009345 funded by the Fogarty International Center, the National Institute of Mental Health, the NIH Office of the Director, the Office of Women’s Health and the Office of AIDS Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or UCGHI.
References
- 1.WHO. Consolidated guidelines on HIV testing services. 2015.
- 2.WHO. GLOBAL HEALTH SECTOR STRATEGY ON SEXUALLY TRANSMITTED INFECTIONS 2016–2021: Towards Ending STIs. 2016.
- 3.Zoni AC, Gonzalez MA, Sjogren HW. Syphilis in the most at-risk populations in Latin America and the Caribbean: a systematic review. Int J Infect Dis. 2013;17(2):e84–92. 10.1016/j.ijid.2012.07.021 . [DOI] [PubMed] [Google Scholar]
- 4.Tabet S, Sanchez J, Lama J, Goicochea P, Campos P, Rouillon M, et al. HIV, syphilis and heterosexual bridging among Peruvian men who have sex with men. AIDS. 2002;16(9):1271–7. . [DOI] [PubMed] [Google Scholar]
- 5.Sanchez J, Lama JR, Peinado J, Paredes A, Lucchetti A, Russell K, et al. High HIV and ulcerative sexually transmitted infection incidence estimates among men who have sex with men in Peru: awaiting for an effective preventive intervention. Journal of acquired immune deficiency syndromes. 2009;51 Suppl 1:S47–51. 10.1097/QAI.0b013e3181a2671d ; PubMed Central PMCID: PMC2725017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima N, Park H, Konda KA, Joseph Davey DL, Bristow CC, Brown B, et al. The PICASSO Cohort: baseline characteristics of a cohort of men who have sex with men and male-to-female transgender women at high risk for syphilis infection in Lima, Peru. BMC infectious diseases. 2017;17(1):255 10.1186/s12879-017-2332-x ; PubMed Central PMCID: PMCPMC5387233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Brumer AG, Reisner SL, McLean SA, Silva-Santisteban A, Huerta L, Mayer KH, et al. Leveraging social capital: multilevel stigma, associated HIV vulnerabilities, and social resilience strategies among transgender women in Lima, Peru. J Int AIDS Soc. 2017;20(1):21462 10.7448/IAS.20.1.21462 ; PubMed Central PMCID: PMCPMC5467605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollock L, Silva-Santisteban A, Sevelius J, Salazar X. 'You should build yourself up as a whole product': Transgender female identity in Lima, Peru. Glob Public Health. 2016;11(7–8):981–93. 10.1080/17441692.2016.1167932 . [DOI] [PubMed] [Google Scholar]
- 9.Silva AP, Greco M, Fausto MA, Carneiro M. Loss to follow-up in a cohort of HIV-negative men who have sex with men: Project Horizonte. Rev Saude Publica. 2017;51:60 10.1590/S1518-8787.2017051006681 ; PubMed Central PMCID: PMCPMC5477724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva-Santisteban A, Eng S, de la Iglesia G, Falistocco C, Mazin R. HIV prevention among transgender women in Latin America: implementation, gaps and challenges. J Int AIDS Soc. 2016;19(3 Suppl 2):20799 10.7448/IAS.19.3.20799 ; PubMed Central PMCID: PMCPMC4949309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang EC, Segura ER, Clark JL, Sanchez J, Lama JR. The syphilis care cascade: tracking the course of care after screening positive among men and transgender women who have sex with men in Lima, Peru. BMJ Open. 2015;5(9):e008552 10.1136/bmjopen-2015-008552 ; PubMed Central PMCID: PMCPMC4577872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipsitz MC, Segura ER, Castro JL, Smith E, Medrano C, Clark JL, et al. Bringing testing to the people—benefits of mobile unit HIV/syphilis testing in Lima, Peru, 2007–2009. International journal of STD & AIDS. 2014;25(5):325–31. 10.1177/0956462413507443 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia PJ, Carcamo CP, Chiappe M, Valderrama M, La Rosa S, Holmes KK, et al. Rapid Syphilis Tests as Catalysts for Health Systems Strengthening: A Case Study from Peru. PloS one. 2013;8(6):e66905 10.1371/journal.pone.0066905 ; PubMed Central PMCID: PMC3694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mabey DC, Sollis KA, Kelly HA, Benzaken AS, Bitarakwate E, Changalucha J, et al. Point-of-care tests to strengthen health systems and save newborn lives: the case of syphilis. PLoS medicine. 2012;9(6):e1001233 10.1371/journal.pmed.1001233 ; PubMed Central PMCID: PMC3373627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strasser S, Bitarakwate E, Gill M, Hoffman HJ, Musana O, Phiri A, et al. Introduction of rapid syphilis testing within prevention of mother-to-child transmission of HIV programs in Uganda and Zambia: a field acceptability and feasibility study. Journal of acquired immune deficiency syndromes. 2012;61(3):e40–6. 10.1097/QAI.0b013e318267bc94 . [DOI] [PubMed] [Google Scholar]
- 16.Binnicker MJ, Yao JD, Cockerill FR 3rd. Non-treponemal serologic tests: a supplemental, not confirmatory testing approach. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;52(2):274–5; author reply 5–6. 10.1093/cid/ciq127 . [DOI] [PubMed] [Google Scholar]
- 17.Lafond RE, Lukehart SA. Biological basis for syphilis. Clinical microbiology reviews. 2006;19(1):29–49. 10.1128/CMR.19.1.29-49.2006 ; PubMed Central PMCID: PMC1360276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naidu NK, Bharucha ZS, Sonawane V, Ahmed I. Comparative study of Treponemal and non-Treponemal test for screening of blood donated at a blood center. Asian journal of transfusion science. 2012;6(1):32–5. 10.4103/0973-6247.95048 ; PubMed Central PMCID: PMC3353627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall P, Bradlow ET. A Unified Approach to Conjoint Analysis Models. Journal of the American Statistical Association. Journal of the American Statistical Association. 2002;97(459):674–82. [Google Scholar]
- 20.Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health services research. 2002;37(6):1681–705. 10.1111/1475-6773.01115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Brooks R, Bolan RK, Flynn R. Assessing willingness to test for HIV among men who have sex with men using conjoint analysis, evidence for uptake of the FDA-approved at-home HIV test. AIDS care. 2013;25(12):1592–8. 10.1080/09540121.2013.793272 ; PubMed Central PMCID: PMCPMC3751974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Newman PA, Comulada WS, Cunningham WE, Duan N. Use of conjoint analysis to assess HIV vaccine acceptability: feasibility of an innovation in the assessment of consumer health-care preferences. International journal of STD & AIDS. 2012;23(4):235–41. 10.1258/ijsa.2011.011189 ; PubMed Central PMCID: PMC3372064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhfeld W. Marketing Research Methods in SAS Experimental Design, Choice, Conjoint, and Graphical Techniques. 2010.
- 24.Numbeo. Historical Data about Cost of Living by Year in Lima. Accessed 8 August 2018.
- 25.Dinh TH, Kamb ML, Msimang V, Likibi M, Molebatsi T, Goldman T, et al. Integration of preventing mother-to-child transmission of HIV and syphilis testing and treatment in antenatal care services in the Northern Cape and Gauteng provinces, South Africa. Sexually transmitted diseases. 2013;40(11):846–51. 10.1097/OLQ.0000000000000042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tucker JD, Bien CH, Peeling RW. Point-of-care testing for sexually transmitted infections: recent advances and implications for disease control. Curr Opin Infect Dis. 2013;26(1):73–9. 10.1097/QCO.0b013e32835c21b0 ; PubMed Central PMCID: PMCPMC3635142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klausner JD, E.W. H. Current Diagnosis & Treatment of Sexually Transmitted Diseases. United States: The McGraw-Hill Companies, Inc.; 2007. [Google Scholar]
- 28.Kuhfeld W. Marketing Research Methods in SAS (2nd ed, Vol. 9). Cary, NC: SAS Institute Inc., 2010. [Google Scholar]
- 29.Castro AR, Esfandiari J, Kumar S, Ashton M, Kikkert SE, Park MM, et al. Novel point-of-care test for simultaneous detection of nontreponemal and treponemal antibodies in patients with syphilis. Journal of clinical microbiology. 2010;48(12):4615–9. 10.1128/JCM.00624-10 ; PubMed Central PMCID: PMC3008492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castro AR, Mody HC, Parab SY, Patel MT, Kikkert SE, Park MM, et al. An immunofiltration device for the simultaneous detection of non-treponemal and treponemal antibodies in patients with syphilis. Sexually transmitted infections. 2010;86(7):532–6. 10.1136/sti.2010.042937 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All data are contained in the paper and in the supplementary file S1 Dataset. That is the minimal dataset used to draw the conclusions of our manuscript. Interested researchers will be able to replicate our study's findings using this file.