Abstract
Background
It has been hypothesized that schistosomiasis negatively influences immune reconstitution in people living with HIV starting antiretroviral therapy (ART). In this study, we investigated the effect of schistosomiasis on the course of HIV infection in patients starting ART in a rural part of Tanzania.
Methodology
Retrospective study including patients prospectively enrolled in a HIV cohort in Ifakara, south-central Tanzania between January 1, 2013 and April 1, 2015. Schistosomal circulating anodic antigen (CAA) was assessed in pre-ART cryopreserved plasma. Regression models were utilized to estimate the effect of CAA positivity on virological and immunological failure and a composite outcome of death/loss to follow-up (LFU).
Principal findings
At ART-initiation 19.1% (88/461) of patients were CAA-positive. A tendency of higher CD4 increases was seen in CAA-positive patients (+182 cells/μl, interquartile range (IQR), 87–285 cells/μl) compared to CAA-negative patients (+147 cells/μl, IQR, 55–234 cells/μl, p = 0.09) after 10 months of follow-up. After adjustment for baseline risk factors, CAA-positivity showed no association with virological or immunological failure. In CAA-positive patients, 22.7% (20/88) died or were LFU, compared to 29.5% (110/373) of CAA-negative patients (hazard ratio (HR): 0.76, 95% confidence interval (CI), 0.47–1.22, p = 0.25). After adjustment for age, sex, body mass index, educational attainment, WHO-stage, tuberculosis status, and year of ART initiation, CAA-positivity showed a trend of a decreased hazard of death/LFU (HR: 0.58, 95% CI: 0.32–1.05, p = 0.07), while CD4 count at baseline (HR: 0.86, 95% CI: 0.76–1.00, p = 0.02) and MXD (sum of eosinophils, basophils, and monocytes counts) >1,100 cells/μl (HR: 0.56, 95% CI: 0.34–0.93, p = 0.03) were identified as independently protective factors.
Conclusions/Significance
Schistosomiasis is prevalent in this HIV cohort and may be beneficial for immunological reconstitution, while no effect on virological failure was apparent. A positive effect of schistosomiasis-induced immunomodulation on survival and retention in care needs confirmation in future studies.
Author summary
Infections with HIV and blood flukes (Schistosoma) both exert chronic modulatory effects on the host’s immune system. Coinfections, meaning the host is simultaneously infected with both pathogens, are common in sub-Saharan Africa. In this situation the induced immune modulation of one pathogen may affect the course of the disease induced by the other pathogen. One study showed that coinfection with Schistosoma in people living with HIV who begin antiretroviral therapy (ART) may have deleterious effects on the reconstitution of the HIV-induced immunosuppression. In the current study, we investigated the effect of Schistosoma coinfection on the recovery of the patient’s immune system, on the efficacy of ART to suppress HIV replication, and on a combined endpoint of lost to follow-up or death. We found that schistosomiasis may have beneficial effects on immune reconstitution, while no deleterious effect was detected on HIV-suppressive efficacy of ART. Surprisingly, our data suggest that schistosomiasis-induced immunomodulatory effects might be beneficial for survival and retention in care. Future studies are warranted to confirm these findings. In the era of increasing access to ART in sub-Saharan Africa, the issue of schistosomiasis-HIV coinfection may have major consequences on the outcome of HIV treatment programs.
Introduction
The geographic distributions of HIV/AIDS and schistosomiasis largely overlap in sub-Saharan Africa, where Schistosoma prevalence reaches up to 30% in HIV cohorts [1–4]. In settings of coinfection, Schistosoma and HIV mutually interfere on several levels, which may impact the course of the associated diseases [5,6]. As both infections induce chronic modulations of the host’s immune system, interactions on this level are of particular interest [7]. Schistosomiasis and other helminth infections lead to an upregulation of T-helper cell type 2 (Th-2) immune response and a downregulation of T-helper cell type 1 (Th-1) immune response and of cytolytic activity of CD8 T-cells [8,9]. Properties of Th-1 immune response, which includes the secretion of interferon-γ and interleukin (IL)-2 by Th-1 lymphocytes promoting the activation of macrophages and dendritic cells and thereby enhances the ability to kill intracellular pathogens, are essential for the control of viral infections [10]. In line with these immunological findings, a study in an Ascaris lumbricoides-HIV coinfected population found higher levels of immune activation, HIV-RNA concentrations, and lower CD4 T-cell counts in individuals with Th-2 bias, as indicated by high A. lumbricoides fecal egg counts, eosinophilia, and IgE response, compared to patients with high A. lumbricoides fecal egg counts, low eosinophil count, and low IgE responses [11]. In a study carried out in Zimbabwe, a reduction in viral load with increase of CD4 T-cell count was seen in Schistosoma-HIV coinfected patients after antischistosomal treatment [12], and similar effects have been shown after treatment of other helminth infections [13,14].
To our knowledge, only two studies have investigated the effect of Schistosoma coinfection on the course of HIV infection in people living with HIV (PLWH) under antiretroviral treatment (ART) [15,16]. Efraim et al. showed that in Schistosoma-HIV coinfected patients starting ART, the odds for immunologic treatment failure were four times higher and CD4 cell count increases were significantly lower compared to PLWH without concurrent schistosomiasis [16]. The effect of schistosomiasis on the virological response or on clinical outcomes was not assessed in that study. In settings of high Schistosoma prevalence and massive roll out of ART in HIV cohorts, Schistosoma-induced treatment failure would have major implications for ART programs.
The working hypothesis of the current study was that Schistosoma coinfection has a negative impact on the course of HIV infection in patients starting ART. To test the hypothesis, we aimed to assess the effect of Schistosoma coinfection on patients’ response to ART in terms of (i) loss to follow-up (LFU) or mortality; (ii) immunological failure; and (iii) virological failure. The study was conducted in a well characterized HIV cohort in a rural part of south-central Tanzania and employed a highly sensitive diagnostic approach for schistosomiasis.
Methods
Ethics statement
The Kilombero and Ulanga Antiretroviral Cohort (KIULARCO) received ethics approval from the institutional review board of the Ifakara Health Institute (IHI) and from the National Health Research Ethics Review Committee of the National Institute for Medical Research of Tanzania. All patients provided written informed consent at inclusion in the KIULARCO cohort and parents or guardians provided informed consent on behalf of all participants under the age of 18 years. We re-tested all circulating anodic antigen (CAA)-positive patients for Schistosoma infection. Patients with positive test results were treated with praziquantel (40 mg/kg twice at 4-week interval).
Study site
This study was carried out in Ifakara, a primarily rural region of south-central Tanzania. Patients were recruited from KIULARCO, an ongoing, open, prospective observational HIV cohort of PLWH followed at the Chronic Disease Clinic in Ifakara (CDCI). Further details of the KIULARCO cohort are given elsewhere [17,18]. Within the cohort, venous blood samples are drawn at routine clinic visits before, 3 months after ART initiation, and every 6 months thereafter. Plasma and cell pellets are cryopreserved at ˗80ºC and ˗20°C on site.
Study design and participants
The study was a retrospective analysis of cryopreserved plasma samples and of data collected within the existing HIV cohort. The study included all patients who were enrolled in KIULARCO, started ART between January 1, 2013 and April 1, 2015, were older than 15 years, not pregnant, had a CD4 count performed at a maximum of 90 days before to 1 week after ART-start, and had at least one pre-ART plasma sample stored (at a maximum of 30 days before ART-start).
Diagnostic procedures
The Schistosoma infection status of each study participant at the moment of ART initiation was retrospectively assessed by testing 50 μl of pre-ART cryopreserved plasma for the presence of CAA with a lateral flow (LF) test with SCAA20 dry format [19,20]. Negative controls and CAA standard series indicated an assay threshold of 10 pg CAA per ml below which samples were designated as negative. If available, a second sample from 6–13 months after ART-initiation was tested for the presence of CAA. Of note, detection of active schistosomiasis has been simplified by recently developed assays for the detection of schistosomal CAA [19,20]. CAA originates from the gut of adult worms of Schistosoma mansoni and S. haematobium (the two schistosome species endemic in Tanzania) and is shed into the host circulation during active infection. After successful treatment with praziquantel, antigen levels decrease within days [21]. In addition, antigen levels are good indicators for worm burden. CAA is highly stable, and hence, can be detected in cryopreserved plasma samples for years after sample preparation [21–23]. Previously used enzyme-linked immunosorbent assays (ELISAs) as well as the currently used LF tests for detection of CAA in plasma are sensitive (80–95%) and highly specific (98–100%) for the diagnosis of active schistosomiasis, and the recently developed dry format of the latter facilitates usage in laboratories in endemic countries [20,24].
Because isolated eosinophil count in peripheral blood is not part of routine diagnostic procedures in the KIULARCO, we used MXD (mixed cell count: sum of the absolute number of eosinophils, basophils, and monocytes counts) as approximate value. Cut-off for elevated MXD value was set at 1,100 cells/μl [25].
The association between CAA-positivity and virological failure was assessed in a subgroup of study participants. Patients who were continuously CAA-positive (CAA positive before ART-initiation, and CAA positive 6–13 months after ART-initiation) were frequency-matched with two controls (CAA-negative pre-ART and 6–13 months after ART-initiation) for age, CD4 cell count, and tuberculosis status at baseline. In these patients, plasma HIV RNA levels were tested in a cryopreserved plasma sample drawn 6–13 months after ART-initiation.
Plasma HIV RNA from 400 μl plasma was extracted using the NucleoSpin Virus kit (Macherey-Nagel; Oensingen, Switzerland) according to the manufacturer’s protocol. Viral RNA quantification was performed with the Brilliant III Ultra-Fast QRT-PCR Master Mix (Agilent Technologies; La Jolla, CA, United States of America) using the StepOneTM Real-Time PCR System (Applied Biosystems; Foster City, CA, United States of America), with a detection limit of 60 viral RNA copies/ml of plasma. CD4 counts are routinely obtained after staining fresh whole blood samples with labeled antibodies: CD4, CD3, CD8, and CD45 in TruCount tubes (BD FACSCalibur; Franklin Lakes, NJ, United States of America).
Statistical analysis
Data were extracted from a readily available electronic database of KIULARCO. The baseline was defined as the date of ART initiation. Continuous variables were summarized with medians and interquartile ranges (IQR) and categorical variables with frequencies and percentages.
Association of CAA-positivity at ART-initiation with death or LFU was assessed with a multivariate Cox regression model. LFU was defined as no visit to the outpatient clinic for more than 6 months. The time of the event was defined as the day of the last follow-up visit documented in the database. Results were presented with hazard ratios (HR) and 95% confidence intervals (CIs). The assumption of proportional hazards was confirmed by Schoenfeld’s global test (p = 0.08).
The association of CAA-positivity at ART initiation with immunological failure was assessed with a multivariate logistic regression model. Immunological failure was defined as CD4 count falling below baseline or persistently <100 cells/μl at the time of the first measurement of CD4 cell count ≥6 months after ART-start. Results were presented with odds ratios (ORs) and 95% CIs.
For both models the following variables were considered a priori as potential co-factors/confounders and were included in the multivariable model (no variable selection was done): age (divided by 10), body mass index (BMI), and CD4 count at baseline (divided by 25) were used as continuous variables. Categorical variable included sex, educational attainment (none and primary school vs. secondary school and college/university), and WHO clinical stages of HIV disease (stage 1 and 2 vs. stage 3 and 4). The variable “active tuberculosis” was defined as diagnosis of tuberculosis at baseline or during follow-up. The variable “MXD value” was dichotomized in normal vs. elevated (>1,100 cells/μl, see above). The variable “year of starting ART” was added to the model used to assess the association of CAA-positivity with death/LFU. The variable “delay to CD4 testing” (i.e., time from ART initiation to measurement of CD4 cell count) was added to the model used to assess the association of CAA-positivity with immunological failure. Linearity of the relationship between continuous explanatory variables and the log odds of the dependent variable was tested by adding polynomial terms of each continuous explanatory variable. Polynomial terms showing a significant association with the log odds of the dependent variable were included in the model.
For the analysis of CAA-positivity as a risk factor for virological failure (defined as HIV RNA concentrations above 1,000 copies/ml after 6–12 months of ART initiation), a logistic regression model was employed, controlling for the following potential co-factors/confounders (defined as above): sex, BMI, educational attainment, WHO clinical stages of HIV disease, MXD value, and delay to HIV RNA testing. Results were presented with ORs and 95% CIs.
Data were anonymized and analyzed using STATA version 12.1 (StataCorp; College Station, TX, United States of America).
Results
A total of 461 eligible ART-naive PLWH were included in the study. Table 1 summarizes the pre-ART baseline characteristics, stratified by CAA status. Overall, 66.6% of participants were females with a median age at enrolment of 38.2 years (IQR, 32.5–45.8 years). Median BMI was 20.2 kg/m2 (IQR, 18.4–22.7 kg/m2), and median CD4 count was 173 cells/μl (IQR, 61–299 cells/μl). CAA-positive patients had higher CD4 counts, higher MXD values, and there was a lower proportion of females (Table 1).
Table 1. Baseline characteristics of the study population in KIULARCO, Tanzania between January 2013 and March 2015.
| Variable | Total | CAA- | CAA+ |
|---|---|---|---|
| N | 461 | 373 | 88 |
| Age, years—median (IQR) | 38.2 (32.5–45.8) | 38.7 (32.8–46.4) | 37.3 (32.1–44.4) |
| Female | 307 (66.6%) | 262 (70.2%) | 45 (51.1%) |
| BMI, kg/m2—median (IQR) | 20.2 (18.4–22.7) | 20.3 (18.5–22.9) | 20.0 (18.1–21.5) |
| Educational attainment | |||
| None | 39 (8.5%) | 31 (8.3) | 8 (9.1%) |
| Primary school | 399 (86.6%) | 322 (86.3%) | 77 (87.5%) |
| Secondary school | 21 (4.6%) | 18 (4.8%) | 3 (3.4%) |
| College/university | 2 (0.4%) | 2 (0.5%) | 0 |
| CD4 cells/μl–median (IQR) | 173 (61–299) | 162 (60–294) | 195 (63–304) |
| WHO-stage 3 or 4 | 235 (51.7%) | 192 (51.9%) | 43 (50.6%) |
| Active tuberculosis | 89 (19.4%) | 73 (19.6%) | 16 (18.2%) |
| MXD cells* | |||
| Cells/μl—median (IQR) | 759.5 (527.2–1,133.3) | 728.0 (511.0–1,086.4) | 973.4 (692.9–1,184.4) |
| >1,100 cells/μl | 112 (26.2%) | 83 (24.1%) | 29 (34.9%) |
*MXD: sum of the absolute number of eosinophils, basophils, and monocytes counts
Data are presented as n (%) or median (IQR)
Prevalence of schistosomal antigenemia
Among the 461 patients recruited, 88 (19.1%, 95% CI, 15.6–23.0%) were CAA-positive. The median CAA titre at ART initiation was 163 pg/ml (IQR, 27–760 pg/ml). After a median time of 36 weeks on ART (IQR, 29–42 weeks), 36 remained CAA-positive, 17 became CAA-negative, and 35 had no follow-up plasma sample. In the group of 373 initially CAA-negative patients, 214 patients were continuously CAA-negative at ART-initiation and in the follow-up testing after a median time of 35 weeks (IQR, 29–43 weeks), four became CAA-positive, and 155 had no follow-up plasma sample.
Outcome and predictors of death and LFU
Of 88 CAA-positive patients at ART initiation, 20 (22.7%) died or were LFU, compared to 110 out of 373 (29.5%) of CAA-negative patients (HR: 0.76, 95% CI, 0.47–1.22, p = 0.25). The median survival time in CAA-positive patients was 20.1 months (IQR, 11.9–29.1 months), compared with 19.8 months (IQR, 12.3–26.8 months; p = 0.57) for CAA-negative patients.
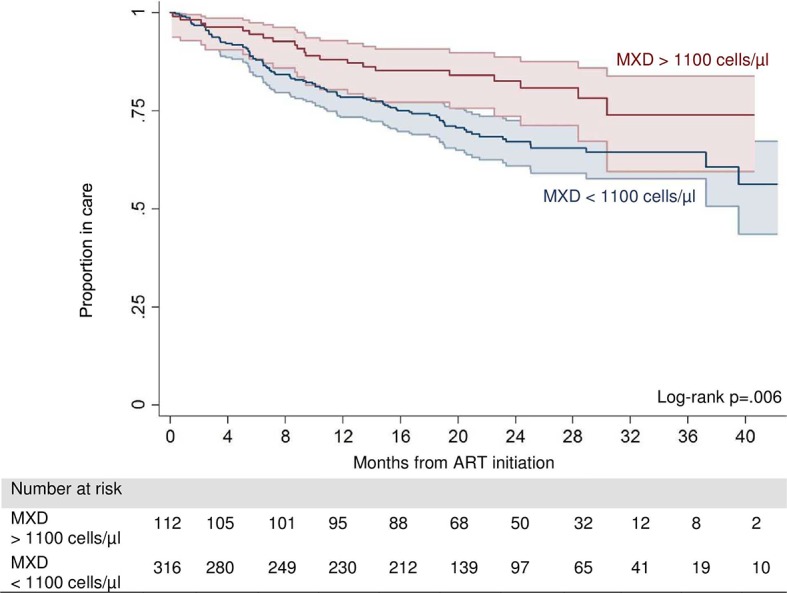
Baseline risk factors for death or LFU are presented in Table 2. After adjusting for age, sex, BMI, educational attainment, baseline CD4 count, WHO-stage, active tuberculosis, and year of ART initiation, CAA positivity showed a trend of a decreased hazard of death or LFU (HR: 0.58, 95% CI: 0.32–1.05, p = 0.07), while CD4 count and MXD >1,100 cells/μl (HR: 0.56, 95% CI: 0.34–0.93–0, p = 0.03) were identified as independent protective factors (Fig 1).
Table 2. Association with death or loss to follow-up, using a Cox regression model.
| Variable | Unadjusted/ univariate model |
Adjusted/ multivariate model (n = 108 events*) |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| CAA-positive | 0.76 (0.47–1.22) | 0.25 | 0.58 (0.32–1.05) | 0.07 |
| Age, per 10 years | 0.96 (0.81–1.12) | 0.60 | 0.98 (0.80–1.19) | 0.83 |
| Female | 0.75 (0.53–1.07) | 0.11 | 0.76 (0.50–1.15) | 0.19 |
| Body mass index (BMI) per kg/m2 |
0.90 (0.85–0.95) | <0.01 | 0.94 (0.88–1.00) | 0.06 |
| Education lower vs. higher** | 0.64 (0.26–1.57) | 0.33 | 0.70 (0.28–1.75) | 0.45 |
| CD4 cell count, per 25/μl | 0.84 (0.75–0.94) | <0.01 | 0.86 (0.76–1.00) | 0.02 |
| CD4 cell count2, per 25/μl | 1.01 (1.00–1.02) | 0.01 | 1.00 (1.00–1.02) | 0.03 |
| CD4 cell count4, per 25/μl | 1.00 (1.00–1.00) | 0.06 | 1.00 (1.00–1.00) | 0.07 |
| WHO-stage 3/4 vs. 1/2 | 2.37 (1.62–3.45) | <0.01 | 1.44 (0.90–2.31) | 0.13 |
| Active tuberculosis | 1.62 (1.08–2.43) | 0.02 | 1.20 (0.74–1.93) | 0.46 |
| MXD >1,100 cells/μl*** | 0.54 (0.34–0.86) | 0.01 | 0.56 (0.34–0.93) | 0.03 |
| ART started 2014 | 1.34 (0.89–2.02) | 0.16 | 1.24 (0.74–2.06) | 0.40 |
| ART started 2015 | 1.91 (1.13–3.22) | 0.02 | 1.75 (0.95–3.22) | 0.07 |
*Event: composite outcome of death/loss to follow-up
**Educational attainment: lower (none/primary) vs. higher than primary
***MXD: sum of the absolute number of eosinophils, basophils, and monocytes counts
Fig 1. Kaplan-Meier survival estimates of death or loss to follow-up by MXD value (MXD value: Sum of the absolute number of eosinophils, basophils, and monocytes counts).
CD4 count reconstitution and immunological failure
A follow-up CD4 count was available for 69 out of 88 (78.4%) of CAA-positive and for 287 out of 373 (76.9%) of CAA-negative patients. CAA-positive patients showed a median difference from baseline in CD4 of +182 cells/μl (IQR, 87–285 cells/μl), compared to +147 cells/μl (IQR, 55–234 cells/μl, p = 0.09) in CAA-negative patients. Median time from ART initiation to measurement of CD4 count was 37.9 weeks (IQR, 32.1–43.7 weeks) for CAA-positive patients, compared to 37.9 weeks (IQR, 28.0–45.1 weeks) in CAA-negative patients. MXD values had no effect on CD4 count changes (MXD <1,100/μl: +151 cells/μl, IQR, 67–236 cells/μl; MXD >1,100/μl: +138 cells/μl, IQR, 51–221 cells/μl, p = 0.60).
Sixty-seven patients (18.8%) met at least one WHO criterion for immunological failure. CAA positivity showed no effect on the risk of immunological failure (OR: 0.78, 95% CI: 0.39–1.59, p = 0.50). Baseline risk factors for immunological failure are presented in Table 3. After adjusting for age, sex, BMI, educational attainment, WHO-stage, active tuberculosis, MXD, and median time from ART initiation to measurement of CD4 count, CAA positivity showed no association with immunological failure (OR: 0.71, 95% CI: 0.32–1.59, p = 0.41), whereas increasing CD4 count at ART initiation was an independent risk factor for immunological failure (OR: 1.08, 95% CI: 1.03–1.13, p = 0.02).
Table 3. Association with immunological failure, using a logistic regression model.
| Variable | Unadjusted/ univariate model |
Adjusted/ multivariate model |
|||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| CAA+ | 0.78 (0.39–1.59) | 0.50 | 0.71 (0.32–1.59) | 0.41 | |
| Age, per 10 years | 1.09 (0.85–1.39) | 0.51 | 1.04 (0.79–1.37) | 0.79 | |
| Female | 1.18 (0.66–2.12) | 0.58 | 0.75 (0.38–1.50) | 0.42 | |
| Body mass index (BMI), per kg/m2 |
1.00 (0.93–1.08) | 0.97 | 0.95 (0.86–1.04) | 0.28 | |
| Education lower vs. higher* | 0.22 (0.28–1.64) | 0.14 | 0.28 (0.04–2.21) | 0.23 | |
| CD4 cell count, per 25/μl | 1.08 (1.04–1.13) | <0.01 | 1.08 (1.03–1.13) | 0.02 | |
| Delay to CD4 testing** | 0.97 (0.95–1.00) | 0.07 | 0.98 (0.95–1.01) | 0.26 | |
| WHO-stage 3/4 vs. 1/2 | 0.87 (0.50–1.48) | 0.60 | 0.88 (0.45–1.71) | 0.71 | |
| Active tuberculosis | 0.63 (0.28–1.40) | 0.25 | 0.38 (0.14–1.05) | 0.06 | |
| MXD >1,100 cells/μl*** | 1.13 (0.62–2.07) | 0.68 | 1.10 (0.58–2.12) | 0.76 | |
| Constant | 3.35 (0.11–100.72) | 0.49 | |||
*Educational attainment: lower (none/primary) vs. higher than primary
**Delay to CD4 testing: median time from ART initiation to measurement of CD4 cell count in weeks
***MXD: cumulative value of eosinophils, basophils, and monocytes
Virological failure
One continuously CAA-positive patient and three continuously CAA-negative patients were excluded from the analysis due to non-availability of plasma samples for HIV PCR. After a median time of 43.4 weeks (IQR, 38.0–49.9 weeks) of ART, eight out of 35 (22.9%) persistent CAA-positive patients had HIV RNA concentrations above 1,000 cp/ml, compared to 12 out of 67 (17.9%) of continuously CAA-negative patients after frequency matching for tuberculosis-status, CD4 count, and age. After adjustment for baseline risk factors, positive CAA status showed no association with virological failure (OR: 1.63, 95% CI: 0.53–4.95, p = 0.39, Table 4).
Table 4. Association with virological failure, using a logistic regression model.
| Variable | Unadjusted/ univariate model |
Adjusted/ multivariate model |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| CAA+ | 0.78 (0.39–1.59) | 0.50 | 1.63 (0.53–4.95) | 0.39 |
| Female | 0.96 (0.35–2.69) | 0.94 | 0.94 (0.30–2.94) | 0.91 |
| Body mass index, per kg/m2 |
1.00 (0.85–1.18) | 1.00 | 1.05 (0.88–1.26) | 0.59 |
| Education higher vs. other* | 0.67 (0.08–5.87) | 0.71 | 0.78 (0.08–7.57) | 0.83 |
| Delay to HIV RNA testing** | 1.23 (0.42–3.66) | 0.69 | 0.98 (0.92–1.03) | 0.44 |
| WHO-stage 3/4 vs. 1/2 | 1.45 (0.54–3.89) | 0.46 | 2.34 (0.71–7.77) | 0.16 |
| MXD >1,100 cells/μl*** | 0.47 (0.14–1.54) | 0.21 | 0.47 (0.13–1.63) | 0.23 |
| Constant | 0.24 (0.00–41.88) | 0.58 | ||
*Educational attainment: lower (none/primary) vs. higher than primary
**Delay to HIV-RNA testing: median time from ART initiation to measurement of HIV RNA
***MXD: cum sum of the absolute number of eosinophils, basophils, and monocytes counts
Discussion
To our knowledge this is the first study analyzing the effect of Schistosoma coinfection on the immunovirological response and the long-term clinical outcome of PLWH starting ART. Highly prevalent active Schistosoma coinfection showed no association with virological and immunological treatment failure but a trend of decreased hazard of death or LFU in patients initiating ART in this mainly rural part of south-central Tanzania. Interestingly, an increase of the MXD value was identified as an independent protective factor against death and LFU in this cohort.
The high prevalence of concomitant active schistosomiasis in this study, as determined by a highly sensitive assay (i.e., CAA) and of schistosomal and helminth infection in general in other studies in PLWH corroborate the potential importance of helminth-HIV coinfection [11,16]. Our findings also highlight that schistosomiasis in adult patients is of special concern as large-scale treatment programs are often school-based and may exclude adults from access to appropriate therapy. An earlier study in KIULARCO yielded a prevalence of 43% for helminth and 11% of Schistosoma coinfection determined by stool and urine microscopy (Cornelia Staehelin, unpublished data)[26]. We do not believe that the higher prevalence of schistosomal infection in our study reflects a true increase of prevalence, but a considerably higher sensitivity of the technique of CAA detection in plasma compared to traditional stool microscopy-based diagnostics [24,26,27]. The low sensitivity of widely used microscopic diagnostics in schistosomal infection may be of special concern in settings were HIV co-exists. Schistosomal maturation and egg excretion are thought to depend of the host’s immune response and some studies suggest that HIV-induced CD4 cell depletion may be linked to a decreased luminal migration of schistosome eggs and an arrest of worm development [28–30]. From a clinical point of view, the implication of a possibly reduced sensitivity of microscopy-based diagnostic in HIV-infected individuals would be preclusion from anthelmintic treatment; in clinical trials on Schistosoma-HIV coinfection falsely negative tested Schistosoma coinfected patients would lead to misclassification and consequently to an underestimation of the effect under investigation. In such circumstances, the use of highly sensitive tests should be considered.
In contrast to the findings of Efraim and colleagues, our study did not show an association of Schistosoma coinfection with an elevated risk for immunological failure or decelerated CD4 count gain in PLWH starting ART [16]. Considering evidence from previous research and pathophysiological mechanisms, a detrimental effect of helminth coinfection on CD4 counts recovery may be expected. Our findings, however, are in line with the findings of Muok and colleagues, who also showed a higher increase of CD4 count one month after initiating ART in patients who were infected with S. mansoni, compared to controls without Schistosoma infection [15]. Our results also suggest that elevated pre-ART CD4 cell counts are a risk factor for immunological failure. This counterintuitive association is a well-known phenomenon in European and sub-Saharan cohort studies and is generally explained by a closer clinical monitoring and better adherence of patients with lower CD4 cell count who are at an increased risk for opportunistic infections [31–33].
The rate of virological failure was substantial, which is a frequent problem, especially in rural settings of sub-Saharan Africa [34–36]. Essential factors for a virological successful ART are an adequate regimen and good adherence to therapy. High HIV RNA concentrations prior to initiation of ART are an additional risk factor for virological treatment failure [37]. Because helminth-induced immunomodulatory effects are associated with elevated HIV RNA levels in ART-naïve individuals, helminth infection could theoretically be associated with an elevated risk of virological failure [11]. Our study did not show an impact of schistosomal infection on virological treatment failure, probably because potential differences in HIV RNA concentrations prior to ART initiation are not important enough to affect efficacy of ART.
The protective effect of schistosomiasis in PLWH starting ART in our study is counterintuitive. Possible explanations of the results include immunomodulatory properties of Schistosoma spp., which may exert beneficial effects in the setting of ART-induced immunological reconstitution. Immune reconstitution inflammatory syndrome (IRIS) is a condition seen in PLWH with profound CD4 T cell depletion and is characterized by an overwhelming inflammatory response to pathogens due to a recovering T cell count after initiation of ART. IRIS is a common cause of early mortality of patients starting ART in sub-Saharan Africa [38]. Schistosoma-induced immunomodulation may have an attenuating effect on IRIS-related morbidity and mortality and by this means, improve the clinical outcome of PLWH starting ART.
The statistically significant association of elevated MXD values with a reduced risk of death and LFU may support this assumption. MXD value is composed of absolute cell counts of monocytes, eosinophilic, and basophilic granulocytes, which are independent from HIV infection and disease status [39–41]. In the current setting in a primarily rural area of south-central Tanzania, elevated MXD values are most likely driven by helminth-induced eosinophilia and may be construed as a surrogate marker of helminth-induced immunomodulation in the HIV-infected host.
Preceding studies in KIULARCO identified an important burden and variety of helminthic infections. Because immunomodulatory effects are a consistent feature of helminth infection, we believe that the weaker effect of schistosomal infection on survival/retention in care in our study may possibly be explained by competing immunomodulatory effects of other undetected helminths as our diagnostics in this study were focused on schistosomiasis. Indeed, about a quarter of PLWH without Schistosoma infection had elevated MXD values.
Our study has several shortcomings that are offered for discussion. First, clinical data of KIULARCO are collected prospectively, but CAA was identified retrospectively in cryopreserved plasma samples with the immanent weaknesses of a retrospective study design. Second, due to a suboptimal ascertainment of mortality in our cohort, we employed a composite outcome of death/LFU, limiting our conclusions regarding the impact of schistosomiasis on mortality. However, LFU is a common issue in ART programs in resource-limited settings and mortality is inversely associated with the rate of LFU [42]. On the basis of an overall LFU of approximately 20% in KIULARCO, mortality of patients and LFU is expected to be about 50% [42]. Third, confounding factors cannot be excluded. In particular, schistosomiasis and elevated MXD values could be associated with other characteristics of the study population, which reduce mortality or LFU, remained unrecognized for which we could not control for (e.g., distance to the clinic or migratory mobility). Fourth, because pre-ART HIV-RNA concentrations were not assessed, it cannot be excluded that uneven distributions of HIV-RNA concentrations between both groups may have introduced bias in the presented results for the effect of CAA-positivity on the virological outcome.
In conclusion, testing for CAA in plasma revealed a 19.1% prevalence of active schistosomiasis in PLWH starting ART in the KIULARCO in rural south-central Tanzania. This observation corroborates concerns of limited sensitivity of microscopy-based diagnostics, which might be additionally compromised in HIV-infected populations and may impede treatment for patients with a potentially life-threatening disease [43]. Our study could not detect detrimental properties of Schistosoma coinfection on immunological and virological response to ART but suggests that helminth-induced immunomodulatory mechanisms might enhance survival of PLWH starting ART.
Our results need confirmation in future studies, which should consider (i) the dynamic aspect of HIV infection and therefore preferentially chose a longitudinal study design, including clinical outcomes; and (ii) that individuals are often coinfected by multiple parasitic worms that may affect the course of the HIV infection by comparable but interindividually variable immunomodulatory effects. To avoid misclassification, comprehensive, highly sensitive helminth diagnostics and markers of the host’s immunological response should be employed. Among these highly sensitive tests, the used CAA-test is a promising candidate and further use and developments may improve the acceptance as a future standard test.
In the era of massive rollout of ART in sub-Saharan Africa and other tropical and sub-tropical countries, the issue of helminth-HIV coinfection may have major ramifications on the outcome of HIV treatment programs. Further research, especially in PLWH initiating ART, is urgently needed.
Supporting information
(DOC)
(DTA)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study received funding from the GILEAD Förderprogramm Infektiologie, GILEAD Science GmbH, Germany (http://gilead-foerderprogramm-infektiologie.de/home.html). The Chronic Diseases Clinic of Ifakara receives financial support from the Government of the Canton of Basel, Switzerland, the Swiss Tropical and Public Health Institute, the Ifakara Health Institute, the Government of Tanzania, and USAID through TUNAJALI-Deloitte. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hotez PJ, Kamath A (2009) Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis 3: e412 10.1371/journal.pntd.0000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bustinduy A, King C, Scott J, Appleton S, Sousa-Figueiredo JC, Betson M, et al. (2014) HIV and schistosomiasis co-infection in African children. Lancet Infect Dis 14: 640–649. 10.1016/S1473-3099(14)70001-5 [DOI] [PubMed] [Google Scholar]
- 3.Mbabazi PS, Andan O, Fitzgerald DW, Chitsulo L, Engels D, Downs JA (2011) Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl Trop Dis 5: e1396 10.1371/journal.pntd.0001396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai Y-S, Biedermann P, Ekpo UF, Garba A, Mathieu E, Midzi N, et al. (2015) Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect Dis 15: 927–940. 10.1016/S1473-3099(15)00066-3 [DOI] [PubMed] [Google Scholar]
- 5.Downs JA, Dupnik KM, van Dam GJ, Urassa M, Lutonja P, Kornelis D, et al. (2017) Effects of schistosomiasis on susceptibility to HIV-1 infection and HIV-1 viral load at HIV-1 seroconversion: a nested case-control study. PLoS Negl Trop Dis 11: e0005968 10.1371/journal.pntd.0005968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secor WE (2012) The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr Opin HIV AIDS 7: 254–259. 10.1097/COH.0b013e328351b9e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obuku AE, Asiki G, Abaasa A, Ssonko I, Harari A, van Dam GJ, et al. (2016) Effect of Schistosoma mansoni infection on innate and HIV-1-specific T-cell immune responses in HIV-1-infected Ugandan fisher folk. AIDS Res Hum Retroviruses 32: 668–675. 10.1089/AID.2015.0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bretscher PA (2014) On the mechanism determining the TH1/TH2 phenotype of an immune response, and its pertinence to strategies for the prevention, and treatment, of certain infectious diseases. Scand J Immunol 79: 361–376. 10.1111/sji.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kullberg MC, Pearce EJ, Hieny SE, Sher A, Berzofsky JA (1992) Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol 148: 3264–3270. [PubMed] [Google Scholar]
- 10.Zanussi S, Simonelli C, D'Andrea M, Caffau C, Clerici M, Tirelli U, et al. (1996) CD8+ lymphocyte phenotype and cytokine production in long-term non-progressor and in progressor patients with HIV-1 infection. Clin Exp Immunol 105: 220–224. 10.1046/j.1365-2249.1996.d01-746.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mkhize-Kwitshana ZL, Taylor M, Jooste P, Mabaso ML, Walzl G (2011) The influence of different helminth infection phenotypes on immune responses against HIV in co-infected adults in South Africa. BMC Infect Dis 11: 273 10.1186/1471-2334-11-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, van Dam GJ, et al. (2005) Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J Infect Dis 192: 1956–1961. 10.1086/497696 [DOI] [PubMed] [Google Scholar]
- 13.Walson JL, Otieno PA, Mbuchi M, Richardson BA, Lohman-Payne B, Macharia SW, et al. (2008) Albendazole treatment of HIV-1 and helminth co-infection: a randomized, double-blind, placebo-controlled trial: AIDS 22: 1601–1609. 10.1097/QAD.0b013e32830a502e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Means AR, Burns P, Sinclair D, Walson JL (2016) Antihelminthics in helminth-endemic areas: effects on HIV disease progression. Cochrane Database Syst Rev 4: CD006419 10.1002/14651858.CD006419.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muok EMO, Simiyu EW, Ochola EA, Ng’ang’a ZW, Secor WE, Karanja DMS, et al. (2013) Association between CD4+ T-lymphocyte counts and fecal excretion of Schistosoma mansoni eggs in patients coinfected with S. mansoni and human immunodeficiency virus before and after initiation of antiretroviral therapy. Am J Trop Med Hyg 89: 42–45. 10.4269/ajtmh.13-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Efraim L, Peck RN, Kalluvya SE, Kabangila R, Mazigo HD, Mpondo B, et al. (2013) Schistosomiasis and impaired response to antiretroviral therapy among HIV-infected patients in Tanzania. J Acquir Immune Defic Syndr 62: e153–156. 10.1097/QAI.0b013e318282a1a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letang E, Kalinjuma AV, Glass TR, Gamell A, Mapesi H, Sikalengo GR, et al. (2017) Cohort profile: The Kilombero and Ulanga Antiretroviral Cohort (KIULARCO)—a prospective HIV cohort in rural Tanzania. Swiss Med Wkly 147: 2728. [DOI] [PubMed] [Google Scholar]
- 18.Vanobberghen F, Letang E, Gamell A, Mnzava DK, Faini D, Luwanda LB, et al. (2017) A decade of HIV care in rural Tanzania: trends in clinical outcomes and impact of clinic optimisation in an open, prospective cohort. PLoS One 12: e0180983 10.1371/journal.pone.0180983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corstjens PLAM De Dood CJ, Kornelis D Tjon Kon Fat EM, Wilson RA Kariuki TM, et al. (2014) Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology 141: 1841–1855. 10.1017/S0031182014000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dam GJ, de Dood CJ, Lewis M, Deelder AM, van Lieshout L, Tanke HJ, et al. (2013) A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp Parasitol 135: 274–282. 10.1016/j.exppara.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jonge N, De Caluwé P, Hilberath GW, Krijger FW, Polderman AM, Deelder AM (1989) Circulating anodic antigen levels in serum before and after chemotherapy with praziquantel in schistosomiasis mansoni. Trans R Soc Trop Med Hyg 83: 368–372. [DOI] [PubMed] [Google Scholar]
- 22.Deelder AM, Qian ZL, Kremsner PG, Acosta L, Rabello AL, Enyong P, et al. (1994) Quantitative diagnosis of Schistosoma infections by measurement of circulating antigens in serum and urine. Trop Geogr Med 46 (4 Spec No): 233–238. [PubMed] [Google Scholar]
- 23.van Lieshout L, Polderman AM, Deelder AM (2000) Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop 77: 69–80. [DOI] [PubMed] [Google Scholar]
- 24.Corstjens PLAM, van Lieshout L, Zuiderwijk M, Kornelis D, Tanke HJ, Deelder AM, et al. (2008) Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J Clin Microbiol 46: 171–176. 10.1128/JCM.00877-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saathoff E, Schneider P, Kleinfeldt V, Geis S, Haule D, Maboko L, et al. (2008) Laboratory reference values for healthy adults from southern Tanzania: laboratory reference values for adults from Tanzania. Trop Med Int Health 13: 612–625. 10.1111/j.1365-3156.2008.02047.x [DOI] [PubMed] [Google Scholar]
- 26.Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S (2015) New diagnostic tools in schistosomiasis. Clin Microbiol Infect 21: 529–542. 10.1016/j.cmi.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 27.Chernet A, Kling K, Sydow V, Kuenzli E, Hatz C, Utzinger J, et al. (2017) Accuracy of diagnostic tests for Schistosoma mansoni infection in asymptomatic Eritrean refugees: serology and point-of-care circulating cathodic antigen against stool microscopy. Clin Infect Dis 65: 568–574. 10.1093/cid/cix366 [DOI] [PubMed] [Google Scholar]
- 28.Karanja DM, Boyer AE, Strand M, Colley DG, Nahlen BL, Ouma JH, et al. (1998) Studies on schistosomiasis in western Kenya: II. Efficacy of praziquantel for treatment of schistosomiasis in persons coinfected with human immunodeficiency virus-1. Am J Trop Med Hyg 59: 307–311. [DOI] [PubMed] [Google Scholar]
- 29.Doenhoff MJ, Pearson S, Dunne DW, Bickle Q, Lucas S, Bain J, et al. (1981) Immunological control of hepatotoxicity and parasite egg excretion in Schistosoma mansoni infections: stage specificity of the reactivity of immune serum in T-cell deprived mice. Trans R Soc Trop Med Hyg 75: 41–53. [DOI] [PubMed] [Google Scholar]
- 30.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Dam GJ van, Erikstrup C, et al. (2005) Schistosomiasis and HIV-1 infection in rural Zimbabwe: implications of coinfection for excretion of eggs. J Infect Dis 191: 1311–1320. 10.1086/428907 [DOI] [PubMed] [Google Scholar]
- 31.Dragsted UB, Mocroft A, Vella S, Viard J-P, Hansen A-BE, Panos G, et al. (2004) Predictors of immunological failure after initial response to highly active antiretroviral therapy in HIV-1-infected adults: a EuroSIDA study. J Infect Dis 190: 148–155. 10.1086/420786 [DOI] [PubMed] [Google Scholar]
- 32.Palladino C, Briz V, Bellón JM, Bártolo I, Carvalho P, Camacho R, et al. (2013) Predictors of attrition and immunological failure in HIV-1 patients on highly active antiretroviral therapy from different healthcare settings in Mozambique. PLoS One 8: e82718 10.1371/journal.pone.0082718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yirdaw KD, Hattingh S (2015) Prevalence and predictors of immunological failure among HIV patients on HAART in southern Ethiopia. PLoS One 10: e0125826 10.1371/journal.pone.0125826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liégeois F, Vella C, Eymard-Duvernay S, Sica J, Makosso L, Mouinga-Ondémé A, et al. (2012) Virological failure rates and HIV-1 drug resistance patterns in patients on first-line antiretroviral treatment in semirural and rural Gabon. J Int AIDS Soc 15: 17985 10.7448/IAS.15.2.17985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallis CL, Papathanasopolous MA, Fox M, Conradie F, Ive P, Orrell C, et al. (2012) Low rates of nucleoside reverse transcriptase inhibitor resistance in a well-monitored cohort in South Africa on antiretroviral therapy. Antivir Ther 17: 313–320. 10.3851/IMP1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusine J, Asiimwe-Kateera B, van de Wijgert J, Boer KR, Mukantwali E, Karita E, et al. (2013) Low primary and secondary HIV drug-resistance after 12 months of antiretroviral therapy in human immune-deficiency virus type 1 (HIV-1)-infected individuals from Kigali, Rwanda. PLoS One 8: e64345 10.1371/journal.pone.0064345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mocroft A, Lundgren JD (2004) Starting highly active antiretroviral therapy: why, when and response to HAART. J Antimicrob Chemother 54: 10–13. 10.1093/jac/dkh290 [DOI] [PubMed] [Google Scholar]
- 38.Letang E, Miró JM, Nhampossa T, Ayala E, Gascon J, Menéndez C, et al. (2011) Incidence and predictors of immune reconstitution inflammatory syndrome in a rural area of Mozambique. PLoS One 6: e16946 10.1371/journal.pone.0016946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centlivre M, Legrand N, Steingrover R, Sluis R van der, Grijsen ML, Bakker M, et al. (2011) Altered dynamics and differential infection profiles of lymphoid and myeloid cell subsets during acute and chronic HIV-1 infection. J Leukoc Biol 89: 785–795. 10.1189/jlb.0410231 [DOI] [PubMed] [Google Scholar]
- 40.Cohen AJ, Steigbigel RT (1996) Eosinophilia in patients infected with human immunodeficiency virus. J Infect Dis 174: 615–618. [DOI] [PubMed] [Google Scholar]
- 41.Sivaram M, White A, Radcliffe KW (2012) Eosinophilia: clinical significance in HIV-infected individuals. Int J STD AIDS 23: 635–638. 10.1258/ijsa.2012.011409 [DOI] [PubMed] [Google Scholar]
- 42.Brinkhof MWG, Pujades-Rodriguez M, Egger M (2009) Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One 4: e5790 10.1371/journal.pone.0005790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knopp S, Corstjens PLAM, Koukounari A, Cercamondi CI, Ame SM, Ali SM, et al. (2015) Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of Schistosoma haematobium in low endemic settings. PLoS Negl Trop Dis 9: e0003752 10.1371/journal.pntd.0003752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DTA)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


