Abstract
Objectives
We aimed to investigate whether socioeconomic position (SEP) in childhood has an effect on the level of cognitive performance and the rate of cognitive decline in older adults.
Methods
We performed a prospective cohort study of individuals enrolled in a multicenter population-based study, SHARE (Survey of Health, Ageing and Retirement in Europe). Interviews were conducted in 6 waves at approximately 2-year intervals and included examinations of cognitive performance (memory, verbal fluency, delayed recall) and measurements of childhood SEP (participants' household characteristics at the age of 10 years). We estimated the associations of SEP with the level of cognitive performance using linear regression and the relation to the rate of cognitive decline with mixed-effects models.
Results
This study included 20,244 participants from 16 European countries (median age at baseline 71 years, 54% women). Adverse childhood SEP was associated with a lower level of baseline cognitive performance. This association was attenuated after adjustment for clinical and social risk factors but remained statistically significant. Childhood SEP was not related to the rate of cognitive decline.
Conclusions
Variation in childhood SEP helps to explain differences in cognitive performance between older people, but not the rate of decline from their previous level of cognition. Strategies to protect cognitive aging should be applied early in life.
The prevalence of cognitive impairment is increasing in Europe as a result of demographic aging.1 A growing body of evidence suggests that cognitive aging is a life-long process that may have its roots in a disadvantaged childhood socioeconomic position (SEP).2–8 Three conceptual frameworks may explain this relationship. First, childhood SEP may determine cognitive abilities in late life by shaping the development of the brain so it tolerates more pathology before it reaches the threshold for manifestation of cognitive decline.9 Conceptualized as passive cognitive reserve, this suggests that individuals with socioeconomic hardship in childhood will have a lower level of cognitive performance in old age.
Second, childhood SEP may form a foundation for an active cognitive reserve of the brain, which would enable the use of preexisting processes or compensatory mechanisms in coping with brain pathology.9 The second mechanism thus suggests that socioeconomic hardship in childhood would be associated with a higher rate of cognitive decline. Third, disadvantages in childhood might set an individual on a pathway toward clinical and social risk factors, such as risky behaviors, cardiovascular risks, depression, and social isolation, which are all associated with an increased risk of cognitive impairment.1 The third mechanism proposes that childhood SEP would not be independently associated with cognition, after adjustment for these clinical and social risk factors.
Our objective was to explore the relationship between childhood SEP, and both the level of cognitive performance as well as the rate of cognitive decline, in a diverse population from 16 European countries.
Methods
We performed a prospective cohort study based on data from the Survey of Health, Ageing and Retirement in Europe (SHARE). SHARE is a study of community-dwelling individuals that collects information about health, social networks, and economic conditions, and has been previously described in detail.10 Briefly, eligible study participants are persons aged 50 years and older and their partners, irrespective of age. The participants are followed over time and refreshment samples of new individuals are enrolled in new waves to compensate for dropout. The first wave of data collection, which was based on computer-assisted personal interviewing, was conducted in 2004, followed by wave 2 in 2006/2007, wave 3 in 2008/2009, wave 4 in 2011/2012, wave 5 in 2013, and wave 6 in 2015.
The sampling frames were allowed to vary between countries according to their particular resources (national or municipal population registers, listings of dwellings, etc.). The sampling designs were based on probability selection methods. To minimize the imprecisions in estimates caused by use of different sampling designs, a number of general advisements on stratification, clustering, variation in selection probabilities, and sample size were provided in each wave to all participating countries.11 The response and retention rates are presented for each country and each wave in detail elsewhere.11–15 For example, the average response rate in wave 1 was 62% and the reported average retention rates in every wave ranged between 60% and 90%.11–15
Standard protocol approvals, registrations, and patient consents
This report uses data from SHARE waves 1, 2, 3, 4, 5, and 6; see elsewhere for details10 (DOIs: 10.6103/SHARE.w1.600, 10.6103/SHARE.w2.600, 10.6103/SHARE.w3.600, 10.6103/SHARE.w4.600, 10.6103/SHARE.w5.600, 10.6103/SHARE.w6.600). SHARE has been repeatedly reviewed and approved by the ethics committee of the University of Mannheim. All participants provided a written consent. Their data were pseudo-anonymized and participants have been informed about the storage and use of the data and their right to withdraw consent. The present analysis was approved by the ethics committee of the National Institute of Mental Health, Czech Republic.
Childhood SEP
Information about childhood SEP is from wave 3 when data about individuals' life histories were collected (SHARELIFE). A reduced version of SHARELIFE was used in wave 5 for those who did not participate in wave 3. A Life History Calendar16 was applied during computer-assisted personal interviewing to obtain information in order to improve the recollection process. Childhood SEP was operationalized according to 3 items concerning participants' household characteristics at the age of 10 years. Housing conditions have frequently been used as indicators of material circumstances related to SEP, with overcrowding indicating hardship.17 We additionally included the number of books in the household in the composite score capturing SEP to improve validity of the metric, a measure that has been used in previous studies to represent SEP.18,19 Validity of the recalled data in SHARELIFE has been previously examined.20
Specifically, participants were asked about the number of rooms (how many rooms their household occupied in their accommodation, including bedrooms but excluding kitchen, bathrooms, and hallways) and household members (how many people, including themselves, lived in their household at their accommodation) and were supposed to give a number. Furthermore, they were asked about the number of books: approximately how many books there were in the place they lived in (excluding magazines, newspapers, or their school books), with 5 possible answers: none or very few (0–10 books)/enough to fill one shelf (11–25 books)/enough to fill one bookcase (26–100 books)/enough to fill 2 bookcases (101–200 books)/enough to fill 2 or more bookcases (more than 200 books).
Using these 3 items, we constructed the variable “childhood socioeconomic hardship” as follows: first, we calculated a ratio “number of household members”/“number of rooms,” assuming that a higher ratio represents a worse SEP in childhood. We created a binary variable specific for each country, where the upper 5% of the distribution of the ratio from each country represents a more adverse SEP (coded as 1), relative to the other 95% of the distribution (coded as 0). Further, the variable “number of books” was transformed into a binary variable where “none or very few books” represented a worse SEP (coded as 1), relative to the rest (coded as 0). Finally, we summed both variables and constructed a binary variable, in which the value 2 stands for “childhood socioeconomic hardship” (coded as 1), in comparison to the rest (coded as 0).
In addition, because overcrowding in the home during childhood has been in particular used as an indicator of adverse childhood SEP,21 we used the ratio “number of household members”/“number of rooms” for a separate set of secondary analyses.
Cognitive functions
Cognitive functions were tested in all waves except for wave 3; thus, the present study uses data from waves 1, 2, 4, 5, and 6. Two brief tests were administered, providing 3 measures of cognitive functions: verbal learning and delayed recall (gained from an adapted 10-word delay recall test22) and verbal fluency (from an animal word fluency test23). Furthermore, we created a composite score of global cognition by converting each of the 3 measures into a z score, using the baseline mean and SD, and averaging them.
Covariates
Information on clinical and social risk factors, as potential mediators in the association of SEP with cognition, was initially identified based on the literature24–30 and availability in the SHARE dataset. From those, we chose variables that were associated with the composite cognitive score at the level of p < 0.05 in at least 4 waves, after adjustment for age, sex, and years of education. The selected factors were body mass index (calculated from the height and weight), depressive symptoms (assessed using the EURO-D scale31), and the following binary variables: cardiovascular disease (obtained from combining information about the history of hypertension, diabetes mellitus, stroke, hypercholesterolemia and coronary disease, and the use of cardiovascular drugs), smoking (yes = ever smoked daily), alcohol use (yes = drinking more than 2 glasses of alcohol almost every day), physical inactivity (yes = hardly ever or never perform activities that are vigorous or require a moderate level of energy), having a partner in the household, and current employment.
Analytical sample
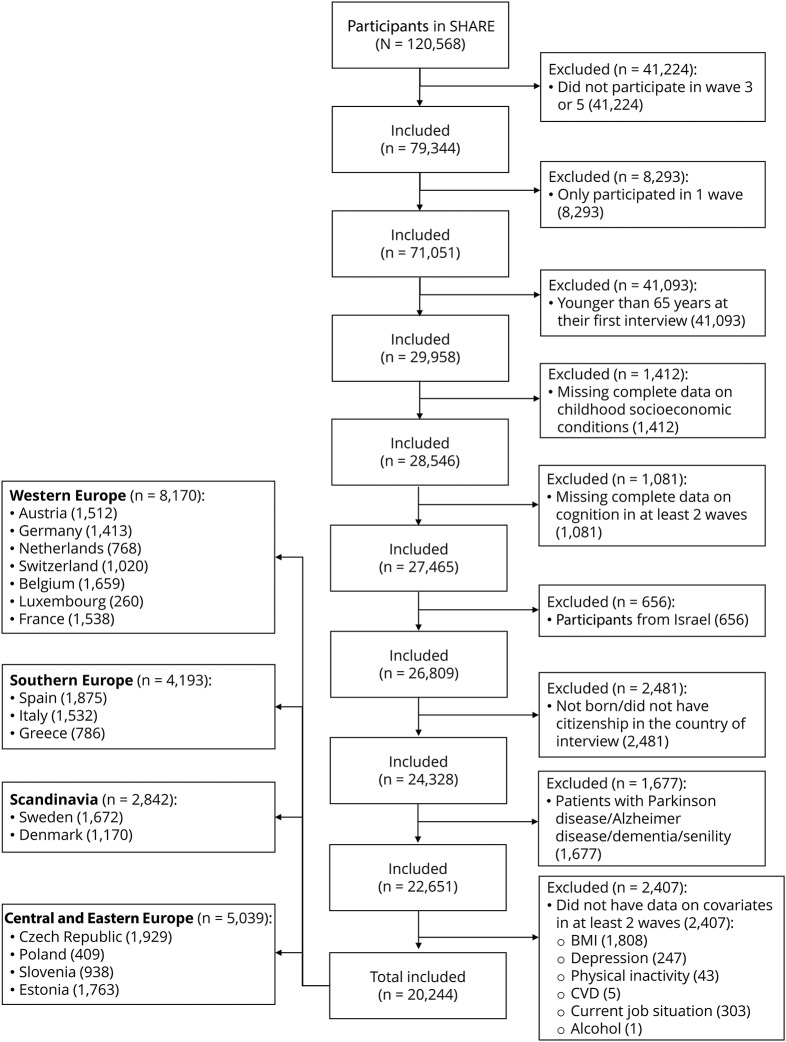
We restricted the analysis to persons who participated in SHARELIFE either in wave 3 or 5, were at least 65 years old at baseline, and gave an interview in at least 2 waves (flowchart presented in the figure). From 29,958 participants who fulfilled these criteria, we excluded persons with missing data on childhood SEP (n = 1,412), those who did not have complete data on cognition in at least 2 waves (n = 1,081), citizens of Israel (n = 656), individuals who were not born/did not have citizenship in the country of interview (n = 2,481), were ever diagnosed with Parkinson disease/Alzheimer disease/dementia/senility (n = 1,677), and did not have data on covariates in at least 2 waves (n = 2,407).
Figure. Selection of the study population.
BMI = body mass index; CVD = cardiovascular disease; SHARE = Survey of Health, Ageing and Retirement in Europe.
The final analytical sample consisted of 20,244 persons from 4 European regions (Western Europe: n = 8,170; Southern Europe: n = 4,193; Scandinavia: n = 2,842; and Central and Eastern Europe: n = 5,039). The average follow-up time of the participants was 4.8 years with an SD of 3.1 years. In 58% of participants, the measures of cognition were available at 3 and more time points (38% of participants 3 times, 11% 4 times, and 9% 5 times), while 42% of individuals had cognition measured twice.
Statistical analysis
We present descriptive data at baseline (the year when information on cognitive tests was first available) as frequency (n [%]), mean ± SD, or median and interquartile range. To compare characteristics of the participants with and without childhood socioeconomic hardship, we used an independent sample t test for continuous variables with normal distribution, Mann-Whitney test for continuous variables with skewed distribution, and χ2 test for binary variables.
We applied linear regression to estimate β with 95% confidence intervals (CIs) for the associations of childhood socioeconomic hardship with the scores in verbal learning, verbal fluency, delayed recall, and global cognition. We fit 2 sets of models. Model 1 was first adjusted for baseline age and sex, and dummy-coded for country of origin. Model 2 was adjusted also for clinical and social risk factors in a way that the variables were kept in the model if they were significantly associated with the dependent variable or improved R2 of the model. To study the strength of individual predictors, we calculated their R2 contribution averaged over orderings among regressors.32 Furthermore, we used linear mixed models to study the relation of socioeconomic hardship to the rate of cognitive decline.33 The models included time (in years since baseline), childhood socioeconomic hardship, and their interaction term (childhood socioeconomic hardship × time), adjusting for covariates as described above.
We performed 3 sets of sensitivity analysis. First, because Europeans lived in profoundly different socioeconomic conditions in the first half of the 20th century3 and great geographical differences in cognitive aging exist in Europe,34 we examined the relation between childhood socioeconomic hardship and cognition separately for European regions. Second, we reran the analysis with imputed values on missing data on cognitive tests. Third, we repeated the analysis when individuals diagnosed with Parkinson disease, Alzheimer disease, dementia, or senility were kept in the analytical sample.
In addition, we performed a set of secondary analyses using a measure of childhood overcrowding (defined as the ratio between household members and rooms, with a higher value indicating more overcrowding). First, we applied linear regression to investigate the association of overcrowding with the baseline level of global cognition as well as linear mixed models to study the relation of overcrowding to the rate of cognitive decline. Second, to detect a potential gradient effect, we divided the variable overcrowding into quartiles and repeated the aforementioned analyses using an ordinal variable. We adjusted for covariates as described above. All tests were 2-sided, and p value <0.05 was taken to indicate statistical significance. Data were analyzed with IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY) and R (version 3.4.0).
Data availability
Access to the SHARE data is provided free of charge on the basis of a release policy that gives quick and convenient access to all scientific users worldwide after individual registration. All details about the application and registration process can be found on this website: share-project.org. The study protocol and syntax of the statistical analysis will be shared upon request from the corresponding author of this study.
Results
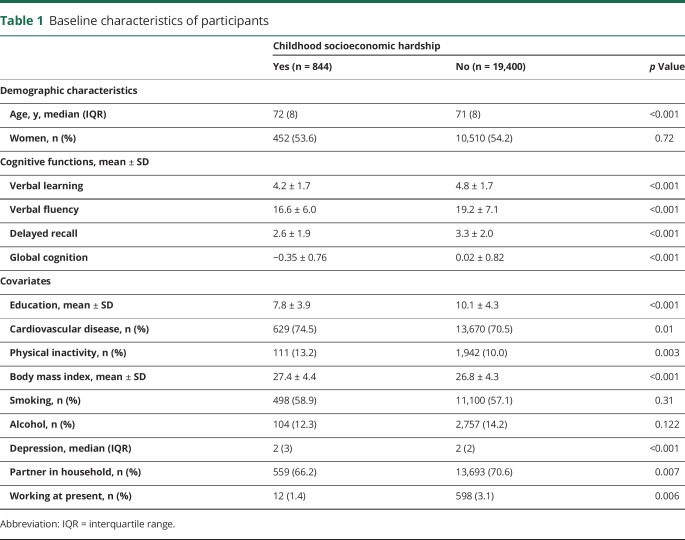
The sample consisted of 20,244 individuals (median age at baseline 71 years, interquartile range 8; 54% women; table 1). At baseline, scores for global cognition ranged from −2.39 to 3.45. From the whole sample, 844 individuals (4%) were characterized as having had socioeconomic hardship in childhood. Compared to the remainder of the cohort, individuals with childhood socioeconomic hardship were less educated, to a lower extent employed, and less frequently had a partner in the household. Furthermore, they scored higher in depressive symptomatology, had more often cardiovascular disease, were less physically active, and had higher body mass index, but did not differ in alcohol use and smoking. Participants with childhood socioeconomic hardship reached lower scores in all cognitive tests (mean −0.35 ± SD 0.76 vs 0.02 ± SD 0.82 for global cognition; p < 0.001).
Table 1.
Baseline characteristics of participants
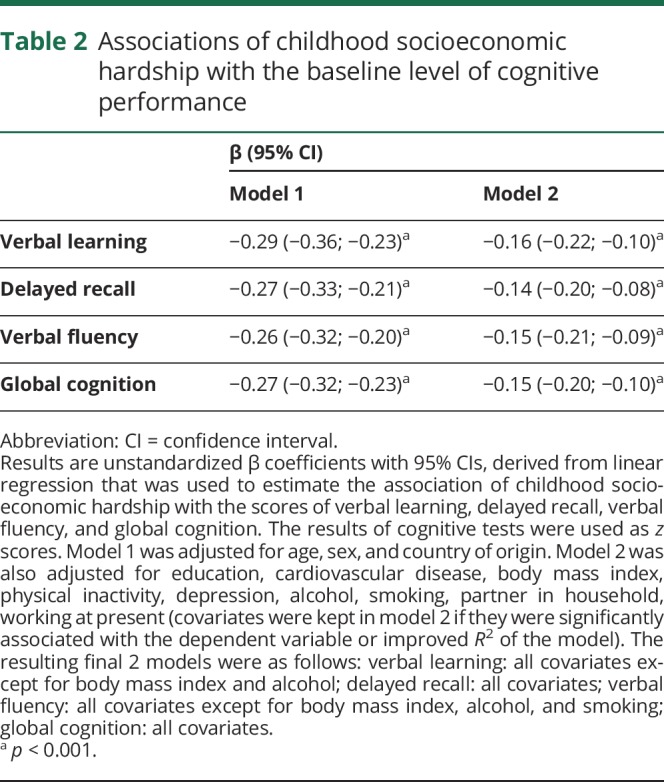
Adjusting for age, sex, and country of origin, the childhood socioeconomic hardship was associated with lower cognitive scores (β −0.274; 95% CI −0.323 to −0.225 for global cognition; model 1, table 2). After adjusting for all clinical and social risk factors, the association between socioeconomic hardship and global cognition score attenuated by approximately 45% (calculated as a difference between the unstandardized β of model 1 and model 2, multiplied by 100) but remained statistically significant (β −0.150; 95% CI −0.196 to −0.103 for global cognition; model 2, table 2). This trend was similar across all cognitive tests. Overall, model 2 explained 33% of variance in global cognitive score (as compared to 25.1% with model 1; Akaike information criterion: 40,316 for model 2 vs 42,483 for model 1). When considering the individual strength of each covariate in model 2, the most variance was explained by the country of origin (11.7%) and education (8.8%). When each covariate was added into the model separately, the association was reduced the most by education and depression, while other risk factors tended to have only small effects (results not presented in tables). Specifically, the association weakened by 35% due to education and by 12% due to depression.
Table 2.
Associations of childhood socioeconomic hardship with the baseline level of cognitive performance
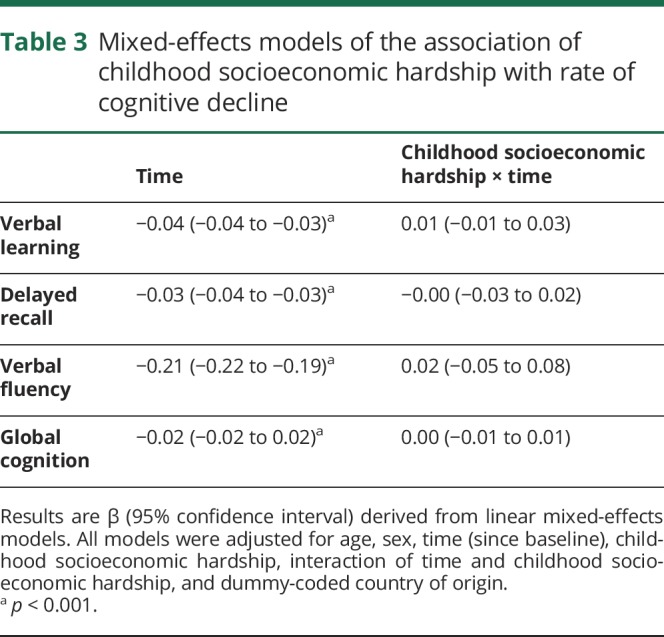
Using mixed models adjusted for baseline age, sex, and country of origin, global cognition decreased yearly by 0.02 points, as shown by the term for time (table 3). Childhood socioeconomic hardship was not related to a rate of decline in any of the cognitive measures, as seen by the interaction term time × childhood socioeconomic hardship (β = 0.00; 95% CI −0.01 to 0.01; p = 0.9 for global cognition).
Table 3.
Mixed-effects models of the association of childhood socioeconomic hardship with rate of cognitive decline
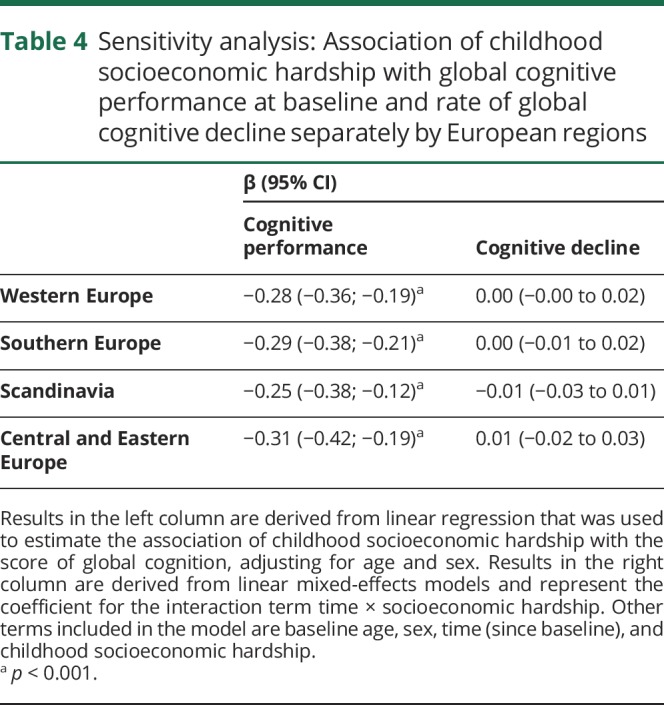
In sensitivity analysis, when modeled separately for European regions (table 4), the association of childhood socioeconomic hardship with the level of global cognitive performance ranged from β = −0.31 (95% CI −0.42 to −0.19) for participants in Central and Eastern Europe to β = −0.25 (95% CI −0.38 to −0.12) for individuals in Scandinavia. The negative result indicating no association between childhood socioeconomic hardships with the rate of cognitive decline was consistent across European regions. Similar results were obtained when imputed data were used for individuals with missing information on cognitive tests and when individuals diagnosed with Parkinson disease, Alzheimer disease, dementia, or senility were kept in the analytical sample (data not presented in tables).
Table 4.
Sensitivity analysis: Association of childhood socioeconomic hardship with global cognitive performance at baseline and rate of global cognitive decline separately by European regions
In secondary analyses, overcrowding in childhood was associated with a lower level of baseline global cognition, adjusting for age, sex, and country of origin (β = −0.04; 95% CI −0.05 to −0.04). After dividing the participants into quartiles and using the first quartile as a reference category, we observed a gradient within the association of overcrowding with baseline global cognition: second quartile β = −0.10 (95% CI −0.13 to −0.08); third quartile β = −0.17 (95% CI −0.20 to −0.14); fourth quartile β = −0.25 (95% CI −0.29 to −0.22), adjusting for age, sex, and country of origin. Overcrowding was not related to the rate of cognitive decline in global cognition, irrespective of the variable being analyzed as a continuous or as an ordinal variable (results not presented in tables).
Discussion
In this population-based, prospective cohort study based on a well-characterized nationally representative sample of more than 20,000 individuals residing in 16 European countries, we found that adverse childhood SEP operationalized by participants' household characteristics was associated with a lower level of cognitive performance. We observed a graded relationship between the degree of SEP and cognitive performance. Social and clinical risk factors significantly attenuated the association but did not account for all the variation in cognition. Over the average follow-up of almost 5 years, adverse childhood SEP was not related to the rate of cognitive decline.
These findings are of interest in the context of the life-course study of cognitive impairment. The current analysis differs from previous studies in 3 ways: (1) it includes by far the largest sample; (2) participants represent a wide range of European regions including countries in Central and Eastern Europe, which have been underrepresented in previous studies on cognitive aging1,35; and (3) it has a longitudinal design, which only a few previous studies have utilized.4,6
Although causality cannot be established based on observational studies, this research builds on previous experiments with animal models that demonstrated lower cognitive functions in subjects that were exposed to less resourceful environments early in life.36,37 In line with previous epidemiologic studies,2–8 the present study suggests that the roots of cognitive ability in old age originate in childhood SEP. Advantaged childhood SEP contributes to better cognitive skills throughout the lifespan and could possibly delay the onset of cognitive deterioration compared with individuals who experienced childhood socioeconomic hardship. This supports the hypothesis of passive cognitive reserve and its role in maintaining cognitive performance in older age and suggests that interventions aimed to improve cognitive aging should include decreasing socioeconomic hardship in childhood.
Past research examining the relationship between childhood SEP and the rate of change in cognition is rare38 and has yielded inconsistent findings. The Chicago Health and Aging Project observed that childhood SEP was associated with differences in cognitive performance of older people, but not with the rate of decline.6 Of note, Barnes et al.4 found that adverse socioeconomic conditions in childhood were related to a slower rate of cognitive decline in African Americans, while no effect was observed for white individuals. In the broader context of childhood adversity and cognitive aging, a study on parental separation during the war from the Helsinki Birth Cohort yielded similar results.39 Individuals exposed to this adverse event during childhood had weaker cognitive skills at the age of 20 and 70 years; however, this event was not associated with their cognitive decline over the 50 years.39 Expanding on this literature, the current study provides the largest investigation into the association of childhood SEP and the rate of cognitive decline.
Considering the robustness of the sample, we can conclude that SEP is an unlikely factor contributing to the rate of cognitive decline in older age and advantages from childhood SEP do not have effects on counteracting age-related pathology. This particular finding could be interpreted as evidence that SEP does not influence active cognitive reserve of the brain that may have a role in slowing the rate of cognitive decline. It could be hypothesized that where passive cognitive reserve of individuals is mirrored in the level of cognitive performance, mechanisms underlying the active cognitive reserve drive the rate of cognitive decline in older age once a threshold for impairment is reached. Further studies exploring the rate of cognitive decline in old age in relation to the active models of reserve will lead to improved understanding of the mechanisms surrounding cognitive decline in old age, in turn, uncovering interventions to diminish the rate and severity of impairment and disability in old age.
Modifiable clinical and social risk factors did not fully explain the relationship between childhood SEP and cognitive performance in old age, but largely mediated it, in particular education. The protective effect of education on cognitive aging is consistent with past literature,40 and adds to the robust foundation supporting education as an intervention to diminish the adverse effects of childhood socioeconomic hardship on cognitive aging. Considering the present findings, adverse household conditions could be an appropriate indicator for identifying at-risk children who would benefit in the long term from targeted educational interventions.
The mechanisms through which education affects cognitive aging are not fully understood, but education could be hypothesized to mitigate cognitive aging by building and conserving brain reserve capacity (BRC).41 This would in turn widen the gap between individuals' standing BRC and the critical threshold of BRC at which point cognitive deficits emerge in old age. Previous literature suggests an association of education with the level of cognitive performance42; however, studies on the relation of education to the rate of cognitive decline yielded mixed results.43,44
In a recent review, research on the influence of education on cognitive decline ranged from showing no effect to a protective effect of education on the rate of cognitive decline, to the effect of education being dependent on subgroups of populations studied, to studies reporting that education mediated some, but not all, cognitive functions measured in the rate of cognitive decline.44 In the present study, we observed an association of more years of education with a higher level of baseline cognitive performance, but not with a rate of cognitive decline (results not presented in tables), which is in line with the passive reserve hypothesis. Of note, the evidence base surrounding the role of education in cognitive aging is substantially limited by the absence of studies examining the relationship between education and the rate of cognitive decline in adults with low levels of education.44
This study is unique in its generalizability to a wide range of European countries. However, there are notable complexities influencing contextualization of the findings of this study within the broader global literature. These include substantial variations in the operationalization and assessment of childhood SEP, ranging from parental occupation, to household and broader community socioeconomic levels.45 Few studies have operationalized SEP using household conditions while examining later-life cognitive aging. In an American population, overcrowding was associated with lower cognitive performance in adulthood, and when exploring variables related to childhood SEP, the father's occupation, parental home status, and overcrowding were all highly correlated.46 In a study from the United Kingdom, which operationalized childhood SEP as the father's occupation, SEP was also found to have a graded association with cognitive aging.47
As a result of social stratification processes, appropriate measures of SEP can differ in low- and middle-income countries (LMICs) than those in high-income countries (HICs),48 and epidemiologic research examining life-course SEP and aging in LMICs is a complex field, which at present is far less researched than in HICs.49 SEP operationalized through household living conditions can be problematic when comparing globally; therefore, weighted analysis of composites or consumption expenditures have been proposed to more accurately reflect SEP in a way that is suitable to comparisons across both LMICs and HICs.48
This study is limited by the retrospective nature of the information on childhood conditions, which is susceptible to recall bias. However, when comparing this recalled information with historical data at a country and cohort level, Havari and Mazzonna20 suggested a good level of internal and external consistency. Another limitation of this study is residual confounding; information about other potentially modifiable social and clinical risk factors, such as levels of blood pressure, cholesterol, social and material deprivation, or loneliness, is not available in the dataset. This could lead to overestimation of the association of childhood SEP on cognitive aging. Simultaneously, this could have underestimated the effect of potentially modifiable risk factors on mitigating the adverse effects of childhood SEP on cognitive aging.
Another limitation of this study is a possible survivor bias. Because individuals who have experienced socioeconomic hardship in childhood face higher risk of death, in particular from cardiovascular causes,50 healthier individuals may be overrepresented in the present study sample. If the study participants had a better cognitive status than individuals not included in this study due to death, inherent selection bias could contribute to underestimation of the effect of childhood SEP on cognitive aging. In addition, the study is limited by different sampling methods, which have not been accounted for by using sampling weights. Our findings should be replicated in future studies with a longer follow-up that could potentially reveal more trends of cognitive decline based on differences in SEP.
Given the aging population and the increasing burden of age-related cognitive decline in Europe and thus far inconclusive evidence that cognitive impairment can be prevented by interventions in late life,1 this study has important implications. The focus of strategies to protect cognitive health should be shifted into early life and take into account the graded influence of SEP, aiming to provide children with proportionally appropriate resources to their disadvantage so they can fully develop their potential. Furthermore, the effects of adverse socioeconomic conditions in childhood may be mitigated by modification of several psychosocial and clinical risk factors, especially by providing opportunities of education. Further research identifying risk and protective factors influencing the rate of cognitive decline, including study populations with low education levels, and replications of similar studies in LMICs will lead to more effective interventions throughout the life course and in older age to decrease the burden of cognitive aging on individuals and carers.
Glossary
- BRC
brain reserve capacity
- CI
confidence interval
- HICs
high-income countries
- LMICs
low- and middle-income countries
- SEP
socioeconomic position
- SHARE
Survey of Health, Ageing and Retirement in Europe
Author contributions
P.C. conceptualized and designed the work, analyzed and interpreted the data, drafted the manuscript, and revised the final version critically for important intellectual content. T.F. made a substantial contribution to the conception and design of the work, analysis and interpretation of the data, and writing of the manuscript. A.K. substantially contributed to the interpretation of the data and writing of the manuscript. P.W. revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
Study funding
The SHARE data collection has been primarily funded by the European Commission through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006-062193; COMPARE: CIT5-CT-2005-028857; SHARELIFE: CIT4-CT-2006-028812) and FP7 (SHARE-PREP: N 211909; SHARE-LEAP: N 227822; SHARE M4: N 261982). Additional funding from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the US National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064, HHSN271201300071C), and from various national funding sources is gratefully acknowledged (see share-project.org). The authors were supported by the project “Sustainability for the National Institute of Mental Health” (grant LO1611), with financial support from the Ministry of Education, Youth and Sports of the Czech Republic.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology February 17, 2018. Accepted in final form July 17, 2018.
References
- 1.Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol 2016;15:455–532. [DOI] [PubMed] [Google Scholar]
- 2.Fors S, Lennartsson C, Lundberg O. Childhood living conditions, socioeconomic position in adulthood, and cognition in later life: exploring the associations. J Gerontol B Psychol Sci Soc Sci 2009;64:750–757. [DOI] [PubMed] [Google Scholar]
- 3.Doblhammer G, van den Berg GJ, Fritze T. Economic conditions at the time of birth and cognitive abilities late in life: evidence from ten European countries. PLoS One 2013;8:e74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes LL, Wilson RS, Everson-Rose SA, Hayward MD, Evans DA, Mendes de Leon CF. Effects of early-life adversity on cognitive decline in older African Americans and whites. Neurology 2012;79:2321–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Gu D, Hayward MD. Childhood nutritional deprivation and cognitive impairment among older Chinese people. Soc Sci Med 2010;71:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. Am J Epidemiol 2003;158:1083–1089. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J Gerontol B Psychol Sci Soc Sci 2005;60:S93–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritze T, Doblhammer G, van den Berg GJ. Can individual conditions during childhood mediate or moderate the long-term cognitive effects of poor economic environments at birth? Soc Sci Med 2014;119:240–248. [DOI] [PubMed] [Google Scholar]
- 9.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 2012;11:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borsch-Supan A, Brandt M, Hunkler C, et al. Data resource profile: the Survey of Health, Ageing and Retirement in Europe (SHARE). Int J Epidemiol 2013;42:992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann M, Kneip T, De Luca G, Scherpenzeel A. Survey Participation in the Survey of Health, Ageing and Retirement in Europe (SHARE), Wave 1–6. Munich: Munich Center for the Economics of Aging; 2017. [Google Scholar]
- 12.Börsch-Supan A, Brugiavini A, Jürges H, et al., editors. First Results from the Survey of Health, Ageing and Retirement in Europe (2004–2007): Starting the Longitudinal Dimension. Mannheim: MEA; 2008. [Google Scholar]
- 13.Schröder M, editor. Retrospective Data Collection in the Survey of Health, Ageing and Retirement in Europe. SHARELIFE Methodology. Mannheim: MEA; 2011:5. [Google Scholar]
- 14.Abduladze L, Balster E, Börsch-Supan A, et al. SHARE Wave 4: Innovations & Methodology. Munich: Munich Center for the Economics of Aging; 2013. [Google Scholar]
- 15.De Luca G, Rossetti C, Malter F. Sample design and weighting strategies in SHARE Wave 5. In: Malter F, Borsch-Supan A, editors. SHARE Wave 5: Innovations & Methodology. Munich: MEA, Max Planck Institute for Social Law and Social Policy; 2015:75–84. [Google Scholar]
- 16.Freedman D, Thornton A, Camburn D, Alwin D, Young-Demarco L. The Life History Calendar: a technique for collecting retrospective data. Sociol Methodol 1988;18:37–68. [PubMed] [Google Scholar]
- 17.Galobardes B, Shaw M, Lawlor DA, Lynch JW. Indicators of socioeconomic position (part 1). J Epidemiol Community Health 2006;60:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedzwiedz CL, Katikireddi SV, Pell JP, Mitchell R. The association between life course socioeconomic position and life satisfaction in different welfare states: European comparative study of individuals in early old age. Age Ageing 2014;43:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 20.Havari E, Mazzonna F. Can we trust older people's statements on their childhood circumstances? Evidence from SHARELIFE. Eur J Popul 2015;31:233–257. [Google Scholar]
- 21.Solari CD, Mare RD. Housing crowding effects on children's wellbeing. Soc Sci Res 2012;41:464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris SJ, Dowson JH. Recall of a 10-word list in the assessment of dementia in the elderly. Br J Psychiatry 1982;141:524–527. [DOI] [PubMed] [Google Scholar]
- 23.Henley NM. A psychological study of the semantics of animal terms. J Verbal Learn Verbal Behav 1969;8:176–184. [Google Scholar]
- 24.Cermakova P, Fereshtehnejad SM, Johnell K, Winblad B, Eriksdotter M, Religa D. Cardiovascular medication burden in dementia disorders: a nationwide study of 19,743 dementia patients in the Swedish Dementia Registry. Alzheimers Res Ther 2014;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cermakova P, Muller M, Armstrong AC, et al. Subclinical cardiac dysfunction and brain health in midlife: CARDIA (Coronary Artery Risk Development in Young Adults) brain magnetic resonance imaging substudy. J Am Heart Assoc 2017:6:e006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cermakova P, Nelson M, Secnik J, et al. Living alone with Alzheimer's disease: data from SveDem, the Swedish Dementia Registry. J Alzheimers Dis 2017;58:1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Ptacek S, Kareholt I, Cermakova P, Rizzuto D, Religa D, Eriksdotter M. Causes of death according to death certificates in individuals with dementia: a cohort from the Swedish Dementia Registry. J Am Geriatr Soc 2016;64:e137–e142. [DOI] [PubMed] [Google Scholar]
- 28.Secnik J, Cermakova P, Fereshtehnejad SM, et al. Diabetes in a large dementia cohort: clinical characteristics and treatment from the Swedish Dementia Registry. Diabetes care 2017;40:1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cermakova P, Szummer K, Johnell K, et al. Management of acute myocardial infarction in patients with dementia: data from SveDem, the Swedish Dementia Registry. J Am Med Dir Assoc 2017;18:19–23. [DOI] [PubMed] [Google Scholar]
- 30.Cermakova P, Eriksdotter M, Lund LH, Winblad B, Religa P, Religa D. Heart failure and Alzheimer's disease. J Intern Med 2015;277:406–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince MJ, Reischies F, Beekman AT, et al. Development of the EURO-D scale—a European Union initiative to compare symptoms of depression in 14 European centres. Br J Psychiatry 1999;174:330–338. [DOI] [PubMed] [Google Scholar]
- 32.Lindeman RH, Merenda PF, Gold RZ. Introduction to Bivariate and Multivariate Analysis. Dallas: Scott Foresman; 1980. [Google Scholar]
- 33.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- 34.Skirbekk V, Loichinger E, Weber D. Variation in cognitive functioning as a refined approach to comparing aging across countries. Proc Natl Acad Sci USA 2012;109:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkler P, Formanek T, Mlada K, Cermakova P. The Czech Mental Health Study (CZEMS): study rationale, design, and methods. Int J Methods Psychiatr Res 2018:e1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marco EM, Valero M, de la Serna O, et al. Maternal deprivation effects on brain plasticity and recognition memory in adolescent male and female rats. Neuropharmacology 2013;68:223–231. [DOI] [PubMed] [Google Scholar]
- 37.Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 2007;32:256–266. [DOI] [PubMed] [Google Scholar]
- 38.Lyu J, Burr JA. Socioeconomic status across the life course and cognitive function among older adults: an examination of the latency, pathways, and accumulation hypotheses. J Aging Health 2016;28:40–67. [DOI] [PubMed] [Google Scholar]
- 39.Pesonen AK, Eriksson JG, Heinonen K, et al. Cognitive ability and decline after early life stress exposure. Neurobiol Aging 2013;34:1674–1679. [DOI] [PubMed] [Google Scholar]
- 40.Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta 2012;1822:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaakov S. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460. [PubMed] [Google Scholar]
- 42.Brayne C, Calloway P. The association of education and socioeconomic status with the Mini Mental State Examination and the clinical diagnosis of dementia in elderly people. Age Ageing 1990;19:91–96. [DOI] [PubMed] [Google Scholar]
- 43.Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology 2000;46:163–177. [DOI] [PubMed] [Google Scholar]
- 44.Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC. Relationship between education and age-related cognitive decline: a review of recent research. Psychogeriatrics 2015;15:154–162. [DOI] [PubMed] [Google Scholar]
- 45.Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis 2007;12:11–22. [DOI] [PubMed] [Google Scholar]
- 46.Packard CJ, Bezlyak V, McLean JS, et al. Early life socioeconomic adversity is associated in adult life with chronic inflammation, carotid atherosclerosis, poorer lung function and decreased cognitive performance: a cross-sectional, population-based study. BMC Public Health 2011;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurst L, Stafford M, Cooper R, Hardy R, Richards M, Kuh D. Lifetime socioeconomic inequalities in physical and cognitive aging. Am J Public Health 2013;103:1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howe LD, Galobardes B, Matijasevich A, et al. Measuring socio-economic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol 2012;41:871–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tollman SM, Norris SA, Berkman LF. Commentary: the value of life course epidemiology in low- and middle-income countries: an ageing perspective. Int J Epidemiol 2016;45:997–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beebe-Dimmer J, Lynch JW, Turrell G, Lustgarten S, Raghunathan T, Kaplan GA. Childhood and adult socioeconomic conditions and 31-year mortality risk in women. Am J Epidemiol 2004;159:481–490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to the SHARE data is provided free of charge on the basis of a release policy that gives quick and convenient access to all scientific users worldwide after individual registration. All details about the application and registration process can be found on this website: share-project.org. The study protocol and syntax of the statistical analysis will be shared upon request from the corresponding author of this study.