Abstract
Objectives:
Serious mental illness is disproportionately common in people with epilepsy and contributes to complications and mortality. Few care approaches specifically target individuals who have epilepsy and severe mental illness. These investigators used an iterative process to refine an existing intervention and tested the novel intervention, Targeted Self-Management for Epilepsy and Mental Illness (TIME) in individuals with epilepsy and comorbid mental illness (E-MI)
Methods:
TIME was developed with input from a community advisory board and then tested for feasibility, acceptability and preliminary efficacy in people with E-MI, using a 16-week prospective randomized controlled design comparing TIME (N=22) vs. treatment as usual (TAU, N=22). Primary outcome was change in depressive symptoms, assessed by the Montgomery Asberg Depression Rating Scale (MADRS). Secondary assessments included global psychiatric symptom severity, seizure frequency, sleep patterns, quality of life, stigma, social support and self-efficacy.
Results:
There were 44 individuals enrolled, mean age 48.25 (SD = 11.82) with 25 (56.8%) African-Americans. The majority, (N=31, 70.5%), were unemployed and most (N=41, 95.5%) had annual income < U.S. $25,000. With respect to study retention, there were 36 individuals (18 in TIME, 18 in TAU) assessed at 12 weeks and 35 individuals (19 in TIME, 16 in TAU) assessed at 16 weeks. There was a significant effect for MADRS (p=0.036; effect size of 0.70), with lower MADRS at 16 weeks in TIME, while TAU MADRS did not change. Differences between most secondary measures were not statistically significant.
Significance:
The TIME intervention engages individuals to actively participate in self-management, and can reduce depression in E-MI. Given the high morbidity and mortality associated with epilepsy complicated by serious mental illness, additional research is needed to better identify how TIME might be implemented in routine care settings.
Keywords: Epilepsy, seizures, comorbidity, mental illness, depression, schizophrenia, bipolar disorder
1. Introduction
Epilepsy affects approximately 3 million Americans and is associated with substantial disability, reduced quality of life, stigma, and premature mortality[1]. In addition to injury and death, seizures in epilepsy are often associated with psychological comorbidity. Rates of serious mental illness (MI), such as schizophrenia, bipolar disorder and severe depression, are disproportionately high in those with epilepsy. It is estimated that 20–30% of people with epilepsy have comorbid mental illnesses.[2–6] Rates of psychotic illness are 6–12 times higher than in the general population, with a prevalence of 7–8% [7]. Rates of bipolar disorder in people with epilepsy may be as high as 12% [8]. Given the stigmatizing nature of both epilepsy and psychiatric illness, individuals with comorbid epilepsy and MI (E-MI) are doubly stigmatized. Heavy stigma burden and under-treated mental illness may at least partially explain the finding that suicide among people with epilepsy is 5 times that of the general population [9, 10].
Active self-management and engagement in care are crucial in minimizing the burden associated with both chronic mental disorders and with epilepsy. Self-management for MI includes adoption of healthy behaviors [11]. Similarly, self-management of epilepsy includes medication treatment as well as lifestyle and seizure management [12].
Since 2009, the Centers for Disease Control and Prevention (CDC) Prevention Research Centers’ Managing Epilepsy Well (MEW) Network has focused on addressing mental health issues in epilepsy[13] with the development and testing of a number of evidence-based practices [13–16]. Thompson and colleagues[15] evaluated UPLIFT (Using Practice and Learning to Increase Favorable Thoughts), a remotely-delivered self-management intervention for people with epilepsy and depression. Using a randomized, controlled crossover design, 128 adults with epilepsy and mild/moderate depressive symptoms were assigned to either UPLIFT or TAU waitlist. Incidence of new or relapsing depressive episodes was significantly lower in UPLIFT compared to TAU. An earlier, single-site RCT of UPLIFT similarly found improvement in depressive severity.[14] In another MEW Network RCT, Ciechanowski and colleagues [16] tested PEARLS, a home-based program for managing epilepsy and depression. Individuals with epilepsy and depression were randomly assigned to PEARLS (N = 40) or TAU (N = 40). Compared to TAU, individuals in PEARLS had lower depression severity (P<0.005) and lower suicidal ideation (P = 0.025) over 12 months.
In spite of the intervention advances developed by the MEW Network and others, approaches for people with epilepsy who are comorbid for chronic psychosis or severe mood disorders are limited. MEW Network investigators at the Case Western Reserve University Prevention Research Center used an iterative, collaborative process to adapt an existing manualized intervention initially developed for people with serious mental illness and comorbid diabetes, [17, 18] and tested the adapted intervention, Targeted Self-Management for Epilepsy and Mental Illness (TIME) for feasibility, acceptability and preliminary efficacy in people with E-MI, using a prospective randomized controlled design comparing TIME vs. treatment as usual (TAU) over a 16-week time period in individuals with E-MI. The primary hypothesis was that at 16 weeks, TIME would be associated with greater improvement in depressive symptoms, as assessed by the Montgomery Asberg Depression Rating Scale (MADRS), compared to TAU.
2. Material and methods
2.1. TIME Intervention:
The TIME intervention is designed as an adjunct to regular medical/neurological care. TIME is based upon social cognitive theory, which posits that individuals learn by observing others, and behave in specific ways to reach goals [19]. Social cognitive theory-derived methods have been successfully applied in MI [20] and in epilepsy [21]. A key feature of TIME is the use of Peer Educators with E-MI to model self-management.
Consistent with community-based participatory research principles that underlie the CDC PRCs, TIME was developed with iterative input from an 11-member community advisory board (CAB) composed of 5 community health professionals, 4 persons with E-MI, and 2 support persons of individuals with E-MI. Mean age for all participants was 51.3 (SD=12.7, range 32–69), 6 female, and 5 male. For those with E-MI, mean years with epilepsy was 31.8 (SD=23.9, range 10–58) and mean years with MI was 10.8 (SD=10.2, range 1–25). The CAB met 3 times. In the first CAB meeting, we described the overall goals of the project, discussed the underlying conceptual model which features education (understanding what needs to be done for optimal self-management), empowerment (gaining confidence in meeting personal goals) and support (modeling and encouragement) from others with E-MI. We also solicited input on barriers and facilitators to care in people with E-MI. We prioritized focus on those factors that are potentially modifiable, and practical for addressing within a self-management framework. Additionally, referring to our underlying conceptual model, we requested input on preferred approaches for people with E-MI. In the second CAB meeting, we presented a first draft of the TIME intervention that incorporated CAB input, and obtained feedback/new input. In the third CAB meeting, we presented a revised draft of TIME that incorporated all previous suggestion and solicited input for a final product.
The final TIME intervention was operationalized in 2 steps. Step 1: Twelve group-format, in-person 60–90 minute sessions (Up to 8 E-MI participants per group), collaboratively delivered by a Nurse Educator-Peer Educator dyad. Groups were held at a central location easily accessible with public transportation. The group-sessions were completed over 12 weeks. The TIME intervention stresses information sharing in a way that is accessible to E-MI participants, and fosters motivation for active self-management. Topics addressed include a summary of facts vs. myths about mental illness and epilepsy, developing an action plan for concurrently coping with mental illness and epilepsy, personal goal-setting, stress management and training to communicate with care providers (see Table 1).
Table 1:
Topics covered in weekly group-format TIME sessions
| Session 1 | Orientation and introductions; Emphasize ground rules; Establishment of a therapeutic relationship; Discuss facts and misconceptions about Mental Illness and about epilepsy. |
| Session 2 | General mental health & epilepsy management principles; Relationship of Mental Illness and of epilepsy symptoms and functioning in response to stress; Introduction to personal goal-setting. |
| Session 3 | Treatments for epilepsy; Complications of epilepsy; Minimizing epilepsy complications. |
| Session 4 | Personal Mental Illness profile (what does worsening illness look like for you); Triggers of Mental Illness relapse; Personal action plan for coping with Mental Illness relapse. |
| Session 5 | Problem-solving skills and the IDEA approach (Identify the problem, Define possible solutions, Evaluate the solutions, Act on the best solution); Talking with your health care providers; Role play of communication with care providers. |
| Session 6 | Stigma and “double stigma”; Strategies to cope with stigma; Nutrition for best physical and emotional health. |
| Session 7 | Substance abuse and its effects on Mental Illness and on epilepsy; Specific stress- management approaches. |
| Session 8 | Effects of exercise and being outdoors on physical and emotional health; The Importance of daily routine and good sleep habits, Medication routines. |
| Session 9 | Medications and psychological treatments for Mental Illness; A personal care plan to take care of the mind and the body. |
| Session 10 | Social supports and using your available supports; Advocacy groups for epilepsy and for Mental Illness. |
| Session 11 | Normalizing your life in spite of having a chronic but unpredictable condition: Prioritizing medication side effects and discussing it with your clinician |
| Session 12 | Self- management as a life-style; Acknowledgement of group progress; Setting the stage for Ongoing Illness Management and Recovery (Step 2). |
Step 2: Following the group sessions, participants had 2 telephone maintenance sessions (spaced approximately 2 weeks apart) with the Peer Educator, and 2 telephone sessions (spaced approximately 2 weeks apart) with the Nurse Educator. Phone sessions emphasized support and ongoing self-management. Nurse Educators provided brief linkage (information sharing, opportunity for questions) to the participants’ clinical providers.
2.2. TAU intervention:
As with TIME, individuals in TAU continued treatment with their regular medical providers. Beyond follow-up research assessments at the same time points as TIME, there was no interaction between participants and the research team.
2.3. Feasibility and fidelity:
Attendance for each TIME session was recorded, and acceptability was evaluated with a brief self-rated questionnaire at the end of each 12-session group series. Following Fraser,[22] fidelity to the TIME intervention was assessed quantitatively (for example, duration and content covered) and qualitatively (for example, participant-interventionist interaction) at each session. Non-interventionist study staff evaluated each fidelity dimension on a 1–10 scale.
2.4. Study participants:
Inclusion criteria included having a DSM IV diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder or chronic/recurrent major depressive disorder, confirmed with the Mini-International Neuropsychiatric Interview (MINI) [23]. Additional inclusion criteria included a diagnosis of epilepsy (told by a doctor that they had epilepsy), ≥ age 18, and able to provide written informed consent. Study entry criteria were purposely broad in order to best represent “real world” E-MI populations. We used the electronic medical record from a large city-wide healthcare system to preliminarily identify people with an epilepsy diagnosis and invited them to participate in the RCT. Actively suicidal/homicidal individuals, those who were too psychiatrically impaired to participate in groups, and those with dementia were excluded, as were pregnant women with E-MI who might need different or more intensive treatments. No individuals from the E-MI CAB were enrolled as participants in the RCT. Sample size was calculated based on preliminary data with the MADRS in pilot work [17] showing an observed mean difference in MADRS scores from baseline of 11.17 (Standard deviation 13.0). For a two-sample t-test comparing mean differences in depression severity between treatment arms, with alpha = 0.05 (two-tailed), power = 0.756, and allowing for attrition, we estimated enrollment of 22 participants per arm, totaling 44 participants. Randomization was allocated using a computer-generated list that was available only after all baseline assessments were complete.
2.5. Measures:
All measures were conducted by dedicated research staff independent of the TIME intervention/TIME interventionists. In addition to demographic and clinical information, general medical burden using the Charlson Comorbidity Index[24] and health literacy[25] were also assessed at baseline. The primary outcome was change from baseline in depressive symptoms, as measured by the rater-administered MADRS [26]. While a variety of standardized tools are used to measure depression in epilepsy, [27–29] the MADRS has been used in recent large cross-sectional and longitudinal studies of people with epilepsy.[30, 31] Secondary outcomes included the Brief Psychiatric Rating Scale (BPRS)[32] for global psychiatric symptoms and the Patient Health Questionnaire (PHQ-9) for self-rated assessment of depressive symptoms [33]. Functional status was evaluated with the Global Assessment of Functioning (GAF)[34] and the World Health Organization Disability Assessment Schedule II [35]. Epilepsy control was assessed via 30-day self-reported seizure frequency. Quality of life was assessed with the Quality of Life in Epilepsy (QOLIE −10) [36]. Sleep pattern was assessed with the Pittsburgh Sleep Quality Index (PSQI) [37]. Self-Efficacy was measured with the Epilepsy Self-Efficacy Scale (ESES) 2000 version [38]. Social support was measured with the Multidimensional Scale of Perceived Social Support (MSPSS) [39]. Stigma for MI was measured with the Internalized Stigma of Mental Illness Scale (ISMI) [40] Stigma for Epilepsy was measured with the 10-item Epilepsy Stigma Scale (ESS) [41]. To assess acceptability of TIME, a brief survey was conducted on perceived benefit vs. burden and suggestions for improvement.
2.6. Quantitative data analysis:
Quantitative descriptive analyses characterized the baseline sample and examined change over time in MADRS as well as secondary outcomes. For the primary outcome of MADRS, Generalized Estimating Equations (GEE) methods were employed, with AR (1) covariance and robust covariance estimation. GEE is a well-established longitudinal analysis approach that allows for the modeling of trajectories of treatment response over time. It provides a formalized hypothesis-testing framework for comparing treatment trajectories, through analysis of treatment-by-time interaction. Statistically significant time-by -treatment interaction indicates different trajectories of depressive symptom severity based upon treatment arm assignment. A hypothesis test for treatment by time interaction was the primary inferential focus. Two-sided Type I error level of 0.05 was adopted. Gender, age, and education level were considered as covariates, and gender was included in a final model, as it had a significant effect, along with the time points as a categorical variable. Presence or non-presence of missing MADRS scores at 16 weeks also was modeled, through logistic regression, taking into account treatment group, age, gender, and educational level. Secondary/exploratory analyses of change from baseline to 12 and 16 weeks were assessed for BPRS, GAF, WHODAS, seizure frequency, QOLIE-10, PSQI, ESES, MSPSS, ESS and ISMI. Type I error of 0.05 was used for all these tests as well. For these variables, differences in values between the baseline and follow up time periods were compared across treatment groups using two sample t-tests.
2.7. Qualitative sample and analysis:
A complementary qualitative component evaluated TIME from the viewpoint of E-MI participants and Peer Educators (patients with epilepsy who have been trained to co-deliver TIME). We conducted in-depth interviews with a sub-set of TIME participants (N=8) as well as the 3 interventionists (2 Peer Educators, Nurse Educator). The qualitative sample (N=11) is within the recommended number of 5–25 individuals who have all experienced the same phenomenon[42]. We attempted to balance the qualitative sample by gender and race/ethnicity according to overall study enrollment. The in-depth interviews were audio recorded, transcribed, and imported into NVivo. Analysis was conducted using the general inductive approach. Detailed readings of the raw data were the basis for a thematic coding dictionary. The goal was to identify the core meanings and categories with relevance to understanding the processes through which the TIME intervention either did or did not succeed. Two researchers (AP and MS) generated themes and reviewed the assignment of quotations to codes in order to maintain internal consistency of coding. In this mixed methods study, we highlight the extent to which findings from qualitative and quantitative results converge, in order to support the validity of findings through triangulation.
3. Results
3.1. Study enrollment and flow:
Figure 1 illustrates overall study flow. Use of the electronic health record to identify and pre-screen people with epilepsy as possible candidates resulted in substantial numbers of individuals who did not fit other study criteria or who were not interested in research. There were 58 individuals screened in-person and 44 individuals eventually enrolled and randomized.
Figure 1:

CONSORT Diagram illustrating TIME and TAU enrollment and participation
3.2. Baseline sample:
Table 2 shows entire sample, TIME and TAU demographic and clinical variables. Mean age was 48.25 (SD = 11.82) with 25 (56.8%) African-Americans. Consistent with the heavy social and socioeconomic burden of epilepsy and comorbid serious mental illness, 31 (70.5%) were unemployed and 42 (95.5%) had an annual income of less than $25,000. Half of individuals (N=22) lived alone and 3 (6.8%) were living in a homeless shelter at time of study entry. The MADRS and BPRS scores suggest a substantial baseline level of depressive symptoms and only mild psychotic or manic symptoms.
Table 2:
Baseline demographic and clinical variables in adults with epilepsy and comorbid serious mental illness
| Variable | Entire sample N= 44 |
TIME group N=22 |
TAU group N=22 |
Statistic |
|---|---|---|---|---|
| Age (Mean, SD) | (48.25, 11.82) | (52.00, 7.58) | (45.10, 14.18) | t=1.98,df=30.25, p=.06 |
|
Race [n (%)] White/Caucasian Black/African Amer. Other |
16 (36.4%) 25 (56.8%) 3 (6.8%) |
8 (36.4%) 13 (59.1%) 1 (4.5%) |
8 (38.1%) 11 (52.4%) 2 (9.5%) |
Chi-square = 0.48, df=2, p=.79 |
|
Ethnicity [n (%)] Hispanic/Latino Not Hispanic/Latino |
3 (6.8%) 41 (93.2%) |
2 (9.1%) 20 (90.9%) |
1 (4.8%) 20 (95.2%) |
Fisher’s exact, p=1.00 |
|
Gender [n (%)] Male Female |
18 (40.9%) 26 (59.1%) |
9 (40.9%) 13 (59.1%) |
9 (42.9%) 12 (57.1%) |
Fisher’s exact, p=1.00 |
|
Education level [n (%)] Less than a high school education High school graduate, GED, or higher |
11 (25.0%) 33 (75.0%) |
7 (31.8%) 15 (68.2%) |
4 (19.0%) 17 (81.0%) |
Fisher’s exact, p=.49 |
|
Employment [n (%)] Unemployed Unable to work Other |
12 (27.2%) 19 (43.2%) 13 (29.6%) |
7 (31.8%) 9 (40.9%) 7 (27.2%) |
4 (19.0%) 10 (47.6%) 7 (33.3%) |
Chi-square = 0.93, df=2, p=.63 |
|
Marital status [n (%)] Married Other |
4 (9.1%) 40 (90.8%) |
4 (18.2%) 18 (81.8%) |
0 (0.0%) 21 (100.0%) |
Fisher’s exact, p=.11 |
|
Living situation [n (%)] Lives alone Only adult in household Lives with other adults Homeless |
22 (50.0%) 3 (6.8%) 16 (36.4%) 3 (6.8%) |
14 (63.6%) 2 (9.1%) 5 (22.7%) 1 (4.5%) |
8 (38.1%) 1 (4.8%) 10 (47.6%) 2 (9.5%) |
N/A** |
|
Income level [n (%)] Less than $25,000 $25,000-$50,000 More than $50,000 |
42 (95.5%) 2 (4.5%) 0 (0.0%) |
21 (95.5%) 1 (4.5%) 0 (0.0%) |
20 (95.2%) 1 (4.8%) 0 (0.0%) |
Fisher’s exact, p=1.00 |
|
Seizure type [n (%)]++ Generalized Partial Unclassified Other Don’t know/Not sure |
30 (68.2%) 11 (25.0%) 3 (6.8%) 4 (9.1%) 5 (11.4%) |
16 (72.7%) 5 (22.7%) 1 (4.5%) 1 (4.5%) 1 (4.5%) |
14 (66.7%) 6 (28.6%) 2 (9.5%) 3 (14.3%) 4 (19.0%) |
N/A** |
|
Mental health diagnosis [n (%)] Depression Bipolar Disorder Schizophrenia |
13 (29.5%) 16 (36.4%) 15 (34.1%) |
7 (31.8%) 7 (31.8%) 8 (36.4%) |
6 (28.6%) 8 (38.1%) 7 (33.3%) |
Chi-square = 0.19, df = 2, p= .91 |
|
Duration of mental disorder in
years (Mean, SD) |
(26.14, 15.76) | (30.27, 17.35) | (22.85, 12.33) | t=1.58,df=40, p=.12 |
|
Duration of epilepsy in years
(Mean, SD) |
(26.27, 15.74) | (27.55, 16.48) | (25.43, 15.47) | t=0.43,df=41, p=.67 |
| CCI (Mean, SD) | (2.45, 2.44) | (3.14, 2.47) | (1.86, 2.29) | t=1.76,df=41, p=.09 |
| REALM-R (Mean, SD) | (5.77, 2.69) | (5.18, 2.77) | (6.29, 2.57) | t=−1.35,df=41, p=.18 |
| MADRS (Mean, SD) | (19.69, 11.17) | (16.75, 10.28) | (22.94, 11.81) | t=−1.73,df=36, p=.09 |
| PHQ-9 (Mean, SD) | (10.83, 5.62) | (9.70, 5.55) | (11.76, 5.72) | t=−1.17,df=39, p=.25 |
| BPRS (Mean, SD) | (37.43, 10.87) | (36.90, 11.78) | (37.85, 10.39) | t=−0.27,df=39, p=.79 |
| GAF (Mean, SD) | (56.68, 15.41) | (59.23, 17.06) | (54.57, 13.66) | t=0.99,df=41, p=.33 |
| WHODAS-II (Mean, SD) | (81.80, 20.83) | (80.86, 18.76) | (82.83, 23.39) | t=−0.30,df=38, p=.31 |
|
Seizure Frequency- Past 30 days
(Mean, SD) |
(5.73, 23.00) | (3.67, 14.08) | (8.79, 32.01) | t=−0.61,df=30, p=.55 |
| QOLIE-10 (Mean, SD) | (3.09, 1.06) | (2.85, 0.82) | (3.34, 1.25) | t=−1.52,df=41, p=.14 |
| PSQI (Mean, SD) | (11.63, 4.55) | (10.55, 4.49) | (12.95, 4.38) | t=−1.71,df=38, p=.87 |
CCI = Charlson Comorbidity Index. Scores from 0–24 with higher scores indicating more medical comorbidity.
REALM-R. Scores from 0–8 with scores ≤6 indicating risk for poor health literacy.
MADRS = Montgomery and Asberg Depression Rating Scale. Scores from 0–60 with higher scores indicating more severe depressive symptoms.
PHQ-9 = Patient Health Questionnaire. Scores from 0–27 with higher scores indicating more severe depressive symptoms.
BPRS = Brief Psychiatric Rating Scale. Scores from 18–126 with higher scores indicating worse symptoms.
GAF = Global Assessment of Functioning. Scores from 0–100 with higher scores indicating better functioning.
WHODAS-II = World Health Organization Disability Assessment Schedule II. Scores from 32–180 with higher scores indicating greater
degree of disability.
QOLIE-10= 10-item Quality of Life in Epilepsy. Scores from 1–5 with lower scores indicating the least amount of problems.
PSQI = Pittsburgh Sleep Quality Index. Scores from 0–21 with higher scores indicating worse sleep quality.
statistic not calculated due to high number of categories with small sample sizes
self-reported seizure type
GEE analysis adjusted for gender, significant treatment by time interaction effect (p=0.036).
TIME 16-week MADRS Mean = 11.21, SD = 9.36
TAU 16- week MADRS: Mean = 21.81, SD = 10.50
3.3. TIME vs. TAU
3.3.1. Adverse events, attendance and study retention:
As noted in Figure 1, there were 9/44 (20%) individuals (3 in TIME and 6 in TAU) who dropped out prematurely. Of those terminating prematurely, one cited privacy concerns and another cited illness as reasons to withdraw. Seven were lost to follow-up. Serious adverse events (SAEs) occurred in 7/44 (15%), and included worsening seizures, worsening heart condition and irritable bowel syndrome, asthma, hypotension, and attempted suicide. No adverse events were related to study participation.
For individuals in TIME, average group session participation was 90% (including make-up sessions). While in-person attendance was highly encouraged, telephone participation was permitted when in-person attendance was impossible. There was only 1 individual assigned to TIME who did not attend a single session. Six individuals attended 11/12 sessions and 8 individuals attended all 12 sessions. With respect to study retention, there were 36 individuals (18 in TIME and 18 in TAU) able to be assessed at 12-weeks and 35 individuals (19 in TIME and 16 in TAU) able to be assessed at 16-weeks.
3.3.2. Primary outcome:
The GEE analysis conducted for our primary, a priori outcome of change in MADRS at 16 weeks showed a significant treatment-by-time interaction effect (p=0.036). Sample treatment-by-time MADRS means are illustrated in Figure 2, and show decreasing TIME MADRS scores, and no change in TAU. For the missing data analysis, among the covariates considered, neither treatment group, age, gender nor educational level was statistically significant. For an estimated MADRS effect size, considering the mean differences from baseline to 4 months in MADRS between treatment groups, divided by respective standard deviation of this difference between means, yielded a moderate/large effect size of 0.70. There were no MADRS differences between individuals with schizophrenia, bipolar disorder and depression.
Figure 2:
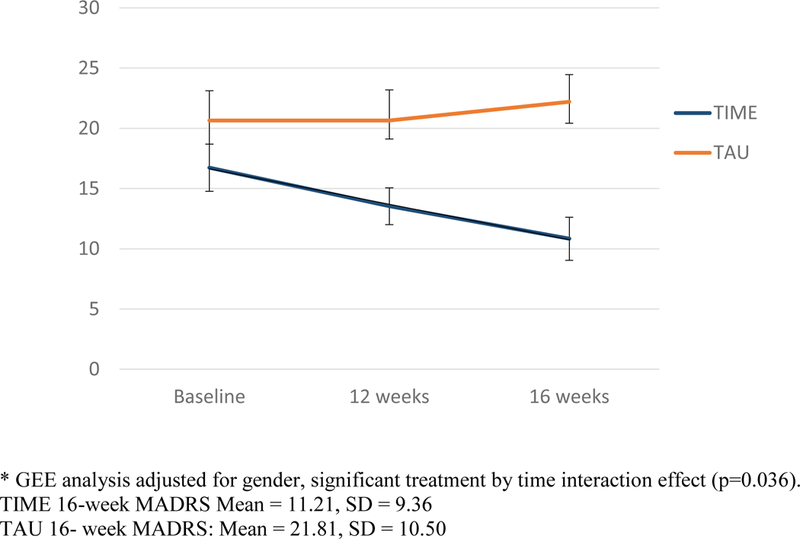
Change in Montgomery Asberg Depression Rating Scale (MADRS) over time among individuals with epilepsy and comorbid mental illness receiving TIME vs. Treatment as usual (TAU)*
3.3.3. Secondary outcomes
Table 3 shows change in TIME vs. TAU on secondary measures. Although group means suggested greater numerical improvement for TIME vs. TAU on most secondary measures, none except for PHQ-9, a self-reported measure of depressive severity, were statistically significant. Seizure frequency interpretations were limited by a generally low frequency of seizures. When categories for seizure count were compared by treatment arm (above or below a cut-off of 1 seizure in the last 30 days), there was a trend at 12-weeks towards a smaller percentage of TIME subjects being below the cutoff (≤1). For TIME, it was 88.9% vs. TAU 60% (two-sided Fishers exact test p-value of 0.10). At 16 weeks, the trend is not apparent, with percentages below the cutoff (≤1) being 76.5% for TIME and 68.8% for TAU.
Table 3:
Secondary outcome group mean values for TIME vs. TAU participants over 12 and 16 weeks follow-up
| Variable | TIME | TAU | Statistic |
|---|---|---|---|
|
PHQ-9 (Mean, SD) n Baseline 12-week 16-week |
(9.70, 5.55) 20 (9.24, 4.97) 17 (6.38, 4.44) 16 |
(11.76, 5.72) 21 (13.19, 5.67) 16 (12.87, 6.63) 15 |
p= .128 p= .015 |
|
BPRS (Mean, SD) n Baseline 12-week 16-week |
(36.90, 11.78) 21 (33.89, 9.67) 18 (29.59, 11.36) 17 |
(37.85, 10.39) 20 (35.81, 11.24) 16 (35.73, 9.63) 15 |
p= .775 p= .433 |
|
GAF (Mean, SD) n Baseline 12-week 16-week |
(59.23, 17.06) 22 (62.83, 15.98) 18 (69.50, 18.27) 18 |
(54.57, 13.66) 21 (59.00, 16.78) 17 (59.94, 18.38) 16 |
p= .936 p= .600 |
|
WHODAS-II (Mean, SD) n Baseline 12-week 16-week |
(80.86, 18.76) 21 (67.68, 13.29) 17 (73.39, 24.94) 18 |
(82.83, 23.39) 19 (79.87, 24.44) 15 (81.50, 21.34) 14 |
p= .042 p= .204 |
|
Seizure Frequency – Past 30 days (Mean, SD) n Baseline 12-week 16-week |
(3.67, 14.08) 18 (1.50, 2.68) 18 (1.00, 1.97) 17 |
(8.79, 32.01) 14 (18.40, 42.36) 15 (6.44, 15.21) 16 |
p= .560 p= .305 |
|
QOLIE-10 (Mean, SD) n Baseline 12-week 16-week |
(2.85, 0.82) 22 (2.38, 0.60) 18 (2.56, 0.89) 18 |
(3.34, 0.25) 21 (3.03, 0.79) 17 (2.84, 0.73) 15 |
p= .129 p= .978 |
|
PSQI (Mean, SD) n Baseline 12-week 16-week |
(10.55, 4.49) 20 (8.88, 3.30) 16 (6.85, 3.80) 13 |
(12.95, 4.38) 20 (12.21, 2.64) 14 (11.45, 3.27) 11 |
p= .759 p= .471 |
PHQ-9 = Patient Health Questionnaire. Scores from 0–27 with higher scores indicating more severe depressive symptoms.
BPRS = Brief Psychiatric Rating Scale. Scores from 18–126 with higher scores indicating worse symptoms.
GAF = Global Assessment of Functioning. Scores from 0–100 with higher scores indicating better functioning.
WHODAS-II = World Health Organization Disability Assessment Schedule II. Scores from 32–180 with higher scores indicating greater degree of disability.
QOLIE-10= 10-item Quality of Life in Epilepsy. Scores from 1–5 with lower scores indicating the least amount of problems.
PSQI = Pittsburgh Sleep Quality Index. Scores from 0–21with higher scores indicating worse sleep quality.
NOTE: p-values correspond to two-sided two sample t-tests, comparing respective 12-week and 16-week difference values from baseline between TIME and TAU
3.4. Qualitative analysis results:
3.4.1. Acceptability survey:
At TIME group completion, 13 participants responded to the acceptability survey. All (100%, N= 13) strongly agreed or agreed that TIME was useful. All (100%, N= 13) strongly agreed or agreed that TIME covered most of the important issues while 91.7% (N=11/12) strongly agreed or agreed that TIME addressed issues important to their particular situation. A majority (83.3%, N=10/12) strongly agreed or agreed that the benefit of TIME exceeded the burden or hassle of attending. Additionally, 53.8% (N=7) felt that the number of sessions was about right, while 84.6% (N=11) felt that the length of each TIME session was about right.
3.4.2. Qualitative interviews:
A total of 11 transcripts (8 participants and 3 interventionists) were coded. Table 4 presents themes and illustrative quotations from patients and TIME interventionists. In addition to the generally positive comments, feedback was focused on the importance of Peer Educators and the benefits of sharing and hearing the perspectives of others. Participants felt that the TIME program gave them a set of useful tools and knowledge. Table 5 lists thematic categories of described mechanisms through which the TIME program was felt to have been effective. The TIME program provided a safe environment in which participants could support each other. Participants did not simply describe adversity; they shared, learned, and practiced strategies for overcoming problems specific to care of epilepsy and of MI. Of particular value was the sequential process through which participants attempted to implement newly learned skills and shared their ongoing successes or challenges. Group encouragement for continuing to stick with attempts at change facilitated momentum for TIME participants to pursue healthier lives.
Table 4.
Participant and Interventionist In-Depth Interview Comments about the Intervention (N=11)
| Thematic Category (n with responses coded) |
Illustrative Quotations |
|---|---|
| Positive Evaluation of the Intervention (11) | |
| General Positive Comments |
100: That was one of the best educational experiences that I’ve ever had |
| Value of Peer Educators |
032: I felt real comfortable, that you had somebody there that been through it but it was like, he was one of us. It makes people feel comfortable coming to class. This is the peer educator, he’s been through this class. |
| PE1: It’s pretty much you have-you have two people running the session. You have book smarts, and you have street smarts. You got book smarts who’s the nurse educator that knows what epilepsy is, know what mental illness is. But you have street smarts, that’s the peer educator that knows what it feels like to have a seizure. Knows what it feels like to have mental illness. | |
| NE:It was really eye-opening to hear their stories. | |
| Shared Learning/Struggle |
031: It was like experiencing life with my issues through someone else’s eyes…what I learned is a lot more valuable. Can’t really put a price tag on it. |
| 016: Understanding the people around me, understand what they was goin’ through, how they was able to cope with it like I was able to cope with it. | |
| PE1: Other people’s stories penetrate you and it’s inspiring…the input that people give – it’s fireworks every session. | |
|
Constructive Suggestions (4) |
002: they need to get more material… and give it to the ones that was in the group, they can keep on studying it [after the study]. |
| 100: I think that we shoulda’ talked a little bit more about the side effects, instead of just what medicines are you on. | |
| NE: we could barely cover everything in the time…there were several that probably could’ve been combined with another one, so maybe we could have done it in fewer than twelve. | |
Table 5.
TIME Mechanisms of Action Described by Participants and Interventionists (N=11)
| Thematic Category (N= participants with responses coded) |
Illustrative Quotations |
|---|---|
|
Illness Knowledge (7) |
032: .there’s a whole learning process once people say disease, I’m thinking contagious right off the bat. Straight out the door. I learned through this program that it’s not contagious. You can’t get it from shaking hands. |
| PE1: No one puts two and two together because it’s…two totally opposite worlds. You don’t even know that your epilepsy affects your mental illness and your mental illness affects your epilepsy | |
|
Physician Communication (10) |
016: I tell the doctor to talk English to me ..lot of doctors talk that journal talk …tell me straight forward, what’s wrong, what I need, what I have to do |
| 021: you gotta’ print it out ‘cause once I leave this office, I’m done. I will forget what you said soon as I leave this room. I’m not gonna’ remember when I get home that you lowered my insulin. it’s no joke when I go to take my insulin. Oh, I better get this on paper. | |
|
Sharing Experiences of Stigma (7) |
NE:….the stigma or lack of acceptance. From family members. Or friends, or whoever….people talked a lot about how they lost a job because of it. |
| 002: I don’t like when people make fun of everybody that has epilepsy…. when I was diagnosed with it, my parents, told me they didn’t want no epilepsy person in the house. And that made me like an outcast. | |
| 025: Sometimes I’m treated differently at work. Sometimes I feel like they treat me differently than everybody else. | |
| Self-Efficacy (7) | 021: I’m very confident now, with this study, and seeing how I’m not the only one… I see the effects. |
| 031: it just gives me a feeling of self-worth and that I am accomplishing something. I think I’m doing pretty well, I would say on a scale one to a hundred percent, I would say, I’m about eighty percent taking care of everything. | |
|
Positive Mental Health Strategies (4) |
032: S: Now I can just think my way out of it. Breathe. Slow my breathing down, slow my heart rate down, slow my thinking down…look at my situation and my surrounding. And take my fear away. I learned how to deal with my mental illness in a more positive way. |
4. Discussion
The results of this RCT, suggest that Targeted Self-Management for Epilepsy and Mental Illness (TIME) is feasible to deliver, acceptable to people with epilepsy and significant psychiatric comorbidity, and can improve depressive symptoms. As Kanner and Meador have recently emphasized,[43] the psychological consequences of epilepsy and high rates of psychiatric comorbidity deserve further consideration given their substantial impact on people with epilepsy. People with epilepsy are socioeconomically disadvantaged compared to the general population, and a recent U.S. national survey found that nearly one in five people with epilepsy lives alone [44]. Self-management may be particularly critical for those with fewer social supports.
People with E-MI are at elevated risk for epilepsy-related complications such as emergency room visits and hospitalizations [45]. Our TIME study sample may reflect the additional negative effects of psychiatric comorbidity on epilepsy with high rates of unemployment, poverty and social isolation. Half of the individuals in this sample lived alone and another 7% were homeless. We are not aware of any other evidence-based interventions that specifically focus on individuals with epilepsy and comorbid serious psychiatric conditions such as schizophrenia, bipolar disorder or severe or recurrent major depressive disorder. While this pilot RCT had a number of limitations, findings are promising and have potential to fill a current gap in care for a vulnerable and hard-to-reach sub-group with epilepsy.
Individuals with epilepsy and comorbid serious mental illnesses such as schizophrenia, bipolar disorder and depression might be expected to have severe or particularly difficult-to- manage depression. Baseline average PHQ-9 score in the TIME study was 10.8 (SD 5.6), which is above the established PHQ-9 cut-off of 10 for moderate or severe depression. A MEW Network study by Fraser and colleagues,[46] which established the evidence-based PACES intervention, excluded individuals with active serious mental illness. In PACES, the baseline mean PHQ-9 was 8.4 (SD 6.1). In the multi-site UPLIFT Prevention trial, mean baseline PHQ-9 scores were 6.7 in UPLIFT participants and 5.8 in TAU participants, although depression severity in the single-site UPLIFT trial were in the high-mild to severe range. In the TIME study, in spite of moderate to severe depression, E-MI participants were readily able to engage in the program and moreover, could serve as Peer Educators to help deliver the intervention. The study attrition rate of 20% is only slightly higher than the recently published MEW Network PACES trial which excluded people with epilepsy complicated by severe mental illness.[46]
With the exception of self-reported depression severity, our RCT did not find statistically significant differences in other secondary outcomes. It is possible that small sample size and short study duration precluded ability to observe significant measurable differences. Individuals in our study sample had, on average, lived with E-MI for over 2–3 decades. It might require more than 16 weeks for change to occur among individuals with very long-standing chronic conditions. In contrast, our qualitative data suggested that TIME participants gained confidence in the efforts to live healthier, knowledge of their co-morbid illnesses and skill in communicating with their clinicians. (See Table 4) TIME participants and interventionists perceived increased health knowledge and being able to implement behavior change as key elements leading to improved outcomes.
This pilot study had a number of limitations including small sample size and a single-site setting which may not represent the larger population with E-MI. People with E-MI who agree to participate in a clinical trial may be more help-seeking than individuals with E-MI more broadly and our study excluded individuals too psychiatrically ill to participate in groups. Additional limitations included the fact that raters were not blind to randomization assignment, we did not collect information on psychotropic medication changes or adherence and epilepsy diagnosis was not verified with EEG evidence. However, the TIME study also had some key strengths including patient-centered input in its development, rigorous psychiatric diagnostic assessment, use of a detailed intervention manual, and outcome assessments consistent with other clinical trials involving people with serious mental illness.
A key challenge to disseminating the TIME approach more widely is the in-person delivery format. In addition to an emphasis on addressing mental health comorbidity, the MEW Network has also promoted the use of technology-facilitated interventions. Remotely-delivered interventions may be optimal for people with epilepsy who do not drive and are dependent on public transportation. To enhance future generalizability, these investigators have adapted the TIME intervention to be delivered remotely via the internet or telephone and are currently testing the approach in a larger-scale trial.
In conclusion, TIME is a self-management approach that can reduce depression in people with epilepsy and long-standing serious mental illness. Given the disproportionate burden associated with epilepsy that is complicated by serious mental illness, additional research is needed to better identify how TIME and related programs might be implemented in routine clinical settings.
Highlights:
Serious mental illness is disproportionately common in people with epilepsy and contributes to complications and mortality
Few care approaches specifically target individuals with comorbid epilepsy and severe mental illness.
This randomized controlled trial tested Targeted Self-Management for Epilepsy and Mental Illness (TIME) vs treatment as usual for epilepsy and mental illness
The TIME approach engages individuals in self-management and can reduce depression in people with epilepsy and mental illness.
Acknowledgment
This publication was supported by the Grant or Cooperative Agreement Number U48DP001930 (SIP12–057) under the Health Promotion and Disease Prevention Research Centers Program, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Author Disclosures
Dr. Sajatovic has received research grants from Pfizer, Merck, Ortho-McNeil Janssen, Reuter Foundation, Woodruff Foundation, Reinberger Foundation, National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC). Dr. Sajatovic has been a consultant to Bracket, Prophase, Otsuka, Sunovion, Pfizer, and Amgen. Dr. Sajatovic has received royalties from Springer Press, Johns Hopkins University Press, Oxford Press, UpToDate, Lexicomp and compensation for CME activities from American Physician’s Institute, MCM Education, and CMEology.
Abbreviations:
- TIME
Targeted Self-Management for Epilepsy and Mental Illness
- TAU
Treatment as usual
- E-MI
Epilepsy and Serious Mental Illness
Contributor Information
Martha SAJATOVIC, Department of Psychiatry and of Neurology, Case Western Reserve University School of Medicine, Neurological and Behavioral Outcomes Center, University Hospitals Case Medical Center, Cleveland, OH, USA.
Curtis TATSUOKA, Department of Neurology, Case Western Reserve University School of Medicine, Neurological and Behavioral Outcomes Center, University Hospitals Case Medical Center, Cleveland, OH, USA.
Elisabeth WELTER, Case Western Reserve University School of Medicine and Neurological and Behavioral Outcomes Center, University Hospitals Case Medical Center, Cleveland, OH, USA.
Adam T. PERZYNSKI, Center for Health Care Research and Policy. Case Western Reserve University, MetroHealth Medical Center, Cleveland, OH, USA.
Kari COLON- ZIMMERMANN, Case Western Reserve University School of Medicine and Neurological and Behavioral Outcomes Center, University Hospitals Case Medical Center, Cleveland, OH, USA.
James R. VAN DOREN, Case Western Reserve University School of Medicine and Neurological and Behavioral Outcomes Center, University Hospitals Case Medical Center, Cleveland, OH, USA.
Ashley BUKACH, Case Western Reserve University School of Medicine and Neurological and Behavioral Outcomes Center, University Hospitals Case Medical Center, Cleveland, OH, USA.
Mary Ellen LAWLESS, Center for Health Care Research and Policy, Case Western Reserve University and MetroHealth Medical Center, Cleveland, OH, USA.
Eleanor R. RYAN, Neurological and Behavioral Outcomes Center, University Hospitals Case Medical Center, Cleveland, OH, USA.
Katherine STURNIOLO, Case Western Reserve University Department of Psychology, Case Western Reserve University School of Medicine and Neurological and Behavioral Outcomes Center, University Hospitals Case Medical Center, Cleveland, OH, USA.
Samden LHATOO, Department of Neurology, Case Western Reserve University School of Medicine, Neurological and Behavioral Outcomes Center, University Hospitals Case Medical Center, Cleveland, OH, USA.
REFERENCES
- [1].Centers for Disease Control and Prevention. Epilepsy Fast Facts [updated 2016. February 2) Available from: http://www.cdc.gov/epilepsy/basics/fast_facts.htm
- [2].Vuilleumier P, Jallon P. [Epilepsy and psychiatric disorders: epidemiological data]. Rev Neurol (Paris) 1998;154: 305–17. [PubMed] [Google Scholar]
- [3].Barry JJ, Lembke A, Gisbert P, Gilliam F. Affective disorders in epilepsy Philadelphia, PA: Lippincott Williams & Williams; 2006. [Google Scholar]
- [4].Tellez-Zenteno JF, Patten SB, Jette N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia 2007;48: 2336–44. [DOI] [PubMed] [Google Scholar]
- [5].Ettinger A, Reed M, Cramer J. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology 2004;63: 1008–14. [DOI] [PubMed] [Google Scholar]
- [6].Kobau R, DiIorio CA, Price PH, Thurman DJ, Martin LM, Ridings DL, Henry TR. Prevalence of epilepsy and health status of adults with epilepsy in Georgia and Tennessee: Behavioral Risk Factor Surveillance System, 2002. Epilepsy Behav 2004;5: 358–66. [DOI] [PubMed] [Google Scholar]
- [7].Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia 1999;40 Suppl 10: S2–20. [DOI] [PubMed] [Google Scholar]
- [8].Ettinger AB, Reed ML, Goldberg JF, Hirschfeld RM. Prevalence of bipolar symptoms in epilepsy vs other chronic health disorders. Neurology 2005;65: 535–40. [DOI] [PubMed] [Google Scholar]
- [9].Rafnsson V, Olafsson E, Hauser WA, Gudmundsson G. Cause-specific mortality in adults with unprovoked seizures. A population-based incidence cohort study. Neuroepidemiology 2001;20: 232–6. [DOI] [PubMed] [Google Scholar]
- [10].Wiegartz P, Seidenberg M, Woodard A, Gidal B, Hermann B. Co-morbid psychiatric disorder in chronic epilepsy: recognition and etiology of depression. Neurology 1999;53: S3–8. [PubMed] [Google Scholar]
- [11].Faulkner G, Cohn TA. Pharmacologic and nonpharmacologic strategies for weight gain and metabolic disturbance in patients treated with antipsychotic medications. Can J Psychiatry 2006;51: 502–11. [DOI] [PubMed] [Google Scholar]
- [12].Dilorio C, Henry M. Self-management in persons with epilepsy. J Neurosci Nurs 1995;27: 338–43. [DOI] [PubMed] [Google Scholar]
- [13].DiIorio CK, Bamps YA, Edwards AL, Escoffery C, Thompson NJ, Begley CE, Shegog R, Clark NM, Selwa L, Stoll SC, Fraser RT, Ciechanowski P, Johnson EK, Kobau R, Price PH. The prevention research centers’ managing epilepsy well network. Epilepsy Behav 2010;19: 218–24. [DOI] [PubMed] [Google Scholar]
- [14].Thompson NJ, Walker ER, Obolensky N, Winning A, Barmon C, DiIorio C, Compton MT. Distance delivery of mindfulness-based cognitive therapy for depression: Project UPLIFT. Epilepsy & Behavior 2010;19: 247–254. [DOI] [PubMed] [Google Scholar]
- [15].Thompson NJ, Patel AH, Selwa LM, Stoll SC, Begley CE, Johnson EK, Fraser RT. Expanding the efficacy of Project UPLIFT: Distance delivery of mindfulness-based depression prevention to people with epilepsy. J Consult Clin Psychol 2015;83: 304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ciechanowski P, Chaytor N, Miller J, Fraser R, Russo J, Unutzer J, Gilliam F. PEARLS depression treatment for individuals with epilepsy: a randomized controlled trial. Epilepsy Behav 2010;19: 225–31. [DOI] [PubMed] [Google Scholar]
- [17].Sajatovic M, Dawson NV, Perzynski AT, Blixen CE, Bialko CS, McKibbin CL, Bauer MS, Seeholzer EL, Kaiser D, Fuentes-Casiano E. Best practices: Optimizing care for people with serious mental illness and comorbid diabetes. Psychiatr Serv 2011;62: 1001–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sajatovic M, Gunzler D, Kanuch S, Cassidy K, Tatsuoka C, McCormick R, Blixen C, Perzynski A, Einstadter D, Thomas C, Lawless M, Martin S, Falck-Ytter C, Seeholzer E, Dawson N. A 60-week Prospective Randomized Controlled Trial of Targeted Training in Illness Management vs. Treatment as Usual in Individuals with Serious Mental Illness and Diabetes Mellitus. . In: 169th APA Annual Meeting Atlanta, GA; 2016. [Google Scholar]
- [19].Bandura A Self-regulation of motivation and action through goal systems . Dordrecht: Kluwer Academic Publishers; 1988. [Google Scholar]
- [20].Patterson TL, McKibbin C, Taylor M, Goldman S, Davila-Fraga W, Bucardo J, Jeste DV. Functional adaptation skills training (FAST): a pilot psychosocial intervention study in middle-aged and older patients with chronic psychotic disorders. Am J Geriatr Psychiatry 2003;11: 17–23. [PubMed] [Google Scholar]
- [21].DiIorio C, Bamps Y, Walker ER, Escoffery C. Results of a research study evaluating WebEase, an online epilepsy self-management program. Epilepsy Behav 2011;22: 469–74. [DOI] [PubMed] [Google Scholar]
- [22].Fraser MW, Richman J, MJ G, SH D. Intervention Research. Developing Social Programs New York, NY: Oxford University Press; 2009. [Google Scholar]
- [23].Sheehan DV, Lecubier Y, Sheehan KH, Amorim P, Janaus J, Weiller E, Hergueta T, Baker R, Dunbar GC. Development and Validation of a Structured Diagnosis Psychiatric Interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 1998;59: 22–23. [PubMed] [Google Scholar]
- [24].Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care 2005;43: 607–15. [DOI] [PubMed] [Google Scholar]
- [25].Wallace L Patients’ health literacy skills: the missing demographic variable in primary care research. Ann Fam Med 2006;4: 85–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134: 382–9. [DOI] [PubMed] [Google Scholar]
- [27].Mazza M, Martini A, Scoppetta M, Mazza S. Effect of levetiracetam on depression and anxiety in adult epileptic patients. Prog Neuropsychopharmacol Biol Psychiatry 2008;32: 539–543. [DOI] [PubMed] [Google Scholar]
- [28].Grabowska-Grzyb A, Jedrzejczak J, Naganska E, Fiszer U. Risk factors for depression in patients with epilepsy. Epilepsy Behav 2006;8: 411–7. [DOI] [PubMed] [Google Scholar]
- [29].Lopez-Gomez M, Espinola M, Ramirez-Bermudez J, Martinez-Juarez IE, Sosa AL. Clinical presentation of anxiety among patients with epilepsy. Neuropsychiatr Dis Treat 2008;4: 1235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hufnagel A, Ben-Menachem E, Gabbai AA, Falcao A, Almeida L, Soares-da-Silva P. Long-term safety and efficacy of eslicarbazepine acetate as adjunctive therapy in the treatment of partial-onset seizures in adults with epilepsy: results of a 1-year open-label extension study. Epilepsy Res 2013;103: 262–9. [DOI] [PubMed] [Google Scholar]
- [31].Garcia ME, Garcia-Morales I, Gil-Nagel A. Prevalence of depressive symptoms and their impact on quality of life in patients with drug-resistant focal epilepsy (IMDYVA study). Epilepsy Res 2015;110: 157–65. [DOI] [PubMed] [Google Scholar]
- [32].Overall JA, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10: 799–812. [Google Scholar]
- [33].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16: 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry 1995;166: 654–9. [DOI] [PubMed] [Google Scholar]
- [35].Ustun TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, Saxena S, von Korff M, Pull C. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ 2010;88: 815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cramer JA, Perrine K, Devinsky O, Meador K. A brief questionnaire to screen for quality of life in epilepsy: the QOLIE-10. Epilepsia 1996;37: 577–82. [DOI] [PubMed] [Google Scholar]
- [37].Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 1998;45: 5–13. [DOI] [PubMed] [Google Scholar]
- [38].Managing Epilepsy Well Network [updated 2015. December 2]. Available from: http://web1.sph.emory.edu/ManagingEpilepsyWell/documents/instruments/Epilepsy%20Self-Efficacy%20Scale%20and%20Description.pdf.
- [39].Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess 1990;55: 610–7. [DOI] [PubMed] [Google Scholar]
- [40].Ritsher JB, Otilingam PG, Grajales M. Internalized stigma of mental illness: psychometric properties of a new measure. Psychiatry Res 2003;121: 31–49. [DOI] [PubMed] [Google Scholar]
- [41].Managing Epilepsy Well Network [updated 2015. December 2]. Available from: http://web1.sph.emory.edu/ManagingEpilepsyWell/documents/instruments/Epilepsy%20Stigma%20Scale%20and%20Description.pdf
- [42].Polkinghorne DE. Phenomenological research methods New York, NY: Plenum Press; 1989. [Google Scholar]
- [43].Kanner AM, Meador KJ. Remember…there is more to epilepsy than seizures! Neurology 2015;85: 1094–5. [DOI] [PubMed] [Google Scholar]
- [44].Kobau R, Epil UCDCP. Nearly one in five adults with active epilepsy lives alone based on findings from the 2010 and 2013 US National Health Interview Surveys. US Centers for Disease Control and Prevention, Epilepsy Program Epilepsy & Behavior; 2015;51: 259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sajatovic M, Welter E, Tatsuoka C, Perzynski AT, Einstadter D. Electronic medical record analysis of emergency room visits and hospitalizations in individuals with epilepsy and mental illness comorbidity. Epilepsy Behav 2015;50: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fraser RT, Johnson EK, Lashley S, Barber J, Chaytor N, Miller JW, Ciechanowski P, Temkin N, Caylor L. PACES in epilepsy: Results of a self-management randomized controlled trial. Epilepsia 2015;56: 1264–74. [DOI] [PubMed] [Google Scholar]


