Figure 4.
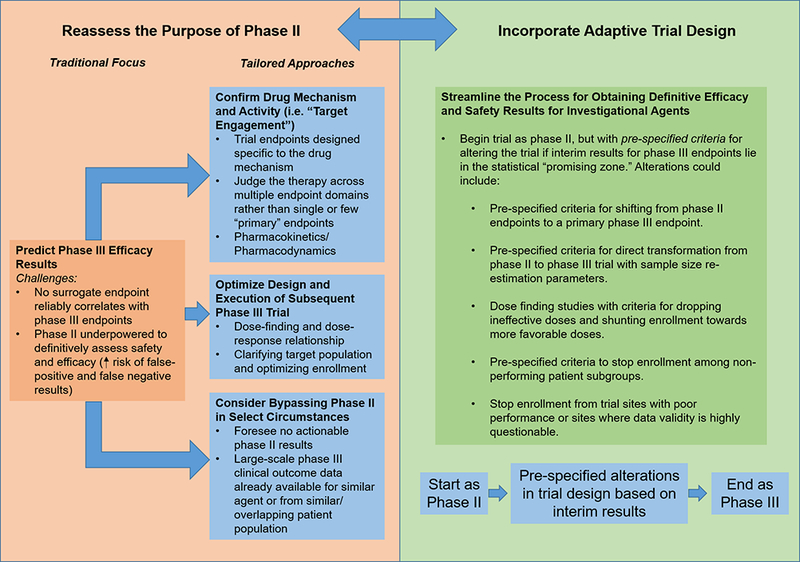
Potential framework for re-appraising the future design, purpose, and execution of phase II studies in HF drug development. Recognizing that there are no reliable surrogate outcomes for phase III approval endpoints, we propose that the central goal of phase II should not be to predict phase III results but to optimize phase III execution and to clarify the drug mechanism and drug-patient interaction. Adaptive trial design may allow for a more efficient drug development process and may more directly allow information gained from phase II to shape the phase III trial.
