Abstract
Objective:
Prospective memory (PM) is described as the capacity to form and maintain an intention that is executed in response to a specific cue. Neural injury and associated neurocognitive disorders are common among persons living with HIV disease, who might therefore be susceptible to impairment in PM.
Method:
This literature review utilized a structured qualitative approach to summarize and evaluate our current understanding of PM functioning in people living with HIV disease. 33 studies of PM in HIV+ persons met criteria for inclusion.
Results:
Findings showed that HIV is associated with moderate deficits in PM, which appear to be largely independent of commonly observed comorbid factors. The pattern of PM deficits reveals dysregulation of strategic processes that is consistent with the frontal systems pathology and associated executive dysfunction that characterizes HIV-associated neural injury. The literature also suggests that HIV-associated PM deficits present a strong risk of concurrent problems in a wide range of health behaviors (e.g., medication non-adherence) and activities of daily living (e.g., employment). Early attempts to improve PM in HIV disease have revealed that supporting strategic processes might be effective for some individuals.
Conclusions:
HIV-associated PM deficits are common and exert a significant adverse effect on the daily lives and health of infected persons. Much work remains to be done to understand the cognitive architecture of HIV-associated PM deficits and the most efficient means to enhance PM functioning and improve health outcomes in persons living with HIV.
Keywords: AIDS, HIV, memory for intentions, prospective memory
Introduction
Prospective memory (PM) is a form of episodic memory that entails the capacity to form and maintain an intention that is ultimately executed in response to a specific cue, such as the occurrence of an event or a specific time. In simpler terms, PM is defined as our ability to “remember to remember.” Everyday examples of PM range from common household tasks, such as remembering to switch off the lights upon leaving a room, to vitally important health-related tasks, such as remembering to take a prescribed medication after a meal or attend a healthcare appointment at 2pm. This review focuses on PM functioning in persons living with HIV disease, including: (1) the frequency, severity, and cognitive architecture of HIV-associated PM deficits; (2) HIV disease correlates of PM deficits; (3) the impact of common clinicodemographic comorbidities (e.g., older age and depression) on the expression of HIV-associated PM deficits; (4) the unique contribution of PM deficits to everyday functioning in HIV; and (5) ways to enhance PM functioning in HIV. To orient the reader, we begin with a brief overview of the central nervous system complications of HIV infection and summarize our theoretical framework for the review, which adopts the Multiprocess Model (McDaniel & Einstein, 2000)
NeuroAIDS and HIV-associated Neurocognitive Disorders (HAND)
HIV-1 enters the central nervous system quickly, sometimes within days of infection (Davis et al., 1992). While the virus does not directly infect neurons, it can nevertheless cause neural and glial injury by both direct (i.e., viral proteins, such as gp120 and tat) and indirect (e.g., inflammatory and vascular) mechanisms (Saylor et al., 2016). The adverse effects of HIV are apparent throughout the neocortex and various white matter tracts, but neuropathological changes are most commonly observed in frontostriatal circuits (Ellis et al., 2007). HIV-associated neurocognitive disorders are evident in approximately one-third to one-half of persons infected with HIV, with the characteristic pattern of impairment reflecting a preferential disruption of prefrontostriatal circuits (Heaton et al., 2014; Reger et al., 2002). Deficits in motor coordination, information processing speed, working memory, and executive functions are frequently observed in people with HIV (e.g., Heaton et al., 2010), whereas deficits in neurocognitive functions associated with the posterior neocortex, such as visuoperception, are less frequently seen (e.g., Reger et al., 2002).
Episodic retrospective memory impairment is quite common among HIV+ persons, with most prevalence estimates ranging between 40 and 60% (e.g., Rippeth et al. 2004). The etiology of the retrospective memory impairment in HIV is likely multifactorial, primarily reflecting the adverse effects of the virus on frontostriatal circuits (e.g., Ellis et al., 2007), as well as mediotemporal structures (e.g., Maki et al., 2009). The profile of HIV-associated retrospective memory deficits is characterized as a mixed encoding and retrieval problem; that is, diminished free recall and limited use of higher-order organizational strategies in the setting of relatively spared retention and recognition (e.g., Delis et al., 1995; Gongvatana et al., 2009; Woods et al., 2006). Although there is heterogeneity in the expression of HIV-associated retrospective memory deficits (e.g., Murji et al., 2003; Obermeit et al., 2015), most research portrays its expression as one of dysfunction in the strategic (i.e., executive) aspects of encoding and retrieval. Of clinical relevance, HIV-associated retrospective memory deficits are reliably associated with poorer everyday functioning outcomes, including medication non-adherence (for a review see Blackstone et al., 2013). Drawing from this literature, our laboratory has taken a theoretical approach to understanding the nature, extent, functional impact, and remediability of PM deficits in HIV disease.
Multiprocess Model of Prospective Memory
There are a few viable conceptual models of PM in the literature (for a review see Kliegel et al., 2008b), all of which tend to agree on some cognitive processes involved in successful execution of a future intention, which include: (1) forming an intention, (2) associating the intention with a retrieval cue (e.g., a certain time or the occurrence of a specific event), (3) retaining this association over a delay interval during which PM cue monitoring may occur when one is engaged in an ongoing task, (4) detecting the PM retrieval cue, (5) disengaging from the ongoing task and retrieving the appropriate intention from retrospective memory, and (6) executing the planned PM action (for a review see Kliegel et al., 2008b). The Multiprocess Model (McDaniel & Einstein, 2000) of PM was chosen to guide this critical review since it is among the most influential theories across the literature, and in HIV specifically. In brief, the Multiprocess Model suggests that PM is a complex, multidimensional cognitive faculty with varying degrees of automatic and strategic processes. The strategic component of PM is guided by a resource-demanding executive system served by frontostriatal circuits whereby individuals voluntarily and effortfully engage in prospective remembering. The automatic component of PM is a more spontaneous process served by mediotemporal and posterior regions that explains why sometimes an intended action simply ‘pops into mind’ on its own (Einstein & McDaniel, 1990) or reflexively when encountering a strong cue (Ellis & Nimmo-Smith, 1993). The neuroimaging studies with nonclinical population have consistently demonstrated the involvement of rostral prefrontal cortex in PM (e.g., Burgess et al., 2001), although there is also evidence for the broader role of the frontoparietal network (e.g., Cona et al., 2015), medial temporal cortex (e.g., Gordon et al., 2011), and insula (Cona et al., 2015).
One of the most widely investigated aspects of PM is whether the retrieval cue is time-based or event-based. That is, PM cues may take the form of an event, such as remembering to buy milk when driving by a grocery store (i.e., event-based), or the passage of time, such as taking a medication at 8pm (i.e., time-based). Whereas successful event-based PM performance is believed to be relatively automatic because cue detection is more reflexive given the salience of environmental cues, time-based PM is hypothesized to be more strategic because it is dependent on cognitive control processes involved in time monitoring. In other words, time-based tasks tend to require greater voluntary, strategic monitoring processes that are self-initiated than do event-based tasks (McDaniel & Einstein, 2000). It is important to recognize however, that there is considerable variability in the automatic versus strategic demands of event-based tasks. For example, highly salient event-based cues (e.g., a loud alarm) rely on more automatic processes and result in high PM performance whereas less salient cues (e.g., a subtle, non-focal visual cue) require more strategic monitoring processes and result in lower PM success (Einstein et al., 2000; McDaniel & Einstein, 2000).
Viewed through the Multiprocess framework, one therefore might predict that: 1) persons living with HIV disease would evidence greater impairment the strategic as compared to the automatic aspects of PM; 2) such strategic PM deficits would confer an increased risk of poorer everyday functioning outcomes; and 3) supporting strategic processing would improve PM performance in HIV disease. Below we review the available published evidence that has examined these hypotheses.
Methods
Due to the relatively circumscribed number of studies on the topic of PM in HIV disease, we were able to include all the published papers and take a hybrid approach to reviewing and summarizing the literature. Specifically, we: 1) conducted a structured qualitative review; 2) report effect sizes when possible and relevant; and 3) provide PM data from a large, well-characterized HIV+ cohort. It was our view that this hybrid approach to reviewing the literature provided a more rigorous, comprehensive, and informative view on the topic than would be provided by any individual approach alone.
Study Identification
To identify articles for inclusion in this review, we adhered to PRISMA guidelines (Liberati, 2009). Specifically, we reviewed PubMed/ MEDLINE, Web of Science, and SCOPUS. Each published database was searched separately across the years 2000 to 2016 using the following key words: prospective memory, memory for intentions, HIV, and AIDS. To be included, an article must have used a self-report or performance-based measure of prospective memory in human participants with verified HIV seropositivity. Full text was retrieved for any articles with inclusion potential. Upon inspection of the content, we included all of the published studies in this review. This approach minimizes our risk of bias. We also reviewed each article identified for additional published articles that met inclusion criteria. The search strategy generated a total of 33 published articles on the topic of PM in HIV disease (see Table 1).
Table 1.
Study details and key findings from manuscripts included in the systematic review
| Authors | Title | Sample(s) | PM Task(s) | Key finding |
|---|---|---|---|---|
| Carey et al. (2006) | Prospective memory in HIV-1 infection |
42HIV+ 29HIV- |
MIST, MIST 24-hr | The HIV+ participants demonstrated deficits in time- and event-based PM, as well as more frequent 24-hr delay PM failures. |
| Woods et al. (2006) | Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 |
35 HIV+ | MIST | Higher levels of MCP-1 in plasma, and soluble receptor for tumor necrosis factor type II and tau in cerebrospinal fluid were associated with lower PM but not with retrospective memory. |
| Martin et al. (2007) | Characteristics of prospective memory deficits in HIV- seropositive substance- dependent individuals: Preliminary observations |
31 HIV+ 35 HIV- (all substance- Dependent individuals) |
Habitual event- and time- based tasks with the clinical neuropsychological battery as the ongoing task |
HIV+ participants showed deficits in time- but not event- based PM. Time-based PM performance was a significant predictor of risky sexual practices in entire sample. |
| Woods et al. (2007) | Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV |
75 HIV + 60 HIV- |
MIST, PRMQ | Self reported PM symptoms were elevated in HIV+ persons, but did not correspond to actual PM abilities. |
| Woods et al. (2008a) |
HIV-associated prospective memory impairment increases risk of dependence in everyday functioning |
66 HIV+ | MIST, PRMQ | HIV-associated PM impairment and elevated PM symptoms were each independently associated with greater dependence in instrumental activities of daily living. |
| Woods et al. (2008b) |
Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? |
87 HIV+ on ART | MIST, MIST 24-hr, PRMQ |
PM was associated with self- reported medication management, independent of other cognitive functions, psychiatric, psychosocial, and environmental factors. |
|
Woods et al. (2009) |
Timing is everything: Antiretroviral non-adherence is associated with impairment in time-based prospective memory |
79 HIV+ on ART | MIST, MIST 24-hr, PRMQ |
Individuals classified as non- adherent to ARTs using electronic medication monitoring had significantly lower PM performance, particularly on time-based PM, but did not differ on PRMQ or MIST 24-hr. |
| Contardo et al. (2009) | Relationship of prospective memory to neuropsychological function and antiretroviral adherence |
97 HIV+ on ART (all substance-dependent individuals) |
MIST | PM loaded separately from retrospective memory and executive functions and was the only correlate of ART adherence. |
| Gupta et al. (2010) | Is prospective memory a dissociable cognitive function in HIV infection? |
162 HIV+ | MIST, AAIM | PM loaded on a unique factor, apart from tests of retrospective memory, executive functions, and motor skills. |
| Woods et al. (2010) | The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection |
35 HIV+ (≤ 40 yrs) 48 HIV+ (≥ 50 yrs) 20 HIV- (≤ 40 yrs) 15 HIV- (≥ 50 yrs) |
MIST, AAIM | Significant additive effects of aging and HIV on event-based PM, especially when PM cue was not semantically related to intention. |
| Zogg et al. (2010) | HIV-associated prospective memory impairment in the laboratory predicts failures on a semi-naturalistic measure of health care compliance |
139 HIV+ | MIST, MIST 24-hr, PRMQ |
MIST, but not PRMQ, was a significant predictor of MIST 24-hr after controlling for demographics, retrospective memory and executive functions, and psychiatric factors. |
| Iudicello et al. (2011) | Misremembering future intentions in methamphetamine dependent individuals |
20 HIV + 19 HIV- (all Methamphetamine- dependent individuals) |
MIST, MIST 24-hr | No effect of HIV on time- or event- based PM in persons with methamphetamine dependence. |
| Weber et al. (2011) | An examination of the age- prospective memory paradox in HIV-infected adults |
53 HIV+ (≤40 yrs) 88 HIV+ (≥ 50 yrs) 59 HIV- (≤40 yrs) 54 HIV- (≥ 50 yrs) |
MIST time-based scale, MIST 24-hr, PRMQ |
Older HIV+ adults performed worse than their younger HIV+ counterparts on laboratory tasks of PM but performed comparably on a naturalistic PM task. |
| Woods et al. (2011) | Prospective memory deficits are associated with unemployment in persons living with HIV infection |
59 HIV+ Unemployed 49 HIV+ Employed |
MIST, MIST 24-hr, PRMQ |
MIST, but not PRMQ or MIST 24- hr, was independently associated with employment status. |
| Zogg et al. (2011) | Are time- and event-based prospective memory comparably affected in HIV infection? |
143 HIV+ 43 HIV- |
MIST | HIV+ individuals demonstrated deficits in time-based PM compared to HIV- adults, as well as a trend- level difference on event-based PM, which was driven by the participants with HAND. |
| Doyle et al. (2012) | Aging, prospective memory, and health-related quality of life in HIV infection |
41 HIV+ (≤40 yrs) 72 HIV+ (≥50 yrs) |
MIST, MIST 24-hr, PRMQ |
Self-reported PM was an independent predictor of health- related QoL in the overall sample; however, time-based PM was only associated with health-related QoL in the younger group. |
| Hoare et al. (2012) | A diffusion tensor imaging and neuropsychological study of prospective memory impairment in South African HIV positive individuals |
128 HIV+ (40 DTI) 32 HIV- (10 DTI) |
MIST, PRMQ | PM associated with fractional anisotropy values in all regions. HIV effect for time-, but not event-based, PM. |
| Morgan et al. (2012) | Longer ongoing task delay interval exacerbate prospective memory deficits in HIV- associated Neurocognitive Disorders (HAND) |
49 HAND 78 HIV- |
MIST, PRMQ | HAND group performed worse on long-delay time-based PM tasks and reported more long-delay PM symptoms but there was no group effect on short-delay PM performance or symptoms. |
| Poquette et al. (2013) | Prospective memory and antiretroviral medication non- adherence in HIV: An analysis of ongoing task delay length using the memory for intentions screening test |
74 HIV+ on ART | MIST | Poor long-delay time-based PM was uniquely predictive of poor ART adherence as measured by electronic monitoring. |
| Blackstone et al. (2013) | Memory-Based Strategies for Antiretroviral Medication Management: An Evaluation of Clinical Predictors, Adherence Behavior Awareness, and Effectiveness |
223 HIV+ on ART | MIST |
Poor event-based PM performance was associated more frequent use of ART adherence strategies. |
| Doyle et al. (2013) | Prospective memory in HIV- associated Neurocognitive Disorders (HAND): The neuropsychological dynamics of time monitoring |
55 HAND 108 HIV- |
MIST, MIST 24-hr | HAND group demonstrated poorer strategic time monitoring, which was related to lower time-based PM. |
| Casaletto et al. (2014) | Self-predictions of prospective memory in HIV-Associated Neurocognitive Disorders: Evidence of a metamemory deficit |
72 HAND+ 131 HAND- 150 HIV- |
MIST, PRMQ | HAND+ group reported more everyday PM symptoms but were inaccurately overconfident in their PM task predictions compared to HAND- and HIV-. PM overconfidence was associated with executive dysfunction and ART nonadherence. |
| Coulehan et al. (2014) | The role of decision-making ability in HIV/AIDS: impact on prospective memory |
89 HIV+ | MIST, MIST 24-hr | Risky decision-making performance predicted PM independent of executive functions. |
| Loft et al. (2014) | Allowing brief delays in responding improves event- based prospective memory for young adults living with HIV disease |
57 HIV+ young adults | Ongoing lexical decision-making task with an embedded focal and non-focal task with varying onset PM response time. |
Delaying the execution of PM responses increased PM performance accuracy, particularly for non-focal targets. |
| Woods et al. (2014) | Task importance affects event- based prospective memory performance in adults with HIV-associated neurocognitive disorders and HIV-infected young adults with problematic substance use |
Study 1: 15 HAND+, 35 HAND-, 31 HIV- Study 2: 58 HIV+ youth (33 with SUD) |
Ongoing lexical decision-making task with a non- focal event-based PM paradigm. |
Emphasizing PM task importance vs. the ongoing task normalized PM accuracy in HAND and SUD. |
| Doyle et al. (2015a) | Time estimation and production in HIV-associated neurocognitive disorders (HAND) |
53 HAND+ 120 HAND- 113 HIV− |
MIST | Time estimation and production correlated with time-, but not event- based PM in HIV |
| Doyle et al. (2015b) | Habitual prospective memory in HIV disease |
36 HAND+ 70 HAND- 115 HIV– |
Computerized habitual time-based PM task with an ongoing cognitive and emotional Stroop task. |
HAND+ individuals had elevated PM repetition errors. Worse habitual PM was associated with poorer learning and dependence in real- world outcomes, including ART nonadherence. |
| Sheppard et al. (2015) | Does older age confer an increased risk of incident neurocognitive disorders among persons living with HIV disease? |
56 HIV+ (≥ 50 yrs) | MIST | PM performance at baseline was associated with incident neurocognitive disorder at one-year follow-up. |
| Avci et al. (2016) | The effects of HIV disease and older age on laboratory-based, naturalistic, and self-perceived symptoms of prospective memory: Does retrieval cue type and delay interval matter? |
48 HIV+ (≤40 yrs) 112 HIV+(≥ 50 yrs) 77 HIV- (≤40 yrs) 77 HIV- (≥ 50 yrs) |
MIST, MIST 24-hr, PRMQ |
Older age and HIV had independent effects on long-delay time-based PM in the laboratory, whereas on a naturalistic PM task older HIV− adults performed better than older HIV+ adults and younger persons. Older age, but not HIV, was associated with a relative sparing of self-perceived PM failures in daily life across longer delay self-cued intervals. |
| Sheppard et al. (2016) | Pill burden influences the association between time-based prospective memory and antiretroviral therapy adherence in younger but not older HIV-infected adults |
117 HIV+(≥ 50 yrs) 82 HIV+ (≤40 yrs) |
MIST | In the older group, better time-based PM performance was associated with higher likelihood of ART adherence, irrespective of pill burden. Within the younger sample, time-based PM was positively related to adherence only in participants with lower pill burden. |
| Sirois et al. (2016) | Associations of memory and executive functioning with academic and adaptive functioning among youth with perinatal HIV exposure and/or infection |
258 HIV+ youth | PROMACY | PM was uniquely predictive of verbal and mathematical academic achievement. |
| Faytell et al. (in press-a) | Visualisation of future task performance improves naturalistic prospective memory for some younger adults living with HIV disease |
60 HIV+ youth | MIST | Visualization at encoding improved naturalistic event-based PM for HIV+ youth with low time-based PM and normal learning. |
| Faytell et al. (in press-b) | Calendaring and alarms can improve naturalistic time-based prospective memory for youth infected with HIV |
47 HIV+ youth | MIST | Calendaring and alarms improved naturalistic time-based PM performance, which was not associated with the MIST. |
Note. AAIM = Abbreviated Assessment of Intentional Memory; ART = Antiretroviral Therapy; DTI = Diffusion Tensor Imaging; HAND = HIV-associated Neurocognitive Disorders; MCP-1 = Monocyte Chemoattractant Protein-1; MIST = Memory for Intentions Screening Test; MIST 24-hr = 24-hour delay trial of Memory for Intentions Screening Test; PRMQ = Prospective and Retrospective Memory Questionnaire; PROMACY = Prospective Memory Assessment for Children and Youth; PM = Prospective Memory; QoL = quality of life; SUD = Substance Use Disorder.
Effect Size Extraction
Although this review primarily adopts a structured, qualitative method to summarizing the extant literature, we also made an effort to adhere to long-standing recommendations in neuropsychology to consider effect sizes to supplement the findings of null hypothesis significance testing (Bezeau & Graves, 2001). Thus, we extracted, report, and summarize effect size data wherever possible throughout this review to supplement our simple tallying of “significant” findings per standard null hypothesis testing (i.e., p values). If a given study did not include an effect size estimate, we calculated effect sizes by drawing the relevant data (e.g., M and SD) from the paper or by contacting the study authors. For summary purposes, all effect sizes were converted to Cohen’s d values using standard methods. Note that this approach was not intended to serve the same function as a rigorous meta-analysis; rather we strove to incorporate effect sizes in a systematic way in an effort to better understand and summarize the magnitude of the null hypothesis significance testing findings across this literature.
Cohort Data
Finally, we also drew from a cohort study of 427 HIV+ persons and 203 HIV- participants with PM measures in an effort to provide empirical data to illustrate specific points in this review and/or address controversial issues (e.g., HIV disease severity) that may have been underpowered across the individual studies reviewed. All participants in this cohort provided informed, written consent and were assessed under IRB-approved protocols at the UCSD HIV Neurobehavioral Research Program in between 2005 and 2013. Participants with histories of serious neurological (e.g., seizure disorders, stroke) or psychiatric (e.g., psychosis) conditions, estimated verbal IQ scores < 70, and active substance use disorders were excluded. All participants completed the research version (Woods, Moran, Dawson et al., 2008) of the Memory for Intentions Screening Test (Raskin et al., 2010) as part of a comprehensive neurocognitive and medical evaluation (see Woods et al., 2009 for details). Basic descriptive data on this cohort is provided in Table 2.
Table 2.
Participant demographic and disease characteristics
| Total Sample (N = 630) |
HIV+ (n = 427) |
HIV- (n = 203) |
p | Group differences |
|
|---|---|---|---|---|---|
| Age (years) | 42.5 (13.9) | 42.3 (13.4) | 42.9 (15) | - | - |
| Education (years) | 13.6 (2.6) | 13.3 (2.5) | 14.2 (2.7) | <.0001 | H+ < HIV- |
| Ethnicity/Race | |||||
| African-American | 26% | 27.6% | 22.7% | - | - |
| Asian | 1.6% | 1.4% | 2% | - | - |
| Hispanic | 18.1% | 18.3% | 17.7% | - | - |
| White | 53.3% | 52% | 56.2% | - | - |
| Gender (% men) | 79.5% | 86.2% | 65.5% | < .0001 | H+ >HIV- |
| Major depression | 49.6% | 57.8% | 34.7% | < .0001 | H+ >HIV- |
| Generalized anxiety | 10.6% | 14% | 4.5% | .0002 | H+ >HIV- |
| Substance dependence | 49.3% | 54.5% | 39.9% | .0008 | H+ >HIV- |
| Hepatitis C Virus | - | 17.9% | 9.5% | .005 | H+ >HIV- |
| Estimated duration of Infection (months) |
- | 140.3 (100.2) | - | - | - |
| AIDS (%) | - | 50.9% | - | - | - |
| CD4 count (cells/mL) | - | 566.6 (284.9) | - | - | - |
| Nadir CD4 (cells/mL) | - | 231.1 (190.6) | - | - | - |
| cART status (% rx) | - | 84.1% | - | - | - |
| Plasma RNA (detectable) | - | 30.5% | - | - | - |
| On cART | - | 19.8% | - | - | - |
Note: AIDS = Acquired immune deficiency syndrome; CD4 = Cluster of differentiation 4; cART = combination antiretroviral therapy; Major depression, generalized anxiety, and substance dependence include lifetime diagnoses.
Measurement of PM
The laboratory-based PM measures in the studies included in this review always included an ongoing task and prospective tasks. As the participant worked on the ongoing task, s/he was required to remember to execute certain behavior (i.e., PM) as a response to a cue (i.e., event-based) or after the passage of time (i.e., time-based). The majority of the studies reviewed utilized the research version (Woods, Moran, Dawson et al., 2008) of the Memory for Intentions Screening Test (MIST; Raskin et al., 2010) to study PM in HIV. The MIST is a standardized measure where participants are asked to perform eight different PM tasks over a 30-min period, during which time they are engaged in a word-search puzzle (i.e., ongoing task). The eight PM tasks are balanced in terms of delay interval (i.e., 2-min or 15-min delay), cue (i.e., time-based or event-based cue), and response modality (i.e., verbal or a physical response). Incorrect responses are coded in terms of errors of omission (e.g., lack of response) and commission (e.g., task substitution errors).
The MIST also has a naturalistic PM component (i.e., MIST 24-hour task) that was used by several studies reviewed in this paper. Participants are instructed to leave a telephone message for the researcher the following day specifying the number of hours slept the night after the assessment. The use of mnemonic strategies such as electronic calendars is permitted on this naturalistic component, but is not explicitly encouraged.
Finally, the Prospective and Retrospective Memory Questionnaire (PRMQ; Smith et al., 2000) is another commonly used measure of PM, which appeared in several studies reviewed here. The PRMQ consists of 16 questions about everyday memory errors including eight PM complaints, which are separated into further subscales: four short-term items, half of which are self-cued (e.g., “Do you decide to do something in a few minutes’ time and then forget to do it?”) and half environmentally cued (e.g., “Do you intend to take something with you before leaving a room or going out, but minutes later leave it behind, even though it’s there in front of you?”). There also are four long-term PM items, half of which are self-cued (e.g., “Do you forget appointments if you are not prompted by someone else or by a reminder such as a calendar or diary?”) and the other half of which are environmentally cued (e.g., “Do you forget to buy something you planned to buy, like a birthday card, even when you see the shop?”). Participants are asked to rate how often each type of memory failure has occurred on a 5-point scale.
Cognitive Neuropsychology of PM in HIV
Studies consistently show that HIV is associated with mild-to-moderate deficits in PM, including elevations in self-reported PM symptoms (e.g., Woods et al., 2007), diminished PM performance in the laboratory (e.g., Carey et al., 2006), and PM failures in daily life (e.g., Avci et al., 2016; Carey et al., 2006). A total of 12 studies examined group differences on PM performance by serostatus, with all 12 reporting significant effects. The average effect size was .47 (d ranged between −.35 and .96). Importantly, across these studies, the observed PM deficits in HIV disease are not better explained by demographics (e.g., age, education), various medical and psychiatric comorbidities (e.g., depression), or general cognitive impairment (e.g., Avci et al., 2016). As detailed below, some of these clinicodemographic factors can modulate the expression of HIV-associated PM deficits, but they do not appear to be interpretive confounds per se.
Consistent with the predictions of the Multiprocess theory, HIV-associated PM deficits are primarily by strategic rather than automatic processes. We tend to observe larger deficits on time-based PM tasks as compared to event-based tasks (Carey et al., 2006; Martin et al., 2007; Morgan, Weber et al., 2011; Zogg et al., 2010). This observation is supported by the examination of effect sizes reported by studies investigating time- and event-based PM in HIV+ and HIV- persons (K = 12; HIV+ N = 787, HIV- N = 763). The average effect size for time-based PM was .61 and for event-based was .37 (Figure 1). Even when we restricted to sample of studies to those that included both time- and event-based PM (K = 6; HIV+ N = 484, HIV- N = 443), as opposed to just one or the other, the effect size differences persisted (d = .63 for time-based PM and d = .31 for event-based PM). There are several possible reasons why time-based PM may be more sensitive to HAND than event-based PM, including psychometric factors such as differential task difficulty (see Chapman & Chapman, 1973). For example, ceiling effects are not uncommon on event-based PM tasks involving more automatic processes, especially in healthy adults (see Raskin et al., 2011). Another potential explanation is that by disrupting frontostriatal systems, HIV is more likely to affect strategic PM processes, which are more closely linked to time- versus event-based PM. This more theoretical explanation of the pattern of findings is explored in detailed below.
Figure 1.
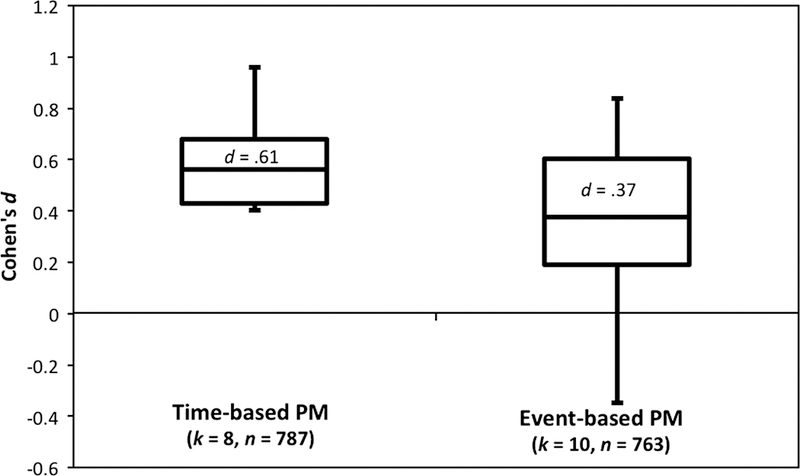
Box-and-whisker plot of effect sizes (Cohen’s d) for time-event based prospective memory (PM) in HIV+ versus HIV- samples. k = number of effect sizes, N = total sample size.
HIV-associated deficits on time-based PM appear to be driven by failures in strategic monitoring, as supported by findings of increased omission errors in the setting of normal performance on the ongoing task and post-test recognition trials. Further evidence concerning the strategic resource demands of HIV-associated deficits in time-based PM was reported by Doyle et al. (2013) who demonstrated that individuals with HAND checked the clock (i.e., time monitoring) significantly less often than the comparison group during a time-based PM task. Subsequent analyses revealed that the frequency of clocking checking was positively related to overall time-based (but not event-based) PM performance, as well as executive functions (i.e., verbal fluency). It was also reported that time perception was associated with the time-based PM but not event-based PM in another study of HIV disease (Doyle et al., 2015b). The third line of evidence supporting a primary strategic time-based PM deficit in HIV is provided by studies on the delay effect: namely the notion from the Multiprocess theory that longer delays place greater demands on limited executive resources supporting cue detection and monitoring (McDaniel & Einstein, 2000). In 2012, Morgan, Weber et al. reported that persons with HAND performed more poorly than seronegatives on longer (i.e., 15-min) but not shorter (i.e., 2-min) PM delay tasks, particularly for time-based cues. In this study, long-delay time-based PM was associated with executive functions (e.g., cognitive flexibility and planning), thus further supporting the strategic hypothesis of HIV-associated PM deficits.
It is also important to recognize that event-based PM deficits can also be observed in HIV disease and, when present, tend to reflect impairment in both strategic and automatic processes. As reviewed above, while time-based tasks are almost invariably strategically demanding, event-based PM tasks can vary in their strategic versus automatic processing demands. Although event-based PM deficits are evident (Carey et al., 2006), especially in persons with HAND (Zogg et al., 2010), these deficits tend to show up as intrusion errors (e.g., responding to the PM cue with the incorrect intent content) and correlate more strongly with retrospective memory (Zogg et al., 2010). However, Woods et al. (2014) and Loft et al. (2014) suggest that event-based PM deficits are a function of poor allocation of attention during monitoring. Moreover, Woods et al. (2010) showed that event-based PM deficits are exacerbated by semantically unrelated cue-intention pairings, again suggesting that strategic processes are also affected.
Although these findings add support to the growing body of research elucidating strategic monitoring processes in the event-based PM functioning of HIV+ adults, fewer studies have specifically looked at the role of highly automatic processes. Examining HIV effects using classic paradigms from the experimental literature, such as focal (i.e., spontaneous/automatic) versus non-focal cues (Kliegel et al., 2008a) informed by the Multiprocess theory, ongoing task load as informed by the multinomial model (Smith & Bayen, 2006), contextual factors (e.g., emotional salience), or PM deactivation/aftereffects (e.g., Walser et al., 2016) may be useful in elucidating underlying mechanisms.
Disease Factors and PM in HIV
HIV disease (e.g., nadir CD4 counts) and treatment (e.g., the CNS penetrance of combination antiretroviral therapy [cART]) factors can moderate the expression of HIV-associated neurocognitive disorders (Ellis et al., 2007). However, our review of the PM literature in HIV suggests that clinical disease severity does not play a strong role in the nature or extent of HIV-associated PM deficits. Indeed, such associations, if present, are usually derived from simple univariate analyses that do not consider confounds and tend to be both quite small and elusive. Specifically, we identified a total of 31 analyses in 9 studies with 827 HIV+ participants that examined the relationship between clinical HIV disease variables and PM. Effect sizes were not reliably available across these analyses, which were exclusively secondary or tertiary subanalyses. With regard to HIV treatment factors, five studies have reported null associations between cART status and PM (e.g., e.g., Doyle et al., 2015a; Martin et al., 2007; Weber et al., 2011; Woods et al., 2010; Zogg et al., 2010), which was consistent with analyses from our own cohort (see Figure 2). Of course, such findings are quite limited in the cART era given that most participants in research are prescribed therapy. We did not identify any prospective studies of the benefits of cART initiation on PM nor the possible association between CNS penetrance of cART regimens (e.g., Letendre et al., 2008) and PM in HIV. Similarly, none of the five studies that have evaluated the association between plasma or CSF viral load have reported significant associations, which aligns with findings from our cohort (see Figure 2). Investigations of the relationship of current CD4 cell counts to PM revealed a similar null pattern that parallels our cohort findings (see Figure 2), with only 1 of the 7 analyses in extant literature reporting a significant association.
Figure 2.
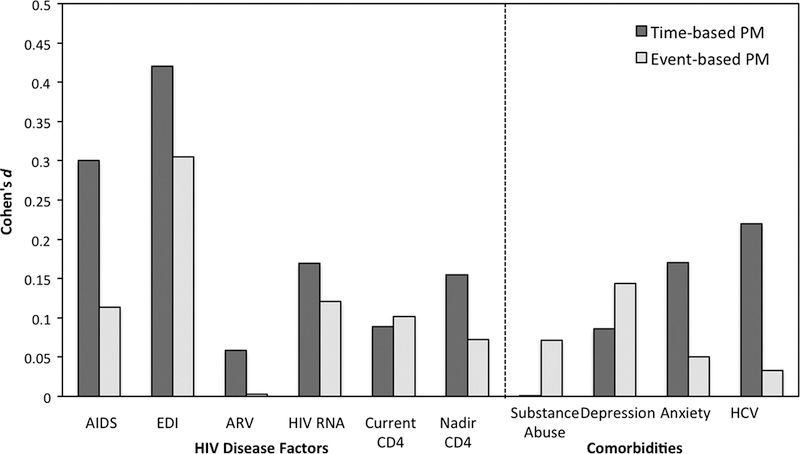
Effect size estimates (Cohen’s d) of the association between prospective memory (PM) and HIV disease characteristics and common comorbidities (N = 416). AIDS = Acquired Immune Deficiency Syndrome; EDI = Estimated Duration of Infection; CD4 = Cluster of Differentiation 4; ARV = Antiretroviral therapy; HCV = Hepatitis C Virus.
With regard to historical disease indicators (viz., infection duration, nadir CD4 count, and AIDS status), we see slightly more mixed, but still largely null findings reported across the literature. Specifically, only 3 of three out of 10 analyses showed significant associations between PM and AIDS status or nadir CD4 count, while zero of four studies found relationships between PM and estimated duration of infection. In our cohort, we see moderate effect size associations between PM and longer duration of infection and having a historical AIDS diagnosis (see Figure 2); however, both of these associations are confounded by older age. That is, persons with longer durations of infection and AIDS diagnoses are older, which – as noted below in this review – is a major contributor to HIV-associated PM deficits. In fact, when infection duration, AIDS, and age were entered simultaneously as predictors of PM in our cohort, only age emerges as an independent contributor (p < .05).
Only two studies to date have directly examined potential neurobiological underpinnings of PM impairment in HIV using more sophisticated biomarkers rather than standard clinical indicators. In 2006, Woods and colleagues reported that poorer PM performance was associated with higher levels of monocyte chemoattractant protein-1 (MCP-1) in plasma (d = 1.1), and soluble receptor for tumor necrosis factor type II (d = 1.5) and tau in cerebrospinal fluid (d = 1.3), which in turn were not associated with retrospective memory scores (mean d = 0.2). PM was not associated with immunological variables, viral RNA, astrocytosis, or growth factors (mean d = 0.3). Taken together, such findings were interpreted to suggest that PM impairment in HIV may reflect specific neuropathogenic mechanisms of macrophage activation and neuroaxonal injury. More recently, Hoare et al., (2012) investigated the relationship of white matter integrity to PM among 40 HIV+ individuals using diffusion tensor imaging (DTI). Results revealed that PM functioning was associated with decreased fractional anisotropy in the white matter tracts that relay through the anterior prefrontal cortical regions, including the genu of the corpus callosum, the cingulum and the superior corona radiata.
Taken together, these findings seem to suggesting that peripheral immunovirological factors may not play a primary role in the neuropathogenesis of HIV-associated PM deficits. Biomarkers of neuroaxonal integrity (e.g., tau, DTI) show stronger associations with PM in HIV-infected persons, but such findings should still be considered preliminary and await replication and extension with other methods, including volumetric MRI indices and functional MRI (task-based and resting state), in order to better understand the neural architecture of HIV-associated PM deficits. Such studies might include seronegative participants and/or other neuropsychological populations (e.g., traumatic brain injury) in order to determine whether the associations between neuroaxonal injury and PM are unique in HIV. In terms of neuroimaging, much work remains to be done in order to determine the brain structural and functional underpinnings of HIV-associated PM deficits, with studies needed to map the neuropathophysiology of performance-based PM impairments onto brain volumes, white matter pathways, metabolism, neurochemical abnormalities (e.g., spectroscopy), as well as functional studies to understand the relevant networks of neuronal activity associated with PM in HIV disease. Investigators might also examine the possible role of host (e.g., APOE) and viral genetics, proteomics, and other novel biomarker approaches in the expression of PM deficits in HIV.
Clinicodemographic Comorbidities and PM in HIV
Aging
Age is an important factor in understanding the effects of HIV on PM for two reasons: (1) The prevalence and incidence of older adults living with HIV is on the rise (Smit et al., 2015; Smith et al., 2010); and (2) Among seronegative populations, older age is strongly associated with declines in PM (Henry et al., 2004). The last two decades have witnessed significant advances in the effectiveness of cART, which has resulted in a tremendous rise in the number of older adults (aged ≥ 50) living with HIV disease (Smit et al., 2015). As HIV-infected individuals age, they are at increased risk for acquiring HIV-associated, Non-AIDS (HANA) conditions (e.g., cardiovascular disease), including HAND (Valcour et al., 2004). The downstream effects of HAND are particularly important for older HIV+ adults, who are more vulnerable to the adverse impact of HAND on retention in HIV care (Jacks et al., 2015) and everyday functioning (e.g., Morgan, Iudicello et al., 2012), including and medication management capacity (Thames et al., 2011). Importantly, lower baseline PM in the laboratory is strongly predictive of incident HAND at one-year follow-up among older HIV+ adults (d = .96; Sheppard et al., 2015). Independent of HIV, aging processes have significant adverse impacts on PM functioning (Henry et al., 2004). Consistent with MP theory, aging in the absence of HIV carries mild-to-moderate effects on PM tasks with high strategic demands (for review, see McDaniel & Einstein, 2011), such as time-based cues and semantically unrelated cue-intention pairings on event-based tasks (Henry et al., 2004). PM deficits are also uniquely predictive of poorer everyday functioning in older adults (e.g., Woods et al., 2011; 2012). Thus, there is overlap in the severity, profile, and functional impact of PM deficits in aging and HIV disease that raises questions regarding the possible additive or synergistic effects of these risk factors in combination.
Laboratory findings of PM and age in HIV
Previous investigations of PM among middle-aged HIV+ individuals show that older age is associated with poorer overall PM performance (Poquette et al., 2013) and time monitoring (i.e., clock checking) during PM tasks (Doyle et al., 2013). Three studies to date have shown independent effects of aging (cohen’s d values range .52-.86) and HIV (d range .40-.79) on PM, which appear to be more prominent among strategically demanding tasks (Avci et al., 2016; Weber et al., 2011; Woods et al., 2010). For example, Weber et al. (2011) reported evidence of additive effects of HIV (d = .40) and aging (d = .86) on time-based PM (see also Figure 3). Using an event-based PM paradigm, Woods et al. (2010) showed additive effects of HIV (d = .79) and aging (d = .52) on when the retrieval cue and intention were semantically unrelated (e.g., “When I show you a picture of a cow, snap your fingers.”), suggesting that older HIV+ adults may be particularly impaired on the strategic aspects of PM. Indeed, lower semantically unrelated event-based PM performance was associated with executive dysfunction (i.e., cognitive flexibility and planning; d = .67) in older HIV+ adults (Woods et al., 2010). A more recent study by Avci et al. (2016) manipulated PM cue type (event- vs. time-based) and delay interval (i.e., time between cue intention and execution of the PM action) to show that older age (d = .75) and HIV (d = .67) have additive adverse effects on strategically demanding PM (e.g., time-based PM, longer delay intervals). In each of these studies the independent effects of aging (d range .52-.86) and HIV (d range .40-.79) were independent from global neurocognitive functioning and HIV disease characteristics. While these three studies provide support for additive independent effects of both HIV and aging on PM—particularly for strategically demanding PM—there does not appear to be an interaction between HIV and aging on PM. In other words, as shown in Figure 3 using data from our cohort, while HIV infection and older age each appear to confer increased risk for poorer PM performances, there is no evidence showing a disproportionate risk for PM problems due to the combined effects of HIV and aging compared to that of HIV or older age infection alone.
Figure 3.
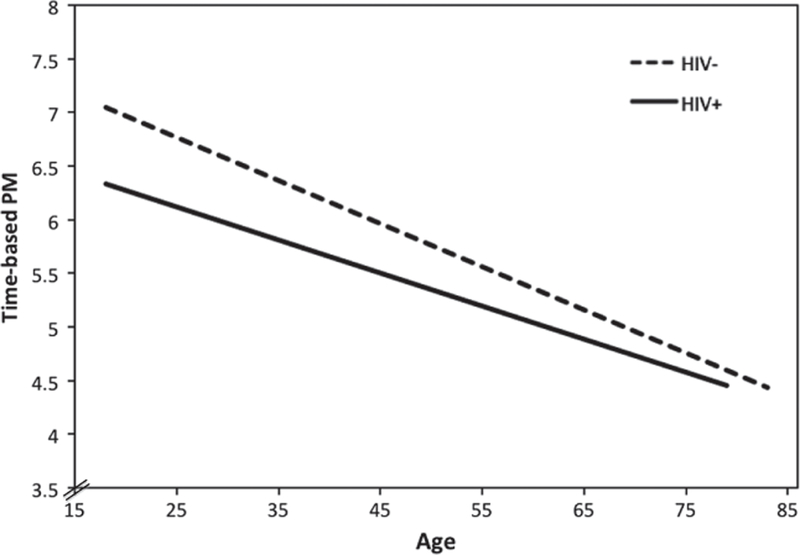
Relationship between age and strategically demanding time-based prospective memory (PM) in HIV+ (n = 427) and HIV- (n = 203) adults.
Naturalistic findings of PM and age in HIV
While studies in the laboratory provide insights into the mechanisms underlying PM performance in a well-controlled environment, there remain important external demands and modulating variables (e.g., compensatory strategies) that can affect how PM is expressed in the real world. A representative example of such a phenomena is the “age-PM” paradox first described in seronegative adults (Rendell & Thomson, 1999), wherein older adults perform more poorly on PM tasks in the laboratory but perform the better than their younger counterparts when it comes to PM tasks in the real world (e.g., medication adherence). However, in HIV disease it appears that there is no strong evidence for such an age advantage in naturalistic tasks. For example, older HIV+ adults often demonstrate largely comparable adherence to their cART regimes as compared to younger adults (e.g., Hinkin et al., 2004). Similarly, Weber et al. (2011) showed that older HIV+ adults performed comparably to their younger counterparts on a naturalistic PM task in which they were asked to call the examiner 24hrs after their study visit and report how many hours they slept the previous night (d = .08). In a more recent study using the same naturalistic paradigm, Avci et al. (2016), showed that older seronegative individuals performed better than older HIV+s and younger persons irrespective of HIV status (odds ratios range = 1.9 [1.1, 3.7] to 2.4 [1.3, 4.5]). There was a similar lack of an age effect on self-reported PM symptoms in daily life among the HIV+ persons in this study (d = .11). One possible explanation for lack of an aging benefit for naturalistic PM tasks vis-à-vis a clear age-related advantage in the laboratory in HIV is insufficient use of compensatory strategies. For example, Weber et al. (2011) showed that older, but not younger, HIV+ adults showed a relationship between naturalistic PM performance and self-reported use of PM-based (d = .77) and external compensatory strategies (e.g., using salient reminders; d = .64). Future studies should investigate the possible moderating and mediating effects of compensatory strategy use in older adults to better understand how the aging HIV+ population will perform on PM tasks in the laboratory and in the real world. We also know little about the effects of HIV on PM in children, which represents an important area for further investigation. In the one study to date, PM was uniquely predictive of verbal and mathematical academic achievement (Sirois et al., 2016).
Other Demographic Factors
We know little about the role of non-age-related demographic factors, such as sex, education, and ethnicity, on the expression of HIV-associated PM deficits. No prospective studies have expressly evaluated these potentially important questions and only a handful of studies examined here reported incidental associations (e.g., Zogg et al., 2010). Data from our HIV+ cohort suggest that these three factors are not strongly related to laboratory-based, self-reported, and naturalistic PM at the univariate level (ps > .05), with a few exceptions: 1) Higher education is weakly related to laboratory-based PM (d = .2); 2) Women tend to report more PM symptoms than men in daily life (d = .39); and 3) Caucasians are slightly more likely than non-Caucasians to complete the MIST 24hr naturalistic trial (d = .37; see also Zogg et al., 2010). It remains to be determined whether these other demographic factors mediate or moderate the expression of HIV-associated neurocognitive deficits (or their relationships with everyday functioning outcomes).
Psychiatric and Medical Comorbidities
Co-occurring conditions are common in HIV infection, which can take the form of long-standing risk factors for acquisition of the virus (e.g., mood disorders), shared risk factors for infectious disease (e.g., substance abuse, hepatitis C virus [HCV]), and possible consequences of chronic HIV disease itself (e.g., vascular disease). To fully understand PM functioning in HIV, it may be important to not only examine the neurocognitive and disease factors that contribute to performance decline, but to also explore relevant comorbidities which may influence PM abilities. Our review of the literature revealed 32 analyses in 10 studies with 958 HIV+ participants that examined the relationship of specific comorbidities (i.e., substance use, HCV, and neuropsychiatric symptoms) to PM in HIV.
Neuropsychiatric comorbidities
The lifetime prevalence estimates of neuropsychiatric comorbidities such as anxiety, apathy, fatigue and depression are as high as 50% among the HIV-infected population (e.g., Ciesla & Roberts, 2001). Findings are largely null with regard to the role of such neuropsychiatric comorbidities in the expression of HAND (e.g., Cysique et al., 2007), with a few larger studies reporting significant effects that are accompanied by small effect sizes (e.g., Castellon et al., 2006). To date, formal examination of these neuropsychiatric conditions in relation to PM in HIV has been similarly null in the literature and in our cohort (see Figure 2). For example, none of the nine analyses investigating the effects of comorbid anxiety (k = 7; Mean Cohen’s d = 0.35) or fatigue (k = 2; Mean Cohen’s d = −0.32) on laboratory-based PM function have reported significant associations per standard null hypothesis testing. Ten analyses have been published regarding the relationship of depression to PM, with only one reporting significant findings: Zogg et al., (2010) found that HIV+ individuals with greater levels of self-reported depression (but not those with diagnoses of major depressive disorder) were more likely to fail a semi-naturalistic PM task (d = −1.52). However, formal prospective studies are needed to more definitively answer these questions, using rigorous diagnostic methods for these complex neuropsychiatric syndromes and careful consideration of potentially confounding factors (e.g., syndrome overlap, comorbidities). Moreover, we do not yet know the influence of apathy on PM in HIV. Apathy is observed in approximately 40% of HIV-infected persons, is an independent predictor of poorer health and everyday functioning outcomes in HIV, and is associated with the integrity of the same frontal-subcortical networks (for a review see Lanctôt et al., 2016 and Kamat et al., 2012) that support PM. Not surprisingly, mood symptoms are positively related to self-reported PM symptoms in daily life in HIV (e.g., Avci et al., 2016; Woods et al., 2007). Thus, it is possible that apathy may influence the expression of PM complaints, performance deficits, and/or symptoms in daily life.
Substance Use Disorders (SUD)
The estimated lifetime prevalence rate of SUD in HIV disease is nearly 70% (Heaton et al., 2010) and as many as 20% of HIV-infected adults meet recent criteria for SUD (U.S.; Pence et al., 2006). SUDs confer additive risk of neurocognitive disorders (e.g., Rippeth et al., 2004) and adverse functional outcomes (e.g., Blackstone et al., 2013) in the setting of HIV disease. Moreover, there is growing evidence that SUD are independently associated with elevated PM symptoms and laboratory-based PM deficits (e.g., Weinborn et al., 2011), which can increase engagement in HIV transmission risk behaviors (e.g., Weinborn et al., 2013). However, our review of the literature yielded only nine analyses examining the relationship of substance use to PM in HIV, eight of which reported null findings (Coulehan et al., 2014; Doyle et al., 2015; Iudicello et al., 2011; Weber et al., 2011; Woods et al., 2007; Woods et al., 2014; Zogg et al., 2010). Only results of Woods and colleagues (2014) showed that PM accuracy improved significantly among individuals with SUD when the importance of the PM study task was emphasized, suggesting that SUD may increase the amount of cognitive attentional resources needed to support PM performance in young adults with HIV infection (d = .91). Indeed the Woods et al., (2014) investigation is unique in its inclusion of participants with current substance dependence. Interestingly, the exclusion criteria regarding substance use for the analyses that reported null effects of SUD on PM ranged from reports of dependence within 1 month to 5 years, which may increase risk of Type II error. Further limitations of this literature include absence of factorial designs and the lack of consideration of different substances of abuse, which may vary in their neurotoxicity and subsequent effects on PM (Verdejo-Garcia et al., 2005).
Hepatitis C (HCV)
It is estimated that one-third of individuals living with HIV are also infected with HCV (Mohsen et al., 2005). Akin to HIV, HCV is neurovirulent (e.g., Forton et al., 2005) and is associated with increased prevalence (e.g., Hilsabeck et al., 2003) and incidence (Sheppard et al., 2015) of neurocognitive impairment reflecting the traditional pattern of frontosubcortical involvement. In the setting of HIV, HCV co-infection can have additive effects on the prevalence and severity of neurocognitive dysfunction (e.g., Miller et al., 2016), although the literature on this topic is mixed (see Clifford et al., 2015). With regard to PM specifically, we discovered four analyses that evaluated whether HCV co-infection worsens HIV-associated PM deficits. Three of the four studies reported null results. Only Avci et al. (2016) reported a significant association between HCV and PM, but this was in a mixed sample of persons with and without HIV disease. In our HIV+ cohort, co-infection with HCV was associated with significantly PM (see Figure 2); however, as with the historical HIV disease analyses reported above, the HCV co-infected group was markedly older (p < .0001) and the inclusion of age in a multivariable regression eradicated the apparent HCV effects on PM (p > .10). Thus, there is currently no strong evidence that co-infection with HCV exacerbates HIV-associated PM deficits. However, prospective factorial studies that include persons with and without HCV and HIV are needed to more definitively answer this question. Indeed, we currently do not know if HCV alone has an impact on PM and, if so, what is the profile and everyday functioning implications of such deficits? Such studies may also wish to examine the possible associations between PM and HCV RNA, as well as biomarkers of liver disease severity (e.g., fibrosis).
Everyday Functioning and PM
One of the more compelling reasons to study PM in HIV is that this construct is held to be ecologically relevant. In fact, everyday examples are commonly used to drive home the clinical importance of PM, such as remembering to take one’s medication as prescribed. In this way, PM is purported to capture unique aspects of daily life that are not measured by traditional neurocognitive tasks of retrospective memory or executive functions and thus may be of tremendous clinical value. Our review of the literature uncovered 12 published studies (N = 1185) that used PM to predict an everyday functioning outcome in HIV-positive participants. All of these studies used performance-based measures of PM, and the majority of them (k = 10) used the MIST. The everyday functioning outcomes measured in these studies included activities of daily living (k = 2), medical compliance (k = 8), employment (k = 1), health-related quality of life (k = 1), and risk taking (k = 1). We derived effect size estimates from all of these studies, which showed an average Cohen’s d value of .45 (SD = .22), suggesting generally small-to-medium effect size associations between PM and everyday functioning outcomes. As shown in Figure 4, the associations were somewhat stronger for time-based PM (mean d = .56) than for event-based PM (mean d = .36), which was more comparable to effect size estimates for measures of retrospective memory (mean d = .36, typically measured with tasks of list and story recall) and executive functions (mean d = .36, typically measured with tasks of cognitive flexibility and planning). In the following section, we review each of the study findings in greater detail.
Figure 4.
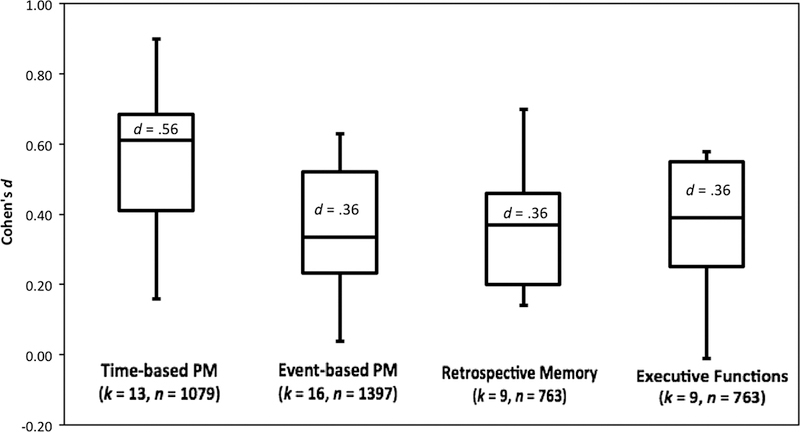
Box-and-whisker plot of effect sizes (Cohen’s d) for the associations between everyday functioning outcomes and neurocognitive domains of time-based PM, event-based PM, retrospective memory, and executive functions. k = number of effect sizes, N = total sample size, PM = prospective memory.
Activities of Daily Living (ADL)
Everyday functioning deficits are highly prevalent among individuals infected with HIV, and a recent study found that 61% of HIV-positive participants were functionally dependent (Blackstone et al., 2013). The association between activities of daily living (ADLs) and PM in HIV has been investigated in two studies thus far (Doyle et al., 2015; Woods, Iudicello et al., 2008). Both studies reported a significant (and independent) association between PM and functional status. In one study (n = 106), Doyle et al. (2015) found an association (d = .28) between laboratory performance on an experimental habitual PM task (i.e., repetitions) and a global functioning composite score that incorporated employment status, self-reported declines in basic and instrumental ADLs, clinician-rated functional impairment, and self-reported cognitive symptoms. This association was independent of demographic, psychiatric, and HIV disease characteristics. Another study (n = 66) found that declines in instrumental ADLs were moderately associated with performance on both time-based (d = .61) and event-based (d = .49) PM tasks, even after controlling for retrospective memory and affective distress (Woods, Iudicello et al., 2008). This literature provides preliminary evidence for a moderate independent association between PM and self-reported declines in ADLs in HIV disease. These findings await extension to a longitudinal design to determine whether baseline PM predicts incident dependence in ADLs and/or whether fluctuations in PM and ADLs travel together. Furthermore, future studies may wish to take advantage of performance-based ADL measures rather than exclusively relying on self-report. For example, skills-based measures of household tasks (e.g., cooking) and/or virtual reality simulators may be fruitful approaches to determine the extent to which PM is associated with functional capacity.
Medical Compliance
Medication Adherence
Adherence to cART regimens is crucial to disease management in HIV, and non-adherence can lead to increased viral loads, greater risk of HIV transmission, and faster disease progression (e.g., Bangsberg et al., 2001). Unfortunately, estimates from a recent meta-analysis suggest that over 40% of adults with HIV disease are non-adherent with their medication (Ortego et al., 2011). Our review yielded 5 published studies on the topic of PM and HIV medication adherence, all of which reported some significant associations independent of demographic, psychiatric, and disease factors. These studies measured adherence with the Medication Event Monitoring System (MEMS; Aprex Corporation, Union City, CA), which uses pill bottles with microchips to collect data anytime a participant opens or closes a pill bottle. Participants are considered adherent if they take at least 90% of their prescribed doses over four weeks. One study (N = 79) reported that adherent participants performed better than the non-adherent group on overall (d = .54) and time-based (d = .60) measures of PM in the laboratory; however, they did not significantly differ on event-based PM tasks (d = .28; Woods et al., 2009). Another study (N = 74) found that adherent and non-adherent groups differed on a time-based PM task with a 15-min delay (d = .77), which was mostly driven by errors of omission (Poquette et al., 2013). However, the groups did not differ on the event-based task with a 15-min delay (d = .23) or the PM tasks with a 2-min delay (d = .18). Additionally, one study (N = 106) found that performance on a habitual laboratory-based PM task was significantly correlated with adherence above and beyond relevant clinicodemographic factors (d = .39) (Doyle et al., 2015). A recent study (N = 199) also found that time-based PM was associated with medication adherence in older adults (age ≥ 50 years) irrespective of pill burden (d = .55; Sheppard et al., 2016). However, in younger participants, the association between time-based PM and adherence (d = .65) was only significant among those with a low pill burden. A final study (N = 97) used a neuropsychological testing battery to determine which cognitive domains were most strongly associated with adherence in HIV-positive participants with a history of substance abuse (Contardo et al., 2009). An exploratory factor analysis of these domains found that only one factor, comprised of performance-based PM scores, was significantly correlated with adherence (d = .47). Overall, the literature provides consistent evidence for a significant relationship between performance-based PM (and in particular, time-based PM) and medication adherence in HIV as measured by MEMS.
Medication management.
Instead of measuring adherence with the MEMS, one research study investigated the association between PM and responses to a medication management questionnaire. While the questionnaire measures participants’ perceptions of their medication-taking behaviors rather than their objective performance, self-reported adherence has been shown to be reliably associated with other measures of adherence, including electronic drug monitoring (e.g., MEMS) and HIV RNA viral load (Simoni et al., 2006). In this study of HIV-positive participants who were prescribed cART (n = 87), both time-based (d = .72) and event-based (d = .63) measures of laboratory PM performance were independently associated with self-reported medication management (Woods, Moran, Carey et al., 2008). Even when accounting for other medical, psychosocial, environmental, and cognitive factors, performance-based PM remained a significant predictor of medication management, thus supporting the incremental ecological relevance of PM in HIV disease.
Medical Instructions
Besides medication adherence, general health care compliance (e.g. following instructions after a medical appointment) is also important for HIV disease management. Two research studies have looked at PM and health care compliance using a semi-naturalistic task in which participants were asked to make a telephone call 24 hours after their laboratory session. In the first study (N = 139), researchers found that compliance with the semi-naturalistic task was associated with both time-based (d = .41) and event-based (d = .55) PM performance in the laboratory (Zogg et al., 2010). After controlling for demographic (e.g., education) and neurocognitive (e.g., working memory) factors, overall performance-based PM was an independent predictor of task compliance. Similarly, the second study (N = 106) found that performance on a habitual PM task was an independent predictor of compliance with the semi-naturalistic health care task (d = .63; Doyle et al., 2015). Such findings may be particularly relevant considering recent efforts to examine the role of HAND across the treatment spectrum in HIV disease, from initial engagement to retention in healthcare (e.g., Jacks et al., 2015).
Employment
Recent research from the Centers for Disease Control and Prevention (2016) estimates that 47% of HIV+ adults in the United States are unemployed. Unemployment may be associated with PM deficits in several ways. For example, PM may be important for job performance (e.g. remembering to complete a task) and may also impact the job search process (e.g. meeting application deadlines and attending interviews). One study has examined this question in 108 HIV+ (Woods et al., 2011), finding that lower scores on overall (d = .58) and event-based (d = .53) PM were associated with a higher rate of unemployment, while time-based PM performance was associated with unemployment at the trend level (d = .41). Overall PM performance remained an independent predictor of unemployment even after controlling for demographic, neurocognitive, psychiatric, and HIV disease factors. Thus, PM abilities may independently impact individuals’ ability to engage in gainful employment, although these relationships were cross-sectional and thus await further rigorous longitudinal studies to determine whether baseline PM predicts incident unemployment or return-to-work (e.g., van Gorp et al., 1999). Furthermore, studies are needed to examine the role of PM in work-related function in currently employed individuals, for example on-the-job performance and productivity, with consideration of potential mediating and moderating factors (e.g., level of functioning, compensatory strategies, oversight).
Health-Related Quality Of Life
Individuals with HIV disease tend to report lower health-related quality of life (HRQoL) than the general population (e.g., Hays et al., 2000). We are aware of only one study (N = 113) that has investigated the role of PM in health-related quality of life (HRQoL) among younger (age ≤ 40 years) and older (age ≥ 50 years) individuals with HIV (Doyle et al., 2012). In this study, time-based PM performance was a significant predictor of HRQoL in younger (d = .90) but not older (d = .16) adults. Among the younger group, the association between PM and mental HRQoL was independent of other related factors (i.e., neurocognitive disorders, current major depressive disorder, lifetime substance dependence, and CD4 count); however, the association between PM and physical HRQoL was reduced to the level of a trend (p = .05) after controlling for these factors. Event-based PM was not significantly associated with HRQoL in either the younger (d = .41) or older (d = .04) group. Doyle et al. (2012) proposed that the age discrepancy in the relationship between PM and HRQoL may be a result of older adults using more compensatory strategies than younger adults. Thus, further research is needed to examine the role of PM in HRQoL and health perceptions across the lifespan in HIV disease, including a careful examination of possible mediating and moderating factors. For example, it is possible that PM deficits adversely affect HRQoL through their association with everyday functioning outcomes such as medical compliance and employment, as has been shown in healthy older adults (see Woods et al., 2015).
Risk-Taking
PM may also relate to risk-taking, since individuals with impaired PM may not remember their intentions to abstain from risky behavior, as has been shown in seronegative youth and substance abusers (Weinborn et al., 2013). Risk-taking is particularly relevant to HIV disease because the virus can be contracted through risky sexual and drug use practices. Participants in a preliminary study of risk behaviors (N = 66) included HIV+ and seronegative substance-dependent individuals (Martin et al., 2007). Performance on a time-based PM task (d = .80), but not an event-based PM task (d = .20), was significantly associated with a self-report questionnaire of high-risk sexual and drug injection behaviors (Martin et al., 2007). Unfortunately, this is the only study that has investigated risk-taking and PM in HIV, and future research is needed to replicate these findings and determine potential mediating and moderating factors.
PM Symptoms
Although this review focuses on performance-based PM measures as predictors of everyday functioning in HIV, we would be remiss to omit the literature on self-reported PM symptoms. HIV is associated with elevations in self-reported PM symptoms, which relate more strongly to affective distress than to actual PM performance (e.g., Woods et al., 2007). However, the potential relevance of self-reported PM complaints should not necessarily be dismissed out of hand, as they are independently associated with some functional outcomes in HIV disease. Indeed, what we measure in the lab does not always translate easily to daily life. To our knowledge, six HIV studies have looked at the association between everyday functioning and self-reported PM complaints on the Prospective and Retrospective Memory Questionnaire (Smith et al., 2000). Three of these studies found significant correlations between PM complaints and functional outcomes, and importantly, these associations were independent of affective distress (Woods, Moran, Dawson et al., 2008; Woods, Iudicello, et al., 2008; Doyle et al., 2012). However, the remaining three studies failed to find significant associations between PM complaints and functioning (Woods et al., 2009; Zogg et al., 2010; Woods et al., 2011). Although the six studies looking at self-reported PM and everyday functioning reported a high effect size on average (mean d = .85), the results were highly variable (interquartile range = .22, 1.38). Interestingly, the association between PM complaints and everyday functioning was unrelated to cue type, with studies reporting the same overall effect sizes for both strategic (d = .85) and more automatic (d = .85) PM complaints. In general, there seems to be a strong, yet inconsistent, relationship between PM complaints and everyday functioning. Therefore, although PM complaints do not necessarily reflect performance-based PM ability, both measures are affected in HIV disease and may be relevant to real-world outcomes.
Meta-PM
Given the discrepancy between self-reported complaints and performance on laboratory tasks, one study investigated metacognitive awareness of PM abilities, or meta-PM, in individuals with HIV disease. In this study, participants with HAND demonstrated worse meta-PM for time-based, but not event-based, PM, compared to participants without HAND (Casaletto et al., 2014). This study also found that overconfidence in time-based PM abilities among participants with HAND (n = 51) was a significant predictor of medication non-adherence. Therefore, in addition to the clinical relevance of performance-based and self-reported PM abilities, the discrepancy between these measures may also be a significant predictor of functional outcomes in individuals with HIV.
Future Directions for PM and Everyday Functioning Research in HIV
Overall, the literature provides evidence that PM deficits are associated with poorer everyday functioning among individuals with HIV disease, and time-based PM performance may be a more consistent predictor of functional ability than event-based PM. Since performance-based PM tasks and self-reported PM complaints reflect two different but clinically relevant constructs, future studies of PM should include both performance-based and self-report measures. The research regarding PM and medication adherence is relatively robust, but investigations into other domains of everyday functioning are still in their infancy. Indeed, there is still much to learn about the association between PM and performance-based functional outcomes (e.g., medication management skills). For example, we do not know whether improvements in PM translate into improvements in daily life, which would have important implications for the treatment of individuals with HIV. The literature on PM and everyday functioning would also benefit from a wider variety of studies addressing the complex relationship between these variables in HIV. In particular, analysis of critical mediating and moderating factors is needed, including consideration of such variables as motivation, insight, real-world task demands, and use of compensatory strategies. Additionally, longitudinal studies could shed light on the progression of PM deficits and functional dependence over time. Interestingly, two studies (Doyle et al., 2012; Sheppard et al., 2016) have reported different associations between PM and everyday outcomes in younger versus older adults. Given the continued growth of the older HIV+ population, longitudinal studies in this area would be highly relevant.
Enhancing PM in HIV
Although much of the present literature has been focused on the mechanisms and functional impact of HIV-associated PM deficits, an emerging body of work is examining techniques and strategies to bolster PM performance in HIV disease. A review of the PM mechanisms literature in HIV with an eye toward remediation lends some empirical support to prospective hypothesis testing for remediation. Namely, HIV is associated with impairment in more strategically demanding PM processes, but spares relatively automatic PM functions. Therefore, one might expect that supporting strategic processes and/or automatizing the demands of PM at encoding, monitoring, or cue detection phases could enhance PM performance for persons with HIV disease. The four studies that have taken a prospective approach to improving PM in HIV+ persons are reviewed below (Faytell et al., in press a and b; Loft et al., 2014; Woods et al., 2014) and are summarized in Figure 5.
Figure 5.
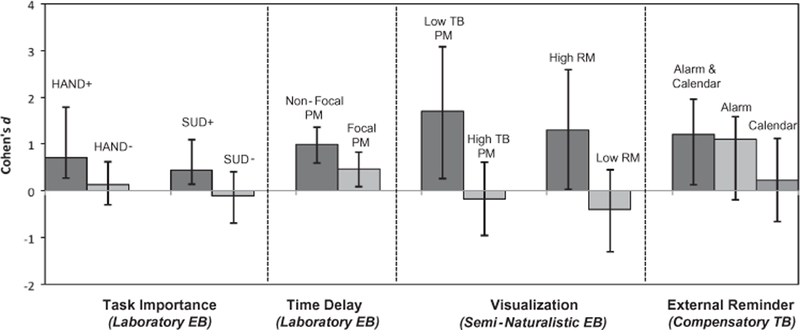
Effect size estimates (Cohen’s d) and moderators of approaches to improve prospective memory (PM) across 6 samples of HIV+ adults. Note: HAND = HIV-Associated Neurocognitive Disorders; SUD = Substance Use Disorders; TB = time-based; EB = event-based; RM = retrospective memory
Two studies have focused on enhancing monitoring to event-based cues as a means of improving PM in HIV disease. One avenue for improving PM performance is by increasing the salience of cues to one’s attention, thereby reducing attentional constraints and triggering spontaneous automatic processes. Using an experimental paradigm in a sample of HIV+ young adults, Loft et al. (2014) tested the hypothesis that imposing a mandatory time delay between the cue onset and the response of a task can improve accuracy in an event-based PM task. The authors found that a delay as short as 600 milliseconds between cue onset and response time significantly improved accuracy in the PM task, particularly when the PM cue was non-focal to the task (see Figure 5). This delay arguably allowed participants to sufficiently assess the ongoing task stimuli for PM-related features. Results from the study have implications for everyday life, for example, by suggesting that by slowing down the surrounding environment, HIV+ young adults allow themselves to fully process stimuli and engage in appropriate behaviors to complete PM goals. In a second study, Woods et al. (2014) tested the hypothesis that drawing attention to the importance of a PM task relative to a concurrent ongoing task would enhance strategic allocation of cognitive resources to the PM cue and thus improve PM task performance. In 2 parallel experiments, the authors demonstrated that individuals with HAND (Experiment 1) or substance use disorders (Experiment 2) showed significantly lower event-based PM accuracy when the importance of the ongoing task was emphasized (i.e., a high strategic demand condition), but improved significantly and no longer evidenced impairment when the importance of the PM task was emphasized (see Figure 5). Such findings suggest that increasing attentional allocation to PM cues may mitigate HIV-associated event-based PM deficits.
Faytell et al. (in press-a) focused on improving HIV-associated PM deficits in the encoding phase by drawing upon visualization literature. They hypothesized that enhancing the strength of the cue-intention pairing by way of a brief visualization exercise would provide support for impaired strategic PM processes and thus improve performance. In this study, young HIV+ adults were randomized to either visualize themselves completing semi-naturalistic event-based PM task (i.e., manage their medications upon seeing the grooved pegboard test that was administered as part of a standard neuropsychological battery) or to more simply repeat the PM task instructions (i.e., an active control condition). The visualization intervention resulted in improved semi-naturalistic PM performance relative to the control condition, but only in individuals who demonstrated poor initial time-based PM and those who had intact retrospective learning ability (see Figure 5). Interestingly, individuals with good initial time-based performance had an 80% accuracy rate on the semi-naturalistic task and did not show substantial benefit of the visualization exercise. Thus, individual differences in neurocognitive ability moderate the response to visualization (and perhaps other interventions) in HIV+ young adults.
To our knowledge, only one study has examined the effectiveness of behavioral strategies to improve naturalistic time-based PM. Faytell et al. (in-press b) investigated two simple methods for improving time-based PM performance in a sample of young adults with HIV: 1) programming a mobile telephone alarm; and 2) using a calendar function on a mobile device. Participants were randomly assigned to use one, both, or neither of these approaches, which were thought to provide behavioral support to impaired strategic processes. The naturalistic PM task outcome was to contact the examiner daily for a week and report the number of hours the participant had slept the night prior. Results showed large effect sizes on the naturalistic PM task for the groups of participants who employed the alarm (with or without calendaring) as compared to those who used only the calendar or neither strategy (see Figure 5). This study demonstrated the effectiveness of supportive behavioral (i.e., compensatory) strategies to improve naturalistic time-based PM in HIV+ young adults.
Collectively, the reviewed work has shown promising evidence for the efficacy of PM remediation strategies in HIV. The present findings suggest that that both time-based and event-based PM deficits seen in HIV are amenable to remediation. However, as illustrated in Figure 5, moderating factors are seen in each of the reviewed studies and predominate the effects in an inconsistent manner. That is, effects are variable across disease factors (e.g., HAND, substance use), aspects of the PM task (e.g., presence of a delay, aspects of the cue), and individual differences (e.g., baseline retrospective learning and PM). Additionally, three of the four studies examined behavioral strategies almost exclusively in HIV+ young adults. Future work may wish to examine the effectiveness of like strategies in older adults, as this population is more susceptible to deficits in PM (Avci et al., 2016). In fact, some research suggests that older adults with HIV may respond differently to PM cues (Woods et al., 2010), thus a thorough investigation of strategies in this susceptible population is warranted.
Some PM-enhancing methods that have been examined outside of the HIV literature may speak to future research directions. In line with the aforementioned effects of visualization on PM, the actual physical enactment of a task has similarly been shown to improve PM in healthy adults (Schult & Steffens, 2016) as well as individuals with mild cognitive impairment (Pereira, et al., 2015). Errorless learning is an encoding-enhancing method during which participants are prevented from making any mistakes when learning a task. Errorless learning has been shown to improve retrospective learning by way of encouraging implicit learning and lessening the potential of cognitive interference of incorrect responses, thereby reducing demands of the memory retrieval process. Recently, errorless learning has been shown to be more effective than traditional errorful learning in improving event-based, but not time-based, prospective memory in neurologically compromised individuals (Fish et al., 2015). Others have taken cognitive training approaches to improving PM in various populations. Sweeney et al., (2016) tested the effectiveness of a novel brief fifty minute combined PM and working memory training program in improving PM in an outpatient substance use treatment sample. The authors found that their single session training was associated with validated measures of PM and working memory, and once again, the effect of training was more robust for event-based compared to time-based PM. Similarly, Richter et al., (2015) found that, among participants with compromised memory, training in working memory and practice in semantic structuring resulted in transferred effects to improved PM performance. Further, future research may also wish to examine in effectiveness of such PM remediation strategies, in eliciting change in health behaviors (e.g., adherence) and downstream effects on clinical outcomes (e.g., CD4 count) and other indicators of everyday functioning.
Concluding Remarks
This structured literature review aimed to summarize and critique our current understanding of PM functioning in people living with HIV. Overall findings from this review suggest that HIV disease is associated with moderate deficits in PM, which appear to be largely independent of commonly observed comorbid factors (e.g., depression, hepatitis C co-infection) and do not vary strongly with traditional clinical markers of HIV disease severity. The pattern of PM deficits reveals dysregulation of strategic processes (e.g., resource-demanding monitoring for the cue) that is consistent with the frontal systems pathology and associated executive dysfunction that characterizes HIV-associated neural injury. In turn, there is a relative sparing of more spontaneous/automatic PM processes that are reliant on more posterior brain regions that are typically less affected in HIV disease. Older age exacerbates the effects of HIV on PM, particularly for strategic tasks as measured in the laboratory; however, age does not appear to influence more naturalistic PM performance, which may be partly due to the relative increase in compensatory strategy use that commonly accompanies older age. Of great clinical relevance, HIV-associated PM deficits confer a strong risk of concurrent problems in a wide range of health behaviors (e.g., medication non-adherence) and ADLs (e.g., employment). The clinical predictive value of PM in HIV disease is independent of sociodemographics, psychiatric comorbidity, HIV disease severity, and global neurocognitive decline, thus suggesting that the construct has some incremental ecological validity. Early attempts to improve PM in HIV disease have revealed that supporting strategic processes at encoding (e.g., visualization), monitoring (e.g., attention allocation), and cue detection (e.g., salient cues) can be effective for some individuals. Indeed, this literature highlights the importance of considering moderating factors (e.g., comorbidities, interindividual variability in neurocognitive functions) when attempting to improve PM in clinical samples. Much work remains to be done, however, in understanding the cognitive architecture of HIV-associated PM deficits, the ways in which they express themselves in daily life, and the most effective and impactful means by which clinicians can manage and improve PM functioning in persons living with HIV disease.
Acknowledgement
This work was partially supported by National Institutes of Health grants R01-MH073419 and R01-DA034497. The authors have no financial conflicts of interest related to this work. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Contributor Information
Gunes Avci, Department of Psychology, University of Houston, TX, USA, 3695 Cullen Boulevard, Suite 204, Houston, TX 77004-5022, 713-743-7226.
David P. Sheppard, Department of Psychology, University of Houston, TX, USA 3695 Cullen Boulevard, Suite 204, Houston, TX 77004-5022, 713-743-6415
Savanna M. Tierney, Department of Psychology, University of Houston, TX, USA, 3695 Cullen Boulevard, Suite 204, Houston, TX 77004-5022
Victoria M. Kordovski, Department of Psychology, University of Houston, TX, USA, 3695 Cullen Boulevard, Suite 204, Houston, TX 77004-5022
Kelli L. Sullivan, Department of Psychology, University of Houston, TX, USA, 3695 Cullen Boulevard, Suite 204, Houston, TX 77004-5022
Steven Paul Woods, Department of Psychology, University of Houston, TX, USA, 126 Heyne Building, Suite 239D, Houston, TX 77004-5022, 713-743-2255.
References
- Avci G, Loft S, Sheppard D, Woods SP, & HIV Neurobehavioral Research Program (HNRP) Group (2016). The effects of HIV disease and older age on laboratory-based, naturalistic, and self-perceived symptoms of prospective memory: Does retrieval cue type and delay interval matter? Aging, Neuropsychology & Cognition, 23, 716–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, & Moss A (2001). Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS, 15(9), 1181–1183. 10.1097/00002030-200106150-00015 [DOI] [PubMed] [Google Scholar]
- Bezeau S, & Graves R (2001). Statistical power and effect sizes of clinical neuropsychology research. Journal of Clinical and Experimental Neuropsychology (Neuropsychology, Development and Cognition: Section A), 23(3), 399–406. 10.1076/jcen.23.3.399.1181 [DOI] [PubMed] [Google Scholar]
- Blackstone K, Iudicello JE, Morgan EE, Weber E, Moore DJ, Franklin DR, Ellis RJ, Grant I, Woods SP, Translational Methamphetamine AIDS Research Center Group. (2013). Human immunodeficiency virus infection heightens concurrent risk of functional dependence in persons with long-term methamphetamine use. Journal of Addiction Medicine, 7(4), 255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K, Woods SP, Weber E, Grant I, & Moore DJ (2013). Memory-Based Strategies for Antiretroviral Medication Management: An Evaluation of Clinical Predictors, Adherence Behavior Awareness, and Effectiveness. AIDS and Behavior, 17(1), 74–85. 10.1007/s10461-012-0308-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, & Frith CD (2001). Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia, 39(6), 545–555. 10.1016/s0028-3932(00)00149-4 [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I, & HIV Neurobehavioral Research Center (HNRC) Group. (2006). Prospective memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology, 28(4), 536–548. 10.1080/13803390590949494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Doyle KL, Weber E, Woods SP, & The HIV Neurobehavioral Research Program (HNRP) Group. (2014). Self-predictions of prospective memory in HIV-associated neurocognitive disorders: Evidence of a metamemory deficit. Archives of Clinical Neuropsychology, 29, 818–827. 10.1093/arclin/acu061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon SA, Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, … Moore L (2006). Components of depression in HIV-1 Infection: Their differential relationship to neurocognitive performance. Journal of Clinical and Experimental Neuropsychology, 28(3), 420–437. 10.1080/13803390590935444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2016). Behavioral and clinical characteristics of persons receiving medical care for HIV infection—Medical Monitoring Project, United States, 2014 Cycle (June 2014–May 2015) (HIV Surveillance Special Report 17). Retrieved from http://www.cdc.gov/hiv/library/reports/ hiv-surveillance.html.
- Chapman LJ, & Chapman JP (1973). The measurement of differential deficit. Journal of Psychiatric Research, 14, 303–311. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, & Roberts JE (2001). Meta-analysis of the relationship between HIV infection and risk for depressive disorders. The American Journal of Psychiatry, 158(5), 725–730. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Vaida F, Kao YT, Franklin DR, Letendre SL, … Collier AC (2014). Absence of neurocognitive effect of hepatitis C infection in HIV-coinfected people. Neurology, 84(3), 241–250. 10.1212/wnl.0000000000001156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona G, Scarpazza C, Sartori G, Moscovitch M, & Bisiacchi PS (2015). Neural bases of prospective memory: a meta-analysis and the “Attention to Delayed Intention” (AtoDI) model. Neuroscience and Biobehavioral Reviews, 52, 21–37. 10.1016/j.neubiorev.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Contardo C, Black AC, Beauvais J, Dieckhaus K, & Rosen M (2009). Relationship of prospective memory to neuropsychological function and antiretroviral adherence. Archives of Clinical Neuropsychology, 24, 547–554. 10.1093/arclin/acp046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulehan K, Byrd D, Arentoft A, Monzones J, Fuentes A, Fraser F, Rosario A, Morgello S, & Mindt MR (2014). The role of decision-making ability in HIV/AIDS: impact on prospective memory. Journal of Clinical and Experimental Neuropsychology, 36(7), 730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Deutsch R, Atkinson JH, Young C, Marcotte TD, Dawson L, ... Heaton RK (2007). Incident major depression does not affect neuropsychological functioning in HIV-infected men. Journal of the International Neuropsychological Society, 13(01), 1–11. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, … Wiley CA (1992). Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology, 42(9), 1736–1736. 10.1212/wnl.42.9.1736 [DOI] [PubMed] [Google Scholar]
- Delis CD, Peavy G, Heaton R, Butters N, Salmon DP, Taylor M, et al. (1995). Do patients with HIV-associated minor cognitive/motor disorder exhibit a “subcortical” memory profile? Evidence using the California verbal learning test. Assessment, 2, 151–165. 10.1177/107319119500200205 [DOI] [Google Scholar]
- Doyle KL, Loft S, Morgan EE, Weber E, Cushman C, Johnston E, … The HIV Neurobehavioral Research Program (HNRP) Group (2013). Prospective memory in HIV-associated neurocognitive disorders (HAND): The neuropsychological dynamics of time monitoring. Journal of Clinical and Experimental Neuropsychology, 35(4), 359–372. 10.1080/13803395.2013.776010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KL, Morgan EE, Weber E, & Woods SP (2015b). Time Estimation and Production in HIV-Associated Neurocognitive Disorders (HAND). Journal of the International Neuropsychological Society, 21(02), 175–181. 10.1017/s1355617715000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KL, Weber E, Morgan EE, Loft S, Cushman C, Villalobos J, … The HIV Neurobehavioral Research Program (HNRP) Group (2015a). Habitual prospective memory in HIV disease. Neuropsychology, 29, 909–918. 10.1037/neu0000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle K, Weber E, Atkinson JH, Grant I, Woods SP, & The HIV Neurobehavioral Research Program (HNRP) Group (2012). Aging, prospective memory, and health-related quality of life in HIV infection. AIDS & Behavior, 16, 2306–2318. 10.1007/s10461-011-0121-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein GO, & McDaniel MA (1990). Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 16(4), 717–726. 10.1037/0278-7393.16.4.717 [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Manzi M, Cochran B, & Baker M (2000). Prospective memory and aging: Forgetting intentions over short delays. Psychology and Aging, 15(4), 671–683. 10.1037/0882-7974.15.4.671 [DOI] [PubMed] [Google Scholar]
- Ellis JA, & Nimmo-Smith I (1993). Recollecting naturally-occurring intentions: A study of cognitive and affective factors. Memory, 1(2), 107–126. 10.1080/09658219308258227 [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, & Masliah E (2007). HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nature Reviews Neuroscience, 8(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Faytell MP, Doyle KL, Naar-King S, Outlaw AY, Nichols SL, Casaletto KB, & Woods SP (in press a). Visualisation of future task performance improves naturalistic prospective memory for some younger adults living with HIV disease. Neuropsychological Rehabilitation, 0–14. 10.1080/09602011.2015.1122636 [DOI] [PMC free article] [PubMed]
- Faytell MP, Doyle K, Naar-King S, Outlaw A, Nichols S, Twamley E, & Woods SP (in press b). Calendaring and alarms can improve naturalistic time-based prospective memory for youth infected with HIV. Neuropsychological Rehabilitation, 1–14. 10.1080/09602011.2016.1236733 [DOI] [PMC free article] [PubMed]
- Fish JE, Manly T, Kopelman MD, & Morris RG (2014). Errorless learning of prospective memory tasks: An experimental investigation in people with memory disorders. Neuropsychological Rehabilitation, 25(2), 159–188. 10.1080/09602011.2014.921204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton DM, Allsop JM, Cox IJ, Hamilton G, Wesnes K, Thomas HC, & Taylor-Robinson SD (2005). A review of cognitive impairment and cerebral metabolite abnormalities in patients with hepatitis C infection. AIDS, 19 Suppl 3, S53–S63. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, … The CHARTER Group. (2009). White matter tract injury and cognitive impairment in human immunodeficiency virus–infected individuals. Journal of Neurovirology, 15(2), 187–195. 10.1080/13550280902769756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Shelton JT, Bugg JM, McDaniel MA, & Head D (2011). Structural correlates of prospective memory. Neuropsychologia, 49(14), 3795–3800. 10.1016/j.neuropsychologia.2011.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Paul Woods S, Weber E, Dawson MS, Grant I, & The HIV Neurobehavioral Research Ce. (2010). Is prospective memory a dissociable cognitive function in HIV infection? Journal of Clinical and Experimental Neuropsychology, 32(8), 898–908. 10.1080/13803391003596470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Cunningham WE, Sherbourne CD, Wilson IB, Wu AW, Cleary PD, … Bozzette SA (2000). Health-related quality of life in patients with human immunodeficiency virus infection in the United States: Results from the HIV cost and services utilization study. The American Journal of Medicine, 108(9), 714–722. 10.1016/s0002-9343(00)00387-9 [DOI] [PubMed] [Google Scholar]
- Heaton MP, Leymaster KA, Kalbfleisch TS, Kijas JW, Clarke SM, … McEwan J (2014). SNPs for parentage testing and traceability in globally diverse breeds of sheep. PLoS ONE, 9(4), e94851 10.1371/journal.pone.0094851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, ... Grant I (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology, 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, & Crawford JR (2004). A meta-analytic review of prospective memory and aging. Psychology and Aging, 19(1), 27–39. 10.1037/0882-7974.19.1.27 [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Hassanein TI, Carlson MD ., Zegler EA, & Perry W (2003). Cognitive functioning and psychiatric symptomatology in patients with chronic Hepatitis C. Journal of the International Neuropsychological Society, 9(6), 847–854. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, & Stefaniak M (2004). Medication adherence in HIV-infected adults. AIDS, 18(Supplement 1), 19–25. 10.1097/00002030-200401001-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J, Westgarth-Taylor J, Fouche JP, Spottiswoode B, Paul R, Thomas K, Stein D, Joska J (2012). A diffusion tensor imaging and neuropsychological study of prospective memory impairment in South African HIV positive individuals. Metabolic Brain Disease, 27(3), 289–97. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Weber E, Grant I, Weinborn M, Woods SP, & The HIV Neurobehavioral Research Center (HNRC) Group (2011). Misremembering future intentions in methamphetamine-dependent individuals. The Clinical Neuropsychologist, 25(2), 269–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks A, Wainwright DA, Salazar L, Grimes R, York M, Strutt AM, … Hasbun R (2015). Neurocognitive deficits increase risk of poor retention in care among older adults with newly diagnosed HIV infection. AIDS, 29(13), 1711–1714. 10.1097/qad.0000000000000700 [DOI] [PubMed] [Google Scholar]
- Kamat R, Woods SP, Marcotte TD, Ellis RJ, & Grant I (2012). Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Archives of Clinical Neuropsychology, 27(5), 520–531. 10.1093/arclin/acs055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M, Jäger T, & Phillips LH (2008a). Adult age differences in event-based prospective memory: A meta-analysis on the role of focal versus nonfocal cues. Psychology and Aging, 23(1), 203–208. 10.1037/0882-7974.23.1.203 [DOI] [PubMed] [Google Scholar]
- Kliegel M, McDaniel MA, & Einstein GO (Eds.) (2008b). Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives Mahwah, NJ, Erlbaum. [Google Scholar]
- Lanctôt KL, Agüera-Ortiz L, Brodaty H, Francis PT, Geda YE, Ismail Z, … Abraham EH (2017). Apathy associated with neurocognitive disorders: Recent progress and future directions. Alzheimer’s & Dementia, 13(1), 84–100. 10.1016/j.jalz.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Letendre S (2008). Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Archives of Neurology, 65(1), 65 10.1001/archneurol.2007.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine, 151(4), W. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- Loft S, Doyle KL, Naar-King S, Outlaw AY, Nichols SL, Weber E, ... & Woods SP (2014). Allowing brief delays in responding improves event-based prospective memory for young adults living with HIV disease. Journal of Clinical and Experimental Neuropsychology, 36(7), 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft S, & Yeo G (2007). An investigation into the resource requirements of event-based prospective memory. Memory & Cognition, 35(2), 263–274. 10.3758/bf03193447 [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, … Martin E (2009). Impairments in memory and hippocampal function in HIV-positive vs HIV- negative women: a preliminary study. Neurology, 72(19), 1661–1668. 10.1212/WNL.0b013e3181a55f65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Nixon H, Pitrak DL, Weddington W, Rains NA, Nunnally G, … Bechara A (2007). Characteristics of prospective memory deficits in HIV-seropositive substance-dependent individuals: Preliminary observations. Journal of Clinical and Experimental Neuropsychology, 29(5), 496–504. 10.1080/13803390600800970 [DOI] [PubMed] [Google Scholar]
- McDaniel MA, & Einstein GO (2000). Strategic and automatic processes in prospective memory retrieval: A Multiprocess framework. Applied Cognitive Psychology, 14(7), S127–S144. 10.1002/acp.775 [DOI] [Google Scholar]
- McDaniel MA, & Einstein GO (2011). The neuropsychology of prospective memory in normal aging: A componential approach. Neuropsychologia, 49(8), 2147–2155. 10.1016/j.neuropsychologia.2010.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TR, Weiss JJ, Bräu N, Dieterich DT, Stivala A, & Rivera-Mindt M (2016). Greater decline in memory and global neurocognitive function in HIV/hepatitis C co-infected than in hepatitis C mono-infected patients treated with pegylated interferon and ribavirin. Journal of NeuroVirology, 23(2), 260–272. 10.1007/s13365-016-0494-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsen A, Murad S, & Easterbrook P (2005). Prevalence of hepatitis C in an ethnically diverse HIV‐1‐infected cohort in south London. HIV Medicine, 6(3), 206–215. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Weber E, Rooney A, Grant I, Woods SP, & HIV Neurobehavioral Research Program (HNRP) Group (2012). Longer ongoing task delay intervals exacerbate prospective memory deficits in HIV-associated Neurocognitive Disorders (HAND). Journal of Clinical and Experimental Neuropsychology, 34, 416–427. 10.1080/13803395.2012.654764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L, … HIV Neurobehavioral Research Program (HNRP) Group. (2012). Synergistic effects of HIV infection and older age on daily functioning. Journal of Acquired Immune Deficiency Syndromes (1999), 61(3), 341–348. 10.1097/QAI.0b013e31826bfc53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murji S, Rourke SB, Donders J, Carter SL, Shore D, & Rourke BP (2003). Theoretically derived CVLT subtypes in HIV-1 infection: Internal and external validation. Journal of the International Neuropsychological Society, 9(01). 10.1017/s1355617703910010 [DOI] [PubMed] [Google Scholar]
- Obermeit LC, Morgan EE, Casaletto KB, Grant I, Woods SP, & HIV Neurobehavioral Research Program Group. (2015). Antiretroviral Non-Adherence is Associated With a Retrieval Profile of Deficits in Verbal Episodic Memory. The Clinical Neuropsychologist, 29(2), 197–213. 10.1080/13854046.2015.1018950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego C, Huedo-Medina TB, Vejo J, & Llorca FJ (2011). Adherence to highly active antiretroviral therapy in Spain. A meta-analysis. Gaceta Sanitaria, 25(4), 282–289. 10.1016/j.gaceta.2010.10.016 [DOI] [PubMed] [Google Scholar]
- Pence BW, Miller WC, Whetten K, Eron JJ, & Gaynes BN (2006). Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the southeastern United States. Journal of Acquired Immune Deficiency Syndromes, 42(3), 298–306. [DOI] [PubMed] [Google Scholar]
- Pereira A, de Mendonça A, Silva D, Guerreiro M, Freeman J, & Ellis J (2015). Enhancing prospective memory in mild cognitive impairment: The role of enactment. Journal of Clinical and Experimental Neuropsychology, 37(8), 863–877. 10.1080/13803395.2015.1072499 [DOI] [PubMed] [Google Scholar]
- Poquette AJ, Moore DJ, Gouaux B, Morgan EE, Grant I, & Woods SP (2012). Prospective memory and antiretroviral medication non-adherence in HIV: An analysis of ongoing task delay length using the memory for intentions screening test. Journal of the International Neuropsychological Society, 19(02), 155–161. 10.1017/s1355617712001051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin S, Buckheit C, & Sherrod C (2010). Memory for intentions test (MIST) Lutz: Psychological Assessment Resources, Inc. [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, & Boone KB (2002). A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society, 8(03), 410–424. 10.1017/s1355617702813212 [DOI] [PubMed] [Google Scholar]
- Rendell PG, & Thomson DM (1999). Aging and prospective memory: differences between naturalistic and laboratory tasks. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 54(4), 256–269. [DOI] [PubMed] [Google Scholar]
- Richter KM, Mödden C, Eling P, & Hildebrandt H (2015). Working memory training and semantic structuring improves remembering future events, not past events. Neurorehabilitation and Neural Repair, 29(1), 33–40. 10.1177/1545968314527352 [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, ... Grant I (2004). Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society, 10(01), 1–14. [DOI] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, … McArthur JC (2016). HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment. Nature Reviews Neurology, 12(4), 234–248. 10.1038/nrneurol.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schult JC, & Steffens MC (2016). The effects of enactment and intention accessibility on prospective memory performance. Memory & Cognition, 45(4), 625–638. 10.3758/s13421-016-0677-9 [DOI] [PubMed] [Google Scholar]
- Sheppard DP, Weber E, Casaletto KB, Avci G, Woods SP, & The HIV Neurobehavioral Research Program (HNRP) Group. (2016). Pill burden influences the association between time-based prospective memory and antiretroviral adherence in younger but not older HIV-infected adults. Journal of the Association of Nurses in AIDS Care, 27, 595–607. 10.1016/j.jana.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Woods SP, Bondi MW, Gilbert PE, Massman PJ, Doyle KL, & HIV Neurobehavioral Research Program Group. (2015). Does older age confer an increased risk of incident neurocognitive disorders among persons living with HIV disease? The Clinical Neuropsychologist, 29(5), 656–677. 10.1080/13854046.2015.1077995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, & Frick PA (2006). Self-Report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS and Behavior, 10(3), 227–245. 10.1007/s10461-006-9078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois PA, Chernoff MC, Malee KM, Garvie PA, Harris LL, Williams PL, … Memory and Executive Functioning Study of the Pediatric HIV/AIDS Cohort Study. (2016). Associations of Memory and Executive Functioning With Academic and Adaptive Functioning Among Youth With Perinatal HIV Exposure and/or Infection. Journal of the Pediatric Infectious Diseases Society, 5(suppl 1), S24–S32. 10.1093/jpids/piw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A van, … Hallett TB (2015). Future challenges for clinical care of an ageing population infected with HIV: A modeling study. The Lancet Infectious Diseases, 15(7), 810–818. 10.1016/s1473-3099(15)00056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Della Sala S, Logie RH, Maylor EA (2000). Prospective and retrospective memory in normal ageing and dementia: a questionnaire study. Memory, 8(5):311–2. [DOI] [PubMed] [Google Scholar]
- Smith RD, Delpech VC, Brown AE, & Rice BD (2010). HIV transmission and high rates of late diagnoses among adults aged 50 years and over. AIDS, 24(13), 2109–2115. 10.1097/qad.0b013e32833c7b9c [DOI] [PubMed] [Google Scholar]
- Smith RE, & Bayen UJ (2006). The source of adult age differences in event-based prospective memory: A multinomial modeling approach. Journal of Experimental Psychology: Learning, Memory, and Cognition, 32(3), 623–635. 10.1037/0278-7393.32.3.623 [DOI] [PubMed] [Google Scholar]
- Sweeney MM, Rass O, Johnson PS, Strain EC, Berry MS, Vo HT, … Johnson MW (2016). Initial feasibility and validity of a prospective memory training program in a substance use treatment population. Experimental and Clinical Psychopharmacology, 24(5), 390–399. 10.1037/pha0000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, … Hinkin CH (2011). Medication and finance management among HIV-infected adults: the impact of age and cognition. Journal of Clinical and Experimental Neuropsychology, 33(2), 200–209. 10.1080/13803395.2010.499357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, … Sacktor N (2004). Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology, 63(5), 822–827. 10.1212/01.wnl.0000134665.58343.8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorp WG, Baerwald JP, Ferrando SJ, Mcelhiney MC, & Rabkin JG (1999). The relationship between employment and neuropsychological impairment in HIV infection. Journal of the International Neuropsychological Society, 5(06). 10.1017/s1355617799566071 [DOI] [PubMed] [Google Scholar]
- Verdejo-García AJ, López-Torrecillas F, Aguilar de Arcos F, & Pérez-García M (2005). Differential effects of MDMA, cocaine, and cannabis use severity on distinctive components of the executive functions in polysubstance users: A multiple regression analysis. Addictive Behaviors, 30(1), 89–101. 10.1016/j.addbeh.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Walser M, Goschke T, Möschl M, & Fischer R (2016). Intention deactivation: effects of prospective memory task similarity on aftereffects of completed intentions. Psychological Research 10.1007/s00426-016-0795-9 [DOI] [PubMed]
- Weber E, Woods SP, Delano-Wood L, Bondi MW, Gilbert PE, Grant I, & HIV Neurobehavioral Research Program (HNRP) Group (2011). An examination of the age-prospective memory paradox in HIV-infected adults. Journal of Clinical and Experimental Neuropsychology, 33(10), 1108–1118. 10.1080/13803395.2011.604027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinborn M, Bucks R, Stritzke W, Leighton A, & Woods SP (2013). Time-based prospective memory predicts engagement in risk behaviors amongst substance users: Results from clinical and nonclinical samples. Journal of the International Neuropsychological Society, 19, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinborn M, Paul Woods S, Nulsen C, & Park K (2011). Prospective memory deficits in Ecstasy users: Effects of longer ongoing task delay interval. Journal of Clinical and Experimental Neuropsychology, 33(10), 1119–1128. 10.1080/13803395.2011.614595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Carey CL, Moran LM, Dawson MS, Letendre SL, Grant I, & The HIV Neurobehavioral Research Center (HNRC) Group (2007). Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Archives of Clinical Neuropsychology, 22, 187–195. 10.1016/j.acn.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH, & The HIV Neurobehavioral Research Center (HNRC) Group (2009). Timing is everything: Antiretroviral non-adherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society, 15, 42–52. 10.1017/S1355617708090012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I, & HIV Neurobehavioral Research Center (HNRC) Group (2010). The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology, 32(4), 398–407. 10.1080/13803390903130737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Doyle KL, Morgan EE, Naar-King S, Outlaw AY, Nichols SL, & Loft S (2014). Task importance affects event-based prospective memory performance in adults with HIV-associated neurocognitive disorders and HIV-infected young adults with problematic substance use. Journal of the International Neuropsychological Society, 20(6), 652–662. 10.1017/s1355617714000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I, & The HIV Neurobehavioral Research Center (HNRC) Group (2008). HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology, 22, 110–117. 10.1037/0894-4105.22.1.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, Gibson S, & The HIV Neurobehavioral Research Center (HNRC) Group. (2008). Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Archives of Clinical Neuropsychology, 23, 257–270. 10.1016/j.acn.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Dawson MS, Carey CL, Grant I, & HIV Neurobehavioral Research Center (HNRC) Group. (2008). Psychometric characteristics of the memory for intentions screening test. The Clinical Neuropsychologist, 22(5), 864–878. 10.1080/13854040701595999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Marquie-Beck J, Carey CL Grant I, Letendre SL, & The HIV Neurobehavioral Research Programs (HNRP) Group (2006). Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cognitive and Behavioral Neurology, 19, 217–221. 10.1097/01.wnn.0000213916.10514.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weber E, Weisz B, Twamley EW, Grant I, & The HIV Neurobehavioral Research Programs (HNRP) Group (2011). Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation Psychology, 56, 77–84. 10.1037/a0022753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weinborn M, Li YR, Hodgson E, Ng ARJ, & Bucks RS (2015). Does prospective memory influence quality of life in community-dwelling older adults? Aging, Neuropsychology, and Cognition, 22(6), 679–692. 10.1080/13825585.2015.1027651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weinborn M, Velnoweth A, Rooney A, & Bucks RS (2012). Memory for intentions is uniquely associated with instrumental activities of daily living in healthy older adults. Journal of the International Neuropsychological Society : JINS, 18(1), 134–138. 10.1017/S1355617711001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Weber E, Iudicello JE, Dawson MS, & Grant I (2010). HIV-associated prospective memory impairment in the laboratory predicts failures on a semi-naturalistic measure of health care compliance. The Clinical Neuropsychologist, 24(6), 945–962. 10.1080/13854046.2010.501343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Weber E, Doyle K, Grant I, … Atkinson JH (2011). Are Time- and Event-based Prospective Memory Comparably Affected in HIV Infection? Archives of Clinical Neuropsychology, 26(3), 250–259. 10.1093/arclin/acr020 [DOI] [PMC free article] [PubMed] [Google Scholar]


