Abstract
Background and Purpose:
Prehospital routing algorithms for patients with suspected stroke due to large vessel occlusions (LVO) should account for likelihood of benefit from endovascular therapy (EVT), risk of alteplase delays, and transport times. We built a mathematical model to give a real-time, location-based optimal EMS routing location based on local resources, transport times, and patient characteristics.
Methods:
Using location, onset time, age, sex, and pre-hospital stroke severity, we calculated odds of a favorable outcome for a patient with suspected LVO under 2 scenarios: direct to EVT-capable hospital vs. transport to the nearest alteplase-capable hospital with transfer to EVT-capable hospital if appropriate. We project lifetime outcomes incorporating disability, quality of life utility, and cost. Multiple parameter sets of center-specific times (e.g., door to alteplase) were randomly selected within a clinically plausible range to account for the model sensitivity to these estimates; for each iteration, the optimal strategy was defined as the most cost-effective outcome (threshold $100,000/QALY gained). After 1000 simulations, the most frequently occurring optimal strategy was the final recommendation, with its strength measured as the proportion of runs for which it was optimal.
Results:
Routing recommendations were highly sensitive to small changes in model input parameters. Under many scenarios, the recommendations for direct transfer to the EVT site increased with increasing stroke severity and geographic proximity, but did not vary substantially with respect to sex, age, or onset time.
Conclusions:
We present a mathematical decision model that determines ideal prehospital routing recommendations for patients with suspected stroke due to LVO, with consideration of patient characteristics and location at onset. This model may be further refined by incorporating real-time data on traffic patterns and actual EVT and alteplase timeliness performance. Further studies are needed to verify model predictions.
Keywords: stroke, endovascular procedure, mathematical model, emergency medical services, triage, Cost-Effectiveness, Quality and Outcomes, Ischemic Stroke
Introduction
In the pre-hospital setting, emergency medical services (EMS) personnel typically transport patients with suspected stroke to the closest alteplase-capable center (acute stroke ready hospital or primary stroke center, PSC), most of which are not endovascular thrombectomy-capable centers (CSC). Yet some patients may benefit from routing past a nearby PSC directly to a CSC, at the potential cost of delayed alteplase administration.
Previous work has found that routing recommendations are highly sensitive to transport and door-to-needle (DTN) times.1, 2 However, those studies assumed presence of acute ischemic stroke (AIS) although, in practice, a patient’s stroke diagnosis is unknown.3 Furthermore, while prior models predicted likelihood of good outcome, the clinical difference may be negligible. Our primary objective was to build a mathematical model that simulates hypothetical patients with stroke-like symptoms in the pre-hospital setting to determine the optimal pre-hospital EMS routing strategy based on long-term outcomes, incorporating real-time transport times and geographical location.
Methods
The authors declare that all supporting data, including model source code, are available within the article (and its online supplementary files).
We constructed a mathematical model to determine the optimal transport decision for pre-hospital routing of patients with suspected stroke. The simulation starting point is at the scene when the EMS provider first reaches the patient with the presumed stroke. Inputs are patient characteristics measurable by EMS providers and geographical location to inform real-time transit times to nearby primary and comprehensive centers. Primary and comprehensive centers are modeled with separate distributions for door-to-needle times, which affects all ischemic stroke patients. Prehospital stroke severity is measured using the Rapid Arterial oCclusion Evaluation (RACE) score which is strongly correlated with the National Institute of Health Stroke Scale (NIHSS)4 and probability of large vessel occlusions (LVO).5
The model output is a recommendation for initial transport to a PSC or CSC. The two primary outcomes simulated per strategy are lifetime cost and quality-adjusted life years (QALYs). Scenarios are compared using a cost-effectiveness approach with a maximum threshold of $100,000 per QALY gained. To account for the model’s sensitivity to center-specific performance (e.g., DTN times), each scenario is run multiple times with randomly chosen time values within a plausible distribution; the final recommendation is the most frequently occurring optimal destination. The model is written in Python 3.6.1 (see supplementary material).6
Because the value of this model is in its application to specific patient scenarios, we selected a few varied hypothetical but realistic clinical situations in which the model might be used. For each vignette, we identified an approximate location for the scene of the stroke and to estimated travel times using certification data from the Joint Commission to identify stroke centers (PSC and CSCs). Vignette inputs are summarized in Table 1. Details about vignette map construction are provided in the supplementary material.
Table 1:
Hypothetical stroke patient located in three US locations for illustrative purposes
| Location | Transport scenarios as of 2/6/2018 | Age | Sex | Time from onset at EMS scene arrival (minutes) |
Stroke severity, RACE (NIHSS) |
|---|---|---|---|---|---|
| Kingston, IL |
Option 1: 31 minutes to PSC with 62-minute transfer to CSC Option 2: 53 minutes direct to CSC PSC: OSF Saint Anthony Medical Center in Rockford, IL CSC: Alexian Brothers in Elk Grove Village, IL |
70 | F | 30 vs. 70 |
3 (6.8) vs. 5 (11.6) |
| Brookfield, CT |
Option 1: 14 minutes to PSC with 60-minute transfer to CSC Option 2: 59 minutes direct to CSC PSC: Danbury Hospital in Danbury, CT CSC: Hartford Hospital in Hartford, CT |
80 | M | 45 vs. 90 |
4 (9.2) vs. 6 (14.0) |
| Beaver Dam, AZ |
Option 1: 37 minutes to PSC with 117-minute transfer to CSC Option 2: 91 minutes direct to CSC PSC: Dixie Regional Medical Center in St George, UT CSC: Sunrise Hospital and Medical Center in Las Vegas, NV |
65 | F | 10 vs. 70 |
3 (6.8) vs. 8 (18.7) |
We did not obtain IRB approval as no individual patient data was used.
Results
Our mathematical model provides real-time, location-based optimal EMS routing destinations for patients with suspected stroke based on local resources, transport times, and patient characteristics. Overall, we found that routing recommendations were sensitive to small changes in input parameters and, in general, that severe strokes are more frequently routed to CSCs. Sites were chosen for these illustrative vignettes based on varied local resources. Results are summarized in Table 2, with graphical illustrations are in Figure 1 (Arizona), Supplementary Figure I (Illinois) and Supplementary Figure II (Connecticut).
Table 2:
Vignette results
| Location | Time from onset (minutes) |
Stroke severity (RACE) |
Optimal Destination | Distribution | |
|---|---|---|---|---|---|
| % CSC | % PSC | ||||
| IL | 30 | 3 | CSC | 58.8 [55.7, 61.9] | 41.2 [38.1, 44.3] |
| 5 | CSC | 84.9 [82.6, 87.2] | 15.1 [12.8, 17.4] | ||
| 70 | 3 | CSC | 54.2 [51.1, 57.4] | 45.8 [42.6, 48.9] | |
| 5 | CSC | 81.9 [79.5, 84.3] | 18.1 [15.7, 20.5] | ||
| CT | 45 | 4 | PSC | 4.9 [3.5, 6.3] | 95.1 [93.7, 96.5] |
| 6 | PSC | 23.2 [20.6, 25.9] | 76.8 [74.1, 79.4] | ||
| 90 | 4 | PSC | 3.6 [2.5, 4.8] | 96.4 [95.2, 97.5] | |
| 6 | PSC | 18.1 [15.8, 20.5] | 81.9 [79.5, 84.2] | ||
| AZ | 10 | 3 | PSC | 12.0 [10.0, 13.9] | 88.0 [86.1, 90.0] |
| 8 | CSC | 99.6 [99.2, 100.0] | 0.4 [0.0, 0.8] | ||
| 70 | 3 | PSC | 15.0 [12.8, 17.1] | 85.0 [82.9, 87.2] | |
| 8 | CSC | 99.4 [98.9, 99.9] | 0.6 [0.1, 1.1] | ||
Figure 1:
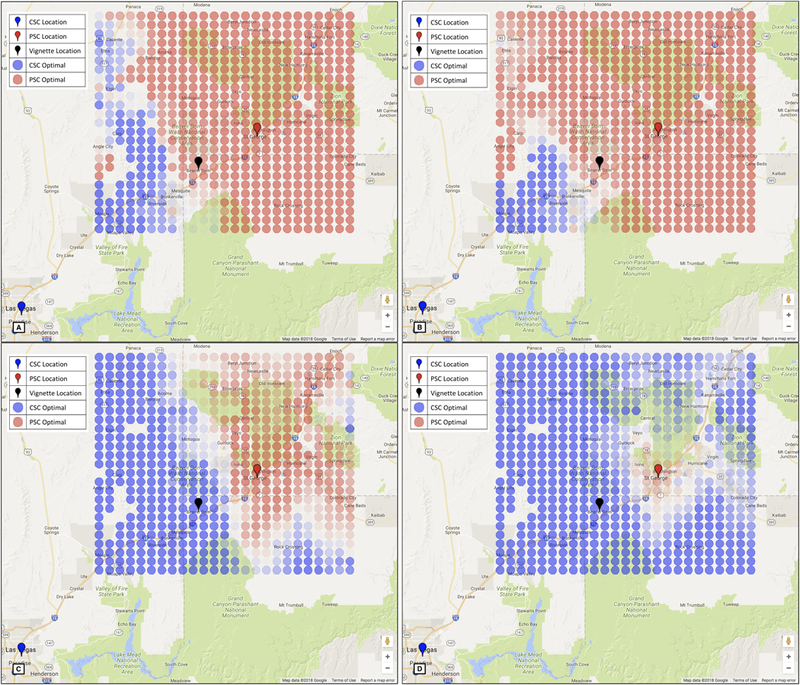
Optimal strategies for a 65-year-old woman 10 minutes from symptom onset with RACE 3 (panel A) and RACE 8 (panel C) and 70 minutes from symptom onset with RACE 3 (panel B) and RACE 8 (panel D). Red circles indicate PSC is optimal and blue circles indicate CSC is optimal. More lightly shaded locations indicate less certainty in the optimal destination. Red markers show primary stroke centers, blue markers show comprehensive stroke centers, and the black marker indicates Beaver Dam, the vignette location. Map data are provided by Google.
Discussion
We report the development of a novel, comprehensive mathematical model which builds on prior published work with the addition of critical prehospital elements and economic and probabilistic outcome modeling. This more nuanced approach to the question of optimal prehospital EMS routing reflects a realistic approach to the real-world challenges encountered by EMS personnel. The model also allows for further refinement with actual hospital-level performance if these data become available. The freely available, open-source platform of the model also enables further use for academic and on-the-ground applications, for example in refining stroke systems of care that are fine tuned to local environments.
This model incorporates local resources (proximity of primary and comprehensive stroke centers), transport times based on real-time traffic data, and patient characteristics including time of onset and severity of symptoms. Since our model is designed to give specific and actionable outputs for any given input scenario, it is specifically not intended to make broad generalizations about destination routing plans since the model clearly shows that the outputs are exquisitely sensitive to minor variations in the input parameters. An important finding from our model is that small changes in time from symptom onset or distance to a nearby CSC lead to significant changes in routing recommendations, as illustrated by the case vignettes. We presented the model outputs as specific case vignettes to demonstrate the ease of personalization of recommendations compared to simple uniform rules that are designed to be “one size fits all”.
Our model has limitations, including reliance on sparse data for long-term outcomes, not accounting for emergency department crowding, and the use of a single PSC and CSC in our algorithm. These limitations are further detailed in the supplementary material.
Our results are similar to prior work by Holodinsky and colleagues, who reported that transport decisions for AIS patients were highly sensitive to transportation times between centers and to DTN times.2 Our version of the model adds these 4 additional key features: 1) it does not assume that AIS status can be determined with certainty in the field, 2) it incorporates the diagnostic uncertainty at the time of EMS transport decision-making and assigns probabilities of hemorrhagic stroke and stroke mimic diagnoses; 3) in contrast to previous studies focusing on good-outcome probabilities, we used QALYs and costs to quantify differences between transport strategies and to provide a clear clinical interpretation to an otherwise mathematical and probabilistic answer; and 4) the open-source and dynamic nature of the model lends itself to incorporation of new data as it becomes available, such as institutional DTN or door-to-puncture times.
Supplementary Material
Acknowledgments
Funding:
This work was funded by a mini-grant from the American Heart Association’s Northeast Cerebrovascular Consortium (NECC) and won additional funding at the 2017 NECC Meeting’s “Stroke Tank” competition. KSZ reports funding from the Agency for Healthcare Quality and Research K08HS024561–01.
Footnotes
Disclosures:
LHS reports serving as Stroke Systems of Care Consultant to the Massachusetts Department of Public Health (modest), as Chair of the AHA Hospital Accreditation Science Subcommittee and Co-chair of Mission-Lifeline Stroke (unpaid), and as a consultant to Penumbra (significant) and Medtronic (significant). In addition, LHS has received significant grants from the NINDS and Genentech. CH reports equity (modest) in Atlas 5D. The other authors have no relationships to disclose.
References
- 1.Schlemm E, Ebinger M, Nolte CH, Endres M, Schlemm L. Optimal transport destination for ischemic stroke patients with unknown vessel status: Use of prehospital triage scores. Stroke. 2017;48:2184–2191 [DOI] [PubMed] [Google Scholar]
- 2.Holodinsky JK, Williamson TS, Kamal N, Mayank D, Hill MD, Goyal M. Drip and ship versus direct to comprehensive stroke center: Conditional probability modeling. Stroke. 2017;48:233–238 [DOI] [PubMed] [Google Scholar]
- 3.Dozois A, Hampton L, Kingston CW, Lambert G, Porcelli TJ, Sorenson D, et al. Plumber study (prevalence of large vessel occlusion strokes in mecklenburg county emergency response). Stroke. 2017;48:3397–3399 [DOI] [PubMed] [Google Scholar]
- 4.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the nih stroke scale using video training. Ninds tpa stroke study group. Stroke. 1994;25:2220–2226 [DOI] [PubMed] [Google Scholar]
- 5.Perez de la Ossa N, Carrera D, Gorchs M, Querol M, Millan M, Gomis M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: The rapid arterial occlusion evaluation scale. Stroke. 2014;45:87–91 [DOI] [PubMed] [Google Scholar]
- 6.Python Core Team (2018). Python: A dynamic, open source programming language. Python Software Foundation URL https://www.python.org/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


