Abstract
Inflammation promotes adverse ventricular remodeling. T1 mapping has been used to non-invasively assess interstitial myocardial fibrosis. We examined the association of baseline markers of systemic inflammation with interstitial myocardial fibrosis measured by extracellular volume fraction and native T1 mapping at 10 years’ follow-up in the Multi-Ethnic Study of Atherosclerosis. 772 participants had complete baseline data and underwent cardiac magnetic resonance imaging. All analyses were stratified by sex. Multivariable linear regression models were constructed to assess the associations of baseline C-Reactive Protein, interleukin 6 and fibrinogen with native T1 time and extracellular volume fraction. Longer native T1 times and higher percentages of extracellular volume fraction represent increasing myocardial fibrosis. A one standard deviation increment of log-transformed interleukin 6 levels was associated with 0.4% higher extracellular volume fraction in males (β= 0.4; p= 0.05). C-Reactive Protein and fibrinogen were not associated to extracellular volume fraction. A one standard deviation increment in the log-transformed CRP levels was associated with 4.9 ms higher native T1 (β = 4.9; p= 0.03). In females, the inflammatory markers did not demonstrate association with native T1 nor extracellular volume fraction. Higher interleukin 6 and C-Reactive Protein levels are associated with increased interstitial myocardial fibrosis assessed by cardiac magnetic resonance in males. However, no inflammatory markers were associated to myocardial fibrosis in females.
Keywords: inflammation, left ventricle, cardiac remodeling, myocardial fibrosis, cardiac magnetic resonance imaging
Introduction
Interstitial myocardial fibrosis (IMF) has been repeatedly identified as a usual feature of cardiac remodeling despite differences in the underlying etiology. 1 Recent advances in non-invasive cardiac imaging, particularly magnetic resonance imaging (MRI), have made possible detailed tissue characterization and identification of diverse patterns of myocardial fibrosis. 2 Myocardial T1 mapping enables quantification of exact T1 values of myocardial tissue and can detect subtle and diffuse changes in the myocardial extracellular matrix (ECM), characteristic of IMF3.
Multiple complex and overlapping pathways including inflammation, neuro-hormonal activation and ongoing myocardial injury orchestrate the process of cardiac remodeling that leads to IMF.4 Despite pharmacotherapeutic advances in targeting traditional risk factors and blockades of the renin-angiotensin-aldosterone and adrenergic systems, the incidence of heart failure (HF) is still high. Additionally, disappointing results for the treatment of HF with preserved ejection fraction highlights that the current therapeutic paradigm for HF is missing one or more key pathophysiological mechanisms. 5 Exploring alternate novel pathways associated with IMF and myocardial remodeling will allow us to better understand the pathogenesis of HF and develop more successful and targeted therapeutic interventions.
There is accumulating evidence supporting the role of inflammation in the initiation and promotion of various cardiovascular disease. 4, 6, 7 Circulating inflammatory markers have been correlated with molecular markers of fibrosis, 8 and histologic analyses of areas of IMF have found a preponderance of immune cells. 4 In a population-based cohort free of known cardiovascular disease at enrollment, we hypothesized that baseline serum markers of systemic inflammation, including C-reactive protein (CRP), interleukin 6 (IL6) and fibrinogen, are associated in a sex-specific manner with the extent of IMF measured by MRI T1 mapping at 10 years’ follow-up.
Material and Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing or expanding on the results after application to and approval by the MESA Publications and Presentations Committee (described at http://www.mesa-nhlbi.org).
Study Design and Population
The Multi-Ethnic Study of Atherosclerosis (MESA), is a multi-center prospective cohort study that recruited 6,814 participants over two years (2000 – 2002), aged 45 to 84 years old and free from known cardiovascular disease with the aim of better understanding subclinical cardiovascular disease processes. In the longitudinal follow-up of this cohort, spanning approximately 10 years, extensive phenotyping of the cohort population was performed over five examinations. 4,716 participants of the 6,814 originally enrolled participated in the fifth examination (MESA 5, 2010 – 2012). Of those, 3,015 underwent cardiac MRI (Figure 1). All participants provided signed informed consent. The study design and protocol were approved by the Institutional Review Boards of all participating institutions. T1 mapping using the modified Look-Locker inversion recovery sequence (MOLLI) was performed in 1,345 participants at 5 clinical sites were able to perform the protocol (Johns Hopkins University, Baltimore, Maryland; University of Minnesota, Minneapolis, Minnesota; Northwestern University, Chicago, Illinois; Wake Forest University, Winston-Salem, North Carolina; and University of California, Los Angeles, California). For the purposes of this analysis, patients with a self-reported condition that could modulate levels of non-specific inflammatory markers and render them non-representative of chronic baseline levels of systemic inflammation were excluded (N=550). These conditions included self-reported of a history of fever, upper respiratory tract infection, pneumonia, bronchitis, seasonal allergy, tooth infection, urinary tract infection, gout flare or arthritis flare within two weeks prior to baseline serum collection. Furthermore, 23 (1.7%) participants who had T1 measures of diffuse fibrosis at exam 5 had missing variables and were additionally excluded from this analysis, leaving 772 participants to constitute the final study population (Figure 1).
Figure 1.
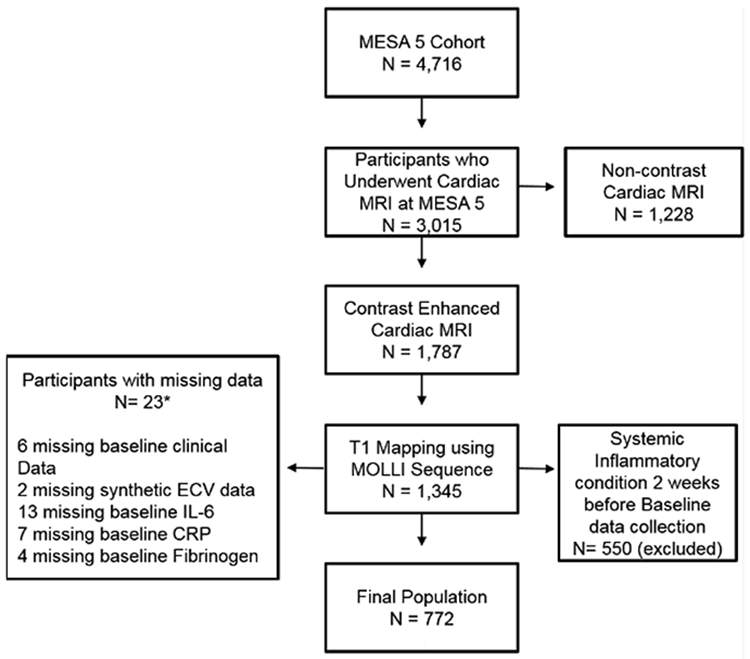
Flowchart delineating inclusion and exclusion criteria of the study population.
CRP: C-Reactive Protein, ECV: Extracellular Volume Fraction; IL6: Interleukin 6, MESA: Multi-Ethnic Study of Atherosclerosis, MOLLI: Modified Look-Locker Inversion MRI: Magnetic Resonance Imaging.
*Some participants had data for more than one variable missing.
Cardiac Magnetic Resonance Imaging Protocol at MESA 5
The MRI protocol used for evaluation of myocardial fibrosis in MESA exam 5 has been previously described.9 Briefly, participants with estimated glomerular filtration rate ≥ 45 mL/min (≥ 60 mL/min for participants enrolled at Northwestern University) and with no history of allergic reactions to contrast agents underwent a contrast enhanced MRI examination using 1.5 Tesla scanners (Avanto and Espree, Siemens Medical Systems, Erlangen, Germany) with a 6-channel anterior phased array torso coil and corresponding posterior coil elements. Left ventricular function, mass and dimensions were determined using a cine steady state free precession sequence where 12 short-axis slices, 1 four-chamber view and 1 two-chamber view were acquired. The MOLLI Sequence was used to assess diffuse myocardial fibrosis.10 1 short-axis MOLLI sequence was performed during end-expiratory apnea before contrast administration and 12 and 25 minutes following an intravenous bolus injection of Gadolinium–diethylene triamine pentaacetic acid (0.15 mmol/kg [Magnevist, Bayer Healthcare Pharmaceuticals, Montville, New Jersey]). The MOLLI sequence consisted of 3 consecutive inversion recovery-prepared, electrocardiography-synchronized Look-Locker trains. Each train was preceded by an inversion pulse at a specific inversion time (100 ms, 200 ms and 350 ms) and was followed by multiple single shot steady state free precession images in consecutive heartbeats. A total of 11 images in 17 heartbeats were acquired per sequence. The same trigger delay used in end diastole was applied for all acquired images. Uniform scanning parameters were deployed across the five centers and included: flip angle = 35º; echo time = 1.1 ms; repetition time = 2.2 ms; field of view: 360 × 360 mm; matrix = 192 × 183; slice thickness = 8 mm; and generalized auto calibrating partially parallel acquisitions factor = 2.
Image Analysis and T1 Measures
All acquired images were transferred to the central core MRI lab (Johns Hopkins University, Baltimore, Maryland) where post-processing and analysis of images was performed in a blinded fashion by MRI-proficient researchers at dedicated workstations. T1 maps were constructed offline using MASS research software (Department of Radiology, Leiden University Medical Center, Leiden, Netherlands). Using the Levenberg-Marquardt algorithm, a 3-parameter curve of the MOLLI source images was fitted and T1 times calculated. For each of the three time points (1 precontrast and 2 postcontrast), a region of interest was manually drawn around the core myocardium, excluding both the epicardium and blood pool at either end, which was used to calculate the myocardial T1 time for each subject. As a result, three myocardial T1 measures were calculated reflecting native T1 and postcontrast T1 at 12 minutes and at 25 minutes. A similar method was used to calculate T1 blood times with regions of interest encircling the blood pool at end diastole. The slope of the line determined by plotting 1/T1myo vs. 1/T1blood at the three predetermined time points was used to calculate the partition coefficient. The extracellular volume fraction (ECV) was then calculated by multiplying the partition coefficient by [1-hematocrit]. Data on hematocrit levels at the time of the MRI exam was only available for 369/772 (47.8 %) of the participants included in this study which restricted ECV measurements, thus we generated the synthetic ECV based on the longitudinal relaxation rate of blood.11 Synthetic ECV has a great correlation to conventional ECV and is associated to cardiovascular outcomes.11 We opted to use native T1 and synthetic ECV as our reference to estimate diffuse IMF.12 The greater the ECV percentage and T1 time, the greater the IMF amount.2
Biomarkers
CRP, IL6 and fibrinogen were measured in fasting serum samples drawn at baseline in all participants enrolled in MESA. All serum sample handling and storage as well as biomarker measurements were completed at the Laboratory for Clinical Biochemistry Research at the University of Vermont using standardized procedures.13 Previous studies have demonstrated a correlation between serum and myocardial cytokine levels indicating that non-invasive serum biomarkers can be used as surrogate measurements for the cardiac inflammatory milieu.14, 15
CRP was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL), which uses a particle enhanced immunonephelometric assay to measure levels of CRP. The reported assay range is 0.175 – 1,100 mg/L with reported intra-assay and inter-assay coefficient of variation ranging from 2.3–4.4 % and 2.1–5.7%, respectively. Normal expected values of CRP in healthy adults, per the manufacturer, are ≤ 3 mg/L.
IL6 was measured by ultra-sensitive sandwich ELISA (Quantikine HS Human IL6 Immunoassay; R&D Systems, Minneapolis, MN) using antibody-based colorimetric detection. The detection range is 0.156–10.0 pg/mL with a lower detection limit of <0.0094 pg/mL and a laboratory coefficient of variation of 6.3%. According to the manufacturer, normal expected values of IL6 in healthy adults range from 0.24 to 12.5 pg/mL.
Fibrinogen antigen was measured using the BNII nephelometer (N Antiserum to Human Fibrinogen; Dade Behring Inc., Deerfield, IL). The amount of fibrinogen present in the sample was quantitatively determined by an immunochemical reaction. The reported intra-assay and inter-assay coefficient of variation are 2.7% and 2.6%, respectively. Normal expected values for fibrinogen in healthy adults, per the manufacturer, are between 180 – 350 mg/dL.
Statistical Analysis
The cohort was stratified by sex for all analyses. Continuous variables were assessed for normality using the Shapiro-Wilk test and graphical plots. Accordingly, in order to better fit a linear relationship between the variables, CRP and IL6 were logarithmic transformed for this analysis. Continuous variables with a normal Gaussian distribution are summarized as mean ± SD. Discrete variables are presented as count and relative frequency (%). Locally weighted scatterplot smoothing method was used to assess the linearity of the association of the three baseline inflammatory markers with ECV and native T1. Multivariable linear regression was implemented to assess the association between ECV and baseline fibrinogen, Log-transformed CRP and Log-transformed IL6 as well as the association between native T1 and the same variables modeled as continuous variables. Models were constructed in which the covariates included reflect baseline clinical measurements at the time of measurement of inflammatory markers and MRI measurements at MESA 5. Additional models are available in the supplemental material (unadjusted, adjusted for demographics, adjusted for demographics and traditional risk factors and a full adjusted model stratified by ethnicity, please see http://hyper.ahajournals.org.). A two-sided p <0.05 was considered statistically significant. All analyses were done using STATA 15 (College Station, TX: StataCorp LP).
Results
The MESA sub-population included in this analysis was comprised of 772 participants who had complete baseline data as well as follow-up measures of myocardial T1 time, including 446 (57.8%) males and 326 (42.2 %) females. Baseline characteristics of the study population, stratified by sex, are presented in Table 1. Mean age of males was 59.3 with 54.5%, 21.7%, 9.9% and 13.9% being Caucasian, African American, Chinese American and Hispanic, respectively. Females had a mean age of 58.3 and a racial distribution of: 50.9 % Caucasians, 23% African American, 13.5 % Chinese American and 12.6 % Hispanic. Females had a higher percentage of non-diabetics than male (85.6% and 76.7%, respectively, p<0.05), lower percentage of impaired fasting glucose and diabetic, had better smoking profile (58.9% never smoking and 41.5% in males, p<0.05), lower diastolic blood pressure (67.6 mmHg and 75.3 mmHg in males, p<0.05) and higher high-density lipoprotein levels than male (56.4 mg/dl and 44.2 mg/dL, respectively, p<0.05). Moreover, cardiac MRI measures showed higher LV ejection fraction, ECV percentage and native T1 values in females and higher LV end systolic and diastolic volumes and LV mass in males (p<0.05). Females had higher baseline CRP and fibrinogen levels than males (3.9 mg/L, 337.7 mg/dL and 2.1 mg/L, 318.3 mg/dL, respectively) (Table 1).
Table 1. Baseline Characteristics Stratified by Sex.
| Characteristics | Males (N=446) | Females (N=326) |
|---|---|---|
| Age (years) | 59.3 (SD 8.9) | 58.3 (SD 8.9) |
| Body Mass Index (kg/m2) | 28.1 (SD 4.1) | 27.6 (SD 5.7) |
| Race (%) | ||
| Caucasian | 243 (54.5) | 166 (50.9) |
| African American | 97 (21.7) | 75 (23.0) |
| Chinese American | 44 (9.9) | 44 (13.5) |
| Hispanic | 62 (13.9) | 41 (12.6) |
| Cigarette Smoking (%)* | ||
| Never | 185 (41.5) | 192 (58.9) |
| Former | 208 (46.6) | 98 (30.1) |
| Current | 53 (11.9) | 36 (11.0) |
| Diabetes Status (%)* | ||
| Non-diabetic | 342 (76.7) | 279 (85.6) |
| Impaired Fasting Glucose | 61 (13.7) | 27 (8.3) |
| Untreated Diabetes | 14 (3.1) | 4 (1.2) |
| Treated Diabetes | 29 (6.5) | 16 (4.9) |
| Systolic Blood Pressure (mmHg) | 122.0 (SD 16.1) | 120.6 (SD 22.9) |
| Diastolic Blood Pressure (mmHg) * | 75.3 (SD 8.9) | 67.6 (SD 10.3) |
| Hypertension Medication (%) | 123 (27.6) | 86 (26.4) |
| Cholesterol (mg/dL) * | 188.7 (SD 34.6) | 200.7 (SD 36.5) |
| LDL (mg/dL) | 117.9 (SD 30.4) | 117.9 (SD 30.4) |
| HDL (mg/dL) * | 44.2 (SD 10.6) | 56.4 (SD15.1) |
| Triglycerides (mg/dL) | 134.6 (SD 78.3) | 125.9 (SD 74.1) |
| Lipid Lowering Medication (%)* | 86 (19.3) | 43 (13.2) |
| LV Ejection Fraction (%) *† | 59.3 (SD 7.5) | 63.9 (SD 5.9) |
| LV End Systolic Volume index (mL/ m2) *†‡ | 28.6 (SD 10.4) | 22.5 (SD 5.7) |
| LV End Diastolic Volume index (mL/ m2) *†‡ | 69.3 (SD 15.7) | 62.1 (SD 10.7) |
| LV Mass index (g/m2) *†‡ | 73.1 (SD 11.9) | 57.7 (SD 9.0) |
| ECV (%)*† | 26.7 (SD 3.1) | 27.4 (SD 2.5) |
| Native T1 (ms) *† | 970.6 (SD 39.6) | 985.8 (SD 44.2) |
| Interleukin 6 (pg/mL) | 1.3 (SD 1.1) | 1.3 (SD 1.0) |
| C Reactive Protein (mg/L) * | 2.1 (SD 3.2) | 3.9 (SD 4.4) |
| Fibrinogen (mg/dL) * | 318.3 (SD 57.6) | 337.7 (SD 65.8) |
HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; LV: Left Ventricle; ECV: Extracellular Volume fraction; SD: Standard deviation
p value < 0.05 comparing men and women characteristics
Variables were measured during MESA 5 exam; All other variables reflect MESA 1 baseline values
LV volumes and mass are indexed to body surface area
Significant associations between IL6 levels and ECV percentages were found in males. One standard deviation increment of log-transformed IL6 levels was associated with 0.4% higher ECV (β= 0.4; p=0.05). However, no significant association was found between the other inflammation markers tested (fibrinogen and CRP) and ECV in males. Among males without myocardial scar, previous myocardial infarction or clinical HF, a one standard deviation increment in the log-transformed CRP levels was associated with 4.9 ms higher native T1 (β= 4.9; P=0.03). (Table 2). However, there was no association between inflammatory markers levels and ECV or native T1 values in females (Table 2) and the associations between markers of systemic inflammation and IMF in males and females were independent of body mass index.
Table 2a.
Multivariable association between baseline markers of systemic inflammation and T1 mapping measures (Native T1mapping and Extracellular Volume Fraction in males (n=446)
| Inflammatory Markers | ECV (%) | Native T1 (ms) | |||
|---|---|---|---|---|---|
| Β-Coefficient (95% CI) | p-Value | Β-Coefficient (95% CI) | p-Value | ||
| Log IL6 * (pg/mL) | Model A | 0.6 (0.1 – 1.0) | 0.01 | 3.1 (−3.0–9.3) | 0.32 |
| Model B | 0.4 (0.01 – 0.9) | 0.05 | 2.8 (−4.0–9.6) | 0.42 | |
| Fibrinogen (mg/dL) | Model A | −0.0002 (−0.005–0.005) | 0.94 | −0.005 (−0.07–0.06) | 0.88 |
| Model B | 0.001 (−0.003–0.006) | 0.61 | 0.01 (−0.06–0.08) | 0.77 | |
| Log CRP * (mg/L) | Model A | 0.07 (−0.2–0.4) | 0.66 | 3.7 (−0.2–7.7) | 0.06 |
| Model B | 0.2 (−0.1–0.5) | 0.23 | 4.9 (0.5–9.2) | 0.03 | |
CRP: C-Reactive Protein; ECV: Extracellular Volume fraction; IL6: Interleukin 6; CI: Confidence interval
CRP and IL6 had a right-skewed distribution leading to logarithmic transformation of these variables for this analysis
Model A is adjusted for age, race, body mass index, baseline cigarette smoking status, diabetes classification, systolic blood pressure, total cholesterol, high density lipoprotein, hypertension medication use and lipid lowering medication use, left ventricular ejection fraction, left ventricular mass index and end diastolic volume index at the time of the MRI exam in MESA 5
Model B: Model A and excluding those with myocardial scar using late gadolinium enhancement, history of myocardium infarction or clinical heart failure (n=372).
Discussion
To our knowledge, this is the first multi-center and multi-ethnic population-based study to address the association between systemic inflammation and IMF estimated by T1 mapping, shedding light on alternate pathologic pathways related to IMF. Increased Interlukin-6 and C-reactive protein levels were associated with higher IMF measures in males. There was no association of the three biomarkers of systemic inflammation (CRP, IL6 and fibrinogen) and IMF in females. IMF measured by T1 mapping is a novel and highly sensitive marker of early cardiovascular remodeling with established prognostic significance for cardiovascular outcomes.2, 12 Identifying inflammation as a potential pathogenic pathway associated with IMF in a population free of known cardiovascular disease may help in better understanding the early pathways contributing to the pathogenesis of HF and point out possible novel therapeutic targets to curtail the rising incidence of HF.
Inflammation and Interstitial Myocardial Fibrosis
The myocardial syncytium is embedded in a rich ECM. An intricate interaction of the cellular components of the ECM, including supporting fibroblasts, resident inflammatory cells, endothelial cells and cardiomyocytes, dictates the composition and volume of the underlying proteinaceous matrix. Pathologic states associated with increased IMF are characterized by expansion of the ECM. This may occur as a primary process mediated by cellular constituents of the ECM or as a response to apoptotic or necrotic myocardial cell loss.1 Multiple molecular pathways such as the renin-angiotensin-aldosterone system, the adrenergic axis, inflammatory cytokines, transforming growth factor beta/Smad signaling and other growth factor mediated pathways have been shown to contribute to the fibrogenic process.4
The pro-inflammatory cytokines, interleukin 1, 6 and tumor necrosis factor-alpha have been reported in multiple studies as integral components of the fibrogenic pathway in the heart 16. IL6 levels in post myocardial infarction individuals were associated with infarct size as well as ventricular remodeling remote from the infarct site. Furthermore, administration of IL6 neutralizing antibodies following myocardial infarction in mice was shown to attenuate LV remodeling both at the infarct site and remotely.17 Similarly, elevated levels of CRP have been associated with increased LV remodeling post myocardial infarction18 with subsequent reduction of LV remodeling following selective CRP apheresis in a porcine model.19 Plasma levels of CRP and fibrinogen have been shown to be associated with incidence of symptomatic HF.20 Additionally, fibrinogen has been associated with subclinical diastolic dysfunction in hypertensive patients.21 In population-based studies correlations between circulating markers of fibrosis and inflammatory markers have been reported,8 and were associated with poorer cardiovascular outcomes.22
IL6 was positively associated with myocardial fibrosis assessed by ECV in males. However, no significant association between ECV and CRP or fibrinogen levels was demonstrated in males. Additionally, native T1 levels were positively associated with CRP but not IL6 or fibrinogen in males. The discrepancy in associations between markers of systemic inflammation and measures of IMF namely, ECV and native T1 time is intriguing. Notably, native T1 and ECV, despite a tight correlation, are affected differentially by changes in the intra- and extra-cellular compartments. ECV measures the extracellular volume fraction, while native T1 measures both the intra and extracellular spaces2, 12. Thus ECV, may be more sensitive and specific to changes in the extracellular space. Native T1 time, albeit not a perfect measure of changes in the extracellular volume, may serve as a surrogate marker of IMF in participants with renal dysfunction who are unable to receive intravenous gadolinium-based contrast which precludes measurement of ECV in these individuals12. Changes in T1 time are usually presumed to be secondary to changes in the extracellular compartment and for these reasons native T1 was included as an endpoint in this analysis. The utility of native T1 time has been corroborated in recent studies.23, 24 In addition, previous studies associating inflammatory markers and myocardial fibrosis used individuals with known inflammatory diseases, showing a strong correlation between disease activity, IMF and subclinical impaired ventricular function.25, 26 MESA was designed primarily to evaluate subclinical cardiovascular diseases and a lower systemic inflammation level is expected in its participants than the aforementioned samples. A greater and sustained inflammatory status may be necessary to identify an association between inflammatory markers and all T1 mapping measures, which is unlikely in our cohort, and more resembles the general population regarding systemic and subclinical inflammation status.
Sex, Inflammation and Interstitial Myocardial Fibrosis
Despite having higher CRP and fibrinogen levels at baseline, these inflammatory markers were not associated to myocardial fibrosis in females. The mechanisms for development and progression of myocardial fibrosis in females are not completely known and probably result from multiple and combined factors. Gender differences in myocardial fibrosis patterns have been attributed to genetic, hormonal and environmental factors. Genes with prominent roles in ventricle remodeling have distinct regulation between sexes. Kararigas et al. showed genes suppression in hypertrophied women’s heart leading to a repression of chemokine- and cytokine-related and inflammatory pathways.27 Furthermore, estrogen has been described to suppress vascular inflammation by downregulating proinflammatory molecules as cytokines and adhesion molecules, subsequently protecting the myocardial from inflammation and fibrosis.7, 28 Besides that, there are major sex differences in heart structure (size, physiology and contractile properties), response to volume or pressure overload and response to aging.9, 27, 29 Finally, there are other variables significantly different between sexes which could influence ventricle remodeling such as external exposures and cardiovascular risk factors as a consequence of lifestyle.7, 27 The effect of all those elements together might modulate or overlap inflammation’s role in myocardial fibrosis in female.
Interstitial Fibrosis and Cardiovascular Disease
IMF is a phenotypic expression of an underlying disease process and has been associated with subclinical myocardial dysfunction as well as a prognostic marker for cardiovascular events dysfunction.30–32 Myocardial fibrosis in animal and human studies is associated with increased ventricular stiffness, worsening ventricular diastolic and systolic function as well as abnormal cardiac remodeling. In a recent population-based study increased IMF has been associated with reduced systolic LV shortening independent of LV mass and volume30. Furthermore, IMF in females correlated with greater diastolic dysfunction and preserved ejection fraction whereas in males it was associated with reduced LV torsion and lower ejection fraction.30 IMF is a reversible intermediate phenotype that precedes adverse cardiovascular outcomes if left untreated.31, 32 Diagnostic strategies using reliable and noninvasive tools such as native T1 and ECV could enable the early identification of IMF and development of targeted therapies to block contributing pathogenic pathways will provide an opportunity to meaningfully impact the HF epidemic.
Limitations
Limitations affecting our analysis include the fact that inflammatory markers were only measured at baseline and thus we were not able to account for possible changes of levels of circulating inflammatory markers during follow-up. Furthermore, despite comprehensive adjustment for multiple established cardiovascular risk factors, we cannot certainly exclude the presence of unmeasured confounders that we could not account for them. Finally, native T1 and ECV have been used to measure myocardial fibrosis but they are not specific for single diseases and their results might be evaluated in the context of specific clinical scenarios.12 ECM expansion usually is secondary to increased fibrosis, but may in some cases be due to other causes such as edema, hypertrophy or other infiltrative cardiac disorders.2 On the other hand, these techniques are reliable and noninvasive options to evaluate myocardial fibrosis and native T1 does not need contrast injection, being an option for individuals with renal impairment.
Conclusion
This study demonstrates a positive association between IL6 and CRP levels and diffuse myocardial fibrosis, as evaluated by cardiac MRI after a ten-year follow-up exam in a large multi-ethnic population cohort (MESA study). This association was present in males after adjustment for age, ethnicity, traditional cardiovascular risk factors and for LV structural and functional parameters.
Perspectives
Cardiac remodeling is an important factor in the development of cardiomyopathies and is related to HF and cardiovascular events. IMF is an important remodeling feature and is associated with poor prognosis. The pathways to developing IMF and the associated factors are not completely understood and there are few population and multiethnic studies on this subject. Our study demonstrated the association of inflammatory markers and myocardial fibrosis detected by cardiac magnetic resonance imaging. This study stimulates future investigation to determinate the role of chronic inflammation in generating IMF in humans and therefore focus efforts in avoiding its development.
Supplementary Material
Table 2b. Multivariable association between baseline markers of systemic inflammation and T1 mapping measures (Native T1mapping and Extracellular Volume Fraction) in females (n=326).
| Inflammatory Markers | ECV (%) | Native T1 (ms) | |||
|---|---|---|---|---|---|
| Β-Coefficient (95% CI) | P-Value | Β-Coefficient (95% CI) | P-Value | ||
| Log IL6 * (pg/mL) | Model A | 0.05 (−0.4 – 0.5) | 0.83 | 4.9 (−3.9 – 13.7) | 0.28 |
| Model B | −0.03 (−0.5 – 1.0) | 0.91 | 3.6 (−5.4 – 12.6) | 0.43 | |
| Fibrinogen (mg/dL) | Model A | −0.0008 (−0.005 – 0.003) | 0.71 | 0.02 (−0.06 – 0.1) | 0.69 |
| Model B | −0.001 (−0.005 – 0.003) | 0.55 | 0.006 (−0.07 – 0.08) | 0.88 | |
| Log CRP * (mg/L) | Model A | −0.08 (−0.3 – 0.2) | 0.53 | 1.7 (−3.0 – 6.3) | 0.48 |
| Model B | −0.1 (−0.3 – 0.1) | 0.45 | 0.9 (−3.7 – 5.6) | 0.70 | |
CRP: C-Reactive Protein; ECV: Extracellular Volume fraction; IL6: Interleukin 6 CI: Confidence interval
CRP and IL6 had a right-skewed distribution leading to logarithmic transformation of these variables for this analysis
Model A is adjusted for age, race, body mass index, baseline cigarette smoking status, diabetes classification, systolic blood pressure, total cholesterol, high density lipoprotein, hypertension medication use and lipid lowering medication use, left ventricular ejection fraction, left ventricular mass index and end diastolic volume index at the time of the MRI exam in MESA 5
Model B: Model A and excluding those with myocardial scar using late gadolinium enhancement, history of myocardium infarction or clinical heart failure (n=317).
Novelty and Significance
What is new?
This is the first population based multiethnic study evaluating the association between inflammatory markers and IMF assessed by cardiac MRI with a ten year’s follow up.
What is relevant?
The role of inflammation in the pathogenesis of hypertension has been progressively recognized and hypertension is associated with cardiac remodeling features.
Our results demonstrate the association between inflammatory markers and IMF, both related to hypertension.
Summary
This study demonstrated the association between inflammatory markers and IMF in a large multiethnic study. Both factors play a role in cardiomyopathies pathophysiology, determining a worse prognosis and suggesting that controlling the chronic inflammation could decrease adverse cardiac remodeling features.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, by grants UL1-TR-000040 and UL1-TR-001079 from National Center for Research Resources the NIH Intramural Research Program, and by a grant from Bayer Healthcare for the use of gadolinium contrast agent.
Disclosures
No author has anything to disclose.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
References
- 1.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26 [DOI] [PubMed] [Google Scholar]
- 2.Ambale-Venkatesh B, Lima JA. Cardiac mri: A central prognostic tool in myocardial fibrosis. Nat Rev Cardiol. 2015;12:18–29 [DOI] [PubMed] [Google Scholar]
- 3.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced t1 mapping. J Am Coll Cardiol. 2008;52:1574–1580 [DOI] [PubMed] [Google Scholar]
- 4.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2017 acc/aha/hfsa focused update of the 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of america. Circulation. 2017;136:e137–e161 [DOI] [PubMed] [Google Scholar]
- 6.Suthahar N, Meijers WC, Sillje HHW, de Boer RA. From inflammation to fibrosis-molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep. 2017;14:235–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairweather D. Sex differences in inflammation during atherosclerosis. Clin Med Insights Cardiol. 2014;8:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph J, Pencina MJ, Wang TJ, Hayes L, Tofler GH, Jacques P, Selhub J, Levy D, D’Agostino RB Sr., Benjamin EJ, Vasan RS. Cross-sectional relations of multiple biomarkers representing distinct biological pathways to plasma markers of collagen metabolism in the community. J Hypertens. 2009;27:1317–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JAC. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced t1 mapping: Mesa (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2013;62:1280–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified look-locker inversion recovery (molli) for high-resolution t1 mapping of the heart. Magn Reson Med. 2004;52:141–146 [DOI] [PubMed] [Google Scholar]
- 11.Treibel TA, Fontana M, Maestrini V, et al. Automatic measurement of the myocardial interstitium: Synthetic extracellular volume quantification without hematocrit sampling. JACC Cardiovasc Imaging. 2016;9:54–63 [DOI] [PubMed] [Google Scholar]
- 12.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of t1, t2, t2* and extracellular volume: A consensus statement by the society for cardiovascular magnetic resonance (scmr) endorsed by the european association for cardiovascular imaging (eacvi). Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the cardiovascular health study. Clin Chem. 1995;41:264–270 [PubMed] [Google Scholar]
- 14.Gerling IC, Ahokas RA, Kamalov G, Zhao W, Bhattacharya SK, Sun Y, Weber KT. Gene expression profiles of peripheral blood mononuclear cells reveal transcriptional signatures as novel biomarkers for cardiac remodeling in rats with aldosteronism and hypertensive heart disease. JACC Heart Fail. 2013;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toli K, Paraskevas KI, Poulakou MV, Agrogiannis G, Kavantzas N, Xanthopoulos V, Iliopoulos DG, Mantas I, Papachristodoulou A, Patsouris E, Mikhailidis DP, Perrea DN. Association between plasma levels and immunolocalization of cytokines in heart valve lesions: A possible target for treatment? Expert Opin Ther Targets. 2008;12:1209–1215 [DOI] [PubMed] [Google Scholar]
- 16.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553 [DOI] [PubMed] [Google Scholar]
- 17.Kobara M, Noda K, Kitamura M, Okamoto A, Shiraishi T, Toba H, Matsubara H, Nakata T. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc Res. 2010;87:424–430 [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, Anzai T, Kaneko H, Mano Y, Anzai A, Nagai T, Kohno T, Maekawa Y, Yoshikawa T, Fukuda K, Ogawa S. Increased c-reactive protein expression exacerbates left ventricular dysfunction and remodeling after myocardial infarction. American journal of physiology. Heart and circulatory physiology. 2010;299:H1795–1804 [DOI] [PubMed] [Google Scholar]
- 19.Slagman AC, Bock C, Abdel-Aty H, et al. Specific removal of c-reactive protein by apheresis in a porcine cardiac infarction model. Blood purification. 2011;31:9–17 [DOI] [PubMed] [Google Scholar]
- 20.Eisen A, Benderly M, Behar S, Goldbourt U, Haim M. Inflammation and future risk of symptomatic heart failure in patients with stable coronary artery disease. Am Heart J. 2014;167:707–714 [DOI] [PubMed] [Google Scholar]
- 21.Catena C, Colussi G, Fedrizzi S, Sechi LA. Association of a prothrombotic state with left-ventricular diastolic dysfunction in hypertension: A tissue-doppler imaging study. J Hypertens. 2013;31:2077–2084 [DOI] [PubMed] [Google Scholar]
- 22.Agarwal I, Glazer NL, Barasch E, Biggs ML, Djousse L, Fitzpatrick AL, Gottdiener JS, Ix JH, Kizer JR, Rimm EB, Sicovick DS, Tracy RP, Mukamal KJ. Fibrosis-related biomarkers and incident cardiovascular disease in older adults: The cardiovascular health study. Circulation. Arrhythmia and electrophysiology. 2014;7:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S. T(1) mapping for the diagnosis of acute myocarditis using cmr: Comparison to t2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging. 2013;6:1048–1058 [DOI] [PubMed] [Google Scholar]
- 24.Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S. Native t1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2014;16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ntusi NAB, Piechnik SK, Francis JM, Ferreira VM, Matthews PM, Robson MD, Wordsworth PB, Neubauer S, Karamitsos TD. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from cmr t1 mapping. JACC Cardiovasc Imaging. 2015;8:526–536 [DOI] [PubMed] [Google Scholar]
- 26.Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM, Robson MD, Moon J, Wordsworth PB, Neubauer S, Karamitsos TD. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis--a clinical study using myocardial t1-mapping and extracellular volume quantification. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2014;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, Knosalla C, Golz S, Hetzer R, Regitz-Zagrosek V. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 2014;16:1160–1167 [DOI] [PubMed] [Google Scholar]
- 28.Novella S, Heras M, Hermenegildo C, Dantas AP. Effects of estrogen on vascular inflammation: A matter of timing. Arterioscler Thromb Vasc Biol. 2012;32:2035–2042 [DOI] [PubMed] [Google Scholar]
- 29.Treibel TA, Kozor R, Fontana M, Torlasco C, Reant P, Badiani S, Espinoza M, Yap J, Diez J, Hughes AD, Lloyd G, Moon JC. Sex dimorphism in the myocardial response to aortic stenosis. JACC Cardiovasc Imaging. Published online November 15, 2017; DOI: 10.1016/j.jcmg.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donekal S, Venkatesh BA, Liu YC, Liu CY, Yoneyama K, Wu CO, Nacif M, Gomes AS, Hundley WG, Bluemke DA, Lima JA. Interstitial fibrosis, left ventricular remodeling, and myocardial mechanical behavior in a population-based multiethnic cohort: The multi-ethnic study of atherosclerosis (mesa) study. Circ Cardiovasc Imaging. 2014;7:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


