Abstract
Objective
Signaling that activates nuclear factor κB (NFκB) in smooth muscle cells (SMCs) is integral to atherosclerosis and involves reversible ubiquitination that activates proteins downstream of pro-atherogenic receptors. Deubiquitination of these proteins is mediated by ubiquitin-specific protease 20 (USP20), among other deubiquitinases. We sought to determine whether USP20 activity in SMCs decreases atherosclerosis.
Approach and Results
To address this question, we used male Ldlr−/− mice without (control) or with SMC-specific expression of murine USP20 (SMC-USP20-Tg) or its dominant-negative (C154S/H643Q) mutant (SMC-DN-USP20-Tg). Prior to the appearance of intimal macrophages, NFκB activation in aortic medial SMCs was greater in SMC-DN-USP20-Tg than in control mice. After 16 weeks on a Western diet, SMC-DN-USP20-Tg mice had 46% greater brachiocephalic artery atheroma area than control mice. Congruently, aortic atherosclerosis assessed en face was 21% greater than control in SMC-DN-USP20-Tg mice and 13% less than control in SMC-USP20-Tg mice. In response to TNF, SMCs from SMC-DN-USP20-Tg mice showed ~3-fold greater NFκB activation than control SMCs. Silencing USP20 in SMCs with siRNA augmented NFκB activation by ~50% in response to either TNF or interleukin-1β. Co-immunoprecipitation experiments revealed that USP20 associates with several components of the TNF receptor-1 signaling pathway including receptor-interacting protein kinase 1 (RIPK1), a critical checkpoint in TNF-induced NFκB activation and inflammation. TNF evoked ~2-fold more RIPK1 ubiquitination in SMC-DN-USP20-Tg than in control SMCs, and RIPK1 was deubiquitinated by purified USP20 in vitro.
Conclusions
USP20 attenuates TNF- and interleukin-1β-evoked atherogenic signaling in SMCs, by deubiquitinating RIPK1, among other signaling intermediates.
Keywords: K63 Polyubiquitination, De-ubiquitination, RIPK1, NFκB, TRAF2
Subject codes: Animal Models of Human Disease, Cell Signaling/Signal Transduction, Inflammation, Atherosclerosis
Introduction
Activated by IL-1 and oxidized LDL,1 respectively, the IL-1R and the TLR4-CD14 complex bind a group of signaling adaptors that include MyD88 (myeloid differentiation primary response gene 88) and members of the IL-1 receptor-associated kinase (IRAK) family (IRAK1 and IRAK4).2 IRAK triggers the oligomerization, activation and auto-ubiquitination of the E3 ubiquitin ligase TRAF6. The synthesis of K63-linked polyubiquitin chains by TRAF6 permits the scaffolding of signaling intermediates that include the transforming growth factor-β-activated kinase 1, which activates IKKβ.2 IKKβ phosphorylates the inhibitor of NFκB, IκBα, and thereby triggers K48-linked polyubiquitination, which tags IκB for proteasomal degradation.2 IκBα degradation promotes nuclear translocation of p65/p50 NFκB heterodimers, which mediate upregulation of pro-inflammatory target genes.2,3 IKKβ also phosphorylates the p65 subunit of NFκB on Ser536, and thereby activates its transcriptional activity.4
In response to TNF, the TNFR1 also activates NFκB through cascades of polyubiquitination that culminate in the activation of IKK and phosphorylation of IκBα.5 Activated TNFR1 promotes the association of a multi-protein complex that includes receptor-interacting serine/threonine-protein kinase 1 (RIPK1), the ubiquitin E3 ligase and scaffolding protein TRAF2 (or TRAF5) and the ubiquitin E3 ligases cIAP1 or cIAP2. In this complex, autoubiquitinated cIAP1 (or cIAP2) ubiquitinates RIPK1 and provides a polyubiquitin platform for scaffolding of transforming growth factor-β-activated kinase 1, which can activate IKKβ. This polyubiquitin platform also associates with and activates the tripartite E3 ligase complex known as LUBAC.6 LUBAC appends linear ubiquitin moieties to TNFR1, RIPK1 and IκB kinase-γ and can thereby promote IKKβ activation.6
We previously demonstrated that the deubiquitinase called USP20 inhibits TLR4-triggered NFκB activation by deubiquitinating TRAF6 in SMCs.7 Concordantly, SMC USP20 activity reduces neointimal hyperplasia induced by carotid endothelial denudation, as shown by transgenic mice expressing a dominant-negative USP20 mutant in SMCs.7 Because SMC gene expression is known to affect atherogenesis,8,9 we used these SMC-specific transgenic mice to ask whether USP20 activity in SMCs affects atherosclerosis.
Materials and Methods
The authors declare that all supporting data are available within the article [and its online supplementary files].
Materials
Chemicals, reagents, and antibodies are listed in the online-only Data Supplement.
Mice
All animal experiments were performed in accordance with protocols approved by Duke University Institutional Animal Care and Use Committee. All mice were congenic on the C57BL/6 genetic background. SM22-driven (SMC-specific) transgenic mice overexpressing mouse USP20 (SMC-USP20) or its catalytically inactive mutant, dominant negative USP20 (SMC-DN-USP20) have been described.7 These mice were crossed with Ldlr−/− mice to obtain the mice that were used for atherosclerosis studies. Verification of SMC-specific transgene expression was achieved with RT-PCR (Supplemental Figure I).
Atherosclerosis studies
These experiments adhered to the guidelines for experimental atherosclerosis studies described in the AHA Statement (ATVB 2017;37:e131. PMID: 28729366). Male mice from the age of 8 wk were fed a Western diet for either 1 wk (“pre-atherosclerotic”) or 16 wk (“atherosclerotic”), as indicated, and then euthanized. Aorta and brachiocephalic artery preparation, staining and quantitative assessment10 are detailed in the online-only Data Supplement. All quantitation was performed by observers blinded to specimen identity.
Mouse physiologic parameters
Serum total and high-density lipoprotein cholesterol were assayed as described.10 Blood pressure and heart rate were measured as described.10
Carotid artery interposition grafting
This procedure was performed as we described.11
Histology
Histochemical and immunofluorescence staining of tissue sections were performed on 5-µm sections and analyzed as we described.7,11 Quantitation was performed by observers blinded to specimen identity, as we described7,11 and as detailed in the online-only Data Supplement.
Cell Lines
Primary aortic SMCs were isolated from male and female mice as we reported.10 Human embryo kidney (HEK-293) cells from the American Type Culture Collection were cultivated as reported.7 HEK-293 cells with stable knockdown of USP20 were generated as described in the online-only Data Supplement.
RNA Interference
SMC transfection with siRNA was performed as described,7 and as detailed in the online-only Data Supplement.
Adenovirus-mediated Transduction
Recombinant adenoviruses expressing HA-tagged USP20 constructs were generated with the AdEasy system (Agilent Technologies). Procedures are detailed in the online-only Data Supplement.
Immunoprecipitation and Immunoblotting
SMCs and HEK-293 cells were solubilized and cellular proteins were immunoprecipitated as described,7 and as detailed in the online-only Data Supplement.
Polyubiquitinated Protein Pull-down
Pulldown of polyubiquitinated proteins was performed using tandem ubiquitin binding entities (TUBEs 1, UM401) covalently linked to agarose beads, according to the manufacturer’s protocol; details appear in the online-only Data Supplement.
USP20 Purification
USP20 was purified by immunoaffinity chromatography, as we reported,12 and as detailed in the online-only Data Supplement.
Deubiquitination of RIPK1 by Purified USP20
293-USP20 shRNA cells were transiently transfected with FLAG-tagged human RIPK1 (pCDNA3/ FLAG-RIPK1, obtained from Addgene).13 Twenty-four h post-transfection, the cells were serum-starved for 4 h and stimulated with 10 ng/ml TNF for 10 min. FLAG immunoprecipitation and deubiquitination reactions were performed as described in the online-only Data Supplement.
Statistical Analyses
All experiments were performed at least three independent times. Data from three or more independent experiments were averaged and presented as means ± SEM. Statistical comparisons between 2 groups were made with t tests as noted in Figure legends. Comparisons among more than two groups and analyses of time course data were made with two-way ANOVA followed by the Sidak post-hoc test for multiple comparisons, unless otherwise noted in the legend, using GraphPad Prism 7.03 statistics software (GraphPad, Inc.). Statistical significance was set at p < 0.05.
Results
SMC USP20 Activity Reduces Inflammation in Pre-Atherosclerotic Aortas
To study the effects of SMC USP20 on atherogenesis, we employed male Ldlr−/− mice with SMC-specific transgenic expression of either mouse USP20 (SMC-USP20) or the dominant-negative mutant of USP20 (SMC-DN-USP20).7 Non-Tg/Ldlr−/−, SMC-USP20-Tg/Ldlr−/−, and SMC-DN-USP20-Tg/Ldlr−/− mice (10–15 mice per group) were equivalent with regard to a variety of physical and metabolic parameters, including: (a) body weight (32±2, 31±3, 31±3 gm, respectively); (b) systolic blood pressure (124±6, 119±6, 122±4 mm Hg, respectively); (c) heart rate (680±30, 680±20, 670±20 beats/min, respectively); (d) serum total cholesterol concentration on a Western diet (23±5, 21±5, 23±5 mmol/L, respectively); serum high-density lipoprotein cholesterol concentration on a Western diet (1.0±0.3, 0.9±0.3, 0.9±0.4 mmol/L, respectively); serum amyloid A concentration after just one week on a Western diet (32±6, 30±10, and 30±10 µg/ml, respectively).
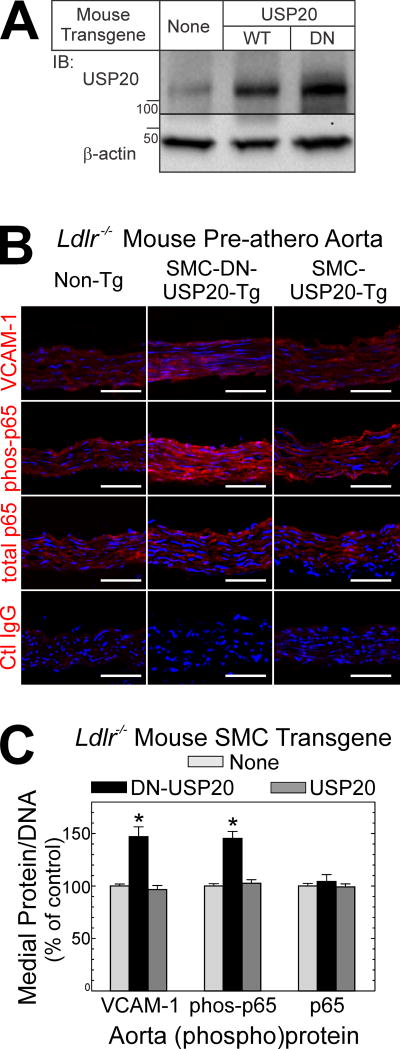
In the initial stages of atherogenesis, endothelial cells and SMCs express on their plasma membranes adhesion molecules like VCAM-1, which facilitates the accumulation of monocyte/macrophages in the intima.3 Expression of VCAM-1 is regulated by NFκB,14 and USP20 regulates NFκB activation downstream of the atherogenic TLR4.7 Accordingly, we asked whether altering SMC USP20 activity would alter the activation of NFκB or the expression of VCAM-1 in the aortas of atherogenic mice prior to the appearance of monocyte/macrophages in the intima. To address this question, we employed our SMC-USP20-Tg/Ldlr−/− and SMC-DN-USP20-Tg/Ldlr−/− mice.7 USP20 expression was 2- or 3-fold greater than endogenous, respectively, in SMC-USP20-Tg/Ldlr−/− and SMC-DN-USP20-Tg/Ldlr−/− mice (Figure 1A).
Figure 1.
USP20 activity in SMCs reduces NFκB activation in the aorta. A, Aortas from Non-Tg/Ldlr−/−, SMC-USP20-Tg/Ldlr−/− and SMC-DN-USP20-Tg/Ldlr−/− mice were solubilized, and 30 µg of aortic protein were immunoblotted serially for USP20 and actin. Shown is an immunoblot from a single experiment, representative of 3 performed. B, Male mice of the indicated genotype were fed a Western diet for 1 wk from the age of 8 wk, and then sacrificed. Aorta frozen sections were immunostained with isotype control (“Ctl”) IgG or IgG specific for VCAM-1, the NFκB p65 subunit phosphorylated on Ser536 (“phos-p65”), or total p65; all specimens were counterstained with Hoechst 33342 (DNA). Fluorescence photomicrographs are shown (original magnification ×400); scale bars = 50 µm. C, For each aortic section, protein immunofluorescence was normalized to DNA fluorescence (see Methods); within each staining cohort, these values were normalized to those obtained for Non-Tg/Ldlr−/− aortas, and plotted as means ± SE of 4 specimens per group. Compared with Non-Tg/Ldlr−/−: *, p<0.05.
To accelerate atherogenesis we fed these Ldlr−/− mice a Western diet,10 but harvested ascending aortas after only 7 days, at a time when intimal macrophages could not be detected (Supplemental Figure II).15 These pre-atherosclerotic aortas from Non-Tg/Ldlr−/− and SMC-USP20-Tg/Ldlr−/− mice showed equivalent activation of NFκB, assessed by immunofluorescence microscopy as phosphorylation of the NFκB subunit p65 on Serine 536 (phospho-p65)7 in the tunica media, which comprises exclusively SMCs. Congruently, Non-Tg/Ldlr−/− and SMC-USP20-Tg/Ldlr−/− mouse aortas also showed equivalent expression of VCAM-1. In contrast, the tunica media of pre-atherosclerotic aortas from SMC-DN-USP20-Tg/Ldlr−/− mice demonstrated 40–50% higher levels of phospho-p65 and VCAM-1 than Non-Tg/Ldlr−/− and SMC-USP20-Tg/Ldlr−/− aortas (Figure 1B, C). Similarly, the level of IL-1β was 50% higher in the tunica media of pre-atherosclerotic aortas from SMC-DN-USP20-Tg/Ldlr−/− mice (Supplemental Figure III), even though SMC-DN-USP20-Tg/Ldlr−/− and Non-Tg/Ldlr−/− pre-atherosclerotic aortas showed equivalent levels of MCP-1, TNF, and RIPK1 (an important effector downstream of the TNF receptor-1). Thus, antagonizing USP20 activity in SMCs augmented inflammatory signaling in the earliest stages of atherogenesis.
SMC USP20 Attenuates Atherosclerosis
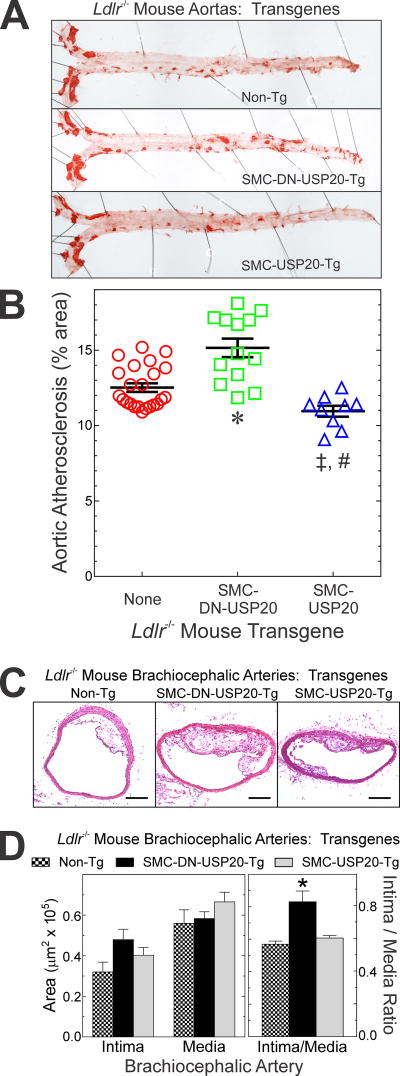
To determine whether USP20-dependent changes in SMC inflammation correlated with congruent effects on atherosclerosis, we studied Non-Tg/Ldlr−/−, SMC-USP20-Tg/Ldlr−/− and SMC-DN-USP20-Tg/Ldlr−/− mice after 16 wk on a Western diet. Analyzed en face, aortic atherosclerosis was 13% less extensive in SMC-USP20-Tg/Ldlr−/− than in Non-Tg/Ldlr−/− mice (11.0% vs 12.5% aortic area, respectively). Conversely, atherosclerosis was 21% more extensive in SMC-DN-USP20-Tg/Ldlr−/− (15.1% aortic area) than in Non-Tg/Ldlr−/− mice (Figure 2A, B). Furthermore, brachiocephalic arteries of SMC-DN-USP20-Tg/Ldlr−/− mice developed 50±20% greater atheroma area than Non-Tg/Ldlr−/− mice, which were indistinguishable from SMC-USP20-Tg Ldlr−/− mice (Figure 2C, D). Thus, in the SMCs of Ldlr−/− mice, low-level overexpression of WT USP20 reduces atherosclerosis whereas low-level overexpression of DN-USP20 augments atherosclerosis.
Figure 2.
USP20 activity in SMCs reduces atherosclerosis. A, Non-Tg/Ldlr−/−, SMC-USP20-Tg/Ldlr−/− and SMC-DN-USP20-Tg/Ldlr−/− male mice were fed a Western diet for 16 wk and then sacrificed. Aortas were stained with Sudan IV (red), sliced longitudinally and then pinned to white wax. Shown are individual aortas representative of ≥9 analyzed per group. B, The percentage of aortic area staining with Sudan IV was measured by planimetry, to obtain “% area”. Values for each mouse are plotted, along with means±SE for each group. Compared with Non-Tg Ldlr−/−: *, p<0.0001; ‡, p<0.05. Compared with SMC-DN-USP20-Tg: #, p<0.0001 (one-way ANOVA with Tukey post-hoc test for multiple comparisons). C, Brachiocephalic arteries from the mice used in “A” were sectioned and stained with hematoxylin and eosin; scale bars=100 µm. D, Brachiocephalic artery cross sections, intimal and medial areas were measured by planimetry. These areas as well as the ratio of these areas (“Intima/Media”) are plotted are the means ± SE for 6 mice/group. Compared with Non-Tg/Ldlr−/−: *, p<0.05 (one-way ANOVA with Tukey post-hoc test for multiple comparisons).
The larger atherosclerotic lesions in SMC-DN-USP20-Tg/Ldlr−/− mice, by cross-sectional area, comprised a 2-fold greater proportion of SMCs than the smaller lesions of Non-Tg/Ldlr−/− mice (Supplemental Figure IVA, B)—even though macrophages and necrotic cores constituted similar percentages of lesion areas in the two mouse groups (Supplemental Figure IVA, B). Congruently, the proportion of PCNA-positive, proliferating SMCs in atherosclerotic lesions was ~2-fold greater in SMC-DN-USP20-Tg/Ldlr−/− mice, while the prevalence of apoptotic SMCs was equivalent in both mouse groups (Supplemental Figure IVA, C). In addition, both phospho-p65(Ser 536) and VCAM-1 levels were higher in SMCs of SMC-DN-USP20-Tg/Ldlr−/− brachiocephalic arteries (Supplemental Figure V). Collectively, these findings accord with greater NFκB activation in DN-USP20-Tg SMCs (Figure 1), and the role that NFκB plays in promoting SMC proliferation.16 Although DN-USP20 augmented SMC signaling via NFB in vitro and in vivo, it had no apparent effect on the SMC expression of hypoxia-inducible factor-1α (HIF-1α), which is a USP20 substrate in overexpression systems17 (Supplemental Figure VI).
To corroborate our atherosclerosis findings in a parallel system, we transplanted carotid arteries from congenic Non-Tg (WT) and SMC-DN-USP20-Tg mice into the carotid arteries of atherogenic Apoe−/− mice; the foam cell-rich atherosclerosis that ensues mirrors results obtained by en face analysis of aortic atherosclerosis.10,11 Six weeks after transplantation, SMC-DN-USP20-Tg arteries demonstrated 2.0±0.5-fold more atherosclerotic neointimal area and 2.0±0.6-fold less luminal area than Non-Tg control arteries (Supplemental Figure VII). In accord with our findings in brachiocephalic arteries (Supplemental Figure V), the larger atherosclerotic lesions in SMC-DN-USP20-Tg carotids comprised a 2.4±0.6-fold greater proportion of SMCs than lesions of Non-Tg carotids (Supplemental Figure VIID). Conversely, the proportion of lesion area comprising macrophages was 12±4% less in SMC-DN-USP20-Tg carotids than in Non-Tg carotids (Supplemental Figure VIID). Thus, the anti-atherogenic effects of endogenous SMC USP20 activity obtain in more than one mouse model of atherosclerosis.
USP20 Reduces NFκB Activation in vitro
At a cellular level, we previously found that LPS-induced NFκB activation was diminished in SMC-USP20-Tg SMCs and augmented in SMC-DN-USP20-Tg SMCs, compared with non-Tg SMCs.7 To confirm these results in an independent system, we overexpressed USP20 or DN-USP20 in HEK-293T cells and assayed TNF-induced, NFκB promoter-driven luciferase activity (Supplemental Figure VIII). TNF-induced NFκB activity was reduced by 40±8% in USP20-overexpressing cells. Conversely, it was increased by 40±4% in DN-USP20-overexpressing cells and by 50±7% in cells in which endogenous USP20 was silenced with siRNA (Supplemental Figure VIII). Thus, our WT and DN USP20 constructs exert reciprocal effects on TNF-induced NFκB activation, and DN-USP20 overexpression phenocopies the silencing of endogenous USP20.
USP20 Inhibits IL-1R-evoked Inflammatory Signaling in SMCs
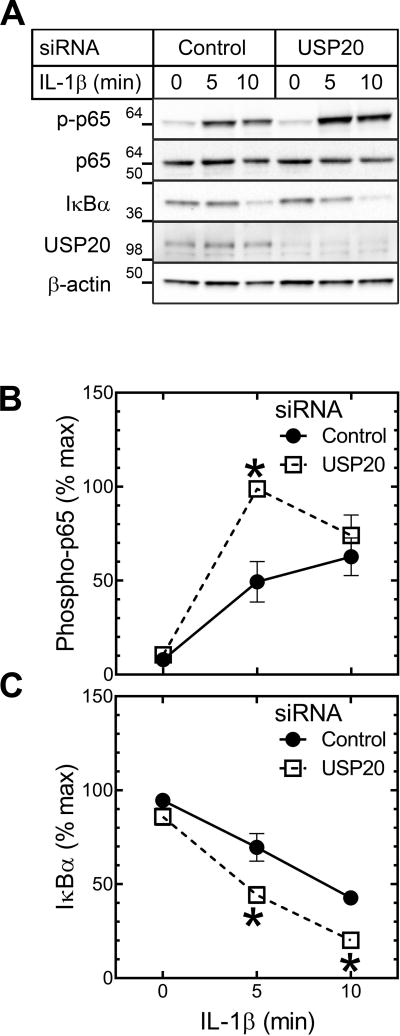
We have previously shown that USP20 inhibits both TLR4-mediated SMC inflammation in vitro and neointimal hyperplasia induced by carotid endothelial denudation in vivo.7 Like TLR4, the IL-1R evokes canonical NFκB activation through MyD88 and the ubiquitin E3 ligase TRAF6.18 Also like TLR4,19 the IL-1R promotes atherosclerosis.20 We therefore silenced USP20 expression in SMCs with siRNA to test whether USP20 attenuates NFκB activation in response to IL-1β; we used NFκB p65 phosphorylation on Ser536 as a read-out.7 In response to IL-1β, p65 phosphorylation on Ser536 occurred more quickly and to a greater extent in USP20-silenced than in control-siRNA-treated SMCs (Figure 3A, B), and in DN-USP20-expressing SMCs compared with control SMCs (Supplemental Figure IX). Concordantly, IκBα degradation was faster in USP20-silenced than in control SMCs (Figure 3A, C). Thus, USP20 inhibits SMC inflammation in response to the atherogenic cytokine IL-1β, as it does to the atherogenic activation of TLR4.7
Figure 3.
USP20 inhibits IL-1β-induced inflammatory signaling in SMCs. WT mouse aortic SMCs were transfected with USP20-targeting or control siRNA; 48 h later, SMCs were treated with IL-1β (10 ng/ml) for the indicated times, and then solubilized. A, SMC lysates were sequentially immunoblotted for the indicated proteins (“phospho-p65” designates NFκB p65 phosphorylated on Ser536). Immunoblots from single experiments are shown. B, Band densities for phospho-p65 were normalized to cognate β-actin bands. These ratios were normalized to that obtained in USP20-silenced SMCs stimulated with TNF for 10 min, to obtain “percent maximum”, plotted as means±SE for 6 independent experiments. Compared with control-siRNA-transfected SMCs: *, p<0.001. C, Data for IκBα band densities were processed as in “B”, except that the maximum IκBα/β-actin ratio for normalization was in control siRNA-transfected SMCs (6 independent experiments). Compared with control-siRNA-transfected SMCs: * p<0.01.
USP20 Attenuates TNF-induced Inflammatory Signaling in SMCs
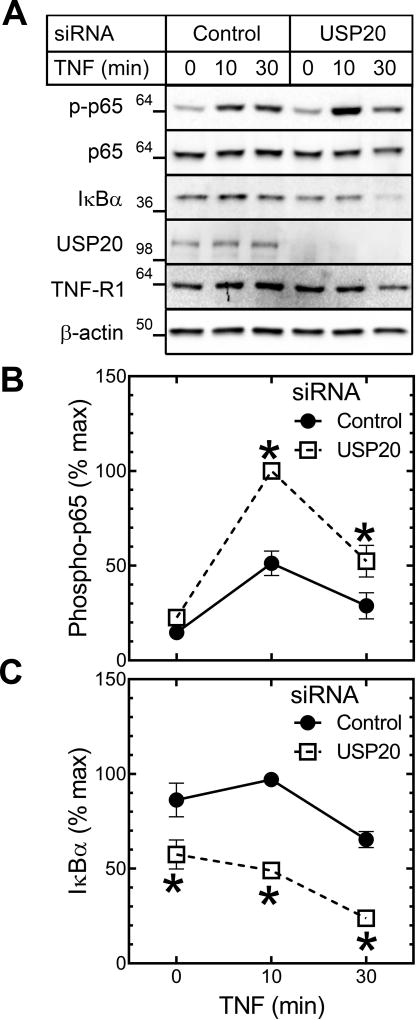
Pro-inflammatory, pro-atherogenic signaling engaged by TNF emanates primarily from TNFR1,11 which triggers canonical NFκB activation through ubiquitin E3 ligases distinct from TRAF6: TRAF2 or TRAF5, cIAP1 and cIAP2, and the ubiquitin ligase complex LUBAC.21 The substrates for these E3 ligases are distinct from those of TRAF6, and we therefore asked whether USP20 could regulate TNFR1-dependent NFκB activation in SMCs. To this end, we used both USP20-silencing siRNA and DN-USP20-Tg SMCs. TNF-evoked p65 phosphorylation was 2-fold greater in USP20-silenced than in control SMCs, and ~2.7-fold greater in DN-USP20-Tg than in Non-Tg SMCs (Figure 4A, B; Supplemental Figure X). Concordantly, IκBα degradation was ~2-fold greater and VCAM-1 up-regulation was ~3-fold greater in USP20-silenced than in control SMCs (Figure 4A, C; Supplemental Figure XI). Even in unstimulated SMCs, IκBα levels were ~20% lower in USP20-silenced SMCs (Figure 4A, C), as we observed previously7—consistent with a role for USP20 in constraining tonic canonical NFκB activation. Taken together, these data suggest that USP20 regulates TNF-induced NFκB activation in SMCs.
Figure 4.
USP20 attenuates TNF-induced inflammatory signaling in SMCs. WT mouse aortic SMCs were transfected with the indicated siRNA and challenged 48 h later with murine TNF (25 ng/ml) for the indicated times, and solubilized. A, SMC lysates were sequentially immunoblotted as in Figure 3. B, Data for phospho-p65 immunoblot bands were processed as in Figure 3, for 3 independent experiments. Compared with control-siRNA-transfected SMCs: * p<0.05. C, Data for IκBα were processed as in Figure 3, for 3 independent experiments. Compared with control-siRNA-transfected SMCs: * p<0.01.
USP20 Associates with Proteins that Mediate TNF-induced NFκB Activation
Although USP20 appears to inhibit TNFR1-dependent NFκB activation, the target or targets of USP20 deubiquitinase activity downstream of TNFR1 activation remain unknown. Indeed, the activated TNFR1 initiates dynamic ubiquitination of many proteins that collectively engender canonical NFκB activation. To identify potential substrates of USP20 and elucidate mechanisms by which USP20 inhibits TNF-evoked NFκB activity, we immunoprecipitated HA-tagged USP20 from HEK-293 cells and immunoblotted for endogenous proteins that constitute TNFR1-activated signaling intermediates. With these co-immunoprecipitation assays, we found that USP20 associated with the ubiquitin E3 ligases TRAF2, cIAP1, and two components of LUBAC6: HOIL-1-interacting protein (HOIP, or RNF31) and SHARPIN (also known as SIPL1) (Supplemental Figure XIIA). In addition, USP20 associated with RIPK1, IκBα, and β-arrestin2 (Supplemental Figure XIIB). Of these USP20-associating proteins, only β-arrestin2 has previously been described.7 In contrast, we could not detect specific association of USP20 with TNFR1, cIAP2, or IKKγ (Supplemental Figure XIIC). Thus, USP20 can associate with at least a substantial subset of TNFR1-dependent signaling intermediates.
Having found that USP20 can associate with several TNFR1-activated signaling intermediates in HEK-293 cells, we next asked whether USP20 associates with any of these proteins in SMCs, and whether USP20 association was affected by TNF stimulation. To address these questions, we transduced primary WT SMCs with our HA-USP20-encoding adenovirus. USP20 immunoprecipitates demonstrated endogenous RIPK1 and SHARPIN upon immunoblotting (Supplemental Figure XIIIA, C), as well as TRAF2 and β-arrestin2 (data not shown). However, the association of USP20 with these proteins did not differ between unstimulated and TNF-stimulated SMCs (Supplemental Figure XIII). Thus, it appears that USP20 constitutively associates with components of the TNFR1 signaling complex to regulate NFκB signaling and inflammation.
USP20 Deubiquitinates RIPK1
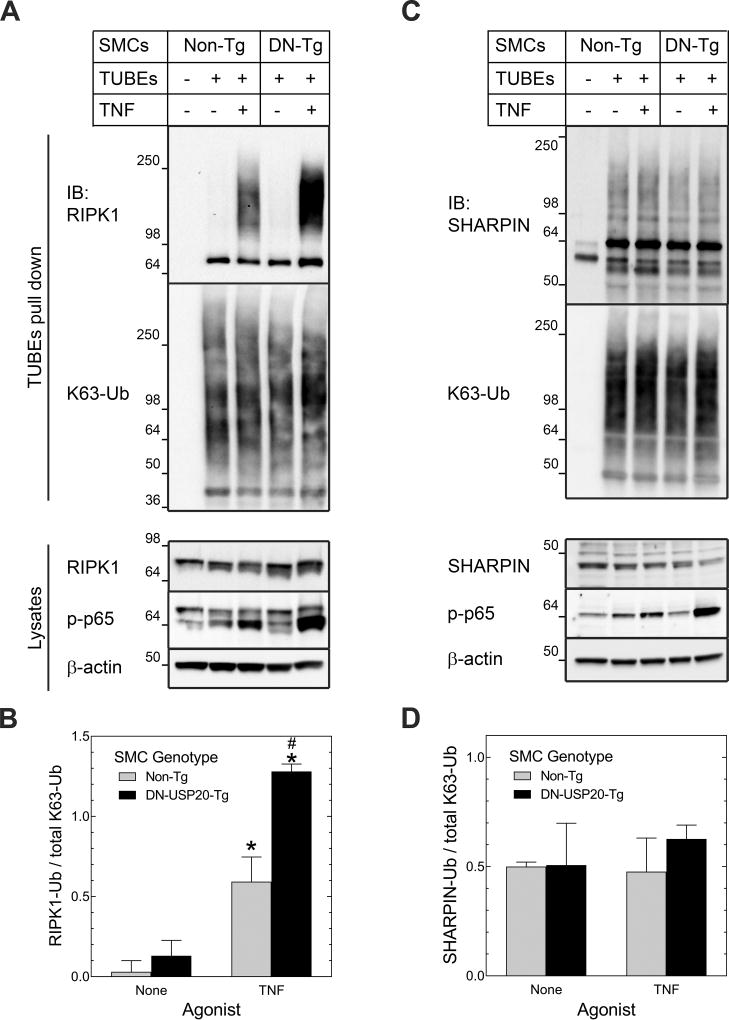
Among the TNFR1-activated, dynamically ubiquitinated proteins that associated with USP20 (Supplemental Figures XII and XIII), which ones are deubiquitinated by USP20? To address this question, we pulled down polyubiquitinated endogenous proteins from TNF-stimulated WT and DN-USP20-Tg SMCs by using tandem ubiquitin binding entities (TUBEs). TUBEs are fusion proteins comprising 4 linearly arrayed ubiquitin-associated domains.22 By immunoblotting SMC TUBEs pull-downs for RIPK1, we found that TNF engendered a 4-fold increase in RIPK1 ubiquitination (Figure 5A, B). Importantly, this TNF-dependent RIPK1 ubiquitination was 2-fold greater in DN-USP20-Tg SMCs than in Non-Tg SMCs—even though DN-USP20-Tg and WT SMCs demonstrated equivalent levels of total ubiquitinated proteins in the TUBEs pull-downs (Figure 5A, B). Immunoblotting TUBEs pull-downs for the LUBAC component SHARPIN yielded contrasting results: SHARPIN was constitutively ubiquitinated in SMCs, and there was no evidence of TNF-induced SHARPIN ubiquitination (Figure 5C, D). Furthermore, SHARPIN ubiquitination was equivalent in WT and DN-USP20-Tg SMCs (Figure 5C, D). Taken together, these data suggest that whereas SHARPIN is not a substrate for USP20 in SMCs, RIPK1 is a USP20 substrate downstream of TNFR1 activation in SMCs.
Figure 5.
USP20 deubiquitinates TNFR1-activated RIPK1. SMCs from Non-Tg and SMC-DN-USP20-Tg (“DN-Tg”) mice were treated ±murine TNF (25 ng/ml, 10 min, 37 °C), then solubilized. Ubiquitin was pulled down with agarose beads conjugated (or not, “-”) to tandem ubiquitin binding entities (TUBEs). A, TUBEs pull-downs (top) and SMC lysates (bottom) were immunoblotted serially for RIPK1 and K63-linked polyubiquitin (“K63-Ub”) (pull-downs), or RIPK1, phospho(“p”)-p65 and β-actin (lysates). B, Densities of the ubiquitinated RIPK1 smears (“RIPK1-Ub”, Mr > 98) were normalized to densities of the K63-ubiquitin smears in cognate TUBEs pulldowns; these ratios were plotted as means ± SE of 3 experiments with independent SMC lines. Compared with unstimulated SMCs from the same group: *, p<0.01; compared with TNF-stimulated Non-Tg SMCs: #, p<0.001. C, TUBEs pull-downs (top) and SMC lysates (bottom) were immunoblotted serially as in “A”, except that the primary immunoblot was for SHARPIN instead of RIPK1. D, Densities of the ubiquitinated SHARPIN smears (Mr > 50) were normalized to densities of the K63-ubiquitin smears in cognate TUBEs pulldowns; data were processed as in “B”, and plotted as the means ± SE of 3 experiments with independent SMC lines.
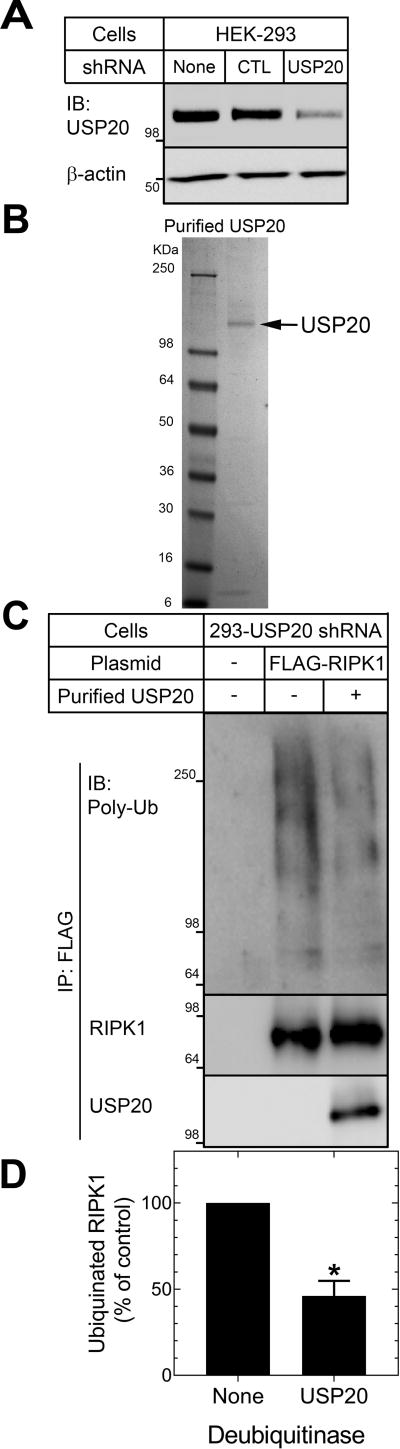
Antagonizing USP20 activity in SMCs by low-level overexpression of DN-USP20 augmented TNF-induced RIPK1 ubiquitination (Figure 5). However, it remained possible that USP20 reduced RIPK1 ubiquitination indirectly, rather than directly—for example, by deubiquitinating (and thereby deactivating) upstream E3 ubiquitin ligases and scaffolds like TRAF223 or HOIL-1-interacting protein24, with which USP20 associates in intact cells (Supplemental Figure XII). To test whether USP20 directly deubiquitinates RIPK1 that has undergone TNF-induced ubiquitination in intact cells, we used purified USP20 to deubiquitinate RIPK1 immunoprecipitated from HEK-293 cells in which USP20 expression was reduced by RNAi (Figure 6A). Our USP20 was >80% pure, as assessed by SDS-PAGE (Fig 6B). Purified UPS20 deubiquitinated RIPK1, as indicated by a 54±9% decrease in the ubiquitinated RIPK1 smear evident by immunoblotting RIPK1 immunoprecipitates for polyubiquitin (Figure 6C, D). Thus, RIPK1 itself appears to be a substrate of USP20—and, by directly deubiquitinating RIPK1, USP20 can inhibit TNFR1-triggered NFκB signaling.
Figure 6.
Purified USP20 deubiquitinates RIPK1. A, Human embryo kidney 293 cells were transduced (or not) with lentiviruses encoding shRNAs targeting either no known mRNA (control, “CTL”) or USP20 (“293-USP20 shRNA”). Protein extracts from these cells (20 µg) were serially immunoblotted for USP20 and β-actin. Shown is a single immunoblot (IB), representative of 3 performed. B, One µg of purified USP20 protein was resolved on 4–20% SDS-PAGE followed by Coomassie staining. USP20 (arrow) migrates with Mr ~110. C, 293-USP20 shRNA cells were transfected (or not, “-”) with a plasmid encoding a FLAG-tagged human RIPK construct. Cells were stimulated with TNF (10 ng/ml) for 10 min (37 °C), solubilized, and then FLAG-RIPK1 was immunoprecipitated. Immunoprecipitates were incubated at 37 °C without (“-”, control) or with (“+”) 0.2 µg of purified USP20 for 1 h, and then subjected to SDS-PAGE and immunoblotting. Nitrocellulose filters were probed sequentially for total polyubiquitin, RIPK1, and USP20, as indicated. Shown are results from a single experiment. D, The densities of polyubiquitin smears (Mr ≥ 64) were normalized to cognate RIPK1 band densities; these ratios were normalized to those obtained from incubations containing no deubiquitinase, to obtain “% of control”, plotted as means±SE of 3 experiments. Compared with control: *, p<0.05 (one-sample t test).
Discussion
This work demonstrates for the first time that the deubiquitinase USP20 impedes canonical NFκB activation by regulating ubiquitination-dependent signaling initiated by the IL-1R and the TNFR1 in SMCs. Furthermore, by impeding canonical NFκB activation in vivo, SMC USP20 activity attenuates atherosclerosis in Ldlr−/− mice. Assessed in intact cells or in preparations of purified proteins, USP20 deubiquitinates RIPK1 that signals downstream of TNFR1. These findings expand the previously known substrate specificity of USP20 beyond TRAF6 and HIF-1α, and expand the previously appreciated range of receptor signaling regulated by USP20.17,25–27
Specific roles in atherogenesis remain obscure for the ~85 deubiquitinases in the mammalian proteome.28 USP15 deubiquitinates IκBα in HeLa cells and in purified protein preparations,29 and thereby may inhibit NFκB activity as it appears to do in RAW 264.7 cells;30 however, whether USP15 regulates NFκB activity in physiologic systems remains an open question. USP31 can reduce NFκB activity when overexpressed in HEK-293 cells, but its physiologic role and protein substrates remain obscure.31 Although the ovarian tumor protease deubiquitinase subfamily member A20 can deubiquitinate TRAF6, diminish NFκB-dependent gene expression and reduce atherosclerosis in Apoe−/− mice,32 it appears that the deubiquitinase activity of A20 is not necessary for its ability to antagonize NFκB activity.33 The USP-family deubiquitinase known as cylindromatosis protein can inhibit NFκB activation when it is overexpressed,34 and RNAi-mediated silencing of cylindromatosis protein augments NFκB activity in HEK-293 cells.35 However, at physiologic levels of expression cylindromatosis protein promotes aneurysm formation by deubiquitinating the NADPH oxidase subunit NOX4.36 The deubiquitinase known as ubiquitin C-terminal hydroxylase L1 (UCHL1) attenuates TNF-induced NFκB activity assessed in A7r5 cells, but its substrates and physiologic role remain enigmatic.37 Because multiple loci in both canonical and non-canonical NFκB signaling pathways are regulated by ubiquitin-dependent mechanisms, it is not surprising that multiple deubiquitinases are involved in attenuating NFκB activity.38 It remains to be seen whether USP20 coordinates with or complements substrate deubiquitination engendered by other deubiquitinases linked with NFκB signaling.
Atherogenesis is augmented by diverse signaling pathways that converge to activate IKKβ and thus NFκB. This general principle is illustrated by diminished atherosclerosis observed in mice lacking TNFR1,11 cIAP2,39 TLR4,19 IL-1R,40 MyD88,19 IRAK4,41 IKKγ 42 or the NFκB p50 subunit in bone marrow cells.43 However, although ablation of NFκB p50 in bone marrow cells reduces atherosclerosis, overexpressing NFκB p65 in macrophages also reduces atherosclerosis.44 Furthermore, although ablating IKKβ reduces NFκB activation, ablating IKKβ in macrophages exacerbates atherosclerosis.45 Similarly paradoxical data arise from investigations of TRAF6: endothelial cell TRAF6 ablation reduces atherosclerosis, but myeloid cell TRAF6 ablation augments atherosclerosis.46 It therefore appears that the effects of NFκB activation on atherogenesis vary with both signaling inputs and cell type.46,47 Thus, the effects of deubiquitination on atherogenic signaling are likely to vary with the substrates of specific deubiquitinases. We have identified TRAF6, β-arrestin27 and RIPK1 as substrates of USP20 in SMCs. Whether USP20 deubiquitinates other substrates in NFκB signaling cascades and whether USP20 activity affects TRAF6 and RIPK1 ubiquitination in a cell-specific manner remain to be determined.
RIPK1 rapidly associates with the TNF-activated TNFR1 signaling complex, and undergoes polyubiquitination with multiple linkages.5,48 RIPK1 K63 polyubiquitination is critical for scaffolding the TAK/TAB kinase complex that phosphorylates IKKβ.48 Linear, methionine1-linked polyubiquitination of RIPK1 (effected by LUBAC) augments recruitment of IKKγ complexed to IKKα and IKKβ, which is then phosphorylated and thereby activated by transforming growth factor-β-activated kinase 1.5 Thus, deubiquitination of RIPK1 diminishes downstream NFκB activation.
Deubiquitination of RIPK1 has been attributed to several deubiquitinases, in model cell lines and overexpression systems. In HEK-293T cells, the bifunctional A20 can remove K63-linked polyubiquitin from RIPK1 and also ubiquitinate RIPK1 with K48-linked polyubiquitin, to promote proteasomal degradation of RIPK1 and consequent attenuation of NFκB activation.49 Other deubiquitinases linked to RIPK1 in overexpression and other model systems include Cezanne,50 USP2,23 USP4,51 and USP21.52 Whether these deubiquitinases regulate RIPK1 in physiologic systems or vascular cells remains to be explored.
USP20 can deubiquitinate HIF-1α and thereby increase steady-state HIF-1α expression, at least in overexpression systems.17 However, we found that neither augmenting nor antagonizing SMC USP20 activity affected SMC HIF-1α expression in vivo, even though we found the expected53 up-regulation of HIF-1α in the context of atherogenesis (Supplemental Figure VI). It may be that USP20 activity on HIF-1α requires levels of USP20 expression higher than those obtaining in SMCs, or that SMCs lack the protein scaffold(s) required for USP20/HIF-1α interaction. Because HIF-1α activity in SMCs promotes atherosclerosis,53 however, USP20-mediated deubiquitination of HIF-1α in SMCs would be expected to increase, rather than decrease atherosclerosis.
Our data reveal that USP20 associates with additional mediators of TNFR-induced NFκB signaling such as TRAF2, cIAP1, HOIL-1-interacting protein, IκBα and β-arrestin2. It is thus possible that USP20 affects the dynamic ubiquitination of these signaling intermediates, just as USP20 deubiquitinates RIPK1 (Figures 5, 6) and β-arrestin2,7 a multifunctional adaptor that regulates signaling downstream of both TNFR1 and TLR4.7,54 By deubiquitinating β-arrestin2, USP20 enables β-arrestin2 to scaffold a ternary complex of USP20, β-arrestin2, and TRAF6 and thereby facilitate deubiquitination of TRAF6 by USP20.7 Perhaps because TLR4-promoted, K63-linked polyubiquitination of β-arrestin2 abrogates its binding to USP20,7 β-arrestin2 promotes neointimal hyperplasia as well as atherosclerosis in Ldlr−/− mice.55 Thus, the anti-atherogenic effect of SMC USP20 may result from regulating β-arrestin2-mediated scaffolding as well as from deubiquitinating other signaling intermediates downstream of TNFR1, IL-1R and TLR4. For example, both β-arrestin254 and USP20 (Supplemental Figure XII) associate with IκBα; therefore, it is possible that a ternary complex of USP20, β-arrestin2 and IκBα facilitates deubiquitination of IκBα—a possibility made more plausible by the observation that β-arrestin2 and its homolog β-arrestin1 can inhibit IκBα degradation and NFκB signaling downstream of TNFR1.54
Our data demonstrate the anti-atherogenic role of USP20 in SMCs, and in so doing add to the evidence demonstrating that SMC-specific gene expression affects atherosclerosis.8,9,53 However, some limitations of our data merit further scrutiny. First, although antagonizing SMC USP20 increased NFκB activity in pre-atherosclerotic aortas, augmenting SMC USP20 activity failed to reduce NFκB activity (Figure 1). This apparent paradox may be attributable to the very modest (~2-fold) level of USP20 overexpression in SMC-USP20-Tg/Ldlr−/− mice, and/or to the very short Western diet exposure (1 wk) prior to harvesting the pre-atherosclerotic aortas. Plausibility of the latter hypothesis derives from our observation that SMC-USP20-Tg/Ldlr−/− mice developed less aortic atherosclerosis than Non-Tg/Ldlr−/− control mice after an additional 15 weeks’ exposure to Western diet. Second, there were relatively modest effects on atherosclerosis when we antagonized or augmented USP20 activity in SMCs. The magnitude of effects we observed was constrained by the modest levels of both WT USP20 and DN-USP20 transgene expression in mouse SMCs (~2- and 3-fold, respectively). Furthermore, restriction of transgene expression to just SMCs likely further constrained the effects of USP20 transgenes on atherosclerosis. Our current data prompt the question of whether systemic or cell-type-specific ablation of USP20 will further demonstrate anti-atherogenic efficacy of USP20, and thereby suggest therapeutic implications for augmenting USP20 activity.
Supplementary Material
Highlights.
USP20 activity reduces inflammatory signaling in the arterial media.
USP20 activity in SMCs attenuates atherosclerosis.
USP20 deubiquitinates RIPK1, a key signaling intermediate required for TNF-evoked inflammatory signaling.
Acknowledgments
None
Sources of Funding
This work was supported by National Institutes of Health Grants HL118369 (to S. K. S. and N. J. F.), HL121689 (to N. J. F.), HL112901 and HL121531 (to J. A. S.), as well as grants from the Edna and Fred L. Mandel Jr. Foundation.
Nonstandard Abbreviations and Acronyms
- cIAP
cellular inhibitor of apoptosis
- DN
dominant-negative
- HA
hemagglutinin
- HIF-1α
hypoxia-inducible factor-1α
- IκBα
inhibitor of NF-κB
- IKK
IκB kinase
- IL-1β
interleukin-1β
- IL-1R
IL-1 receptor
- IRAK
IL-1R-associated kinase
- LUBAC
linear ubiquitin chain assembly complex
- PCNA
proliferating cell nuclear antigen
- RIPK1
receptor-interacting protein kinase 1
- SHARPIN
SHANK associated RH domain interacting protein
- SMC
vascular smooth muscle cell
- Tg
transgenic
- TLR4
Toll-like receptor 4
- TNF
tumor necrosis factor
- TNFR1
TNF receptor-1
- TRAF
TNFR-associated factor
- USP
ubiquitin-specific protease
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Disclosures
Conflict of Interest: None
References
- 1.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 2.Chen ZJ. Ubiquitination in signaling to and activation of IKK. Immunol Rev. 2012;246:95–106. doi: 10.1111/j.1600-065X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 5.Peltzer N, Darding M, Walczak H. Holding RIPK1 on the ubiquitin leash in TNFR1 signaling. Trends Cell Biol. 2016;26:445–461. doi: 10.1016/j.tcb.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Walczak H, Iwai K, Dikic I. Generation and physiological roles of linear ubiquitin chains. BMC Biol. 2012;10:23. doi: 10.1186/1741-7007-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jean-Charles PY, Zhang L, Wu JH, Han SO, Brian L, Freedman NJ, Shenoy SK. Ubiquitin-specific protease 20 regulates the reciprocal functions of β-arrestin2 in Toll-like receptor 4-promoted nuclear factor-κB (NF-κB) activation. J Biol Chem. 2016;291:7450–7464. doi: 10.1074/jbc.M115.687129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian V, Golledge J, Ijaz T, Bruemmer D, Daugherty A. Pioglitazone-induced reductions in atherosclerosis occur via smooth muscle cell-specific interaction with PPARγ. Circ Res. 2010;107:953–958. doi: 10.1161/CIRCRESAHA.110.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JH, Zhang L, Fanaroff AC, Cai X, Sharma KC, Brian L, Exum ST, Shenoy SK, Peppel K, Freedman NJ. G protein-coupled receptor kinase-5 attenuates atherosclerosis by regulating receptor tyrosine kinases and 7-transmembrane receptors. Arterioscler Thromb Vasc Biol. 2012;32:308–316. doi: 10.1161/ATVBAHA.111.239608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Peppel K, Sivashanmugam P, Orman ES, Brian L, Exum ST, Freedman NJ. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1087–1094. doi: 10.1161/ATVBAHA.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. β-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci U S A. 2009;106:6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo J, Lee EW, Sung H, et al. CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3. Nat Cell Biol. 2016;18:291–302. doi: 10.1038/ncb3314. [DOI] [PubMed] [Google Scholar]
- 14.Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1) J Biol Chem. 1992;267:16323–16329. [PubMed] [Google Scholar]
- 15.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114:1757–1771. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peppel K, Zhang L, Orman ES, Hagen PO, Amalfitano A, Brian L, Freedman NJ. Activation of vascular smooth muscle cells by TNF and PDGF: overlapping and complementary signal transduction mechanisms. Cardiovasc Res. 2005;65:674–682. doi: 10.1016/j.cardiores.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Wang D, Messing EM, Wu G. VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1alpha. EMBO Rep. 2005;6:373–378. doi: 10.1038/sj.embor.7400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joosten LA, Abdollahi-Roodsaz S, Dinarello CA, O'Neill L, Netea MG. Toll-like receptors and chronic inflammation in rheumatic diseases: new developments. Nat Rev Rheumatol. 2016;12:344–357. doi: 10.1038/nrrheum.2016.61. [DOI] [PubMed] [Google Scholar]
- 19.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res. 2005;66:583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Dondelinger Y, Darding M, Bertrand MJ, Walczak H. Poly-ubiquitination in TNFR1-mediated necroptosis. Cell Mol Life Sci. 2016;73:2165–2176. doi: 10.1007/s00018-016-2191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hjerpe R, Aillet F, Lopitz-Otsoa F, Lang V, England P, Rodriguez MS. Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO Rep. 2009;10:1250–1258. doi: 10.1038/embor.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahul-Mellier AL, Pazarentzos E, Datler C, Iwasawa R, AbuAli G, Lin B, Grimm S. De-ubiquitinating protease USP2a targets RIP1 and TRAF2 to mediate cell death by TNF. Cell Death Differ. 2012;19:891–899. doi: 10.1038/cdd.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerlach B, Cordier SM, Schmukle AC, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 25.Yasunaga J, Lin FC, Lu X, Jeang KT. Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-κB signaling. J Virol. 2011;85:6212–6219. doi: 10.1128/JVI.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kommaddi RP, Jean-Charles PY, Shenoy SK. Phosphorylation of the deubiquitinase USP20 by protein kinase A regulates post-endocytic trafficking of β2-adrenergic receptors to autophagosomes during physiological stress. J Biol Chem. 2015;290:8888–8903. doi: 10.1074/jbc.M114.630541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate β2-adrenergic receptor recycling and resensitization. EMBO J. 2009;28:1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clague MJ, Heride C, Urbe S. The demographics of the ubiquitin system. Trends Cell Biol. 2015;25:417–426. doi: 10.1016/j.tcb.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Schweitzer K, Bozko PM, Dubiel W, Naumann M. CSN controls NF-κB by deubiquitinylation of IκBα. EMBO J. 2007;26:1532–1541. doi: 10.1038/sj.emboj.7601600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenblatt MB, Park KH, Oh H, et al. CHMP5 controls bone turnover rates by dampening NF-κB activity in osteoclasts. J Exp Med. 2015;212:1283–1301. doi: 10.1084/jem.20150407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzimas C, Michailidou G, Arsenakis M, Kieff E, Mosialos G, Hatzivassiliou EG. Human ubiquitin specific protease 31 is a deubiquitinating enzyme implicated in activation of nuclear factor-κB. Cell Signal. 2006;18:83–92. doi: 10.1016/j.cellsig.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Wolfrum S, Teupser D, Tan M, Chen KY, Breslow JL. The protective effect of A20 on atherosclerosis in apolipoprotein E-deficient mice is associated with reduced expression of NF-κB target genes. Proc Natl Acad Sci U S A. 2007;104:18601–18606. doi: 10.1073/pnas.0709011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De A, Dainichi T, Rathinam CV, Ghosh S. The deubiquitinase activity of A20 is dispensable for NF-κB signaling. EMBO Rep. 2014;15:775–783. doi: 10.15252/embr.201338305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takami Y, Nakagami H, Morishita R, et al. Potential role of CYLD (Cylindromatosis) as a deubiquitinating enzyme in vascular cells. Am J Pathol. 2008;172:818–829. doi: 10.2353/ajpath.2008.070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 36.Yu B, Liu Z, Fu Y, Wang Y, Zhang L, Cai Z, Yu F, Wang X, Zhou J, Kong W. CYLD deubiquitinates nicotinamide adenine dinucleotide phosphate oxidase 4 contributing to adventitial remodeling. Arterioscler Thromb Vasc Biol. 2017;37:1698–1709. doi: 10.1161/ATVBAHA.117.309859. [DOI] [PubMed] [Google Scholar]
- 37.Takami Y, Nakagami H, Morishita R, et al. Ubiquitin carboxyl-terminal hydrolase L1, a novel deubiquitinating enzyme in the vasculature, attenuates NF-κB activation. Arterioscler Thromb Vasc Biol. 2007;27:2184–2190. doi: 10.1161/ATVBAHA.107.142505. [DOI] [PubMed] [Google Scholar]
- 38.Harhaj EW, Dixit VM. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 2011;21:22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sleiman L, Beanlands R, Hasu M, Thabet M, Norgaard A, Chen YX, Holcik M, Whitman S. Loss of cellular inhibitor of apoptosis protein 2 reduces atherosclerosis in atherogenic apoE−/− C57BL/6 mice on high-fat diet. J Am Heart Assoc. 2013;2:e000259. doi: 10.1161/JAHA.113.000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 41.Rekhter M, Staschke K, Estridge T, et al. Genetic ablation of IRAK4 kinase activity inhibits vascular lesion formation. Biochem Biophys Res Commun. 2008;367:642–648. doi: 10.1016/j.bbrc.2007.12.186. [DOI] [PubMed] [Google Scholar]
- 42.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-κB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Kanters E, Gijbels MJ, van der Made I, Vergouwe MN, Heeringa P, Kraal G, Hofker MH, de Winther MP. Hematopoietic NF-κB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood. 2004;103:934–940. doi: 10.1182/blood-2003-05-1450. [DOI] [PubMed] [Google Scholar]
- 44.Ye X, Jiang X, Guo W, Clark K, Gao Z. Overexpression of NF-κB p65 in macrophages ameliorates atherosclerosis in apoE-knockout mice. Am J Physiol Endocrinol Metab. 2013;305:E1375–1383. doi: 10.1152/ajpendo.00307.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanters E, Pasparakis M, Gijbels MJ, et al. Inhibition of NF-κB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polykratis A, van Loo G, Xanthoulea S, Hellmich M, Pasparakis M. Conditional targeting of tumor necrosis factor receptor-associated factor 6 reveals opposing functions of Toll-like receptor signaling in endothelial and myeloid cells in a mouse model of atherosclerosis. Circulation. 2012;126:1739–1751. doi: 10.1161/CIRCULATIONAHA.112.100339. [DOI] [PubMed] [Google Scholar]
- 47.Boesten LS, Zadelaar AS, van Nieuwkoop A, Gijbels MJ, de Winther MP, Havekes LM, van Vlijmen BJ. Tumor necrosis factor-α promotes atherosclerotic lesion progression in APOE*3-Leiden transgenic mice. Cardiovasc Res. 2005;66:179–185. doi: 10.1016/j.cardiores.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Witt A, Vucic D. Diverse ubiquitin linkages regulate RIP kinases-mediated inflammatory and cell death signaling. Cell Death Differ. 2017;24:1160–1171. doi: 10.1038/cdd.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wertz IE, O'Rourke KM, Zhou H, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 50.Enesa K, Zakkar M, Chaudhury H, Luong le A, Rawlinson L, Mason JC, Haskard DO, Dean JL, Evans PC. NF-κB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. J Biol Chem. 2008;283:7036–7045. doi: 10.1074/jbc.M708690200. [DOI] [PubMed] [Google Scholar]
- 51.Hou X, Wang L, Zhang L, Pan X, Zhao W. Ubiquitin-specific protease 4 promotes TNF-α-induced apoptosis by deubiquitination of RIP1 in head and neck squamous cell carcinoma. FEBS Lett. 2013;587:311–316. doi: 10.1016/j.febslet.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 52.Xu G, Tan X, Wang H, et al. Ubiquitin-specific peptidase 21 inhibits tumor necrosis factor α-induced nuclear factor-κB activation via binding to and deubiquitinating receptor-interacting protein 1. J Biol Chem. 2010;285:969–978. doi: 10.1074/jbc.M109.042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu D, Lei L, Desir M, Huang Y, Cleman J, Jiang W, Fernandez-Hernando C, Di Lorenzo A, Sessa WC, Giordano FJ. Smooth muscle hypoxia-inducible factor-1α links intravascular pressure and atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:442–445. doi: 10.1161/ATVBAHA.115.306861. [DOI] [PubMed] [Google Scholar]
- 54.Freedman NJ, Shenoy SK. Regulation of inflammation by β-arrestins: not just receptor tales. Cell Signal. 2018;41:41–45. doi: 10.1016/j.cellsig.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Zhang L, Peppel K, Wu JH, Zidar DA, Brian L, DeWire SM, Exum ST, Lefkowitz RJ, Freedman NJ. β-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res. 2008;103:70–79. doi: 10.1161/CIRCRESAHA.108.172338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.