Abstract
Background and Purpose
Type-2 diabetes mellitus (T2DM) is a major comorbidity that exacerbates ischemic brain injury and worsens functional outcome after stroke. T2DM is known to aggravate white matter impairment but the underlying mechanism is not completely understood. This study was designed to test the hypothesis that T2DM impedes post-stroke white matter recovery by suppressing both oligodendrogenesis and beneficial microglia/macrophage responses.
Methods
Permanent distal middle cerebral artery occlusion was performed in wild-type, homozygous diabetic db/db, and heterozygous db/+ mice. The adhesive removal, open field, and Morris water maze tests were used to assess neurobehavioral outcomes. Neuronal tissue loss, white matter damage, oligodendrogenesis, and microglia/macrophage responses were evaluated up to 35d after stroke. The functional integrity of white matter was measured by electrophysiology. Primary microglia-oligodendrocyte co-cultures were used for additional mechanistic studies.
Results
T2DM exacerbated structural damage and impaired conduction of compound action potentials in white matter 35d after stroke. The deterioration in white matter integrity correlated with poor sensorimotor performance. Furthermore, T2DM impaired the proliferation of oligodendrocyte precursor cells (OPCs) and the generation of new myelinating oligodendrocytes. T2DM also promoted a shift of microglia/macrophage phenotype toward the pro-inflammatory modality. Co-culture studies confirmed that microglia/macrophage polarization toward the pro-inflammatory phenotype under high glucose conditions suppressed OPC differentiation.
Conclusions
Deterioration of white matter integrity and impairments in oligodendrogenesis after stroke are associated with poor long-term functional outcomes in experimental diabetes mellitus. High glucose concentrations may shift microglia/macrophage polarization toward a pro-inflammatory phenotype, significantly impairing OPC differentiation and white matter repair.
Keywords: diabetes, white matter, oligodendogenesis, microglia/macrophage polarization, neurological function
According to the 2017 report from Centers for Disease Control and Prevention, 30.3 million Americans have diabetes, and more than 90% of these subjects have insulin-resistant type 2 diabetes mellitus (T2DM). In addition, 84.1 million have prediabetes that, if left untreated, will lead to T2DM within five years. T2DM patients have an almost four-fold increased risk of stroke compared to non-diabetic control subjects.1 The diabetic condition worsens stroke outcomes, as prospective studies confirm poorer functional outcomes after ischemic stroke in diabetic patients.1 Although T2DM does not initially affect stroke severity at ischemia onset, it is an independent factor associated with worse functional outcomes at the time of discharge.2
White matter (WM) plays an essential role in signal transduction across brain regions and is critical for the functional integrity of the entire neuraxis. The severity of WM injury is known to correlate with poor functional prognosis in stroke patients.3 Greater disruptions in WM integrity have been observed in diabetic patients compared to age-matched non-diabetic controls.4 However, the effect of diabetes on WM injury after stroke and its subsequent influence on functional outcomes remain elusive. Our prior studies document a M2 to M1 phenotypic shift in microglia associated with WM injury after stroke in both young and aged mice.5 An anti-inflammatory M2 microglial status promoted, whereas a pro-inflammatory M1 phenotype impaired the survival of oligodendrocytes and the differentiation/maturation of oligodendrocyte precursor cells (OPCs).6, 7 Elevated levels of glucose in culture media influence monocyte/macrophage responses and shift their polarization toward a M1-like inflammatory phenotype.8 However, it is not known whether hyperglycemia during and after stroke, as experienced in T2DM, modifies microglia/macrophage polarization, and if the resulting polarization state influences WM injury and functional deficits.
In the current study, we assessed post-stroke WM integrity and microglia/macrophage polarization in the db/db homozygous mouse model of T2DM, db/+ heterozygous mice, and wild-type (WT) mice at various time points after permanent distal middle cerebral artery occlusion (dMCAO). We observed that T2DM exacerbated WM damage and led to prolonged functional deficits after stroke. The presence of a high glucose environment promoted a pro-inflammatory phenotypic shift in microglia/macrophages, and this pro-inflammatory shift impaired oligodendrogenesis and WM repair.
Materials and Methods:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animal model
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the principles outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Male C57/BL6 mice, db/+ mice (Leprdb/+), and db/db mice (Leprdb/db) (10 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were randomly divided into the sham surgery or ischemic injury group using a lottery drawing box. Mice in the latter group were subjected to dMCAO, as described previously.5 Cerebral blood flow (CBF) was measured using the two-dimensional laser speckle imaging system and laser Doppler flowmetry. Mice with less than 70% reduction of blood flow in the ischemic core or that died during surgery were excluded from further analyses. Mice in the sham surgery group were similarly anesthetized and operated on but not subjected to MCA cauterization. All efforts were made to minimize animal suffering. All outcome endpoints were measured by investigators blinded to experimental group assignments. A total of 243 mice (29 sham-operated and 197 ischemic mice) were used, including 17 mice that were excluded from further assessments due to death after ischemia. The mortality rate was 0% in WT mice (0/66), 0% in db/+ mice (0/67) and 22.97% in db/db mice (17/74).
Primary microglia and OPC cultures
Primary microglia and OPCs were prepared from the whole brains of 1-day-old Sprague-Dawley rat pups, as previously described.6 Microglia were cultured for 48h in media with a normal concentration of glucose (5.5mM) or high glucose (15mM), in the presence or absence of low concentrations of lipopolysaccharides (LPS, 2.5ng/mL). For co-culture experiments, pretreated microglia were co-cultured with OPCs for 3d.
Statistical analyses
All data are presented as mean ± standard error (SEM). Differences between groups were compared using the Student’s t test (for single comparison) or one-way analyses of variance (ANOVA) for continuous variables with normal distributions. Differences in means across groups with repeated measurements over time were analyzed using repeated measures ANOVAs. When the ANOVA revealed significant differences, the post hoc Bonferroni test was used for pairwise comparisons between means. Linear correlation analyses were performed using Pearson product-moment correlation analyses. Statistical differences were deemed significant when p≤0.05.
Results:
Diabetic and non-diabetic mice exhibit similar rCBF after ischemic injury
In all groups, regional CBF (rCBF) in the left MCA territories decreased slightly after left CCA ligation and significantly dropped after occlusion of the left MCA (Supplementary Fig. IA and Fig. IB). Thereafter, rCBF recovered gradually and returned to approximately 80% of baseline values at 7d after dMCAO. There was no significant difference in rCBF between WT, db/+, and db/db groups at any time points measured. Further analyses revealed no difference among three groups in the cortical areas where the rCBF decreased to less than 30% or between 30–50% of baseline values (Supplementary Fig. IC). Physiological parameters, including blood pH, blood gases, and concentrations of electrolytes, were comparable between WT, db/+, and db/db groups before and after surgery (Supplementary Table I).
Diabetic mice exhibit worse sensorimotor performance compared to non-diabetic mice after dMCAO
The post-stroke mortality rate (Fig. 1A) and blood sugar levels (Fig.1B) of db/db mice were significantly higher than WT and db/+ mice. The increased body weight in sham db/db mice (WT: 24.74±2.349g,db/+: 27.9±1.769g, db/db: 45.41±2.681g; WT vs. db/db, p < 0.0001; db/+ vs. db/db, p < 0.0001; one-way ANOVA followed by Bonferroni post hoc) appeared to impair their performance in multiple behavior tests, including the rotarod and footfault tests (data not shown). The adhesive removal test, which is not affected by differences in body weight, was therefore used to assess post-stroke sensorimotor function (Fig.1C [a-b]). The db/db mice exhibited impaired sensorimotor performance, as revealed by significantly longer latencies until contact (Fig.1Cc) and removal (Fig.1Cd) of the tape from the right forepaw.
Figure 1. The db/db mice exhibit impaired sensorimotor performance but similar cognitive functions compared to db/+ mice and WT mice up to 35d after dMCAO.
A, Post-stroke mortality rates were higher in db/db mice than WT and db/+ mice. Note that the data points for the db/+ mice (blue triangles) overlap those of the WT mice (black circles). Logrank test. n=12–13/group. B, The blood sugar levels of db/db mice were significantly higher than WT and db/+ mice. n=8/group. C, Sensorimotor functions were evaluated by the adhesive removal test. The latencies to contact and remove the tape were recorded. n=6/sham group. n=8–12/dMCAO group. D, In the open field test, total distances travelled in the arena and the time spent in the central area of the open field was recorded. n=4/sham group. n=8–12/dMCAO group. *p<0.05, **p<0.01, ***p<0.001, WT vs. db/db; +p<0.05, ++p<0.01, +++p<0.001, WT vs. db/+; #p<0.05, ##p<0.01, ###p<0.001, db/+ vs. db/db; two-way repeated measures ANOVA followed by the Bonferroni post hoc. Both main effects and pairwise comparisons (with corrections) are displayed.
In the open field test, the db/db mice were significantly less active than WT and db/+ mice and traveled shorter total distances in the arena (Fig.1Da). These data suggest a reduction in overall exploratory/locomotor activity in diabetic mice under sham injury conditions. Interestingly, locomotor activity exhibited a significant gene dosage effect in that db/+ mice travelled shorter distances compared to WT mice but longer distances than db/db mice. After stroke, the total distance traveled decreased in both WT and db/+ groups, and remained at very low levels in db/db mice compared to the other two groups (Fig.1Dc). As a result, the reduction in locomotor activity was more dramatic in WT and db/+ stroke groups vs diabetic stroke mice due to the low basal activity levels in the latter. In addition, the db/db and db/+ mice spent much less time exploring the central area of the open field than WT mice after either sham operation (Fig.1Db) or ischemic stroke (Fig.1Dd), suggesting anxiety-like behavior in diabetic animals.
The Morris water maze was used to assess long-term cognitive function after dMCAO (Supplementary Fig.II). The swimming speed of db/db mice was much slower than WT and db/+ mice after either sham operation or dMCAO, probably due to the increased body weight in db/db mice. Spatial learning capacity was therefore quantified using path efficiency, calculated as the Euclidean distance between the starting location and the final location divided by the total distance travelled,9 to adjust for differences in swimming speed. The WT mice, db/+ mice, and db/db mice demonstrated comparable path efficiency after sham operation and dMCAO (Supplementary Fig.II). There was also no difference in memory function among the three lines of mice after either sham operation or stroke surgery (Supplementary Fig.II).
Diabetic mice exhibit greater neuronal tissue loss compared to non-diabetic mice after ischemic injury
Neuronal tissue loss was measured by NeuN immunostaining at 3 and 35d after dMCAO. Infarct volume was normalized to the contralateral hemisphere. The db/db mice demonstrated significantly enlarged areas of neuronal tissue loss at 3d after stroke (Fig.2A). Although neuronal tissue loss was larger in db/db mice than the other two lines at 35d after dMCAO, the effect did not reach statistical significance (Fig.2B, WT vs. db/db, p = 0.08; db/+ vs. db/db, p = 0.06). Correlation analyses revealed a weak but statistically significant correlation between neuronal tissue loss at 35d post-stroke and behavioral performance in the adhesive removal test from days 14–35 post-stroke (Fig.2C).
Figure 2. The db/db mice exhibit greater neuronal tissue loss compared to WT mice and db/+ mice after dMCAO.

A, Volume of neuronal tissue loss on NeuN-stained coronal sections 3d after dMCAO. Dotted lines indicate infarct areas. n=10–14/group. B, Volume of neuronal tissue loss on NeuN-stained coronal sections 35d after dMCAO. Dotted lines indicate infarct areas. n=7–10/group. *p<0.05, WT vs. db/db; ##p<0.01, db/+ vs. db/db; ANOVA followed by the Bonferroni post hoc. C, Pearson correlation between volume of neuronal tissue loss at 35d after dMCAO and average latency to contact or remove adhesive tape from 14 to 35d after dMCAO.
Deterioration of WM integrity after ischemic injury in diabetic mice is associated with impairments in long-term neurological functions
Axons and myelin were immunostained with NF200 and MBP, respectively, to evaluate differences in WM integrity in WT mice, db/+ mice, and db/db mice. While db/db mice showed no significant differences in MBP loss early (3d) after stroke, greater loss of MBP immunostaining in the external capsule (EC) was observed in db/db mice compared to WT mice and db/+ mice 35d after dMCAO (Supplementary Fig.III). The WT mice and db/+ mice demonstrated proportional loss in NF200 and MBP levels as a percentage of their respective contralateral levels in the EC and cortex after dMCAO, rendering a NF200/MBP ratio of ~1.0 for both groups (Fig.3B). The ratio of NF200/MBP was comparable in the EC and the cortex of WT mice, db/+ mice and db/db mice at 3d after dMCAO. In contrast, the ratio of NF200/MBP was significantly higher in the EC of db/db mice at 14d and 35d after dMCAO (Fig.3A-3B), suggesting greater loss of myelin (MBP staining) than axons (NF200 staining) compared to WT mice and db/+ mice. These data reveal greater demyelination in diabetic mice after dMCAO. A negative correlation was observed between MBP staining intensity and the latency to contact and remove the tape in the adhesive removal test, suggesting a robust association between WM integrity and sensorimotor function (Fig.3C). Electrophysiological studies showed that the db/db mice exhibited similar reduction in CAP amplitudes in myelinated N1 fibers and unmyelinated N2 fibers in the EC, compared to db/+ and WT mice at 3d after stroke (Fig.3Db), indicating comparable initial white mater injury among the three mouse lines. However, diabetic mice exhibited lower amplitudes in myelinated N1 fibers and unmyelinated N2 fibers in the EC when the stimulation strength exceeded 750 μA at 35d after stroke (Fig.3Dc). Sham-injured WT mice, db/+ mice, and db/db mice showed no difference in N1 or N2 CAP amplitude (Fig.3Da). No difference was observed in CAP N1 velocity between diabetic and non-diabetic mice (Fig.3D).
Figure 3. Deterioration of WM injury in db/db mice is associated with impairments in long-term neurological functions after dMCAO.
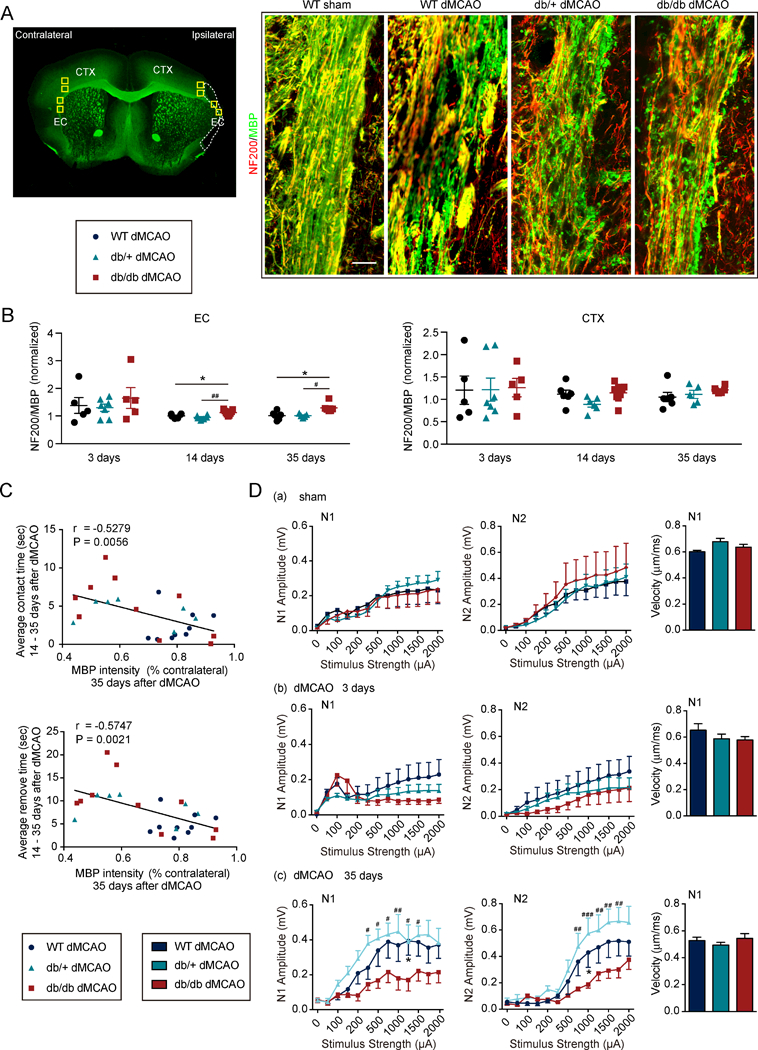
A, Left: Yellow rectangles illustrate the anatomical location of microscopic images captured in the ipsilateral peri-infarct regions and corresponding contralateral areas. Right: Representative images of NF200 and MBP double-immunostained coronal sections 35d after dMCAO or sham operation. Scale bar: 40 μM. B, Ratio of NF200 (red) and MBP (green) staining intensities in the external capsule (EC) or cortex (CTX) 3d, 14d and 35d after dMCAO. n=5 – 9/group. one-way ANOVA followed by Bonferroni post hoc. C, Pearson correlation between MBP levels in the EC 35d after dMCAO and average latency to contact or remove adhesive tape from 14d to 35d after dMCAO. n=7–10/group. D, Electrophysiological studies were performed on brain slices prepared from WT mice, db/+ mice and db/db mice at 3d (n=6–8/group) and 35d (n=3–4/group) after dMCAO or in sham animals (n=4/group). (a-c) Quantification of amplitude and velocity in myelinated N1 fibers and amplitude in unmyelinated N2 fibers in sham mice (a) and 3d (b) and 35d (c) after dMCAO. *p<0.05, WT vs. db/db; #p<0.05, ##p<0.01, ###p < 0.001, db/+ vs. db/db; two-way ANOVA followed by Bonferroni post hoc.
Post-stroke oligodendrogenesis is inhibited in diabetic mice
BrdU was injected at 3–6d after dMCAO to label newly generated cells. The total number of BrdU+ cells in the EC and cortex was decreased in db/db mice compared to non-diabetics at 14d and 35d after stroke (Supplementary Fig.IVA), suggesting that diabetic conditions impair the proliferation of CNS cells after stroke. Compared to non-diabetic mice, immunostaining revealed significant lower numbers of NG2+BrdU+ OPCs in the EC and cortex in db/db mice 14d and 35d after dMCAO (Fig.4A). Adenomatous Polyposis Coli (APC)+BrdU+ oligodendrocytes in the EC and cortex were also lower in db/db mice at 14d and 35d (Fig.4B) after dMCAO. The percentages of newly proliferated BrdU+ cells expressing NG2 or APC in the EC and cortex were significantly lower in diabetic mice (Supplementary Fig.IVB). There was no significant difference in the number of NG2+ cells or in the number of APC+ cells in the EC and cortex 3d after stroke (Supplementary Fig.IVC), consistent with the comparable electrophysiological measurements in the CC/EC areas across the three mouse lines. The total numbers of NG2+ cells and APC+ cells were significantly reduced in the EC and cortex of db/db mice compared to non-diabetics 14 days and 35 days after stroke (Supplementary Fig.IVC). The numbers of NG2+ cells, APC+ cells, NG2+BrdU+ cells and APC+BrdU+ cells in the contralateral EC and contralateral CTX exhibited no difference between WT mice, db/+ mice, and db/db mice 35d after dMCAO (Supplementary Fig.IVD). The numbers of newly generated NG2+BrdU+ OPCs and APC+BrdU+ oligodendrocytes were positively correlated with MBP intensities in the EC but not in the CTX (Fig.4C). Interestingly, the number of pre-existing BrdU- OPCs (NG2+BrdU-) or oligodendrocytes (APC+BrdU-) showed no correlation with MBP staining intensity in the EC or cortex (Supplementary Fig.V). The total number of NG2 cells, but not APC cells, displayed a positive correlation with MBP staining intensity in the EC (Supplementary Fig.V). These data suggest that newly generated OPCs and oligodendrocytes are important for WM integrity after stroke, while the pre-existing oligodendrocytes have marginal effects.
Figure 4. Post-stroke oligodendrogenesis is inhibited in db/db mice compared to WT mice and db/+ mice.
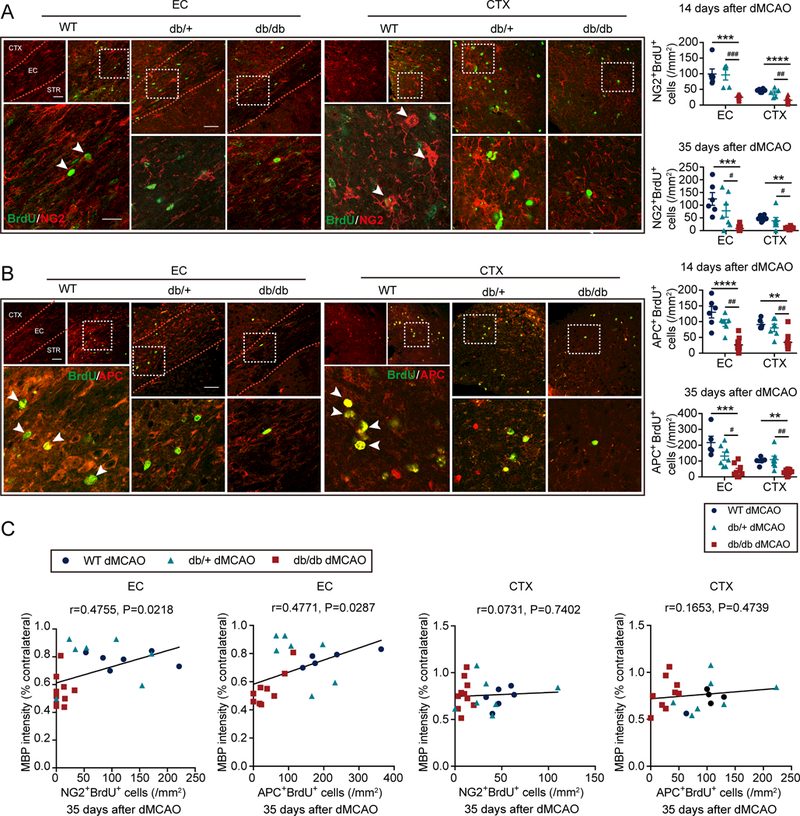
BrdU and NG2 or APC double immunostaining was performed to detect oligodendrogenesis in the external capsule (EC) and cortex (CTX) of WT mice, db/+, mice and db/db mice 14d and 35d after dMCAO. A, Quantification of the number of BrdU and NG2 double-immunostained cells. n=6–10/group. B, Quantification of the number of BrdU and APC double-immunostained cells. n=5–10/group. Scale bar: 40 μM. **p<0.01, ***p<0.001, ****p<0.0001, WT vs. db/db; #p<0.05, ##p<0.01, ###p <0.001, db/+ vs. db/db; one-way ANOVA followed by Bonferroni post hoc. C, Pearson correlation between the number of NG2+ BrdU+ OPCs or APC+BrdU+ oligodendrocytes and MBP staining intensities in the EC or CTX 35d after dMCAO.
Diabetic mice exhibit enhanced inflammatory microglia/macrophage responses
The numbers of CD206+Iba1+ M2-like microglia/macrophages were significantly reduced (Fig.5A), whereas the numbers of CD16+Iba1+ M1-like microglia/macrophages were significantly increased (Fig.5A) in the EC of db/db mice compared to WT and db/+ mice at 3d and 14d after stroke. Similar changes in the microglia/macrophage phenotypes were observed at 3d after dMCAO in the CTX (Supplementary Fig.VIB), but these changes did not persist to day 14 (Supplementary Fig.VIB). The number of M1-like or M2-like microglia were similar in WT mice, db/+ mice, and db/db mice under sham conditions (Supplementary Fig.VIC). Flow cytometry analyses demonstrated that the expression of the M2 marker Arg1 was significantly reduced in CD11b+CD45+ populations in db/db mice 3d after stroke (Supplementary Fig.VIIA-VIIBa), which represent activated microglia and infiltrated macrophages. In addition, expression of the M1 marker CD16 was increased in CD11b+CD45+ populations in db/db mice (Supplementary Fig.VIIA,VIIBb). The total numbers of CD11b+CD45+ cells did not differ between diabetic and non-diabetic mice (Supplementary Fig.VIIBc).
Figure 5. Diabetic mice exhibit enhanced M1-like inflammatory microglia/macrophage responses, which are associated with impaired oligodendrogenesis.
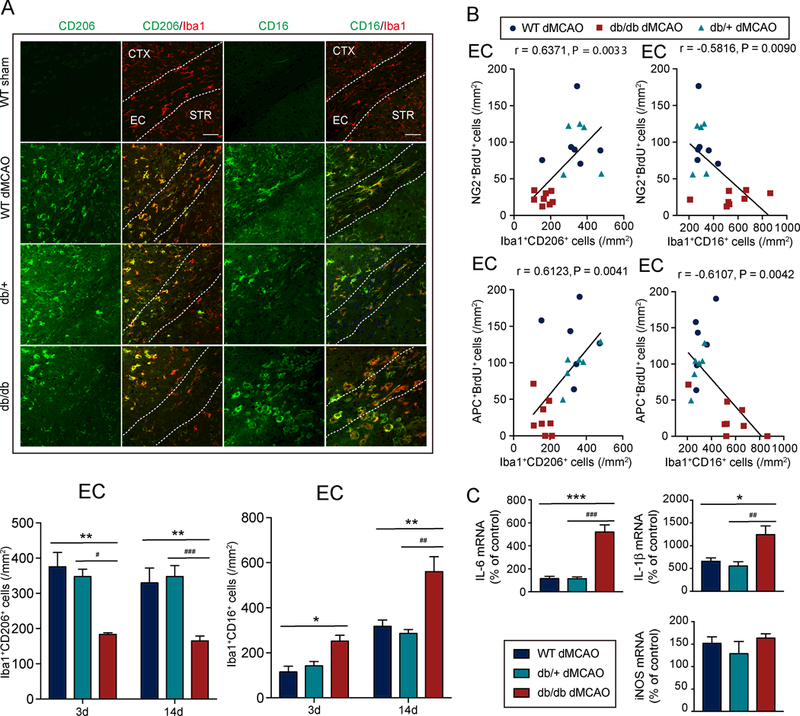
A, Upper: Representative images of Iba1 and CD16 or CD206 double-immunostaining in the external capsule (EC), cortex (CTX), and striatum (STR) of WT mice, db/+ mice, or db/db mice 14d after dMCAO. Scale bar: 40 μM. Lower: Quantification of the number of CD206+Iba1+ M2-like microglia/macrophages and CD16+Iba1+ M1-like microglia/macrophages in the peri-infarct area in the EC 3d and 14d after dMCAO. Three days after dMCAO: n=3–4/group, 14d after dMCAO: n=5–8/group. B, Pearson correlation between the number of BrdU+NG2+ OPCs or BrdU+APC+ oligodendrocytes and the number of CD16+Iba1+ M1-like microglia/macrophages or CD206+Iba1+ M2-like microglia/macrophages in the EC 14d after dMCAO. C, mRNA expression of inflammatory mediators (IL-1β, IL-6, and iNOS) in the ischemic brains of db/db mice, db/+ mice, and WT mice was measured at 14d after dMCAO using RT-PCR. n=4/group. Data are % of corresponding contralateral brains. *p<0.05, **p<0.01, ***p<0.001, WT vs. db/db; #p<0.05, ##p<0.01, ###p<0.001, db/+ vs. db/db; one-way ANOVA followed by Bonferroni’s posthoc.
A positive linear correlation was observed between the numbers of M2-like microglia/macrophages and the numbers of BrdU+NG2+ OPCs (Fig.5B), as well as between M2-like microglia/macrophages and the numbers of BrdU+APC+ oligodendrocytes in the EC (Fig.5B). There was no correlation between these measures in the CTX (Supplementary Fig.VIA). The numbers of M1-like microglia/macrophages, in contrast, were negatively correlated with the numbers of BrdU+NG2+ OPCs (Fig.5B) and the numbers of BrdU+APC+ oligodendrocytes in the EC (Fig.5B), but not in the CTX (Supplementary Fig.VIA).
Consistent with the shift of microglia/macrophage phenotype toward a pro-inflammatory modality in diabetic mice, the production of inflammatory cytokines (IL-1β and IL-6) was significantly elevated in the db/db mice compared to WT or db/+ mice at 14d after stroke (Fig.5C).
Exposure to high glucose levels shifts microglial polarization toward the pro-inflammatory state and impairs OPC differentiation under both physiological and inflammatory conditions
Primary cultures were used to assess the effects of high glucose levels on microglial phenotypic changes and the subsequent impact on OPC differentiation (Fig.6A). Microglia were cultured in media for 48h with a standard concentration of glucose (5.5mM) or high glucose (15mM—analogous to a diabetic condition within the cell culture system). Exposure to high glucose slightly increased expression of the M1 marker CD16 (Fig.6B). When microglia were exposed to a low-concentration of LPS, the high glucose levels shifted microglia toward the M1 polarity, increasing the expression of three M1 markers (CD16, iNOS, and TNFα), and inhibiting the expression of two M2 markers (CD206 and TGFβ) (Fig.6B). Co-culture of the unstimulated microglia in standard glucose concentrations increased OPC differentiation, as revealed by increased mRNA expression of maturation markers mbp, mog and plp compared to OPC cultures without microglia (dashed lines in Fig.6C). Co-cultures of either normal or LPS-stimulated microglia in high glucose with OPCs significantly inhibited mRNA expression of mbp, mog and plp in the OPCs (Fig.6C). High glucose alone had no direct effect on OPC differentiation (data not shown). These results suggest that high glucose-treated microglia inhibit OPC differentiation either under physiological or inflammatory conditions. Immunofluorescent staining confirmed decreases in the percentages of MBP+ oligodendrocytes when co-cultured with microglia under high glucose conditions, either in the absence or presence of LPS (Fig.6D).
Figure 6. High glucose shifts microglial polarization toward M1, which impairs oligodendrocyte precursor cell differentiation under both physiological and inflammatory conditions.
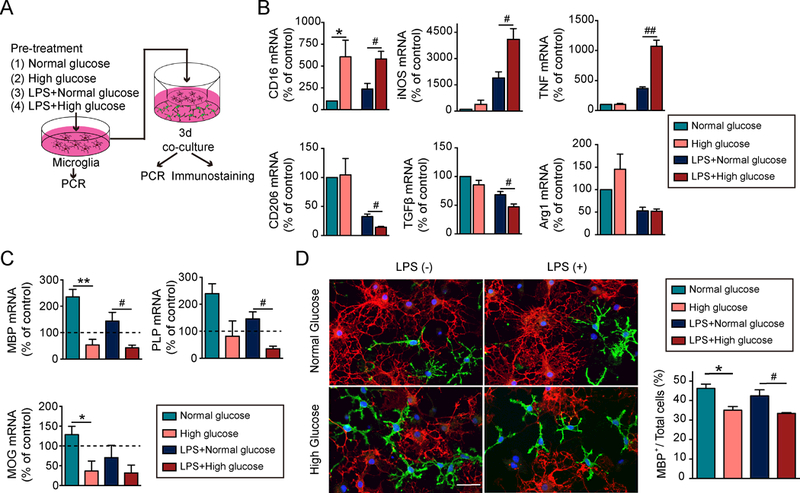
A, Experimental design: Microglia in transwells were exposed to media with a normal concentration of glucose (5.5 mM) or high glucose (15 mM) for 48h in the presence or absence of a low concentration of LPS (2.5 ng/mL). Pretreated microglia were then co-cultured with oligodendrocyte precursor cells (OPCs) for 3d. B, The RNA expression of M1 and M2-like markers in microglia in transwells were measured by qRT-PCR. Expression of gapdh was used as an internal control. Data are fold-change relative to non-treated microglia. C, Expression of mbp, plp and mog in OPCs was assessed by qRT-PCR. Expression of gapdh was used as an internal control. Data are fold-change relative to OPCs without microglia co-cultures (dashed line). D, Double immunostaining for mature oligodendrocyte marker MBP (red) and OPC marker NG2 (green) in OPCs co-cultured for 3d with microglia-in-transwells. OPC differentiation was quantified by the percentage of MBP+ cells out of total cells (MBP++NG2+). Data were collected from 3 independent experiments. Scale bar: 50 μm. *p<0.05, **p<0.01, ***p<0.001; one way ANOVA and Bonferroni post hoc.
Discussion
This study is the first to demonstrate that the condition of T2DM exacerbates long-term functional deficits after stroke, coincident with greater disruption in the structural and functional integrity of WM. Indeed, long-term functional deficits after ischemic stroke in T2DM mice (db/db) correlated with greater loss of MBP+ WM as well as NeuN+ gray matter compared to non-diabetic stroke mice (WT and db/+). However, sensorimotor dysfunction was more strongly correlated with loss of WM than parallel loss of gray matter. Consistent with these findings, clinical magnetic resonance imaging studies also report a positive association between the deterioration in WM structure in stroke patients and poor neurological outcomes and prognosis.4, 10 Interestingly, the db/db mice exhibited similar loss of MBP and reductions in fiber tract conduction properties compared to db/+ and WT mice early after stroke, indicating comparable initial white mater injury among the three mouse lines. Therefore, the deterioration in WM integrity in diabetic mice 35d after stroke might be better associated with impaired recovery rather than with greater initial injury.
Previous animal studies have suggested several mechanisms that may underlie the deterioration of WM after brain ischemia in diabetic mice.11, 12 One potential mechanism is impairment in the proliferation and survival of OPCs in WM, as shown in diabetic mice after bilateral CCA stenosis.11 We demonstrated here that the deterioration of WM integrity in diabetic mice is accompanied by reduced numbers of proliferating OPCs and fewer newly generated mature oligodendrocytes. These results suggest that diabetes has a profound negative impact on OPC differentiation and maturation after stroke. Although db/db mice exhibited impaired oligodendrogenesis in both EC and cortex, the number of newly generated NG2+BrdU+ OPCs and APC+BrdU+ oligodendrocytes was positively correlated with MBP staining intensity only in the EC and not in the cortex (Fig.4). This discrepancy might be related to the lack of differences in WM integrity in the cortex in db/db mice versus WT or db/+ mice after stroke, as measured by MBP staining intensities (Supplementary Fig.3) and NF200/MBP ratios (Fig.3B). These data are consistent with previous observations that OPCs from different CNS regions exhibit distinct temporal patterns of oligodendrocyte generation and myelinogenesis. 13
Aside from the abovementioned observations, we also found that the impaired oligodendrogenesis in diabetic mice is partially attributed to pro-inflammatory microglia/macrophage responses in the ischemic diabetic brain. Microglia/macrophage phenotype alterations exert profound influences on OPC survival, differentiation, and maturation.6, 7 In our study, low concentration LPS-treated M1 microglia exerted minimal effects on MBP expression in OPCs within 3d of co-culturing. However, the mRNA expression of mbp, mog and plp genes were dramatically inhibited by LPS-treated microglia, suggesting potentially lower expression of these maturation markers in OPCs at subsequent time points not assessed in the present report. Elevated glucose levels, as seen in T2DM, induce prolonged periods of microglia activation.14 The transient surge of blood glucose during acute ischemic stroke, even in those subjects without preexisting diabetes, may drive the polarization of monocyte/macrophages toward the pro-inflammatory phenotype.15 The present in vitro studies confirm a direct effect of glucose on microglial polarization. Exposure to media with high glucose levels shifted microglia under both physiological and LPS-primed conditions toward an inflammatory M1-like phenotype. This pro-inflammatory phenotype impaired the differentiation and maturation of OPCs, as expected. Accordingly, inflammation-prone microglia/macrophage responses in the ischemic brain of diabetic mice may obstruct oligodendrogenesis and WM repair.
Brain repair and regeneration after stroke requires coordinated communication between various central and peripheral cell types. The current study focused on the impact of diabetes on microglia-OPC interactions and WM integrity. Additional evidence from the literature supports the involvement of other mechanisms in the inferior long-term outcomes in diabetic stroke mice. For example, diabetes impairs reparative neovascularization and results in a dramatic decline in the cerebrovascular network after stroke.16 Diabetic mice exhibit diminished leptomeningeal collateral flow compensation compared with non-diabetic mice.17 Furthermore, diabetes exacerbates BBB damage and facilitates macrophage infiltration, which impair cognitive functions.18 In addition, diabetes results in a significant reduction in the proliferation of doublecortin+ neuroblasts, and impairs dendritic/spine plasticity after stroke.19 Whether these reparative mechanisms contribute to long-term sensorimotor or cognitive deficits in diabetic stroke victims, either independently or involving interplay with microglia/macrophages, warrants further exploration.
The point mutation in the leptin receptor lepr renders db/db mice incapable of gauging caloric limits, resulting in high blood glucose and insulin levels. Being obese and hyperglycemic, the db/db mouse is an established animal model for the obese variant of T2DM and insulin resistance.20 However, it is important to acknowledge that db/db mice may have symptoms that are not specific to diabetic patients but are more reflective of obesity or reduced leptin function. For example, the anxiety-like behavior in the open field in db/db animals with or without ischemic injury might be related to obesity or leptin blockage, as the db/+ with normal blood glucose also demonstrated a trend toward anxiety. A second limitation of the current study is that we used only young adult male mice, while stroke mainly afflicts the aged population. Sex differences in ischemic injury have also been reported in diabetes mice and estrogen might be a mechanism of brain protection in diabetic females.21 Therefore, further studies in aged male and female diabetic mice are warranted. However, the db/db mice are not suitable for long-term studies due to their development of infectious complications with aging. In addition, although our in vivo studies were performed in a mouse model, we conducted the in vitro studies with rat OPCs because of the higher OPC yield from rat brains. Nevertheless, in agreement with our current genetic model of diabetes, recent studies using the nicotinamide–streptozotocin-induced diabetic model in middle-aged rats also described impaired WM integrity and OPC differentiation after stroke in diabetic rats.19, 22 These studies support the view that diabetes has a detrimental impact on WM repair after stroke in both rats and mice. While the present study focused on elucidating the effects of T2DM on WM integrity after stroke, treatment strategies that target WM injury in diabetic stroke victims are not available and require further investigation. To this end, both permanent and reperfusion models of stroke should be explored, as the recent clinical success of endovascular thrombectomy is expected to increase the chances of post-stroke reperfusion in the near future.23
In summary, the present study suggests that diabetes elicits deterioration in functional deficits after stroke. These functional deficits are robustly correlated with loss of WM integrity. High glucose exposure shifts microglia/macrophage polarization toward a pro-inflammatory phenotype, which impairs OPC differentiation. Thus, our data support the view that therapeutic modulation of microglia/macrophage polarization towards an oligodendrogenesis-enhancing phenotype may be a fruitful strategy to promote WM repair and functional recovery after stroke in diabetic patients.
Supplementary Material
Acknowledgments
Acknowledgements: We thank Dr. Amanda Smith for editorial assistance.
Funding Sources: J. C. is supported by NIH/National Institute of Neurological Disorders and Stroke (NINDS) and Veterans Health Administration. Jun Chen is a recipient of the Veterans Affairs Senior Research Career Scientist Award.
Footnotes
Disclosures: None.
Full descriptions of experimental procedures are available in the online-only Data Supplement file.
References
- 1.Kuwashiro T, Sugimori H, Ago T, Kuroda J, Kamouchi M, Kitazono T. The impact of predisposing factors on long-term outcome after stroke in diabetic patients: The fukuoka stroke registry. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2013;20:921–927 [DOI] [PubMed] [Google Scholar]
- 2.Tziomalos K, Spanou M, Bouziana SD, Papadopoulou M, Giampatzis V, Kostaki S, et al. Type 2 diabetes is associated with a worse functional outcome of ischemic stroke. World journal of diabetes. 2014;5:939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oksala NK, Oksala A, Pohjasvaara T, Vataja R, Kaste M, Karhunen PJ, et al. Age related white matter changes predict stroke death in long term follow-up. Journal of neurology, neurosurgery, and psychiatry. 2009;80:762–766 [DOI] [PubMed] [Google Scholar]
- 4.Ben Assayag E, Eldor R, Korczyn AD, Kliper E, Shenhar-Tsarfaty S, Tene O, et al. Type 2 diabetes mellitus and impaired renal function are associated with brain alterations and poststroke cognitive decline. Stroke; a journal of cerebral circulation. 2017;48:2368–2374 [DOI] [PubMed] [Google Scholar]
- 5.Suenaga J, Hu X, Pu H, Shi Y, Hassan SH, Xu M, et al. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol. 2015;272:109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1864–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during cns remyelination. Nature neuroscience. 2013;16:1211–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres-Castro I, Arroyo-Camarena UD, Martinez-Reyes CP, Gomez-Arauz AY, Duenas-Andrade Y, Hernandez-Ruiz J, et al. Human monocytes and macrophages undergo m1-type inflammatory polarization in response to high levels of glucose. Immunology letters. 2016;176:81–89 [DOI] [PubMed] [Google Scholar]
- 9.Illouz T, Madar R, Louzon Y, Griffioen KJ, Okun E. Unraveling cognitive traits using the morris water maze unbiased strategy classification (must-c) algorithm. Brain, behavior, and immunity. 2016;52:132–144 [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Song R, Jiaerken Y, Yuan L, Huang P, Lou M, et al. White matter injury induced by diabetes in acute stroke is clinically relevant: A preliminary study. Diabetes & vascular disease research. 2017;14:40–46 [DOI] [PubMed] [Google Scholar]
- 11.Yatomi Y, Tanaka R, Shimada Y, Yamashiro K, Liu M, Mitome-Mishima Y, et al. Type 2 diabetes reduces the proliferation and survival of oligodendrocyte progenitor cells in ishchemic white matter lesions. Neuroscience. 2015;289:214–223 [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017;37:1326–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Power J, Mayer-Proschel M, Smith J, Noble M. Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions. Developmental biology. 2002;245:362–375 [DOI] [PubMed] [Google Scholar]
- 14.Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, et al. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via n-methyl-d-aspartate-dependent mechanisms. Molecular pharmacology. 2006;70:1246–1254 [DOI] [PubMed] [Google Scholar]
- 15.Khan MA, Schultz S, Othman A, Fleming T, Lebron-Galan R, Rades D, et al. Hyperglycemia in stroke impairs polarization of monocytes/macrophages to a protective noninflammatory cell type. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:9313–9325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ergul A, Abdelsaid M, Fouda AY, Fagan SC. Cerebral neovascularization in diabetes: Implications for stroke recovery and beyond. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akamatsu Y, Nishijima Y, Lee CC, Yang SY, Shi L, An L, et al. Impaired leptomeningeal collateral flow contributes to the poor outcome following experimental stroke in the type 2 diabetic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:3851–3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stranahan AM, Hao S, Dey A, Yu X, Baban B. Blood-brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice. J Cereb Blood Flow Metab. 2016;36:2108–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Chopp M, Zhang Y, Xiong Y, Li C, Sadry N, et al. Diabetes mellitus impairs cognitive function in middle-aged rats and neurological recovery in middle-aged rats after stroke. Stroke; a journal of cerebral circulation. 2016;47:2112–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King AJ. The use of animal models in diabetes research. British journal of pharmacology. 2012;166:877–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakata A, Mogi M, Iwanami J, Tsukuda K, Min LJ, Jing F, et al. Female type 2 diabetes mellitus mice exhibit severe ischemic brain damage. Journal of the American Society of Hypertension : JASH. 2011;5:7–11 [DOI] [PubMed] [Google Scholar]
- 22.Ding G, Chen J, Chopp M, Li L, Yan T, Davoodi-Bojd E, et al. White matter changes after stroke in type 2 diabetic rats measured by diffusion magnetic resonance imaging. J Cereb Blood Flow Metab. 2017;37:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. The New England journal of medicine. 2018;378:11–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



