Abstract
Objective:
Revealing patterns of associations between circulating protein and lipid levels could improve biological understanding of cardiovascular disease. In this study, we investigated associations between proteins related to cardiovascular disease, and triglyceride (TG), total cholesterol (TC), LDL and HDL cholesterol (LDL-C and HDL-C) levels in individuals from the general population.
Approach and Results:
We measured plasma protein levels using the Olink ProSeek CVD I or II+III arrays and analyzed 57 proteins available in three population-based cohorts: EpiHealth (n=2,029 52% women, median age 61 years), PIVUS (n=790 51% women, all aged 70 years) and ULSAM (n=551, all men aged 77 years). A discovery analysis was performed in EpiHealth in a regression framework (adjusted for sex, age, BMI, smoking, glucose levels, systolic blood pressure, blood pressure medication, diabetes medication and CVD history) and associations with FDR<0.05 were further tested in PIVUS and ULSAM, where p-value of 0.05 was considered a successful replication (validation FDR of 0.1%). We used summary statistics from a genome-wide association study (GWAS) on each protein biomarker (meta-analysis of EpiHealth, PIVUS, ULSAM and IMPROVE) and publicly available data from Global Lipids Genetics Consortium (GLGC) to perform Mendelian randomization (MR) analyses to address possible causality of protein levels. Out of 57 tested proteins, 42 demonstrated an association with at least one lipid fraction; 35 were associated with TG, 15 with TC, 9 with LDL-C, and 24 with HDL-C. Among these associations, we found kidney injury molecule (KIM-1), tumor necrosis factor receptor 1 and 2 (TNF-R1 and TNF-R2), TNF-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) and resistin (RETN) to be associated with all four lipid fractions. Further, 15 proteins were related to both TG and HDL-C in a consistent and biologically expected manner, i.e. higher TG and lower HDL-C or vice versa. Another common pattern of associations was concomitantly higher TG, TC and LDL-C, which is associated with higher CVD risk. We did not find evidence of causal links for protein levels.
Conclusions:
Our comprehensive analysis of plasma proteins and lipid fractions of 3,370 individuals from the general population provides new information about lipid metabolism.
Keywords: Total cholesterol, HDL, LDL, triglycerides, protein biomarker, general population, mendelian randomization, Biomarkers, Lipids and Cholesterol, Proteomics, Epidemiology
INTRODUCTION
Triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), important lipid fractions, attract clinical attention when present in abnormal concentrations. This is not only due to their strong association with coronary heart disease, the leading cause of death worldwide, but increased TG and decreased HDL-C are also key metabolic abnormalities in patients with obesity, insulin resistance (IR) and type 2 diabetes (T2D),1 increasing public health burdens.
Central roles of lipids in physiology and disease, and their metabolic and signaling functions often arise from interactions with proteins.2 For instance, fatty acid-binding protein 4 (FABP4) acts as a cytoplasmic lipid chaperone, suppressing adipose tissue lipogenesis and promoting lipolysis, with direct effects on the composition of the local and circulating free fatty acid pool.3, 4 Similarly growth hormone (GH), in addition to its primary function to control and promote skeletal growth, also stimulates lipolysis in adipose tissue, resulting in increased fatty free acids levels in the blood.5 Kidney injury molecule (KIM-1), is a phosphatidylserine receptor that recognizes apoptotic cells and directs them to lysosomes, but also serves as a receptor for oxidized lipoproteins.6 Recent advances in highly multiplexed immunoassays allow measuring simultaneously a wide range of plasma proteins selected for their role in cardiovascular disease; thus facilitating studies of the broad picture of lipid-protein associations in the circulatory system, which may provide valid nodes for drug action to treat human diseases.
Observational epidemiological studies provide a wealth of information on associations between disease exposures and outcomes, but these relations should not be interpreted as causal, owing to limitations introduced by confounding and reverse causality.7 Mendelian randomization (MR) is a method using genetic variants as instrumental variables to assess causal relationships from observational data.8 Thus, this design excludes possibility of reverse causation and under certain assumptions, MR methods also avoid confounding.8
The primary aim of this study was to evaluate associations of circulating proteins with lipid fractions in several population-based cohorts. In addition, we investigated causal effects of protein levels on lipid levels using MR methods, as well as used different orthogonal data sources to increase biological understanding of associations between circulating plasma proteins and lipids.
METHODS
Data are made available to researchers who meet the criteria for confidential data access as stipulated by participant informed consent and institutional review board. Data access in ULSAM is granted through the Interdisciplinary Collaboration Team on Uppsala Longitudinal Studies (ICTUS; http://www.pubcare.uu.se/ulsam/Research/Proposals). Data from the PIVUS study are available after application to the PIVUS steering committee (http://www.medsci.uu.se/pvus/). Data from the EpiHealth study are granted after application to the EpiHealth steering committee (https://www.epihealth.se/For-scientists/Research-Application-/).
Study samples
EpiHealth
The EpiHealth study was initiated in 2011 with the goal of recruiting 300,000 men and women aged 45–75 years in the Swedish towns of Uppsala and Malmö.9 The study consists of three parts: a collection of self-reported data on lifestyle by internet-based questionnaires; a clinical visit where blood samples are collected and physiological parameters recorded; and follow-up for occurrence of outcomes using nationwide medical registers. The present study is based on a random sample of 2,467 individuals with measured protein profile. Of this sample, seven individuals were excluded due to unsuccessful protein profiling (not passing the quality control), 141 due to fasting time < six hrs, 240 due to being on lipid-lowering medication, and 50 due to missing information on covariates used in the main analysis; thus, leaving 2,029 individuals for the main analysis (Table 1).
Table 1.
Clinical characteristics of the study samples.
| Variable | EpiHealth (n=2,029) | PIVUS (n=790) | ULSAM (n=551) |
|---|---|---|---|
| Female sex | 1051 (52) | 404 (51) | 0 (0) |
| Age (years) | 61 (45-75) | 70 | 78 (76-80) |
| Current smoking | 148 (7) | 84 (11) | 45 (8) |
| BMI (kg/m2) | 26.0 (16.4-50.7) | 26.5 (16.6-49.7) | 25.8 (16.4-41.3) |
| SBP (mmHg) | 134 (88-240) | 148 (90-230) | 150 (102-230) |
| Triglycerides (mmol/L) | 1.1 (0.4-15.0) | 1.1 (0.1-4.2) | 1.2 (0.3-6.1) |
| Total cholesterol (mmol/L) | 6.0 (3.1-13.0) | 5.5 (1.8-9.4) | 5.5 (2.8-10.2) |
| LDL (mmol/L) | 4.0 (1.5-9.7) | 3.5 (0.8-6.9) | 3.6 (1.4-7.6) |
| HDL (mmol/L) | 1.5 (0.7-3.7) | 1.4 (0.6-3.8) | 1.3 (0.6-2.6) |
| Blood glucose (mmol/L) | 5.8 (4.3-19.7) | 5.0 (2.8-19.9) | 5.5 (2.9-17.2) |
| Diabetes treatment | 22 (1.1) | 37 (4.7) | 61 (10.2) |
| Antihypertensive treatment | 365 (18.0) | 214 (27.1) | 205 (37.2) |
| Myocardial infarction | 18 (0.9) | 23 (2.9) | 49 (8.9) |
| Angina | 15 (0.8) | 31 (3.9) | 39 (7.1) |
| Stroke | 11 (0.5) | 20 (2.5) | 47 (8.5) |
| Heart failure | 11 (0.5) | 24 (3.0) | 65 (11.8) |
| Atrial Fibrillation | 42 (2.1) | 19 (2.4) | 52 (9.4) |
Data are medians (range) or number (%).
Prospective Study of the Vasculature in Uppsala Seniors (PIVUS)
The PIVUS study has been described previously10 and on the Internet (www.medsci.uu.se/pivus/pivus.htm). All 70-year old individuals residing in Uppsala, Sweden, in 2001–2005 were eligible for the study, and 2,025 individuals were invited in a randomized order within two months of their 70th birthday. Of these, 1,016 (50.2%) participated and 510 (50.2%) of them were women. We excluded 25 individuals due to unsuccessful protein profiling, 21 individuals due to C-reactive protein (CRP) levels above 20 mg/L (to exclude individuals with possible ongoing infection and other inflammatory processes), 154 due to being on lipid-lowering medication and 26 individuals due to missing information on covariates in the main analysis. Hence, the eligible sample size for the present study was 790 individuals (Table 1).
Uppsala Longitudinal Study of Adult Men (ULSAM)
The ULSAM study has been described previously11 and on the Internet (http://www.pubcare.uu.se/ulsam). The study was initiated in the period 1970–1973 by inviting all men living in Uppsala, Sweden, born between 1920 and 1924. The invitation letter was sent to 2,841 men and 2,322 (81.7%) of them participated in the initial examination. The participants have been re-examined at multiple time points. The present study was based on the re-examination at age 77, when 748 of the original participants had died, and another 176 men were not eligible for other reasons. From 1997 to 2001, the remaining 1,398 individuals were re-invited, and 839 (60.0%) of them participated. We excluded 77 individuals due to unsuccessful protein profiling, 21 individuals due to CRP levels above 20 mg/L, 139 due to lipid lowering medication and 51 individuals due to missing information on covariates in the main analysis. Hence, the eligible sample size for the present study was 551 men.
The EpiHealth, PIVUS and ULSAM studies were approved by the Ethics Committee of Uppsala University in agreement with the Helsinki Declaration, and all subjects gave their informed written consent.
Lipids measurement
In EpiHealth, at the test center, 10ml of the blood sample were used for determinations of fasting glucose, LDL-C and HDL-C, and TG at the hospital laboratory using an Architect Ci8200 analyzer (Abbott Laboratories, Abbott Park, IL, USA)9. In PIVUS, lipid variables were measured by standard laboratory techniques at the Uppsala University hospital10. In ULSAM, cholesterol and triglyceride concentrations were analyzed in serum and isolated lipoprotein fractions by enzymatic techniques using IL Test Cholesterol Trinder’s Method 181618–10 and IL Test Triglyceride Enzymatic-colorimetric Method 181610–60 for use in a Monarch apparatus (Instrumentation Laboratories, Lexington, USA). HDL particles were separated by precipitation with magnesium chloride and phosphotungstate. LDL cholesterol was calculated using Friedewald’s formula.12
Other covariates
Anthropometric measures (height and weight) of PIVUS, ULSAM and EpiHealth participants were recorded at the time of blood draw used for proteomic analysis and were measured by trained staff. For PIVUS and ULSAM, medical history, smoking habits, and regular medication were collected at time of blood draw by self-administered questionnaires or interviews by a study nurse and national registries. The same data for EpiHealth were collected prior to the clinical visit by Internet-based questionnaires or by national registries.
Proteomic analysis
Proteomic profiling in EpiHealth was performed using the Olink Proseek® Multiplex cardiovascular disease (CVD) II and III96×96 kits (http://www.olink.com/products/cvd-ii-panel/ and http://www.olink.com/products/cvd-iii-panel/) measuring 92 selected cardiovascular disease-related proteins simultaneously. The kits are based on the proximity extension assay (PEA) technology, where 92 oligonucleotide-labelled antibody probe pairs are allowed to bind to their respective target present in the sample. The PEA technique is exceptionally high specific and very sensitive13, 14. The platform provides normalized protein expression (NPX) data where a high protein value corresponds to a high protein concentration, but not an absolute quantification. Samples that (i) failed for technical reasons on both chips; or (ii) a sample call rate <0.95 on either chip were excluded. Proteins with ≥10% missingness were also excluded. Each protein was normalized by plate (by setting the mean = 0 and standard deviation = 1 within each plate).
Details on PIVUS and ULSAM blood samples have been described previously.15, 16 Analyses were performed using the Olink Proseek® Multiplex CVD I96×96 kit (http://www.olink.com/products/document-download-center/). All samples and runs for PIVUS and ULSAM passed technical quality control. Twelve proteins with a call rate <85% (i.e. <85% of the individuals had a valid measurement of that specific protein) in PIVUS and/or ULSAM were removed from further analysis, including IL-4, melusin, BNP, Beta-NGF, SIRT2, NEMO, mAmP, PTX3, NT-pro-BNP, MMP-7, cystatin-B and heat shock 27 kDa protein. Individuals with excess missingness based on visual inspection of a histogram were excluded (>5% and >3% in PIVUS and ULSAM, respectively). Each protein was normalized by plate and further by storage time (correction based on the observed values and predicted values from a spline model). We analyzed 57 proteins that overlapped between the CVD I, II and III arrays and that passed quality control in all three cohorts. The full lists of analyzed proteins are presented in Supplementary Table I.
Statistical Analysis
Primary Analysis
We evaluated the distribution of protein values and clinical covariates using the Shapiro-Wilk normality test and qq-plots. All protein and lipid variables were normalized by rank transformation. The EpiHealth study was a priori designated as the discovery cohort as it was largest, and had equal proportions of males and females. Linear regression models were used for all analyses. Lipids levels were used as the independent variables and the 57 proteins were used as dependent variables in separate models (one per lipid fraction and protein marker). The analysis of the protein profiling data was performed in an age- and sex-adjusted model (only age in ULSAM as all participants were males), and a multivariable-adjusted model. We considered the multivariable-adjusted models as our main models, both when deciding which protein markers to pursue for replication, and which to annotate as significantly associated. The multivariable-adjusted model included age, sex, body mas index (BMI), smoking (a factor variable with three levels: never, former and current smoker), glucose levels, systolic blood pressure, antihypertensive medication, diabetes medication and prior myocardial infarction, angina, stroke, heart failure and atrial fibrillation. Prior cardiovascular diseases were defined by self-report (EpiHealth and ULSAM) or the International Classification of Diseases (ICD) codes (ULSAM). Protein markers with a false discovery rate (FDR; estimated using the Benjamini & Hochberg method)17 < 5% in EpiHealth, were selected for further replication in PIVUS and ULSAM. Study-specific estimates from PIVUS and ULSAM were combined using an inverse variance-weighted mixed-effects meta-analysis, and associations with the same direction as in EpiHealth at p-value < 0.05 were considered successful replication. We have previously shown that this replication strategy is conservative and indeed, the risk of false positive findings in the replication stage (vFDR) was calculated to 0.1% for each lipid fraction separately (http://fafner.meb.ki.se/personal/yudpaw/rdr/18). The study design is depicted in Figure 1.
Figure 1.
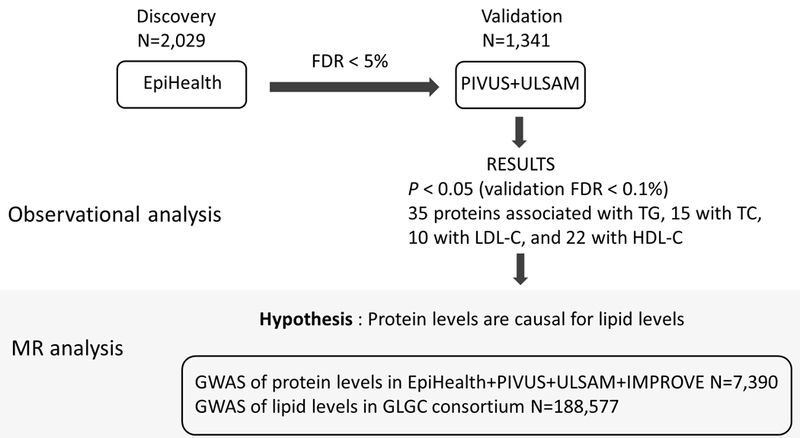
Study design.
Orthogonal data sources
We queried Search Tool for the Retrieval of Interacting Genes/Proteins (STRING)19 for protein-protein interaction networks, filtering on an interaction score of strong evidence (0.7). Then, we used data from the Genotype-Tissue Expression (GTEx) project20 to depict expression patterns in physiologically relevant tissues, specifically whole blood (as the protein biomarkers in our study were measured in blood samples); coronary artery, aorta, tibial artery, heart left ventricle and heart atrial appendage (representing cardiovascular system since the protein biomarkers were selected based on their relation to cardiovascular disease; and subcutaneous adipose tissue, visceral adipose tissue, liver and skeletal muscle (tissues involved in lipid metabolism and insulin resistance). Finally, we searched Catalog of published genome-wide association studies (GWAS Catalog)21 inquiring gene names corresponding to protein biomarkes. This allowed us to find associations in GWAS (P<5×10−8) if variants were mapped to the inquired genes or the inquired genes were in the neighborhood of associated loci.
Mendelian Randomization
In MR analyses, an association between a genetic instrument and the outcome is indicative of a causal effect of the risk factor on the outcome.22 In this study, we performed two-sample MR analyses using genetic summary statistics where we tested the hypothesis that different protein levels affect lipid fractions. The analyses were performed for all lipid-protein associations that were replicated in the observational analyses.
Summarized data used in MR
Genome-wide association studies (GWAS), which typically report regression coefficients summarizing the associations of many genetic variants with various traits, are potentially a powerful source of data for MR investigations.23 We used summary statistics from GWAS performed on each protein biomarker, as described in Folkersen et al.;24 but in the present study, we obtained results from a meta-analysis of EpiHealth, PIVUS, ULSAM and IMPROVE (total number of subjects n=7,390). The IMPROVE study is a multicentre, observational study, which recruited 3,711 men and women aged between 55 to 79 years with at least three cardiovascular risk factors but without symptoms of CVD.25 The study was conducted in accordance with the declaration of Helsinki and all participants gave written informed consent. The ethics and sampling of this cohort have been further documented in prior publications, e.g.26.
For lipid fractions we used summary statistics data from Global Lipids Genetics Consortium (GLGC), the largest (n=188,577), publicly available GWAS of lipid traits.27
As instrumental variables, we selected all independent variants (r2<0.01) associated with protein levels at genome-wide significant level (P<5×10−8) and having cis effects on protein-coding gene expression, i.e. variants in the same locus as the protein coding gene (Supplementary Table III). Out of the 42 proteins associated with at least one lipid fraction, we were able to create genetic instruments for 30 protein biomarkers. There were no genome-wide significant variants associated with GH and LEP; no cis single nucleotide polymorphisms (SNPs) associated with levels of FGF23, IL-27, IL-6, MCP-1, PDGF subunit B, SCF, tPA, VEGF-D; and no proxies in the GWAS data on lipids in GLGC for variants associated with CD40L and FABP4 (presumably due to low minor allele frequency, 1.2% for the single variants associated with these proteins). For outcomes, we used data from GLGC.27 We used the Wald method when only one SNP was used as an instrument, while the inverse-variance weighted method was used for combinations of more than one variant.28
All MR analyses were performed with the TwoSampleMR package in R, which allows evaluating the causal effect of an exposure on an outcome using only summary statistics from GWAS.29 To minimalize risk of false positive findings due to high number of tests, we applied Bonferroni correction of the nominal alpha threshold of 0.05. Using an online tool (https://sb452.shinyapps.io/power/), we estimated statistical power for the different MR analyses using the outcome sample sizes, effect sizes observed using traditional regression, variance explained for the instrumental variable and the Bonferroni-corrected P-value, all specific for each analysis.
RESULTS
The clinical characteristics of the study samples are presented in Table 1. Compared to the EpiHealth (discovery cohort), the PIVUS and ULSAM populations were older, and more likely to be taking antihypertensive and diabetes medication and have prior CVD. Also, importantly, ULSAM consisted exclusively of males.
Observational analyses
Out of 57 investigated protein biomarkers, 42 demonstrated a replicated association with at least one of the lipid fractions; 35 were associated with TG, 15 with TC, 10 with LDL-C, and 22 with HDL-C (Figure 2 and 3; Table 2). Among these associations, we found kidney injury molecule (KIM-1), tumor necrosis factor receptor 1 and 2 (TNF-R1 and TNF-R2), TNF-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) and resistin (RETN) to be associated with all four lipid fractions. Further, 15 proteins were related to both TG and HDL-C in a consistent and biologically expected manner, i.e. higher TG and lower HDL-C or vice versa. Out of these, stem cell factor (SCF) and GH represent a pattern generally associated with lower insulin resistance and CVD risk, as they were associated with lower TG and higher HDL-C. FABP4, tissue-type plasminogen activator (t-PA), interleukin-1 receptor antagonist protein (IL-1RA), C-C motif chemokine 3 (CCL3), fibroblast growth factor 23 (FGF-23), interleukin 16 (IL-16), interleukin 18 (IL-18), matrix metalloproteinase-12 (MMP-12), placenta growth factor (PlGF), RETN, TNF-R1, TNF-R2 and TRAIL-R2 were associated with higher TG and lower HDL-C, thus a pattern consistent with insulin resistance. Another common pattern of associations was concomitantly higher TG, TC and LDL-C, which often is associated with higher CVD risk. This was observed for higher C-X-C motif chemokine 16 (CXCL16), Gal3 (Galectin-3) and monocyte chemotactic protein 1 (MCP-1). Moreover, there was a set of proteins that were associated only with TG, all of them in a positive fashion (Figure 2). Results from the age- and sex-adjusted analyses are presented in Supplementary Table I. Overall, some associations from age- and sex-adjusted model were no longer significant in the fully adjusted analyses (Supplementary Table II).
Figure 2.
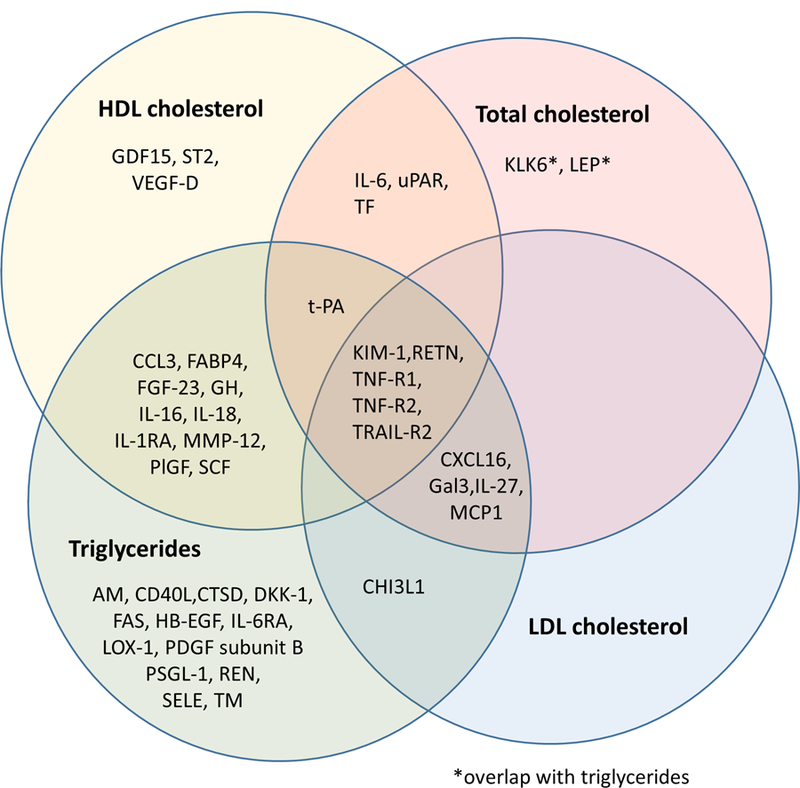
Overlap of protein biomarkers associated with lipid fractions.
Figure 3.
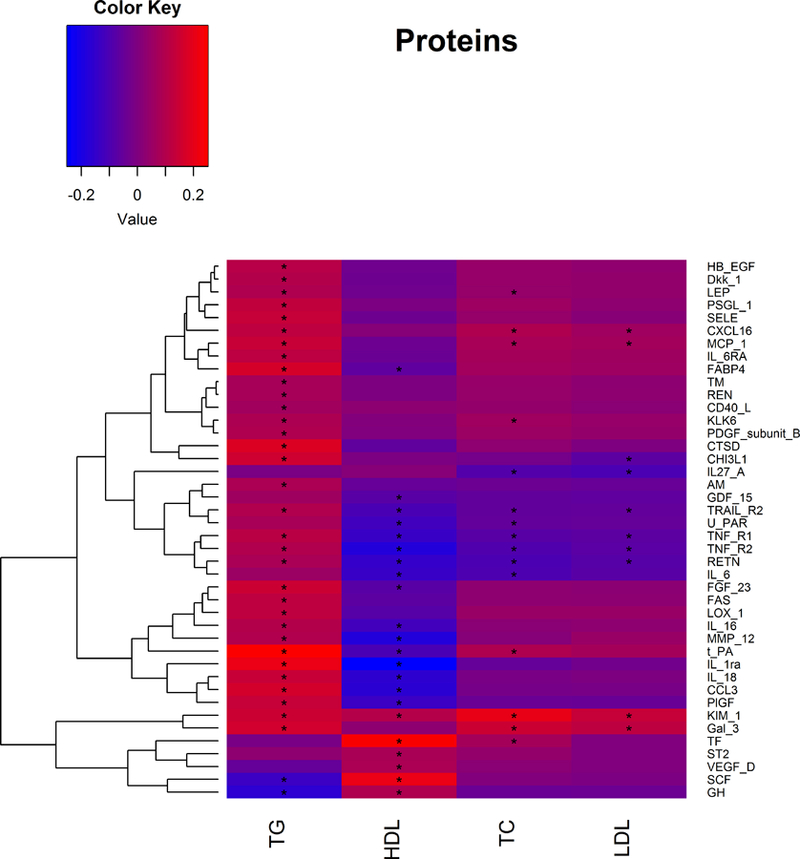
Directions of the associations between protein biomarkers and lipid levels. Asterisks indicate significant associations. Effects for protein levels are given for a 1-SD increase in lipid levels.
Table 2.
Significant associations between protein biomarkers and lipid fractions in the multivariable model.*
| TRIGLYCERIDES (TG) | ||||||
|---|---|---|---|---|---|---|
| EpiHealth | PIVUS+ULSAM | |||||
| Marker | Beta | SE | P | Beta meta | SE meta | P meta |
| Fatty acid-binding protein 4 (FABP4) | 0.153 | 0.017 | 9.27E-19 | 0.193 | 0.022 | 5.65E-18 |
| Tissue-type plasminogen activator (t-PA) | 0.263 | 0.020 | 7.68E-37 | 0.216 | 0.026 | 2.95E-16 |
| Interleukin-1 receptor antagonist protein (IL-1RA) | 0.220 | 0.021 | 3.68E-25 | 0.198 | 0.026 | 2.60E-14 |
| Cathepsin D (CTSD) | 0.171 | 0.023 | 2.13E-13 | 0.197 | 0.027 | 4.70E-13 |
| Galectin-3 (Gal-3) | 0.149 | 0.023 | 1.30E-10 | 0.181 | 0.027 | 4.32E-11 |
| C-C motif chemokine 3 (CCL3) | 0.159 | 0.023 | 1.31E-11 | 0.169 | 0.028 | 3.19E-09 |
| TNF-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) | 0.057 | 0.022 | 8.86E-03 | 0.162 | 0.028 | 7.58E-09 |
| Matrix metalloproteinase-12 (MMP-12) | 0.057 | 0.023 | 1.09E-02 | 0.161 | 0.028 | 1.09E-08 |
| Kidney Injury Molecule-1 (KIM-1) | 0.150 | 0.022 | 1.20E-11 | 0.148 | 0.027 | 7.82E-08 |
| Tumor necrosis factor receptor 1 (TNF-R1) | 0.091 | 0.022 | 4.92E-05 | 0.148 | 0.028 | 9.94E-08 |
| Chitinase-3-like protein 1 (CHI3L1) | 0.156 | 0.022 | 4.45E-12 | 0.151 | 0.028 | 1.40E-07 |
| E-selectin (SELE) | 0.135 | 0.023 | 8.09E-09 | 0.145 | 0.028 | 2.47E-07 |
| Placenta growth factor (PlGF) | 0.140 | 0.022 | 3.02E-10 | 0.142 | 0.028 | 4.11E-07 |
| Interleukin-18 (IL-18) | 0.143 | 0.024 | 1.62E-09 | 0.140 | 0.028 | 8.39E-07 |
| Stem cell factor (SCF) | −0.131 | 0.024 | 7.65E-08 | −0.139 | 0.028 | 9.83E-07 |
| Tumor necrosis factor receptor 2 (TNF-R2) | 0.072 | 0.023 | 1.96E-03 | 0.136 | 0.028 | 1.75E-06 |
| Heparin-binding EGF-like growth factor (HB-EGF) | 0.093 | 0.025 | 1.54E-04 | 0.135 | 0.029 | 3.49E-06 |
| Leptin (LEP) | 0.097 | 0.014 | 7.62E-12 | 0.084 | 0.018 | 4.56E-06 |
| Growth hormone (GH) | −0.184 | 0.021 | 6.62E-18 | −0.125 | 0.027 | 5.35E-06 |
| Fibroblast growth factor 23 (FGF-23) | 0.170 | 0.024 | 1.14E-12 | 0.124 | 0.028 | 8.44E-06 |
| Tumor necrosis factor receptor superfamily member 6 (FAS) | 0.124 | 0.023 | 5.38E-08 | 0.127 | 0.029 | 9.59E-06 |
| Monocyte chemotactic protein 1 (MCP-1) | 0.150 | 0.023 | 1.74E-10 | 0.127 | 0.029 | 1.08E-05 |
| Lectin-like oxidized LDL receptor 1 (LOX-1) | 0.128 | 0.024 | 6.95E-08 | 0.122 | 0.029 | 1.95E-05 |
| C-X-C motif chemokine 16 (CXCL16) | 0.129 | 0.024 | 6.79E-08 | 0.119 | 0.028 | 3.14E-05 |
| Interleukin-6 receptor subunit alpha (IL-6RA) | 0.123 | 0.024 | 5.24E-07 | 0.115 | 0.029 | 6.46E-05 |
| Resistin (RETN) | 0.061 | 0.024 | 1.16E-02 | 0.115 | 0.029 | 7.08E-05 |
| Kallikrein-6 (KLK6) | 0.077 | 0.024 | 1.51E-03 | 0.106 | 0.028 | 1.69E-04 |
| Platelet-derived growth factor subunit B (PDGF subunit B) | 0.084 | 0.025 | 7.85E-04 | 0.109 | 0.029 | 1.77E-04 |
| Interleukin-16 (IL-16) | 0.101 | 0.024 | 2.82E-05 | 0.106 | 0.029 | 2.06E-04 |
| Renin (REN) | 0.058 | 0.023 | 1.13E-02 | 0.099 | 0.027 | 2.34E-04 |
| Dickkopf-related protein 1 (DKK-1) | 0.102 | 0.025 | 3.79E-05 | 0.105 | 0.029 | 3.25E-04 |
| P-selectin glycoprotein ligand 1 (PSGL-1) | 0.148 | 0.024 | 1.80E-09 | 0.100 | 0.029 | 5.45E-04 |
| Thrombomodulin (TM) | 0.070 | 0.024 | 3.73E-03 | 0.088 | 0.029 | 2.17E-03 |
| Adrenomedullin (AM) | 0.092 | 0.021 | 8.95E-06 | 0.072 | 0.028 | 9.91E-03 |
| CD40 ligand (CD40L) | 0.065 | 0.025 | 8.86E-03 | 0.070 | 0.029 | 1.62E-02 |
| TOTAL CHOLESTEROL (TC) | ||||||
| Kidney Injury Molecule-1 (KIM-1) | 0.188 | 0.020 | 5.79E-20 | 0.232 | 0.027 | 7.44E-18 |
| Galectin-3 (Gal-3) | 0.140 | 0.021 | 6.03E-11 | 0.166 | 0.027 | 1.02E-09 |
| Tumor necrosis factor receptor 2 (TNF-R2) | −0.080 | 0.021 | 2.11E-04 | −0.110 | 0.028 | 9.12E-05 |
| Tissue factor (TF) | 0.060 | 0.022 | 7.16E-03 | 0.092 | 0.028 | 1.02E-03 |
| Tumor necrosis factor receptor 1 (TNF-R1) | −0.071 | 0.021 | 6.80E-04 | −0.086 | 0.028 | 1.80E-03 |
| C-X-C motif chemokine 16 (CXCL16) | 0.096 | 0.022 | 1.46E-05 | 0.087 | 0.028 | 2.04E-03 |
| Resistin (RETN) | −0.109 | 0.022 | 1.08E-06 | −0.087 | 0.028 | 2.30E-03 |
| Interleukin-6 (IL-6) | −0.106 | 0.021 | 3.34E-07 | −0.077 | 0.027 | 5.12E-03 |
| Tissue-type plasminogen activator (t-PA) | 0.107 | 0.019 | 4.52E-08 | 0.068 | 0.026 | 9.54E-03 |
| TNF-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) | −0.052 | 0.020 | 9.94E-03 | −0.068 | 0.028 | 1.44E-02 |
| Monocyte chemotactic protein 1 (MCP-1) | 0.089 | 0.022 | 4.60E-05 | 0.065 | 0.028 | 2.17E-02 |
| Interleukin-27 subunit alpha (IL27-A) | −0.101 | 0.023 | 1.15E-05 | −0.063 | 0.028 | 2.41E-02 |
| Urokinase plasminogen activator surface receptor (uPAR) | −0.054 | 0.020 | 6.89E-03 | −0.060 | 0.028 | 3.20E-02 |
| Kallikrein-6 (KLK6) | 0.067 | 0.023 | 3.02E-03 | 0.059 | 0.028 | 3.52E-02 |
| Leptin (LEP) | 0.048 | 0.013 | 3.03E-04 | 0.037 | 0.018 | 4.28E-02 |
| LDL CHOLESTEROL (LDL-C) | ||||||
| Kidney Injury Molecule-1 (KIM-1) | 0.132 | 0.020 | 7.77E-11 | 0.154 | 0.027 | 8.95E-09 |
| Galectin-3 (Gal-3) | 0.113 | 0.021 | 1.03E-07 | 0.134 | 0.027 | 5.95E-07 |
| Chitinase-3-like protein 1 (CHI3L1) | −0.055 | 0.021 | 8.19E-03 | −0.094 | 0.028 | 8.24E-04 |
| Tumor necrosis factor receptor 2 (TNF-R2) | −0.066 | 0.021 | 1.81E-03 | −0.083 | 0.028 | 2.86E-03 |
| Tumor necrosis factor receptor 1 (TNF-R1) | −0.064 | 0.021 | 1.93E-03 | −0.077 | 0.027 | 4.64E-03 |
| Interleukin-27 subunit alpha (IL27-A) | −0.128 | 0.023 | 1.65E-08 | −0.066 | 0.028 | 1.73E-02 |
| Resistin (RETN) | −0.088 | 0.022 | 7.55E-05 | −0.067 | 0.028 | 1.77E-02 |
| Monocyte chemotactic protein 1 (MCP-1) | 0.076 | 0.022 | 4.53E-04 | 0.062 | 0.028 | 2.70E-02 |
| TNF-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) | −0.056 | 0.020 | 5.33E-03 | −0.057 | 0.027 | 3.71E-02 |
| C-X-C motif chemokine 16 (CXCL16) | 0.071 | 0.022 | 1.35E-03 | 0.057 | 0.028 | 3.99E-02 |
| HDL CHOLESTEROL (HDL-C) | ||||||
| Interleukin-1 receptor antagonist protein (IL-1RA) | −0.282 | 0.023 | 7.97E-34 | −0.208 | 0.027 | 3.65E-14 |
| Matrix metalloproteinase-12 (MMP-12) | −0.160 | 0.025 | 8.30E-11 | −0.214 | 0.029 | 4.98E-13 |
| Tumor necrosis factor receptor 2 (TNF-R2) | −0.158 | 0.025 | 4.74E-10 | −0.208 | 0.030 | 2.80E-12 |
| Tissue factor (TF) | 0.262 | 0.025 | 2.74E-24 | 0.206 | 0.030 | 5.86E-12 |
| Stem cell factor (SCF) | 0.231 | 0.026 | 4.62E-18 | 0.188 | 0.030 | 3.19E-10 |
| Tumor necrosis factor receptor 1 (TNF-R1) | −0.122 | 0.024 | 7.08E-07 | −0.162 | 0.029 | 3.79E-08 |
| TNF-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) | −0.068 | 0.024 | 4.43E-03 | −0.157 | 0.029 | 1.09E-07 |
| C-C motif chemokine 3 (CCL3) | −0.169 | 0.026 | 7.02E-11 | −0.154 | 0.030 | 3.18E-07 |
| Kidney Injury Molecule-1 (KIM-1) | 0.075 | 0.024 | 2.29E-03 | 0.146 | 0.029 | 5.47E-07 |
| Resistin (RETN) | −0.145 | 0.026 | 4.02E-08 | −0.151 | 0.030 | 6.79E-07 |
| Interleukin-18 (IL-18) | −0.172 | 0.026 | 3.85E-11 | −0.146 | 0.030 | 1.33E-06 |
| Placenta growth factor (PlGF) | −0.135 | 0.024 | 3.43E-08 | −0.140 | 0.029 | 2.26E-06 |
| Interleukin-6 (IL-6) | −0.152 | 0.024 | 4.39E-10 | −0.121 | 0.029 | 3.93E-05 |
| Vascular endothelial growth factor D (VEGF-D) | 0.071 | 0.026 | 7.01E-03 | 0.103 | 0.029 | 4.26E-04 |
| Fibroblast growth factor 23 (FGF-23) | −0.062 | 0.026 | 1.90E-02 | −0.097 | 0.029 | 8.80E-04 |
| Growth/differentiation factor 15 (GDF-15) | −0.061 | 0.021 | 3.64E-03 | −0.095 | 0.029 | 9.48E-04 |
| Growth hormone (GH) | 0.090 | 0.024 | 1.35E-04 | 0.095 | 0.029 | 1.06E-03 |
| Interleukin-16 (IL-16) | −0.136 | 0.026 | 2.43E-07 | −0.098 | 0.030 | 1.20E-03 |
| Fatty acid-binding protein 4 (FABP4) | −0.051 | 0.019 | 7.36E-03 | −0.076 | 0.024 | 1.64E-03 |
| Urokinase plasminogen activator surface receptor (uPAR) | −0.144 | 0.023 | 8.14E-10 | −0.086 | 0.030 | 4.22E-03 |
| ST2 protein (ST2) | 0.084 | 0.025 | 8.20E-04 | 0.078 | 0.030 | 8.28E-03 |
| Tissue-type plasminogen activator (t-PA) | −0.130 | 0.023 | 1.64E-08 | −0.064 | 0.028 | 2.39E-02 |
Adjusted for age, sex, BMI, smoking, glucose levels, systolic blood pressure, antihypertensive medication, diabetes medication and myocardial infarction, angina, stroke, heart failure and atrial fibrillation.
We performed additional bioinformatics analyses of the five proteins associated with all lipid fractions. Four of them, TNF-R1, TNF-R2, TRAIL-R2 and RETN, displayed protein-protein interactions with high confidence (probability of 0.7) according to the STRING database. These interactions included interactions from curated databases of biological pathway knowledge such as Kyoto Encyclopedia of Genes and Genomes (KEGG), interactions determined in experiments, co-expression and protein homology (Figure 4). TNF-R1, TNF-R2 and TRAIL-2 share some of the functional partners including TRADD (TNFRSF1A Associated Via Death Domain) which is involved in apoptosis modulation and signaling; RIPK-1 (Receptor Interacting Serine/Threonine Kinase 1) that plays a role in inflammation and cell death in response to tissue damage; and TRAF2 (TNF Receptor Associated Factor 2) which plays a central role in the regulation of cell survival and apoptosis. The only partner for RETN identified by STRING was NR3C1, a glucocorticoid receptor; whereas there were no functional partners identified for KIM-1. TNFRSF1A, TNFRSF1B and TNFRSF10B, encoding for TNF-R1, TNF-R2 and TRAIL-R2 respectively, are expressed in whole blood, coronary artery, aorta, tibial artery, heart left ventricle, heart atrial appendage, subcutaneous and visceral adipose tissue, liver and skeletal muscle; whereas RETN is detected mostly in the whole blood (Figure 5). There is no expression of HAVCR1 encoding for KIM-1 in the examined tissues. GWAS results from previous studies show that intronic variant rs1553318 in HAVCR1, as well intergenic SNPs in the HAVCR1 locus (rs6882076 and rs1501908) have been associated with TG, TC and LDL-C levels, which is consistent with our findings from observational analyses. Intronic variants in TNFRSF1A (rs1800693, rs4149576, rs2284344, rs4149577, rs1860545) have been associated with autoimmune and inflammatory diseases, such as multiple sclerosis, primary biliary cirrhosis or ankylosing spondylitis. A variant mapped to the chromosome region of TNFRSF1B has been associated with tuberculosis, and a variant mapped to the TNFRSF10B locus (encoding for TRAIL-R2) has been associated with renal cell carcinoma. A variant rs1423096 in 3’-region of RETN which is also in high linkage disequilibrium with variation in the promoter of RETN, as well as variant rs3219175 in 5’ near RETN have been associated with RETN levels (Supplementary Table IV).
Figure 4.
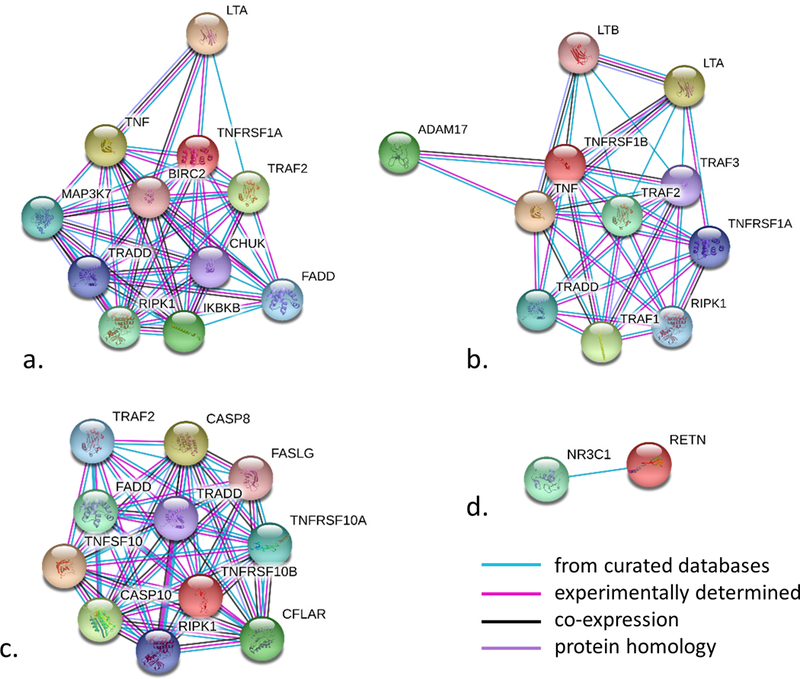
STRING networks for proteins associated with all four lipid fractions a. TNF-R1 (UniProt symbol TNFRSF1A), b. TNF-R2 (UniProt symbol TNFRSF1B), c. TRAIL-R2 (UniPort symbol TNFRSF10B) and d. RETN. Inquired proteins are depicted in red and interactions are shown for scores of strong evidence (the estimated likelihood of a given interaction being biologically meaningful, specific and reproducible, given the supporting evidence is 0.7).
Figure 5.
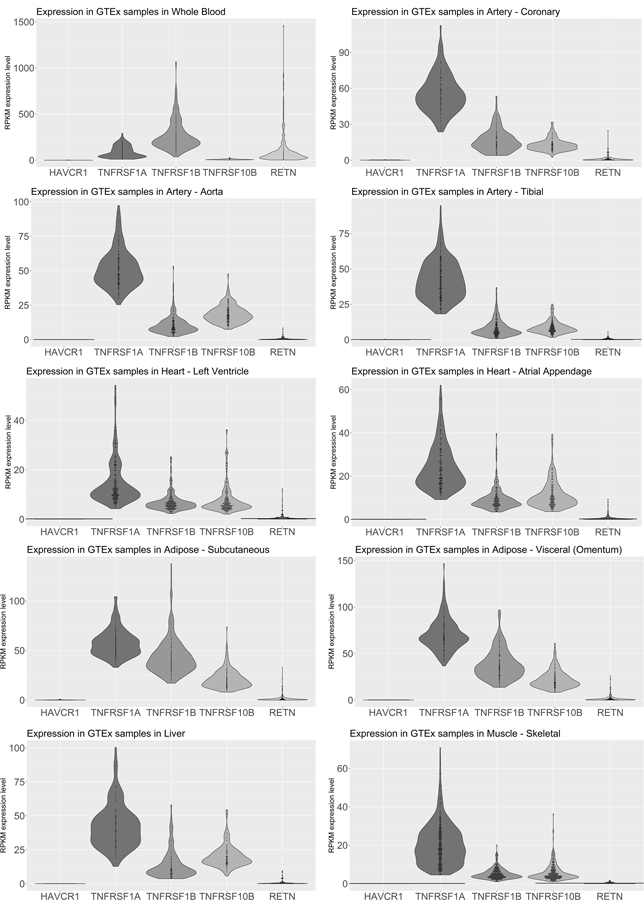
Expression of genes encoding proteins associated with four lipid fractions in relevant tissues based on GTEx data. HAVCR1 is a gene encoding for KIM-1, TNFRSF1A encoding for TNF-R1, TNFRSF1B encoding for TNF-R2 and TNFRSF10B encoding for TRAIL-R2.
Mendelian Randomization
The number of SNPs included in each instrument is indicated in Table 3. In total, we performed 59 tests in these MR analyses (many of the 30 proteins were associated with several lipid fractions) resulting in an alpha threshold of 0.0008 (after Bonferroni correction). We had at least 80% statistical power to detect an effect size of 0.07 standard deviation (SD) change in protein level per one SD change in lipid level with variance explained for the instrumental variable of 1.5%, or 100% statistical power for an effect size of 0.06 standard deviation (SD) change in protein level per one SD change in lipid level with variance explained for the instrumental variable of 15%. For 45 of the 59 analyses, we had >80% power to detect the effect size from the observational analyses (Table 3).
Table 3.
Results of MR analyses, shown if the statistical power was at least 80%.
| Exposure | Outcome | N SNPs | B | SE | P |
|---|---|---|---|---|---|
| ADM | Triglycerides | 2 | −0.067 | 0.025 | 0.006 |
| CCL3 | HDL cholesterol | 1 | 0.005 | 0.015 | 0.717 |
| CCL3 | Triglycerides | 1 | 0.003 | 0.014 | 0.833 |
| CHI3L1 | LDL cholesterol | 3 | 0.021 | 0.019 | 0.269 |
| CHI3L1 | Triglycerides | 3 | 0.003 | 0.019 | 0.892 |
| CSTD | Triglycerides | 2 | 0.024 | 0.015 | 0.099 |
| CXCL16 | LDL cholesterol | 2 | −0.041 | 0.052 | 0.428 |
| CXCL16 | Total cholesterol | 2 | −0.006 | 0.069 | 0.926 |
| CXCL16 | Triglycerides | 2 | 0.050 | 0.045 | 0.269 |
| Dkk-1 | Triglycerides | 4 | −0.065 | 0.047 | 0.166 |
| FAS | Triglycerides | 2 | 0.017 | 0.016 | 0.267 |
| Gal3 | LDL cholesterol | 3 | −0.015 | 0.010 | 0.129 |
| Gal3 | Total cholesterol | 3 | −0.017 | 0.010 | 0.070 |
| Gal3 | Triglycerides | 3 | −0.015 | 0.009 | 0.090 |
| GDF15 | HDL cholesterol | 2 | −0.056 | 0.022 | 0.010 |
| IL-16 | HDL cholesterol | 2 | 0.007 | 0.008 | 0.384 |
| IL-16 | Triglycerides | 2 | 0.005 | 0.010 | 0.587 |
| IL-18 | HDL cholesterol | 4 | 0.016 | 0.016 | 0.321 |
| IL-18 | Triglycerides | 4 | 0.003 | 0.016 | 0.866 |
| IL-1RA | HDL cholesterol | 2 | 0.013 | 0.017 | 0.458 |
| IL-1RA | Triglycerides | 2 | 0.022 | 0.013 | 0.099 |
| IL-6RA | Triglycerides | 2 | −0.024 | 0.041 | 0.553 |
| KIM-1 | HDL cholesterol | 4 | 0.000 | 0.008 | 0.974 |
| KIM-1 | LDL cholesterol | 4 | −0.016 | 0.020 | 0.432 |
| KIM-1 | Total cholesterol | 4 | −0.017 | 0.020 | 0.400 |
| KIM-1 | Triglycerides | 4 | −0.004 | 0.013 | 0.737 |
| LOX-1 | Triglycerides | 1 | 0.031 | 0.034 | 0.374 |
| MMP-12 | HDL cholesterol | 3 | −0.016 | 0.015 | 0.271 |
| MMP-12 | Triglycerides | 3 | −0.001 | 0.013 | 0.964 |
| PlGF | HDL cholesterol | 1 | 0.001 | 0.029 | 0.967 |
| PlGF | Triglycerides | 1 | −0.052 | 0.028 | 0.064 |
| PSGL-1 | Triglycerides | 1 | −0.017 | 0.010 | 0.089 |
| RETN | HDL cholesterol | 1 | −0.019 | 0.034 | 0.564 |
| RETN | LDL cholesterol | 1 | 0.006 | 0.035 | 0.876 |
| RETN | Total cholesterol | 1 | 0.014 | 0.034 | 0.675 |
| SELE | Triglycerides | 1 | 0.038 | 0.036 | 0.289 |
| ST2 | HDL cholesterol | 5 | −0.007 | 0.011 | 0.507 |
| TF | HDL cholesterol | 2 | 0.012 | 0.033 | 0.711 |
| TF | Total cholesterol | 2 | 0.017 | 0.019 | 0.373 |
| TM | Triglycerides | 2 | 0.004 | 0.009 | 0.613 |
| TRAIL-R2 | HDL cholesterol | 1 | −0.025 | 0.016 | 0.109 |
| TRAIL-R2 | LDL cholesterol | 1 | 0.004 | 0.017 | 0.817 |
| TRAIL-R2 | Total cholesterol | 1 | −0.012 | 0.017 | 0.486 |
| TRAIL-R2 | Triglycerides | 1 | 0.022 | 0.016 | 0.157 |
| uPAR | HDL cholesterol | 1 | −0.034 | 0.035 | 0.337 |
DISCUSSION
The aim of this study was to investigate the associations between circulating cardiovascular-related protein biomarkers and triglycerides, total cholesterol, LDL and HDL cholesterol. The data obtained from three Swedish populations provide a broad picture of lipid-protein associations in the circulatory system, showing amongst other things that kidney injury molecule (KIM-1), tumor necrosis factor receptor 1 and 2 (TNF-R1 and TNF-R2), TNF-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) and resistin (RETN) were associated with all four lipid fractions. To the best of our knowledge, this study represents the most comprehensive analysis of the circulating lipid fractions and protein biomarkers. We studied a broad set of different proteins such as cytokines, enzymes, growth factors, hormones and receptors in healthy, general population. This is of great importance, since it could provide ideas for possible strategies of intervention before cardiovascular disease develop. Moreover, it is well-established that alterations in glucose metabolism are strongly associated with cardiovascular disease.30 In the current study, we found 15 of the cardiovascular-related protein biomarkers to be associated with higher TG and lower HDL-C (or vice versa), a phenotype reflecting insulin resistance.
Potential biological explanations to our observations
TNF-α, a proinflammatory cytokine produced mainly by macrophages, has the capacity to induce dyslipoproteinemia.31–33 TNF-α, exerts its action through two receptors, soluble TNF receptor 1 (sTNFR1) and sTNFR2, both of which are expressed in membrane-bound form on the surface of most cells.34 From the cell surface, both receptors are shed into the circulation, partly as a response to TNF. In inflammatory states, the levels of soluble TNFRs usually parallel the circulating levels of TNF.34, 35 It has been shown that TNF-α decreases HDL cholesterol levels,36 which is in line with our results showing negative associations between HDL and TNFR1 and TNFR2. TG levels were associated with the activities of TNF-α, sTNFR1 and sTNFR2 in patients with systemic lupus erythematosus (SLE).37 Thus, enhanced activity in the TNFα/TNFR system may be one underlying factor contributing to both dyslipoproteinemia and promotion of chronic inflammation not only in SLE patients,37 but also in healthy individuals.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a death ligand, a cytokine that activates apoptosis through cell surface death receptors. The levels of circulating TRAIL correlate with total body fat and serum lipid levels.38 Its receptor, TRAIL-R2 is expressed on the cellular surface of human preadipocytes and is still present in significant amounts on lipid-laden adipocytes.39 In the previous study, activation of TRAIL-R2 with recombinant human TRAIL resulted in a robust inhibition of insulin-stimulated glucose uptake and insulin-stimulated de novo lipogenesis.39 By binding to TRAIL-R2, TRAIL activates the cleavage of caspase-8 and caspase-3, which in turn cleaves and inactivates peroxisome proliferator activated receptor gamma (PPARγ). This causes changes in gene expression of lipogenic genes and finally leads to the inhibition of insulin-stimulated glucose uptake and lipogenesis. In the current study TRAIL-R2 levels were associated with higher TG levels, but lower TC, LDL-C and HDL-C levels.
KIM-1 is a phosphatidylserine receptor that recognizes apoptotic cells directing them to lysosomes. It also serves as a receptor for oxidized lipoproteins and hence is adept at recognizing apoptotic cell ‘eat me’ signals.40 In previous studies, higher urinary KIM-1 levels have been associated with increased risk for heart failure, lower insulin sensitivity and higher risk of cardiovascular mortality.41–43 In our study, circulating KIM-1 was associated with high levels of all four lipid fractions.
Resistin (RETN), named after its insulin resistance effect observed in mice after resistin injection, is produced and released from adipose tissue to serve endocrine functions.44 In our study it was associated with IR phenotype, i.e. high TG and low HDL. RETN increases the production of LDL-C and also degrades LDL receptors in the liver, thus decreases liver’s ability to clear LDL cholesterol from circulation.45 Moreover, resistin accelerates the accumulation of LDL in arteries, increasing the risk of heart disease. Interestingly in our study, RETN was associated with lower LDL-C and this needs more attention in future studies to evaluate the effect of RETN on circulating lipids in healthy individuals, as most of the previous studies was performed in obese or diabetics subjects.
TNF-R1, TNF-R2 and TRAIL-2 represent members of the tumor necrosis factor (TNF) family of cytokines that induce apoptosis in a wide variety of cells, and according to STRING their share some functional partners. For instance, TRADD (TNFRSF1A Associated Via Death Domain), RIPK-1 (Receptor Interacting Serine/Threonine Kinase 1) and TRAF2 (TNF Receptor Associated Factor 2) are all involved in response to tissue damage, regulation of cell survival and apoptosis. Further, a predicted partner for RETN is glucocorticoid receptor (NR3C1) and glucocorticoids are potent inducers of apoptosis in many cell types and tissues. There were no functional partners identified by STRING for KIM-1, however KIM-1 is a phosphatidylserine receptor that recognizes apoptotic cells and directs them to lysosomes. Taken together, it seems like proteins associated with all four lipid fractions in our study are involved in apoptosis. Whereas apoptosis is a normal mechanism for cellular turnover, it may be accelerated by cellular stresses caused by lipid accumulation. Lipotoxicity is an increased accumulation of lipid intermediates into non-adipose tissue, notably, skeletal muscle, liver, and heart, leading to cellular dysfunction and cell death, i.e. lipoapoptosis.46 Using orthogonal data, we indeed showed that genes encoding for TNF-R1, TNF-R2 and TRAIL-2 are expressed in heart, skeletal muscle and liver.
Among proteins associated with higher TG and lower HDL-C; FABP4, t-PA and IL-1RA have been previously linked to insulin resistance measured by homeostasis model assessment (HOMA) in two community-based cohorts.47 For the remaining 12 proteins showing this pattern, less is known about their relation to insulin resistance and dyslipidemia. That said, several proteins have known biology that could imply a role in insulin resistance. For example, IL-16 plays a role in initiating and/or sustaining an inflammatory response via stimulation of IL-1B and IL-6 expression in human monocytes.48 Neutralization of IL-16 protects non-obese diabetic mice from autoimmune type 1 diabetes,49 but more studies are needed to explain the association between TG, HDL-C and IL-6 in general human population.
In MR analyses, we used genetic variants to explore possible causal effects of protein levels on lipid levels and we had at least 80% power to detect most of the possible effects None of MR analyses revealed causal relationships, but there may still exist smaller causal effects than what we had no power to test for. We cannot rule out the possibility that lipid levels might be causal for protein levels, as we were unable to test it due to lack of statistical power.
Strengths & Limitations
A major strength of the study is the design in which findings from the discovery cohort were confirmed in other general population-based cohorts. Moreover, with strict multiple testing correction the risk of false positive findings in the replication stage was calculated to 0.1%. Another strength is the large number of available protein biomarkers in all three cohorts (57 proteins) which allowed us for a comprehensive analysis. Moreover, in this study we present not only an observational analysis, but we also attempted to investigate causality between the observed associations. However, as a limitation of the MR analyses, there is a possibility that a non-synonymous variant may affect binding of the protein-detecting antibodies, thus affecting the quantification. This could result in an invalid instrument since the instrument will not really reflect the protein levels, but occurs as synthetic association due to a technical artefact. Although this risk is probably very small, it has been reported for a genetic association study of adiponectin analyzed with enzyme-linked immunosorbent assay (ELISA).50 We also want to acknowledge that in contrast to mass spectrometry methods (MS), the Olink protein immunoassay does not allow profiling of different protein isoforms or posttranslational modifications. However, MS methods have the limitation of lower throughput making it difficult to analyze samples from 3,370 individuals.Finally, our study samples were middle-aged to elderly and of Northern European descent, so the generalizability to other ethnicities and younger individuals is unknown.
CONCLUSION
In summary, we report a comprehensive lipid-protein association map, rich in cytokines, enzymes, receptors and growth factors. Some of these proteins, such as KIM-1, RETN, TNFR1, TNFR2 and TRAIL-2, could be good candidates to follow up in longitudinal studies to determine their ability to predict changes to lipids levels. Further, future studies should address lipoapoptotic properties of these proteins, as well as potential causality of lipid metabolism on protein levels.
Supplementary Material
HIGHLIGHTS:
In our comprehensive study of circulating protein biomarkers, we found 42 proteins to be associated with at least one lipid fraction.
We found kidney injury molecule (KIM-1), tumor necrosis factor receptor 1 and 2 (TNF-R1 and TNF-R2), TNF-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) and resistin (RETN) to be associated with all four lipid fractions.
Comprehensive studies of lipid-protein associations may provide novel nodes for drug action to treat human diseases.
Our studies reveal new insights to the pathophysiology underlying dyslipidemia.
ACKNOWLEDGEMENTS
S.M. Figarska, T. Fall and E. Ingelsson conceived and designed the research. J. Sundström, A. Mälarstig, L. Lind and E. Ingelsson acquired the data. J. Ärnlöv, L. Lind and E. Ingelsson handled funding and supervision S.M. Figarska, A. Mälarstig, and S. Gustafsson performed statistical analysis. S.M. Figarska, E. Ingelsson and S. Elmståhl analyzed and interpreted the data. S.M. Figarska drafted the manuscript. S. Gustafsson, J. Sundström, J. Ärnlöv, A. Mälarstig, S. Elmståhl, T. Fall, L. Lind and E. Ingelsson made critical revision of the manuscript for important intellectual content. The authors would like to thank the participants of the EpiHealth, PIVUS and ULSAM cohort for their generous contribution.
SOURCES OF FUNDING
This study was conducted with support from National Institutes of Health (1R01DK106236–01A1) and Knut och Alice Wallenberg Foundation (2013.0126). T. Fall has grants from Swedish Research Council, Swedish Heart- and Lung Foundation and the Göran Gustafsson Foundation.
ABBREVIATIONS
- BMI
body mass index
- CCL3
C-C motif chemokine 3
- CXCL16
C-X-C motif chemokine 16
- CRP
C-reactive protein
- CVD
cardiovascular disease
- ELISA
enzyme-linked immunosorbent assay
- FABP4
fatty acid-binding protein 4
- FDR
false discovery rate
- FGF-23
fibroblast growth factor 23
- Gal3
Galectin-3
- GH
growth hormone
- GTEx
genotype-tissue expression
- GWAS
genome-wide association study
- ICD
International Classification of Diseases
- IL-6, 8 and 18
interleukin 6, 8 and 18
- HDL-C
high-density lipoprotein cholesterol
- HOMA
homeostasis model assessment
- IL-1RA
interleukin-1 receptor antagonist protein
- IR
insulin resistance
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KIM-1
(HAVCR1) kidney injury molecule
- LDL-C
low-density lipoprotein cholesterol
- MCP-1
monocyte chemotactic protein 1
- MMP-12
matrix metalloproteinase 12
- MR
mendelian randomization
- MS
mass spectrometry
- NPX
normalized protein expression
- NR3C1
nuclear receptor subfamily 3 group C member 1, glucocorticoid receptor
- PEA
proximity extension assay
- PIVUS
Prospective Study of the Vasculature in Uppsala Seniors
- PPARγ
peroxisome proliferator activated receptor gamma
- RETN
resistin
- SCF
stem cell factor
- SLE
systemic lupus erythematosus
- STRING
search tool for the retrieval of interacting genes/proteins
- TC
total cholesterol
- TG
triglycerides
- T2D
type 2 diabetes
- TNF-R1
(TNFRSF1A) tumor necrosis factor receptor 1
- TNF-R2
(TNFRSF1B) tumor necrosis factor receptor 2
- t-PA
tissue-type plasminogen activator
- TRADD
TNFRSF1A associated via death domain
- TRAF2
TNF receptor associated factor 2
- TRAIL-R2
(TNFRSF10B ) TNF-related apoptosis-inducing ligand receptor 2
- ULSAM
Uppsala Longitudinal Study of Adult Men
Footnotes
DISCLOSURES
E. Ingelsson has received consulting fees from Olink Proteomics for work unrelated to the present project. The company had no influence over design, analysis or interpretation of data in the present study, and did not provide any funding for the study.
REFERENCES
- 1.Kannel WB, Vasan RS, Keyes MJ, Sullivan LM and Robins SJ. Usefulness of the triglyceride-high-density lipoprotein versus the cholesterol-high-density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham Offspring Cohort). Am J Cardiol 2008;101:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niphakis MJ, Lum KM, Cognetta AB 3rd, Correia BE, Ichu TA, Olucha J, Brown SJ, Kundu S, Piscitelli F, Rosen H and Cravatt BF. A Global Map of Lipid-Binding Proteins and Their Ligandability in Cells. Cell 2015;161:1668–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuhashi M, Saitoh S, Shimamoto K and Miura T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clinical Medicine Insights Cardiology 2014;8:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil GS and Bernlohr DA. Metabolic functions of FABPs--mechanisms and therapeutic implications. Nature reviews Endocrinology 2015;11:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moller N and Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocrine reviews 2009;30:152–77. [DOI] [PubMed] [Google Scholar]
- 6.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS and Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 2008;118:1657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes MV, Ala-Korpela M and Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nature reviews Cardiology 2017;14:577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess S and Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 2015;181:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lind L, Elmstahl S, Bergman E, Englund M, Lindberg E, Michaelsson K, Nilsson PM and Sundstrom J. EpiHealth: a large population-based cohort study for investigation of gene-lifestyle interactions in the pathogenesis of common diseases. European journal of epidemiology 2013;28:189–97. [DOI] [PubMed] [Google Scholar]
- 10.Lind L, Fors N, Hall J, Marttala K and Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol 2005;25:2368–75. [DOI] [PubMed] [Google Scholar]
- 11.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B and Lind L. Insulin resistance and risk of congestive heart failure. Jama 2005;294:334–41. [DOI] [PubMed] [Google Scholar]
- 12.Sundstrom J, Lind L, Nystrom N, Zethelius B, Andren B, Hales CN and Lithell HO. Left ventricular concentric remodeling rather than left ventricular hypertrophy is related to the insulin resistance syndrome in elderly men. Circulation 2000;101:2595–600. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg M, Eriksson A, Tran B, Assarsson E and Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic acids research 2011;39:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson AC, Lindstedt P, Stenvang J, Gullberg M and Fredriksson S. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang B, Svensson P, Arnlov J, Sundstrom J, Lind L and Ingelsson E. Effects of cigarette smoking on cardiovascular-related protein profiles in two community-based cohort studies. Atherosclerosis 2016;254:52–58. [DOI] [PubMed] [Google Scholar]
- 16.Cornelis MC, Gustafsson S, Arnlov J, Elmstahl S, Soderberg S, Sundstrom J, Michaelsson K, Lind L and Ingelsson E. Targeted proteomic analysis of habitual coffee consumption. Journal of internal medicine 2018;283:200–211. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y and Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. R Stat Soc Series B Methodol 1995;55:289–300. [Google Scholar]
- 18.Ganna A, Lee D, Ingelsson E and Pawitan Y. Rediscovery rate estimation for assessing the validation of significant findings in high-throughput studies. Briefings in bioinformatics 2015;16:563–75. [DOI] [PubMed] [Google Scholar]
- 19.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ and von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic acids research 2015;43:D447-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Consortium GTEx. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science (New York, NY) 2015;348:648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, Junkins H, McMahon A, Milano A, Morales J, Pendlington ZM, Welter D, Burdett T, Hindorff L, Flicek P, Cunningham F and Parkinson H. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic acids research 2017;45:D896-d901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S and Harshfield E. Mendelian randomization to assess causal effects of blood lipids on coronary heart disease: lessons from the past and applications to the future. Current opinion in endocrinology, diabetes, and obesity 2016;23:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess S, Butterworth A and Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genetic epidemiology 2013;37:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folkersen L, Fauman E, Sabater-Lleal M, Strawbridge RJ, Franberg M, Sennblad B, Baldassarre D, Veglia F, Humphries SE, Rauramaa R, de Faire U, Smit AJ, Giral P, Kurl S, Mannarino E, Enroth S, Johansson A, Enroth SB, Gustafsson S, Lind L, Lindgren C, Morris AP, Giedraitis V, Silveira A, Franco-Cereceda A, Tremoli E, Gyllensten U, Ingelsson E, Brunak S, Eriksson P, Ziemek D, Hamsten A and Malarstig A. Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLoS genetics 2017;13:e1006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valensi P, Benroubi M, Borzi V, Gumprecht J, Kawamori R, Shaban J, Shah S, Shestakova M and Wenying Y. The IMPROVE study--a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. International journal of clinical practice 2008;62:1809–19. [DOI] [PubMed] [Google Scholar]
- 26.Baldassarre D, Nyyssonen K, Rauramaa R, de Faire U, Hamsten A, Smit AJ, Mannarino E, Humphries SE, Giral P, Grossi E, Veglia F, Paoletti R and Tremoli E. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: the IMPROVE study. European heart journal 2010;31:614–22. [DOI] [PubMed] [Google Scholar]
- 27.Willer CJ, Schmidt EM, Sengupta S et al. Discovery and refinement of loci associated with lipid levels. Nature genetics 2013;45:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess S, Small DS and Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Statistical methods in medical research 2017;26:2333–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierce BL and Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol 2013;178:1177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low Wang CC, Hess CN, Hiatt WR and Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation 2016;133:2459–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotamisligil GS, Shargill NS and Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (New York, NY) 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 32.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U and Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 1999;145:33–43. [DOI] [PubMed] [Google Scholar]
- 33.Chen G and Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science (New York, NY) 2002;296:1634–5. [DOI] [PubMed] [Google Scholar]
- 34.Bazzoni F and Beutler B. The tumor necrosis factor ligand and receptor families. The New England journal of medicine 1996;334:1717–25. [DOI] [PubMed] [Google Scholar]
- 35.Horiuchi T, Mitoma H, Harashima S, Tsukamoto H and Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford, England) 2010;49:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Field FJ, Watt K and Mathur SN. TNF-alpha decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2. Journal of lipid research 2010;51:1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svenungsson E, Gunnarsson I, Fei GZ, Lundberg IE, Klareskog L and Frostegard J. Elevated triglycerides and low levels of high-density lipoprotein as markers of disease activity in association with up-regulation of the tumor necrosis factor alpha/tumor necrosis factor receptor system in systemic lupus erythematosus. Arthritis and rheumatism 2003;48:2533–40. [DOI] [PubMed] [Google Scholar]
- 38.Choi JW, Song JS and Pai SH. Associations of serum TRAIL concentrations, anthropometric variables, and serum lipid parameters in healthy adults. Annals of clinical and laboratory science 2004;34:400–4. [PubMed] [Google Scholar]
- 39.Keuper M, Wernstedt Asterholm I, Scherer PE, Westhoff MA, Moller P, Debatin KM, Strauss G, Wabitsch M and Fischer-Posovszky P. TRAIL (TNF-related apoptosis-inducing ligand) regulates adipocyte metabolism by caspase-mediated cleavage of PPARgamma. Cell death & disease 2013;4:e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonventre JV. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc 2014;125:293–9; discussion 299. [PMC free article] [PubMed] [Google Scholar]
- 41.Carlsson AC, Calamia M, Riserus U, Larsson A, Helmersson-Karlqvist J, Lind L and Arnlov J. Kidney injury molecule (KIM)-1 is associated with insulin resistance: results from two community-based studies of elderly individuals. Diabetes research and clinical practice 2014;103:516–21. [DOI] [PubMed] [Google Scholar]
- 42.Carlsson AC, Larsson A, Helmersson-Karlqvist J, Lind L, Ingelsson E, Larsson TE, Bottai M, Sundstrom J and Arnlov J. Urinary kidney injury molecule-1 and the risk of cardiovascular mortality in elderly men. Clinical journal of the American Society of Nephrology : CJASN 2014;9:1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlsson AC, Larsson A, Helmersson-Karlqvist J, Lind L, Ingelsson E, Larsson TE, Sundstrom J and Arnlov J. Urinary kidney injury molecule 1 and incidence of heart failure in elderly men. European journal of heart failure 2013;15:441–6. [DOI] [PubMed] [Google Scholar]
- 44.Jamaluddin MS, Weakley SM, Yao Q and Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. British journal of pharmacology 2012;165:622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melone M, Wilsie L, Palyha O, Strack A and Rashid S. Discovery of a new role of human resistin in hepatocyte low-density lipoprotein receptor suppression mediated in part by proprotein convertase subtilisin/kexin type 9. Journal of the American College of Cardiology 2012;59:1697–705. [DOI] [PubMed] [Google Scholar]
- 46.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr., Ory DS and Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proceedings of the National Academy of Sciences of the United States of America 2003;100:3077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowak C, Sundstrom J, Gustafsson S, Giedraitis V, Lind L, Ingelsson E and Fall T. Protein Biomarkers for Insulin Resistance and Type 2 Diabetes Risk in Two Large Community Cohorts. Diabetes 2016;65:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathy NL, Scheuer W, Lanzendorfer M, Honold K, Ambrosius D, Norley S and Kurth R. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 2000;100:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meagher C, Beilke J, Arreaza G, Mi QS, Chen W, Salojin K, Horst N, Cruikshank WW and Delovitch TL. Neutralization of interleukin-16 protects nonobese diabetic mice from autoimmune type 1 diabetes by a CCL4-dependent mechanism. Diabetes 2010;59:2862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Y, Li Y, Lange EM, Croteau-Chonka DC, Kuzawa CW, McDade TW, Qin L, Curocichin G, Borja JB, Lange LA, Adair LS and Mohlke KL. Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Human molecular genetics 2010;19:4955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


