Abstract
Electrical neuromodulation of spinal networks improves the control of movement of the paralyzed limbs after spinal cord injury (SCI). However, the potential of noninvasive spinal stimulation to facilitate postural trunk control during sitting in humans with SCI has not been investigated. We hypothesized that transcutaneous electrical stimulation of the lumbosacral enlargement can improve trunk posture. Eight participants with non-progressive SCI at C3-T9, American Spinal Injury Association Impairment Scale (AIS) A or C, performed different motor tasks during sitting. Electromyography of the trunk muscles, three-dimensional kinematics, and force plate data were acquired. Spinal stimulation improved trunk control during sitting in all tested individuals. Stimulation resulted in elevated activity of the erector spinae, rectus abdominis, and external obliques, contributing to improved trunk control, more natural anterior pelvic tilt and lordotic curve, and greater multi-directional seated stability. During spinal stimulation, the center of pressure (COP) displacements decreased to 1.36 ± 0.98 mm compared with 4.74 ± 5.41 mm without stimulation (p = 0.0156) in quiet sitting, and the limits of stable displacement increased by 46.92 ± 35.66% (p = 0.0156), 36.92 ± 30.48% (p = 0.0156), 54.67 ± 77.99% (p = 0.0234), and 22.70 ± 26.09% (p = 0.0391) in the forward, backward, right, and left directions, respectively. During self-initiated perturbations, the correlation between anteroposterior arm velocity and the COP displacement decreased from r = 0.5821 (p = 0.0007) without to r = 0.5115 (p = 0.0039) with stimulation, indicating improved trunk stability. These data demonstrate that the spinal networks can be modulated transcutaneously with tonic electrical spinal stimulation to physiological states sufficient to generate a more stable, erect sitting posture after chronic paralysis.
Keywords: : neuromodulation, paralysis, seated posture, transcutaneous electrical spinal cord stimulation, trunk stability and control
Introduction
Sensory-motor impairments that affect trunk, upper extremity, and lower extremity functions are common in individuals with spinal cord injury (SCI).1,2 The severity of these impairments primarily relates to the neurological level and degree of completeness of the lesion sustained by the spinal cord. As a result, the complex synergies required to regulate postural stability while performing routine daily tasks, such as reaching from a wheelchair to lift objects, or transferring from a wheelchair to a bed or into a car, can be affected to varying degrees.3,4 Individuals with SCI are consequently exposed to a higher risk of instability and falling even in a quiet seated posture, increasing the probability of fall-related pain, bone fractures, and other injuries.5,6 In addition to restoring seated quiet and dynamic abilities,7 clinical and physiological benefits of improving trunk posture and control have been shown to include a decrease in neck and back pain,8,9 the ability to perform pressure relief,10 improvements in bimanual workspace,4 pelvic tilt, lateral vertebral alignment,11,12 enhanced forward reaching and active pulling,4,11 and improvements in diaphragmatic and deep breathing.13 Hence, reaching an optimal level of quiet and dynamic seated postural capability is generally one of the key objectives in SCI rehabilitation, with the ultimate goal of enhancing affected individuals' health as well as performance and independence in daily activities.14
Postural regulation of trunk is also one of the key elements of locomotor control.15–19 Although the importance of studying seated postural control is frequently acknowledged, the success of restoring stability during quiet and dynamic sitting in individuals with SCI is limited. Current clinical practice has established an emphasis on the reduction of pressure sores, the control of abnormal tone, and skeletal alignment, and utilizes almost exclusively repetitive reaching movements toward a target while sitting on the edge of a treatment table.20,21 In addition, such practice often focuses on the use of compensatory strategies that emphasize the engagement of stronger muscles, rather than on the restoration of function of the weaker and/or paretic muscles.22,23 As such, instead of promoting the recovery of motor control,24–26 these rehabilitation strategies are aimed at strengthening muscles above the spinal lesion and using leverage, momentum, and substitution to move weak or paralyzed parts of the body.22
Prior efforts have clearly demonstrated that the spinal neuronal circuitries below a paralyzing site of injury have a functional potential that far exceeds what has been thought possible, allowing significant levels of voluntary control of standing, stepping, and leg movements.27–31 These functional outcomes depend on multiple factors, including the stimulation location, intensity, and frequency, as well as the specific motor task practiced. Recently, a noninvasive electrical neuromodulatory technique was proposed as an alternative to the invasive epidural approach, to augment the functional state of the spinal locomotor-specific networks, enabling the recovery of rhythmic stepping movements in individuals with paralysis.32–36 Neural regulation of locomotion and posture may occur in a synergistic manner because both systems rely on similar peripheral (visual, vestibular, neck and trunk proprioception) input sources, and afferent inputs are projected to anatomically overlapping neural networks comprising projections to the lower trunk and leg muscles.17,18,37,38 In this light, the objective of this study was to quantify the electrophysiological, kinematic, and kinetic characteristics of postural control of the trunk during sitting in response to noninvasive electrical neuromodulation of postural-specific networks of the lumbosacral enlargement. We hypothesize that: (1) the physiological states of the lumbosacral spinal networks can be electrically modulated, resulting in improved seated trunk control and stability of individuals with SCI; and (2) postural-specific networks can perform multiple motor control strategies when appropriately neuromodulated spinally.
Methods
Participants
Experiments were conducted in eight participants as outlined in Table 1 (see details in Supplementary Table 1; www.liebertpub.com/neu). Each participant gave written informed consent to the experimental procedure, which was approved by the Institutional Review Board of the University of California Los Angeles in accordance with the Declaration of Helsinki on the use of human subjects in experiments.
Table 1.
Clinical Characteristics and Anthropometrics of Study Participants
| Stimulation intensity threshold (mA) | Stimulation intensity used (mA) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant ID | Sex | NLI | AIS grade | Age (yr) | Height (cm) | Body mass (kg) | Post-SCI (yr) | T11 30 Hz | L1 15 Hz | T11 30 Hz | L1 15 Hz |
| P1 | M | T4 | A | 25 | 180 | 91 | 7 | 50 | 60 | 40 | 50 |
| P2 | M | T2 | A | 23 | 169 | 80 | 5 | 140 | 120 | 100 | 80 |
| P3 | M | T9 | A | 26 | 156 | 50 | 2 | 110 | 80 | 100 | 60 |
| P4 | M | T2 | A | 26 | 157 | 57 | 8 | 40 | 15 | 25 | 5 |
| P5 | M | C4 | C | 26 | 183 | 79 | 7 | 55 | 60 | 50 | 50 |
| P6 | M | T3 | A | 30 | 188 | 84 | 10 | 70 | 70 | 60 | 65 |
| P7 | F | C5 | C | 32 | 160 | 51 | 13 | 30 | 25 | 25 | 20 |
| P8 | M | T3 | A | 47 | 188 | 84 | 6 | 80 | 40 | 75 | 35 |
NLI, neurological level of injury; AIS, American Spinal Injury Association Impairment Scale; SCI, spinal cord injury.
Experimental protocol
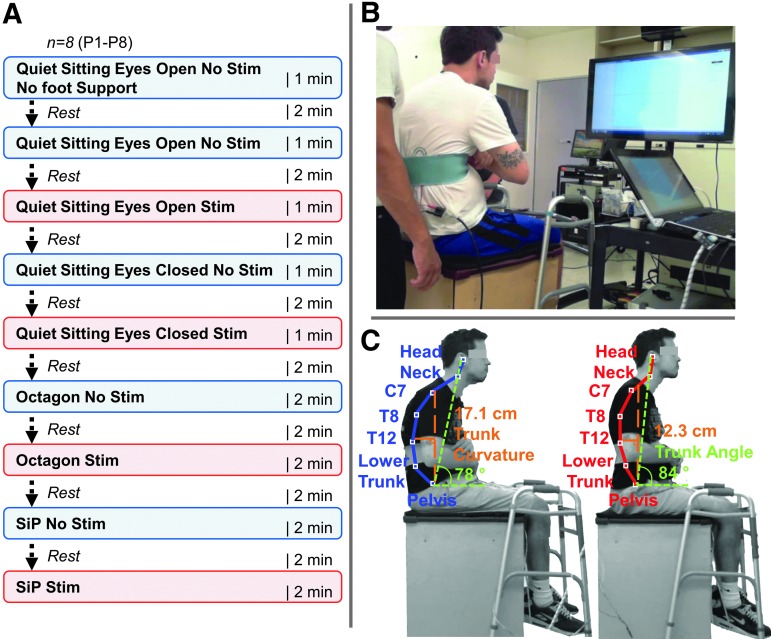
Prior to the experimental session, the participants were asked to empty their bladders. All participants were examined during sitting with and without using multi-site spinal stimulation over a range of intensities between the spinous processes of the 11th and 12th thoracic (T11 and T12) and the first and second lumbar (L1 and L2) vertebrae, hereafter referred to as T11 and L1. Stimulated and non-stimulated conditions alternated in a pseudo-random sequence at the individual level to achieve a balanced protocol. Each participant received each condition once within an experimental session. Each session was a maximum of 90 min long, including setup, calibration, and testing. The duration of each exercise varied from 1 to 2 min, and 2 min of rest were provided between tests. A schematic representation of the experimental design and interventions is shown in Figure 1A.
FIG. 1.
(A) Schematic representing the experimental design protocol and interventions. Eight individuals were tested in a single experimental session without and in the presence of transcutaneous electrical spinal cord stimulation. (B) Testing room layout and experimental setup. (C) Representative participant (P5) without (left) and with submotor threshold spinal stimulation (right). Note the decrease in trunk curvature (orange), increase in trunk angle (green), and improvement in upright sitting posture and spinal alignment. Key anatomical landmarks are shown without (blue) and with stimulation (red).
Assessment of postural control
Three primary tests were performed: quiet sitting, multi-directional leaning (“octagon”), and self-initiated perturbations. During each test, participants were instructed to sit unsupported on the force plate and attempt to actively maintain their trunk as upright as possible, maintain balance, and minimize the use of their arms and heads. One researcher was standing behind each participant to ensure the safety of the participants and to provide assistance to prevent falls if needed. During the quiet sitting test, participants were instructed to sit as quietly as possible for 60 sec with eyes open. After 2 min of rest, the task was repeated with the participant's eyes closed. During the multi-directional leaning test, the ability to voluntarily displace the center of pressure (COP) to a maximum distance without losing balance was assessed.39 Participants were instructed to lean from the center position as far as possible in the indicated direction without losing balance, while keeping their backs extended. The COP position registered by a force plate was visualized on the monitor to provide feedback on body displacement. Eight targets were presented at 45 degree angle increments around the center, starting in the forward right direction. The participant was asked to move the COP indicator to each target, which was present for 5 sec, hold the position during that time, and return to the starting position.
Then, a self-initiated perturbation test was performed in seven participants (P1–4, P6–8) to assess the efficiency of postural corrective responses. The data from participant P5 were omitted from the group analysis because of incomplete video and three-dimensional (3D) kinematic recordings during the self-initiated perturbation test. In agreement with previous literature,40 the participants were instructed to rapidly raise their extended right arm forward to a horizontal position and then return the arm back to the initial position based on auditory cues.
Transcutaneous electrical spinal cord stimulation
A custom-built, three channel constant-current stimulator with a range of 0–250 mA was used to deliver transcutaneous spinal cord stimulation. The stimulation was administered using two self-adhesive electrodes (ValuTrode, Axelgaard Ltd., Fallbrook, CA) with a diameter of 3.2 cm placed as cathodes on the skin over T11 and L1. In addition, two 7.5 × 13 cm self-adhesive electrodes serving as anodes (ValuTrode) were placed symmetrically on the skin over the iliac crests. A foam rubber pad was placed over the cathodes and secured using adhesive tape, and an elastic belt was wrapped tightly around the trunk above the waist to ensure a constant pressure between the electrodes and the skin. The stimulation waveform consisted of monophasic, rectangular 1 ms pulses at a frequency of 30 Hz during stimulation over T11, and 15 Hz during stimulation over L1, with each pulse filled with a carrier frequency of 10 kHz.41 In the beginning of each experimental session, the participants were instructed to maintain a “relaxed” sitting posture; that is, without actively trying to extend the trunk. During these preliminary tests, the stimulation intensity, ranging from 10 to 150 mA at each location, was gradually increased to generate threshold motor outputs as detected via the COP movements recorded by the force plate and/or via changes in trunk extension and improved trunk curvature as confirmed by visual inspection (see Supplementary Video 1) (see online supplementary material at http://www.liebertpub.com).
After that, the stimulation intensity at each location was adjusted to a submotor threshold level, which was then kept constant during the main tests with stimulation.
Electromyography (EMG)
EMG signals were recorded using bipolar surface electrodes. Signals from the right rectus abdominis (RA), external obliques (Obl), erector spinae at the levels of the seventh thoracic vertebra (E-T7) and the third lumbar vertebra (E-L3), rectus femoris (RF), and anterior deltoid (AD) muscles were recorded using a PowerLab 16/35 series DAQ system (ADInstruments, Australia) with a low-noise, high-gain differential amplifier (Octal Bio Amp, ADInstruments, Australia). A reference electrode was placed over the sternum. EMG signals were differentially amplified with a band-pass filter with a bandwidth between 10 and 2000 Hz (−3 dB) and digitized at a sampling frequency of 2 kHz.
Force plate system
Experiments were performed using the ‘‘Stabilan-01’’ (Rhythm, Russia) force plate analysis system. Participants were asked to sit on the force plate covered with a silicon mat, which was implemented into an elevated surface (Fig. 1B). During the multi-directional leaning test, they were instructed to look at the monitor that was placed at eye level. Force plate data were sampled at 50 Hz.
3D Kinematics
Kinematic data were collected using the Xbox One Kinect (Microsoft Corp., Redmond, WA). The Xbox One Kinect is a markerless computer vision system that uses an RGB camera and an infrared laser. Three-dimensional positioning was determined via a point cloud generated by the infrared laser and a triangulation process described by Freedman and coworkers.42 Essentially, a laser light source is split via refraction after exiting a specialized lens that creates a point cloud on an object. These refractions are transmitted back to the Kinect receiver, which then processes the data using Onboard firmware.
The Xbox One Kinect was placed ∼1.5 m in front of the participant at ∼2 m height from ground level and angled 60 degrees downward. Video (RGB camera) and kinematic (infrared depth-finding camera) data were both acquired at 640 × 480 pixels of resolution and 30 Hz. The kinematic model output of the Xbox One Kinect consisted of 24 segments of which the head, neck, intervertebral space between the VII cervical and I thoracic vertebrae (T8), XII thoracic and lumbar vertebrae (T12), III and IV lumbar vertebrae (L3), V lumbar vertebra and sacrum (lower trunk), and pelvis (midpoint between the left and right hip joint locations) landmarks were used to calculate trunk curvature, angle, and displacements during the quiet and dynamic seated tests. Trunk curvature was determined as the distance between the vertical line projecting from the anterior-superior iliac crest (ASIS), and a point corresponding to the maximal T12 landmark displacement in the anteroposterior (A-P) direction for forward and backward movements, or in the mediolateral (M-L) direction for left and right movements. The trunk angle (degrees) was determined as the angle between the horizontal line intersecting the ASIS and a vertical line from that to the head landmark from the Kinect sensor (Fig. 1C).
Experimental data processing and analysis
COP trajectory data from the force plate were analyzed in the A-P and M-L directions. The COP characteristics were quantified via total excursion, mean displacement, and mean acceleration. COP excursions were calculated where (xi,yi) was the COP position at frame i and N the total number of samples:
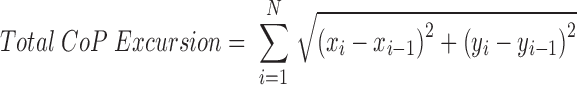 |
The maximum COP displacement for both the quiet and dynamic sitting tasks was defined for each direction as the mean COP position maintained for 0.24 sec at the extreme points of a given direction; the primary directions (forward, backward, right, and left) were used to define the limits of stability.
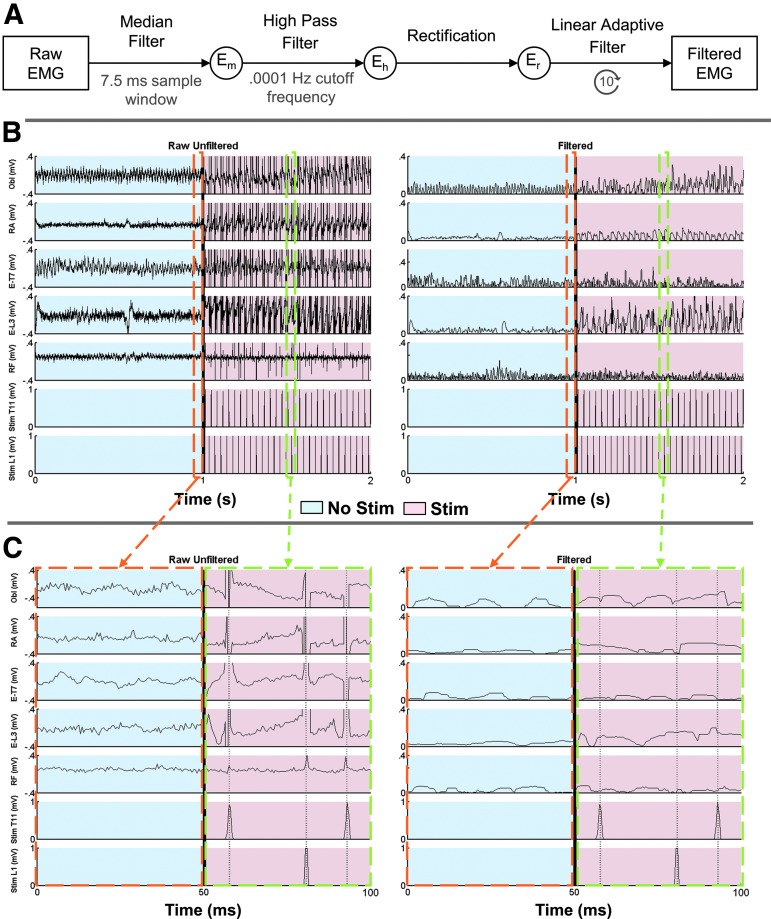
The digitized EMG time series were full-wave rectified and filtered with custom Matlab code as outlined in Figure 2A. First, each individual, raw trunk muscle EMG (Obl, RA, E-T7, E-L3) was passed through a median filter with a 7.5 ms sample window to reduce the magnitude of the noise signal and remove any significant outliers while retaining prominent muscle activation features; for example, muscle activation peaks. This signal was then passed through a high-pass filter with a 0.0001 Hz cutoff frequency (Eh) to bring the EMG signal to baseline (0 DC component), and then full-wave rectified (Er). The filtered and rectified signal, Er, was finally passed 10 times through a 15th order linear adaptive filter with Δ set to 1 × 10−9 sec.43 The input signal was Er, and the reference signal was the summation of both stimulation channels (Stim T7 and Stim L3). The linear adaptive filter was used to isolate the EMG artifacts caused by stimulation from both the T-7 and L-3 stimulation electrodes. An example of the EMG time series prior to and following filtering and the same signal post-filtering is shown in Figure 2B and C. Muscle activity was quantified by calculating the mean EMG signal amplitude, after measurement windows were normalized in duration for each trial. Incidences of lost balance were excluded from analysis. The EMG data of participant P3 were excluded from the group analysis because of the relatively low, T9 neurological level of SCI.
FIG. 2.
(A) Schematic representing the filtering of the trunk muscle's electromyography (EMG) time series. Note that for the linear adaptive filter, the reference signal was the sum of the T11 and L1 stimulation signals. (B) Representative raw unfiltered (left) and filtered (right) EMG sample from P1 without (light blue) and with (light red) the presence of stimulation. (C) Zoomed in, 50 ms sample of raw unfiltered (left) and filtered (right) EMG without (light blue) and with (light red) stimulation. The orange and green dashed lines indicate the enlarged segments without and with stimulation, respectively.
Custom source code with a graphical interface was used to extract image, joint, and depth data from the Xbox One Kinect motion capture system. Custom Matlab (Mathworks, USA) code was used to plot and analyze the COP and kinematic joint location data and associated trajectories to determine the limits of stability, quantify spinal alignment, and graph EMG data during each test. The 3D kinematic data was used to determine the trunk angle and curvature. These displacements were then used to determine the velocity and acceleration profiles of each joint via single and double derivatives of the position profile, respectively.
Statistical analysis
Statistical analyses were performed using a within-subject statistical design. Comparisons among force plate data, EMG, and 3D kinematic positional data for all assessment tasks without and with the presence of stimulation were performed using the two tailed nonparametric Wilcoxon signed- rank test (α < 0.05). The results for the pooled data are presented as mean values and standard deviations (SD). In addition to EMG comparisons within each of the quiet sitting, multi-directional leaning, and self-initiated perturbation tasks, comparisons between no stimulation in quiet sitting versus no stimulation in multidirectional leaning as well as stimulation in quiet sitting versus stimulation in multi-directional leaning were performed. Correlation coefficients between arm velocity and A-P COP displacement for the self-initiated perturbation were determined using the nonparametric Spearman's rank correlation coefficient, with rho and p values indicated (α < 0.05). A statistical comparison of the correlation coefficients, with the null hypothesis implying that the difference between no stimulation and stimulation conditions is 0, was performed. For this purpose, we used the cocor R package web-based interface (http://comparingcorrelations.org/) developed by the CRAN project (http://cran.r-project.org/package=cocor), using an α = 0.05, and a confidence interval of 95%.44 Pearson and Filon's z test results are reported.
Results
Quiet sitting
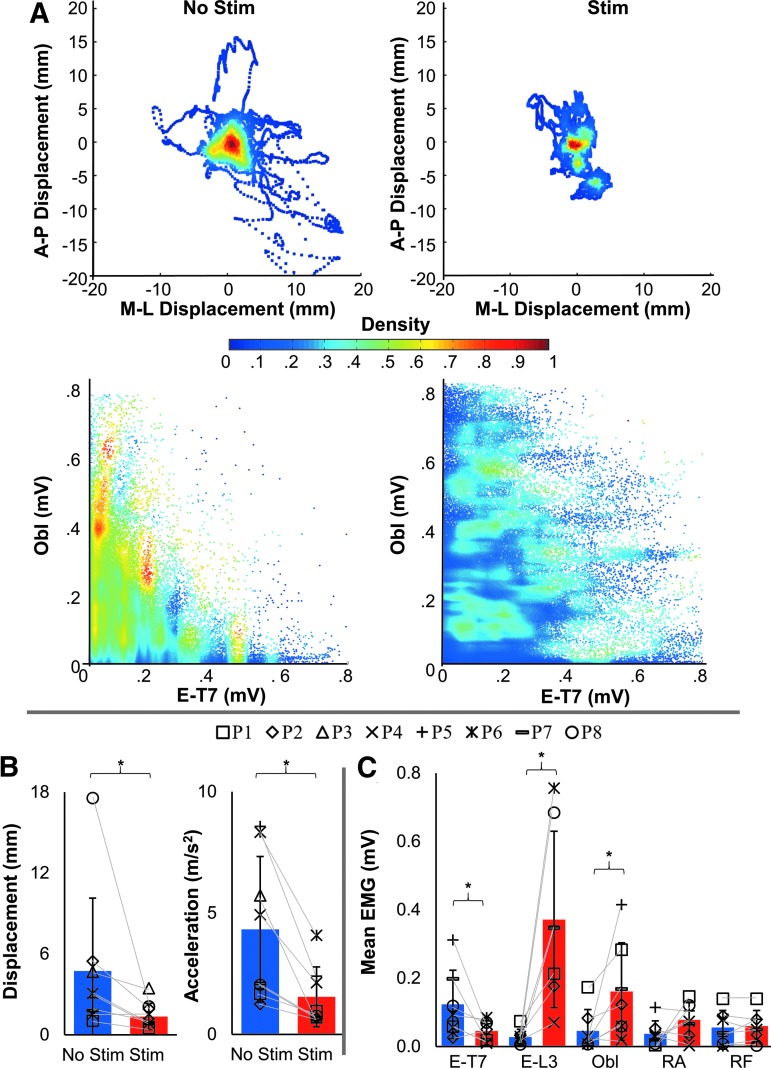
With a gradual increase of spinal stimulation intensity during preliminary test in “relaxed” sitting, the participant assumed a more erect posture once the intensity reached the supramotor threshold levels (Supplementary Video 1). During the main test, when participants were instructed to actively maintain their upright posture and stimulation was delivered at the submotor threshold intensity, the COP displacements decreased to 1.36 ± 0.98 mm compared with 4.74 ± 5.41 mm without stimulation (Z = −2.418, p = 0.0156). The COP acceleration decreased from 4.33 ± 3.00 m/sec2 without stimulation to 1.55 ± 1.24 m/sec2 in the presence of stimulation (Z = −2.661, p = 0.0078) (Fig. 3A, B). There was also an increase in the coactivation of Obl and E-T7, as shown in the normalized pooled scattergram (Fig. 3B), and a change in the distribution of the trunk muscle activation (Fig. 3C).
FIG. 3.
Acute effects of submotor threshold spinal stimulation on center of pressure (COP) parameters during unsupported quiet sitting for eight participants. (A) Normalized COP excursion density for all eight participants with their eyes open without (left) and with submotor threshold spinal stimulation (right). The density indicates time spent at each position for all participants. Below, a scattergram between the erector spinae at the T7 level (E-T7) and external obliques (Obl) without and with stimulation in one representative participant (P2) is shown. (B) Mean amplitude and standard deviation of mean COP displacement (left) and mean COP acceleration (right) without (blue) and with (red) stimulation; A two tailed nonparametric Wilcoxon signed-rank test was used for assessing differences between stimulation conditions; n = 8, *statistical significance, α < 0.05. A lower COP amplitude and acceleration are indicative of better control. (C) Mean electromyography (EMG) between stimulation conditions of E-T7, erector spinae at the L-3 level (E-L3), Obl, rectus abdominus (RA), and rectus femoris (RF). Note the significant change in activity of E-T7, E-L3, and Obl; two tailed nonparametric Wilcoxon signed-rank test in which n = 7 and α < 0.05. P3 was omitted from EMG calculations because of the neurological level of injury of T9. Note that there is no significant change in the RF.
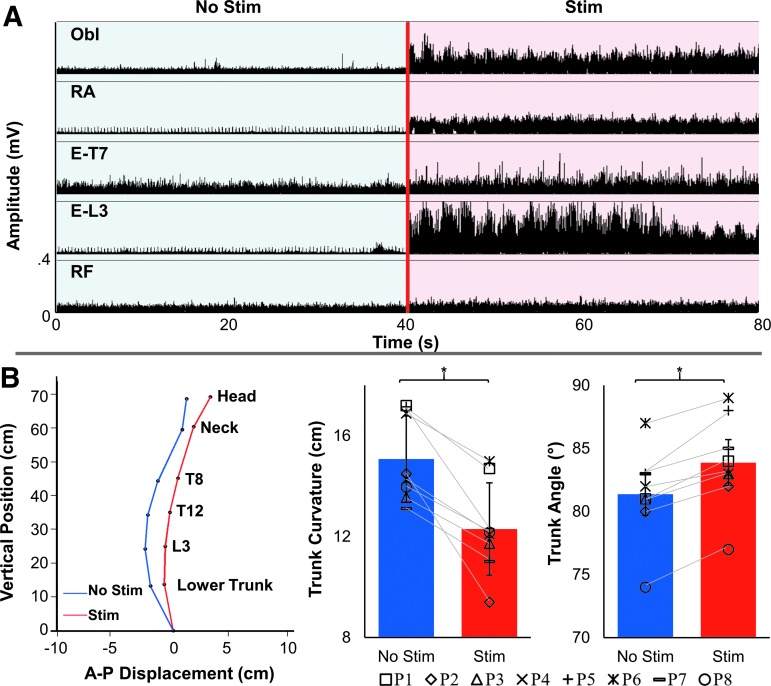
The EMG activity of E-T7, E-L3, and Obl changed from 0.12 ± 0.10 mV, 0.03 ± 0.02 mV, and 0.05 ± 0.06 mV without stimulation, to 0.05 ± 0.03 mV (Z = −1.9872, p = 0.0.0469), 0.37 ± 0.26 mV (Z = 2.418, p = 0.0156), and 0.16 ± 0.14 mV (Z = 2.153, p = 0.0313) in the presence of stimulation, respectively. There were no significant changes in the RA and RF EMG activity (Fig. 3C). Greater extension was observed in the lower trunk during stimulation; therefore, sitting became more upright, with a mean trunk curvature of 12.30 ± 1.83 cm compared with 15.08 ± 1.71 cm without stimulation (Z = −2.4181, p = 0.0117); the mean trunk angle increased from 83.88 ± 3.72 degrees with stimulation compared with 81.38 ± 3.66 degrees without stimulation (Z = 2.305, p = 0.0106) (Figs. 1C and 4).
FIG. 4.
Characteristics of spinal stimulation during quiet sitting. (A) Electromyography (EMG) recordings of four trunk muscles from a representative participant (P2) without (blue) and with (red) submotor threshold stimulation during unsupported quiet sitting. The external obliques (Obl), rectus abdominis (RA), erector spinae at levels T7 (E-T7) and L3 (E-L3), and rectus femoris (RF) are shown. (B) Spinal alignment (left), mean trunk curvature (middle), and trunk angle (right) during quiet sitting without (blue) and with (red) spinal stimulation. A 5 sec window was used (4–9 sec after trial onset) to determine the mean trunk curvature, horizontal distance between the hip (anterior-superior iliac crest), and maximal trunk displacement during quiet upright sitting. The pelvis is assumed to be fixed at the origin. Higher values in trunk curvature indicate a decrease in trunk extension and more kyphotic (C-shaped) sitting. Higher values of trunk angle indicate more upright sitting. Individual data for all participants are shown via symbols. *statistical significance, two-tailed nonparametric Wilcoxon signed-rank test, in which n = 8 and α < 0.05.
Multi-directional leaning
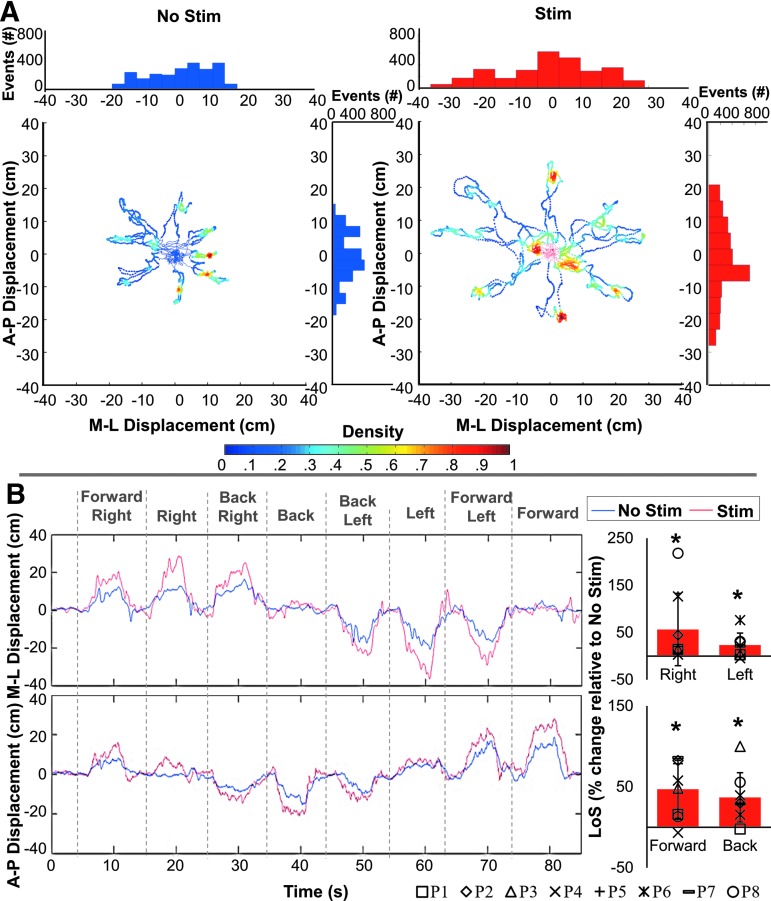
During the multi-directional leaning (“octagon”) test (Fig. 5, Supplementary Video 2), participants were asked to lean as far as possible in the indicated direction without losing balance, hold the position, and return to the initial position based on visual cues while minimizing movement of the arms and head to maintain balance and keeping the back extended (see online supplementary material at http://www.liebertpub.com). In the presence of stimulation, the mean limits of stability increased by 46.92 ± 35.66% (Z = 2.4181, p = 0.0156), 36.92 ± 30.48% (Z = 2.4181, p = 0.0156), 54.67 ± 77.99% (Z = 2.267, p = 0.0234), and 22.70 ± 26.09% (Z = 2.063, p = 0.0391) in the forward, backward, right, and left directions, respectively. The limits of stability increase was also associated with a less multi-modal and a more uniformly shaped distribution of the A-P and M-L movement profiles of the COP displacements (Fig. 5A), indicating better temporospatial coordination; that is, participants were able to perform a more continuously controlled trunk leaning movement, hold the extended position for a greater duration of time, and return to the starting position without falling (Fig. 5B).
FIG. 5.
Acute effects of submotor threshold spinal stimulation on the limits of stability (LoS) during the “octagon” multi-directional leaning test. (A) Density plot of the center of pressure (COP) excursions during the “octagon” test for a representative participant (P2) without (left) and with (right) spinal stimulation. The distribution of directional displacement events, occurrence of a magnitude of displacement binned into uniform time windows, is shown in both the mediolateral (M-L) direction above each density plot and in the anteroposterior (A-P) direction to the right of each density plot. Note the distribution of the bins; the presence of spinal stimulation results in a more normal distribution of movement. (B) The M-L and A-P time series, with positive values representing the directions right and forward, and negative values representing the directions left and backward. The pooled percent increases in the LoS in the presence of spinal stimulation compared with no stimulation for all participants (n = 8) are shown to the right of each time series plot. Note the significant increase in all leaning directions. *statistical significance; two tailed nonparametric Wilcoxon signed-rank test in which n = 8 and α < 0.05.
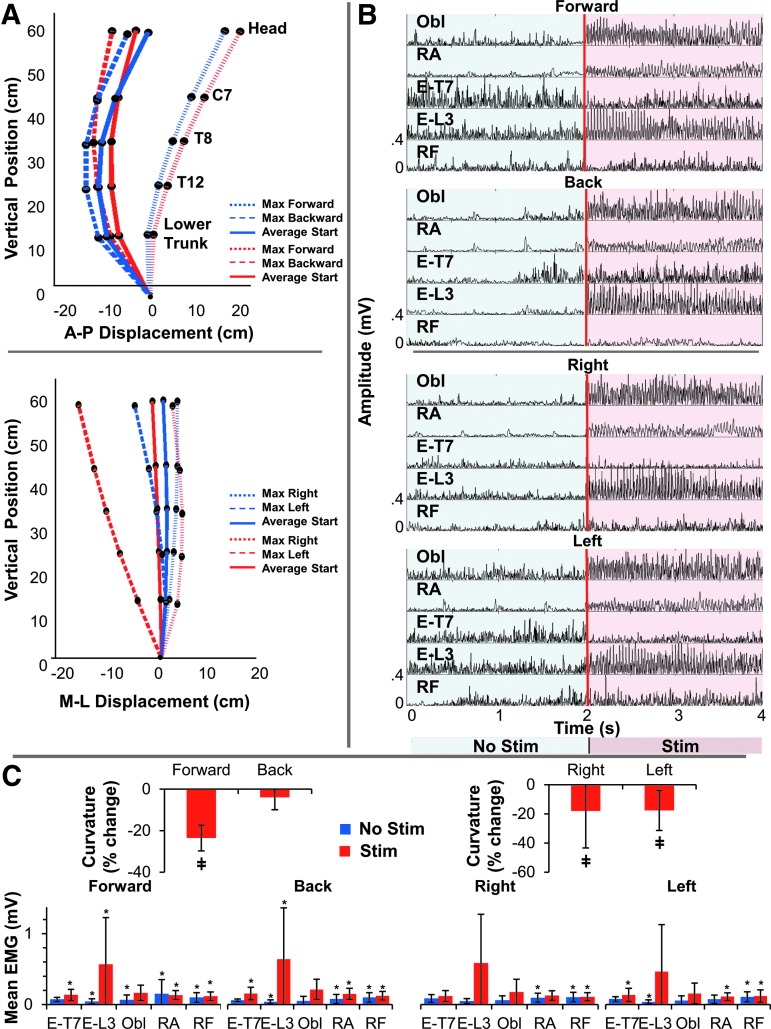
Individual EMG data during multi-directional leaning are presented in Supplementary Table 2 (see online supplementary material at http://www.liebertpub.com). During multi-directional leaning, more neutral spinal alignment and decreased trunk curvature occurred and were maintained in both of the leaning directions (Fig. 6A) in the presence of spinal stimulation when compared with no stimulation. This was most notable for trunk extension; that is, the backward leaning direction in the sagittal plane where “leaning with extension” was the newly adopted strategy as opposed to “slouching.” The improvement in seated spinal stability occurred as a result of increases in trunk extensor (E-T7 and E-L3) and flexor (Obl and RA) activity in the presence of spinal stimulation (Fig. 6B).
FIG. 6.
Acute effects of submotor threshold spinal stimulation on the limits of stability (LoS) during the “octagon” multi-directional leaning. (A) Three-dimensional joint kinematics showing the spinal alignment during directional leaning without (blue) and with (red) spinal stimulation in a representative participant (P6): leaning in the anteroposterior (A-P) direction (top) and mediolateral (M-L) direction (bottom). Note three-dimensional joint kinematics during the averaged start and extreme forward, backward, right, and left positions. The pelvis is assumed to be fixed at the origin. (B) Electromyographic (EMG) data from a representative participant without (blue) and with (red) stimulation for four trunk muscles in the forward, backward, right, and left directions. The external obliques (Obl), rectus abdominis (RA), erector spinae at levels T7 (E-T7) and L3 (E-L3), and rectus femoris (RF) are shown. Note the change in magnitude of muscle activity. (C) The change in trunk curvature in the presence of stimulation during the A-P (left) and M-L (right) directions (n = 8, α < 0.05). A decrease in trunk curvature indicates more upright neutral spinal alignment. ‡statistical significance. The mean EMG activity levels and their standard deviations during each directional leaning without (blue) and with (red) spinal stimulation are shown (n = 7, α < 0.05, P3 was omitted from EMG calculations because of an NLI of T9). *statistical significance between the corresponding values during quiet sitting and leaning in the indicated direction.
During leaning forward, there was a significant increase in E-L3 mean EMG activity from 0.05 ± 0.03 mV without stimulation to 0.57 ± 0.66 mV with stimulation (Z = 2.4181, p = 0.0156). Without stimulation, the activity in E-L3, Obl, RA, and RF increased compared with quiet sitting (Z = 2.4181, p = 0.0156; Z = 2.4181, p = 0.0156; Z = 2.1532, p = 0.0313; and Z = 2.1532, p = 0.0313, respectively). In the presence of stimulation, the activity in E-T7, E-L3, RA, and RF was higher during leaning forward than during quiet sitting (Z = 2.4181, p = 0.0156; Z = 2.1532, p = 0.0313; Z = 2.4181, p = 0.0156; and Z = 2.1532, p = 0.0313, respectively).
During leaning backwards, mean EMG activity increased in E-T7, E-L3, Obl, and RF from 0.06 ± 0.02, 0.04 ± 0.03, 0.05 ± 0.06, and 0.09 ± 0.06 mV without stimulation to 0.14 ± 0.08 (Z = 2.1532, p = 0.0313), 0.59 ± 0.66 mV (Z = 2.4181, p = 0.0156), 0.19 ± 0.13 mV (Z = 2.1532, p = 0.0313), and 0.11 ± 0.05 mV (Z = 2.1532, p = 0.0313) in the presence of stimulation, respectively. Without stimulation, the activity in E-L3, RA, and RF increased compared with quiet sitting (Z = 2.4181, p = 0.0156; Z = 2.4181, p = 0.0156; and Z = 1.9872, p = 0.0469, respectively). In the presence of stimulation, the activity in E-T7, E-L3, RA, and RF was higher during leaning backward than during quiet sitting (Z = 2.4181, p = 0.0156; Z = 2.4181, p = 0.0156; Z = 2.4181, p = 0.0156; and Z = 2.1532, p = 0.0313, respectively).
During leaning to the right, mean EMG activity increased in E-L3, Obl, RA, and RF from 0.08 ± 0.05, 0.06 ± 0.06, 0.09 ± 0.06, and 0.09 ± 0.06 mV without stimulation to 0.11 ± 0.07 mV (Z = 2.4181, p = 0.0156), 0.16 ± 0.16 mV (Z = 2.4181, p = 0.0156), 0.12 ± 0.06 mV (Z = 2.1532, p = 0.0313), and 0.10 ± 0.06 mV (Z = 2.4181, p = 0.0156) in the presence of stimulation, respectively. Without stimulation, the activity in RA and RF increased compared with quiet sitting (Z = 2.4181, p = 0.0156 and Z = 2.4181, p = 0.0156, respectively). In the presence of stimulation, the activity in RF was higher during leaning to the right than during quiet sitting (Z = 2.1532, p = 0.0313).
Lastly, during leaning to the left, there was a significant increase in the E-L3 mean EMG activity from 0.03 ± 0.03 without stimulation to 0.43 ± 0.61 mV in the presence of stimulation (Z = 2.4181, p = 0.0156). Without stimulation, the activity in E-L3 and RF increased compared with quiet sitting (Z = 2.1532, p = 0.0313 and Z = 1.9872, p = 0.0469, respectively). In the presence of stimulation, the activity in E-T7, RA, and RF was higher during leaning to the left than during quiet sitting (Z = 1.9872, p = 0.0469; Z = 1.9872, p = 0.0469; and Z = 1.9872, p = 0.0469, respectively). This finding is consistent with the results during quiet upright sitting, in which the EMG contribution supported an altered spinal alignment and sitting posture that was more spine neutral and upright, and less kyphotic. The percent change in curvature was −23.55 ± 0.34% (Z = −2.3863, p = 0.017), −3.96 ± 1.68% (Z = −1.2951, p = 0.1953), −18.05 ± 2.65% (Z = −2.2014, p = 0.0277), and −17.65 ± 0.62% (Z = −2.1004, p = 0.0391) during leaning in the forward, back, right, and left directions, respectively. Increased lordosis and decreased kyphosis were maintained throughout multi-directional leaning in the presence of stimulation when compared with no stimulation (Fig. 6C). This improved posture can be attributed to increased tonic activity of the trunk flexors and extensors, especially in E-L3.
Self-initiated perturbation
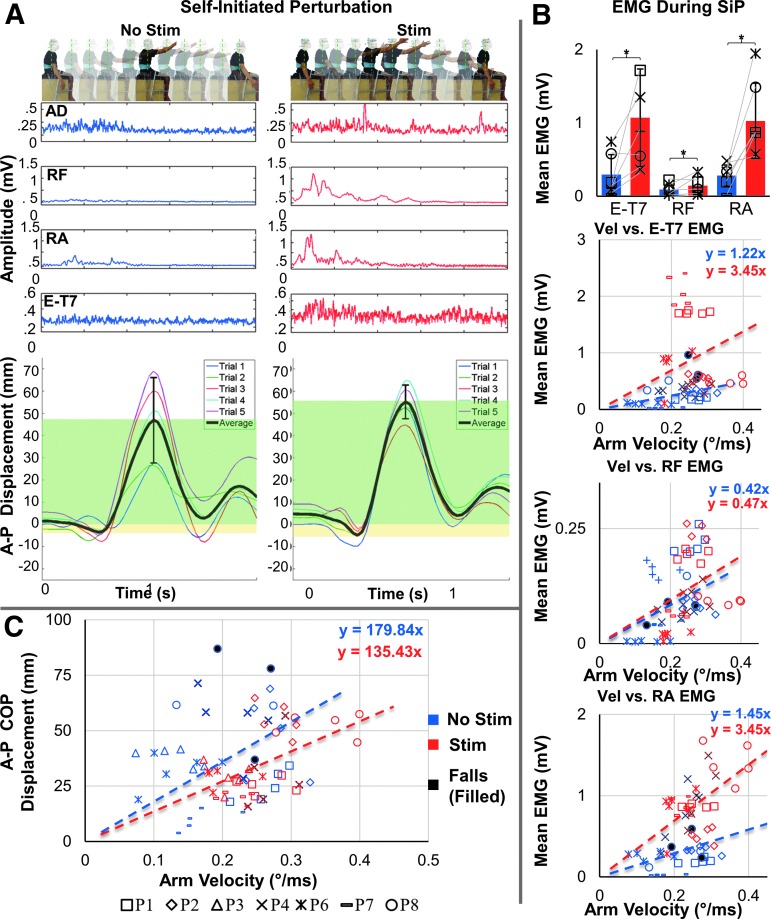
During the self-initiated perturbation, participants rapidly raised their extended right arm forward to a horizontal position and then returned the arm back to the initial position based on auditory cues. Without spinal stimulation, the participants' arm extension was often slower and more limited in range than with stimulation, because the participants had to interrupt the motion in order to maintain balance and not fall. The magnitude of the COP displacement during the perturbation varied across the trials to a great extent, and was dependent on the arm velocity/angular displacement. EMG responses from the trunk muscles were of lower amplitudes without spinal stimulation (Fig. 7A), and the relationship between their magnitude and the arm velocity/angular displacement was low (Fig. 7B).
FIG. 7.
Acute effects of transcutaneous spinal stimulation on the anteroposterior center of pressure (COP) excursion during the self-initiated perturbation (SiP) test. (A) Self-initiated perturbation. Below, the average electromyographic activity of the anterior deltoid (AD), rectus femoris (RF), rectus abdominis (RA), and erector spinae at level T7 (E-T7) along with the anteroposterior (A-P) COP displacement of one representative participant (P2) without (left) and with (right) spinal stimulation is shown. Individual (colored traces) as well as the average COP displacements (black trace) are shown. The magnitude of average anterior (green) and posterior (yellow) displacement are shown. (B) Pooled electromyography activity (EMG) of the E-T7, RF, and RA during the self-initiated perturbation without (blue) and with (red) stimulation. Note the change in EMG activity in the indicated muscles. *statistical significance between the mean EMG with stimulation and the mean EMG without stimulation. (C) Mean angular arm velocity versus A-P COP for all trials. Note the increase in arm velocity with stimulation (red) compared with without stimulation (blue) (n = 6, α < 0.05).
In the presence of spinal stimulation, the effectiveness of postural corrections in response to the self-initiated perturbations was considerably enhanced (Fig. 7). Consistent with the quiet and dynamic seated tests, the participants' upright seated posture was improved as revealed by decreased trunk curvature; during the perturbations, the arm extension was faster, with a larger range of motion, and the participants were able to maintain balance during the test (Supplementary Video 3) (see online supplementary material at http://www.liebertpub.com). The EMG responses in the trunk muscles were significantly higher during the self-initiated perturbation, with a pronounced activity in E-T7 and RA following the arm extension, which somewhat depended on the arm velocity/angular displacement. Interestingly, there was also an emergence of RF activity in response to the self-initiated perturbation (Fig. 7A, B). Correlation between arm velocity and mean EMG of E-T7, RF, and RA shifted from 0.46 (p = 0.0105), 0.30 (p = 0.1072), and 0.30 (p = 0.1072) without to 0.50 (p = 0.0048), 0.53 (p = 0.0026), and 0.26 (p = 0.1653) with stimulation. Although a tendency toward a larger dependence between arm velocity and mean EMG in each muscle can be seen in Figure 7B, the Pearson and Filon's z test failed to reveal significant changes in the correlation coefficient between no simulation and stimulation: E-T7 (Z = 0.2593, p = 0.7954), RF (Z = 1.3405, p = 0.1801), and RA (Z = 1.1204, p = 0.2625). In addition, the amplitude of the mean COP displacement during and following the perturbation was lower and less correlated with the arm velocity/angular displacement with stimulation (Fig. 7C). During self-initiated perturbations, the correlation between arm velocity and A-P COP displacement decreased from r = 0.5821 (p = 0.0007) without to r = 0.5115 (p = 0.0039) with stimulation (Z = −2.0402, p = 0.0413). In the presence of stimulation, larger arm velocities occurred as well as a smaller A-P COP displacement, indicating that a higher degree of stability is maintained in the presence of stimulation, as the participants are able to exhibit a faster and larger range of motion and maintain balance without falling.
Discussion
Our results indicate the feasibility and effectiveness of noninvasive spinal stimulation in regaining postural control during sitting following chronic SCI. We demonstrated that kinematics, kinetics, and neuromuscular activity patterns are modified in the presence of spinal stimulation, resulting in enhanced performance during quiet sitting and dynamic tasks. Our data show that improved postural control during sitting can be achieved within a single experimental session in individuals with complete or partial paralysis diagnosed >2 years earlier.
Potential mechanisms of improved seated postural control during spinal stimulation
Neuromodulation in combination with performing a motor task can facilitate restoration of supraspinal-spinal connectivity and reactivation of spinal networks even after chronic, severe spinal injury.45–48 Regaining of significant levels of clinically relevant functions, such as stepping, standing, and voluntary leg movements, after SCI has been demonstrated using both invasive49–51 and noninvasive33–36 spinal stimulation. In the present study, we observed that multiple features of seated postural control, including quiet and dynamic tasks, improved during spinal stimulation. With neuromodulation, the participants could actively maintain a more upright seated posture, and improve various balance strategies, both quantitatively and qualitatively.
The primary spinal stimulation parameters chosen to neuromodulate spinal networks in the present study were consistent with previous studies performed during supine position, standing, or stepping.30,41,52,53 For example, it has been demonstrated that stimulation of the rostral portion of the lumbosacral enlargement (corresponding approximately to the T11–T12 vertebral level) at a frequency of 30 Hz is more specific for facilitating voluntary movements,33,34,51,54,55 whereas stimulation delivered over the caudal area of the lumbosacral enlargement (corresponding approximately to the L1–L2 vertebral level) at a frequency of 15 Hz results in facilitation of tonic extensor activity specific for postural control.49,50,56 Finally, our stimulation locations approximated the myotomal maps of the lumbosacral spinal cord projecting to E-L2, RA, Obl, and the internal oblique from the rostral segments, and to E-L2, Obl, and the internal oblique from the caudal segments.57 The average activity of the trunk muscles during multi-directional leaning was not characterized by muscle specificity that depended on movement direction. However, we observed improved motor performance, which was accompanied by the trunk muscles' co-contraction; for example, of Obl and E-L3 during any direction. Such lack in muscle specificity may be attributed to impaired function of the sublesional networks, and/or to adaptive compensatory strategies aimed to overcome decreased postural steadiness by increasing trunk core stiffness. This is similar to what has been shown in the lower leg muscles during standing in the elderly,58 or during stepping in stroke survivors.59
With spinal stimulation, corrections to self-initiated seated balance perturbations were more successful than when performed without stimulation. When the postural control system was challenged by a rapid arm extension, the corrective responses during the first 200 ms were characterized by increased muscle activation and the emergence of hip- and trunk-based synergies. Although not significant on the individual muscle level, there was a positive relationship between the magnitude of the muscle response and perturbation intensity (i.e., arm velocity). Consequently, the increased muscle recruitment resulted in better trunk stability, which was revealed by lower COP displacements in response to increased arm velocity. It is possible that a feed-forward mechanism38 allowed the spinal networks to process, in real time, the more extensive range of sensory inputs and motor commands for the successful execution of the intended task and to maintain a more stable sitting posture.
It has been thought that automatic balance recovery strategies are attributed only to spinal mechanisms, and are not associated with supraspinal control.60 However, growing evidence suggests that supraspinal adaptations contribute significantly to improved balance performance even following externally challenged balance training,17,61–63 and have been shown to be facilitated by feed-forward mechanisms.38 If the connections spared following SCI can be reinforced by spinal cord stimulation, functional improvement is possible by amplifying the potential of interneurons to plastically reorganize after SCI.46 These observations are indicative that spinal postural-specific neural networks are highly flexible and adaptable, can function predictably as a feed-forward mechanism, and can effectively maintain equilibrium, synergistically at sub- and potentially supralesional levels, when enabled by spinal stimulation
Spinal stimulation versus functional electrical stimulation (FES) to facilitate postural control of trunk
There is a question as to whether the observed enhanced postural control can be primarily attributed to direct neuromuscular activation of paraspinal muscles adjacent to the stimulation sites, similar to the inducing effects of FES. FES has been proposed as a means to facilitate and restore trunk and postural control during sitting and standing in the SCI population.4,64–68 Intramuscular stimulation delivered bilaterally at the L1–L2 spinal nerves coupled with erector spinae and gluteus maximus muscles stimulation showed the increased isometric trunk extension moment essential for forward reaching to different heights.69 Low-intensity FES delivered bilaterally over the rectus abdominis and erector spinae70 muscles at the thoracic and lumbar regions have shown directional-dependent increases in trunk stiffness, specifically in the A-P direction associated with the stimulated muscles.70 FES can also improve A-P stability during sitting,70 increase bimanual workspace,11 improve A-P vertebral alignment and posture,11,12 improve active upright sitting, and increase shoulder height. However, these inducing strategies were tested in a single static posture, and substitute one statically stable posture for another requiring the use of the upper limb to transition postures.69,71
In our study, the activity of the erector spinae, rectus abdominis, and external obliques, all contributors to seated posture, were elevated during voluntary efforts to sit upright, when combined with stimulation of the lumbosacral enlargement. These elevated activities and increased equilibrium during sitting were associated with a more natural anterior pelvic tilt and lordotic curve, and greater stability. In contrast to the direction-dependent effects noted previously using FES in a static posture, it appears that spinal stimulation improved multi-directional leaning and postural corrections during self-initiated perturbations within the first experimental session. These effects occurred using the same stimulation locations and frequencies for each individual. FES is typically delivered over several trunk muscles, resulting in increased co-activation of antagonistic muscles, which, in turn, contributes to kinematically improved upright sitting,70,72 although not necessarily to improvement in energy efficiency. In the present study, however, spinal stimulation was delivered over the spine, and resulted in improved trunk postural regulation through facilitation of postural synergies, independent of the motor tasks or direction of active movements. Previous electrophysiological73–75 and computational76–78 studies demonstrated that the structures, stimulated electrically by epidural or transcutaneous spinal lumbar spinal cord stimulation, are primarily afferent fibers of the posterior roots. For lumbar and sacral roots, the exits of the spinal nerves from the spinal canal are relatively far from the electrode, and, therefore, it is possible to activate predominantly sensory posterior root fibers during stimulation over the L1. However, the intervertebral foramina of some thoracic roots are close to the T11 stimulation electrode and, as such, mixed afferent and efferent fibers could be directly electrically activated.79 Examining the properties of individual spinally evoked motor potentials in various trunk muscles could be revealing in characterizing afferent or efferent fibers versus interneuronal circuitry targeted during spinal stimulation. However, because of the proximity of the stimulating and recording electrodes, spinally evoked potentials from the trunk muscles were obscured by a prominent stimulation artifact, which did not allow us to investigate the specific properties and contributions of the individual motor pools to trunk postural control. In addition, it has been shown that depending on the body position, different neural structures may be involved in the response during spinal stimulation.79,80 It is possible that different postural tasks during sitting have similar effects. This limits our understanding of the targeted neural structures during spinal stimulation.
Finally, FES often requires feedback controllers or manual assistance when transitioning between static postures, such as upright and leaning sitting.2,81,82 The spinal stimulation delivered in our study (at constant intensity and frequency) facilitated postural control and improved stability in a variety of static and dynamic motor tasks. Based on these critical differences in the effects of FES and spinal stimulation, we suggest that the facilitatory effects of spinal stimulation can be attributed to its ability to potentiate the processing and projections of proprioceptive and descending inputs to broader components of the neural networks that contribute to sitting, including multi-segmental projections to the trunk and lower limb musculature, which execute corresponding motor tasks.
Limitations and methodological considerations
A balanced, within-subject crossover study design has been used, wherein each participant received an equal number of stimulation and no stimulation conditions within an experimental session. The blinded design was intended, however, participants with complete paralysis could perceive the facilitatory effects of spinal stimulation on upright posture because of increased tone in the trunk musculature. “Dose-dependent” effects of the stimulation intensity on EMG responses and the level of trunk extension, as well as the effects of “sham” stimulation delivered, for example, distant from the lumbosacral enlargement may provide further evidence on cause and effect in defining the efficacy of a given intervention. Further research with larger sample sizes is warranted to test, using a multi-factorial ANOVA design, the effects of factors such as the participants' body composition, level and duration of injury, and presence of hardware in the spine, as well as training or practice. Paired comparisons of individual variables between no stimulation and stimulation conditions, not multiple comparisons, were utilized in this exploratory study
Conclusion
We demonstrated that, after >2 years following the onset of complete or partial paralysis, stimulation over the lumbosacral enlargement improved self-governing control of upright sitting. Spinal stimulation targeted similar networks to those that control rhythmic movements and upright standing, suggesting important functional and anatomical overlap between these systems. From a functional perspective, any strategy that can improve or enable them to regain sitting and postural control represents a substantial advancement for individuals with paralysis in their daily life activity.83 The physiological implications of our findings are consistent with qualities that reflect independence, enhanced function of sensorimotor and autonomic systems, and improved quality of life of individuals with SCI. From a clinical perspective, our approach provides further evidence of the acute neuroplastic capacity of spinal and potentially supraspinal networks, and provides a conceptional rationale for the development of a longer-term seated postural control protocol enabled by noninvasive spinal stimulation. The present results are indicative for planning an activity-based rehabilitation program for improving balance. Finally, this study highlights the importance of including assessments of postural-specific networks while subjects are seated, as outcomes that might be extrapolated to the potential to control standing.
Supplementary Material
Acknowledgments
We thank Bree Navarro, Nicole Sassounian, and the other research student volunteers for their valuable contributions to this study. We also thank Amanda Turner for invaluable administrative support and assistance with this project. This work was supported by the Paralyzed Veterans of America (PVA) Research Foundation (Grant #3068), National Institutes of Health (NIH) Small Business Innovation Research (SBIR) grant R43EB018232, and the Russian Foundation for Fundamental Research (Grant No. 16-29-08173-ofi-m). The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
Author Disclosure Statement
V.R.E., Y.P.G., and J.B., researchers on the study team, hold shareholder interest in NeuroRecovery Technologies. They hold certain inventorship rights on intellectual property licensed by the regents of the University of California to NeuroRecovery Technologies and its subsidiaries. The other authors have nothing to disclose.
References
- 1.Seelen H.A., Potten Y.J., Drukker J., Reulen J.P., and Pons C. (1998). Development of new muscle synergies in postural control in spinal cord injured subjects. J. Electromyogr. Kinesiol. 8, 23–34 [DOI] [PubMed] [Google Scholar]
- 2.Seelen H.A., Potten Y.J., Huson A., Spaans F., and Reulen J.P. (1997). Impaired balance control in paraplegic subjects. J. Eectromyogr. Kinesiol. 7, 149–160 [DOI] [PubMed] [Google Scholar]
- 3.Desroches G., Gagnon D., Nadeau S., and Popovic M.R. (2013). Effects of sensorimotor trunk impairments on trunk and upper limb joint kinematics and kinetics during sitting pivot transfers in individuals with a spinal cord injury. Clin. Bomech. (Bristol, Avon) 28, 1–9 [DOI] [PubMed] [Google Scholar]
- 4.Triolo R.J., Boggs L., Miller M.E., Nemunaitis G., Nagy J., and Bailey S.N. (2009). Implanted electrical stimulation of the trunk for seated postural stability and function after cervical spinal cord injury: a single case study. Arch. Phys. Med. Rehabil. 90, 340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper R.A. (1998). Wheelchair Selection and Configuration. Springer Publishing Company, New York, NY [Google Scholar]
- 6.Crawford A., Armstrong K., Loparo K., Audu M., and Triolo R. (2018). Detecting destabilizing wheelchair conditions for maintaining seated posture. Disabil. Rehabil. Assist. Technol. 13, 178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figura J.C. (1985) Positioning technique for abdominal strengthening suggestion from the field. Phys. Ther. 65, 45. [DOI] [PubMed] [Google Scholar]
- 8.Caneiro J.P., O'Sullivan P., Burnett A., Barach A., O'Neil D., Tveit O., and Olafsdottir K. (2010). The influence of different sitting postures on head/neck posture and muscle activity. Man. Ther. 15, 54–60 [DOI] [PubMed] [Google Scholar]
- 9.Schuldt K., Ekholm J., Harms-Ringdahl K., Nemeth G., and Arborelius U.P. (1986). Effects of changes in sitting work posture on static neck and shoulder muscle activity. Ergonomics 29, 1525–1537 [DOI] [PubMed] [Google Scholar]
- 10.Minkel J.L. (2000). Seating and mobility considerations for people with spinal cord injury. Phys. Ther. 80, 701–709 [PubMed] [Google Scholar]
- 11.Kukke S.N., and Triolo R.J. (2004). The effects of trunk stimulation on bimanual seated workspace. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 177–185 [DOI] [PubMed] [Google Scholar]
- 12.Wu G.A., Lombardo L., Triolo R.J., and Bogie K.M. (2013). The effects of combined trunk and gluteal neuromuscular electrical stimulation on posture and tissue health in spinal cord injury. PM R 5, 688–696 [DOI] [PubMed] [Google Scholar]
- 13.Sinderby C., Ingvarsson P., Sullivan L., Wickstrom I., and Lindstrom L. (1992). The role of the diaphragm in trunk extension in tetraplegia. Paraplegia 30, 389–395 [DOI] [PubMed] [Google Scholar]
- 14.Milosevic M., Masani K., Kuipers M.J., Rahouni H., Verrier M.C., McConville K.M., and Popovic M.R. (2015). Trunk control impairment is responsible for postural instability during quiet sitting in individuals with cervical spinal cord injury. Clin. Biomech. (Bristol, Avon) 30, 507–512 [DOI] [PubMed] [Google Scholar]
- 15.Thorstensson A., Nilsson J., Carlson H., and Zomlefer M.R. (1984). Trunk movements in human locomotion. Acta Physiol. Scand. 121, 9–22 [DOI] [PubMed] [Google Scholar]
- 16.Veneman J.F., Menger J., van Asseldonk E.H., van der Helm F.C., and van der Kooij H. (2008). Fixating the pelvis in the horizontal plane affects gait characteristics. Gait Posture 28, 157–163 [DOI] [PubMed] [Google Scholar]
- 17.Deliagina T.G., Zelenin P.V., and Orlovsky G.N. (2012). Physiological and circuit mechanisms of postural control. Curr. Opin. Neurobiol. 22, 646–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horak F.B., and Nashner L.M. (1986). Central programming of postural movements: adaptation to altered support-surface configurations. J. Neurophysiol. 55, 1369–1381 [DOI] [PubMed] [Google Scholar]
- 19.Earhart G.M. (2013). Dynamic control of posture across locomotor tasks. Mov. Disord. 28, 1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reft J., and Hasan Z. (2002). Trajectories of target reaching arm movements in individuals with spinal cord injury: effect of external trunk support. Spinal Cord 40, 186–191 [DOI] [PubMed] [Google Scholar]
- 21.Healy A., Ramsey C., and Sexsmith E. (1997). Postural support systems: their fabrication and functional use. Dev. Med. Child Neurol. 39, 706–710 [DOI] [PubMed] [Google Scholar]
- 22.Behrman A.L., and Harkema S.J. (2007). Physical rehabilitation as an agent for recovery after spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 18, 183–202 [DOI] [PubMed] [Google Scholar]
- 23.Bromley I., and Rose L. (2006). Wheelchairs and wheelchair management, in: Tetraplegia and Paraplegia (Sixth Edition). Churchill Livingstone: Edinburgh, pps. 149–183 [Google Scholar]
- 24.Galea M.P., Dunlop S.A., Davis G.M., Nunn A., Geraghty T., Hsueh Y.S., and Churilov L. (2013). Intensive exercise program after spinal cord injury (“Full-On”): study protocol for a randomized controlled trial. Trials 14, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung D.H., Yoon S.D., and Park G.D. (2015). The effect of complex rehabilitation training for 12 weeks on trunk muscle function and spine deformation of patients with SCI. J. Phys. Ther. Sci. 27, 951–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirth B., van Hedel H.J., Kometer B., Dietz V., and Curt A. (2008). Changes in activity after a complete spinal cord injury as measured by the Spinal Cord Independence Measure II (SCIM II). Neurorehabil. Neural Repair 22, 279–287 [PubMed] [Google Scholar]
- 27.Illis L.S. (2012). Central nervous system regeneration does not occur. Spinal Cord 50, 259–263 [DOI] [PubMed] [Google Scholar]
- 28.Brown J.M., Deriso D.M., and Tansey K.E. (2012). From contemporary rehabilitation to restorative neurology. Clin. Neurol. Neurosurg. 114, 471–474 [DOI] [PubMed] [Google Scholar]
- 29.Taccola G., Sayenko D., Gad P., Gerasimenko Y., and Edgerton V.R. (2018). And yet it moves: Recovery of volitional control after spinal cord injury. Prog. Neurobiol. 160, 64–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minassian K. and Hofstoetter U.S. (2016). Spinal cord stimulation and augmentative control strategies for leg movement after spinal paralysis in humans. CNS Neurosci. Ther. 22, 262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgerton V.R. and Harkema S. (2011). Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev. Neurother. 11, 1351–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minassian K., Hofstoetter U.S., Danner S.M., Mayr W., McKay W.B., Tansey K., and Dimitrijevic M.R. (2013). Mechanisms of rhythm generation of the human lumbar spinal cord in response to tonic stimulation without and with step-related sensory feedback. Biomed. Tech. 58, Suppl. 1 [DOI] [PubMed] [Google Scholar]
- 33.Hofstoetter U.S., Krenn M., Danner S.M., Hofer C., Kern H., McKay W.B., Mayr W. and Minassian K. (2015). Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals. Artif. Organs 39, E176–186 [DOI] [PubMed] [Google Scholar]
- 34.Gerasimenko Y.P., Lu D.C., Modaber M., Zdunowski S., Gad P., Sayenko D.G., Morikawa E., Haakana P., Ferguson A.R., Roy R.R., and Edgerton V.R. (2015). Noninvasive reactivation of motor descending control after paralysis. J. Neurotrauma 32, 1968–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofstoetter U.S., Hofer C., Kern H., Danner S.M., Mayr W., Dimitrijevic M.R., and Minassian K. (2013). Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed. Tech. 58, Suppl. 1 [DOI] [PubMed] [Google Scholar]
- 36.Minassian K., Hofstoetter U.S., Danner S.M., Mayr W., Bruce J.A., McKay W.B., and Tansey K.E. (2016). Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord-injured individuals. Neurorehabil. Neural Repair 30, 233–243 [DOI] [PubMed] [Google Scholar]
- 37.Hultborn H., and Nielsen J.B. (2007). Spinal control of locomotion—from cat to man. Acta Physiol. (Oxf.) 189, 111–121 [DOI] [PubMed] [Google Scholar]
- 38.Gerasimenko Y., Sayenko D., Gad P., Liu C.T., Tillakaratne N.J., Roy R.R., Kozlovskaya I., and Edgerton V.R. (2016). Feed-Forwardness of Spinal Networks in Posture and Locomotion. Neuroscientist 53, 441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sayenko D.G., Alekhina M.I., Vette A.H., Nakazawa K., and Popovic M.R. (2009). Balance training with visual feedback can improve balance abilities in people with spinal cord injury, in: The 19th International Conference of the ISPGR Bologna, pps. 105–106 [Google Scholar]
- 40.Reft J., and Hasan Z. (2002). Trajectories of target reaching arm movements in individuals with spinal cord injury: effect of external trunk support. Spinal Cord 40, 186. [DOI] [PubMed] [Google Scholar]
- 41.Gerasimenko Y., Gorodnichev R., Moshonkina T., Sayenko D., Gad P., and Reggie Edgerton V. (2015). Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 58, 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freedman B., Shpunt A., Machline M. and Arieli Y. (2013). US Patent No 8, 493, 496. Washington, DC: US Patent and Trademark Office [Google Scholar]
- 43.Marque C., Bisch C., Dantas R., Elayoubi S., Brosse V., and Perot C. (2005). Adaptive filtering for ECG rejection from surface EMG recordings. J. Electromyogr. Kinesiol. 15, 310–315 [DOI] [PubMed] [Google Scholar]
- 44.Diedenhofen B., and Musch J. (2015). Cocor: A comprehensive solution for the statistical comparison of correlations. PLoS One 10, e0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edgerton V.R., Tillakaratne N.J., Bigbee A.J., de Leon R.D., and Roy R.R. (2004). Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 27, 145–167 [DOI] [PubMed] [Google Scholar]
- 46.Pohland M., and Glumm J. (2015). Propriospinal interneurons in the spotlight for anatomical and functional recovery after spinal cord injury. Neural Regen. Res. 10, 1737–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Brand R., Mignardot J.B., von Zitzewitz J., Le Goff C., Fumeaux N., Wagner F., Capogrosso M., Martin Moraud E., Micera S., Schurch B., Curt A., Carda S., Bloch J., and Courtine G. (2015). Neuroprosthetic technologies to augment the impact of neurorehabilitation after spinal cord injury. Ann. Phys. Rehabil. Med. 58, 232–237 [DOI] [PubMed] [Google Scholar]
- 48.Field-Fote E.C. (2015). Exciting recovery: augmenting practice with stimulation to optimize outcomes after spinal cord injury. Prog. Brain Res. 218, 103–126 [DOI] [PubMed] [Google Scholar]
- 49.Rejc E., Angeli C., and Harkema S. (2015). Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS One 10, e0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grahn P.J., Lavrov I.A., Sayenko D.G., Van Straaten M.G., Gill M.L., Strommen J.A., Calvert J.S., Drubach D.I., Beck L.A., Linde M.B., Thoreson A.R., Lopez C., Mendez A.A., Gad P.N., Gerasimenko Y.P., Edgerton V.R., Zhao K.D., and Lee K.H. (2017). Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin. Proc. 92, 544–554 [DOI] [PubMed] [Google Scholar]
- 51.Angeli C., Edgerton V.R., Gerasimenko Y.P., and Harkema S.J. (2014). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jilge B., Minassian K., Rattay F., and Dimitrijevic M.R. (2004). Frequency-dependent selection of alternative spinal pathways with common periodic sensory input. Biol. Cybern. 91, 359–376 [DOI] [PubMed] [Google Scholar]
- 53.Harkema S., Gerasimenko Y., Hodes J., Burdick J., Angeli C., Chen Y., Ferreira C., Willhite A., Rejc E., Grossman R.G., and Edgerton V.R. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dimitrijevic M.M., Dimitrijevic M.R., Illis L.S., Nakajima K., Sharkey P.C., and Sherwood A.M. (1986). Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: I. Clinical observations. Cent. Nerv. Syst. Trauma 3, 129–144 [DOI] [PubMed] [Google Scholar]
- 55.Barolat G., Myklebust J.B., and Wenninger W. (1986). Enhancement of voluntary motor function following spinal cord stimulation—case study. Appl. Neurophysiol. 49, 307–314 [DOI] [PubMed] [Google Scholar]
- 56.Jilge B., Minassian K., Rattay F., Pinter M.M., Gerstenbrand F., Binder H., and Dimitrijevic M.R. (2004). Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp. Brain Res. 154, 308–326 [DOI] [PubMed] [Google Scholar]
- 57.Kendall F., McCreary E., Provance P., Rogers M., and Romani W. (1993). Muscles Testing and Function with Posture and Pain. Physiological Therapy. Lippincott Williams & Wilkins: Baltimore [Google Scholar]
- 58.Vette A.H., Sayenko D.G., Jones M., Abe M.O., Nakazawa K. and Masani K. (2017). Ankle muscle co-contractions during quiet standing are associated with decreased postural steadiness in the elderly. Gait Posture 55, 31–36 [DOI] [PubMed] [Google Scholar]
- 59.Lamontagne A., Richards C.L., and Malouin F. (2000). Coactivation during gait as an adaptive behavior after stroke. J. Electromyogr. Kinesiol. 10, 407–415 [DOI] [PubMed] [Google Scholar]
- 60.Dietz V. (1992). Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiol. Rev. 72, 33–69 [DOI] [PubMed] [Google Scholar]
- 61.Taube W., Gruber M., Beck S., Faist M., Gollhofer A., and Schubert M. (2007). Cortical and spinal adaptations induced by balance training: correlation between stance stability and corticospinal activation. Acta Physiol. (Oxf.) 189, 347–358 [DOI] [PubMed] [Google Scholar]
- 62.Sayenko D.G., Alekhina M.I., Masani K., Vette A., Obata H., Popovic M., and Nakazawa K. (2010). Positive effect of balance training with visual feedback on standing balance abilities in people with incomplete spinal cord injury. Spinal Cord 48, 886–893 [DOI] [PubMed] [Google Scholar]
- 63.Deliagina T.G., Beloozerova I.N., Zelenin P.V. and Orlovsky G.N. (2008). Spinal and supraspinal postural networks. Brain Res. Rev. 57, 212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fong A.J., Roy R.R., Ichiyama R.M., Lavrov I., Courtine G., Gerasimenko Y., Tai Y.C., Burdick J., and Edgerton V.R. (2009). Recovery of control of posture and locomotion after a spinal cord injury: solutions staring us in the face. Prog. Brain Res. 175, 393–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ho C.H., Triolo R.J., Elias A.L., Kilgore K.L., DiMarco A.F., Bogie K., Vette A.H., Audu M.L., Kobetic R., Chang S.R., Chan K.M., Dukelow S., Bourbeau D.J., Brose S.W., Gustafson K.J., Kiss Z.H., and Mushahwar V.K. (2014). Functional electrical stimulation and spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 25, 631–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Popovic M.R., Masani K., and Micera S. (2012). Functional electrical stimulation therapy: recovery of function following spinal cord injury and stroke, in: Neurorehabilitation Technology. Dietz V., Nef T., Rymer W.Z., (eds.). Springer: London, pps. 105–121 [Google Scholar]
- 67.Ragnarsson K.T. (2008). Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord 46, 255–274 [DOI] [PubMed] [Google Scholar]
- 68.Wilkenfeld A.J., Audu M.L., and Triolo R.J. (2006). Feasibility of functional electrical stimulation for control of seated posture after spinal cord injury: a simulation study. J. Rehabil. Res. Dev. 43, 139–152 [DOI] [PubMed] [Google Scholar]
- 69.Triolo R.J., Bailey S.N., Miller M.E., Lombardo L.M., and Audu M.L. (2013). Effects of stimulating hip and trunk muscles on seated stability, posture, and reach after spinal cord injury. Arch. Phys. Med. Rehabil. 94, 1766–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vette A.H., Wu N., Masani K. and Popovic M.R. (2015). Low-intensity functional electrical stimulation can increase multidirectional trunk stiffness in able-bodied individuals during sitting. Med. Eng. Phys. 37, 777–782 [DOI] [PubMed] [Google Scholar]
- 71.Yang Y.S., Koontz A.M., Triolo R.J., Cooper R.A., and Boninger M.L. (2009). Biomechanical analysis of functional electrical stimulation on trunk musculature during wheelchair propulsion. Neurorehabil. Neural Repair 23, 717–725 [DOI] [PubMed] [Google Scholar]
- 72.Watanabe S., Eguchi A., Kobara K., and Ishida H. (2007). Influence of trunk muscle co-contraction on spinal curvature during sitting for desk work. Electromyogr. Clin. Neurophysiol. 47, 273–278 [PubMed] [Google Scholar]
- 73.Hunter J.P., and Ashby P. (1994). Segmental effects of epidural spinal cord stimulation in humans. J. Physiol. 474, 407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maertens de Noordhout A., Rothwell J.C., Thompson P.D., Day B.L., and Marsden C.D. (1988). Percutaneous electrical stimulation of lumbosacral roots in man. J. Neurol. Neurosurg. Psychiatry 51, 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minassian K., Persy I., Rattay F., Dimitrijevic M.R., Hofer C., and Kern H. (2007). Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 35, 327–336 [DOI] [PubMed] [Google Scholar]
- 76.Rattay F., Minassian K., and Dimitrijevic M.R. (2000). Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord 38, 473–489 [DOI] [PubMed] [Google Scholar]
- 77.Ladenbauer J., Minassian K., Hofstoetter U.S., Dimitrijevic M.R., and Rattay F. (2010). Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 18, 637–645 [DOI] [PubMed] [Google Scholar]
- 78.Danner S.M., Hofstoetter U.S., Ladenbauer J., Rattay F., and Minassian K. (2011). Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif. Organs 35, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Danner S.M., Krenn M., Hofstoetter U.S., Toth A., Mayr W., and Minassian K. (2016). Body position influences which neural structures are recruited by lumbar transcutaneous spinal cord stimulation. PLoS One 11, e0147479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sayenko D.G., Angeli C., Harkema S.J., Edgerton V.R., and Gerasimenko Y.P. (2014). Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 111, 1088–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murphy J.O., Audu M.L., Lombardo L.M., Foglyano K.M., and Triolo R.J. (2014). Feasibility of closed-loop controller for righting seated posture after spinal cord injury. J. Rehabil. Res. Dev. 51, 747–760 [DOI] [PubMed] [Google Scholar]
- 82.Audu M.L., Lombardo L.M., Schnellenberger J.R., Foglyano K.M., Miller M.E., and Triolo R.J. (2015). A neuroprosthesis for control of seated balance after spinal cord injury. J. Neuroeng. Rehabil. 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson K.D. (2004). Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.