Abstract
Purpose
Elevations in intraocular pressure (IOP) are associated with the development of glaucoma and loss of sight. High transforming growth factor-β (TGF-β) 1 levels in the eye’s anterior chamber can lead to dysfunctional contractions through RhoA signaling in trabecular meshwork (TM) cells and IOP spikes. Sustained high TGF-β levels leads to TM fibrosis and sustained increases in IOP. We investigated whether inhibiting RhoA, using a siRNA-mediated RhoA (siRhoA), controls IOP by altering TM expression of fibrosis and contractility-related proteins in a rodent model of glaucoma.
Methods
TGF-β was injected intracamerally twice a week into adult Sprague Dawley rats, and IOP was recorded with tonometry. Animals were euthanized on day 7 and 35 with TM expression of fibrosis and contractility-related proteins, as well as survival of retinal ganglion cells (RGCs) assessed with immunohistochemistry. siRNA against RhoA or enhanced green fluorescent protein (EGFP) was also injected intracamerally into select animals. Successful RhoA knockdown was determined with quantitative reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry, and the effects of the knockdown on the parameters above analyzed.
Results
TGF-β caused increased TM contractile proteins and IOP spikes by day 7, sustained increases in IOP from day 15, and TM fibrosis at day 35. siRhoA abolished the transient 7 day IOP rise but not the later sustained IOP increase (due to fibrosis). At 35 days, TGF-β-related RGC loss was not prevented with siRhoA treatment.
Conclusions
We conclude that RhoA signaling mediates the early IOP rise induced by TM cellular changes associated with contractility but not the sustained IOP elevation caused by TM fibrosis. Thus, RhoA therapies offer a clinically relevant opportunity for IOP management, likely through the modulation of TM contractility, but appear to be ineffective in the amelioration of fibrosis.
Introduction
Glaucoma refers to a group of optic neuropathies characterized by the selective and progressive degeneration of retinal ganglion cells (RGCs) with associated visual field defects. The most common form of glaucoma is chronic open angle glaucoma (OAG) in which the iridocorneal angle remains open, and intraocular pressure (IOP) elevation is caused by stiffening of the trabecular meshwork (TM) and associated ciliary muscles (CMs) due to the build-up of plaques and fibrosis in and around the drainage canals, reducing aqueous humor (AqH) outflow. Elevated IOP is the main modifiable risk factor for the development of glaucoma leading to RGC death and loss of vision [1].
Most AqH drains through the TM in the iridocorneal angle, into Schlemm’s canal (SC) and then the episcleral veins. Smooth muscle contractions between the TM and CMs mostly control AqH outflow through being able to alter the stiffness and tone of the TM [2]. The extracellular matrix (ECM) also determines outflow resistance through the juxtacanalicular region of the TM [3]. Pathologically high resistance to AqH outflow maybe due to the transformation of TM cells to myofibroblast-like cells [4] (leading to a loss of TM cell numbers) and from the development of sheath-derived plaques and increased ECM deposition within the TM, which all contribute to decreasing AqH outflow [5]. The sheath-derived plaques may be the more important cause of high outflow resistance that correlates with the severity of optic nerve (ON) damage [6]. In current clinical practice, IOP is reduced by (1) impeding AqH production using β-blockers [7], α-agonists, and carbonic anhydrase inhibitors [8] and increasing AqH outflow by either prostaglandin analogs or α-agonist treatment and surgery to reduce the resistance to AqH drainage, for example, by trabeculectomy [9]. Current medical treatments slow but often do not arrest disease progression, and surgery carries considerable risks. Therefore, there is an unmet clinical need for new therapies that target the disease etiology.
Transforming growth factor-β (TGF-β) 1 [10] and TGF-β2 isoforms induce TM and CM contraction and ECM deposition in patients with OAG, with levels significantly elevated in the AqH of patients with OAG [9] compared to control patients with cataract [11] or primary closed angle glaucoma [12,13]. In patients with secondary OAG, higher levels of the TGF-β1 isoform occur as seen most commonly in pseudoexfoliation glaucoma, while in those with primary OAG, the TGF-β2 isoform is raised. TGF-β1 and TGF-β2 induce ECM deposition in cultured Tenon’s capsule fibroblasts [14] and TM cells derived from patients with glaucoma [15] by signaling through the Smad pathway [16]. That expression of fibronectin and collagen (associated with reduced AqH outflow in vivo) is increased by these growth factors in a concentration-dependent manner as shown in human and porcine-derived TM cells [14,17]. TGF-β1 also induces ECM remodelling in the TM by altering the metalloproteinase (MMP) to tissue inhibitors of the MMP (TIMP) ratio to favor deposition of ECM [18,19].
Activation of the RhoA/Rho-kinase (ROCK) signaling pathway within TM cells induces actin filament contraction and polymerization through phosphorylation of LIM kinase-2 and myosin light chain (MLC) [20], causing the TM to contract (i.e., become more stiff) altering resistance to outflow in the TM. Inhibiting contractile machinery (i.e., reducing the stiffness) in TM cells has been shown to effectively lower IOP in human TM cells [21,22] and in rats [21] through its ability to enhance TM permeability by increasing intracellular spaces for AqH to flow more easily [23]. RhoA is a small G-protein activated by phosphorylation of guanosine diphosphate (GDP) into guanosine triphosphate (GTP) [24] after receptor binding by the ECM proteins laminin [25], collagen [26], and fibronectin [27] and by endothelin-1 [28], thrombin [29], and TGF-β [24,30]. When TGF-β binds receptor 1 (ALK5) on TM cells there is activation of a downstream signal transduction cascade so that RhoA-GTP and ROCK are activated. This is associated with raised expression of alpha smooth muscle action (α-SMA) and MLC-p and correlated with increased TM contractility, reduced AqH drainage, and a rapid IOP rise [31]. In rabbits, intracameral (IC) injection of the ROCK inhibitor Y-27632 reduces IOP, probably by reducing the RhoA signaling cascade leading to TM cell contraction [2].
The relative contributions of TGF-β/RhoA-induced TM cell contractility to the pathogenesis of OAG remains unclear [32], as does the potential of RhoA/ROCK inhibitors to affect the underlying disease process [33]. In the present study, we aimed to define the relative contributions of responsive TM contractility and fibrosis to the raised IOP and associated RGC death that occur in OAG, by measuring changes in a rat model that raises IOP through biweekly IC injections of TGF-β1 and evaluating the impact on IOP of local siRNA-mediated RhoA (siRhoA) knockdown.
Methods
TGF-β1 was purchased from Peprotech (Cat #100–21, London, UK). Nuclease stable siRhoA and enhanced green fluorescent protein–specified siRNA (siEGFP) were manufactured and provided as a generous gift by Quark Pharmaceuticals Inc. (Ness Ziona, Israel), and Quark Pharmaceuticals has all proprietary rights. All other reagents were purchased from Sigma (Poole, UK) unless otherwise specified.
Experimental design
All animal procedures were performed in accordance with guidelines described in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, the ARRIVE guidelines and UK Home Office Animals Scientific Procedures Act 1986 and approved by the University of Birmingham Animal Welfare and Ethical Review Board. Thirty-five Sprague Dawley rats weighing 180–200 g (Charles River, Kent, UK) were housed at 21 °C and 55% humidity under a 12 h:12 h light-dark cycle, given food and water ad libitum, and supervised by qualified staff. Anaesthesia was induced with 5% isoflurane 1.5 l/min with O2 (National Veterinary Supplies, Stoke, UK) and maintained at 3.5%. The design of each experimental component of the study is summarized in Table 1. The two time points of 7 days and 35 days were chosen based on previous studies demonstrating considerable RGC death after 30 days, as well as the observation that TGF-β injections could be stopped and pressure would remain high [34], suggesting that fibrosis has been established. The 7 day time point was chosen to represent an earlier time point to observe the early phase changes. At this time point, if TGF-β injections stops, the pressure returns to normal, suggesting the presence of a transient mechanism unlikely to be fibrosis.
Table 1. Experimental design.
| Number of rats | Treatment group (n=number of eyes/group) | Measured endpoints |
|---|---|---|
| 12 rats |
IV Vehicle (n=4)
5 ul IV siEGFP (20ng; n=4)
5 ul IV siRhoA (10ng; n=4)
5 ul IV siEGFP (15ng; n=4)
5 ul IV siRhoA (20ng; n=4)
5 ul IV siRhoA (30ng; n=4)
5 ul IV siRhoA (40ng; n=4) |
RT–PCR (to assess knockdown efficiency and the activation of innate immunity) |
| 3 Rats |
Intact control eyes (n=3) |
IHC (contractility & fibrosis) |
| 4 Rats |
3.5 ul IC of 5ng/µl TGFβ1 (n=4) |
7d IHC (contractility & fibrosis) |
| 3.5 ul IC 3.5ul PBS (n=4) |
7d IHC (contractility & fibrosis) |
|
| 4 Rats |
3.5 ul IC 5ng/µl TGFβ1 (n=4) |
35d IHC (contractility & fibrosis) |
| 3.5 ul IC PBS (n=4) |
35d IOP; IHC (contractility & fibrosis) |
|
| 6 Rats |
3.5 ul IC 5ng/µl TGFβ1+5ng/µl siRhoA (n=6) |
7d IOP; IHC (contractility & fibrosis) |
| IC 5 ng/µl TGFβ1+5ng/µl siEGFP (n=6) |
7d IOP; IHC (contractility & fibrosis) |
|
| 6 Rats | IC 5 ng/µl TGFβ1+5ng/µl siRhoA (n=6) |
35d IOP; IHC (contractility & fibrosis) |
| IC 5 ng/µl TGFβ1+5ng/µl siEGFP (n=6) | 35d IOP; IHC (contractility & fibrosis) |
Demonstration of RhoA knockdown by siRhoA
Rat retinas were used to demonstrate the potency of siRhoA knockdown in the eye, because TM tissues are small and difficult to isolate precisely. Therefore, increasing doses of siRhoA (10, 15, 20, 30, and 40 ng) based on previous work with other siRNAs of this type [35], dissolved in a final volume of 5 µl of PBS (1X; Potassium Phosphate monobasic-KH2PO4; 1 mM, Sodium Chloride-NaCl; 155 mM, Sodium Phosphate dibasic-Na2HPO4-7H2O; 3 mM, pH 7.4), were injected intravitreally using glass micropipettes. Animals were allowed to survive for 7 days before euthanasia by exposure to rising CO2 concentrations. The retinas were harvested, immediately frozen in RNAlater (Invitrogen, Paisley, UK) in liquid nitrogen, and stored at −80 ºC. Total RNA was extracted using TRIzol and purified according to the manufacturer’s instructions (Qiagen, Manchester, UK), and quantitative reverse transcription (RT)–PCR was performed. Briefly, 40 ng of the reverse transcribed cDNA template was mixed with Universal MasterMix and either the human 18S rRNA endogenous control probe or the relevant TaqMan Gene Expression Assay and amplified on an ABI PRIZM 7700 (all from Applied Biosystems, Foster City, CA) set at 95 ºC for 10 min, followed by 40 cycles of 95 ºC for 15 s and then 60 ºC for 1 min. PCR data were analyzed using the 2-ΔΔCt method. TaqMan gene expression assays were used to measure relative mRNA levels of genes, including rhoa, oas1, mx1, casp7, and ifit.
Ocular effects of siRhoA treatment in a rat glaucoma model
Eyes were injected IC twice a week. The eyes of eight rats were injected with 3.5 µl of activated TGF-β1 (5 ng/µl; n=4 eyes) and 3.5 µl of PBS (n=4 eyes). Four rats were euthanized at 7 days and four at 35 days. The eyes of an additional 12 rats were injected IC twice a week with a total volume of 7 µl consisting of either 3.5 µl of activated TGF-β1 and 3.5 µl of siEGFP (5 ng/µl; n=12) or 3.5 µl of activated TGF-β1 and 3.5 µl of siRhoA (5 ng/µl; n=12). Six of these 12 rats were euthanized at 7 days (referred to as the early phase), and six were euthanized at 35 days (referred to as the chronic phase). In addition, for the immunohistochemical (IHC) analysis, three intact eyes from three untreated rats were used as intact controls. The study was designed to confirm the effects of TGF-β1 on (i) IOP elevation and TM ECM production (previously reported elsewhere [34]) and contractile protein expression within the TM by comparing TGF-β-injected eyes with PBS-injected eyes and intact eyes and to assess the relative contribution of RhoA-mediated TM contraction during the early and chronic stages of TGF-β-induced IOP elevation.
IOP measurements
Immediately after induction of anesthesia, IOP was recorded using an iCare Tonolab rebound tonometer (iCare, Helsinki, Finland) as described previously [34]. Mean IOP values were derived from three consecutive sets of six recordings taken between 21:00 and 24:00 (to minimize circadian variability) from each eye immediately before each IC injection. Accurate tonometry readings are dependent on a healthy cornea, and in the present study, no thickening or calcifications were observed throughout the study. IOP readings are displayed as mean ± standard error of the mean (SEM).
Surgical procedures
At day 0, after the first IOP reading, the heads of anaesthetized rats were fixed in a stereotactic frame. The cornea was incised with a disposable 15 degree blade to create a self-sealing two-step incision as previously described [34]. The first and all subsequent IC injections were administered through the incision using a sterile glass micropipette, produced in-house from a glass capillary rod (Harvard Apparatus, Kent, UK).
Immunohistochemistry
Dissected eyes were prepared for immunohistochemistry (using antibodies listed in Table 2) after the rats were euthanized at 7 days and 35 days by exposure to rising CO2 concentrations, as described previously [34]. Briefly, animals were perfused intracardially with 4% paraformaldehyde (PFA; TAAB, Reading, UK). The eyes were removed and immersion fixed in 4% PFA for a further 2 h at 4 °C, then cryoprotected by sequential immersion in 10%, 20%, and 30% sucrose solution in PBS, and blocked up in optimal cutting temperature (OCT) embedding medium (Thermo Shandon, Runcorn, UK) in peel-away molds (Agar Scientific, Essex, UK). Radial eye cryosections were cut at 20 µm thick in a standard plane through the ON head on a cryostat (Bright Instruments Ltd, Huntingdon, UK), adhered on charged glass slides (SuperFrost Plus, Fisher Scientific, Pittsburgh, PA), and stored at −20 ºC. For subsequent analyses, sections were thawed at room temperature (RT), hydrated in PBS, permeabilized in 0.1% Triton X-100, washed in PBS, and encircled with a hydrophobic PAP pen (Immuedge pen; Vector Laboratories, Peterborough, UK). Non-specific protein binding sites were blocked by incubating sections in blocking buffer (75 µl; 0.5% bovine serum albumin (BSA; g/ml), 0.3% Tween-20, and 15% normal goat/donkey serum (Vector Laboratories, Peterborough, UK) in PBS) in a humidified chamber for 30 min at RT, drained, and incubated with primary antibodies (Table 2) diluted in antibody diluting buffer (ADB; 0.5% BSA, 0.3% Tween-20 in PBS) overnight at 4 ºC. Sections were then washed and incubated with Alexa Fluor 488 or Alexa Fluor 594 conjugated secondary antibodies (Table 2) diluted in ADB for 1 h in a hydrated incubation chamber at RT. The sections were washed in PBS, mounted in Vectorshield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Peterborough, UK), and stored at 4 ºC in the dark before the microscopic analysis. No immunoreactive (IR) staining was seen in the negative antibody controls in which the primary antibody was omitted (data not shown).
Table 2. Antibodies used for immunohistochemistry.
| Antigen | Dilution | Supplier | Catalogue no. |
|---|---|---|---|
| Laminin |
1:200 |
Sigma (Poole, UK) |
#L9393 |
| Fibronectin |
1:200 |
Sigma (Poole, UK) |
#F3648 |
| αSmooth Muscle Actin (αSMA) |
1:100 |
Sigma (Poole, UK) |
#A5228 |
| Phosphorylated Myosin Light Chain (MLCp) |
1:400 |
Abcam (Cambridge, UK) |
#ab2480 |
| RhoA |
1:200 |
Abcam (Cambridge, UK) |
#ab54835 |
| Brn3a |
1:100 |
Santa Cruz (Santa Cruz, CA) |
#SC-31984 |
| Mouse IgG (Fluor 488) |
1:400 |
Molecular Probes (Paisley, UK) |
#A-21202 |
| Rabbit IgG (Fluor 488) |
1:400 |
Molecular Probes (Paisley, UK) |
#A-21206 |
| Rabbit IgG (Fluor 594) |
1:400 |
Molecular Probes (Paisley, UK) |
#A-21207 |
| Goat IgG (Fluor 594) | 1:400 | Molecular Probes (Paisley, UK) | #A-11058 |
Microscopy and analysis
Immunostained eye sections were photographed at 20X magnification using a Zeiss Axioplan-2 fluorescent microscope equipped with an Axiocam HRc camera and Axiovision software (all from Carl Zeiss, Ltd., Hertfordshire, UK). All images were equivalently contrast-enhanced using Photoshop CS3 (Adobe Systems, Inc., San Jose, CA). Brn3a+ RGC were counted in 20 µm thick standard radial sections along a 250 µm linear region of the ganglion cell layer (GCL) at the same distance from either side of the ON head as validated previously in this glaucoma model and others [34,36]. As the TM has a very small tissue volume and is intimately related to the surrounding connective tissue, western blot and RT–PCR were inappropriate analyses for TM RhoA. RhoA expression and TM fibrosis, however, were quantified by measuring IR in the same standardized TM quadrant in all eyes using ImageJ software (National Institutes of Health, Bethesda, MD) to measure percentage changes in pixels compared to the threshold reference level of brightness in intact control eyes as previously described [34]. All image analysis was conducted by an operator masked to the group identifications.
Adult rat retinal cultures
As TM cells are difficult to isolate and culture, mixed adult rat retinal cultures were used as a representative ocular cell type to demonstrate that RhoA knockdown using siRhoA had no off-target effects, such as activating the innate immune response. Cultures were established from the dissected retinas of Sprague Dawley rats (180–200 g; Charles River). Briefly, retinal cells were dissociated into single cell suspensions using as Papain Dissociation kit, according to the manufacturer’s instructions (Worthington Biochemicals, Lakewood, NJ). Then 125,000 retinal cells were plated in eight-well chamber slides (Beckton Dickinson, Watford, UK) precoated with 100 µg/ml poly-D-lysine and 20 µg/ml laminin, in supplemented Neurobasal-A (Invitrogen). Cells were allowed to settle overnight and then transfected in a final volume of 300 μl of supplemented Neurobasal-A with increasing concentrations (10–100 μg/ml) of siRhoA or a T7-transcribed sip75NTR, an siRNA that shows statistically significant upregulation of the innate immune response [37] using Lipofectamine 2000 (Invitrogen) following the manufacturer’s protocol. Cells were transfected for 5 h and then topped up with fresh Neurobasal-A and incubated at 37 ºC and 5% CO2 for a further 3 days before extraction of total RNA for quantitative RT-PCR (qRT-PCR) analysis.
Statistical analysis
Statistical tests were performed using SPSS 21.0 (IBMM SPSS, Inc., Chicago, IL), and data were presented as mean±SEM. The Shapiro-Wilk test was used to ensure all data were normally distributed before a one-way ANOVA (ANOVA) was applied with a Tukey post-hoc test for IHC analysis. For the IOP data, generalized estimated equations were used to determine the statistical significance to accommodate for the measurement dates not always being the same between groups. A p value of less than 0.05 was considered statistically significant.
Results
siRhoA induces knockdown of RhoA expression in ocular tissues without inducing an immune response
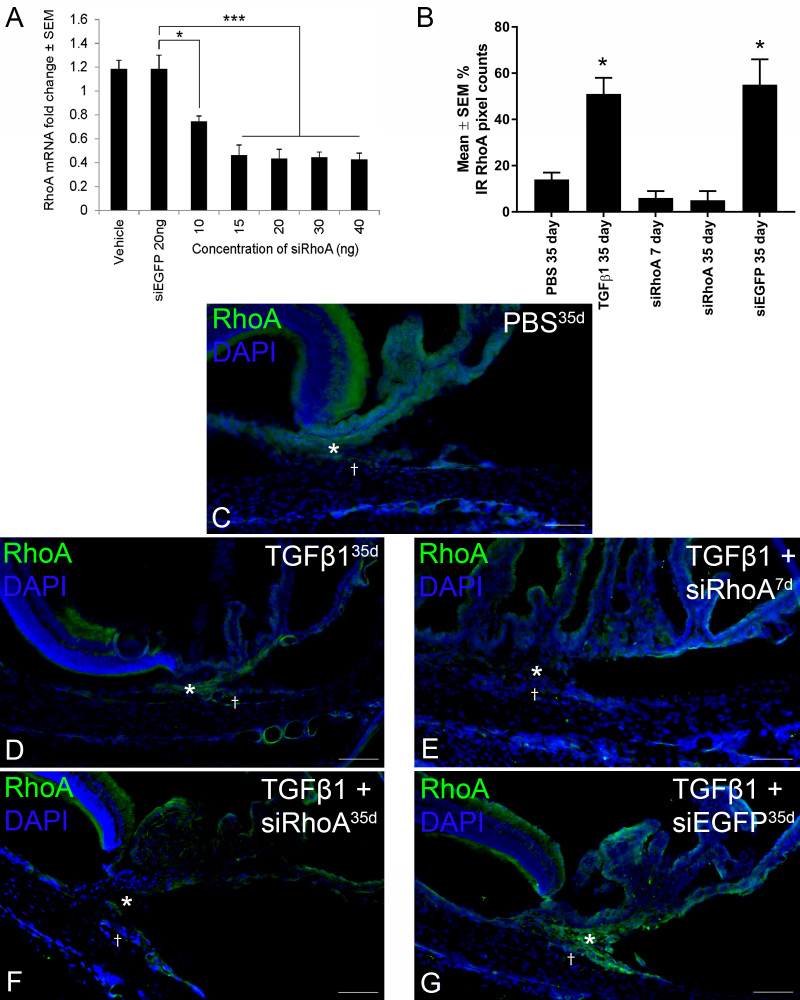
Due to the very small volume of TM, we first analyzed the knockdown of RhoA expression by siRhoA in whole eyes after intravitreal injection of siRNA. Increasing concentrations of siRhoA significantly knocked down endogenous RhoA mRNA extracted from retinas, with a maximum reduction observed with an effective dose of 15 ng of siRNA, where RhoA mRNA levels were reduced by 64% (Figure 1A). Knockdown efficiency did not increase further using siRhoA concentrations above 15 ng. This study guided our selection of 17.5 ng per injection for the in vivo TGF-β experiments.
Figure 1.
RT–PCR and immunohistochemistry demonstrate RhoA knockdown. A: Reverse transcription polymerase chain reaction (RT-PCR) shows a statistically significant (*p<0.05; ***p<0.0001) reduction in endogenous RhoA mRNA in eyes treated with increasing concentrations of siRhoA. The percentage immunoreactivity (IR) pixel counts of RhoA expression in the trabecular meshwork (TM) for the groups are defined in C–G. B: Note the statistically significant (*p<0.0001 difference from PBS/siRhoA) upregulation of RhoA protein in the TGF-β1-treated eyes and downregulation after knockdown with siRhoA. Radial rat eye sections immunohistochemically stained for RhoA (green) nuclear counterstaining with 4′,6-diamidino-2-phenylindole (DAPI) (blue) after intracameral (IC) injection with PBS for 35 days (C), TGF-β1 for 35 days (D), TGF-β1+siRhoA for 7 days (E) and 35 days (F), and TGF-β1+siEGFP for 35 days (G). Images were taken of the iridocorneal angle (C–G; *TM;†Schlemm’s canal). Note the successful knockdown of RhoA in the TM of the IC siRhoA-treated eyes (E and F). All images are representative of the eight images taken per treatment group (scale bar=50 µm).
In the mixed adult rat retinal cultures, the siRhoA at any of the concentrations tested between 50 and 100 μg/ml did not upregulate the expression of MX1 (Appendix 1 panel A), IFIT (Appendix 1 panel B), OAS1 (Appendix 1 panel C), or Casp7 (Appendix 1 Panel D), which are all markers of the innate immune response. However, a control T7 transcribed siRNA to p75NTR, which we have shown previously to upregulate the innate immune markers above [37] significantly increased MX1 (Appendix 1 panel A), IFIT (Appendix 1 panel B), OAS1 (Appendix 1 panel C), and Casp7 (Appendix 1 panel D) by ten- to 18-fold compared to the control and siRhoA-treated cultures. Taken together, these results show that siRhoA significantly knocks down RhoA expression in ocular tissues in vivo and in retinal cell cultures but does not induce an innate immune response at doses tested well above those used in the in vivo studies.
Intracameral siRhoA induces knockdown of RhoA protein in the TM
Pixel counts of the immunoreactivity (IR) of RhoA expression in the TM are shown (Figure 1B) demonstrating a relatively low abundance of RhoA protein in the control PBS-injected eyes (14±3.0% IR pixel count; Figure 1C) and statistically significant upregulation after IC TGF-β1 injection (51±7.0% IR pixel count; Figure 1D). Successful knockdown of RhoA IR was apparent in the TM at 7 days (6±3% IR pixel count; Figure 1E) and 35 days (5±4% IR pixel count; Figure 1F) after IC injections of TGF-β1+siRhoA when compared to TGF-β1+siEGFP (55±11% IR pixel count; Figure 1G). Although it cannot be assumed that the successful mRNA knockdown in the retina (Figure 1A) will translate into successful knockdown in the TM, combined with the reduced IR/pixel counts in the TM, siRhoA delivered IC over 35 days suppressed the expression of RhoA protein in the TM for the duration of the experiment. IC siRhoA treatment did not affect RhoA expression in the retinal GCL (data not shown).
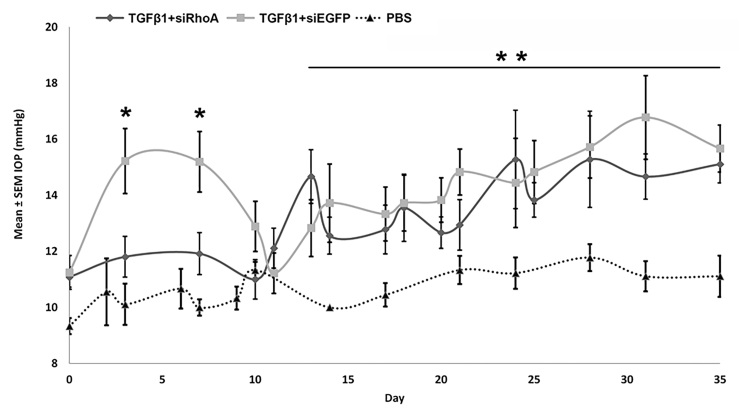
IC TGF-β1 injections induce an early and sustained rise in IOP, of which the first phase is RhoA dependent
In the TGF-β1+siEGFP-injected eyes, there was an early rise in the IOP between 3 and 7 days followed by a second sustained rise in the IOP appearing by 13 days and progressively incrementing up to 35 days (Figure 2). In the TGF-β+siRhoA-injected eyes, RhoA knockdown abolished the early rise in IOP between 3 and 7 days (p<0.05). The later raised IOP measurements between 13 and 35 days were unaffected by RhoA knockdown, which persisted throughout this time interval and was significantly different from that of the PBS-injected eyes which remained consistently low. RhoA signaling in the TM, therefore, mediates the initial IOP response to intracameral TGF-β injection but not the later sustained IOP elevation.
Figure 2.
IOP (mmHg) measurements in rat eyes receiving biweekly IC injections of TGF-β1+siRhoA, TGF-β1+siEGFP, and PBS. Note the statistically significant acute intraocular pressure (IOP) rise between 3 days and 7 days in the TGF-β1+siEGFP-injected group compared to the TGF-β1+siRhoA- and PBS-injected groups (*p<0.05) and the lack of a statistically significant difference between the TGF-β1+siRhoA- and the TGF-β1+siEGFP-injected eyes in the chronic phase (13 days to 35 days). The TGF-β1+siRhoA- and TGF-β1+siEGFP-injected eyes were statistically significantly different from control PBS-injected eyes in the chronic phase (13 days to 35 days;** p<0.05). TGF-β1+siEGFP intraocular pressure (IOP) values were not statistically significantly different from those in rats receiving TGF-β1 alone (IOP for TGF-β1 alone was previously reported [34]).
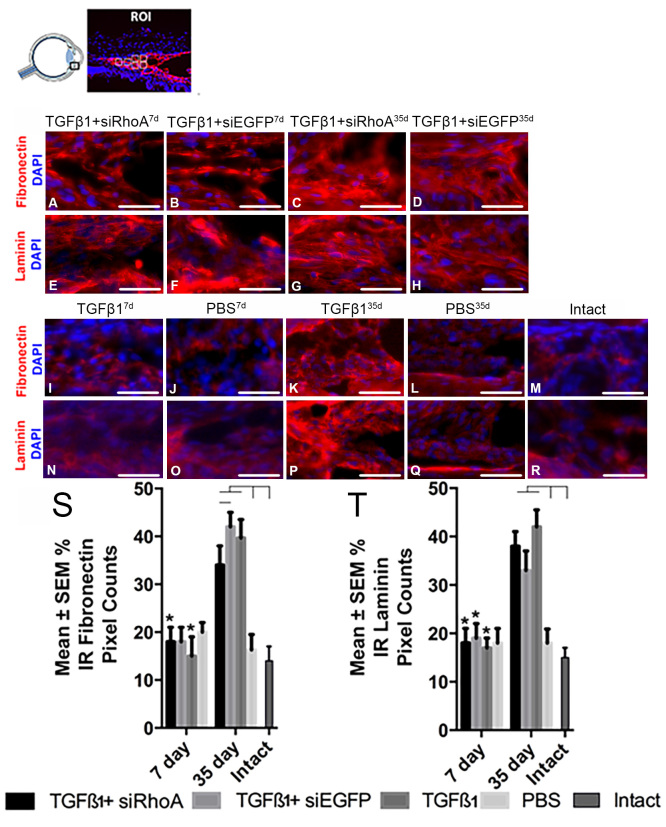
IC TGF-β1 injection increases fibrosis of the TM at 35 days but not at 7 days
The percentage above-threshold pixel counts in the TM of IR laminin and fibronectin were similar at 7 days in all groups indicating that TGF-β1-induced fibrosis was not established by this time point, despite the early phase IOP elevation onset by 3 days (Figure 3). However, at 35 days in the TGF-β1-, TGF-β1+siRhoA- and TGF-β1+siEGFP-injected eyes, IR laminin and fibronectin immunofluorescence was raised statistically significantly by 40% above those recorded in the PBS-injected and intact eyes and above that seen at the 7 day time point in any treatment group (Figure 3), with no detectable difference between the siRhoA and siEGFP eyes.
Figure 3.
Percentage of IR fibronectin and laminin pixel counts in the TM of radial eye sections. Images were taken of defined areas centered on the trabecular meshwork (TM) from radial sections of eyes that were immunohistochemically stained for fibronectin or laminin (red). Samples were assessed after intracameral (IC) injection with TGF-β1+siRhoA for 7 days (A and E), TGF-β1+siEGFP for 7 days (B and F), TGF-β1+siRhoA for 35 days (C and G), TGF-β1+siEGFP for 35 days (D and H), TGF-β1 for 7 days (I and N), PBS for 7 days (J and O), TGF-β1 for 35 days (K and P), and PBS for 35 days (L and Q), as well as untreated Intact eyes (M and R). All images are representative of eight images taken per retina from each treatment group (scale bar=50 µm). All sections were nuclear counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). The percentage immunoreactivity (IR) pixel counts in the TM are presented for fibronectin (S) and laminin (T) from the groups defined in A–R. Asterisks indicate the statistically significant differences at p<0.01 between the same treatments at 7 days and the 35 days, whereas the black lines indicate statistically significant differences at p<0.05 between groups at 35 days.
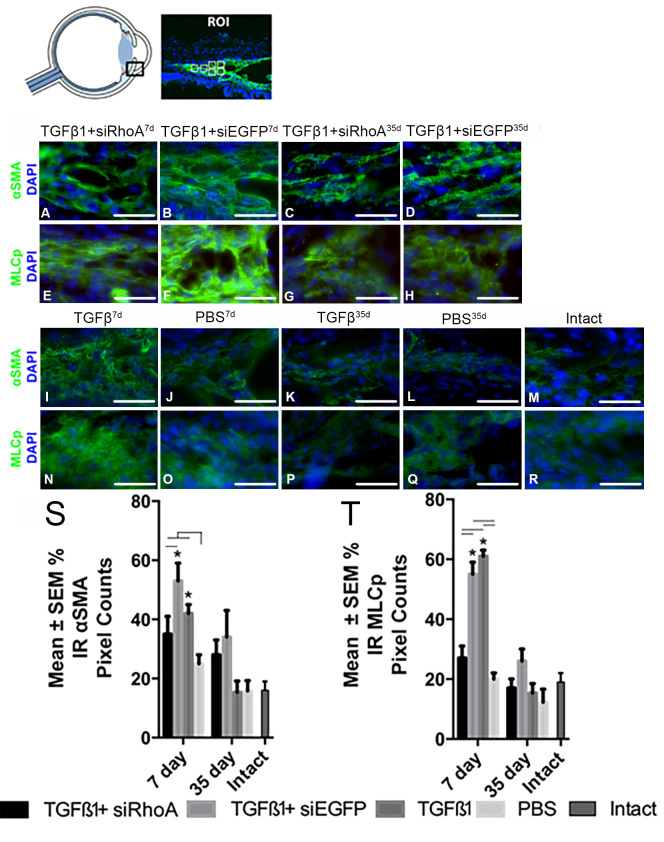
IC TGF-β1 injection induces an increase in TM contractility proteins in the TM at 7 days but not at 35 days
TGF-β1 injections increased IR α-SMA and MLC-p levels in the TM when compared to the levels in the PBS-injected and intact eyes at the 7 day time point but not at the 35 day time point. The injection of siRhoA abrogated the TGF-β-mediated increase in IR α-SMA and MLC-p at 7 days but not at 35 days, an observation that is consistent with increased TM contractility being an early response leading to acutely raised IOP. Accordingly, at 7 days, the IR α-SMA and MLC-p pixel counts were statistically significantly higher in the TM after the TGF-β1+siEGFP treatment compared to the TGF-β1+siRhoA-injected eyes (53±5.0% and 55±5.0% versus 35±5.0% and 27±5.0%, respectively; Figure 4). IC TGF-β1 induced significantly greater IR α-SMA and MLC-p levels in the TM compared to IC PBS alone (42% and 61%, respectively) at 7 days. At 35 days, the IR α-SMA and MLC-p pixel counts in the TM were statistically significantly lower when compared to 7 days in all TGF-β1+siRNA treatment groups and to the levels in the PBS-injected and intact eyes. Accordingly, at 35 days, the IR α-SMA and MLC-p pixel counts in the TM were 28±5.0% and 17±5.0% after TGF-β1+siRhoA treatment compared to 34±5.0% and 26±5.0% after TGF-β+siEGFP injections, respectively, values that were not statistically significantly different from each other. In addition, at 35 days, eyes receiving TGF-β1 alone demonstrated low IR α-SMA and MLC-p pixel counts in the TM (15±4.0% and 15±3.0%, respectively), as did the PBS-injected eyes (16±3.0% and 12±4.0%, respectively).
Figure 4.
Percentage of IR α-SMA and MLCp pixel counts in the TM of radial eye sections. Images are of defined areas centered on the trabecular meshwork (TM) taken from sections of radially sectioned eyes that were immunohistochemically stained for alpha smooth muscle action (α-SMA) and myosin light chain (MLC) p (MLCp; green). Samples were assessed from rats intracameral (IC) injected with TGF-β1+siRhoA for 7 days (A and E), TGF-β1+siEGFP for 7 days (B and F), TGF-β1+siRhoA for 35 days (C and G), TGF-β1+siEGFP for 35 days (D and H), TGF-β1 for 7 days (I and N), PBS for 7 days (J and O), TGF-β1 for 35 days (K and P), and PBS for 35 days (L and Q), as well as untreated Intact eyes (M and R). All images are representative of the eight images taken per retina from each treatment group (scale bar=50 µm). All sections were nuclear counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). The percentage immunoreactivity (IR) pixel counts in the TM are presented for α-SMA (S) and MLCp (T) from the groups defined in A–R. Asterisks indicate the statistically significant differences at p<0.05 between the same treatments at 7 days and 35 days, whereas the black lines indicate statistically significant differences (p<0.05) between groups at 7 days.
RhoA knockdown does not prevent the RGC death caused by TGF-β-induced raised IOP
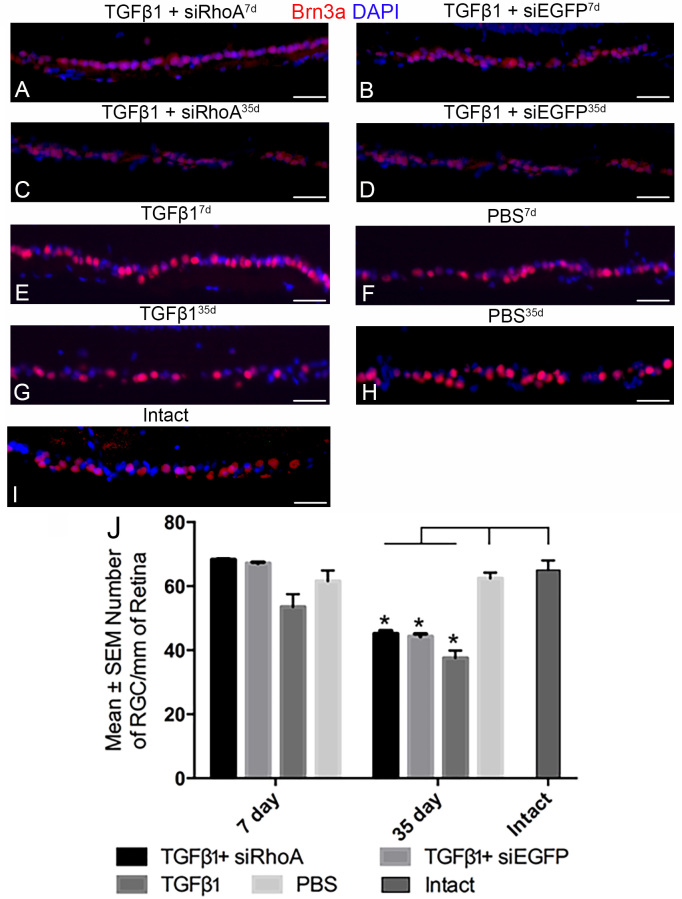
At 7 days, the RGC counts were not statistically significantly different among the Intact control, TGF-β1, TGF-β1+siRhoA, TGF-β1+siEGFP, and PBS treatments (65±3.0, 54±4.0, 68.3±0.20, 67.2±0.30, and 61.6±3.30 RGC/mm of the retina, respectively; Figure 5). At 35 days, there was equivalent and statistically significant RGC death (p<0.01) after the IC TGF-β1, TGF-β1+siEGFP, and TGF-β1+siRhoA injections when compared to all groups at 7 days, and to the 35 day PBS group and the Intact control group (38±2.3 after TGF-β1, 45.2±0.90 after TGF-β1+siRhoA, and 44.4±0.80 at 35 days after TGF-β1+siEGFP IC injections). There was no statistically significant difference in the RGC counts at 35 days between the TGF-β1+siRhoA- and TGF-β1+siEGFP-treated groups or between groups treated with TGF-β1 alone, suggesting that in this model RGC loss was initiated by the later and more sustained second phase of raised IOP.
Figure 5.
Brn3a+ RGC counts in radial eye sections. Standardized areas of the ganglion cell layer are shown in sections immunohistochemically stained for Brn3a (red) prepared from rats intracamerally (IC) injected with TGF-β1+siRhoA for 7 days (A), TGF-β1+siEGFP for 7 days (B), TGF-β1+siRhoA for 35 days (C), TGF-β1+siEGFP for 35 days (D), TGF-β1 for 7 days (E), PBS for 7 days (F), TGF-β1 for 35 days (G), and PBS for 35 days (H), as well as untreated Intact eyes (I). All images are representative of the eight images taken per retina from six different animals (scale bar=50 µm). All images are nuclear counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). In (J), the mean number of Brn3a+ RGC/mm of retina is presented with counts from the control PBS-treated eyes shown by a dashed line. Asterisks indicate the significantly lower (p<0.01) RGC counts in the 35 day groups compared to the counts made from their respective 7 day groups as well as the PBS-treated and Intact control groups.
Discussion
Knockdown of RhoA was initially confirmed in retinal tissues as extraction specifically of TM tissue is surgically difficult and available in limited amounts. For example, in the retina, 15–40 ng of siRhoA delivered intravitreally was sufficient to significantly reduce RhoA mRNA, a result that was similar to other siRNAs of this nature [35]. As it cannot be assumed that successful knockdown in the retina will translate to knockdown in the TM, effective RhoA knockdown was also confirmed with IHC after IC injections of siRhoA, illustrated by low levels of RhoA IR in the TM after siRhoA treatment, confirming that IHC can be used to qualitatively determine the knockdown of target genes [35,38].
In siRNA-based knockdown technologies, many studies have reported off-target effects and in particular, activation of the innate immune response by the siRNA itself or the delivery methods [39-42]. The siRNA structure, sequence, and delivery methods all contribute to activation of the innate immune response, leading to undesired effects and misinterpretation of results [43]. For example, interferon stimulated genes, such as OAS1 (Gene ID 192281, OMIM 164350), MX1 (Gene ID 24575, OMIM 147150), IFIT (Gene ID 56824, OMIM 147690), and Casp7 (Gene ID 64026, OMIM 601761), are typically upregulated in response to siRNA [44-47]. However, the siRhoA used in this study has been modified by proprietary methods (Quark Phamaceutical Inc., Israel) and has been made stable to resist nuclease degradation such that activation of interferon response genes is minimal or nonexistent. Therefore, the apparent effects of the siRhoA are effects mediated by target-specific knockdown of RhoA and not related to off-target effects, as has been observed for other similarly modified siRNAs [35,48].
An early and sustained rise in IOP was induced in IC TGF-β1-injected eyes (with or without siEGFP) characterized by an early rise in IOP over 4–7 days and then a gradual later rise in IOP over 8–35 days. The angle resembled that of OAG suggesting that the IOP increases induced were due to cellular effects of TGF-β and not to angle closure (IHC morphology data not shown). No early (1–7 days) spike in IOP was observed in the TGF-β1+siRhoA-treated eyes, but the later rises in IOP still developed over 8–35 days, confirming that RhoA mediates the early but not late TGF-β-induced rise in IOP. This suggests that the early phase IOP response to IC TGF-β1 is not associated with immunohistochemically detectable TM fibrosis, and the TM fibrosis seen by 35 days after IC TGF-β1 injections and associated with sustained IOP elevation is not mediated through RhoA-dependent signaling pathways. There was increased IR for the contractile proteins α-SMA and MLC-p in the TM of the TGF-β1- and TGF-β1+siEGFP-treated eyes during the early rise in IOP, which was suppressed by siRhoA. The TM IR for markers of fibrosis (laminin and fibronectin) at 35 days was associated with the chronic rise in IOP in the TGF-β1-, TGF-β1+siEGFP-, and TGF-β1+siRhoA-treated eyes when contractile protein IR was decreased. Similar numbers of RGCs died at 35 days in the TGF-β1+siRhoA- and TGF-β1+siEGFP-treated eyes, correlated with the consistent induction of the late rise in IOP by TGF-β1 despite the siRNA treatment.
An early and sustained rise in IOP and chronically increased production of ECM by human Tenon’s fibroblasts [14] has been experimentally induced by TGF-β1 and TGF-β2, although in pathology the predominant role of the former may be in inflammation [10], and of the latter in fibrogenesis as shown in other studies from scar tissue in the lesioned spinal cord [14,49]. Aberrant rises in IOP associated with perturbed AqH drainage and TM fibrosis occur in rat eyes after adenoviral transfection of TM cells with TGF-β2 [50,51] and after biweekly IC injection of TGF-β1 and TGF-β2 [34]. Adenoviral gene transfection of TGF-β2 in secreted protein acidic and rich in cysteine (SPARC) knockout mice demonstrated a SPARC-dependent IOP rise and upregulation of collagen IV and fibronectin levels after 8 days [50]. However, the key fibrogenic proteins, laminin, and collagen I/VI were not upregulated at this time point, suggesting that the major fibrotic responses do not develop until after 8 days. However, the TM fibrotic responses of mice and rats may differ as exemplified in the spinal cord of mice which express significantly more laminin than do rats at 8 days post-lesion [52].
The findings reported here are consistent with the observation that TGF-β induces an early phase of rapid activation of RhoA signaling in TM cells so that the actin cytoskeleton is mobilized to cause cellular changes associated with TM contraction and thus, alter the stiffness of TM permitting resistance. This is followed by a later phase in which RhoA becomes downregulated [53], suggesting that the acutely raised IOP, characteristic of early glaucomatous disease, may be RhoA-mediated and amenable to RhoA-targeted treatment. Furthermore, we have shown here and previously [34] that the later phase of IOP elevation seen after IC TGF-β1 is caused by TM fibrosis, but it seems that the initiation of TM fibrosis is multifactorial and therefore, is not solely reliant on RhoA signaling, as the initiation is not modulated by siRhoA knockdown highlighting a need for a multifactorial approach to treatment. It may be beneficial to target the contraction and stiffness of the TM and Schlemm’s canal with antifibrotic agents [54,55]. The results of the present in vivo study demonstrate that siRhoA treatment can suppress the early but not the later rise in IOP induced by elevated TGF-β levels. That is, in this model TGF-β initiates rapid rises in IOP through RhoA-induced cellular changes that are associated with TM contractility and maintains TM resistance by later initiating fibrosis. Cultures of human TM cells expressing a constitutively active form of RhoA express not only increased contractility markers (raised α-SMA and MLC-p levels) but also express fibrotic proteins (laminin and fibronectin) [56]. Expression of a constituently active form of RhoA in the AqH drainage pathway led to overexpression of not only contractility markers but also the fibrosis marker collagen. RhoA kinase inhibitors ameliorated the rise in collagen expression. However, IOP increased by only approximately 2 mmHg in their study suggesting that RhoA overexpression resulted in a small amount of fibrosis relative to other models [57]. We have demonstrated in rat eyes in vivo decreased fibronectin IR intensity at 35 days in the TGF-β1+siRhoA-treated eyes compared to the TGF-β1+siEGFP-treated eyes, suggesting a modest resolution of fibrosis from siRhoA treatment in this group only. Taken together with the laminin levels, however, which did not reduce with siRhoA treatment, we suggest that the TGF-β1-mediated fibrosis shown in this model is unresponsive to RhoA inhibition, suggesting other mechanisms lead to fibrosis or that, once fibrosis is established, RhoA inhibition alone is not sufficient to normalize TM function. Perhaps a combinatorial approach of RhoA inhibitors with antiscarring agents [34] would be beneficial to patients where fibrosis is present.
Rapid rises in IOP are induced by RhoA-mediated TM contraction in enucleated porcine eyes [31,56]. Others have shown upregulation of collagen mRNA over the first 3 days after IC TGF-β1 injection, when an early rise in IOP was also observed, with no associated increase in TM fibrotic protein levels, although mRNA changes are not always associated with protein changes and typically precede them [15,58-60]. Moreover, the transient fall in IOP observed between 8 days and 13 days described in this study, despite continuous TGF-β1 administration, suggests that TM fibrosis is not responsible for the acute phase rise in IOP.
Although undetected at 7 days, RGC death at 35 days was about 45% in all eyes associated with TGF-β1-induced chronically raised IOP, consistent with our previous work [34] and including those in which the early rise in IOP was abolished by siRhoA (TGF-β1+siRhoA- treated eyes). These results suggest that the progression of RGC death up to 35 days is causally related to the development of chronically raised IOP; i.e., that sustained raised IOP resulting from TM fibrosis is the major causal factor for RGC death in this model. RhoA is constitutively expressed in RGC somata and axons [61] and elevated in the optic nerve head in patients with glaucoma [62]. Although retinal RhoA knockdown by intravitreal siRhoA injections is RGC neuroprotective after optic nerve crush in rats [63], this effect was not seen in the TGF-β1-treated eyes that also received IC siRhoA, presumably because neuroprotective doses were not delivered to the GCL due to restricted anterior to posterior chamber transfer and remained within the TM as shown in a previous gene expression study [64]. This observation is consistent with preserved retinal RhoA IR despite immunohistochemically detectable knockdown in TM cells in the anterior segment after IC siRhoA injections. Our assessment of RGC survival used cell counting on sagittal eye sections, which we have previously demonstrated is an accurate method in this and other models of retinal injury [34,36]. However, the sensitivity of this method of assessing RGC survival means that it is unlikely to detect subtle differences in RGC numbers when compared to counts made in retinal whole mounts, and we cannot exclude a modest effect on RGC survival of intracameral siRhoA treatment in this model.
RhoA-mediated transient TM contractility is implicated in the genesis of diurnal variable and progressive day and night IOPs, reported to cause pathological glaucomatous changes [65-69]. Nocturnal spikes in IOP, in association with lowered nocturnal blood pressure, can cause retinal hypoperfusion episodes, optic nerve and retinal ischemia-reperfusion injury, and RGC loss. Of relevance, expression of dominant-negative RhoA in the TM lowers nocturnal IOP spikes in an animal model of glaucoma [70].
The efficacy of RhoA inhibitors currently used in clinical practice for lowering chronically elevated IOP in patients with glaucoma [71-73] suggests that a reversible element of TM contraction remains responsible for a proportion of IOP elevation even in established disease, in contradiction to our rodent model of stable fibrosis-induced IOP elevation, which lacks a reversible element amenable to treatment by RhoA knockdown. This indicates that there are aspects of the TM disease process not captured by its characterization as a TGF-β1-driven TM fibrosis etiology. Thus, more refined models of POAG pathogenesis may be required to fully assess the clinical impact of RhoA inhibitors. In addition, rats have different responses to IOP-lowering medications than humans, with prostaglandin analogs transiently elevating IOP [74], suggesting that outflow pathways and their control mechanisms may differ between species.
In summary, IC TGF-β induced an early and late rise in IOP by elevating TM resistance and reducing AqH drainage. The early rise in IOP over the 4–7 day period was caused by a RhoA-dependent mechanism with concurrent cellular changes associated with TM cell contraction, while the later rise in IOP was attributed to RhoA-independent TM fibrosis. RGC death was causally related to the late but not the early rise in IOP.
Acknowledgments
This study was funded by the Rosetrees Trust, a BBSRC studentship - grant number BB/F017553/1 and the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 749346. All siRNAs used were proprietary to Quark Pharmaceuticals Inc and were provided as a generous gift from the company. Grant information: Rosetrees Trust and BBSRC studentship, grant number BB/F017553/1. This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 749346.
Appendix 1. Supplementary Figure 1.
Reverse transcription PCR shows the expression of the innate immune response markers MX1 (A), IFIT (B), OAS1 (C), and Casp7 (D) in mixed adult rat retinal cultures after siRhoA treatment (0.5-100 μg/ml) in comparison to a positive control T7 transcribed siRNA to p75NTR. Statistical significance (p<0.001) is present at all concentrations of siRNA tested. To access the data, click or select the words “Appendix 1.”
References
- 1.Coleman AL, Miglior S. Risk Factors for Glaucoma Onset and Progression. Surv Ophthalmol. 2008;53:S3–10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Narumiya S, Honda Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–44. [PubMed] [Google Scholar]
- 3.Abu-Hassan DW, Acott TS, Kelley MJ. The Trabecular Meshwork: A Basic Review of Form and Function. Journal of Ocular Bio 2, http://fulltextarticles.avensonline.org/JOCB-2334-838-02-0017.html (2014). [DOI] [PMC free article] [PubMed]
- 4.Tamm ER, Braunger BM, Fuchshofer R. Intraocular Pressure and the Mechanisms Involved in Resistance of the Aqueous Humor Flow in the Trabecular Meshwork Outflow Pathways. Prog Mol Biol Transl Sci. 2015;134:301–14. doi: 10.1016/bs.pmbts.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–75. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Gottanka J, Johnson DH, Martus P, Lutjen-Drecoll E. Severity of optic nerve damage in eyes with POAG is correlated with changes in the trabecular meshwork. J Glaucoma. 1997;6:123–32. [PubMed] [Google Scholar]
- 7.Grieshaber MC, Flammer J. Is the medication used to achieve the target intraocular pressure in glaucoma therapy of relevance? – An exemplary analysis on the basis of two beta-blockers. Prog Retin Eye Res. 2010;29:79–93. doi: 10.1016/j.preteyeres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Lee AJ, Goldberg I. Emerging drugs for ocular hypertension. Expert Opin Emerg Drugs. 2011;16:137–61. doi: 10.1517/14728214.2011.521631. [DOI] [PubMed] [Google Scholar]
- 9.Denniston AKO, Murray PI. Oxford Handbook of Ophthalmology. (Oxford University Press, 2009). [Google Scholar]
- 10.Schlötzer-Schrehardt U, Zenkel M, Küchle M, Sakai LY, Naumann GO. Role of transforming growth factor-β1 and its latent form binding protein in pseudoexfoliation syndrome. Exp Eye Res. 2001;73:765–80. doi: 10.1006/exer.2001.1084. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan AA, Ozdemir N, Canataroglu A. The Aqueous Levels of TGF-β2 in Patients with Glaucoma. Int Ophthalmol. 2004;25:19–22. doi: 10.1023/b:inte.0000018524.48581.79. [DOI] [PubMed] [Google Scholar]
- 12.Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–13. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- 13.Min SH, Lee TI, Chung YS, Kim HK. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean J Ophthalmol. 2006;20:162–5. doi: 10.3341/kjo.2006.20.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kottler UB, Junemann AG, Aigner T, Zenkel M, Rummelt C, Schlotzer-Schrehardt U. Comparative effects of TGF-beta 1 and TGF-beta 2 on extracellular matrix production, proliferation, migration, and collagen contraction of human Tenon’s capsule fibroblasts in pseudoexfoliation and primary open-angle glaucoma. Exp Eye Res. 2005;80:121–34. doi: 10.1016/j.exer.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Ramsey KE, Stephan DA, Russell P. Gene and Protein Expression Changes in Human Trabecular Meshwork Cells Treated with Transforming Growth Factor-β. Invest Ophthalmol Vis Sci. 2004;45:4023–34. doi: 10.1167/iovs.04-0535. [DOI] [PubMed] [Google Scholar]
- 16.Sethi A, Jain A, Zode GS, Wordinger RJ, Clark AF. Role of TGFβ/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalmol Vis Sci. 2011;52:5251–9. doi: 10.1167/iovs.11-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Tripathi BJ, Tripathi RC. Modulation of pre-mRNA splicing and protein production of fibronectin by TGF-beta2 in porcine trabecular cells. Invest Ophthalmol Vis Sci. 2000;41:3437–43. [PubMed] [Google Scholar]
- 18.Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003;77:757–65. doi: 10.1016/s0014-4835(03)00220-3. [DOI] [PubMed] [Google Scholar]
- 19.Schlötzer-Schrehardt U, Lommatzsch J, Kuchle M, Konstas AGP, Naumann GOH. Matrix metalloproteinases and their inhibitors in aqueous humor of patients with pseudoexfoliation syndrome/glaucoma and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2003;44:1117–25. doi: 10.1167/iovs.02-0365. [DOI] [PubMed] [Google Scholar]
- 20.Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124:679–83. doi: 10.1242/jcs.064964. [DOI] [PubMed] [Google Scholar]
- 21.Luna C, Li G, Huang J, Qiu J, Wu J, Yuan F, Epstein DL, Gonzalez P. Regulation of trabecular meshwork cell contraction and intraocular pressure by miR-200c. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu M, Chen X, Wang N, Cai S, Li N, Qiu J, Brandt CR, Kaufman PL, Liu X. H-1152 effects on intraocular pressure and trabecular meshwork morphology of rat eyes. J Ocul Pharmacol Ther. 2008;24:373–9. doi: 10.1089/jop.2008.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–37. [PubMed] [Google Scholar]
- 24.Inoue T, Tanihara H. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res. 2013;37:1–12. doi: 10.1016/j.preteyeres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Lemons ML, Condic M. Combined integrin activation and intracellular cAMP cause Rho GTPase dependent growth cone collapse on laminin-1. Exp Neurol. 2006;202:324–35. doi: 10.1016/j.expneurol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Huveneers S, Danen EH. J. Adhesion signaling – crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–69. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 27.Peng F, Zhang B, Wu D, Ingram AJ, Gao B, Krepinsky JC. TGFβ-induced RhoA activation and fibronectin production in mesangial cells require caveolae. Am J Physiol Renal Physiol. 2008;295:F153–64. doi: 10.1152/ajprenal.00419.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao L, Dai Y, Zhang J. Mechanism of RhoA/Rho kinase activation in endothelin-1-induced contraction in rabbit basilar artery. Am J Physiol Heart Circ Physiol. 2002;283:H983–9. doi: 10.1152/ajpheart.00141.2002. [DOI] [PubMed] [Google Scholar]
- 29.Anwar KN, Fazal F, Malik AB, Rahman A. RhoA/Rho-Associated Kinase Pathway Selectively Regulates Thrombin-Induced Intercellular Adhesion Molecule-1 Expression in Endothelial Cells via Activation of IκB Kinase β and Phosphorylation of RelA/p65. J Immunol. 2004;173:6965–72. doi: 10.4049/jimmunol.173.11.6965. [DOI] [PubMed] [Google Scholar]
- 30.Peng F, Zhang B, Wu D, Ingram AJ, Gao B, Krepinsky JC. TGFbeta-induced RhoA activation and fibronectin production in mesangial cells require caveolae. Am J Physiol Renal Physiol. 2008;295:F153–64. doi: 10.1152/ajprenal.00419.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pattabiraman PP, Inoue T, Rao PV. Elevated intraocular pressure induces Rho GTPase mediated contractile signaling in the trabecular meshwork. Exp Eye Res. 2015;136:29–33. doi: 10.1016/j.exer.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pervan CL. Smad-independent TGF-beta2 signaling pathways in human trabecular meshwork cells. Exp Eye Res. 2017;158:137–45. doi: 10.1016/j.exer.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Wang SK, Chang RT. An emerging treatment option for glaucoma: Rho kinase inhibitors. Clin Ophthalmol. 2014;8:883–90. doi: 10.2147/OPTH.S41000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill LJ, Mead B, Blanch RJ, Ahmed Z, De Cogan F, Morgan-Warren PJ, Mohamed S, Leadbeater W, Scott RA, Berry M, Logan A. Decorin Reduces Intraocular Pressure and Retinal Ganglion Cell Loss in Rodents Through Fibrolysis of the Scarred Trabecular Meshwork. Invest Ophthalmol Vis Sci. 2015;56:3743–57. doi: 10.1167/iovs.14-15622. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed Z, Kalinski H, Berry M, Almasieh M, Ashush H, Slager N, Brafman A, Spivak I, Prasad N, Mett I, Shalom E, Alpert E, Di Polo A, Feinstein E, Logan A. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis. 2011;2:e173. doi: 10.1038/cddis.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mead B, Thompson A, Scheven BA, Logan A, Berry M, Leadbeater W. Comparative evaluation of methods for estimating retinal ganglion cell loss in retinal sections and wholemounts. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suggate EL, Ahmed Z, Read ML, Eaton-Charnock K, Douglas MR, Gonzalez AM, Berry M, Logan A. Optimisation of siRNA-mediated RhoA silencing in neuronal cultures. Mol Cell Neurosci. 2009;40:451–62. doi: 10.1016/j.mcn.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Morgan-Warren PJ, O’Neill J, de Cogan F, Spivak I, Ashush H, Kalinski H, Ahmed Z, Berry M, Feinstein E, Scott RA, Logan A. siRNA-Mediated Knockdown of the mTOR Inhibitor RTP801 Promotes Retinal Ganglion Cell Survival and Axon Elongation by Direct and Indirect Mechanisms siRTP801 Promotes RGC Survival and Axon Growth. Invest Ophthalmol Vis Sci. 2016;57:429–43. doi: 10.1167/iovs.15-17511. [DOI] [PubMed] [Google Scholar]
- 39.Marques JT, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- 40.Morral N, Witting SR. shRNA-induced interferon-stimulated gene analysis. Methods Mol Biol. 2012;820:163–77. doi: 10.1007/978-1-61779-439-1_10. [DOI] [PubMed] [Google Scholar]
- 41.Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L, McClintock K, MacLachlan I. Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation. Hum Gene Ther. 2008;19:991–9. doi: 10.1089/hum.2008.131. [DOI] [PubMed] [Google Scholar]
- 42.Sioud M. RNA interference and innate immunity. Adv Drug Deliv Rev. 2007;59:153–63. doi: 10.1016/j.addr.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Olejniczak M, Polak K, Galka-Marciniak PJ, Krzyzosiak W. Recent advances in understanding of the immunological off-target effects of siRNA. Curr Gene Ther. 2011;11:532–43. doi: 10.2174/156652311798192770. [DOI] [PubMed] [Google Scholar]
- 44.Bridge AJ, Pebernard S, Ducraux A, Nicoulaz A-L, Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet. 2003;34:263–4. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- 45.Read ML, Mir S, Spice R, Seabright RJ, Suggate EL, Ahmed Z, Berry M, Logan A. Profiling RNA interference (RNAi)-mediated toxicity in neural cultures for effective short interfering RNA design. J Gene Med. 2009;11:523–34. doi: 10.1002/jgm.1321. [DOI] [PubMed] [Google Scholar]
- 46.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, Preece JA, Logan A, Seymour LW. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005;33 doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sledz CA, Holko M, De Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–9. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 48.Vigneswara V, Berry M, Logan A, Ahmed Z. Pigment Epithelium-Derived Factor Is Retinal Ganglion Cell Neuroprotective and Axogenic After Optic Nerve Crush Injury. Invest Ophthalmol Vis Sci. 2013;54:2624–33. doi: 10.1167/iovs.13-11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lagord C, Berry M, Logan A. Expression of TGFbeta2 but not TGFbeta1 correlates with the deposition of scar tissue in the lesioned spinal cord. Mol Cell Neurosci. 2002;20:69–92. doi: 10.1006/mcne.2002.1121. [DOI] [PubMed] [Google Scholar]
- 50.Swaminathan SS, Oh D-J, Kang MH, Shepard AR, Pang I-H, Rhee DJ. TGF-β2–Mediated Ocular Hypertension Is Attenuated in SPARC-Null Mice. Invest Ophthalmol Vis Sci. 2014;55:4084–97. doi: 10.1167/iovs.13-12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shepard AR, Millar JC, Pang I-H, Jacobson N, Wang W-H, Clark AF. Adenoviral Gene Transfer of Active Human Transforming Growth Factor-β2 Elevates Intraocular Pressure and Reduces Outflow Facility in Rodent Eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–76. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- 52.Kundi S, Bicknell R, Ahmed Z. Spinal cord injury: current mammalian models. Am J Neurosci. 2013;4:1–12. doi: 10.1016/j.neures.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–14. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012;23:135–43. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamer WD, Braakman ST, Zhou EH, Ethier CR, Fredberg JJ, Overby DR, Johnson M. Biomechanics of Schlemm’s canal endothelium and intraocular pressure reduction. Prog Retin Eye Res. 2015;44:86–98. doi: 10.1016/j.preteyeres.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–63. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pattabiraman PP, Rinkoski T, Poeschla E, Proia A. Challa, Rao P. RhoA GTPase-Induced Hypertension in a Rodent Model Is Associated with Increased Fibrogenic Activity in the Trabecular Meshwork. Am J Pathol. 2015;185:496–512. doi: 10.1016/j.ajpath.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perl K, Ushakov K, Pozniak Y, Yizhar-Barnea O, Bhonker Y, Shivatzki S, Geiger T, Avraham KB, Shamir R. Reduced changes in protein compared to mRNA levels across non-proliferating tissues. BMC Genomics. 2017;18:305. doi: 10.1186/s12864-017-3683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, Emili A, Xie XS. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–8. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berry M, Ahmed Z, Lorber B, Douglas M, Logan A. Regeneration of axons in the visual system. Restor Neurol Neurosci. 2008;26:147–74. [PubMed] [Google Scholar]
- 62.Goldhagen B, Proia AD, Epstein DL, Rao PV. Elevated levels of RhoA in the optic nerve head of human eyes with glaucoma. J Glaucoma. 2012;21:530–8. doi: 10.1097/IJG.0b013e318241b83c. [DOI] [PubMed] [Google Scholar]
- 63.Koch JC, Tonges L, Michel U, Bahr M, Lingor P. Viral vector-mediated downregulation of RhoA increases survival and axonal regeneration of retinal ganglion cells. Front Cell Neurosci. 2014;8:273. doi: 10.3389/fncel.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Q, Wu K, Qiu X, Yang Y, Lin X, Yu M. siRNA silencing of gene expression in trabecular meshwork: RhoA siRNA reduces IOP in mice. Curr Mol Med. 2012;12:1015–27. doi: 10.2174/156652412802480907. [DOI] [PubMed] [Google Scholar]
- 65.Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603–24. doi: 10.1016/s0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- 66.Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9:134–42. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Spaniol K, Schoppner M, Eter N, Prokosch-Willing V. Diurnal Fluctuations of Intraocular Pressure, Blood Pressure, and Ocular Perfusion Pressure in Glaucoma Patients. Klin Monatsbl Augenheilkd. 2015;232:773–8. doi: 10.1055/s-0034-1396317. [DOI] [PubMed] [Google Scholar]
- 68.Denis P. Effect of intraocular pressure and arterial blood pressure variations on glaucoma progression. J Fr Ophtalmol. 2004;xx:xx–xx. [PubMed] [Google Scholar]
- 69.Pache M, Dubler B, Flammer J. Peripheral vasospasm and nocturnal blood pressure dipping–two distinct risk factors for glaucomatous damage? Eur J Ophthalmol. 2003;13:260–5. doi: 10.1177/112067210301300304. [DOI] [PubMed] [Google Scholar]
- 70.Borrás T, Buie LK, Spiga MG, Carabana J. Prevention of nocturnal elevation of intraocular pressure by gene transfer of dominant-negative RhoA in rats. JAMA Ophthalmol. 2015;133:182–90. doi: 10.1001/jamaophthalmol.2014.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skaat A, Jasien JV, Ritch R. Efficacy of topically administered rho-kinase inhibitor AR-12286 in patients with exfoliation syndrome and ocular hypertension or glaucoma. J Glaucoma. 2016;25:e807–14. doi: 10.1097/IJG.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 72.Wang SK, Chang RT. An emerging treatment option for glaucoma: Rho kinase inhibitors. Clin Ophthalmol. 2014;8:883–90. doi: 10.2147/OPTH.S41000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rao PV, Pattabiraman PP, Kopczynski C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research. Exp Eye Res. 2017;158:23–32. doi: 10.1016/j.exer.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Husain S, Yates PW, Crosson CE. Latanoprost-induced changes in rat intraocular pressure: direct or indirect? J Ocul Pharmacol Ther. 2008;24:367–72. doi: 10.1089/jop.2008.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]