Abstract
Background:
In patients with heart failure (HF), malnutrition and dietary sodium excess are common and may worsen outcomes. No prior studies have provided low-sodium, nutritionally-complete meals following HF hospitalization.
Methods and Results:
The Geriatric OUt-of-hospital Randomized MEal Trial in Heart Failure (GOURMET-HF) study randomized patients discharged from HF hospitalization to four weeks of home-delivered sodium-restricted Dietary Approaches to Stop Hypertension meals (DASH/SRD; 1,500 mg sodium/day) vs. usual care. The primary outcome was the between-group change in the Kansas City Cardiomyopathy Questionnaire (KCCQ) Summary Score from discharge to four weeks post-discharge. Additional outcomes included changes in the KCCQ Clinical Summary Score and cardiac biomarkers. All patients were followed 12 weeks for death/all-cause readmission and potential diet-related adverse events (symptomatic hypotension, hyperkalemia, acute kidney injury). 66 patients were randomized 1:1 at discharge to DASH/SRD vs. usual care (age 71±8 years, 30% female, ejection fraction 39±18%). The KCCQ Summary Score increased similarly between groups (DASH/SRD 46±23 to 59±20 vs. usual care 43±19 to 53±24, p=0.38) but the KCCQ Clinical Summary Score increase tended to be greater in DASH/SRD participants (47±22 to 65±19 vs. 45±20 to 55±26, p=0.053). Potentially diet-related adverse events were uncommon; 30-day HF readmissions (11% vs. 27%, p=0.06) and days rehospitalized within that timeframe (17 vs. 55, p=0.055) trended lower in DASH/SRD participants.
Conclusions:
Home-delivered DASH/SRD following HF hospitalization appeared safe in selected patients, and had directionally favorable effects on HF clinical status and 30-day readmissions. Larger studies are warranted to clarify the effects of post-discharge nutritional support in patients with HF.
Clinical Trial Registration:
Keywords: diet, sodium, nutrition, readmission, elderly
Introduction
Patients hospitalized for acutely decompensated heart failure (HF) commonly suffer functional decline, rehospitalization, and death. With the advent of financial penalties from the Centers for Medicare and Medicaid Services (CMS) for excess 30-day readmissions, most U.S. hospitals have instituted formal programs to reduce preventable rehospitalizations in patients with HF 1. However, resulting declines in 30-day readmissions have plateaued, only 1/3 of readmissions are due to recurrent HF, and 30-day mortality has increased in recent years 2, 3.
Clearly, more effective strategies are needed to improve outcomes for patients with HF during the particularly vulnerable post-discharge period 4 and beyond. Dietary factors are thought to contribute to many HF hospitalizations 5, and dietary recommendations for patients with HF historically have focused on sodium restriction 6. Yet this strategy has been associated with increased readmission rates and even mortality in several large clinical trials 7, 8. One potential reason is that patients with HF instructed to eat less sodium may inadvertently worsen existing nutritional deficits 9. In addition, patients with HF often face significant barriers to following healthy eating patterns 10.
In light of these challenges, we conducted the Geriatric OUt-of-hospital Randomized MEal Trial in Heart Failure (GOURMET-HF) pilot study. GOURMET-HF is the first trial to test the effects of home-delivered, nutritionally complete, sodium-restricted meals in patients following discharge from HF hospitalization. We hypothesized that this strategy would improve disease-specific quality of life at four weeks post-discharge. Given previous studies demonstrating adverse events with a low-sodium diet, important additional goals of GOURMET-HF were to assess the safety of the intervention, including effects on cardiac biomarkers and rehospitalization burden.
Methods
Study design and population
The study design and methods of GOURMET-HF have previously been published 11. The data, methods used in the analysis, and materials used to conduct the research will not be made available to other researchers. In brief, GOURMET-HF was a randomized, controlled trial of 12 weeks total duration conducted at three sites (Michigan Medicine/University of Michigan, Ann Arbor Veterans Affairs Health System, both in Ann Arbor, MI, and Columbia University Medical Center/New York Presbyterian Hospital, in New York, NY). The trial was approved by the institutional review board at each site, and all enrolled participants gave written informed consent.
Patients ≥65 years of age having primary hospitalization for acute decompensated HF and meeting no exclusion criteria at hospital discharge (Table 1) were randomized to usual care vs. receiving four weeks of home-delivered meals. Due to slower-than-expected recruitment, the age of inclusion was later lowered to ≥55 years after discussion with NIH/NIA and the study Data and Safety Monitoring Board.
Table 1:
Inclusion and exclusion criteria
| Inclusion Criteria |
| Age ≥ 55 years old |
| History of systemic hypertension |
| Patient being discharged to home |
| Admission for acute decompensated HF, with criteria: |
| ≥ 1 symptom of HF: |
| • dyspnea, fatigue, orthopnea, PND |
| ≥ 2 signs of HF: |
| • pulmonary congestion on exam or chest x-ray, jugular venous distension, edema or rapid weight gain, elevated BNP (>100 pg/ml) |
| Change in medical treatment specifically targeting HF: |
| • diuretics, vasodilators, and/or neurohormonal agents |
| No other apparent cause of patient’s signs and symptoms |
| Exclusion Criteria |
| Hypotension during hospitalization: persistent systolic BP <100 |
| Hyperkalemia: |
| • ≥ 2 serum potassium > 5.0 mmol/L during hospitalization |
| • History serum potassium > 6.0 mmol/L |
| Severe anemia (hemoglobin < 9.0 g/dL) |
| Length of stay < 48 hours or > 14 days |
| Expected survival < 12 months |
| Active alcohol or substance abuse |
| Dementia or history of nonadherence to treatment |
| Exclusions at Discharge: |
| Blood pressure (persistent within 24 hr prior to discharge): |
| • Systolic BP > 180 mmHg or diastolic BP > 100 mmHg |
| • Hypotension (persistent systolic BP < 110 mmHg) |
| Need for intravenous inotropic therapy |
| Severe renal insufficiency (eGFR < 30 ml/min/1.73 m2) |
The primary outcome of GOURMET-HF was the inter-group change in the Kansas City Cardiomyopathy Questionnaire (KCCQ) Summary Score between discharge and four weeks post-discharge 12. The study diet (DASH/SRD) followed the Dietary Approaches to Stop Hypertension pattern, recommended for patients with hypertension 13, and was sodium-restricted to 1,500 mg/day, as per American Heart Association recommendations 14. Randomization was stratified by gender and by left ventricular ejection fraction <50% vs. ≥50%. Investigators were blinded to treatment assignment. Participants were assessed in-person at hospital discharge and at one, four, and 12 weeks post-discharge, and their care was otherwise managed by their usual medical team.
Physical examinations, blood pressure measurements, and blood samples for renal function and electrolytes were performed at a safety visit one week post-discharge. At this visit, the investigator could adjust HF therapies as needed to maintain clinical stability and/or stop the dietary intervention if potentially diet-related adverse events occurred. These parameters were assessed again, along with the KCCQ, at the four week visit. In participants who were rehospitalized at the time of their four week visit, the KCCQ was administered as an inpatient. In patients who were unable to attend their four week visit, the KCCQ was obtained by mail.
Intervention
The DASH/SRD contains higher intake of whole grains, fruits and vegetables, nuts and legumes, and lower intake of red meat and sweets than the typical American diet 15. As recommended by the National Kidney Foundation 16, the potassium content of the DASH/SRD in GOURMET-HF was reduced from 4,500 mg/day to 3,000 mg/day in patients with: 1) discharge estimated glomerular filtration rate (eGFR) <45 ml/min/1.73 m2, 2) discharge eGFR 45–60 and potassium >4.5 mmol/L, and/or 3) discharge potassium >4.5 mmol/L and potassium-sparing diuretic use.
Meals were prepared, packaged for refrigerator storage, and home-delivered once weekly by Mom’s Meals NourishCare (PurFoods LLC, Ankeny, IA), a commercial entity. Each week participants assigned to the study diet could choose their preferred menu items from a variety of options tailored to DASH/SRD specifications. Three daily meals, snacks, and some beverages were provided (see Supplementary Data for sample menus) for a daily calorie intake of 2,100. Both groups received a standardized educational pamphlet (see Supplementary Data) at hospital discharge with information on how to follow a sodium-restricted diet (standard of care advice at the three study sites). Meal delivery was paused for rehospitalization and resumed at hospital discharge.
Study outcomes
The KCCQ is a self-administered, 23-item instrument that assesses HF-related physical limitations, symptoms, self-efficacy, and social interference. The KCCQ Summary Score (primary outcome) and the individual KCCQ sub-domain scores range from 0–100, with higher scores indicating better quality of life. We also evaluated inter-group change in the KCCQ Clinical Summary Score, a composite of HF-related symptoms and physical limitations that is increasingly used as an endpoint in HF clinical trials 17.
Baseline dietary patterns and nutrient intake were assessed at hospital discharge using the 110-item Block Food Frequency Questionnaire as previously described 18. Adherence to the study diet was assessed by meal delivery records from PurFoods and review of three-day food diaries recorded during weeks one and four post-discharge. Overall adherence was defined as the proportion of the total meals consumed from the home-delivered study food. Cardiac biomarkers (high-sensitivity troponin I and BNP), prealbumin, and C-reactive protein were measured at hospital discharge and at week four. 24-hour urine collections were also performed for sodium and potassium excretion at these time points.
We identified symptomatic hypotension requiring urgent medical attention, hyperkalemia (serum potassium >5.7 mmol/L), and worsening renal insufficiency (defined as decrease in estimated glomerular filtration rate [eGFR] by >50%) as adverse events of special interest that could potentially be related to DASH/SRD. Readmissions, deaths, and the composite of post-discharge days hospitalized or dead through 12 weeks post-discharge were recorded for all participants.
Power calculations and statistical analysis
Pre-study power calculations assumed a standard deviation of the change in KCCQ Summary Score of 8 points and a 10% dropout rate. Based on a 2-sample t-test, 66 randomized participants (33 per group) would provide >80% power to detect a six-point difference between groups in the change in KCCQ Summary Score between discharge and week four 19.
All study outcomes were evaluated in an intent-to-treat analysis. Between-group differences in baseline characteristics were assessed using chi-square tests for dichotomous variables and 2-sample t-tests for continuous variables. Within-group discharge-to-week four changes in KCCQ Summary Scores were evaluated using paired t-tests. Between-group comparisons were made using linear regression with week four KCCQ as the outcome and with treatment group and discharge KCCQ as covariates. The KCCQ Clinical Summary Score, cardiac biomarkers, and other quantitative parameters were similarly evaluated, with log-transformation for B-type natriuretic peptide, high-sensitivity troponin I, and C-reactive protein due to skewed distribution. Missing data due to study withdrawals, missed visits, and a death reduced the sample size for several of the tests.
The probabilities over time since discharge of all-cause rehospitalization and HF rehospitalization were visualized using Kaplan-Meier curves, with between-group comparisons made using log rank tests. The frequencies of potentially diet-related adverse events were compared between groups with Fisher’s exact test. Inter-group comparison of the number of days hospitalized or dead between the index discharge and 30 days post-discharge was performed using 2-sample t-tests after log-x+1 transformation (i.e., in order to avoid zero values, adding one to the number of days for scale of 1–31, then log-transforming). Data were analyzed using SAS software (Cary, NC).
Results
A total of 107 patients were originally consented to participate in the study, and 66 were randomized 1:1 to DASH/SRD vs. usual care at hospital discharge. The flow of participants through the study is shown in Figure 1. The most common reasons for being consented but not randomized at hospital discharge were hypotension, hyperkalemia, and/or renal insufficiency during the index admission. Of the randomized participants, six (9%) did not complete the KCCQ primary outcome measure at week four, three due to formal withdrawal from active participation and three who did not attend the visit and declined to complete the questionnaire by mail. By week 12, one participant died and six others withdrew from active participation in the study. All 66 randomized participants, including those who withdrew from active participation, were followed for 12 weeks by telephone and medical record review for rehospitalization or death.
Figure 1:
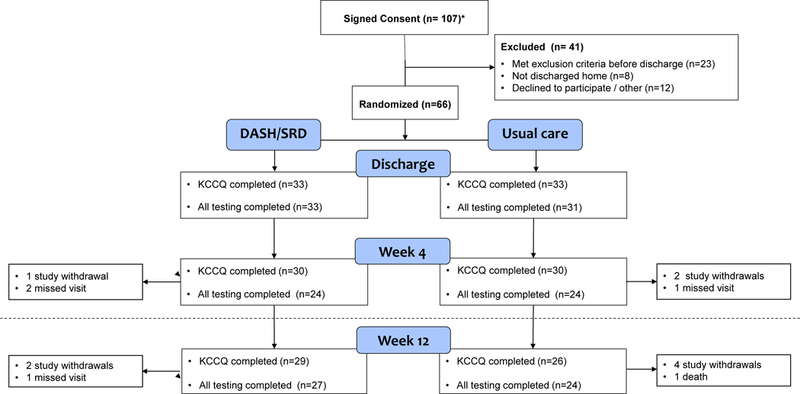
Study participant flow
Baseline characteristics of the study population are shown in Table 2. In general, randomized participants were older adults, frequently were obese, and had multiple comorbid illnesses. Renal dysfunction, anemia, and elevated levels of BNP and high-sensitivity troponin I were common. The enrolled cohort was approximately one-third female and of diverse race and ethnicity. Most patients (64%) had left ventricular ejection fraction <50%. There were no significant between-group differences in baseline characteristics.
Table 2:
Demographics and baseline characteristics
| Parameter | Overall cohort | Usual care | DASH/SRD | p value |
|---|---|---|---|---|
| Age (years) | 71±8 | 70±8 | 71±8 | 0.57 |
| Gender (% female) | 30% | 33% | 27% | 0.59 |
| BMI (kg/m2) | 32.6±7.7 | 34.0±7.9 | 31.3±7.1 | 0.15 |
| Race | ||||
| -White | 36 (55%) | 18 (55%) | 18 (55%) | 1.0 |
| -Black | 20 (30%) | 10 (30%) | 10 (30%) | |
| -Other | 10 (15%) | 5 (15%) | 5 (15%) | |
| Ethnicity | ||||
| - Hispanic | 24 (36%) | 11 (33%) | 13 (39%) | 0.61 |
| - Non-Hispanic | 38 (64%) | 22 (67%) | 20 (61%) | |
| Cardiovascular parameters | ||||
| -Systolic blood pressure (mmHg) | 124±20 | 121±20 | 126±21 | 0.37 |
| -Diastolic blood pressure (mmHg) | 72±11 | 71±11 | 72±11 | 0.84 |
| -Heart rate (beats/minute) | 76±17 | 80±19 | 74±14 | 0.16 |
| -Left ventricular ejection fraction | 39±18 | 39±18 | 39±18 | 0.91 |
| Comorbidities | ||||
| -Hypertension | 65 (98%) | 33 (100%) | 32 (97%) | 1.0 |
| -Diabetes mellitus | 37 (56%) | 20 (61%) | 17 (52%) | 0.46 |
| -Ischemic etiology of heart failure | 30 (45%) | 15 (45%) | 15 (45%) | 1.0 |
| Laboratory parameters | ||||
| -eGFR (ml/min/1.73 m2) | 54±17 | 55±18 | 53±17 | 0.66 |
| -Hemoglobin (g/dl) | 12.4±1.7 | 12.4±1.7 | 12.4±1.7 | 0.94 |
| -BNP (pg/ml)* | 188 (82, 580) | 190 (104, 604) | 176 (68, 501) | 0.43 |
| -hs-Troponin I (pg/ml) * | 148 (86, 253) | 138 (73, 275) | 148 (87, 230) | 0.80 |
Abbreviations: BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; hs-Troponin I, high-sensitivity troponin I
Values expressed as percentage, mean ± standard deviation, or median (interquartile range) for log-transformed variables
Statistical tests were performed on the log scale for BNP and hs-Tropopnin I due to skewness
In available Food Frequency Questionnaire data (n=57 participants) obtained during the index hospitalization, estimated energy intake was 1,602 (IQR 1,192–2,154) kcal/d, sodium intake was 2,987 (2,148–3,561) mg/d, and potassium intake 2,557 (1,911–3278) mg/d. There were no significant between-group differences in estimated calorie, sodium, or potassium intake. Energy and sodium intake were highly correlated (r=0.93, p<0.001).
Participants assigned to DASH/SRD received home-delivered meals for an average of 27±1 days post-discharge. Per review of three-day food diaries, available in 29 of 33 participants assigned to DASH/SRD, 77% of all meals consumed consisted of complete or partial home-delivered study meals. Information on the nutrient content of study meals is shown in the Supplementary Data. Compared with baseline intake, the study diet was ~50% lower in sodium content while providing ~25% more calories and a ~25–45% increase in potassium content. Medication changes between hospital discharge and week 4 were similar between groups (see Supplementary Data). There were no significant changes in urinary sodium or potassium excretion between discharge and week four in either group (see Supplementary Figure).
The baseline KCCQ Summary Score was similar to other previously reported cohorts at hospital discharge 20 and was not statistically different between groups (p=0.38). This was also the case with the KCCQ Clinical Summary Score (p=0.70). The KCCQ results are illustrated in Figure 2. The KCCQ Summary Score increased in both groups from hospital discharge to week four (DASH/SRD: 46±23 to 59±20, change 13±19; usual care: 43±19 to 53±24, change 10±16; both p<0.001). The mean increase in KCCQ Summary Score was three points greater in the DASH/SRD group, but this difference was not statistically significant (p=0.37). The KCCQ Clinical Summary Score also increased in both groups from hospital discharge to week four (DASH/SRD: 47±22 to 65±19, change 18±20; usual care: 45±20 to 55±26, change 10±18; both p<0.001). The mean increase in the KCCQ Clinical Summary Score was nine points greater in the DASH/SRD group, nearing but not achieving statistical significance (p=0.053). Results were similar in patients with left ventricular ejection fraction <50% vs. ≥50% (Supplementary Figure).
Figure 2: KCCQ Summary and Clinical Summary Scores.
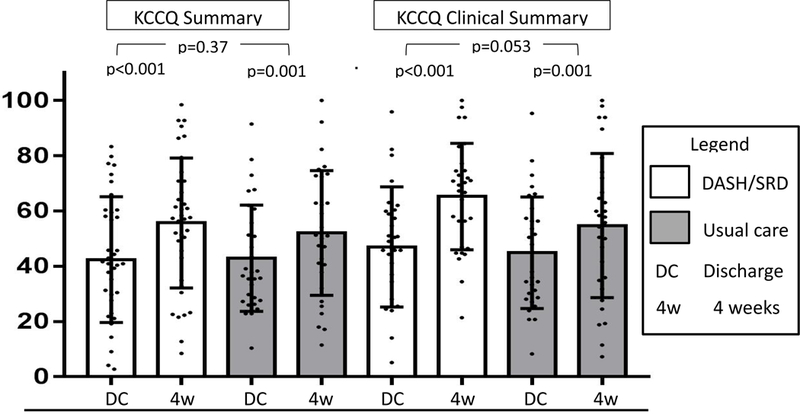
Abbreviations: DASH/SRD, sodium-restricted Dietary Approaches to Stop Hypertension eating pattern; KCCQ, Kansas City Cardiomyopathy Questionnaire
There were no deaths during the first 30 days post-discharge. In the DASH/SRD group at this time point four all-cause rehospitalizations had occurred in four patients, as compared to 12 total all-cause hospitalizations in nine participants in the usual care group (p=0.12). Over the same timeframe, in the DASH/SRD group three patients had three HF rehospitalizations, as compared to nine patients with a total of 11 HF rehospitalizations in the usual care group (p=0.055). Within the first 30 post-discharge days, the DASH/SRD group spent 17 cumulative days rehospitalized, as compared to 55 cumulative days in the usual care group (p=0.06 for between-group comparison after log-transformation of days hospitalized). This relationship, along with a Kaplan-Meier curve showing the probability of avoiding hospitalization or death over time since index hospital discharge, is shown in Figures 3a and 3b. By 12 weeks post-discharge, 11 DASH/SRD patients had 15 total all-cause rehospitalizations, while 14 usual care patients had a total of 22 all-cause hospitalizations and one death (p=0.45 for comparison). At 12 weeks there were eight HF rehospitalizations in seven DASH/SRD patients, as compared to 18 HF rehospitalizations in 13 usual care patients (p=0.11).
Figure 3: (A) Kaplan-Meier curve for freedom from hospitalization following index hospital discharge, (B) Boxplots by treatment group of days hospitalized from index discharge to day 30.
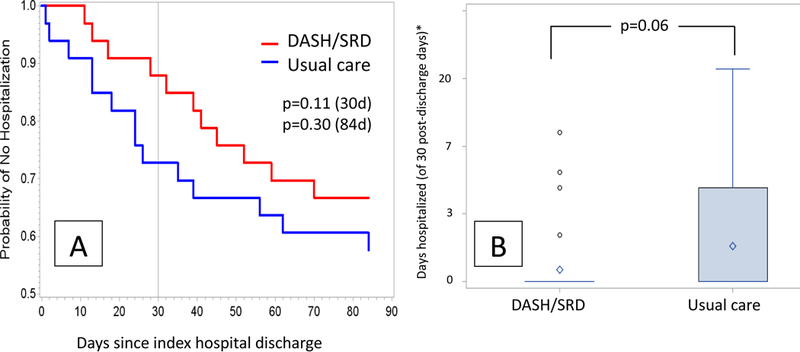
*Tick marks correspond to one-unit increase in log-days hospitalized, numbers on axis indicate days hospitalized rounded to the nearest day; diamond indicates mean, box inter-quartile range, whiskers 5–95%, and circles outliers
Changes in safety measures and serological biomarkers over the four-week intervention period in participants with paired samples at both time points are shown in Table 3. There were no significant changes in serum potassium, serum creatinine, or systolic blood pressure between discharge and week four in either group. B-type natriuretic peptide (BNP) levels were elevated, as expected in this cohort, and increased from baseline to week four in the DASH/SRD group while not changing in the usual care group. High-sensitivity troponin I decreased from baseline to week four in the DASH/SRD group, but not in the usual care group. Prealbumin levels increased in both groups; C-reactive protein levels decreased significantly in the control group, but not in the DASH/SRD cohort. There were no significant between-group changes in any of these parameters between discharge and week four.
Table 3:
Selected clinical and laboratory values (paired samples)
| Parameter | Usual Care | DASH/SRD | Between group difference DASH/SRD vs. Usual Care |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Discharge | Week 4 | p | Within group change | Discharge | Week 4 | p | Within group change | p |
Estimate (SE) or
Log Effect (%) |
|
| Systolic blood pressure (mmHg) | 123±22 | 123±22 | 0.99 | 0±20 | 126±22 | 121±19 | 0.17 | -5±18 | 0.46 | -3.6 (4.8) |
| Serum potassium (mmol/L) | 4.3±0.4 | 4.2±0.5 | 0.84 | 0.0±0.5 | 4.3±0.4 | 4.3±0.7 | 0.98 | 0.0±0.8 | 0.60 | 0.09 (0.16) |
| Serum creatinine (mg/dL) | 1.4±0.4 | 1.3±0.5 | 0.53 | 0.0±0.3 | 1.4±0.4 | 1.4±0.4 | 0.49 | 0.0±0.3 | 0.87 | 0.01 (0.08) |
| BNP (pg/mL)* | 190 (125,624) |
237 (143,917) |
0.44 | 61 (−94,133) |
176 (68,501) |
232 (112,606) |
0.041 | 36 (−13,169) |
0.59 | 11% higher* |
| hs-Troponin I (pg/mL)* | 162 (76,362) |
183 (76,347) |
0.84 | -7.5 (−30,76) |
150 (109,230) |
131 (74,219) |
0.025 | -21 (−65,31) |
0.23 | 20% lower* |
| C-reactive protein (mg/L)* | 14.2 (5.4,28.5) |
5.2 (2.8,9.2) |
0.001 | -6.6 (−19.3,−2.0) |
6.2 (4.6,14.3) |
4.9 (1.5,9.0) |
0.10 | -0.9 (−3.9,0.5) |
0.35 | 37% higher* |
| Prealbumin (mg/dL) | 21.2±6.3 | 25.9±8.3 | 0.008 | 4.7±7.1 | 23.2±6.6 | 25.6±6.5 | 0.025 | 2.5±5.2 | 0.36 | -1.6 (1.8) |
Abbreviations: BNP; B-type natriuretic peptide; hs-troponin I; high sensitivity troponin I. Within-group changes are Week 4 minus Discharge, with values expressed as mean ± standard deviation, or median (interquartile range) for log-transformed variables.
Statistical tests were performed on the log scale for BNP and hs-Tropopnin I and on the log(x+1) scale for C-reactive protein; all transformations were made due to skewness. Estimates are presented on the percent scale (approximate percent scale for C-reactive protein). Because confidence intervals are asymmetrical and thus SEs are not useful, we present 95% confidence intervals for estimates: BNP (−25% lower to 55% higher); hs-Troponin I (43%lower to 14% higher); C-reactive protein (29% lower, 162% higher)
Three potentially diet-related adverse events occurred in the DASH/SRD group within the first 30 days post-discharge (one each of presyncope, acute renal insufficiency, and hyperkalemia) and zero such events in the usual care group (p=0.24 for comparison by Fisher’s exact test). None of the events directly resulted in rehospitalization. The presyncopal episode occurred during an upper respiratory infection, and rapid heart rate was noted on home blood pressure monitoring. The participant sought medical attention three days later and was hospitalized for atrial fibrillation with rapid ventricular response. These events were judged unlikely related to diet. The acute renal insufficiency event was noted on post-discharge day one in a participant who was seen for concern of dehydration. The patient had not yet received study meals. Loop diuretics were discontinued, angiotensin-converting enzyme inhibitor was temporarily held, and renal function returned to baseline two days later. The hyperkalemia episode was identified at the week 1 safety visit and treated in the emergency department. The intervention was stopped by the blinded site primary investigator. After review of the event, the patient had been randomized to meals but mistakenly not been assigned by the study team to the lower-potassium version of DASH/SRD.
Discussion
The GOURMET-HF study is the first randomized trial to evaluate the safety and efficacy of home-delivered DASH/SRD meals post-discharge from HF hospitalization. Home delivery of meals was feasible, participants largely adhered to the study diet, and diet-related adverse events were uncommon. While not meeting its primary outcome, this pilot study demonstrated trends for efficacy in several domains important to recently hospitalized patients with HF and their providers. These outcomes included symptoms and physical limitations related to HF as well as rehospitalization burden.
Most previous dietary interventions in patients with HF have focused on sodium restriction as the primary intervention. In a series of such studies performed by Italian investigators, patients with HF and reduced ejection fraction were assigned at hospital discharge to dietitian-guided moderate vs. intensive sodium restriction (120 vs. 80 mmol, or 2,800 vs. 1,800 mg/day) at hospital discharge. Contrary to initial expectations, patients assigned to lower sodium intake had higher readmission and mortality rates 8, 21. These concerning findings were reinforced by the Heart Failure Adherence and Retention Trial (HART). In HART, 902 U.S. patients with HF randomized to self-management counseling vs. usual care self-reported their sodium intake with a questionnaire. Following propensity-matching, patients with estimated sodium intake <2500 mg/day had a 44% higher risk of HF hospitalization 7.
One possible reason for these results would be if dietary sodium restriction compromises overall nutritional status, an aspect not considered in the studies above. When assessed with validated screening instruments, the prevalence of malnutrition in patients hospitalized for HF is at least 15%, and is up to 90% in patients with advanced HF. Malnourishment has significant clinical consequences in hospitalized patients with HF, increasing length of stay and readmission rate while approximately tripling long-term mortality 22–24. In addition, malnutrition contributes to and overlaps with frailty, sarcopenia, and reduced mobility, each individually associated with poor outcomes in patients with HF 25–28. National survey data indicate that the correlation between sodium and calorie intake is ~0.7 in most U.S. populations 29. The substantially higher correlation in GOURMET-HF implies that patients with HF attempting to restrict sodium may consume insufficient calories for daily energy needs. Well-intentioned sodium restriction may also inadvertently contribute to micronutrient deficiencies in patients with HF 9, another risk factor for poor outcomes 30.
When compared with healthy older adults, patients with HF have additional factors affecting food intake and hence nutritional status. Mobility limitations, transportation difficulties, economic concerns, and symptoms such as dyspnea, nausea, anxiety, depression, and fatigue can greatly impact the motivation and ability to follow healthy eating patterns 10, 31. We hypothesized that home delivery of DASH/SRD meals during the particularly vulnerable post-discharge period 4 could overcome some of these challenges. The results of the GOURMET-HF pilot study suggest that this strategy is feasible and associated with a low rate of adverse events. Overall, there was no significant change in blood pressure, serum creatinine, or serum potassium in either group. In contrast to prior studies of sodium restriction post-discharge, we did not observe progressive neurohormonal activation or azotemia 21. This may be because medical therapy in GOURMET-HF was guided by clinical status rather than a standardized protocol, or could be related to the relatively short duration of the DASH/SRD intervention.
The GOURMET-HF study was not powered or intended to determine the effect of home-delivered meals on readmission rates. However, the trend toward fewer 30-day HF readmissions and total days rehospitalized in the DASH/SRD group is encouraging. These findings are consistent with previous literature both in plausibility and magnitude. In particular, the PICNIC study (Nutritional Intervention Program in Hospitalised Patients with Heart Failure who are Malnourished) provides important context. The PICNIC study randomized 120 patients discharged from HF hospitalization at two large Spanish hospitals to an intensive, team-based six-month nutritional intervention vs. usual care. At 12 months post-discharge, the combined mortality and HF rehospitalization in the nutritional intervention group was substantially lower (27 vs. 61%, p<0.001). These results were consistent across key subgroups and the number needed to treat was 2.5 to prevent one event; the survival curves began to separate within the first 30 days post-discharge and continued to diverge throughout the six-month intervention period 32. The Kaplan-Meier curves in GOURMET-HF (Figure 3a) diverge during the meal delivery period, but converge after this period, suggesting that a longer intervention might be needed for sustained benefits of nutritional support.
The GOURMET-HF results are supported by the recent Mediterranean DieT in Acute Heart Failure (MEDIT-AHF) analysis. In this prospective cohort study, Spanish patients hospitalized for acutely decompensated HF who were more adherent to a Mediterranean diet, which shares many characteristics with DASH, had a lower risk of death and rehospitalization for HF over the subsequent 12 months 33. Our findings are also generally aligned with large cohort studies indicating that the DASH dietary pattern is associated with reduced incidence of HF and improved long-term outcomes in HF 34–36.
The mechanisms underlying any potential benefits of post-discharge nutritional support with DASH/SRD in HF are not clear from our results. Although controversy exists, reduced sodium and increased potassium intake (as expected with DASH/SRD) have been generally associated with improved cardiovascular function and outcomes 37. Protein-calorie malnutrition, also potentially addressed by the study diet, is a strong risk factor for death and readmission in older patients hospitalized for HF 38, 39. We did not note a differential increase in prealbumin levels in the DASH/SRD group, but this commonly used biomarker may not be an accurate index of nutritional status during acute illness 40. Cardiac biomarkers did not demonstrate a clear trend, with BNP increasing and high-sensitivity troponin I decreasing in the DASH/SRD group and no change in either parameter in usual care patients.
Limitations
The standard deviation of the between-group change in KCCQ Summary Score was more than twice as large as predicted, reducing the power of the study for its primary outcome. In addition, the magnitude of the between-group change in KCCQ Summary Score (3 points) was not in a range considered clinically significant (typically >5 points). However, the magnitude of change in the DASH/SRD group was substantially greater in the domains most likely to respond to nutritional support, i.e. HF symptoms and physical limitations, and negligible in less directly related domains (general quality of life and social limitations).
In most cases, food diary records were sufficient to gauge the proportion of home-delivered meals consumed by participants assigned to DASH/SRD. We could not definitively analyze the nutrients consumed during GOURMET-HF participation, as some three-day food records had inadequate detail despite prompting from study personnel. Because of this limitation, we cannot provide direct comparison between the provided meals and the diet consumed post-discharge by usual care group participants.
We hypothesized that urinary sodium would decrease and urinary potassium would increase in the DASH/SRD group and serve as a supplementary measure of adherence, but did not find any changes in these parameters. Others have found poor reproducibility of 24-hour urine electrolytes over a one-month period in stable outpatients with HF 41, and this issue may be compounded by diuretic dose and administration route changes prior to discharge in hospitalized patients.
Any small treatment trial carries the risk of important between-group differences despite randomization. While there were no statistically significant differences in baseline characteristics, it is possible that the trend toward fewer hospitalizations relates to a lower inherent risk in the DASH/SRD cohort. Due to limited prior safety and efficacy data with the DASH diet in HF 42, 43, we enrolled only participants with a history of systemic hypertension, the focus of most prior DASH diet studies. While most patients with HF have a history of hypertension, our findings may not be generalizable to patients who do not have a history of hypertension.
The GOURMET-HF results are hypothesis-generating, and establish the rationale for conducting a larger study of direct dietary support in patients with HF following hospital discharge. Such a trial would ideally be powered to assess this strategy’s impact on hospital readmission burden. In addition, the complexity and costs of this intervention, as well as its feasibility and efficacy across patients of diverse demographics and socioeconomic status, would be important considerations in a future trial.
Conclusions
Home delivery of low-sodium, nutrient-dense meals following discharge from HF hospitalization is feasible and safe. While not meeting its primary outcome, the GOURMET-HF pilot study suggests that post-discharge nutritional support has the potential to improve HF symptoms and reduce readmissions. Larger studies are warranted to explore these concepts further.
Supplementary Material
What is new?
Dietary factors are believed to be an important cause of hospitalizations in patients with heart failure (HF), but few dietary interventions have been performed in this population.
Malnutrition is common in HF and associated with adverse outcomes, and the standard recommendation to restrict dietary sodium could contribute to dietary nutritional deficiencies.
The GOURMET-HF pilot study is the first randomized trial to examine the effects of direct dietary support in patients with HF following hospital discharge.
What are the clinical implications?
GOURMET-HF randomized patients with HF at hospital discharge to four weeks of home-delivered, low sodium, nutritionally complete meals vs. usual care.
Disease-specific quality of life (primary outcome) did not improve more in patients assigned to home-delivered meals.
However, secondary analyses suggest potential benefits of this strategy on HF symptoms, physical limitations, and readmission reduction.
Larger studies are warranted to clarify the effects of home-delivered meals on rehospitalization burden and quality of life in recently hospitalized patients with HF.
Acknowledgments
Sources of Funding
The GOURMET-HF pilot study was jointly funded by the National Institute of Aging/National Institutes of Health (R21-AG047939) and PurFoods, LLC (Ankeny, IA).
Footnotes
Disclosures
None.
References
- 1.Bradley EH, Curry L, Horwitz LI, Sipsma H, Thompson JW, Elma M, Walsh MN and Krumholz HM. Contemporary Evidence About Hospital Strategies for Reducing 30-Day Readmissions: A National Study. J Am Coll Cardiol. 2012;60:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonarow GC, Konstam MA and Yancy CW. The Hospital Readmission Reduction Program Is Associated With Fewer Readmissions, More Deaths: Time to Reconsider. J Am Coll Cardiol. 2017;70:1931–1934. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Aziz AA, Hayward RA, Aaronson KD and Hummel SL. Association between medicare hospital readmission penalties and 30-day combined excess readmission and mortality. JAMA Cardiology. 2017;2:200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krumholz HM. Post-Hospital Syndrome — An Acquired, Transient Condition of Generalized Risk. N Engl J Med. 2013;368:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalsen A, Konig G and Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. [See comments.]. Heart. 1998;80:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ and Wilkoff BL. 2013. ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 7.Doukky R, Avery E, Mangla A, Collado FM, Ibrahim Z, Poulin M- F, Richardson D and Powell LH. Impact of Dietary Sodium Restriction on Heart Failure Outcomes. JACC: Heart Failure. 2016;4:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterna S, Fasullo S, Parrinello G, Cannizzaro S, Basile I, Vitrano G, Terrazzino G, Maringhini G, Ganci F, Scalzo S, Sarullo FM, Cice G and Di Pasquale P. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study). Am J Med Sci. 2011;342:27–37. [DOI] [PubMed] [Google Scholar]
- 9.Jefferson K, Ahmed M, Choleva M, Mak S, Allard JP, Newton GE and Arcand J. Effect of a Sodium-Restricted Diet on Intake of Other Nutrients in Heart Failure: Implications for Research and Clinical Practice. J Card Fail. 2015;21:959–962. [DOI] [PubMed] [Google Scholar]
- 10.Lennie TA, Moser DK, Heo S, Chung ML and Zambroski CH. Factors influencing food intake in patients with heart failure: a comparison with healthy elders. J Cardiovasc Nurs. 2006;21:123–9. [DOI] [PubMed] [Google Scholar]
- 11.Wessler JD, Maurer MS and Hummel SL. Evaluating the safety and efficacy of sodium-restricted/Dietary Approaches to Stop Hypertension diet after acute decompensated heart failure hospitalization: design and rationale for the Geriatric OUt of hospital Randomized MEal Trial in Heart Failure (GOURMET-HF). Am Heart J. 2015;169:342–348 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauser K, Spertus JA, Pierchala L, Davis E and Pang PS. Quality of Life Assessment for Acute Heart Failure Patients From Emergency Department Presentation Through 30 Days After Discharge: A Pilot Study With the Kansas City Cardiomyopathy Questionnaire. J Card Fail. 2014;20:18–22. [DOI] [PubMed] [Google Scholar]
- 13.Eckel RH, Jakicic JM, Ard JD, Hubbard VS, de Jesus JM, Lee I- M, Lichtenstein AH, Loria CM, Millen BE, Miller NH, Nonas CA, Sacks FM, Smith SC, Svetkey LP, Wadden TW and Yanovski SZ. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013. [Google Scholar]
- 14.Appel L, Frohlich E, Hall J, Pearson T, Sacco R, Seals D, Sacks F, Smith S, Vafiadis D and Van Horn L. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the american heart association. Circulation. 2011;123:1138–1143. [DOI] [PubMed] [Google Scholar]
- 15.Karanja NM, Obarzanek E, Lin PH, McCullough ML, Phillips KM, Swain JF, Champagne CM and Hoben KP. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99:S19–27. [DOI] [PubMed] [Google Scholar]
- 16.Foundation NK. K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease. Am J Kidney Dis. 2004;43 (suppl 1):S1–S290. [PubMed] [Google Scholar]
- 17.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K and Zile MR. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 18.Mellen PB, Gao SK, Vitolins MZ and Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–14. [DOI] [PubMed] [Google Scholar]
- 19.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P and Spertus JA. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115:1975–81. [DOI] [PubMed] [Google Scholar]
- 20.Allen LA, Gheorghiade M, Reid KJ, Dunlay SM, Chan PS, Hauptman PJ, Zannad F, Konstam MA and Spertus JA. Identifying Patients Hospitalized With Heart Failure at Risk for Unfavorable Future Quality of Life. Circulation: Cardiovascular Quality and Outcomes. 2011;4:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paterna S, Gaspare P, Fasullo S, Sarullo FM and Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond). 2008;114:221–30. [DOI] [PubMed] [Google Scholar]
- 22.Lin H, Zhang H, Lin Z, Li X, Kong X and Sun G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev. 2016;21:549–565. [DOI] [PubMed] [Google Scholar]
- 23.Fingar KRWA, Barrett ML, Elixhauser A, Steiner CA, Guenter P ,Brown MH. All-Cause Readmissions Following Hospital Stays for Patients With Malnutrition, 2013. Rockville, MD: Agency for Healthcare Research and Quality; 2016. [PubMed] [Google Scholar]
- 24.Adejumo OL, Koelling TM and Hummel SL. Nutritional Risk Index predicts mortality in hospitalized advanced heart failure patients. J Heart Lung Transplant. 2015;34:1385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA and Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18:869–75. [DOI] [PubMed] [Google Scholar]
- 26.Dorner TE, Luger E, Tschinderle J, Stein KV, Haider S, Kapan A, Lackinger C and Schindler KE. Association between nutritional status (MNA®-SF) and frailty (SHARE-FI) in acute hospitalised elderly patients. The journal of nutrition, health & aging. 2014;18:264–269. [DOI] [PubMed] [Google Scholar]
- 27.Onoue Y, Izumiya Y, Hanatani S, Tanaka T, Yamamura S, Kimura Y, Araki S, Sakamoto K, Tsujita K, Yamamoto E, Yamamuro M, Kojima S, Kaikita K and Hokimoto S. A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int J Cardiol. 2016;215:301–6. [DOI] [PubMed] [Google Scholar]
- 28.Curtis JP, Rathore SS, Wang Y and Krumholz HM. The association of 6-minute walk performance and outcomes in stable outpatients with heart failure. J Card Fail. 2004;10:9–14. [PubMed] [Google Scholar]
- 29.Medicine Io. Sodium Intake in Populations: Assessment of Evidence. 2013. [PubMed]
- 30.Song EK, Moser DK, Kang SM and Lennie TA. Association of Depressive Symptoms and Micronutrient Deficiency With Cardiac Event-Free Survival in Patients With Heart Failure. J Card Fail. 2015;21:945–51. [DOI] [PubMed] [Google Scholar]
- 31.Monsivais P, Rehm CD and Drewnowski A. The DASH diet and diet costs among ethnic and racial groups in the united states. JAMA Internal Medicine. 2013;173:1922–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonilla-Palomas JL, Gámez-López AL, Castillo-Domínguez JC, Moreno-Conde M, López Ibáñez MC, Alhambra Expósito R, Ramiro Ortega E, Anguita-Sánchez MP and Villar-Ráez A. Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure. Arch Med Res. 2016;47:535–540. [DOI] [PubMed] [Google Scholar]
- 33.Miró Ò, Estruch R, Martín-Sánchez FJ, Gil V, Jacob J, Herrero-Puente P, Herrera Mateo S, Aguirre A, Andueza JA, Llorens P and Group I- SR. Adherence to Mediterranean Diet and All-Cause Mortality After an Episode of Acute Heart Failure: Results of the MEDIT-AHF Study. JACC Heart Fail 2018;6:52–62. [DOI] [PubMed] [Google Scholar]
- 34.Levitan EB, Lewis CE, Tinker LF, Eaton CB, Ahmed A, Manson JE, Snetselaar LG, Martin LW, Trevisan M, Howard BV and Shikany JM. Mediterranean and DASH Diet Scores and Mortality in Women with Heart Failure: The Women’s Health Initiative. Circ Heart Fail. 2013;6:1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levitan EB, Wolk A and Mittleman MA. Relation of consistency with the dietary approaches to stop hypertension diet and incidence of heart failure in men aged 45 to 79 years. Am J Cardiol. 2009;104:1416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitan EB, Wolk A and Mittleman MA. Consistency With the DASH Diet and Incidence of Heart Failure. Arch Intern Med. 2009;169:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aaron KJ and Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88:987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keenan PS, Normand S- LT, Lin Z, Drye EE, Bhat KR, Ross JS, Schuur JD, Stauffer BD, Bernheim SM, Epstein AJ, Wang Y, Herrin J, Chen J, Federer JJ, Mattera JA, Wang Y and Krumholz HM. An Administrative Claims Measure Suitable for Profiling Hospital Performance on the Basis of 30-Day All-Cause Readmission Rates Among Patients With Heart Failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. [DOI] [PubMed] [Google Scholar]
- 39.Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S and Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–701. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Pereira SL, Luo M and Matheson EM. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients. 2017;9:e829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basuray A, Dolansky M, Josephson R, Sattar A, Grady EM, Vehovec A, Gunstad J, Redle J, Fang J and Hughes JW. Dietary sodium adherence is poor in chronic heart failure patients. J Card Fail. 2015;21:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rifai L, Pisano C, Hayden J, Sulo S and Silver MA. Impact of the DASH diet on endothelial function, exercise capacity, and quality of life in patients with heart failure. Proceedings (Baylor University Medical Center). 2015;28:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovacs SJ and Kolias TJ. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013;6:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


