Abstract
Coronary artery anomalies and variants are relatively uncommon congenital disorders of the coronary artery anatomy and constitute the second most common cause of sudden cardiac death in young competitive athletes. The rapid advancement of imaging techniques, including computed tomography, magnetic resonance imaging, intravascular ultrasound and optical coherence tomography, have provided us with a wealth of new information on the subject. Anomalous origin of a coronary artery from the contralateral sinus is the anomaly most frequently associated with sudden cardiac death, in particular if the anomalous coronary artery has a course between the aorta and the pulmonary artery. However, other coronary anomalies, like anomalous origin of the left coronary artery from the pulmonary artery, atresia of the left main stem and coronary fistulae, have also been implicated in cases of sudden cardiac death. Patients are usually asymptomatic, and in most of the cases, coronary anomalies are discovered incidentally during coronary angiography or on autopsy following sudden cardiac death. However, in some cases, symptoms like angina, syncope, heart failure and myocardial infarction may occur. The aims of this article are to present a brief overview of the diverse coronary variants and anomalies, focusing especially on anatomical features, clinical manifestations, risk of sudden cardiac death and pathophysiologic mechanism of symptoms, as well as to provide valuable information regarding diagnostic workup, follow-up, therapeutic choices and timing of surgical treatment.
Keywords: Ectopic coronary arteries, Coronary artery anomalies, Coronary fistulae, Coronary artery variants, Myocardial bridging, Coronary artery anatomy, Sudden cardiac death
Core tip: Coronary artery anomalies and variants are a diverse group of congenital disorders of the coronary artery anatomy with a wide variety of clinical manifestations. Though relatively uncommon and usually discovered incidentally during coronary angiography, they have garnered interest because they are the second most common cause of sudden cardiac death in young competitive athletes. Though by no means entirely exhaustive, this overview aims to act as a guide for the practicing cardiologist along the complex web of these disorders and may facilitate the assessment, investigation, follow-up and treatment of patients diagnosed with or suspected of having a coronary artery anomaly.
INTRODUCTION
Coronary artery anomalies and variants are a diverse group of congenital disorders of the coronary artery anatomy with a wide variety of clinical manifestations. Though relatively uncommon and usually discovered incidentally during coronary angiography, they have attracted interest as they constitute the second most frequent cause of sudden death in young adults participating in competitive sports[1,2]. Though by no means entirely exhaustive, this overview aims to act as a guide for the practicing cardiologist along the complex web of these disorders and may facilitate the assessment, investigation, follow-up and treatment of patients diagnosed with or suspected of having a coronary artery anomaly. The majority of the figures presented are from our personal dataset.
It is important to have definite morphological criteria to describe normal coronary arteries. Angelini[3] has suggested criteria of normality of coronary anatomy, using several morphological features of the coronary arteries, such as number of ostia, location, course and branches. We also need to distinguish between normal variants of coronary anatomy and proper coronary anomalies. Anatomical features of the coronary arteries should be considered variants rather than congenital anomalies when they are prevalent in more than 1% of general population[4,5].
NORMAL CORONARY ANATOMY
Before venturing to explore the various coronary anatomy variations and anomalies, it is worth going through a brief overview of normal coronary anatomy. Normally there are two main coronary arteries, which stem from the sinuses of Valsalva and descend towards the cardiac apex. The left main stem (LMS) originates from the left sinus of Valsalva and crosses between the main pulmonary artery and the left atrial appendage. LMS has an average length of 2-4 cm and normally bifurcates into the left anterior descending artery (LAD) and the left circumflex artery (LCX). The right coronary artery (RCA) stems from the right sinus of Valsalva. The LAD descends towards the apex of the heart in the epicardial fat across the anterior interventricular sulcus. Its length varies between 10 and 13 cm and gives rise to diagonal and septal branches. It supplies the anterior wall, the apex and a significant portion of the interventricular septum. The LCX has a length of 5-8 cm and crosses the coronary sulcus on the diaphragmatic cardiac surface. It gives rise to the obtuse marginal branches and supplies mostly the lateral wall of the left ventricle. The RCA passes to the right between the pulmonary artery and the right auricle, descends across the right atrioventricular sulcus and continues posteriorly after the acute margin of the heart. Normal length is 12-14 cm. During its course, it may give rise to several branches, like the conus branch, sinoatrial branch, right ventricular branch, atrioventricular nodal branch, posterior descending branch (PDA) and posterolateral branch. It can broadly be considered as the artery that supplies the right side of the heart[1,6]. According to Angelini[3], the coronary anatomy falls within the spectrum of normality when: (1) there are two to four coronary ostia located at the upper midsection of the anterior aspect of the right and left sinuses of Valsalva. This is because sometimes the LAD and LCX may have separate origins from the left sinus, whereas the RCA and the conus branch may have separate origins from the right sinus; (2) a proximal stem bifurcating into major arteries is present only in the left coronary system (LMS bifurcating into LAD and LCX); (3) coronary arteries originate from the aortic wall with an angle of 45°-90°, follow an extramural (subepicardial) course, provide adequate branches for the perfusion of myocardium and terminate at the capillary bed distally; and (4) RCA perfuses the right ventricular free wall, LAD perfuses the anteroseptal wall and the obtuse marginal branch of LCX perfuses the free wall of the left ventricle (essential perfusion territories).
CORONARY ARTERY VARIANTS
Based on the 1% rule mentioned above, frequent variations of the coronary arteries like those involving coronary dominance, take-off and course, supply of the inferior wall, the sinoatrial and atrioventricular nodes as well as the presence of a separate conus branch, a ramus intermedius branch and myocardial bridging fall within the limits of normality. The PDA usually arises from the RCA, in which case we have right coronary dominance (in 70% of the population); however, it may also arise form the LCX (left dominance - 10% of cases) or both (co-dominance - 20%)[7].
Supply of the inferior wall may also present several variations. The PDA may be very small or have an early take-off. In the case of a small PDA, perfusion of the inferior wall is usually provided by RCA, LCX and obtuse marginal branches. Occasionally LAD may be a very long vessel that goes beyond the apex of the heart and reaches the inferior interventricular groove, thus perfusing the apical inferior wall of the heart (wraparound LAD) (Figure 1)[8].
Figure 1.

Wrap-around left anterior descending artery. A, B: Angiographic views of a large left anterior descending artery that wraps around the apex of the heart and supplies blood to part of the inferior wall (arrows) in anteroposterior caudal (A) and cranial view (B). From the authors’ personal dataset.
The sinoatrial branch supplies the sinoatrial node and usually arises from the proximal RCA in 60% of cases (Figure 2). However, it may also arise from the proximal LCX or more rarely from the distal segment of either of these vessels[9]. The atrioventricular nodal branch that provides blood to the atrioventricular node usually arises from the distal segment of whichever artery is dominant, RCA (more frequently) or LCX[7].
Figure 2.
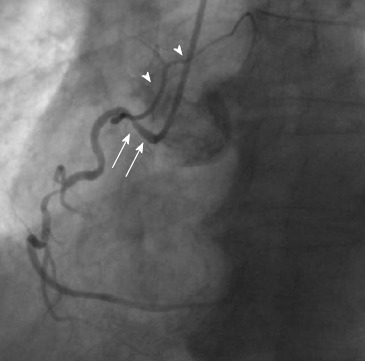
Shepherd’s crook right coronary artery. Arrows show the prominent ascending course of proximal right coronary artery segment and an equally prominent, almost 180° switchback turn (left anterior oblique view). Arrowheads show the sinoatrial branch. From the authors’ personal dataset.
Another frequent normal variant is the presence of a ramus intermedius branch. In this case, the LMS trifurcates into three branches instead of two as usual, the LAD, the LCX and the ramus intermedius branch. This branch usually takes the route of a diagonal or obtuse marginal branch and supplies the lateral and inferior walls of the heart[10].
Other normal variants include an acute take-off of the LCX, in which case there is an angle < 45° between the LMS and the LCX, probably caused by a distal point of origin of the LCX[11]. A “shepherd’s crook” RCA, in which the RCA follows a tortuous and high course with a prominent ascending course of its proximal segment and an equally prominent, almost 180° switchback turn (Figure 2), may comprise a technical challenge in interventional cardiology due to the poor support provided by the guiding catheters[7]. Other variants include separate origin of the conus branch directly from the right sinus of Valsalva[12], presence of a descending septal branch originating from the RCA that supplies part of the basal interventricular septum (Figure 3) and high take-off of a coronary ostium (5 mm or more above the aortic sinotubular junction), in which case the affected coronary artery (usually the RCA) takes an intramural course within the wall of the ascending aorta and is then externalized at its normal origin site[7,13-15].
Figure 3.

Descending septal branch. A branch of proximal right coronary artery (arrows) providing blood to part of the basal interventricular septum (left anterior oblique view with shallow cranial angulation). This branch may occasionally have a separate origin from the right sinus of Valsalva. From the authors’ personal dataset.
All aforementioned variants are clinically benign and pose no threat to patients. Usually they are incidental findings during coronary angiography performed for other reasons and do not require any diagnostic work-up, further investigation or treatment. At most, some of them, like the shepherd’s crook RCA, may present technical challenges during coronary intervention for other issues, due to difficulty in engaging angiographic catheters and guides. In addition, a descending septal branch, which originates from the RCA and supplies part of the interventricular septum, may be used as a target for alcohol septal ablation in symptomatic patients with hypertrophic obstructive cardiomyopathy whose basal septum is supplied by this branch of the RCA. In addition, a descending septal branch from the RCA may be an important source of collateral retrograde filling of a proximally occluded LAD.
MYOCARDIAL BRIDGING: ANOMALY OR VARIANT?
There is some confusion whether myocardial bridges constitute an anomaly or a normal variation. The prevalence of myocardial bridging varies between 0.15%-25% angiographically and 5%-86% at autopsy, and therefore its frequency in the general population suggests that it should be considered a normal variant[1,3,5,16]. A myocardial bridge is defined as an atypical course of a coronary artery intramyocardially, which may result in compression of the vessel during systole (Figure 4). It must be pointed out that myocardial bridging is a fixed defect that is entirely different to the dynamic phenomenon of coronary artery spasm. Coronary spasm entails intense vasoconstriction of an epicardial coronary artery due to hyper-reactivity of vascular smooth muscle cells and occasionally some degree of endothelial dysfunction in the presence of vasoconstrictor stimuli, leading to occlusion or near occlusion of the vessel and symptoms of Prinzmetal angina or acute coronary syndrome. Myocardial bridging usually involves the proximal and mid segment of the LAD and the length of the intramyocardial segment ranges between 10 and 50 mm. Patients with myocardial bridging are usually asymptomatic and the condition is considered benign[17]. However, there have been reports of rare cases of myocardial bridging related to ischemia and atypical angina. Possible causes are endothelial dysfunction, a delay in the diastolic reopening of the intramyocardial segment of the artery that was compressed during systole and a deeper intramyocardial course of the bridge, as opposed to the more benign superficial myocardial bridges[18-20]. Endothelial dysfunction is frequent in cases of myocardial bridging and may be revealed by functional studies, like intracoronary acetylcholine challenge. Any kind of percutaneous intervention on myocardial bridges is not supported at present due to the risks involved (restenosis, crushing and fracture of the stent)[4,21].
Figure 4.
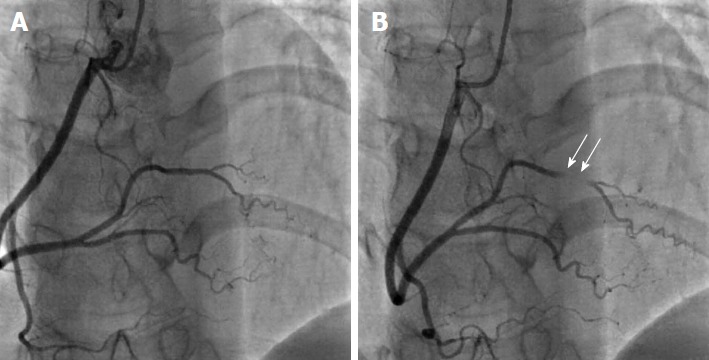
Myocardial bridging. A: Anteroposterior view with cranial angulation shows normal appearance of right coronary artery in diastole; B: Complete obliteration of a short segment of the posterolateral branch in systole (arrows) due to myocardial bridging. From the authors’ personal dataset.
The presence of myocardial bridging in patients with hypertrophic cardiomyopathy (HCM) is a point of contention both in terms of clinical significance and therapeutic management. Myocardial bridging is much more frequent in patients with HCM compared to the general population, with a prevalence of up to 30%[22]. In HCM patients, myocardial bridging tends to be of the deep intramyocardial course of the LAD variety, but it does not seem to affect the prognosis and risk of sudden death in adults[22-24]. Therefore, in adult asymptomatic patients with HCM, myocardial bridging appears to be a benign condition that does not warrant any treatment. However, the issue remains open regarding younger and/or symptomatic patients with HCM. Myocardial bridging has not been ruled out as a possible cause of ischemia and sudden death in younger individuals with HCM and may be associated with angina symptoms in adult patients. Provided there is a positive test for functional ischemia in the LAD territory, treatment with stent implantation, coronary artery bypass surgery or surgical unroofing via supra-arterial myotomy may improve quality of life in adult symptomatic patients with HCM and documented myocardial bridging, as well as reduce risk of sudden cardiac death and alleviate symptoms in younger patients[23].
CLASSIFICATION OF CORONARY ARTERY ANOMALIES
Several classification schemes have been proposed for coronary artery anomalies. Based on clinical significance some authors categorize them as major and minor. Based on functional relevance they can be classified as: (1) anomalies that are associated with definite ischemia (for example, anomalous origin of the LMS from the pulmonary artery or atresia of a coronary artery); (2) anomalies that are not associated with ischemia (for example, separate ostia of LAD and LCX with absence of LMS, split LAD or split RCA); and (3) anomalies with exceptional ischemia (like anomalous origin of coronary artery from the contralateral sinus and coronary fistulae). However, the most detailed and accurate classification has been proposed by Angelini[3] and is based on anatomical features. According to this classification, coronary anomalies can be characterized as: (1) anomalies of origination and course of coronaries (separate ostia of LAD and LCX, anomalous location of coronary ostia within the aortic root, outside the sinuses of Valsalva or at the contralateral sinus, single coronary artery); (2) anomalies of intrinsic coronary anatomy (split RCA/LAD, ostial stenosis/atresia, ectasia, hypoplasia, intramural course/bridging, absent coronary artery, ectopic origin of first septal branch, ectopic origin of PDA from the LAD or a septal branch); (3) anomalies of coronary termination (mainly fistulas); and (4) anomalous collateral vessels.
Table 1 summarizes the most common variations, aberrations and anomalies for coronary anatomy, based on anomalies of origin, course, intrinsic coronary anatomy and termination.
Table 1.
Classification of coronary artery variants, aberrations and anomalies
| Variations/anomalies of origin and course |
| 1 Separate ostia of LAD and LCX (absent left main stem) |
| 2 Separate ostium of conus branch |
| 3 Anomalous location of coronary ostium |
| Within sinuses of Valsalva |
| High, low or commissural |
| From posterior ("non-coronary") sinus |
| Outside sinuses of Valsalva |
| Ascending aorta, aortic arch, innominate artery |
| Descending aorta |
| Bronchial arteries |
| Pulmonary arteries |
| Anomalous origin from contralateral sinus |
| 4 Single coronary artery |
| Variations/anomalies of intrinsic coronary anatomy |
| 1 Trifurcation of left main (presence of ramus branch) |
| 2 Wrap-around LAD (supplying apical inferior wall) |
| 3 Descending septal branch from RCA (supplying basal septum) |
| 4 Split RCA |
| 5 Split LAD |
| 6 Hypoplasia of coronary artery |
| 7 Atresia of coronary artery |
| 8 Ectasia of coronary arteries |
| 9 Myocardial bridging |
| Anomalies of coronary termination |
| 1 Fistulas |
| 2 Small number of arteriolar/capillary ramifications |
LAD: Left anterior descending artery; LCX: Left circumflex artery; RCA: Right coronary artery.
ANOMALIES OF ORIGINATION AND COURSE
Absence of LMS
Absence of the LMS (separate ostia of LAD and LCX) is the most common coronary anomaly, with an incidence ranging between 0.41%-0.67% (Figure 5). It is a benign anomaly that causes no hemodynamic impairment or ischemic consequences[15,25].
Figure 5.
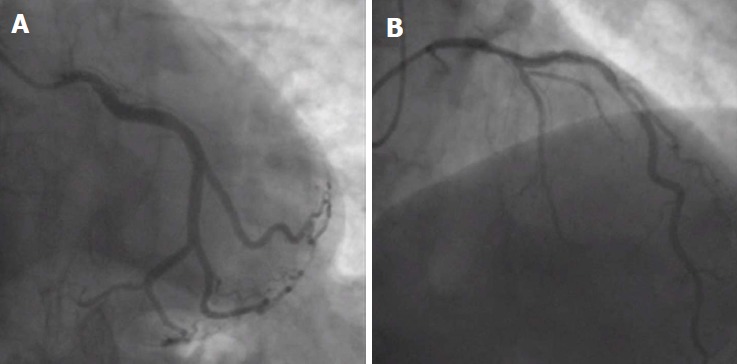
Absence of left main stem. A, B: Separate ostia of left circumflex artery (A, anteroposterior caudal view) and left anterior descending artery (B, shallow right anterior oblique view with cranial angulation) indicating a congenital absence of left main stem. From the authors’ personal dataset.
Anomalous location of coronary ostia
A coronary ostium may have an anomalous origin but may be still located within the proper coronary sinus; in those cases, the coronary ostium may originate from a higher position or a lower position compared to the normal site of origin, or may stem from the commissural level. On the other hand, when the anomalous coronary ostium is located outside the proper coronary sinuses, it can present in a wide variety of locations, like the non-coronary sinus of Valsalva, the ventricles and ectopic sites in the aorta or the large arteries (ascending or descending aorta, anonymous artery, carotid arteries), or even smaller arteries (bronchial arteries, internal thoracic artery, etc). Anomalous origin of a coronary artery from the contralateral sinus of Valsalva is particularly interesting from a clinical point of view, because these anomalies can be associated with sudden cardiac death, especially when the anomalous coronary artery crosses interarterially between the aorta and the pulmonary artery[26-30].
Anomalous origin of coronary ostium from contralateral sinus
A coronary artery that originates from the contralateral sinus of Valsalva can follow five potential paths towards its perfusion territory: (1) pre-pulmonic, anterior to the right ventricular outflow tract (usually benign, though rarely associated with angina); (2) retro-aortic, posterior to the aortic root (no hemodynamic consequences); (3) trans-septal, with a subpulmonic intramyocardial course; (4) retro-cardiac, behind the mitral and tricuspid valves, in the posterior atrioventricular groove; and (5) inter-arterial, between the aorta and the pulmonary artery[1-3].
The inter-arterial path has clinical significance, as it has been associated with an increased risk of sudden cardiac death, especially in young athletes[1-4,31,32]. The causes are not clear and several explanations have been proposed. In some cases, autopsy findings have shown an acute angle in the take-off of the anomalous coronary artery with a slit-like lumen and a proximal course between the aorta and the pulmonary trunk[2]. In other cases, histological examination has also shown an intramural course of the anomalous coronary within the aortic wall. It has been proposed that the acute angulation and slit-like ostium of the anomalous coronary vessel predispose to myocardial ischemia. Exercise-induced expansion of the aortic root and the pulmonary trunk compresses and exacerbates the slit-like ostium, resulting in further ischemia[4,6,33,34]. A scissors-like mechanism that compresses the anomalous coronary between the aorta and the pulmonary artery has also been suggested[3,35]. Intravascular ultrasound studies have demonstrated an intramural proximal intussusception of the ectopic artery at the aortic wall for a variable distance[36,37]. The intussuscepted segment of the vessel is smaller in circumference compared to its more distal segment (circumferential hypoplasia), and its cross-section is ovoid instead of circular. These parameters lead to a lateral luminal compression that is present throughout the whole of the cardiac cycle but even more pronounced during systole[3,37]. Furthermore, the intramural segment has thin inner and outer aortic wall layers. Therefore, the section of the aorta that is penetrated by the ectopic artery is a localized weak spot, prone to more extensive distensibility, which can further exacerbate stenosis and ischemia[37].
Ectopic RCA originating from the left sinus of Valsalva has a frequency of 0.03%-0.92% (Figure 6), and ectopic LAD arising from the RSV has a frequency of 0.03% (Figure 7)[1]. Both of these anomalies may be associated with an intramural inter-arterial course, in which case there is an increased risk of sudden cardiac death. Ectopic origin of the LCX from the right sinus of Valsalva (Figure 8) or from the proximal RCA is the second most common coronary artery anomaly, with a frequency of 0.37%[1,14,38,39], but it is considered benign and the course of the ectopic LCX is usually retroaortic or retrocardiac[4,15].
Figure 6.
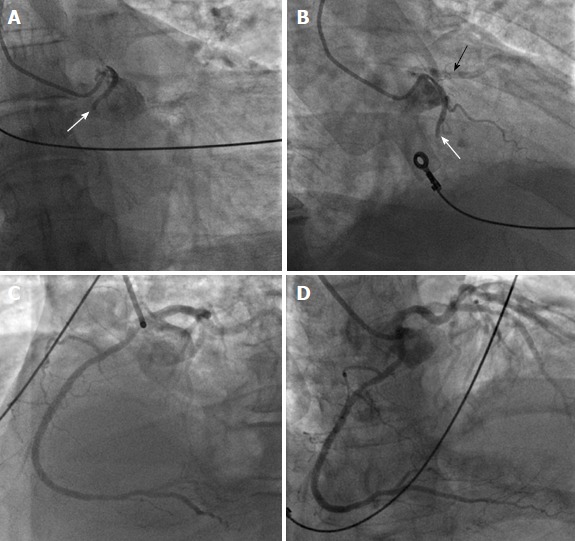
Ectopic right coronary artery originating from the left sinus of Valsalva. A, B: Proximal occlusion of anomalous right coronary artery (RCA) (white arrows) with origin from the left sinus of Valsalva in a patient with inferior wall ST elevation myocardial infarction (A: anteroposterior caudal view; B: right anterior oblique view). The black arrow in panel B shows a patent proximal left anterior descending artery in non-selective angiography of the left coronary artery (LCA); C, D: Left anterior oblique view with cranial angulation (C) and anteroposterior cranial view (D) show the LCA and an excellent final angiographic of the anomalous RCA after successful primary percutaneous coronary intervention. From the authors’ personal data and from Ref.[73] (modified with permission).
Figure 7.
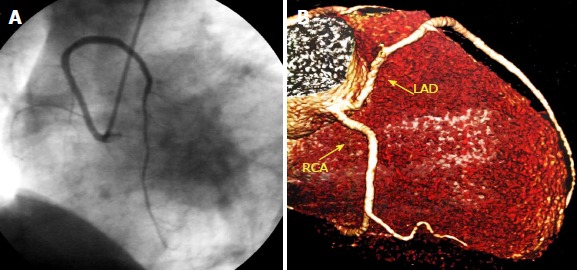
Ectopic left anterior descending artery originating from the right sinus of Valsalva. A: Left anterior oblique view with cranial angulation of the left anterior descending artery (LAD) originating from the right Valsalva sinus in coronary angiogram; B: Reconstructed image from computed tomography coronary angiogram showing both LAD and right coronary artery arising from the right sinus of Valsalva. From the authors’ personal dataset. LAD: Left anterior descending; RCA: Right coronary artery.
Figure 8.
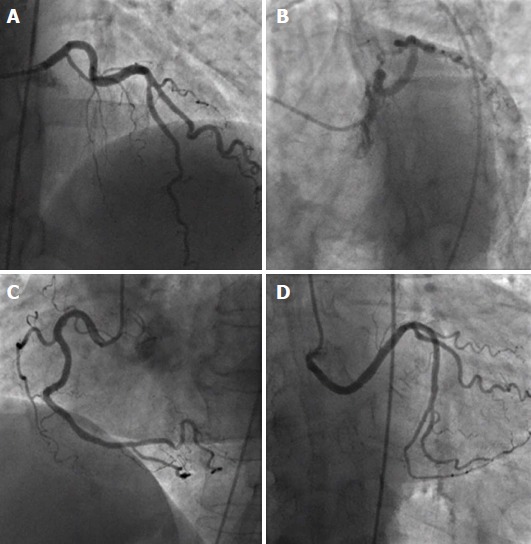
Ectopic left circumflex artery originating from the right sinus of Valsalva. A, B: Left coronary artery providing left anterior descending artery, with absence of left circumflex artery (LCX) (A: anteroposterior cranial view; B: left anterior oblique view with caudal angulation); C, D: Separate origin of right coronary artery (C, left anterior oblique view) and aberrant LCX from the right sinus of Valsava (D, anteroposterior caudal view). From the authors’ personal dataset.
It must be noted that anomalous origin of a coronary artery from an opposite sinus of Valsalva is related with sudden death mostly in young athletes < 35 years old but less frequently in older patients. Symptoms like angina, syncope, heart failure and myocardial infarction may appear in both age groups but are seen more frequently in older patients. It has been proposed that stiffening of the aortic wall in older adults is the reason why sudden death is less frequent in this age group[3,40].
Diagnosis of anomalous origin of a coronary artery from the contralateral sinus is often difficult, since most of the times patients are asymptomatic prior to sudden cardiac death. However, there have been some reports of premonitory symptoms like syncope or chest pain prior to sudden death[2,41,42]. Diagnostic workup in patients with the above symptoms (especially young athletes) must include electrocardiography, Holter monitoring and focused expert echocardiography. If at least two normally located coronary ostia can be identified with echocardiography, no further investigations are needed[3]. However, if echocardiographic findings are inconclusive, further imaging with computed tomography or magnetic resonance imaging is recommended[3,43-45]. Treadmill evaluation is unfortunately hampered by a high incidence of false positive and false negative results[2,37,46]. If the anomaly is confirmed, patients should undergo nuclear stress testing to evaluate exercise-induced ischemia and to establish a baseline for follow-up. Coronary angiography may reveal additional obstructive coronary disease, and intracoronary imaging establishes the severity of the condition[3,4]. Intravascular ultrasound and/or optical coherence tomography findings that determine the severity of an anomalous origin of a coronary artery from an opposite sinus include the length of the intramural segment, the hypoplasia index that quantifies the severity of the circumferential hypoplasia (ratio of the circumference of the intramural segment vs the circumference of the more distal epicardial segment of the vessel), the vessel asymmetry score (ratio of transverse to longitudinal diameter in a cross-sectional image from intravascular ultrasound) and the systolic vs diastolic cross-sectional area of stenosis during a cardiac cycle at rest and during simulated exercise with infusion of saline, atropine and dobutamine (SAD test)[4,47]. When an anomalous left coronary artery (LCA) with origin from the right sinus of Valsalva is diagnosed incidentally during coronary angiography, specific angiographic anatomical findings help differentiate between the inter-arterial and the more benign trans-septal varieties of this anomaly, since in the trans-septal origin the LMS gives rise to the first septal branch, presents a mild concentric myocardial bridge effect at the distal segment and connects with mid-LAD[4]. However, computed tomography remains the most accurate method to characterize the course of ectopic coronary arteries.
Asymptomatic patients with anomalous RCA arising from the left sinus of Valsalva with a negative nuclear stress test need regular follow-up only. Symptomatic patients and asymptomatic patients with a positive nuclear stress test must be further assessed with intracoronary imaging with intravascular ultrasound or optical coherence tomography, and if a significant stenosis or high risk features are found, percutaneous coronary intervention with stent[3,36,48] or surgical repair should be offered. Patients with anomalous LMS arising from the right sinus of Valsalva should undergo surgical repair regardless of symptoms, if younger than 35 years old. Older patients should undergo surgery if they develop symptoms or if they have a positive nuclear stress test[4]. Surgical options include osteoplasty (creation of a new ostium at the end of the ectopic artery’s intramural segment), direct reimplantation of the ectopic artery at the aortic root (a challenging procedure) and unroofing of the intramural segment (excising of the common wall located between the aorta and the anomalous coronary)[4,33,49-51]. Currently, unroofing is the most favored of these procedures. Bypass surgery with an internal mammary artery is not preferred, because of the high risk of regression of the graft lumen due to competitive flow[4,52,53].
Anomalous origin of coronary arteries outside the aortic root
The most dramatic clinical appearance in this group of congenital coronary anomalies occurs when the ectopic coronary arises from the pulmonary artery. The RCA originates from the pulmonary artery in 0.002% of the general population. In this anomaly, blood flows from the LCA to the RCA via collaterals and further back into the pulmonary artery. Most patients with this anomaly are asymptomatic; however, sudden cardiac death, heart failure and syncope have been occasionally reported[1,15,25].
Anomalous origin of the LCA from the pulmonary artery (ALCAPA) is called Bland-White-Garland syndrome and has a low prevalence of 0.008%. The RCA is dilated and provides extensive collaterals to LCA. This condition may coexist with aortic coarctation and a patent ductus arteriosus. Most patients (about 85%) develop symptoms of myocardial ischemia and heart failure in infancy (Figure 9) and die within the first year of life. However, a minority of patients may remain asymptomatic and survive into adulthood, probably because of adequate blood flow to the LCA territory from the RCA collaterals (Figure 10). Treatment is surgical and reimplantation of the LMS onto the aorta is the preferred method. However, the procedure is more technically challenging in adults due to the frailty of the coronary artery. Ligation of the LCA ostium and coronary artery bypass grafting with venous or arterial grafts is a viable alternative in these cases (Figure 10)[14,15,25,54,55]. The LAD arises from the pulmonary artery extremely rarely in 0.0008% of the general population and is associated with myocardial ischemia and sudden cardiac death[1,15,38]. There have also been reports of all coronary arteries originating from the pulmonary artery. Patients die within the first month of life, and the condition frequently coexists with patent ductus arteriosus and other major congenital cardiac anomalies[25,56]. Finally, cases of accessory coronary arteries arising from the pulmonary artery have been documented. The most frequent artery involved is the conus branch, and this anomaly has no clinical significance[1,25].
Figure 9.
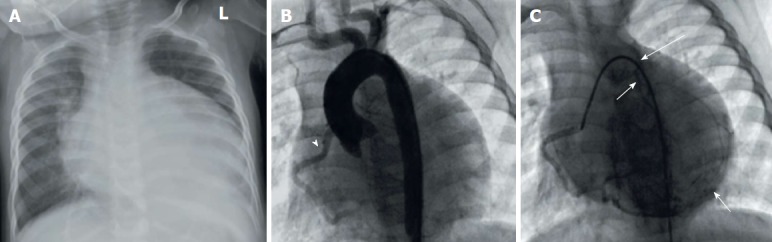
Anomalous origin of the left coronary artery from the pulmonary artery in infant. A: Cardiomegaly in chest X-ray; B: Aortogram showing only right coronary artery (RCA) originating from the aortic sinuses (arrowhead) but no left coronary artery; C: Selective angiography of RCA shows extensive collaterals from the RCA backfilling the left system and the pulmonary artery (arrows). Reproduced from ref. [74].
Figure 10.
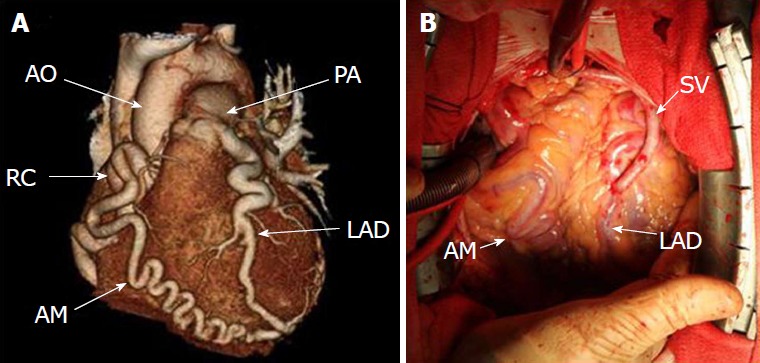
Anomalous origin of the left coronary artery from the pulmonary artery in adult. A: Three-dimensional reconstruction of computed tomography images showing prominent collaterals from right coronary artery to the left anterior descending artery (LAD), while LAD originates from the pulmonary artery; B: Coronary bypass grafting with a saphenous vein graft to the LAD. Reproduced from ref. [75]. SV: Saphenous vein; RC: Right coronary; LAD: Left anterior descending.
Single coronary artery
There have been several reports of presence of a single coronary artery with a variety of anatomies. A single coronary artery may originate either from the left or the right Valsalva sinus and may sometimes coexist with other congenital anomalies[1]. Lipton et al[57] proposed an anatomical classification of single coronary arteries based on location of the ostium (R: right Valsalva sinus, L: left Valsalva sinus), anatomical distribution (I: single coronary artery following course of normal left or RCA, II: a coronary artery with abnormal origin from the proximal segment of the other coronary artery, III: single coronary artery with a origin from the right Valsalva sinus, with the LAD and LCX originating separately from the common trunk) and course of the transverse trunk (A: anterior course to the great vessels, B: between the aorta and the pulmonary artery, P: posterior course, S: septal course, C: combined type).
When the single coronary artery has an origin from the left sinus, the RCA may originate from the proximal or mid segment of the LAD and follows a course anterior to the pulmonary artery or between the aorta and the pulmonary artery towards the right atrioventricular groove (Figure 11). The prevalence of this anomaly is around 0.024%-0.066% in the general population[1,58,59]. This anomaly is usually benign, provided that the course of the anomalous RCA is not inter-arterial. If, however, the anomalous RCA crosses between the aorta and the pulmonary artery, myocardial ischemia and sudden cardiac death may occur[29,58,60,61]. Rarely, the RCA may be supplied by the distal LCX (Figure 12).
Figure 11.
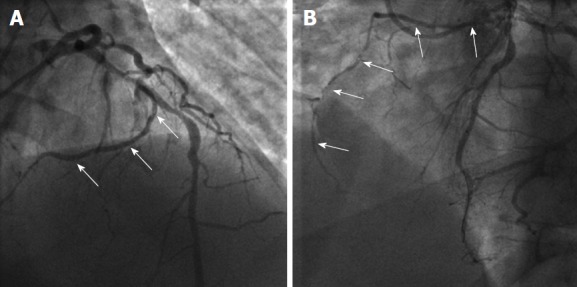
Single coronary artery from the left sinus. A, B: Angiographic images showing the origin (arrows in A, anteroposterior cranial view) and course (arrows in B, left anterior oblique view) of the right coronary artery from the mid segment of left anterior descending artery. From the authors’ personal dataset and from Ref. [76] (modified with permission).
Figure 12.
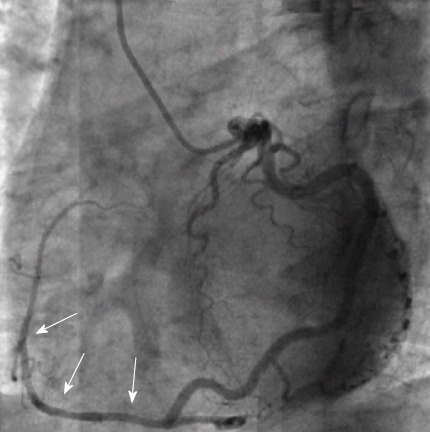
Single coronary artery from the left sinus. Angiographic images showing the origin and course (arrows) of the aberrant right coronary artery from the distal segment of left circumflex artery (left anterior oblique view). From the authors’ personal dataset.
When the single coronary artery originates from the right sinus (Figure 13), the LCA follows an anterior or inter-arterial path towards its perfusion territory. Prevalence is around 0.02%-0.05%, and this condition is associated with sudden cardiac death more frequently than an anomalous single coronary artery originating from the left sinus. The anterior variant is usually - but not always - benign, whereas the inter-arterial variant is the potentially most threatening one[1,15,62].
Figure 13.
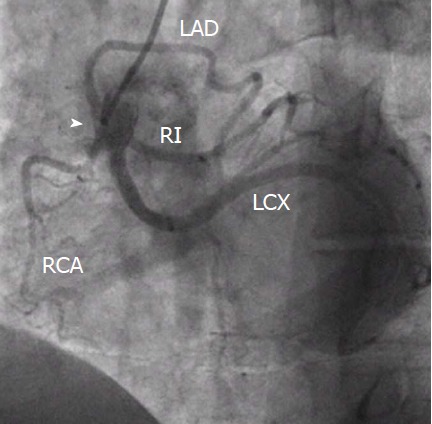
Single coronary artery originating from the right sinus. Common trunk from the right sinus (arrowhead) providing right coronary artery, left anterior descending artery, left circunflex artery and ramus intermedius. From the authors’ personal dataset. RCA: Right coronary artery; LAD: Left anterior descending; LCX: Left circunflex artery; RI: Ramus intermedius.
ANOMALIES OF INTRINSIC CORONARY ANATOMY
Split RCA
A split RCA is the most common type of coronary artery anomaly, with a frequency around 1% in the general population. It is a benign anomaly in which the RCA has a split PDA (Figure 14). The split RCA is divided early into an anterior and posterior branch. The anterior branch runs through the free wall of the right ventricle and supplies a PDA that follows the distal posterior interventricular groove, whereas the posterior branch follows the right atrioventricular groove and leads to a PDA that runs through the proximal segment of the posterior interventricular groove[1,63-65].
Figure 14.
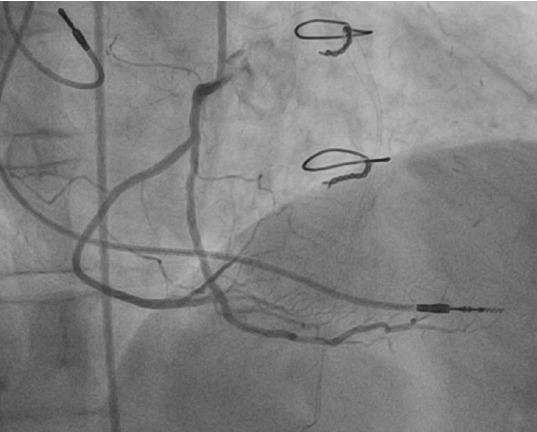
Split right coronary artery. Right coronary artery is divided early into two branches, both of which supply posterior descending arteries. From the authors’ personal dataset.
Split LAD
There have been reported four types of split LAD. In types 1-3, the LAD branches into two subdivisions, a short one that terminates high in the anterior interventricular groove and a longer one that branches off the proper LAD, descends on the left (type 1) or the right (type 2) ventricular side of the anterior interventricular groove or intramyocardially (type 3) and finally reenters the anterior interventricular groove distally. Sometimes in type 3 the intramyocardial LAD never emerges but instead provides septal branches to the apical septum. Type 4 is an entirely different entity, in which a short LAD originates from the LMS and terminates high in the anterior interventricular groove, while a duplicated LAD arises from the RCA and follows a pre-pulmonic, septal or inter-arterial course towards the distal anterior interventricular groove. This anomaly is relatively benign; however, it may complicate significantly surgical intervention[7,66,67].
Atresia of LMS
True atresia of the LMS is an extremely rare congenital disorder in which there is neither left coronary ostium nor LMS. The LAD and the LCX connect but end blindly proximally. The left system receives blood retrogradely via collaterals from the RCA, but in most cases blood flow is inadequate for the needs of the perfusion territory, resulting in symptoms of myocardial ischemia and an increased risk of sudden cardiac death. This condition often coexists with supravalvular aortic stenosis and other congenital cardiac defects. Patients usually become symptomatic during infancy or early childhood, but there have been reports of patients who remained asymptomatic during childhood and young adulthood and developed symptoms later in life. Due to poor prognosis, atresia of the LMS requires surgical intervention with coronary artery bypass grafting and an internal mammary artery graft to the LAD in adults, whereas in children surgical reconstruction of the LMS with a baffle of ascending aorta may be preferable[1,68].
Hypoplasia of coronary arteries
Congenital hypoplasia of coronary arteries presents as a narrowed luminal diameter (less than 1.5 mm) in one or two of the three main coronary arteries with no compensatory branches. Limitations to blood flow caused by the narrow lumen lead to symptoms of myocardial ischemia and sudden cardiac death. Most frequent variants in reported cases are hypoplasia of both LCX and RCA and hypoplasia of the LAD. Treatment options are extremely limited. Transmyocardial revascularization and implantable cardioverter-defibrillator have been suggested in the literature[13,69-71].
ANOMALIES OF CORONARY TERMINATION
Coronary fistulae are defined as abnormal connections between the termination of a coronary artery or its branches and a low-pressure vascular space, like a cardiac chamber or a great vessel (Figure 15). They may present as small discrete fistulae or more complex arteriovenous malformations. Reported prevalence is 0.3%-0.87% in the general population. Most patients are asymptomatic; however, there have been reports of symptoms of myocardial ischemia, heart failure, arrhythmia and sudden cardiac death, pulmonary hypertension, rupture and endocarditis, usually after the age of 50.
Figure 15.
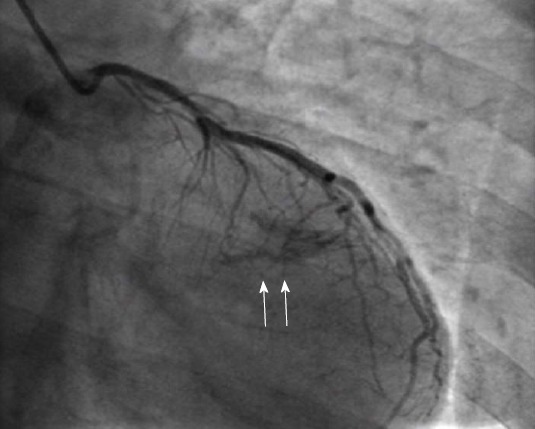
Coronary fistula. Opacification of the left ventricle during injection of the left coronary artery (arrows) indicate the presence of a fistula. From the authors’ personal dataset.
Several mechanisms for the causes of these symptoms have been proposed. Fistulae draining into the right heart chambers (60% of cases) function as left to right shunts and may cause right ventricular volume overload. Termination in a low pressure space causes enlargement and tortuosity of the fistulous coronary artery that leads to vascular wall degeneration, aneurysmatic dilatation and predisposition to rupture. Furthermore, the dilatation of the involved coronary artery may cause distortion of the aortic root and aortic valve disruption and regurgitation. Myocardial ischemia may result from two separate mechanisms: (1) a persistent or episodic steal of blood flow from the normal coronary branches to the competing fistulous low-pressure tract; and (2) stenosis and obstruction of side branches secondary to thrombus formation related to ulceration and atherosclerosis in the aneurysmal coronary artery. Indications for closing a coronary fistula are not well established. Symptomatic patients should definitely be treated as well as patients with a pulmonary to systemic flow ratio that exceeds 1.5 and patients with severe aneurysmal degeneration. Available options are surgical closure at the drainage site or catheter-based repair with catheter occlusion devices. It is generally recommended to intervene before adulthood, since negative postoperative remodeling of the fistulous artery is much more common in children compared to adults[4,15,17,37,38].
Furthermore, we have recently described a fistulous tract from the left ventricle to the Thebesian venous network of the myocardium[72]. Thebesian veins are small valveless veins in the walls of all four heart chambers that act as an alternative channel of nutrition to the myocardium or as venous drainage conduits. They are more prevalent in the right heart chambers, but they can also appear in the left ventricle. We described the case of inadvertent angiography of a large posterior interventricular cardiac vein during a left ventriculogram. This happened because the end-hole of the angiographic catheter inadvertently engaged the endocardial opening of a small Thebesian vein, leading to retrograde opacification of the cardiac vein through the Thebesian network (Figure 16)[72].
Figure 16.
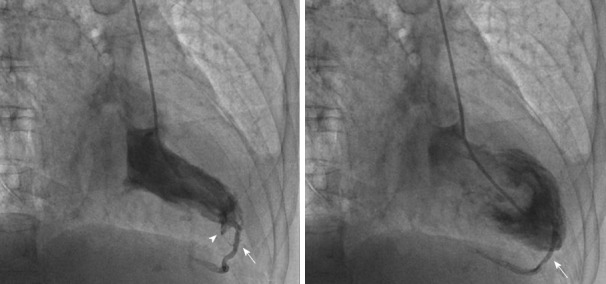
Inadvertent cardiac phlebography through the Thebesian network. A, B: Right anterior oblique views in systole (A) and diastole (B) during left ventriculography show a minor subendocardial staining (arrowhead) and opacification of a posterior interventricular vein (arrows), due to opening of a 'functional' fistula between the left ventricle and the cardiac vein through the Thebesian network. From the authors’ personal dataset and from Ref. [72] (with permission).
CONCLUSION
It becomes apparent that the term “coronary artery anomalies” covers a very wide spectrum of anatomical entities with diverse clinical manifestations and varying degrees of severity. Coronary artery variants have a prevalence of at least 1% in the general population and are mostly clinically benign. On the other hand, coronary artery anomalies are much more uncommon, and their clinical significance varies from benign without ischemic consequences to ischemia-related symptoms and arrhythmias leading to an increased risk of sudden cardiac death. Anomalous origin of a coronary ostium from the contralateral sinus is the anomaly most frequently associated with sudden cardiac death, in particular in the case of anomalous LCA from the right sinus or if the anomalous coronary artery has a course between the aorta and the pulmonary artery or other high-risk features. Other coronary anomalies, like anomalous origin of the LCA from the pulmonary artery or atresia of the LMS, have also been associated with extensive ischemia and high risk of sudden cardiac death early in infancy or in early adulthood. Accurate diagnosis, risk-assessment and appropriate choice of treatment are of utmost importance, since coronary artery anomalies constitute the second most common cause of sudden cardiac death in young competitive athletes. The rapid advancement of imaging techniques, including intravascular ultrasound, optical coherence, computed tomography and magnetic resonance imaging, have provided us with a wealth of new information on the subject as well as more accurate diagnostic criteria regarding the severity of these conditions that allow risk-stratification for sudden cardiac death and provide guidelines for optimal treatment. Consultation of focused experts by individual cardiologists in cases of suspected or diagnosed coronary artery anomalies and systematic referral of diagnosed patients to specialized centers play a significant role in timely diagnosis, appropriate management and successful prevention of sudden cardiac death.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Manuscript source: Invited manuscript
Peer-review started: April 16, 2018
First decision: June 6, 2018
Article in press: August 31, 2018
Specialty type: Cardiac and cardiovascular systems
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Anan R, Barik R, Scicchitano P, Tomizawa N S- Editor: Ji FF L- Editor: Filipodia E- Editor: Wu YXJ
Contributor Information
Stylianos Kastellanos, Cardiology Department, Castle Hill Hospital, Hull and East Yorkshire NHS Trust, Cottingham HU16 5JQ, United Kingdom; Peripheral Vessels Unit and EKKAN (Unit for the athletes and for hereditary cardiovascular diseases), 1st Department of Cardiology, Hippokration Hospital, Medical School of National and Kapodistrian University of Athens, Athens 11527, Greece.
Konstantinos Aznaouridis, Cardiology Department, Castle Hill Hospital, Hull and East Yorkshire NHS Trust, Cottingham HU16 5JQ, United Kingdom; Peripheral Vessels Unit and EKKAN (Unit for the athletes and for hereditary cardiovascular diseases), 1st Department of Cardiology, Hippokration Hospital, Medical School of National and Kapodistrian University of Athens, Athens 11527, Greece. conazna@yahoo.com.
Charalambos Vlachopoulos, Peripheral Vessels Unit and EKKAN (Unit for the athletes and for hereditary cardiovascular diseases), 1st Department of Cardiology, Hippokration Hospital, Medical School of National and Kapodistrian University of Athens, Athens 11527, Greece.
Eleftherios Tsiamis, Peripheral Vessels Unit and EKKAN (Unit for the athletes and for hereditary cardiovascular diseases), 1st Department of Cardiology, Hippokration Hospital, Medical School of National and Kapodistrian University of Athens, Athens 11527, Greece.
Evangelos Oikonomou, Peripheral Vessels Unit and EKKAN (Unit for the athletes and for hereditary cardiovascular diseases), 1st Department of Cardiology, Hippokration Hospital, Medical School of National and Kapodistrian University of Athens, Athens 11527, Greece.
Dimitris Tousoulis, Peripheral Vessels Unit and EKKAN (Unit for the athletes and for hereditary cardiovascular diseases), 1st Department of Cardiology, Hippokration Hospital, Medical School of National and Kapodistrian University of Athens, Athens 11527, Greece.
References
- 1.Villa AD, Sammut E, Nair A, Rajani R, Bonamini R, Chiribiri A. Coronary artery anomalies overview: The normal and the abnormal. World J Radiol. 2016;8:537–555. doi: 10.4329/wjr.v8.i6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 3.Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115:1296–1305. doi: 10.1161/CIRCULATIONAHA.106.618082. [DOI] [PubMed] [Google Scholar]
- 4.Moscucci M. 2005. Grossman and Baim’s Cardiac Catheterization, Angiography, and Intervention. 8th Edition. Lippincott Williams Wilkins (LWW) pp. 335–353. [Google Scholar]
- 5.Angelini P, Villason S, Chan AV, Diez JG. Normal and anomalous coronary arteries in humans. In: Angelini P, ed, editors. Coronary Artery Anomalies: A Comprehensive Approach. Philadelphia: Lippincott Williams Wilkins; 1999. pp. 27–150. [Google Scholar]
- 6.Ali M, Hanley A, McFadden EP, Vaughan CJ. Coronary artery anomalies: a practical approach to diagnosis and management. Heart Asia. 2011;3:8–12. doi: 10.1136/ha.2010.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shriki JE, Shinbane JS, Rashid MA, Hindoyan A, Withey JG, DeFrance A, Cunningham M, Oliveira GR, Warren BH, Wilcox A. Identifying, characterizing, and classifying congenital anomalies of the coronary arteries. Radiographics. 2012;32:453–468. doi: 10.1148/rg.322115097. [DOI] [PubMed] [Google Scholar]
- 8.Apitzsch J, Kühl HP, Mühlenbruch G, Mahnken AH. Unusual malignant coronary artery anomaly: results of coronary angiography, MR imaging, and multislice CT. Cardiovasc Intervent Radiol. 2010;33:389–393. doi: 10.1007/s00270-009-9663-y. [DOI] [PubMed] [Google Scholar]
- 9.Karadag B, Ayan F, Ismailoglu Z, Goksedef D, Ataev Y, Vural VA. Extraordinary cause of ischemic chest pain in a young man: congenital ostial atresia of the right coronary artery. J Cardiol. 2009;54:335–338. doi: 10.1016/j.jjcc.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Chiribiri A, Ishida M, Nagel E, Botnar RM. Coronary imaging with cardiovascular magnetic resonance: current state of the art. Prog Cardiovasc Dis. 2011;54:240–252. doi: 10.1016/j.pcad.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Angelini P, Trujillo A, Sawaya F, Lee VV. “Acute takeoff” of the circumflex artery: a newly recognized coronary anatomic variant with potential clinical consequences. Tex Heart Inst J. 2008;35:28–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Boffano C, Chiribiri A, Cesarani F. Native whole-heart coronary imaging for the identification of anomalous origin of the coronary arteries. Int J Cardiol. 2009;137:e27–e28. doi: 10.1016/j.ijcard.2008.05.075. [DOI] [PubMed] [Google Scholar]
- 13.Menke DM, Waller BF, Pless JE. Hypoplastic coronary arteries and high takeoff position of the right coronary ostium. A fatal combination of congenital coronary artery anomalies in an amateur athlete. Chest. 1985;88:299–301. doi: 10.1378/chest.88.2.299. [DOI] [PubMed] [Google Scholar]
- 14.Frescura C, Basso C, Thiene G, Corrado D, Pennelli T, Angelini A, Daliento L. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol. 1998;29:689–695. doi: 10.1016/s0046-8177(98)90277-5. [DOI] [PubMed] [Google Scholar]
- 15.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 16.Angelini P, Trivellato M, Donis J, Leachman RD. Myocardial bridges: a review. Prog Cardiovasc Dis. 1983;26:75–88. doi: 10.1016/0033-0620(83)90019-1. [DOI] [PubMed] [Google Scholar]
- 17.Young PM, Gerber TC, Williamson EE, Julsrud PR, Herfkens RJ. Cardiac imaging: Part 2, normal, variant, and anomalous configurations of the coronary vasculature. AJR Am J Roentgenol. 2011;197:816–826. doi: 10.2214/AJR.10.7249. [DOI] [PubMed] [Google Scholar]
- 18.Morales AR, Romanelli R, Tate LG, Boucek RJ, de Marchena E. Intramural left anterior descending coronary artery: significance of the depth of the muscular tunnel. Hum Pathol. 1993;24:693–701. doi: 10.1016/0046-8177(93)90004-z. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira AG Jr, Trotter SE, König B Jr, Décourt LV, Fox K, Olsen EG. Myocardial bridges: morphological and functional aspects. Br Heart J. 1991;66:364–367. doi: 10.1136/hrt.66.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumari M, Rha SW, Poddar KL, Park JY, Choi BG, Kim YK, Na JO, Choi CU, Lim HE, Kim JW, et al. Clinical and angiographic characteristics of coronary endothelial dysfunction severity in patients with myocardial bridge as assessed by acetylcholine provocation test. J Am Coll Cardiol. 2011;57:E1513. [Google Scholar]
- 21.Haager PK, Schwarz ER, vom Dahl J, Klues HG, Reffelmann T, Hanrath P. Long term angiographic and clinical follow up in patients with stent implantation for symptomatic myocardial bridging. Heart. 2000;84:403–408. doi: 10.1136/heart.84.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Tajik AJ, Holmes DR. Myocardial bridging in adult patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;42:889–894. doi: 10.1016/s0735-1097(03)00854-4. [DOI] [PubMed] [Google Scholar]
- 23.Basso C, Thiene G, Mackey-Bojack S, Frigo AC, Corrado D, Maron BJ. Myocardial bridging, a frequent component of the hypertrophic cardiomyopathy phenotype, lacks systematic association with sudden cardiac death. Eur Heart J. 2009;30:1627–1634. doi: 10.1093/eurheartj/ehp121. [DOI] [PubMed] [Google Scholar]
- 24.Olivotto I, Cecchi F, Yacoub MH. Myocardial bridging and sudden death in hypertrophic cardiomyopathy: Salome drops another veil. Eur Heart J. 2009;30:1549–1550. doi: 10.1093/eurheartj/ehp216. [DOI] [PubMed] [Google Scholar]
- 25.Roberts WC. Major anomalies of coronary arterial origin seen in adulthood. Am Heart J. 1986;111:941–963. doi: 10.1016/0002-8703(86)90646-0. [DOI] [PubMed] [Google Scholar]
- 26.Corrado D, Thiene G, Nava A, Rossi L, Pennelli N. Sudden death in young competitive athletes: clinicopathologic correlations in 22 cases. Am J Med. 1990;89:588–596. doi: 10.1016/0002-9343(90)90176-e. [DOI] [PubMed] [Google Scholar]
- 27.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–133. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 28.Burke AP, Farb A, Virmani R, Goodin J, Smialek JE. Sports-related and non-sports-related sudden cardiac death in young adults. Am Heart J. 1991;121:568–575. doi: 10.1016/0002-8703(91)90727-y. [DOI] [PubMed] [Google Scholar]
- 29.Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334:1039–1044. doi: 10.1056/NEJM199604183341607. [DOI] [PubMed] [Google Scholar]
- 30.Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998;339:364–369. doi: 10.1056/NEJM199808063390602. [DOI] [PubMed] [Google Scholar]
- 31.Van Camp SP, Bloor CM, Mueller FO, Cantu RC, Olson HG. Nontraumatic sports death in high school and college athletes. Med Sci Sports Exerc. 1995;27:641–647. [PubMed] [Google Scholar]
- 32.Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992;20:640–647. doi: 10.1016/0735-1097(92)90019-j. [DOI] [PubMed] [Google Scholar]
- 33.Mustafa I, Gula G, Radley-Smith R, Durrer S, Yacoub M. Anomalous origin of the left coronary artery from the anterior aortic sinus: a potential cause of sudden death. Anatomic characterization and surgical treatment. J Thorac Cardiovasc Surg. 1981;82:297–300. [PubMed] [Google Scholar]
- 34.Barriales-Villa R, Morís de la Tassa C. Congenital coronary artery anomalies with origin in the contralateral sinus of Valsalva: which approach should we take? Rev Esp Cardiol. 2006;59:360–370. [PubMed] [Google Scholar]
- 35.Grollman JH Jr, Mao SS, Weinstein SR. Arteriographic demonstration of both kinking at the origin and compression between the great vessels of an anomalous right coronary artery arising in common with a left coronary artery from above the left sinus of Valsalva. Cathet Cardiovasc Diagn. 1992;25:46–51. doi: 10.1002/ccd.1810250110. [DOI] [PubMed] [Google Scholar]
- 36.Angelini P, Velasco JA, Ott D, Khoshnevis GR. Anomalous coronary artery arising from the opposite sinus: descriptive features and pathophysiologic mechanisms, as documented by intravascular ultrasonography. J Invasive Cardiol. 2003;15:507–514. [PubMed] [Google Scholar]
- 37.Angelini P. Coronary artery anomalies--current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J. 2002;29:271–278. [PMC free article] [PubMed] [Google Scholar]
- 38.Baltaxe HA, Wixson D. The incidence of congenital anomalies of the coronary arteries in the adult population. Radiology. 1977;122:47–52. doi: 10.1148/122.1.47. [DOI] [PubMed] [Google Scholar]
- 39.Bunce NH, Lorenz CH, Keegan J, Lesser J, Reyes EM, Firmin DN, Pennell DJ. Coronary artery anomalies: assessment with free-breathing three-dimensional coronary MR angiography. Radiology. 2003;227:201–208. doi: 10.1148/radiol.2271020316. [DOI] [PubMed] [Google Scholar]
- 40.Cheitlin MD. Coronary anomalies as a cause of sudden death in the athlete. In: Estes NAM, Salem DN, Wang JJP, eds, editors. Sudden Cardiac Death in the Athlete. Armonk NY: Futura Publishing Co; 1998. pp. 379–391. [Google Scholar]
- 41.Roberts WC, Siegel RJ, Zipes DP. Origin of the right coronary artery from the left sinus of valsalva and its functional consequences: analysis of 10 necropsy patients. Am J Cardiol. 1982;49:863–868. doi: 10.1016/0002-9149(82)91970-1. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh PK, Agarwal SK, Kumar R, Chandra N, Puri VK. Anomalous origin of right coronary artery from left aortic sinus. J Cardiovasc Surg (Torino) 1994;35:65–70. [PubMed] [Google Scholar]
- 43.Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449–2454. doi: 10.1161/01.cir.0000016175.49835.57. [DOI] [PubMed] [Google Scholar]
- 44.Hejmadi A, Sahn DJ. What is the most effective method of detecting anomalous coronary origin in symptomatic patients? J Am Coll Cardiol. 2003;42:155–157. doi: 10.1016/s0735-1097(03)00510-2. [DOI] [PubMed] [Google Scholar]
- 45.Post JC, van Rossum AC, Bronzwaer JG, de Cock CC, Hofman MB, Valk J, Visser CA. Magnetic resonance angiography of anomalous coronary arteries. A new gold standard for delineating the proximal course? Circulation. 1995;92:3163–3171. doi: 10.1161/01.cir.92.11.3163. [DOI] [PubMed] [Google Scholar]
- 46.Waller BF. Exercise-related sudden death in young (age less than or equal to 30 years) and old (age greater than 30 years) conditioned subjects. Cardiovasc Clin. 1985;15:9–73. [PubMed] [Google Scholar]
- 47.Angelini P, Flamm SD. Newer concepts for imaging anomalous aortic origin of the coronary arteries in adults. Catheter Cardiovasc Interv. 2007;69:942–954. doi: 10.1002/ccd.21140. [DOI] [PubMed] [Google Scholar]
- 48.Hariharan R, Kacere RD, Angelini P. Can stent-angioplasty be a valid alternative to surgery when revascularization is indicated for anomalous origination of a coronary artery from the opposite sinus? Tex Heart Inst J. 2002;29:308–313. [PMC free article] [PubMed] [Google Scholar]
- 49.Van Son JA, Haas GS. Anomalous origin of left main coronary artery from right sinus of Valsalva: modified surgical treatment to avoid neo-coronary ostial stenosis. Eur J Cardiothorac Surg. 1996;10:467–469. doi: 10.1016/s1010-7940(96)80118-7. [DOI] [PubMed] [Google Scholar]
- 50.Angelini P, Walmsley RP, Libreros A, Ott DA. Symptomatic anomalous origination of the left coronary artery from the opposite sinus of valsalva. Clinical presentations, diagnosis, and surgical repair. Tex Heart Inst J. 2006;33:171–179. [PMC free article] [PubMed] [Google Scholar]
- 51.García-Rinaldi R, Sosa J, Olmeda S, Cruz H, Carballido J, Quintana C. Surgical treatment of right coronary arteries with anomalous origin and slit ostium. Ann Thorac Surg. 2004;77:1525–1529. doi: 10.1016/j.athoracsur.2003.08.084. [DOI] [PubMed] [Google Scholar]
- 52.Angelini P. Letter by Angelini regarding article, “long-term outcome and impact of surgery on adults with coronary arteries originating from the opposite coronary cusp”. Circulation. 2011;124:e383. doi: 10.1161/CIRCULATIONAHA.111.023614. [DOI] [PubMed] [Google Scholar]
- 53.Krasuski RA, Magyar D, Hart S, Kalahasti V, Lorber R, Hobbs R, Pettersson G, Blackstone E. Long-term outcome and impact of surgery on adults with coronary arteries originating from the opposite coronary cusp. Circulation. 2011;123:154–162. doi: 10.1161/CIRCULATIONAHA.109.921106. [DOI] [PubMed] [Google Scholar]
- 54.Alexi-Meskishvili V, Nasseri BA, Nordmeyer S, Schmitt B, Weng YG, Böttcher W, Hübler M, Berger F, Hetzer R. Repair of anomalous origin of the left coronary artery from the pulmonary artery in infants and children. J Thorac Cardiovasc Surg. 2011;142:868–874. doi: 10.1016/j.jtcvs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Barbetakis N, Efstathiou A, Efstathiou N, Papagiannopoulou P, Soulountsi V, Fessatidis I. A long-term survivor of Bland-White-Garland syndrome with systemic collateral supply: a case report and review of the literature. BMC Surg. 2005;5:23. doi: 10.1186/1471-2482-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation. 1956;14:800–805. doi: 10.1161/01.cir.14.5.800. [DOI] [PubMed] [Google Scholar]
- 57.Lipton MJ, Barry WH, Obrez I, Silverman JF, Wexler L. Isolated single coronary artery: diagnosis, angiographic classification, and clinical significance. Radiology. 1979;130:39–47. doi: 10.1148/130.1.39. [DOI] [PubMed] [Google Scholar]
- 58.Yurtdaş M, Gülen O. Anomalous origin of the right coronary artery from the left anterior descending artery: review of the literature. Cardiol J. 2012;19:122–129. [PubMed] [Google Scholar]
- 59.Sloan K, Majdalany D, Connolly H, Schaff H. Inferior wall myocardial infarction caused by anomalous right coronary artery. Can J Cardiol. 2008;24:e102–e103. doi: 10.1016/s0828-282x(08)70704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isner JM, Shen EM, Martin ET, Fortin RV. Sudden unexpected death as a result of anomalous origin of the right coronary artery from the left sinus of Valsalva. Am J Med. 1984;76:155–158. doi: 10.1016/0002-9343(84)90765-4. [DOI] [PubMed] [Google Scholar]
- 61.Wann S, Schuchard G. Images in clinical medicine. Anomalous origin of the right coronary artery. N Engl J Med. 2006;355:e8. doi: 10.1056/NEJMicm050051. [DOI] [PubMed] [Google Scholar]
- 62.Flessas D, Mamarelis I, Maniatis V, Souretis G, Laschos N, Kotoulas C, Lazaridis K. An unusual pattern of three major components of the cardiovascular system: multimodality imaging and review of the literature. J Cardiothorac Surg. 2013;8:61. doi: 10.1186/1749-8090-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sawaya FJ, Sawaya JI, Angelini P. Split right coronary artery: its definition and its territory. Tex Heart Inst J. 2008;35:477–479. [PMC free article] [PubMed] [Google Scholar]
- 64.Gulel O, Yazici M, Durna K, Demircan S. A rare coronary anomaly: double right coronary artery. Clin Cardiol. 2007;30:309. doi: 10.1002/clc.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erbagci H, Davutoglu V, Turkmen S, Kizilkan N, Gumusburun E. Double right coronary artery: review of literature. Int J Cardiovasc Imaging. 2006;22:9–11. doi: 10.1007/s10554-005-5139-6. [DOI] [PubMed] [Google Scholar]
- 66.Agarwal PP, Kazerooni EA. Dual left anterior descending coronary artery: CT findings. AJR Am J Roentgenol. 2008;191:1698–1701. doi: 10.2214/AJR.08.1193. [DOI] [PubMed] [Google Scholar]
- 67.Spindola-Franco H, Grose R, Solomon N. Dual left anterior descending coronary artery: angiographic description of important variants and surgical implications. Am Heart J. 1983;105:445–455. doi: 10.1016/0002-8703(83)90363-0. [DOI] [PubMed] [Google Scholar]
- 68.Musiani A, Cernigliaro C, Sansa M, Maselli D, De Gasperis C. Left main coronary artery atresia: literature review and therapeutical considerations. Eur J Cardiothorac Surg. 1997;11:505–514. doi: 10.1016/s1010-7940(96)01121-9. [DOI] [PubMed] [Google Scholar]
- 69.Roberts WC, Glick BN. Congenital hypoplasia of both right and left circumflex coronary arteries. Am J Cardiol. 1992;70:121–123. doi: 10.1016/0002-9149(92)91407-u. [DOI] [PubMed] [Google Scholar]
- 70.McFarland CA, Svamy RSA. Hypoplastic coronary artery disease: a rare cause of sudden cardiac death and its treatment with an implantable defibrillator. J Cardiol Cases. 2011;4:e148–e151. doi: 10.1016/j.jccase.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fraisse A, Quilici J, Canavy I, Savin B, Aubert F, Bory M. Images in cardiovascular medicine. Myocardial infarction in children with hypoplastic coronary arteries. Circulation. 2000;101:1219–1222. doi: 10.1161/01.cir.101.10.1219. [DOI] [PubMed] [Google Scholar]
- 72.Aznaouridis K, Masoura C, Kastellanos S, Alahmar A. Inadvertent cardiac phlebography. World J Cardiol. 2017;9:558–561. doi: 10.4330/wjc.v9.i6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aznaouridis K, Alahmar A. Transradial primary angioplasty of anomalous right coronary artery from the left sinus of Valsalva. Indian Heart J. 2017;69:411–413. doi: 10.1016/j.ihj.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farzanah I. ALCAPA: The Al Capone of coronary artery anomalies. SA Journal of Radiology. 2012;16:100–101. [Google Scholar]
- 75.Dionne PO, Poirier N, Forcillo J, Stevens LM, Chartrand-Lefebvre C, Mansour S, Noiseux N. A rare case of anomalous origin of the left main coronary artery in an adult patient. J Cardiothorac Surg. 2013;8:15. doi: 10.1186/1749-8090-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waleed M, Aznaouridis K. Anomalous Origin of the “Nonculprit” Right Coronary Artery From the Left Anterior Descending Artery in a Patient With Anterolateral ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv. 2015;8:e223–e225. doi: 10.1016/j.jcin.2015.07.019. [DOI] [PubMed] [Google Scholar]


