Abstract
Background
Colorectal cancer is the third most common form of cancer and colorectal surgery is the treatment of choice in local disease. Anastomotic leakage following colorectal surgery is a major complication with a high incidence and mortality. Adjuvant hyperbaric oxygen treatment (HBOT) may be associated with reduction of anastomotic leakage. A systematic review was conducted regarding HBOT as an adjunctive therapy to colorectal surgery.
Methods
Systematic review (1900–2017) using PubMed, Cochrane, EMBASE, Web of Science and EMCARE. All original published studies on the effect of HBOT as an adjunctive therapy for colorectal surgery with the creation of an anastomosis were considered.
Results
Thirteen small animal trials were included for qualitative synthesis. We found no human trials. Eleven trials used bursting pressure whilst eight used hydroxyproline levels as a marker for collagen synthesis as primary outcome to assess the strength of the anastomosis. A meta-analysis performed for normal and ischaemic anastomoses showed that postoperative HBOT improves bursting pressure and hydroxyproline levels significantly in both normal (P ≤ 0.001 and P = 0.02 respectively) and ischaemic anastomoses (P ≤ 0.001 and P = 0.04 respectively).
Conclusions
Postoperative HBOT has a positive effect on colorectal anastomoses in rats. Further research should focus on a larger systematic animal study.
Keywords: Surgery, Gastrointestinal tract, Animal model, Hyperbaric research, Systematic review
Introduction
Colorectal cancer is the third most common form of cancer with an incidence of almost 1.4 million cases in 2012 according to the WHO.[ 1] Colorectal surgery is the treatment of choice in local carcinoma.[ 2 , 3] A major complication following colorectal surgery is anastomotic leakage (AL) with a reported incidence of 10–13% and a mortality of up to 33%.[ 4] A recent meta-analysis showed that AL is associated with local recurrence and reduced survival.[ 5] Hyperbaric oxygen treatment (HBOT) has been suggested as adjunct therapy to reduce the risk of AL.
HBOT involves breathing 100 percent oxygen at two to three times normal atmospheric pressure and results in elevated oxygen tension in arteries and tissue.[ 6] HBOT is already being used widely as a treatment for a variety of indications as set out in published recommendations of the Undersea and Hyperbaric Medical Society and the European College of Hyperbaric Medicine.[ 7 , 8] HBOT has a variety of mechanisms of action: it improves tissue oxygenation; inhibits the pro-inflammatory reaction by reducing cytokines; improves neo-vascularization; has a bacteriostatic effect on anaerobic bacteria and stimulates stem cells and growth factors.[ 9] HBOT is considered a low-risk therapy. Described side effects are middle ear barotrauma (up to 43%, usually mild), myopia, aerosinusitis, (acute and chronic) oxygen poisoning including seizures and lung failure.[ 10 , 11]
Preconditioning with HBOT might be useful as an adjunct for various types of surgery. For instance, a better outcome in left ventricular function was demonstrated after on-pump coronary artery bypass surgery after pretreatment with three HBOT sessions,[ 10] whilst in patients undergoing pancreaticoduodenectomy, a single preoperative HBOT appeared to improve outcome.[ 12] Furthermore, preconditioning with HBOT is associated with a reduction in the interleukin inflammatory markers IL-6 and IL-10.[ 12]
The effect of HBOT on cancer depends highly on the type of cancer; it might even have an inhibitory effect on certain types of cancer.[ 13] The current consensus is that there is no scientific evidence that HBOT has a cancer-promoting effect.[ 13 , 14] including in colorectal cancer.[ 15] The latter study concluded that HBOT does not promote the growth or recurrence of colorectal cancer, but that treating colorectal cancer solely with HBOT does not seem to have a beneficial effect.
Although strong evidence is still lacking, HBOT could potentially be an adjunct in the treatment of colorectal cancer. The primary aim of this systematic review and meta-analysis is to provide the best evidence to date regarding the effects of HBOT as an adjunctive therapy on anastomotic healing after colorectal surgery.
Methods
The protocol for objectives, literature search strategies, inclusion and exclusion criteria, outcome measurements, and methods of statistical analysis was prepared a priori, according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement,[ 16 , 17] and is described in this section.
LITERATURE SEARCH STRATEGY
A systematic review (1900–2017) was performed in PubMed, Cochrane, EMBASE, Web of Science and EMCARE. The keywords used in the search were "hyperbaric oxygenation" and its synonyms in combination with "colorectal surgery", "colectomy" and their equivalents. Also, the combination of "surgery" and its synonyms, with "colon", "rectum", "sigmoid" and their equivalents was used. The search was limited to original studies published in English.
Inclusion and exclusion criteria, data extraction and outcomes of interest
Two authors (RJB, ACE) independently identified the studies for inclusion and exclusion and extracted the data. The accuracy of the extracted data was further confirmed by a third author (RH). Studies were included when they used colorectal surgery, including the formation of an anastomosis, in combination with HBOT.
QUALITY ASSESSMENT
The quality of trials was assessed using the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) risk-of-bias tool.[ 17] This tool is designed to assess bias in animal studies and contains ten items to investigate bias in selection, performance, detection, attrition and reporting. Ten points are scored for every item complied with. No points are awarded when the study does not meet the criterium or when documentation is unclear. The total score ranges from 0 to 100 with 0 being the worst, with a high chance of bias, and 100 being the best score, seemingly free from bias.
OUTCOME MEASURES
The main outcome measures of the included studies were bursting pressure (BPR) and hydroxyproline levels (HP). BPR involves a measurement whereby air is instilled in a closed segment of bowel with the anastomosis, and established by means of a sudden decline in pressure or visualization of air bubbles in a submerged anastomosis. Hydroxyproline is formed during the synthesis of collagen and has proven to be a good predictor for AL.[ 18] Other outcomes measured in some studies were histopathological analysis (HA), various biochemical analyses and the total energy of rupture biomechanical test (ETR).
STATISTICAL ANALYSIS
The software package Review Manager 5.3,[ 19] was used to perform a meta-analysis of the primary outcome sources, which was determined after careful study of the results. An inversed variance test was used for the meta-analysis. In all cases, P < 0.05 (two-sided) was considered statistically significant.
Results
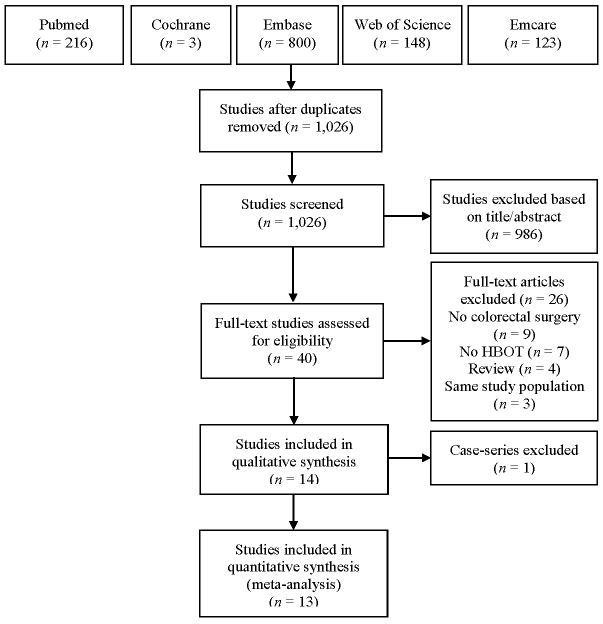
The PRISMA literature search and study selection are shown in Figure 1. Thirteen animal trials were included for qualitative and quantitative synthesis (Table 1).[ 4 , 20 - 31] Appendix A∗ identifies where each item in the PRISMA checklist may be found in this report. In addition, Appendix B∗ presents the full electronic search strategy such that it could be repeated.
Figure 1.
PRISMA flow chart for meta-analysis
Table 1. Study protocol of included animal trials; HBOT − hyperbaric oxygen therapy; M – male; F − female; d – days; ATA − atmosphere absolute; CRT – chemoradiotherapy; CT – Chemotherapy; GH − growth hormone; BPR − bursting pressure; HP – hydroxyproline; PPg-glucan − poly B1-6 glucotriosys B1-3 glucopyranose glucan; PA − pathological analysis; MMP − matrix-metalloproteinase; BA − biochemical analysis; ETR − total energy of rupture biomechanical test .
| Author | n | Species/sex | Pre/postop HBOT | HBOT days/length (min)/pressure (kPa) | Additional interventions | Additional interventions | Measured outcomes |
| Boersema[4] | 10 | Wistar, M | Colectomy | Pre- and postop | 7 preop + 3 postop / 1 x 90 / 243 | None | BPR+PA |
| Hamzaoğlu[20] | 10 | Wistar, M | Left colon resection | Postoperative | 4 / 1 x 60 / 253 | Induced ischaemia | BPR+HP |
| Erenoglu[21] | 10 | Wistar, M | Colectomy | Postoperative | 7 / 2 x 90 / 203 | Preoperative CRT | BPR+HP |
| Guzel[22] | 10 | Wistar, F | Induced ischaemia + colonic anastomosis | Postoperative | 4 / 1 x 60 / 253 | PPg-glucan | BPR+HP |
| Yagci[23] | 10 | Wistar, M | Left colon resection | Preoperative | 2 / 2 x 90 / 284 | Induced ischaemia | BPR+HP+PA |
| Yagci[23] | 10 | Wistar, M | Left colon resection | Postoperative | 4 / 2 x 90 / 284 | Induced ischaemia | BPR+HP+PA |
| Yagci[23] | 10 | Wistar, M | Left colon resection | Pre- and postop | 2 preop + 4 postop / 2 x 90 / 284 | Induced ischaemia | BPR+HP+PA |
| Sucullu[24] | 8 | Wistar, M/F | Induced peritonitis + colectomy | Postoperative | 3 or 7 / 1 x 90 / 253 | None | BPR+HP+PA |
| Azevedoi[25] | 10 | Wistar, ? | Colectomy | Postoperative | 7 / 1 x 90 / 203 | Induced ischaemia | HP+MMP1+MMP9 |
| Rocha[26] | 15 | Wistar, F | Induced peritonitis + colectomy | Postoperative | 4 / 1 x 120 / 203 | None | ETR |
| Adas[27] | 10 | Wistar, M | Left colon resection | Postoperative | 4 / 3 x 60 / 253 | GH | BPR+PA |
| Kemik[28] | 10 | Wistar, F | Left colon resection | Postoperative | 4 / 4 x 80 / 253 | CT | BPR+PA |
| Yildiz[29] | 12 | Wistar, F | Left colon resection | Postoperative | 5 / 2 x 90 / unknown | Preoperative CRT | BPR+HP+PA |
| Poyrazoglu[30] | 7 | Sprague-Dawley, M | Left colon resection | Postoperative | 4 / 1 x 120 / 284 | None | BPR+BA |
| Poyrazoglu[30] | 7 | Sprague-Dawley, M | Left colon resection | Pre- and postop | 2 h preop + 4 postop / 1 x 120 / 284 | None | BPR+BA |
| Emir[31] | 10 | Wistar, M | Laparoscopic left colon resection | Postoperative | 10 / 1 x 60 / 213 | None | BPR+HP+PA |
STUDY PROTOCOLS
All animal trials reported the effect of HBOT on colonic anastomoses in rats (Table 1). Ten studies[ 20 - 22 , 24 - 29 , 31] used postoperative HBOT, one study used a combination of pre-and postoperative (combined) HBOT,[ 4] one had two study groups researching postoperative and combined HBOT[ 30] and the last study had three study groups analyzing preoperative, postoperative and combined HBOT.[ 23] All studies performed open surgery with one exception which used a laparoscopic technique.[ 30] There is wide variation in the HBOT protocols in terms of the treatment intervals, durations of treatment, length of the HBOT courses and pressures.
QUALITY ASSESSMENT
The quality assessment using the SYRCLE tool is shown in Table 2. None of the studies met all quality criteria. Six studies[ 4 , 24 , 26 , 29 - 31] randomized the study and control groups, but none of the studies provided baseline statistics, potentially concealing selection bias. None of the studies randomly selected the animals for outcome assessment or described blinding the outcome assessor. In only one study were the investigators blinded.[ 30] In all but one[ 23] of the seven studies that included pathologic analysis,[ 4 , 23 , 24 , 27 - 29 , 31] the outcome assessor for the analysis was blinded, decreasing the chance of detection bias. Overall, the included studies generally lacked steps in their protocols to minimize the chance of (any kind of) bias.
Table 2. Quality assessment using the SYRCLE risk of bias tool; 1 = yes, 2 = no, 3 = unclear; 10 points are scored for every item complied with and no points are awarded when the study does not meet the criterium or when documentation is unclear; total score ranges from 0−100, 0 being the worst, with a high chance of bias, and 100 being the best score, seemingly free from bias .
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Yes | Unclear | No | Score | |
| Hamzaoğlu[20] | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 1 | 1 | 2 | 2 | 2 | 6 | 20 |
| Erenoglu[21] | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 1 | 1 | 2 | 2 | 2 | 6 | 20 |
| Guzel[22] | 2 | 3 | 2 | 1 | 2 | 3 | 3 | 1 | 1 | 2 | 3 | 4 | 3 | 30 |
| Yagci[23] | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 1 | 2 | 3 | 1 | 6 | 30 |
| Sucullu[24] | 1 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 1 | 2 | 5 | 1 | 4 | 50 |
| Azevedo[25] | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 2 | 2 | 1 | 7 | 20 |
| Rocha[26] | 1 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 2 | 3 | 1 | 6 | 30 |
| Adas[27] | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 1 | 2 | 3 | 1 | 6 | 30 |
| Kemik[28] | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 1 | 1 | 4 | 0 | 6 | 40 |
| Yildiz[29] | 1 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 1 | 2 | 4 | 1 | 5 | 40 |
| Poyrazoglu[30] | 1 | 3 | 3 | 2 | 3 | 3 | 3 | 1 | 1 | 2 | 3 | 2 | 5 | 30 |
| Boersema[4] | 1 | 3 | 3 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 4 | 1 | 5 | 40 |
| Emir[31] | 1 | 3 | 1 | 1 | 1 | 3 | 3 | 1 | 1 | 2 | 6 | 1 | 3 | 60 |
NON-ISCHAEMIC ANASTOMOSIS
Ten[ 4 , 20 , 21 , 23 , 25 , 27 - 31 ] of the thirteen studies focused on non-ischaemic anastomosis in normal conditions (Table 3). One study used three study groups − preoperative, postoperative and combined HBOT,[ 22] whilst another used two study groups − postoperative and combined HBOT,[ 29] resulting in a total of thirteen different study groups. Of these thirteen study groups, five study groups from five different studies reported a significant improvement of the anastomosis after HBOT treatment.[ 4 , 20 , 25 , 27 , 30] In the other eight study groups, analyzed by six different studies, any observed improvement of the anastomosis did not reach statistical significance.[ 21 , 23 , 28 - 31] There was no association between HBOT and anastomosis strength in the study groups assessing preoperative or combined HBOT.[ 4 , 23 , 30]
Table 3. Outcome of studies assessing normal anastomoses; HBOT− hyperbaric oxygen treatment; Prob. − probability; ALF − anastomotic line fibrosis; U − unknown; MMP − matrix-metalloproteinase; ns − not significant; ↑ − significantly increased; ↓ − significantly decreased; NV – neovascularization; CD − collagen deposition; N – necrosis; E – epithelialization; G – granulation; FML − formation of mucosal layer; SI − severity of inflammation; tMDA − tissue malondialdehyde; tMPO − tissue myeloperoxidase; tSOD − tissue superoxide dismutase; tGSH-Px − tissue glutathionperoxidase; sMDA − serum malondialdehyde; sNO − serum nitric oxide; sCAT − serum catalase; tNO − tissue nitric oxide; tCAT − tissue catalase .
| Author | Bursting pressure(mmHg) | Hydroxyproline | Pathology | Other | Improved | |||||
| HBOT | Control | P≤0.05 | HBOT | Control | Units | P≤0.05 | ||||
| Hamzaoğlu[20] | 123±18.4 | 104±18.9 | Yes | 10.12±4 | 7.4±2 | mg·mg tissue-1 | Yes | − | − | Yes |
| Erenoglu[21] | 221±6.05 | 190.2±18.14 | No | 22.88±2.38 | 9.01±2.04 | µg·10 mg tissue-1 | Yes | − | − | No |
| Yagci[23] | 115.5±21.1 | 107.2±37.5 | No | 13.89±3.43 | 9.95±2.65 | µM· mg tissue-1 | Yes | ALF ns | − | No |
| Yagci[23] | 113.6±16.9 | 107.2±37.5 | No | 13.11±4.39 | 9.95±2.65 | µM· mg tissue-1 | Yes | ALF ns | − | No |
| Yagci[23] | 119.2±16.7 | 107.2±37.5 | No | 13.25±3.27 | 9.95±2.65 | µM· mg tissue-1 | Yes | ALF ns | − | No |
| Azevedo[25] | − | − | − | Unknown | Unknown | µg· mg tissue-1 | Yes | − | MMP1 ns; MMP9 ns | Yes |
| Adas[27] | 93.3±20.5 | 81.4±20.1 | Yes | − | − | − | − | CD↑; NV↑; N ns; E ns; G ns | − | Yes |
| Kemik[28] | 93.4±24.8 | 84.5±24.4 | No | − | − | − | − | NV↑; CD ns; N ns; E ns; G ns | − | No |
| Yildiz[29] | 133.7±29.7 | 110.7±18.6 | No | 17.3±6.6 | 15±5.8 | µM·mg-1 tissue-1 | No | ALF ns | − | No |
| Poyrazoglu[30] | 152.9±18 | 122.6±16.7 | Yes | 83±11.1 | 60.4±14.4 | mg·g protien-1 | Yes | − | tMDA↓; tMPO↓; tSOD↑; tGSH-Px↑ | Yes |
| Poyrazoglu[30] | 126±13.6 | 122.6±16.7 | No | 63.7±18.7 | 60.4±14.4 | mg·g protien-1 | No | − | tMPO↓; tMDA ns; tSOD ns; tGSH-Px ns | No |
| Boersema[4] | 162.4±39.7 | 141.1±73.3 | No | − | − | − | − | NV↑; CD206+↑; M2/M1↑; CD ns; iNOS+ ns | − | Yes |
| Emir[31] | 213±27 | 197±9.1 | No | 26.5±4.1 | 26.8±4.36 | µg·10 mg tissue-1 (dry) | No | FML ns; SI ns | sMDA↓; sNO ns; CAT ns; tMDA ns; tNO ns; tCAT ns | No |
∗ Footnote:
Appendices A and B may be obtained from the corresponding author at: rjbrouwer@alrijne.nl
The BPR was measured in twelve study groups, from nine different studies, and was higher in the HBOT group in all study populations.[ 4 , 20 , 21 , 23 , 27 - 31] A significant increase of BPR was observed in three study groups, analysed by three different studies.[ 20 , 27 , 30] HP was measured in ten study groups from seven different studies.[ 20 , 21 , 23 , 25 , 29 - 31] Of these ten study groups, HP was significantly higher in seven study groups, analysed by five different studies.[ 20 , 21 , 23 , 25 , 30] There was a marked variation in HP levels (Table 3). Six studies[ 20 - 22 , 25 , 30 , 31] measured HP in grams in tissue, while two studies[ 23 , 29] measured HP molarity in tissue. One study[ 22] measured HP in wet tissue, whilst another[ 31] dried the tissue for 24 hours before analysis. The remaining six studies[ 20 , 21 , 23 , 25 , 29 , 30] measuring HP did not describe how they prepared the tissue for analysis.
HISTOPATHOLOGICAL ANALYSIS
The histopathological analysis varied between studies. Three studies assessed anastomotic line fibrosis and found no significant difference between any groups.[ 23 , 24 , 29] Another assessed the formation of a mucosal layer and the severity of inflammation at the anastomosis and found no significant differences.30 Three studies found a significant increase in neovascularization in the HBOT group.[ 4 , 27 , 28] The same three studies assessed collagen deposition, but only one found a significant increase in collagen deposition in the HBOT group.26 No significant differences were found in necrosis, epithelialization or granulation.[ 26 , 27] All tissue biochemical markers changed in the study group that received only postoperative HBOT.[ 29] Malondialdehyde (MDA), an indicator of fat oxidation, and myeloperoxidase, an indicator of inflammation, were lowered and superoxide dismutase and glutathione peroxidase, both indicators of the antioxidant response, were elevated.[ 29] In the study group that received both pre- and postoperative HBOT, only MDA was significantly lower.[ 29] In another study measuring nitric oxide, MDA and catalase in serum and tissue, a significant decrease was demonstrated only in serum MDA in the HBOT group.[ 30]
ISCHAEMIC ANASTOMOSES
Seven study groups from five different studies assessed the influence of HBOT on ischaemic anastomoses (Table 4).[ 20 , 22 , 23 , 25 , 27] In six groups from the five studies, HBOT had a positive effect on the anastomosis. The only exception was he group that received preoperative HBOT only.[ 23] Five study groups analyzed by four studies, found a significant improvement in the BPR.[ 20 , 22 , 23 , 27] Three study groups from three of these studies, also found a significant improvement in HP.[ 20 , 22 , 23]
Table 4. Outcome of studies assessing ischemic anastomoses; HBOT− hyperbaric oxygen treatment; ALF− anastomotic line fibrosis; ns − not significant; MMP − matrix-metalloproteinase; ↑ − significantly increased; ↓ − significantly decreased; CD − collagen deposition; NV – neovascularization; N – necrosis; E – epithelialization; G − granulation .
| Author | Bursting pressure(mmHg) | Hydroxyproline | Pathology | Other | Improved | |||||
| HBOT | Control | Prob. ≤ 0.05 | HBOT | Control | Units | Prob. ≤ 0.05 | ||||
| Hamzaoğlu[20] | 102.2±14.8 | 77.5±22.1 | Yes | 6.04±2 | 4.76±2 | mg·mg tissue-1 | Yes | − | Yes | |
| Guzel[22] | 104±19.4 | 69.5±16.7 | Yes | 12.1±1.1 | 4.3±0.6 | mg·100 mg wet tissue-1 | Yes | − | Yes | |
| Yagci[23] | 81.2±9.2 | 79.3±7.7 | No | 9.51±.87 | 8.42±2.1 | µM·mg tissue-1 | No | ALF ns | − | No |
| Yagci[23] | 97.9±17.9 | 79.3±7.7 | Yes | 10.01±1.88 | 8.42±2.1 | µM·mg tissue-1 | No | ALF ns | − | Yes |
| Yagci[23] | 109.0±8.4 | 79.3±7.7 | Yes | 11.06±1.95 | 8.42±2.1 | µM·mg tissue-1 | Yes | ALF ns | − | Yes |
| Azevedo[25] | − | − | − | Unknown | Unknown | µM·mg tissue-1 | No | − | MMP1↑; MMP9↑ | Yes |
| Adas[27] | 109.9±25.3 | 62±21.19 | Yes | − | − | − | − | CD↑; NV↑; N↓; E ns; G ns | − | Yes |
ANASTOMOSES DURING PERITONITIS
Two studies[ 24 , 26] investigated the effect of HBOT on colonic anastomoses created during peritonitis. One[ 24] observed an improvement in the anastomosis during peritonitis with a significantly higher BPR, but this observation using ETR as outcome measure was not supported by the other.[ 25]
META-ANALYSIS
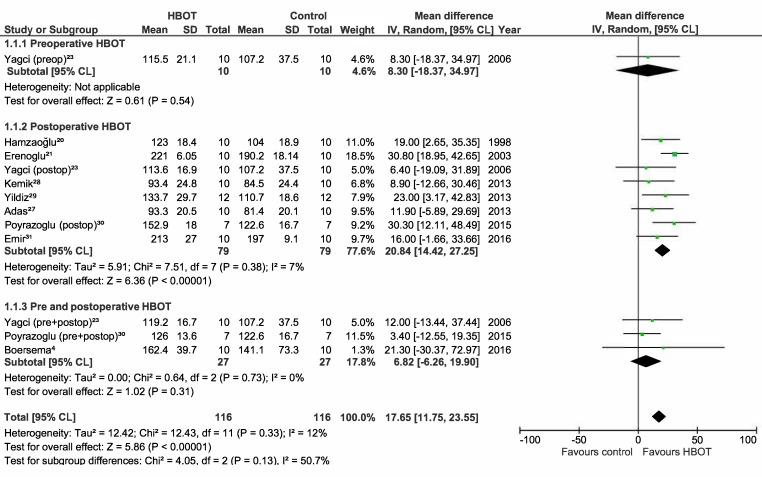
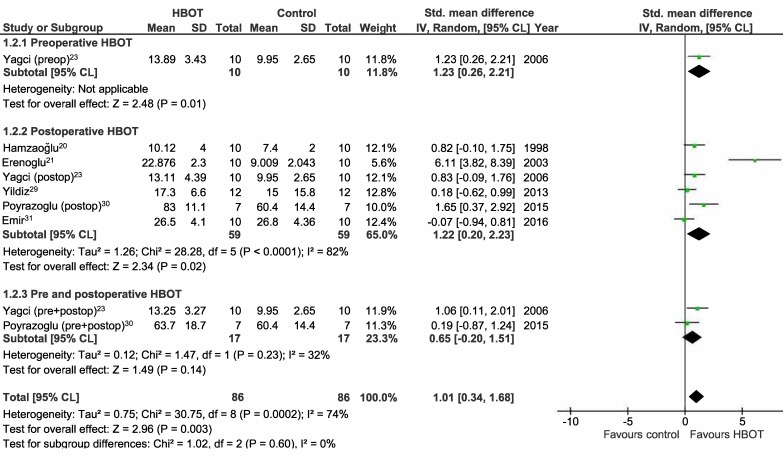
Meta-analyses on the studies using BPR and HP as outcome measures were performed and included the studies assessing normal and ischaemic anastomoses.[ 4 , 20 - 23 , 25 , 27 - 31] The results are displayed as Forest plots in Figures 2, 3, 4, and 5. Only one study analyzed the effect of preoperative HBOT on both normal and ischaemic anastomoses, and the effect of combined HBOT on ischaemic anastomoses.[ 23] Therefore, this meta-analysis will not provide extra insights for these groups. For the BPR group, the mean difference (MD) is displayed. Because of the variety in the test determining HP, a standardized mean difference (SMD) was used and because of the high variance of the HBOT protocols between the studies, a random effect was chosen for this meta-analysis. For meta-analysis including the studies using BPR to assess normal anastomoses, a low statistical heterogeneity was found (I2 = 12%). The other three meta-analyses showed high statistical heterogeneity (I2 = 74%, 88% and 84% respectively) and, therefore, should be interpreted with caution.
Figure 2.
Forest plot showing the effect of HBOT on bursting pressure (BPR) in normal anastomoses; HBOT − hyperbaric oxygen treatment; SD − standard deviation; IV − inverse variance, Random − random effect, CI − confidence interval; preop − preoperative; Z − Z-test; P – probability; postop – postoperative; Chi2 − chi-square test; I2 − I-square test for heterogeneity; df − degrees of freedom
Figure 3.
Forrest plot showing the effect of HBOT on hydroxyproline levels (HP) in normal anastomoses; HBOT − hyperbaric oxygen treatment; SD − standard deviation; IV − inverse variance, Random − random effect, CI − confidence interval; preop − preoperative; Z − Z-test; P – probability; postop – postoperative; Chi2 − chi-square test; I2 − I-square test for heterogeneity; df − degrees of freedom
Figure 4.
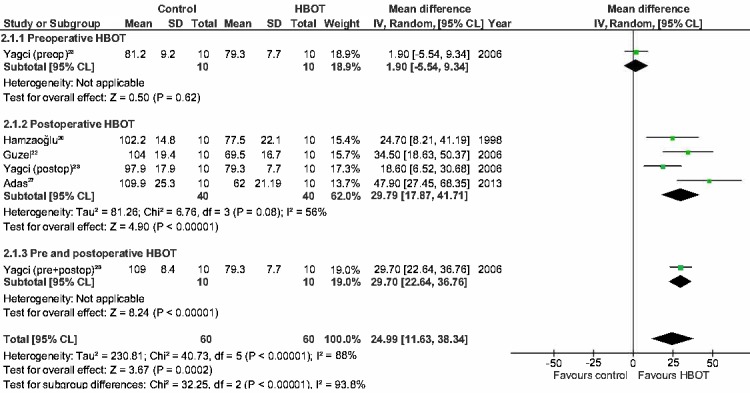
Forest plot showing the effect of HBOT on bursting pressure (BPR) in ischemic anastomoses; HBOT − hyperbaric oxygen treatment; SD − standard deviation; IV − inverse variance, Random − random effect, CI − confidence interval; preop − preoperative; Z − Z-test; P – probability; postop – postoperative; Chi2 − chi-square test; I2 − I-square test for heterogeneity; df − degrees of freedom
Figure 5.
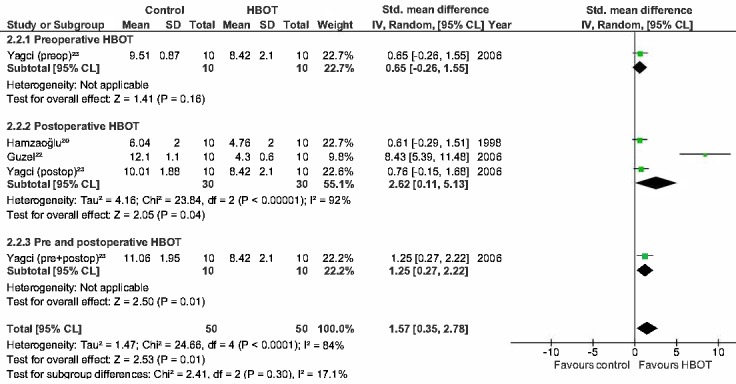
Forest plot showing the effect of HBOT on hydroxyproline levels (HP) in ischemic anastomoses; HBOT − hyperbaric oxygen treatment; SD − standard deviation; IV − inverse variance, Random − random effect, CI − confidence interval; preop − preoperative; Z − Z-test; P – probability; postop – postoperative; Chi2 − chi-square test; I2 − I-square test for heterogeneity; df − degrees of freedom
The BPR and HP in the postoperative group of normal anastomoses are significantly improved as shown in Figure 2 and 3 (MD = 20.8 mmHg (14.4, 27.3), P ≤ 0.001; SMD = 1.2 (0.20, 2.23), P = 0.02). The BPR and HP of the studies performing combined HBOT do not show a significant improvement (MD = 6.8 mmHg (-6.3, 19.9), P = 0.31, SMD = 0.7 (-0.20, 1.51), P = 0.14). The postoperative group of ischaemic anastomoses (Figures 4 and 5) show significant improvement in both BPR and HP (MD = 29.8 mmHg (17.9, 41.7), P ≤ 0.001, SMD = 2.6 (0.11, 5.13), P = 0.04).
Discussion
This is the first meta-analysis describing the effect of HBOT on the outcome in colorectal surgery and shows significant improvement of BPR and HP in both normal and ischaemic anastomoses in rats after postoperative HBOT. HP is considered a reliable marker for the strength of the anastomosis and risk of AL in a rabbit model.[ 18] Therefore, these results could be useful in the complex pathophysiology regarding HBOT and oncology in humans.
The exact mechanism of HBOT in the improvement of colorectal anastomoses is unknown. However, some steps within this pathway are becoming more clearly defined:
HBOT reduces the risk of AL by lowering the pro-inflammatory response;[ 9]
Elevated immune parameters like IL-1, IL-6, IL-10 and tumour necrosis factor (TNF-α) are associated with AL, indicating a connection between AL and a pro-inflammatory response;[ 32]
HBOT reduces the risk of AL by improvement of neovascularization.[ 4 , 26 , 27]
Only three studies used preoperative HBOT as a part of their HBOT protocol.[ 4 , 23 , 30] Of these, a significant difference was only found in the combined HBOT group of one study assessing ischaemic anastomoses,[ 23] but not in the other two.[ 4 , 30] The meta-analysis for postoperative HBOT showed a stronger association between HBOT and the prevention of AL than that for preoperative HBOT. The reasons for this difference are not yet identified. Regarding the results shown in Figure 2, preoperative HBOT might possibly prevent the positive effect of postoperative HBOT.
The major limitation of the current review is the quality of the available evidence. According to the SYRCLE tool there is a risk of bias in most of the included studies. Also, the protocols varied between studies, making it problematic to combine them in a meta-analysis. Different HBOT doses (pressure and time) might influence outcome. Furthermore, the statistical heterogeneity between included studies was high, and only the meta-analysis of the subgroup using BPR as outcome measure for postoperative HBOT in non-ischaemic anastomoses could be regarded as trustworthy. The results of the other three subgroups should be interpreted with caution. Finally, most colorectal resections are performed on patients with a malignancy, whereas these studies are performed on rats without a malignancy. Although the current consensus is that HBOT does not promote cancer, further research might be needed before recommending HBOT as a routine for patients with colorectal cancer.
There is only one reported human HBOT case series of five patients who underwent an ultra-low anterior resection with a temporary loop ileostomy and who developed AL with chronic pelvic sepsis.[ 32] All five received postoperative HBOT (90 minutes at 203–243 kPa, five days per week for six weeks), four also receiving adjuvant chemo-radiotherapy. All the patients showed improvement in the degree of anastomotic separation and sepsis.[ 32]
Conclusions
This meta-analysis provides some evidence to suggest HBOT may be a useful adjunct in colorectal surgery. Postoperative HBOT increases the strength of the colorectal anastomosis in rats without a malignancy, this effect appearing to be stronger in ischaemic anastomoses. To investigate the full potential of HBOT to prevent AL in human patients undergoing colorectal surgery, a pilot study should be performed. Since it would be hard to obtain the large numbers of human patients that would be necessary, further research should focus primarily on a larger systematic animal study using postoperative HBOT and with AL as the primary outcome measure.
Footnotes
Acknowledgements
We thank Professor H Putter, Department of Medical Statistics and Bioinformatics, for statistical support, and CAM van der Hoorn, Walaeus Library, both of the Leiden University Medical Centre, Leiden, The Netherlands and OC Lindner, Library, Alrijne Hospital, Leiderdorp, The Netherlands for their help with the literature search.
Conflicts of interest: nil.
Contributor Information
Robin J Brouwer, Department of Surgery, Alrijne Hospital, Leiderdorp, The Netherlands.
Alexander C Engberts, Department of Surgery, Alrijne Hospital, Leiderdorp, The Netherlands.
Boudewijn LS Borger van der Burg, Department of Surgery, Alrijne Hospital, Leiderdorp, The Netherlands.
Thijs TCF van Dongen, Department of Surgery, Alrijne Hospital, Leiderdorp, The Netherlands; Defense Healthcare Organization, Ministry of Defense, Utrecht, The Netherlands.
Rob A van Hulst, Department of Anesthesiology, Amsterdam Medical Center, Amsterdam, The Netherlands; Maritime Medical Expertise Center, Diving Medical Center, Royal Netherlands Navy, Den Helder, The Netherlands.
Rigo Hoencamp, Department of Surgery, Alrijne Hospital, Leiderdorp, The Netherlands; Defense Healthcare Organization, Ministry of Defense, Utrecht, The Netherlands; Department of Surgery, Leiden University Medical Center, Leiden, The Netherlands.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012 . CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Clinical Practice Guidelines management of colon cancer . [cited 2017 Aug 30]; Available from: https://www.fascrs.org/sites/default/files/downloads/publication/practice_parameters_for_the_management_of_colon.21.pdf.
- Clinical Practice Guidelines management of rectal cancer . [cited 2017 Aug 30]; Available from: https://www.fascrs.org/sites/default/files/downloads/publication/practice_parameters_for_the_management_of_rectal.2.pdf.
- Boersema GS, Wu Z, Kroese LF, Vennix S, Bastiaansen-Jenniskens YM, van Neck JW, et al. Hyperbaric oxygen therapy improves colorectal anastomotic healing . Int J Colorectal Dis. 2016;31:1031–1038. doi: 10.1007/s00384-016-2573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha GW, Kim JH, Lee MR. Oncologic impact of anastomotic leakage following colorectal cancer surgery: a systematic review and meta-analysis . Ann Surg Oncol. 2017;11:3289–3299. doi: 10.1245/s10434-017-5881-8. [DOI] [PubMed] [Google Scholar]
- Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy . N Engl J Med. 1996;334:1642–1648. doi: 10.1056/nejm199606203342506. [DOI] [PubMed] [Google Scholar]
- Mathieu D, Marroni A, Kot J. Tenth European Consensus Conference on Hyperbaric Medicine: recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment . Diving Hyperb Med. 2017;47:24–32. doi: 10.28920/dhm47.1.24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LK, editor. 13th ed. North Palm Beach, FL: Undersea and Hyperbaric Medical Society; 2014. Hyperbaric oxygen therapy indications . [Google Scholar]
- Camporesi EM, Bosco G. Mechanisms of action of hyperbaric oxygen therapy . Undersea Hyperb Med. 2014;41:247–252. [PubMed] [Google Scholar]
- Camporesi EM. Side effects of hyperbaric oxygen therapy . Undersea Hyperb Med. 2014;41:253–257. [PubMed] [Google Scholar]
- Lehm JP, Bennett MH. Predictors of middle ear barotrauma associated with hyperbaric oxygen therapy . SPUMS Journal. 2003;33:127–133. [Google Scholar]
- Bosco G, Casarotto A, Nasole E, Camporesi E, Salvia R, Giovinazzo F, et al. Preconditioning with hyperbaric oxygen in pancreaticoduodenectomy: a randomized double-blind pilot study . Anticancer Res. 2014;34:2899–2906. [PubMed] [Google Scholar]
- Moen I, Stuhr LE. Hyperbaric oxygen therapy and cancer − a review . Target Oncol. 2012;7:233–242. doi: 10.1007/s11523-012-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeier J, Carl U, Hartmann K, Sminia P. Hyperbaric oxygen: does it promote growth or recurrence of malignancy? . Undersea Hyperb Med. 2003;30:1–18. [PubMed] [Google Scholar]
- Daruwalla J, Christophi C. The effect of hyperbaric oxygen therapy on tumour growth in a mouse model of colorectal cancer liver metastases . Eur J Cancer. 2006;42:3304–3311. doi: 10.1016/j.ejca.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement . J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration . PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandall A, Lowndes R, Young HL. Colonic anastomotic healing and oxygen tension . Br J Surg. 1985;72:606–609. doi: 10.1002/bjs.1800720808. [DOI] [PubMed] [Google Scholar]
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre TCC; 2014. [Google Scholar]
- Hamzaoglu I, Karahasanoglu T, Aydin S, Sahin DA, Carkman S, Sariyar M, et al. The effects of hyperbaric oxygen on normal and ischemic colon anastomoses . Am J Surg. 1998;176:458–461. doi: 10.1016/s0002-9610(98)00234-7. [DOI] [PubMed] [Google Scholar]
- Erenoglu C, Uluutku H, Shahrbaf S, Emeksiz S, Akin ML, Foley E, Celenk T. Effect of hyperbaric oxygen on anastomoses created under the influence of 5-FU . Undersea Hyperb Med. 2003;30:321–326. [PubMed] [Google Scholar]
- Guzel S, Sunamak O, As A, Celik V, Ferahman M, Nuri MM, et al. Effects of hyperbaric oxygen and Pgg-glucan on ischemic colon anastomosis . World J Gastroenterol. 2006;12:1421–1425. doi: 10.3748/wjg.v12.i9.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagci G, Ozturk E, Ozgurtas T, Gorgulu S, Kutlu OC, Topal T, et al. Preoperative and postoperative administration of hyperbaric oxygen improves biochemical and mechanical parameters on ischemic and normal colonic anastomoses . J Invest Surg. 2006;19:237–244. doi: 10.1080/08941930600778230. [DOI] [PubMed] [Google Scholar]
- Sucullu I, Sinan H, Filiz AI, Yildiz S, Yucel E, Kurt Y, et al. The effects of hyperbaric oxygen therapy on colonic anastomosis in rats with peritonitis . J Invest Surg. 2008;21:195–200. doi: 10.1080/08941930802155534. [DOI] [PubMed] [Google Scholar]
- Azevedo LA, Parra RS, Da Rocha JJ, Ramalho LN, Ramalho FS, Feres O. Hyperbaric oxygen on the healing of ischemic colonic anastomosis – an experimental study in rats . Undersea Hyperb Med. 2010;37:405–411. [PubMed] [Google Scholar]
- Rocha AA, Leal RF, Ayrizono Mde L, Chung WF, Coy CS, Lee HD, et al. Hyperbaric oxygen therapy and mechanical resistence of the colonics anastomosis in rats with peritonitis . Acta Cir Bras. 2010;25:368–374. doi: 10.1590/s0102-86502010000400013. [DOI] [PubMed] [Google Scholar]
- Adas M, Kemik O, Adas G, Arikan S, Kuntsal L, Kapran Y, et al. Is combined therapy more effective than growth hormone or hyperbaric oxygen alone in the healing of left ischemic and non-ischemic colonic anastomoses? . Clinics (Sao Paulo) 2013;68:1440–1445. doi: 10.6061/clinics/2013(11)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemik O, Adas G, Arikan S, Gurluler E, Dogan Y, Toklu AS, et al. Evaluation of the effects of hyperbaric oxygen treatment and enoxaparin on left colon anastomosis. An experimental study . Eur Rev Med Pharmacol Sci. 2013;17:2286–2292. [PubMed] [Google Scholar]
- Yildiz R, Can MF, Yagci G, Ozgurtas T, Guden M, Gamsizkan M, et al. The effects of hyperbaric oxygen therapy on experimental colon anastomosis after preoperative chemoradiotherapy . Int Surg. 2013;98:33–42. doi: 10.9738/CC130.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyrazoglu Y, Topal T, Yuksel R, Bircan FS, Simsek K, Gocgeldi E, et al. Effects of hyperbaric oxygen and preconditioning on wound healing in colonic anastomoses . J Invest Surg. 2015;28:188–195. doi: 10.3109/08941939.2014.999961. [DOI] [PubMed] [Google Scholar]
- Emir S, Gurdal SO, Sozen S, Bali I, Yesildag E, Celik A, et al. Does hyperbaric oxygen therapy reduce the effects of ischemia on colonic anastomosis in laparoscopic colon resection? . Ann Ital Chir. 2016;87:83–91. [PubMed] [Google Scholar]
- Komen N, de Bruin RW, Kleinrensink GJ, Jeekel J, Lange JF. Anastomotic leakage, the search for a reliable biomarker. A review of the literature . Colorectal Dis. 2008;10:109–115. doi: 10.1111/j.1463-1318.2007.01430.x. [DOI] [PubMed] [Google Scholar]