Abstract
Objective To ascertain if useful criteria for prenatal diagnosis of fetal ventral body wall defects (VBWDs) exists by reviewing published literature on diagnosis of VBWD as compared with our own diagnostic experience.
Study Design A comprehensive literature review of diagnostic criteria of fetal VBWD including pentalogy of Cantrell (POC), omphalocele, exstrophy, imperforate anus, spina bifida (OEIS), cloacal exstrophy, limb–body wall complex (LBWC), and body stalk anomaly was performed followed by a retrospective review of all fetal magnetic resonance imaging (MRI) examinations from our medical center over a 2-year period.
Results Classically, OEIS is omphalocele, bladder exstrophy, imperforate anus, and spina bifida. POC is defects of the supraumbilical abdomen, sternum, diaphragm, pericardium, and heart. LBWC is two of the following: exencephaly or enencephaly with facial clefts, thoracoschisis or abdominoschisis, and limb defects. Twenty-four cases of VBWD on MRI over a 24-month period were identified with seven cases involving defects of additional organ systems. Six of these seven cases demonstrated findings from two or more of the traditional diagnoses POC, OEIS, and LBWC making diagnosis and counseling difficult.
Conclusion There is a lack of consensus on useful diagnostic criteria within the published literature which is reflected in our own diagnostic experience and poses a challenge for accurate prenatal counseling.
Keywords: OEIS, cloacal exstrophy, limb–body wall complex, body stalk anomaly, pentalogy of Cantrell, fetal MRI
Fetal ventral body wall defects (VBWDs) comprise a range of congenital malformations of widely varying severity and prognosis. Some can be isolated and relatively straightforward, involving high rates of survival to delivery and successful postnatal surgical repair, such as uncomplicated gastroschisis. 1 Others involve abnormalities of multiple organ systems and have been described as uniformly fatal, such as limb–body wall complex (LBWC). 2 Several entities previously thought to be unique, such as cloacal exstrophy and omphalocele, exstrophy, imperforate anus, spina bifida (OEIS) complex, are now regarded by many as one and the same. 3 Conversely, some conditions initially thought to be represented by a single phenotype, such as pentalogy of Cantrell (POC), have recently been described as having partial presentations. 4 5 Distinct from uncomplicated omphalocele, which has its own unique developmental pathophysiology and association with abnormal karyotypes, other types of VBWD involve some degree of failure of fusion of the lateral and craniocaudal body folds around the umbilical ring during the 5th and 6th weeks of gestation. While a few sources have suggested there may be a genetic basis for certain types of these VBWD, 6 the majority indicates that these patients nearly always have a normal karyotype. 7 8 9
With improvements in prenatal diagnostic methods, VBWDs are now often detected earlier in utero. Consequently, an interdisciplinary team of maternal–fetal medicine (MFM) physicians, neonatologists, pediatric surgeons, pediatric radiologists, and various other subspecialists can be involved in diagnosis, prognostication, and family counseling. Aberrations of the fetal ventral body wall can be detected in the late first or early second trimester, during screening ultrasound (US). At our center, this typically leads to a referral to MFM and subsequent expert US. Further evaluation with fetal magnetic resonance imaging (MRI) is performed for cases in which the diagnosis is in question, lung and/or herniated organ volume measurement are desired, or suspected neurological anomalies require further characterization.
Diagnosis is often not in question when the VBWD is an isolated defect such as gastroschisis or bowel-only omphalocele. However, when the defect is more complex, involving the thorax, pelvis, neural tube, limbs, or genitourinary system, there can be greater diagnostic difficulty. While there are certainly some published case reports and case series describing patients whose imaging findings fit neatly within established diagnostic criteria, many others do not. For these other cases, the findings may straddle multiple diagnoses or may be best described as “hybrid.” Indeed, many cases initially published as representative of a certain VBWD were later critiqued in subsequent reports as having been misclassified. Similarly, multiple retrospective studies that reanalyzed prenatal imaging found high discrepancy rates between the clinical imaging report and the diagnosis reached during the study. 7 10 11 12 13 This apparent lack of consensus raises questions regarding the benefit of too rigid and adherence to the traditional diagnostic categories within the range of VBWD. Further, a review of recent cases encountered within our own prenatal diagnostic clinic confirms the disparate reports in the literature, and the theme that many patients present with overlapping features of different VBWD. The practice of prenatal diagnosis depends on the ability to counsel families appropriately regarding expected outcomes, and we suggest that the current paradigm is too dependent on rigid diagnostic categories that do not account for those hybrid cases frequently reported in the literature and seen in our own cohort.
Materials and Methods
The Institutional Review Board of our academic health care system approved this retrospective imaging study with waiver of written participant consent.
Literature Review
An extensive review of the literature was performed. First, recent articles pertaining to the radiologic diagnosis of VBWDs were identified through a PubMed search of “diagnosis” AND each key phrase: “pentalogy of Cantrell” (155 results), “OEIS” (60 results), “cloacal exstrophy” (214 results), “limb–body wall complex” (69 results), and “body stalk anomaly” (BSA) (56 results). These were reviewed and a subset of publications which focused on prenatal imaging diagnosis was identified and summarized. Further critical review determined the seminal papers for each diagnosis, from which a narrative of the diagnostic history of each entity was produced. Next, case reports and case series gathered in the initial PubMed search of each entity were systematically reviewed to collate diagnostic criteria, common findings, and reported findings ( Table 1 and Fig. 1) .
Table 1. Reported fetal findings by diagnosis.
| OEIS complex/cloacal exstrophy | |
| Diagnostic criteria | Four key criteria in the acronym |
| Omphalocele | |
| Exstrophy of the bladder | |
| Imperforate anus | |
| Spina bifida | |
| Proposed common findings | Kidney malformations (up to 60% of cases 49 ) |
| Hydroureter 49 50 | |
| Hydronephrosis 49 50 51 | |
| Congenital megaureter 16 52 | |
| Pelvic kidney 16 53 54 | |
| Duplex kidney 16 54 | |
| Renal dysplasia 51 55 | |
| Cystic dysplasia 16 49 50 | |
| Renal agenesis 49 50 52 55 56 57 | |
| Limb anomalies | |
| Clubfoot with vertical talus 50 | |
| Limb length discrepancy 53 | |
| Bilateral clubfeet 16 53 56 | |
| Neural tube defects 57 58 (70% of cases 49 ) | |
| Spina bifida, myelocystocele 49 | |
| Terminal myelocystocele 16 53 54 | |
| Lumbosacral spina bifida 51 56 | |
| Hindbrain herniation, lipomyelomeningocele 16 | |
| Omphalocele 16 52 54 55 56 | |
| Spine abnormalities | |
| Kyphoscoliosis 49 50 | |
| Sacral hemivertebra 55 | |
| Pubic diastasis 51 | |
| Missing bladder 16 51 52 53 54 56 | |
| Single umbilical artery 16 52 54 55 | |
| Abnormality of external genitalia 16 54 | |
| Hypospadias 55 | |
| Bifid corporal bodies 53 | |
| Duplicated vagina, bifid scrotum, micropenis 56 | |
| Ambiguous genitalia 51 | |
| Case reports | Intrinsic cardiac abnormalities |
| Moderate PDA, small PFO, mild tricuspid regurgitation 54 | |
| ASD, atrioventricular valve, unroofed coronary sinus, persistent L SVC, LVH 55 | |
| Single right ventricle 56 | |
| Pentalogy of Cantrell | |
| Diagnostic criteria | Five distinct criteria, partial cases may have fewer than five |
| Intracardiac abnormalities | |
| Anterior pericardial defects | |
| Lower sternal defects | |
| Anterior diaphragmatic defects | |
| Supraumbilical abdominal wall defects 12 | |
| Proposed common findings | Ectopia cordis 4 8 22 24 26 59 |
| Intrinsic cardiac malformations | |
| ASD 4 26 50 | |
| VSD 4 8 24 50 | |
| Tetralogy of Fallot 4 22 24 26 50 | |
| Left ventricular diverticulum 26 50 | |
| Transposition of the great vessels 26 | |
| Tricuspid atresia, dextrocardia, anomalous cardiac venous return 8 | |
| Neural tube defects 26 58 | |
| Midline supraumbilical abdominal wall defect | |
| Omphalocele 4 8 22 26 50 59 | |
| Diastasis recti abdominis, umbilical hernia, epigastric hernia 50 | |
| Ventral hernia, open defect 26 | |
| Supraumbilical hernia 24 | |
| Sternal defects | |
| Bifid sternum 4 26 | |
| Cleft sternum 4 22 24 | |
| Absence of xiphoid 8 | |
| Split sternum 26 | |
| Diaphragmatic defects | |
| Anterior diaphragmatic hernia 4 24 26 59 | |
| Pericardial defects | |
| Pericardial hernia 4 50 | |
| Absent pericardium 26 59 | |
| Umbilical cord defects | |
| Single umbilical artery 8 22 60 | |
| Short cord, hypercoiled cord associated with POC + ectopia cordis 8 | |
| Facial defects | |
| Cleft lip and/or palate 26 | |
| Case reports | Aplastic left limb 61 |
| Spinal defect 62 | |
| Hypoplastic lung 26 | |
| Bronchopulmonary dysplasia, hypoplastic kidney, cleft lip and palate, pulmonary atresia 4 | |
| Gastroschisis, twin reversed arterial perfusion sequence 8 | |
| Nonrotation of the midgut, accessory spleen 22 | |
| Limb–body wall complex | |
| Diagnostic criteria | Two of the following |
| Exencephaly or encephalocele with facial clefts | |
| Thoraco- and/or abdominoschisis | |
| Limb defects 7 63 | |
| Two distinct phenotypes | |
| Craniofacial defects often with cranioplacental adhesion and or amniotic bands | |
| No craniofacial defects but abdominal–placental attachment with short/absent umbilical cord and urogenital anomalies 35 | |
| Body stalk anomaly | |
| Large abdominal wall defect with herniation of abdominal contents into the extraembryonic coelom | |
| Absent or rudimentary umbilical cord 34 | |
| Proposed common findings | Absent/rudimentary/short umbilical cord 32 34 47 58 |
| Limb anomalies | |
| Pseudosyndactyly without amniotic bands, oligodactyly, polydactyly, split hand and foot, single bone forelimb, forebone abnormalities, absent limb and limb girdle, absent muscles and arthrogryposis 7 63 | |
| Clubbed feet, single lower limb 34 | |
| Bilateral clubbed feet 32 | |
| Club foot, rocker bottom foot 47 | |
| Neural tube defects 46 58 | |
| Exencephaly or encephalocele 7 63 | |
| Abdominal wall defects | |
| Abdominoschisis 34 64 | |
| Omphalocele 32 39 47 | |
| Skeletal abnormalities | |
| Kyphoscoliosis 32 34 | |
| Scoliosis 46 47 64 | |
| Craniofacial abnormalities 28 34 | |
| Case reports | Encephalocele, anophthalmia, bilateral cleft lip, thoracic cleft with ectopia cordis, omphalocele, short umbilical cord, single umbilical artery 39 |
| Ectopia cordis 11 | |
| Anencephaly 32 | |
| Abdominoschisis, diaphragmatic defect 39 | |
| Agenesis of the anal canal, agenesis of the genitourinary tract, hypoplastic lungs 64 | |
| Absent diaphragm, bowel atresia, renal agenesis, anal atresia, no external genitalia 44 | |
| Anal atresia, no external genitalia, no urinary bladder, hypoplastic lungs 47 | |
Abbreviations: ASD, atrial septal defect; OEIS, omphalocele, exstrophy, imperforate anus, spina bifida; PDA, patent ductus arteriosus; PFO, patent foramen ovale; SVC, superior vena cava.
Fig. 1.
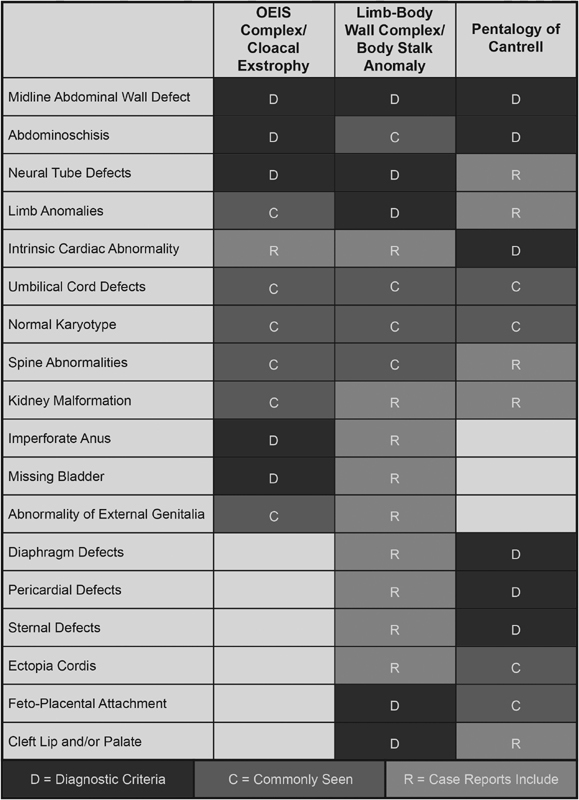
Overlap of findings for OEIS complex/cloacal exstrophy, limb–body wall complex/body stalk anomaly, and pentalogy of Cantrell graphically represented. OEIS, omphalocele, exstrophy, imperforate anus, spina bifida.
Case Series
The institutional radiology database was queried for MRI of pregnant females over a 24-month period from January 2016 to January 2018. A total of 364 studies were identified, 24 (6.5%) of which demonstrated findings of VBWD. Cases of isolated omphalocele and gastroschisis were excluded, leaving a total of 7 out of 24 (29%) studies.
Review of these imaging examinations revealed that MRI was performed for a variety of clinical indications including concern for fetal pathology, suspected morbidly adherent placenta, or concern for maternal intra-abdominal inflammatory process.
Maternal clinical and demographic information were obtained from retrospective chart review, including maternal age, gestational age (GA), parity, race, health conditions, and medications used during pregnancy. Clinical information about the fetus was obtained from chart review and included: maternal parity and pertinent medical history, findings on prenatal US and prenatal MRI, GA at the time of these studies, results of genetic testing, GA at delivery, delivery history, placenta pathologic examination results, intrapartum and neonatal outcomes, surgical interventions and intraoperative findings, mortality, and autopsy results.
US imaging studies were performed via transabdominal technique via GE Voluson 730 (GE Electric Medical System, Milwaukee, WI) with an abdominal convex probe of 3.5 MHz.
MRI studies were obtained from a Siemens 3.0 tesla scanner (MAGNETOM Skyra; Siemens, Erlangen, Germany). Exact pulse sequences differed depending on the indication for MRI, but included half-Fourier acquisition single-shot fast spin-echo, balanced steady-state free precession, T1 spoiled gradient echo, echo planar imaging, and diffusion-weighted imaging. All scans utilized included three planes of imaging.
Results
Seven cases of prenatal MRI were identified for review. The MRI examinations were performed between 25 and 37 weeks' GA. For each case, GA at MRI, prenatal imaging diagnosis, GA at birth, delivery type, Apgar score (when applicable), length of neonatal hospital stay, type of neonatal surgical repair, surgical diagnosis, pathologic diagnosis, and ultimate disposition are discussed later.
Case 1
A 35-year-old G4P2012 patient with two prior cesarean sections had a routine prenatal US at 18 + 4 weeks at an outside hospital showing multiple congenital anomalies including ventral wall defect. A prenatal MRI was performed at 19 + 4 weeks to further refine the diagnosis, and expert US was performed at 29 + 2 weeks which showed: (1) a septated cystic structure at fetal sacrum with no Doppler flow, (2) abdominal wall defect containing liver, and (3) empty right renal fossa with a right pelvic kidney. MRI examinations showed: (1) an abdominoschisis containing liver and bowel, (2) pelvic right kidney, (3) splaying of the bladder into hemimasses, (4) sacral myelocystocele, (5) thoracic kyphosis (butterfly vertebra), and (6) hypogenesis of the corpus callosum. Calculated lung volumes were 70% of predicted and invasive placenta previa was noted. Amniocentesis showed XY with no chromosomal abnormalities and a microarray was normal. Overall, the findings suggested a diagnosis of OEIS, and the patient was counseled accordingly.
Delivery was at 35 + 5 weeks via cesarean section, and the neonate had a birth weight of 2,810 g with Apgar scores of 1, 1, and 5 at 1 minute, 5, and 10 minutes, respectively. There was a three-vessel umbilical cord. Several days after birth, the neonate underwent surgery to tubularize the colon, form an end colostomy, and reapproximate the bladder halves. At that time, the patient was noted to have a short colonic segment with two appendices, giant abdominoschisis, and pelvic findings of OEIS. The patient spent time admitted to the neonatal intensive care unit (NICU) and had a recovery complicated by return to the operating room for resection of a bowel stricture. The patient remains alive.
Case 2
A 29-year-old G3P2 patient with a history of preterm labor had routine prenatal US showing ventral wall defect, which led to prenatal MRI at 28 + 2 weeks ( Fig. 2 ) showing: (1) giant abdominoschisis with fusion of the hernia sac to the placenta, (2) covered lumbosacral myelomeningocele, (3) unilateral renal agenesis, (4) nonvisualized bladder with “elephant trunk” appearance of the ileum between hemibladder masses, (5) narrow thoracic cavity, (6) focal scoliosis with hemivertebra, (7) a shortened umbilical cord, (8) suspected hypoplastic sternum, (9) small defect in the anterior left hemidiaphragm and potentially in the diaphragmatic pericardium with inferior displacement of the heart, (10) complete absence of the right lower extremity, and (11) hypoplastic left foot and partial amputation of the left tibia. Calculated lung volumes were 40% of predicted. The patient was referred to our fetal center, and expert prenatal US at 31 + 1 weeks showed similar findings including: (1) abdominoschisis with membrane adherent to the placenta, (2) a majority of the abdominal contents herniating through the defect, (3) absent right lower extremity and hemipelvis, (4) abnormalities of the distal bones of the left lower extremity, (5) a closed myelomeningocele, and (6) nonvisualized bladder and genitalia. Cell-free DNA analysis suggested a male fetus with low risk for aneuploidy. Given the highly complex and varied findings, and especially given the pulmonary hypoplasia, the patient was counseled that the prognosis was guarded.
Fig. 2.
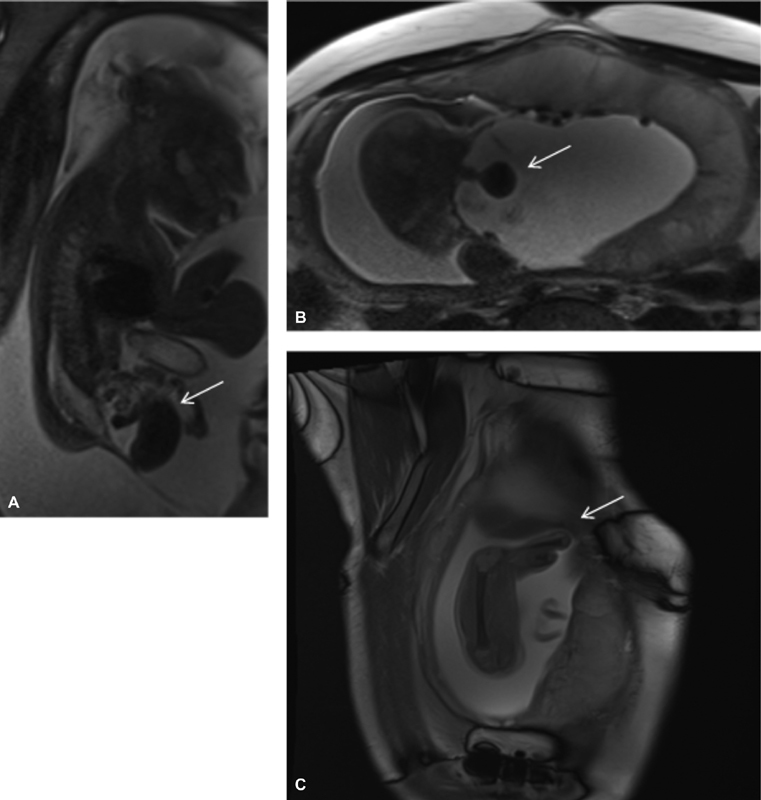
Case 2 sagittal ( A, C ) and axial ( B ) SSFSE MRIs. ( A, B ) No fluid-filled bladder, with “elephant trunk” midline loop of bowel and lateralized hemibladder masses. ( C ) Amputated lower limb in patient with myelomeningocele and nonvisualized bladder. MRI, magnetic resonance imaging; SSFSE, single-shot fast spin-echo.
Delivery was at 33 + 4 weeks via emergent cesarean section at an outside hospital due to premature labor. The neonate was unable to be adequately ventilated, and died at 6 hours after birth secondary to extreme metabolic and respiratory acidosis. An autopsy was not performed.
Case 3
A 32-year-old G2P0101 patient with hypothyroidism and history of preterm premature rupture of membranes was referred to our center for ventral wall defect, and received expert prenatal US at 29 + 3 weeks which showed: (1) splayed lower spine with intact skin, (2) bilateral talipes equinovarus, (3) nonvisualized bladder, and (4) two-vessel cord. Follow-up prenatal MRI at 31 + 5 weeks showed: (1) infraumbilical abdominoschisis, (2) nonvisualized bladder with hemibladder masses externally, (3) poorly formed external genitalia, (4) lumbosacral myelomeningocele, (5) bilateral talipes equinovarus deformity, and (6) two-vessel cord. The patient did not desire genetic testing. The constellation of findings was most suggestive of OEIS, and the patient was counseled accordingly.
Delivery was at 35 + 4 weeks via urgent cesarean section for breech position and preterm labor. The male neonate was transferred to the NICU. At several days of life, the neonate underwent surgery to form an ostomy and reapproximate the bladder halves. Repair of the lipomyelomeningocele and tethered cord was conducted months later. Bilateral pelvic osteotomies with additional iliac closing wedge were done at 13 months of age and surgery to form an ileovesicostomy was done at 14 months of age. The infant is currently alive and continues follow-up in the urology and spina bifida clinics.
Case 4
A 28-year-old G2P1001 patient had a routine prenatal US at 18 + 0 weeks which showed: (1) a ventral wall defect with liver and bowel extrusion, (2) nonvisualized bladder, (3) possible early ectopia cordis with a structural cardiac abnormality, (4) short umbilical cord which traveled directly from the anterior placenta to the herniated organs, (5) motion of fetal extremities but not of the fetal body, (6) scoliosis, and (7) a right talipes equinovarus deformity. A prenatal MRI at 28 + 5 weeks showed: (1) nonvisualized bladder with “elephant trunk” appearance of the ileum between hemibladder masses, (2) infraumbilical abdominoschisis with fusion of the hernia sac to the placenta, (3) lumbosacral myelomeningocele with scoliosis, (4) a right talipes equinovarus deformity, (5) narrowed thoracic cavity, and (6) single umbilical artery with a shortened cord and anomalous fetal insertion (wide separation of the vessels prior to entering the fetal abdominal cavity). Calculated lung volumes were 25% of predicted. Cell-free DNA testing suggested a female fetus with low risk for aneuploidy. Given the highly complex and varied findings, including features of POC, OEIS, and LBWC, and especially given the pulmonary hypoplasia, the patient was counseled that the prognosis was likely to be poor.
Delivery was at 32 + 0 weeks via classical cesarean section for preterm labor, with damage to the ventral hernia sac membrane occurring during delivery, as expected. At immediate neonatal surgical repair, the patient was found to have a large abdominoschisis with exstrophy of bladder and a myelomeningocele covered with skin. She was difficult to ventilate after delivery. A decision for comfort care was made after 6 hours of life and death occurred shortly thereafter. No autopsy was performed.
Case 5
A 25-year-old G2P1 patient with no significant past medical history had a prenatal US at an outside center at 19 + 6 weeks which showed an abdominal wall mass and possible neural tube defect. A prenatal MRI at 22 + 5 weeks showed: (1) an abdominoschisis containing the liver, (2) two-vessel umbilical cord, (3) nonvisualized urinary bladder with hemibladder masses, (4) abnormal male external genitalia, (5) lumbar myelomeningocele, and (6) right renal agenesis. Cell-free DNA testing suggested a male fetus with no other abnormalities. These features were thought to be most compatible with OEIS, and the patient was counseled accordingly. However the pregnancy ended with fetal demise in the second trimester, with delivery at an outside center.
Case 6
A 31-year-old G3P1111 patient had a routine prenatal US at outside center, which showed VBWD. She presented to our center at 24 + 6 weeks and prenatal MRI was performed ( Fig. 3 ), showing: (1) abdominoschisis, (2) myelomeningocele, (3) severe scoliosis, (4) talipes equinovarus deformity bilaterally, (5) short umbilical cord, and (6) small thoracic cavity. A second MRI at 28 + 5 weeks redemonstrated the small thoracic cavity. Calculated lung volumes were 17% of predicted. Karyotype from amniocentesis showed XY with no other additional studies ordered. Given these findings, the patient was counseled regarding OEIS as the most likely diagnosis, and given the severe pulmonary hypoplasia, was told that prognosis was poor. The pregnancy ended with fetal demise in the third trimester, with delivery at an outside center.
Fig. 3.
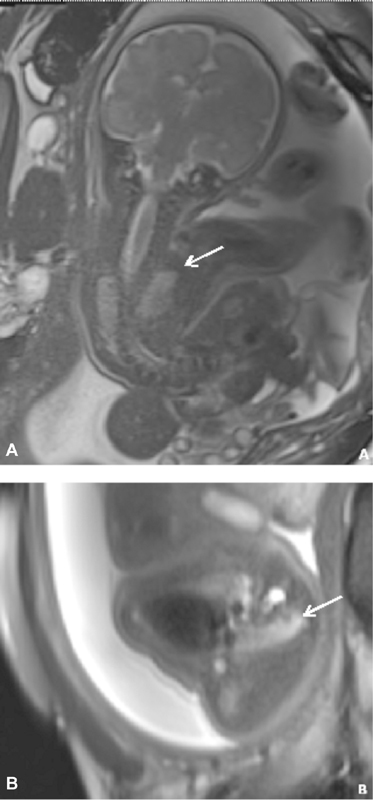
Case 6 coronal ( A ) and axial ( B ) SSFSE MRIs. ( A, B ) Scoliosis and severely narrowed thoracic cavity, with pulmonary hypoplasia. MRI, magnetic resonance imaging; SSFSE, single-shot fast spin-echo.
Case 7
A 24-year-old G1P0 patient with sickle cell trait had a ventral defect found on outside routine prenatal US and underwent prenatal MRI at 21 + 0 weeks which showed: (1) supraumbilical abdominoschisis, (2) suspected mild defect of the anterior diaphragm and lower sternum/chest wall, and (3) small thoracic cavity. Calculated lung volumes were 65% of predicted. An expert prenatal US at 25 + 3 weeks showed: (1) abdominoschisis with liver herniation and (2) malposition of the cardiac apex abnormally anterior in position. No genetic testing was desired. At the time of this case study, the fetus is not yet delivered.
Discussion
One of the earliest published reports of cloacal exstrophy was made in the late 19th century by an Italian teratologist, Dr. C. Taruffi, who also referenced ancient descriptions of perineal congenital anomalies. 14 In 1978, Carey et al published a case series of 10 patients, the largest to that date, suggesting the name “OEIS complex,” as a simple and accurate moniker. 11 Importantly, this article proposed that “OEIS complex is a distinct and clinically recognizable entity of heterogeneous etiology.” 11 Indeed, OEISs represented the consistent findings among the cases, but other malformations, such as clubfoot and ambiguous genitalia, were also reported. In a 2001 editorial, Carey clarified OEIS complex and cloacal exstrophy are meant to be synonymous. 3 In 1992, a case report from Smith et al asserted OEIS may be the worst form of the exstrophy–epispadias sequence but provided no citation for this claim. 6 In 1998, Austin et al proposed a set of diagnostic criteria for US, based heavily on the initial observations of Carey et al and moving OEIS from a postnatal to a prenatal diagnosis. 10 They suggested the following major criteria: nonvisualization of the bladder, a large midline infraumbilical anterior wall defect or cystic anterior wall structure (persistent cloacal membrane), abdominoschisis, and lumbosacral anomalies, and minor criteria: lower extremity defects, renal anomalies, ascites, widened pubic arches, a narrow thorax, hydrocephalus, and a single umbilical artery. 10 In 1999, Hamada et al suggested the midline prolapsed ileum visualized on US between the hemibladder masses, termed the “elephant trunk sign,” should be added to Austin et al's sonographic diagnostic criteria. 15 Calvo-Garcia et al reported a case series of eight patients with cloacal exstrophy suggesting fetal MRI to be useful in the prenatal diagnostic algorithm when US is inconclusive. 16 They also identified specific fetal MRI findings contributing to the diagnosis of cloacal exstrophy. 17
Much of the literature on OEIS is found in urologic journals because these defects are typically repaired and patients are followed up by pediatric urologists. The oft-quoted incidence of OEIS, 1 in 200,000 live births originates in a 1970 five case series with review of cases to date. 18 The incidence of 1 in 400,000 births is from an extrapolation of the rate of bladder exstrophy and relative proportions of vesical exstrophy complex of anomalies in 1986. 19 These incidences have been questioned in case reports. 13 Recently, a 2011 epidemiologic study from the International Clearinghouse for Birth Defects Surveillance and Research suggested an overall prevalence of 1 in 131,579 with variance by country. 20 A survival rate of up to 90% was first reported in a series of 34 patients spanning 1963 to 1986 and published in 1987. 21
POC was first described by Cantrell et al in a 1958 case series of five patients linked to an additional 16 previously published case reports. 12 In it, they described a syndrome of congenital defects: midline supraumbilical abdominoschisis, lower sternal defect, deficiency of the anterior diaphragm, deficiency of the diaphragmatic pericardium, and intracardiac abnormalities. These constitute the five findings necessary for a diagnosis. Notably, ectopia cordis is not necessary for diagnosis but is often seen in POC and is considered a poor prognostic factor. 4 22 In 1972, Toyama published a case report and review of 60 purported cases of POC, in which they suggest diagnosing incomplete expression as a variant of the syndrome. 5 Thus, a neonate with partial POC may present with only two, three, or four of the necessary five defects. In 1998, Vazquez-Jimenez et al conducted an extensive review of the literature, compiling 153 purported cases of POC and reviewing the spectrum of malformations. 23 US, the mainstay of prenatal imaging, is most frequently used to assist in the diagnosis of POC. 22 24 Siles et al noted that pericardial effusion was a helpful indicator of a pericardial defect in a three case series of POC. 25 In 2007, McMahon et al reported the use of combined fetal MRI and fetal echocardiography to guide prenatal planning. 24 US was considered more useful than MRI for assessing sternal and pericardial defects. The incidence of POC, which has been quoted at 5.5 in 1 million live births, was first estimated in a case series of five patients from a population in the Baltimore–Washington, DC region of the United States. 26 The authors qualified this incidence as a regional estimate. Other descriptions in the literature suggest estimates ranging from 1 in 6,500 to 1 in 200,000 births. 22 The latter is derived from the 5.5 in 1 million estimate, while the former originates from a Finnish group who assumed seven cases in 7 years represented the total live birth incidence of POC in Finland. 27
LBWC was first reported in the European literature at the start of the 20th century. Van Allen et al are widely cited as the first to put forward discrete diagnostic criteria for LBWC in a case series of 25, published in 1987. 7 They based their diagnosis on two of the three following findings: (1) exencephaly or encephalocele with facial clefts, (2) thoraco- and/or abdominoschisis, and (3) limb defect. Initially, LBWC was regarding as distinct from a similar diagnosis, BSA. Embryologically, the “body stalk” is seen early in development, connecting the embryo to the placenta in early life and is composed of extraembryonic somatic mesoderm and the three umbilical vessels. 28 Abnormal persistence of this connection has been termed “BSA,” and in a 1992 case report, Giacoia suggested absence of the umbilical cord and fusion of the organ containing membranous sac to the placenta as key findings in BSA. 29 Although not clear in the literature as to when, at some point many authors began to assert that LBWC was equivalent with BSA. 30 31 32 33 Other sources state that these are distinct entities on the same spectrum, and some have presented criteria on how to differentiate the two. Namely, BSA will not have extremity defects. 34
In 1993, based on a review of current literature, Russo et al suggested LBWC presents with two distinct phenotypes. In this description, the first has two specific findings: (1) encephalocele or exencephaly, always associated with facial cleft, and (2) amniotic bands and or broad amniotic adhesion between the cranial defect and the placenta. The second phenotype will not have the aforementioned findings, but often presents with (1) urogenital anomalies, (2) anal atresia, (3) lumbosacral meningocele, and (4) placental abnormalities such as an intact amnion, short cord, and persistence of the extraembryonic coelom. 35 This nomenclature has been invoked as a valid method to classify LBWC defects by multiple subsequent authors. US is frequently cited as an effective prenatal diagnostic modality for this condition, 28 30 32 33 34 and Sahinoglu et al put forth sonographic criteria for three phenotypes of LBWC based on a case series of six. 36 Recently, Aguirre-Pascual et al noted fetal MRI to be useful as an adjunct diagnostic modality to US in the prenatal characterization of LBWC. 37 LBWC has also been included as one manifestation of the amniotic band sequence, an idea first proposed by Torpin in 1965. 38 39 However, in 1989, a case series of four by Hartwig et al challenged this relationship, saying that malformations of LBWC are better explained by a malfunction in the ectodermal placodes. 40 Recently, Moerman et al argued that amniotic band sequence and LBWC represent discrete entities which have pathogenic overlap as opposed to spectrums of the same disease. 41 Given the large number of cases with normal karyotype, many authors have proposed a multiple hit phenomenon. 42 43 However, in 2011, Hunter et al described an overview of the many diverse theories and made a case for a primary mechanism. 43 LBWC is considered by most authors to be uniformly fatal with a purported incidence of 1 in 4,000, though no source is given for this. 44
The precise pathogenesis of these three entities remains unknown, though frequently debated. Many theories have been proposed but none has been validated. Many of the case reports in the literature contain, within a single patient, features from two or more of these diagnoses or overlapping features from multiple diagnoses. Given this, some have proposed that these conditions are less likely to be distinct diagnostic categories with unique pathophysiologic mechanisms, but rather more likely to represent multiple points along a continuous spectrum. This would also mirror proposals by Smrcek et al and Hunter et al who each suggested that the numerous manifestations of VBWD are likely to reflect variations of aberrant cephalic, caudal, and/or lateral folding, thus giving rise to subsequent patterns of maldevelopment. 30 43
As a clear example, consider the following two sets of criteria which have been asserted as diagnostic of OEIS and type 2 LBWC ( Table 2 ). OEIS is described as omphalocele/exstrophy of the bladder/imperforate anus/spina bifida, while type 2 LBWC is described as abdominoschisis/urogenital anomalies/anal atresia/lumbosacral meningocele.
Table 2. Comparison of diagnostic criteria for OEIS complex and type 2 LBWC.
| OEIS complex 11 | Type 2 LBWC 35 |
|---|---|
| Omphalocele | Abdominoschisis |
| Exstrophy of the bladder | Urogenital anomalies |
| Imperforate anus | Anal atresia |
| Spina bifida | Lumbosacral meningocele |
Abbreviations: LBWC, limb–body wall complex; OEIS, omphalocele, exstrophy, imperforate anus, spina bifida.
The remarkable similarity of these two sets of criteria confuses the diagnostic process. Of even greater concern is that the published mortality rates of these two conditions are incredibly divergent, frequently reported as ∼10% for OEIS, and as 100% for LBWC. 2 21 For expectant families, such a wide range of supposed outcomes severely limits the ability of the perinatal care team to provide proper counseling.
Our case series and review of the literature demonstrate that it can be extremely challenging to fit a newly diagnosed fetal VBWD into the existing diagnostic categories. Numerous reported cases of VBWD ( Fig. 4 ), rather than fitting completely into one of the traditional diagnostic silos, instead span across more than one of these diagnoses. 31 42 45 46 47 48 In our own cohort, six of the seven complicated VBWD cases encountered over a 24-month period demonstrated just such a hybrid constellation of findings. The traditional diagnostic categories may obscure more than they reveal.
Fig. 4.
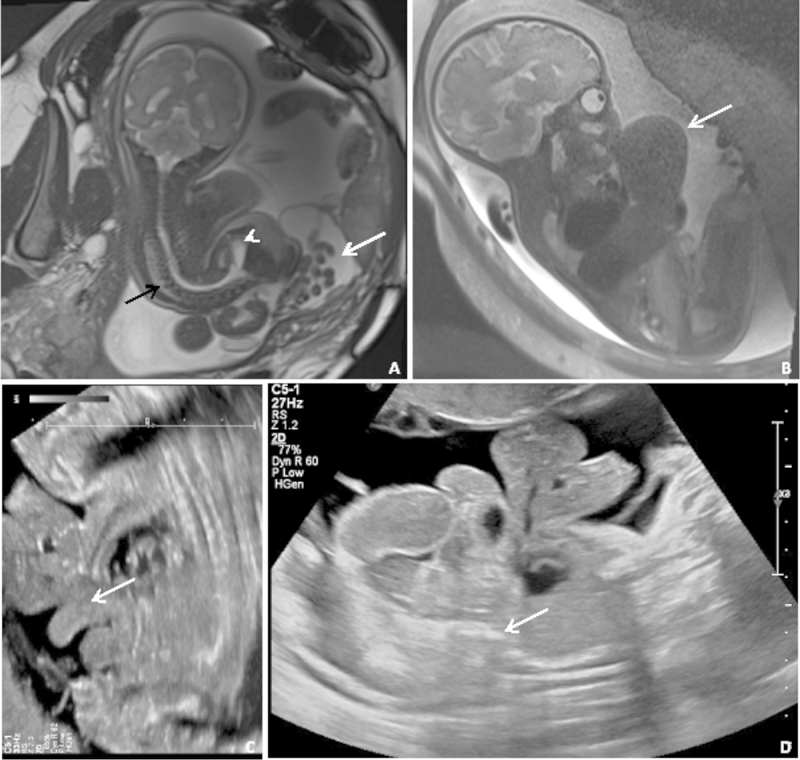
Coronal SSFP ( A ) and sagittal SSFSE ( B ) MRIs and two transabdominal ultrasound images of several older cases from our center. ( A ) Severe scoliosis (black arrow), abdominoschisis (white arrow), and meningocele (short arrow), but without body wall fusion. ( B ) Supraumbilical defect (white arrow) and absent bladder. ( C ) “Elephant trunk” sign representing prolapsed terminal ileum. ( D ) Fetal fusion to the placenta (white arrow) with large abdominoschisis. MRI, magnetic resonance imaging; SSFP, steady-state free precession; SSFSE, single-shot fast spin-echo.
We believe that the current paradigm depends too heavily on creating distinctions, when many cases seem to fall somewhere between two or more of the traditional diagnostic categories. This is especially clear given the published literature on this topic is far from uniform agreement. Perhaps most importantly, we need to consider what approach would be most clinically relevant.
Further, although many of the patients in our cohort exhibited diverse findings related to multiple diagnoses, six of the seven had findings that correlated well with the diagnostic criteria for cloacal exstrophy/OEIS. Given the published incidence of around 1 in 200,000 live births, this would mean that six cases in 24 months would represent a startlingly high rate to a region in the United States which has a total of around 30,000 deliveries annually. An alternative interpretation would be to recognize multiple of the cases in our cohort as hybrid, and not as completely representative of classic OEIS.
More importantly, we found that outcomes correlated much more closely with pulmonary development. In our recent cohort, the prenatal identification of pulmonary hypoplasia was quite useful, given that our two survivors (cases 1 and 3) had no documented hypoplasia on prenatal imaging, and three of the four patients who died (cases 2, 4, and 6) had pulmonary hypoplasia with lung volumes ranging from 17 to 40% of expected.
The size of our patient cohort and the relative infrequency of many of the diagnoses discussed here is a limitation of this review and analysis. Further, there are inherent limitations to any analysis of prenatal diagnosis of VBWD given the lack of diagnostic consensus in the literature, unknown etiology, and absence of confirmatory testing.
Conclusion
Our experience with these complex cases of VBWD reveals that they are most appropriately understood as existing along a spectrum of anomalies arising from failure of the lateral and craniocaudal folds to close appropriately early in gestation. Further, our analysis of the published literature on this topic demonstrates no clear consensus on how to optimally diagnose those cases which straddle the traditional diagnostic categories of OEIS/cloacal exstrophy, LBWD/BSA, and POC.
We propose a prenatal diagnostic process that values prognostication and planning over classification. Key objectives of prenatal diagnosis are to appropriately counsel families, plan for safe delivery, and direct immediate neonatal management. Any diagnostic categorization should be at the service of these goals.
At best, a rigid dependence on formal categories can lead to a confusing misnaming of disease, but at worst, it can result in serious prognostic inaccuracies and lead to increased distress for families seeking care. As an alternative, we call for the development of a more descriptive diagnostic approach, depending on type and volume of organ herniation, degree of pulmonary hypoplasia, and presence of head, body, or hernia membrane fusion to the placenta. Future prospective studies will be needed to further elucidate what imaging findings are most predictive of outcome.
Note
No funding sources supported this work. The authors have no disclosure.
References
- 1.Feldkamp M L, Botto L D, Byrne J LB, Krikov S, Carey J C. Clinical presentation and survival in a population-based cohort of infants with gastroschisis in Utah, 1997-2011. Am J Med Genet A. 2016;170A(02):306–315. doi: 10.1002/ajmg.a.37437. [DOI] [PubMed] [Google Scholar]
- 2.Emanuel P G, Garcia G I, Angtuaco T L. Prenatal detection of anterior abdominal wall defects with US. Radiographics. 1995;15(03):517–530. doi: 10.1148/radiographics.15.3.7624560. [DOI] [PubMed] [Google Scholar]
- 3.Carey J C. Exstrophy of the cloaca and the OEIS complex: one and the same. Am J Med Genet. 2001;99(04):270. doi: 10.1002/ajmg.1211. [DOI] [PubMed] [Google Scholar]
- 4.Kaul B, Sheikh F, Zamora I J et al. 5, 4, 3, 2, 1: embryologic variants of pentalogy of Cantrell. J Surg Res. 2015;199(01):141–148. doi: 10.1016/j.jss.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Toyama W M. Combined congenital defects of the anterior abdominal wall, sternum, diaphragm, pericardium, and heart: a case report and review of the syndrome. Pediatrics. 1972;50(05):778–792. [PubMed] [Google Scholar]
- 6.Smith N M, Chambers H M, Furness M E, Haan E A. The OEIS complex (omphalocele-exstrophy-imperforate anus-spinal defects): recurrence in sibs. J Med Genet. 1992;29(10):730–732. doi: 10.1136/jmg.29.10.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Allen M I, Curry C, Gallagher L. Limb body wall complex: I. Pathogenesis. Am J Med Genet. 1987;28(03):529–548. doi: 10.1002/ajmg.1320280302. [DOI] [PubMed] [Google Scholar]
- 8.Brochut A-CM, Baumann M U, Kuhn A et al. Pentalogy or hexalogy of Cantrell? Pediatr Dev Pathol. 2011;14(05):396–401. doi: 10.2350/10-09-0914-CC.1. [DOI] [PubMed] [Google Scholar]
- 9.Ueda H, Miyamoto T, Minase G, Sengoku K. Prenatal diagnosis of a body stalk anomaly by a combination of ultrasonography and foetal magnetic resonance imaging. J Obstet Gynaecol. 2017;37(07):946–947. doi: 10.1080/01443615.2017.1306842. [DOI] [PubMed] [Google Scholar]
- 10.Austin P F, Homsy Y L, Gearhart J Pet al. The prenatal diagnosis of cloacal exstrophy J Urol 1998160(3 Pt 2):1179–1181. [DOI] [PubMed] [Google Scholar]
- 11.Carey J C, Greenbaum B, Hall B D.The OEIS complex (omphalocele, exstrophy, imperforate anus, spinal defects) Birth Defects Orig Artic Ser 197814(6B):253–263. [PubMed] [Google Scholar]
- 12.Cantrell J R, Haller J A, Ravitch M M. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynecol Obstet. 1958;107(05):602–614. [PubMed] [Google Scholar]
- 13.Keppler-Noreuil K, Gorton S, Foo F, Yankowitz J, Keegan C. Prenatal ascertainment of OEIS complex/cloacal exstrophy - 15 new cases and literature review. Am J Med Genet A. 2007;143A(18):2122–2128. doi: 10.1002/ajmg.a.31897. [DOI] [PubMed] [Google Scholar]
- 14.Taruffi C. Bologna, Italy: Regia Tipografia; 1894. Storia Della Teratologia. Vol 7. [Google Scholar]
- 15.Hamada H, Takano K, Shiina H, Sakai T, Sohda S, Kubo T. New ultrasonographic criterion for the prenatal diagnosis of cloacal exstrophy: elephant trunk-like image. J Urol. 1999;162(06):2123–2124. doi: 10.1016/S0022-5347(05)68138-4. [DOI] [PubMed] [Google Scholar]
- 16.Calvo-Garcia M A, Kline-Fath B M, Rubio E I, Merrow A C, Guimaraes C V, Lim F-Y. Fetal MRI of cloacal exstrophy. Pediatr Radiol. 2013;43(05):593–604. doi: 10.1007/s00247-012-2571-3. [DOI] [PubMed] [Google Scholar]
- 17.Calvo-Garcia M A, Kline-Fath B M, Levitt M A et al. Fetal MRI clues to diagnose cloacal malformations. Pediatr Radiol. 2011;41(09):1117–1128. doi: 10.1007/s00247-011-2020-8. [DOI] [PubMed] [Google Scholar]
- 18.Tank E S, Lindenauer S M. Principles of management of exstrophy of the cloaca. Am J Surg. 1970;119(01):95–98. doi: 10.1016/0002-9610(70)90018-8. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler M, Duckett J, Howell C. Chicago: Year Book Medical Publishers; 1986. Cloacal exstrophy; pp. 764–771. [Google Scholar]
- 20.Feldkamp M L, Botto L D, Amar E et al. Cloacal exstrophy: an epidemiologic study from the International Clearinghouse for Birth Defects Surveillance and Research. Am J Med Genet C Semin Med Genet. 2011;157C(04):333–343. doi: 10.1002/ajmg.c.30317. [DOI] [PubMed] [Google Scholar]
- 21.Hurwitz R S, Manzoni G A, Ransley P G, Stephens F D.Cloacal exstrophy: a report of 34 cases J Urol 1987138(4 Pt 2):1060–1064. [DOI] [PubMed] [Google Scholar]
- 22.Desselle C, Herve P, Toutain A, Lardy H, Sembely C, Perrotin F. Pentalogy of Cantrell: sonographic assessment. J Clin Ultrasound. 2007;35(04):216–220. doi: 10.1002/jcu.20318. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez-Jimenez J F, Muehler E G, Daebritz S et al. Cantrell's syndrome: a challenge to the surgeon. Ann Thorac Surg. 1998;65(04):1178–1185. doi: 10.1016/s0003-4975(98)00089-7. [DOI] [PubMed] [Google Scholar]
- 24.McMahon C J, Taylor M D, Cassady C I, Olutoye O O, Bezold L I. Diagnosis of pentalogy of Cantrell in the fetus using magnetic resonance imaging and ultrasound. Pediatr Cardiol. 2007;28(03):172–175. doi: 10.1007/s00246-006-0032-1. [DOI] [PubMed] [Google Scholar]
- 25.Siles C, Boyd P A, Manning N, Tsang T, Chamberlain P.Omphalocele and pericardial effusion: possible sonographic markers for the pentalogy of Cantrell or its variants Obstet Gynecol 199687(5 Pt 2):840–842. [PubMed] [Google Scholar]
- 26.Carmi R, Boughman J A. Pentalogy of Cantrell and associated midline anomalies: a possible ventral midline developmental field. Am J Med Genet. 1992;42(01):90–95. doi: 10.1002/ajmg.1320420118. [DOI] [PubMed] [Google Scholar]
- 27.Vanamo K, Sairanen H, Louhimo I. The spectrum of Cantrell's syndrome. Pediatr Surg Int. 1991;6(06):429–433. [Google Scholar]
- 28.Goldstein I, Winn H N, Hobbins J C. Prenatal diagnostic criteria for body stalk anomaly. Am J Perinatol. 1989;6(01):84–85. doi: 10.1055/s-2007-999552. [DOI] [PubMed] [Google Scholar]
- 29.Giacoia G P.Body stalk anomaly: congenital absence of the umbilical cord Obstet Gynecol 199280(3 Pt 2):527–529. [PubMed] [Google Scholar]
- 30.Smrcek J M, Germer U, Krokowski M et al. Prenatal ultrasound diagnosis and management of body stalk anomaly: analysis of nine singleton and two multiple pregnancies. Ultrasound Obstet Gynecol. 2003;21(04):322–328. doi: 10.1002/uog.84. [DOI] [PubMed] [Google Scholar]
- 31.Mandrekar S RS, Amoncar S, Banaulikar S, Sawant V, Pinto R GW. Omphalocele, exstrophy of cloaca, imperforate anus and spinal defect (OEIS complex) with overlapping features of body stalk anomaly (limb body wall complex) Indian J Hum Genet. 2014;20(02):195–198. doi: 10.4103/0971-6866.142906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kocherla K, Kumari V, Kocherla P R. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25(01):67–70. doi: 10.4103/0971-3026.150162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11(02):116–120. doi: 10.1016/j.radcr.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy A, Platt L D. First-trimester diagnosis of body stalk anomaly using 2- and 3-dimensional sonography. J Ultrasound Med. 2011;30(12):1739–1743. doi: 10.7863/jum.2011.30.12.1739. [DOI] [PubMed] [Google Scholar]
- 35.Russo R, D'Armiento M, Angrisani P, Vecchione R. Limb body wall complex: a critical review and a nosological proposal. Am J Med Genet. 1993;47(06):893–900. doi: 10.1002/ajmg.1320470617. [DOI] [PubMed] [Google Scholar]
- 36.Sahinoglu Z, Uludogan M, Arik H et al. Prenatal ultrasonographical features of limb body wall complex: a review of etiopathogenesis and a new classification. Fetal Pediatr Pathol. 2007;26(03):135–151. doi: 10.1080/15513810701563728. [DOI] [PubMed] [Google Scholar]
- 37.Aguirre-Pascual E, Epelman M, Johnson A M, Chauvin N A, Coleman B G, Victoria T. Prenatal MRI evaluation of limb-body wall complex. Pediatr Radiol. 2014;44(11):1412–1420. doi: 10.1007/s00247-014-3026-9. [DOI] [PubMed] [Google Scholar]
- 38.Torpin R. Amniochorionic mesoblastic fibrous strings and amniotic bands: associated constricting fetal malformations or fetal death. Am J Obstet Gynecol. 1965;91:65–75. doi: 10.1016/0002-9378(65)90588-0. [DOI] [PubMed] [Google Scholar]
- 39.Zeidler S, Oudesluijs G G, Schoonderwaldt E M, Van Bever Y. Early prenatal disruption; a foetus with features of severe limb body wall sequence, body stalk anomaly and amniotic bands. Genet Couns. 2014;25(03):315–320. [PubMed] [Google Scholar]
- 40.Hartwig N G, Vermeij-Keers C, De Vries H E, Kagie M, Kragt H. Limb body wall malformation complex: an embryologic etiology? Hum Pathol. 1989;20(11):1071–1077. doi: 10.1016/0046-8177(89)90225-6. [DOI] [PubMed] [Google Scholar]
- 41.Moerman P, Fryns J P, Vandenberghe K, Lauweryns J M. Constrictive amniotic bands, amniotic adhesions, and limb-body wall complex: discrete disruption sequences with pathogenetic overlap. Am J Med Genet. 1992;42(04):470–479. doi: 10.1002/ajmg.1320420412. [DOI] [PubMed] [Google Scholar]
- 42.Heyroth-Griffis C A, Weaver D D, Faught P, Bellus G A, Torres-Martinez W. On the spectrum of limb-body wall complex, exstrophy of the cloaca, and urorectal septum malformation sequence. Am J Med Genet A. 2007;143A(10):1025–1031. doi: 10.1002/ajmg.a.31691. [DOI] [PubMed] [Google Scholar]
- 43.Hunter A GW, Seaver L H, Stevenson R E. Limb-body wall defect. Is there a defensible hypothesis and can it explain all the associated anomalies? Am J Med Genet A. 2011;155A(09):2045–2059. doi: 10.1002/ajmg.a.34161. [DOI] [PubMed] [Google Scholar]
- 44.D'Souza J, Indrajit I K, Menon S. Limb body wall complex. Med J Armed Forces India. 2004;60(01):77–80. doi: 10.1016/S0377-1237(04)80169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunapinun N, Treetipsatit J. Discordant anomalies with combined features of pentalogy of Cantrell and OEIS complex: a case report in monochorionic twins. Fetal Pediatr Pathol. 2017;36(05):357–363. doi: 10.1080/15513815.2017.1332122. [DOI] [PubMed] [Google Scholar]
- 46.Vujovic D, Sretenovic A, Raicevic M et al. Thoracoschisis associated with limb body wall complex. APSP J Case Rep. 2017;8(03):19. doi: 10.21699/ajcr.v8i3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasun P, Behera B K, Pradhan M. Limb body wall complex. Indian J Pathol Microbiol. 2008;51(02):255–256. doi: 10.4103/0377-4929.41674. [DOI] [PubMed] [Google Scholar]
- 48.Bijok J, Massalska D, Kucińska-Chahwan A et al. Complex malformations involving the fetal body wall - definition and classification issues. Prenat Diagn. 2017;37(10):1033–1039. doi: 10.1002/pd.5141. [DOI] [PubMed] [Google Scholar]
- 49.Chauvin N A, Epelman M, Victoria T, Johnson A M. Complex genitourinary abnormalities on fetal MRI: imaging findings and approach to diagnosis. AJR Am J Roentgenol. 2012;199(02):W222-31. doi: 10.2214/AJR.11.7761. [DOI] [PubMed] [Google Scholar]
- 50.Torres U S, Portela-Oliveira E, Braga FdelC, Werner H, Jr, Daltro P AN, Souza A S. When closure fails: what the radiologist needs to know about the embryology, anatomy, and prenatal imaging of ventral body wall defects. Semin Ultrasound CT MR. 2015;36(06):522–536. doi: 10.1053/j.sult.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Gobbi D, Fascetti Leon F, Tregnaghi A, Gamba P G, Midrio P. Early prenatal diagnosis of cloacal exstrophy with fetal magnetic resonance imaging. Fetal Diagn Ther. 2008;24(04):437–439. doi: 10.1159/000174570. [DOI] [PubMed] [Google Scholar]
- 52.Yamano T, Ando K, Ishikura R, Hirota S. Serial fetal magnetic resonance imaging of cloacal exstrophy. Jpn J Radiol. 2011;29(09):656–659. doi: 10.1007/s11604-011-0600-z. [DOI] [PubMed] [Google Scholar]
- 53.Clements M B, Chalmers D J, Meyers M L, Vemulakonda V M. Prenatal diagnosis of cloacal exstrophy: a case report and review of the literature. Urology. 2014;83(05):1162–1164. doi: 10.1016/j.urology.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 54.Allam E S, Shetty V S, Farmakis S G. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50(12):2155–2158. doi: 10.1016/j.jpedsurg.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 55.Kant S G, Bartelings M M, Kibbelaar R E, Van Haeringen A. Severe cardiac defect in a patient with the OEIS complex. Clin Dysmorphol. 1997;6(04):371–374. doi: 10.1097/00019605-199710000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Goto S, Suzumori N, Obayashi S, Mizutani E, Hayashi Y, Sugiura-Ogasawara M. Prenatal findings of omphalocele-exstrophy of the bladder-imperforate anus-spinal defects (OEIS) complex. Congenit Anom (Kyoto) 2012;52(03):179–181. doi: 10.1111/j.1741-4520.2011.00342.x. [DOI] [PubMed] [Google Scholar]
- 57.Goyal A, Fishwick J, Hurrell R, Cervellione R M, Dickson A P. Antenatal diagnosis of bladder/cloacal exstrophy: challenges and possible solutions. J Pediatr Urol. 2012;8(02):140–144. doi: 10.1016/j.jpurol.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Chen C-P. Syndromes, disorders and maternal risk factors associated with neural tube defects (III) Taiwan J Obstet Gynecol. 2008;47(02):131–140. doi: 10.1016/S1028-4559(08)60070-4. [DOI] [PubMed] [Google Scholar]
- 59.Liang R I, Huang S E, Chang F M. Prenatal diagnosis of ectopia cordis at 10 weeks of gestation using two-dimensional and three-dimensional ultrasonography. Ultrasound Obstet Gynecol. 1997;10(02):137–139. doi: 10.1046/j.1469-0705.1997.10020137.x. [DOI] [PubMed] [Google Scholar]
- 60.Kubba T, Khalil A, Abu-Rustum R et al. Prenatal diagnosis of pentalogy of Cantrell at 11-13 weeks: evidence for a hexalogy. J Obstet Gynaecol. 2013;33(01):85–86. doi: 10.3109/01443615.2012.730079. [DOI] [PubMed] [Google Scholar]
- 61.Cakiroglu Y, Doger E, Yildirim Kopuk S, Babaoglu K, Caliskan E, Yucesoy G. Prenatal diagnosis of Cantrell's pentalogy associated with agenesis of left limb in a twin pregnancy. Case Rep Obstet Gynecol. 2014;2014:314284. doi: 10.1155/2014/314284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gün I, Kurdoğlu M, Müngen E, Muhcu M, Babacan A, Atay V. Prenatal diagnosis of vertebral deformities associated with pentalogy of Cantrell: the role of three-dimensional sonography? J Clin Ultrasound. 2010;38(08):446–449. doi: 10.1002/jcu.20726. [DOI] [PubMed] [Google Scholar]
- 63.Van Allen M I, Curry C, Walden C E, Gallagher L, Patten R M. Limb-body wall complex: II. Limb and spine defects. Am J Med Genet. 1987;28(03):549–565. doi: 10.1002/ajmg.1320280303. [DOI] [PubMed] [Google Scholar]
- 64.Chikkannaiah P, Dhumale H, Kangle R, Shekar R. Limb body wall complex: a rare anomaly. J Lab Physicians. 2013;5(01):65–67. doi: 10.4103/0974-2727.115930. [DOI] [PMC free article] [PubMed] [Google Scholar]


