Abstract
Invasive lobular carcinoma is the second-most-common subtype of invasive breast carcinoma. Its metastatic pattern is different compared to invasive carcinoma—no special type. It metastasizes more often to the gastrointestinal tract, peritoneum, pleura, and ovaries. The extrahepatic gastrointestinal tract metastases occur mostly in the stomach and small intestine and less often in the colon and rectum.
We present a case description of an 87-year-old woman admitted to our hospital with hematochezia, abdominal discomfort, fatigue, and weight loss. A colonoscopy revealed an exophytic tumor of the sigmoid colon. Metastatic disease was not found in imaging studies. A low anterior resection was performed. The pathologic examination revealed a collision tumor consisting of a poorly differentiated adenocarcinoma of the colon and metastatic lobular carcinoma. The diagnosis was challenging due to the lack of a previous history. Also, the diffuse architectural pattern and signet ring cells found may be in primary signet ring carcinoma of the colon as well as in carcinomas from other anatomical sites. Immunohistochemistry was helpful in making the diagnosis. A review of the literature revealed that this is the fourth case of metastatic breast carcinoma coexisting with colonic adenocarcinoma.
Keywords: lobular carcinoma, metastasis, colon, colorectal carcinoma, gata-3, cdx-2, immunohistochemistry
Introduction
Invasive lobular carcinoma (ILC) is the second-most-common subtype of invasive carcinoma of the breast, accounting for up to 15% of cases. The classical variant displays multifocality, a diffuse architectural, the single-file linear pattern of tumor cells arranged in a concentric or targetoid pattern around normal ducts or structures, and intracellular mucin production either in the form of intracytoplasmic lumina or signet ring cells [1]. Several variants have been reported in the literature besides the classical ILC, including solid, signet ring cell, tubulolobular, alveolar, trabecular, pleomorphic, and a recently described variant with extracellular mucin production [2]. The latter variant shows both intra- and extracellular mucin production, a rare feature among breast carcinomas [3]. The ILC metastatic pattern is different compared to invasive ductal carcinoma, metastasizing more often to the gastrointestinal (GI) tract, peritoneum, pleura, and ovaries [4-5] whereas invasive carcinoma of no special type (NST) metastasizes more often to the lungs, liver, and brain [4]. Extrahepatic GI tract metastasis involves more often the stomach and the small intestine and, very rarely, the colon and rectum [6]. Other rare metastatic sites include the appendix [7], endometrium [8], gallbladder [9], kidney [10], orbit [11], pancreas [12], parotid gland [13], spleen [14], urinary bladder [15], uterine cervix [8], uterus [16], and vulva [17].
We herein describe a case of collision tumor in an 87-year-old woman consisting of a poorly differentiated colonic adenocarcinoma and a metastatic lobular carcinoma as an incidental finding in a colectomy specimen and a review of the literature.
Case presentation
An 87-year-old patient with no previous history was admitted to our hospital due to hematochezia, abdominal discomfort, fatigue, and weight loss. Colonoscopy showed an exophytic tumor of the sigmoid colon. No evidence of metastatic disease was found on computed tomography (CT) scan or MRI. A low anterior resection was performed.
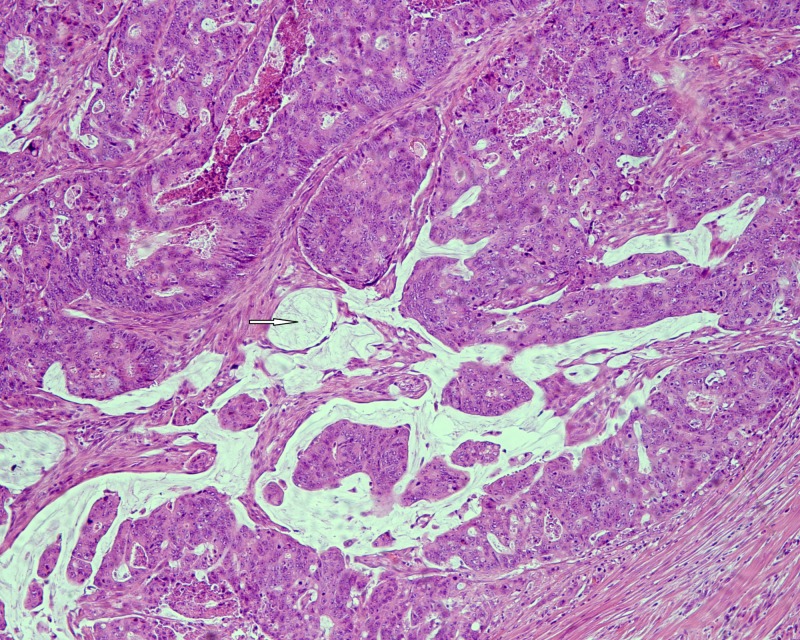
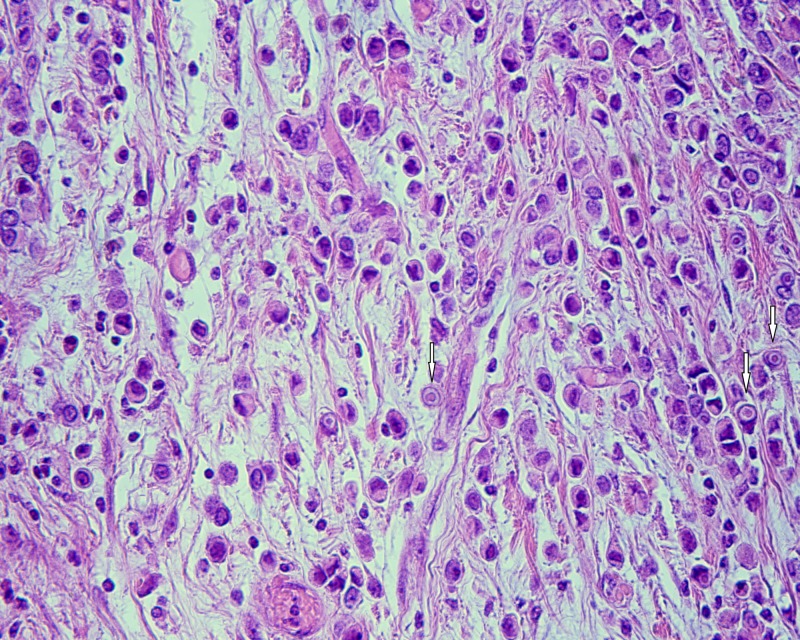
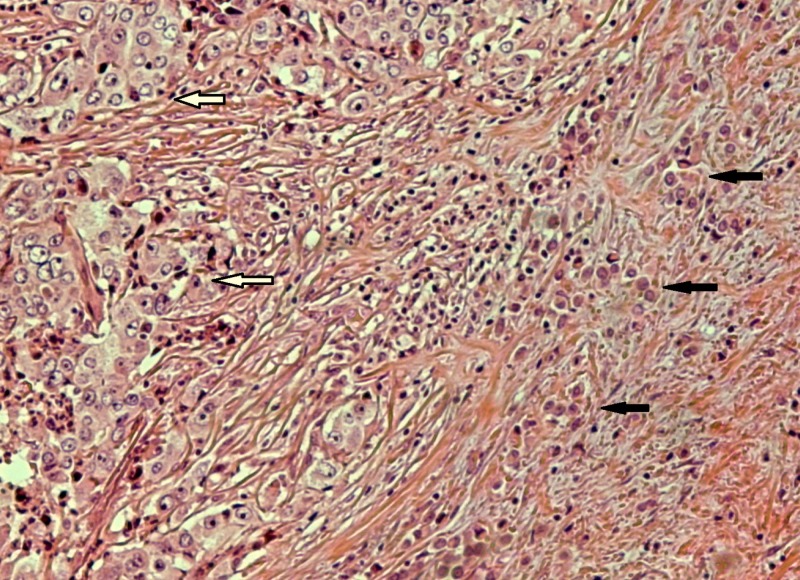
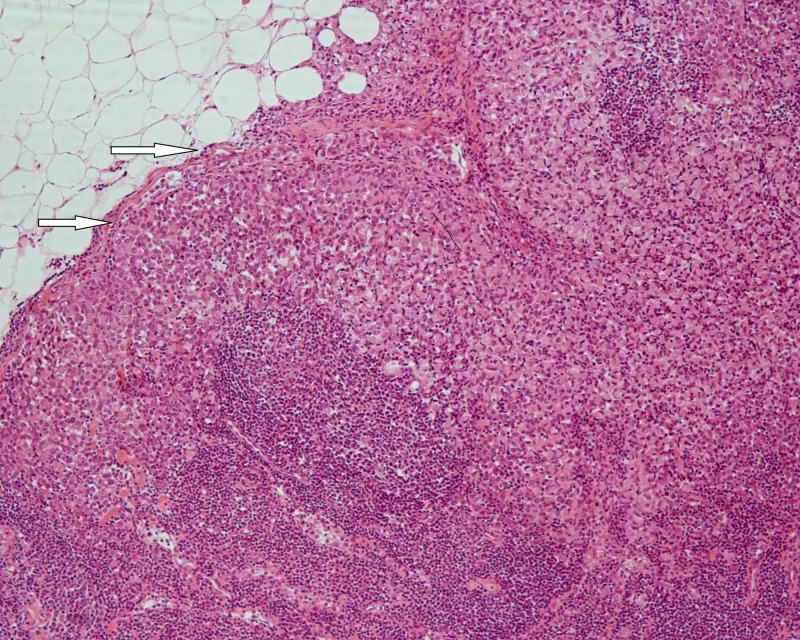
A pathologic evaluation of the resected specimen revealed a poorly differentiated adenocarcinoma of the colon with focal extracellular mucin production (Figure 1) and pericolic fat infiltration. There was a second neoplastic population found mainly near the serosa, consisting of smaller cells with a diffuse architectural pattern, some of them displaying intracytoplasmic lumina (Figure 2). In some areas, both neoplastic populations were found in close proximity (Figure 3). Several signet ring cells were present. Fourteen lymph nodes were infiltrated, five by the colonic carcinoma and nine by the second type of neoplastic cells (Figure 4). Due to the fact that there was no other neoplasm in her history, the second neoplastic population was assumed to be a minor component of primary colonic carcinoma with signet ring morphology. The differential diagnosis also included ILC and other metastatic carcinomas with a diffuse architectural pattern.
Figure 1. Poorly differentiated colonic adenocarcinoma displaying extracellular mucin production (white arrow) (HE x 100).
Abbreviation: HE: hematoxylin and eosin.
Figure 2. Diffuse type carcinoma cells display eccentric nuclei and intracytoplasmic lumina (white arrows) at high magnification (HE x 400).
Abbreviation: HE: hematoxylin and eosin.
Figure 3. Nests of poorly differentiated colonic carcinoma (white arrows) in close proximity to smaller neoplastic cells (black arrows) with a diffuse architectural pattern (HE x 100).
Abbreviation: HE: hematoxylin and eosin.
Figure 4. Lymph node infiltration by diffuse type carcinoma cells (white arrows) (HE x 100).
Abbreviation: HE: hematoxylin and eosin.
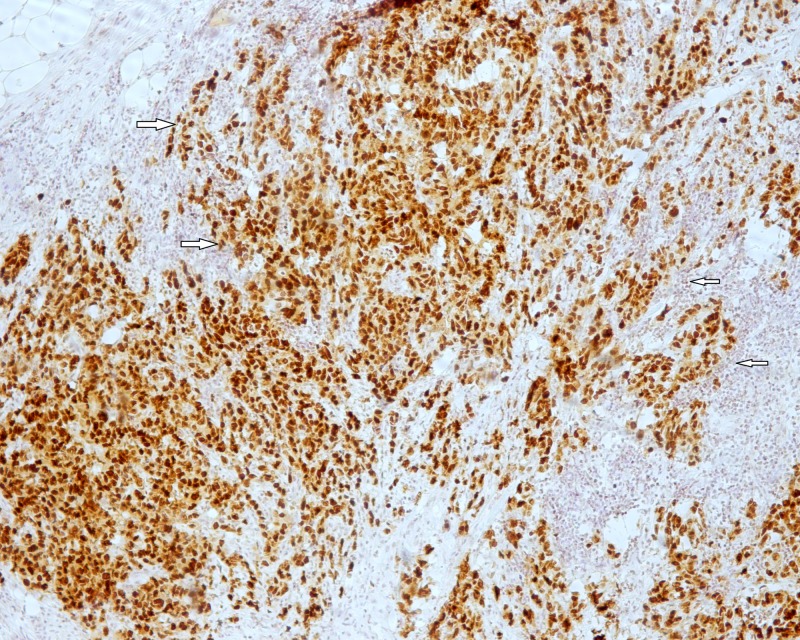
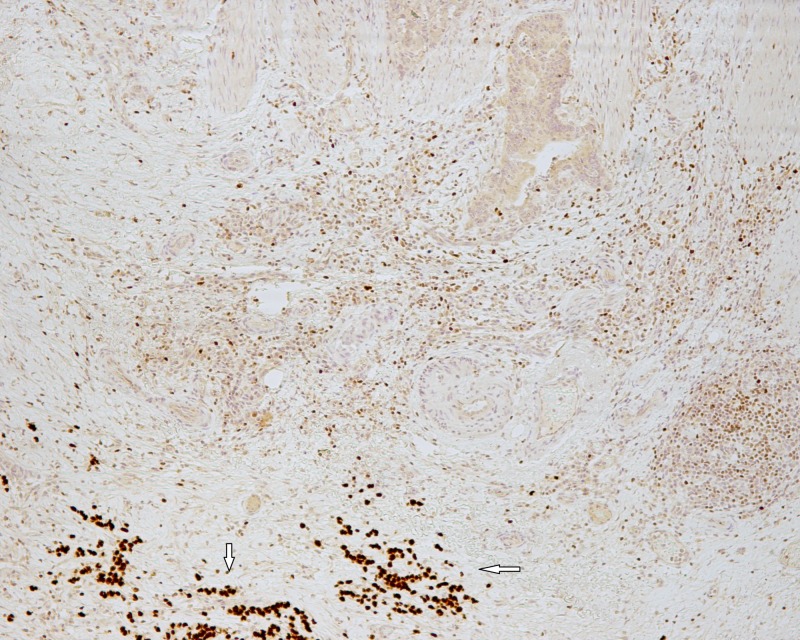
The immunohistochemical study was negative for CK-20 and CDX-2 in the diffuse type carcinoma cells and positive for them in the colonic adenocarcinoma (Figure 5). CK-7 was negative in both. Additional stainings for GATA-3 (Figure 6), mammaglogin, estrogen receptor (ER)/progesterone receptor (PR) were performed, which were negative in the colonic adenocarcinoma and positive in the diffuse type carcinoma. E-cadherin was negative in the diffuse type carcinoma and positive in the colonic adenocarcinoma. Chromogranin and synaptophysin were negative in both. The diagnosis of a poorly differentiated colonic carcinoma with focal extracellular mucin production co-existing with a carcinoma consistent with metastatic ILC was made.
Figure 5. CDX-2 is diffusely positive in colonic carcinoma cells (white arrows) (CDX-2 x 100).
Figure 6. Diffuse type carcinoma cells were positive for GATA-3 (white arrows) while colonic adenocarcinoma cells were negative (GATA-3 x 100).
Imaging studies did not reveal any primary breast tumor. Due to her advanced age, the multidisciplinary tumor board decided to treat her with hormonal therapy. The patient refused further treatment and was lost to follow up.
Discussion
Breast carcinoma is the most frequent cancer in women, accounting for approximately one-third of cancers, and is a significant cause of morbidity and mortality [18]. GI tract metastases usually occur four to five years after primary breast cancer diagnosis but sometimes 20 or even 30 years later. Occasionally, their presentation is concurrent but, rarely, they may be the first clinical manifestation of breast cancer [4,19]. The rate of GI tract involvement in metastatic breast cancer is around 1% and is found in up to 12% of autopsies [18].
The metastatic pattern of ILC is different from that of an NST carcinoma of the breast. The reason for this different metastatic pattern has been suggested to be the loss of E-cadherin [18]. ILC metastasizes more commonly to the GI tract than NST carcinoma of the breast with an incidence of 4.5% and 0.2%, respectively [18] and is usually associated with disseminated disease [4].
Clinical diagnosis is difficult because colonic metastases may present as a mass mimicking a primary GI tumor, other benign GI tract tumors, or as Crohn’s disease [18,20]. The histological diagnosis may be difficult as well. A previous history of breast malignancy can be very helpful in establishing the correct diagnosis. It has been suggested that for any woman presenting with new GI complaints and a history of breast carcinoma, metastasis must be considered [18]. Attention to histological detail paired with the previous history will usually suffice for diagnosis. In difficult cases, immunohistochemistry will provide the solution as breast carcinoma tumor cells are positive for CK-7, GATA-3, mammaglobin, GCDFP-15, and estrogen and progesterone receptors. In our case, despite the fact that CK-7 was negative, the diagnosis of metastatic ILC was made due to the fact that markers considered more specific for mammary carcinoma (GATA-3, mammaglobin, and GCDFP-15) were positive.
Our literature review has revealed three cases of breast carcinoma metastasis to the colon coexisting with colonic adenocarcinoma [4-5,20]. Two of these involved ILC and the third was NST carcinoma. In the first case, the metastatic ILC was undiagnosed until colorectal resection [4] whereas the other two cases had a known history of ILC [5,20]. The clinicopathological features of these cases are presented in Table 1. Interestingly, the patient with colonic metastasis by NST carcinoma had a history of ILC 30 years prior [20]. The lymph nodes were infiltrated by both colorectal and breast carcinoma in the two cases involving ILC [4-5]. As in our case, the primary tumor was not located by further imaging studies in one of the cases [4].
Table 1. Clinicopathological features in cases of breast carcinoma metastasis to the colon co-existing with colonic adenocarcinoma.
Abbreviations: Adj. Therapy: adjuvant therapy; ANED: alive no evidence of disease; Ca: cancer; Chemo: chemotherapy; Conc: concomitant; CRC: colorectal carcinoma; G: grade; Horm: hormonal treatment; ILC: invasive lobular carcinoma; mo: months; NST: no special type carcinoma.
*Grade is not mentioned
**Patient refused treatment
***Patient was lost to follow up
| Case | Year | Age | Timing | Breast Ca | Colon Ca | Adj. Therapy | Outcome (mo) |
| 14 | 2013 | 78 | 9 years | ILC * | CRC G2 | Horm+Chemo | 48 ANED |
| 220 | 2014 | 83 | Conc. | NST G2 | CRC G2 | Horm | 18 ANED |
| 35 | 2015 | 80 | Conc. | ILC G2 | CRC G2 | Horm | 4 ANED |
| Present case | 2017 | 87 | Conc. | ILC G2 | CRC G3 | ** | *** |
There is no consensus regarding the treatment of metastatic breast cancer with intestinal involvement due to its rarity. Systemic treatment with surgery or surgery alone has been suggested to have a favorable outcome in some reports [20].
Conclusions
While breast carcinoma colorectal metastasis is a rare event, primary colonic and metastatic breast carcinoma coexistence is extremely rare. The precise diagnosis of metastatic breast carcinoma in a colectomy specimen may be very challenging, especially in cases lacking a previous history. In such cases, immunohistochemistry usually provides a diagnostic solution. Systemic treatment has been suggested to have a favorable outcome in some reports.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Invasive lobular carcinoma of the breast: Morphology, biomarkers and ’omics. Reed AEM, Kutasovic JR, Lakhani SR, Simpson PT. Breast Cancer Res. 2015;17:12. doi: 10.1186/s13058-015-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Invasive lobular carcinoma with extracellular mucin production—a novel pattern of lobular carcinomas of the breast. Clinico-pathological description of eight cases. Cserni G, Floris G, Koufopoulos N, et al. Virchows Arch. 2017;471:3–12. doi: 10.1007/s00428-017-2147-6. [DOI] [PubMed] [Google Scholar]
- 3.Mucinous cystadenocarcinoma of the breast: the challenge of diagnosing a rare entity. Koufopoulos N, Goudeli C, Syrios J, Filopoulos E, Khaldi L. Rare Tumors. 2017;9:98–100. doi: 10.4081/rt.2017.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unexpected diagnosis of both adenocarcinoma of the colon and metastatic lobular carcinoma of the breast in the gastrointestinal tract. Miller T, Ross C, Al-Rawi H, Taylor B, Al-Jafari M. Case Rep Pathol. 2013;2013:1–3. doi: 10.1155/2013/153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An unusual case of colonic adenocarcinoma development in the region of disseminating lobular breast carcinoma infiltration: Diagnostic approach and review of the literature. Mroz A, Kiedrowski M. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4525989/ Int J Clin Exp Pathol. 2015;8:7470–7474. [PMC free article] [PubMed] [Google Scholar]
- 6.Rectal localization of metastatic lobular breast cancer. Cervi G, Vettoretto N, Vinco A, Cervi E, Villanacci V, Grigolato P, Giulini SM. Dis Colon Rectum. 2001;44:453–455. doi: 10.1007/BF02234749. [DOI] [PubMed] [Google Scholar]
- 7.Metastatic breast carcinoma presenting as perforated appendicitis. Dirksen JL, Souder MG, Burick AJ. Breast Care. 2010;5:409–410. doi: 10.1159/000322656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metastasis of breast cancer to an endometrial polyp, the cervix and a leiomyoma: a case report and review of the literature. Razia S, Nakayama K, Tsukao M, et al. Oncol Lett. 2017;14:4585–4592. doi: 10.3892/ol.2017.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biliary dyskinesia as a rare presentation of metastatic breast carcinoma of the gallbladder: a case report. Markelov A, Taheri H, Vunnamadala K, Ibrahim G. Case Rep Pathol. 2011;2011:1–3. doi: 10.1155/2011/806570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metastatic invasive lobular carcinoma of the breast masquerading as a primary renal malignancy. Al-Jarrah A, Taranikanti V, Sawhney S, Furrukh M, Al-Hosni M, Saparamadu PAM, De Silva MVC. Sultan Qaboos Univ Med J. 2013;13:453–455. [PMC free article] [PubMed] [Google Scholar]
- 11.Unilateral orbital pain and eyelid swelling in a 46-year-old woman: orbital metastasis of occult invasive lobular carcinoma of breast masquerading orbital pseudotumour. Gupta S, Bhatt VR, Varma S. BMJ Case Rep. 2011;2011:1220103580. doi: 10.1136/bcr.12.2010.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pancreatic solitary and synchronous metastasis from breast cancer: a case report and systematic review of controversies in diagnosis and treatment. Molino C, Mocerino C, Braucci A, et al. World J Surg Oncol. 2014;12:2. doi: 10.1186/1477-7819-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parotid gland metastasis of breast cancer: case report and review of the literature. Ando K, Masumoto N, Sakamoto M, et al. Breast Care. 2011;6:471–473. doi: 10.1159/000335222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobular carcinoma of the breast metastatic to the spleen and accessory spleen: report of a case. Groisman GM. Case Rep Pathol. 2016;2016:5160180. doi: 10.1155/2016/5160180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A case of recurrent breast cancer with solitary metastasis to the urinary bladder. Nieder C, Pawinski A. Case Rep Oncol Med. 2014;2014:1–3. doi: 10.1155/2014/931546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uterine metastasis of lobular breast cancer during adjuvant letrozole therapy. Komeda S, Furukawa N, Kasai T, Washida A, Kobayashi H. J Obstet Gynaecol (Lahore) 2013;33:100–101. doi: 10.3109/01443615.2012.721407. [DOI] [PubMed] [Google Scholar]
- 17.Metastatic lobular carcinoma of the breast to the vulva: a case report and review of the literature. Papaioannou N, Zervoudis S, Grammatikakis I, Peitsidis P, Palvakis K, Youssef TF. http://www.nci.cu.edu.eg/Journal/Mar2010/Can_7.pdf. J Egypt Natl Canc Inst. 2010;22:57–60. [PubMed] [Google Scholar]
- 18.Obstructive colon metastases from lobular breast cancer: report of a case and review of the literature. Mistrangelo M, Cassoni P, Mistrangelo M, et al. Tumori. 2011;97:800–804. doi: 10.1177/030089161109700619. [DOI] [PubMed] [Google Scholar]
- 19.Gastro-intestinal metastases as first clinical manifestation of the dissemination of a breast cancer. Clavien PA, Laffer U, Torhost J, Harder F. http://www.ncbi.nlm.nih.gov/pubmed/2157608. Eur J Surg Oncol. 1990;16:121–126. [PubMed] [Google Scholar]
- 20.Synchronous gist, colon and breast adenocarcinoma with double colonic polyp metastases. Jafferbhoy S, Paterson H, Fineron P. Int J Surg Case Rep. 2014;5:523–526. doi: 10.1016/j.ijscr.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]