Abstract
Life has evolved to internalize and depend upon the daily and seasonal light cycles to synchronize physiology and behavior with environmental conditions. The nightscape has been vastly changed in response to the use of artificial lighting. Wildlife is now often exposed to direct lighting via streetlights or indirect lighting via sky glow at night. Because many activities rely on daily and seasonal light cues, the effects of artificial light at night could be extensive, but remain largely unknown. Laboratory studies suggest exposure to light at night can alter typical timing of daily locomotor activity and shift the timing of foraging/food intake to the daytime in nocturnal rodents. Additionally, nocturnal rodents decrease anxiety-like behaviors (i.e., spend more time in the open and increase rearing up) in response to even dim light at night. These are all likely maladaptive responses in the wild. Photoperiodic animals rely on seasonal changes in day length as a cue to evoke physiological and behavioral modifications to anticipate favorable and unfavorable conditions for survival and reproduction. Light at night can mask detection of short days, inappropriately signal long days, and thus desynchronize seasonal reproductive activities. We review laboratory and the sparse field studies that address the effects of exposure to artificial light at night to propose that exposure to light at night disrupts circadian and seasonal behavior in wildlife, which potentially decreases individual fitness and modifies ecosystems.
Keywords: circadian rhythms, light at night, light cycle, photoperiodism, wildlife
1 | INTRODUCTION
Evolution of life over millions of years has depended on the sun. Not unexpectedly, life is intimately tied to the cycles of light and dark produced by the rotation of earth on its axis, as well as the seasonal revolution of Earth around the sun. Much in the same way organisms evolved to rely on atmospheric gases to maintain biochemical and physiological processes, they also internalized the light–dark system. Although it is well known that selection has produced adaptations to spatial niches, selection has produced adaptations to specific temporal niches as well. Survival of individuals depends on being well suited to their environment, and thus physiological and behavioral processes evolved to coordinate with light cycles in order to maximize available resources while minimizing survival risks.
The earth rotates on its axis once every ∼24 h producing a daily light–dark cycle. Sunlight produces up to 100,000 lx of light on a cloudless day, whereas a full moon on a clear night reflects less than 2 lx of light (Weaver, 2011). Daily fluctuations in light levels are dramatic and predictable, and many behaviors and physiological processes in microbes, invertebrates, and vertebrates fluctuate with the daily solar cycle. The circadian system coordinates internal biological rhythms with environmental conditions. Circadian rhythms are self-sustaining, endogenous biological rhythms with periods of approximately 24 h in the absence of entraining cues (e.g., light), however, light entrains these rhythms to precisely 24 h. In vertebrates, circadian rhythms are centrally controlled by a molecular network located in the suprachiasmatic nucleus (SCN) of the hypothalamus (reviewed in Partch, Green, & Takahashi, 2014). Intrinsically photosensitive retinal ganglion cells respond to light and relay the information to the SCN where a transcriptional autoregulatory feedback loop maintains circadian rhythmicity (Partch et al., 2014). The SCN then relays time of day information to other brain regions and peripheral tissues, which allows individuals’ physiological processes and behaviors to synchronize with the environment.
Many organisms at higher latitudes also rely on seasonal changes in day length to determine the time of year. The changing color of leaves and the abundance of insects are obvious seasonal changes in these regions, but many organisms undergo more subtle seasonal changes. Annual changes in day length, or photoperiod, serve as a precise reference for the time of year, and photoperiodic organisms use this information to align life events with the time of year to optimize survival and reproduction. Time of year information is distinguished by the circadian system, which regulates melatonin production from the pineal gland. Light suppresses melatonin production, and darkness releases this suppression, stimulating the synthesis and secretion of melatonin into the bloodstream (Benarroch, 2008). Long days with short nights produce relatively short durations of melatonin secretion, whereas during the long nights of winter, extended durations of melatonin secretion occur. Circulating melatonin signals time of year, and seasonal physiological and behavioral processes depend on this change in the duration of elevated circulating melatonin for accurate timing.
Even minor fluctuations in light levels, such as those produced by moon phase, exert behavioral effects in some species. The natural lunar cycle fluctuates between approximately 0.5 lx during a new moon and 2 lx during a full moon (Weaver, 2011). These subtle changes in light patterns drive dramatic changes in ecosystems such as the vertical rhythms of zooplankton (Last, Hobbs, Berge, Brierley, & Cottier, 2016; Ludvigsen et al., 2018), and many organisms utilize moonless or cloudy nights to avoid predation during foraging, migrating, and mating (Clarke, 1983; Griffin, Griffin, Waroquiers, & Mills, 2005). Moon phase can also affect vigilance behavior; some animals increase vigilance under a bright full moon, whereas others decrease vigilance in brighter moonlight (Beauchamp, 2015; Beauchamp & McNeil, 2003).
In many urban and suburban areas, sky brightness resulting from artificial lighting is much greater than nights with a full moon (Falchi et al., 2016). Because behavior in organisms can be dramatically affected even by natural fluctuations in moonlight, exposure to artificial light at night (ALAN) likely alters behavior in species that typically rely on dark nights. This could reduce fitness both independently and in combination with adverse effects to physiology. Light at night can be the result of direct exposure to artificial light, such as directly in the path of a streetlight, or indirectly from sky glow, artificial light that is scattered and reflected back to earth by the atmosphere (Davies, Bennie, Inger, & Gaston, 2013; Kyba, Ruhtz, Fischer, & Hölker, 2011). Sky glow can illuminate areas located far from urban centers, reaching approximately 23% of earth’s terrestrial ecosystems outside the Arctic and Antarctic, and almost 50% of the United States (Falchi et al., 2016), and emissions from artificial lighting are estimated to be increasing globally at 3–6% per year (Hölker, Wolter, Perkin, & Tockner, 2010). Although artificial lighting provides many benefits to human society, effects on wildlife and ecosystems are mostly unknown.
The theory of natural selection explains how individuals benefit from being well adapted to their environment. Biological fitness of wildlife depends upon the ability to out-compete others for resources, survive to reproductive age, and successfully produce viable and reproductively successful offspring. The specific evolutionary processes that might be affected by ALAN are reviewed elsewhere (Swaddle et al., 2015), but here we discuss ways in which ALAN could alter ecosystem dynamics and negatively affect individual fitness. The effects of ALAN on wildlife and ecosystems are only recently beginning to be understood, and most available studies have been conducted using birds. Evidence from laboratory studies and controlled field experiments in artificial outdoor settings suggest ALAN presents a threat to organisms in their natural habitats. Here, we review laboratory studies of the effects of ALAN on behavior, and discuss the implications of these behavioral alterations in natural wildlife populations. The effects of ALAN on birds has been addressed in several other reviews and books (Dominoni, 2015; Gauthreaux & Belser, 2006; Montevecchi, 2006); therefore, we will mainly focus below on the effects of ALAN in non-avian terrestrial vertebrates.
2 | DAILY BEHAVIORAL CHANGES IN ARTIFICIAL NIGHT LIGHTING
Potential fitness costs of ALAN could occur from daily and seasonal circadian dysregulation, or as an effect of light itself, independent of disrupted biological rhythms.
Light is often used for orientation during nighttime navigation; artificial lighting can attract or repel individuals, or mask natural sources of light (Gaston, Bennie, Davies, & Hopkins, 2013; van Langevelde, Ettema, Donners, WallisDeVries, & Groenendijk, 2011). For example, sea turtles (Caretta caretta and Chelonia mydas) can fail to migrate toward the ocean after hatching because they are instead attracted to shore lights (Tuxbury & Salmon, 2005). Avian migratory patterns can be altered because birds are attracted and remain near artificial lights (La Sorte, Fink, Buler, Farnsworth, & Cabrera-Cruz, 2017; Van Doren et al., 2017), or ALAN directly interferes with the magnetic compass used by birds for navigation (Wiltschko & Wiltschko, 1995; Wiltschko, Munro, Ford, & Wiltschko, 1993). Light can also directly alter predator success. Harbor seals (Phoca vitulina) located near bridges with artificial nighttime lighting were more successful at capturing salmonid smolts when the bridge lights were turned on at night (Yurk & Trites, 2000). Although this was beneficial for the seals in this particular region, Chinook salmon (Oncorhynchus tshawytscha) populations were in decline, and limiting seal predation by removing ALAN could help the population recover. Fitness costs of direct light effects are more apparent, whereas the fitness costs of changes in biological rhythmicity remain largely unspecified.
Changes in ambient illumination levels affect behaviors of both predators and prey (Clarke, 1983; Longcore & Rich, 2004). Nocturnal vigilance, a key component of antipredator behavior especially in diurnal prey animals, can be altered with exposure to ALAN. Some species prefer sleeping under artificial lighting because presumably they can more easily detect predators (Gorenzel & Salmon, 1995). Others prefer sleeping under dark nights putatively to decrease their visibility to predators (Yorzinski et al., 2015). The presence of ALAN increases vigilant behaviors such as head and eye movements and peeking (periodically opening the eyes while sleeping) (Jones, Krebs, & Whittingham, 2007; Lung & Childress, 2006), which allows for rapid detection of predators (Lima & Bednekoff, 1999), but can also disturb sleep in diurnal animals. Bats (Pipistrellus pipistrellus) increased activity when exposed to ALAN, likely in response to an increase in insect activity (Spoelstra et al., 2015).
In laboratory rodents, exposure to ALAN decreased so-called anxiety-like behaviors. For example, exposure to ALAN increased the time rodents spent in the open arms of an elevated plus maze (Bedrosian, Fonken, Walton, Haim, & Nelson, 2011), increased the amount of time spent in a lit chamber compared with a dark chamber in a light–dark box paradigm, and increased the amount of rearing up in an open field paradigm (Aubrecht, Weil, Magalang, & Nelson, 2013). Anxiety-like behaviors were also decreased in two rodents with intact melatonin rhythms, Siberian hamsters (Phodopus sungorus) and C3H/HeNHsd mice (Bedrosian et al., 2011; Hogan, Kovalycsik, Sun, Rajagopalan, & Nelson, 2015). The results of laboratory tests used to assess anxiety-like responses could reflect maladaptive responses in the wild. Although decreased anxiety may seem to be a benefit from a human perspective, it presents a potential threat to individuals in the wild. Neophobia and anxiety-like behaviors are beneficial in wildlife populations and necessary for survival. An animal’s anxiety signals danger, and individuals respond with appropriate defensive behaviors. Therefore, decreased anxiety and the resulting behavioral changes, such as time spent in the open, increased activity in the light, and increased rearing up could increase the visibility of prey, decreasing the fitness of the individual (Figure 1).
FIGURE 1.
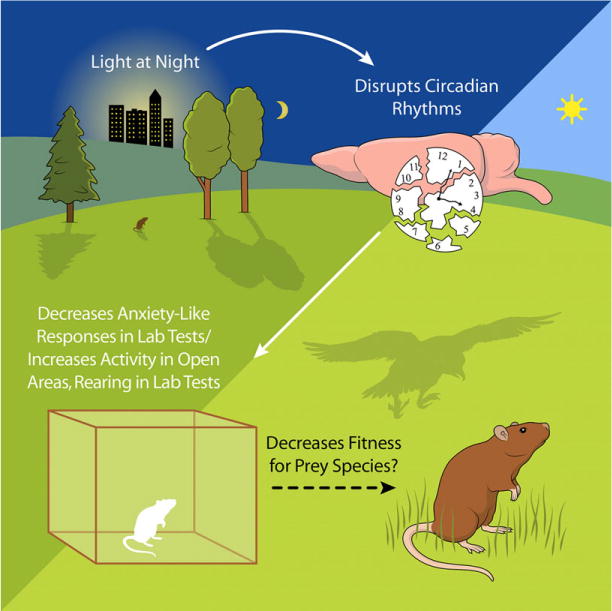
Fitness of prey species in the wild could be decreased with exposure to nighttime lighting [Color figure can be viewed at wileyonlinelibrary.com]
Vertebrates use light patterns to regulate many daily behaviors. Approximately 70% of mammals are nocturnal (Bennie, Duffy, Inger, & Gaston, 2014) and more active in the dark, whereas diurnal mammals are active during the daytime. Besides these two main activity patterns, a smaller proportion of mammals are cathemeral, active during both daylight and darkness, or crepuscular, active mostly during twilight. Each of these strategies for regulating activity over the 24 h daily cycle relies on light cues in order to synchronize appropriate behaviors (Kronfeld-Schor et al., 2013); however, animals using each strategy likely respond differently when exposed to ALAN. Diurnal animals might interpret ALAN as a cue to be more active, nocturnal animals are likely to be less active at night with ALAN (Fonken et al., 2010), and ALAN could extend the period detected as twilight for crepuscular mammals. The overall effects of these changes in activity patterns on populations and ecosystems are complex and difficult to study. However, it is likely that alterations in timing of activities can alter the social network structure of populations, which is important for processes such as communication, transfer of information, reproduction, and the transfer of diseases (Kurvers & Holker, 2015). Additionally, changes in activity patterns can alter animals’ ability to obtain resources through foraging and drastically alter predator–prey interactions.
Aberrant exposures to light at night also disrupt the molecular clock, and consequently dysregulate circadian rhythms (Bedrosian, Vaughn, Weil, & Nelson, 2013b; Fonken et al., 2013). Endogenous biological rhythms that synchronize with the external environment allow organisms to respond proactively to environmental changes and time behaviors appropriately. Thus, disruption of these rhythms could be detrimental if an organism is no longer aligned with the temporal aspects of its environment. Indeed, artificial lighting alters responses in plants, invertebrates, and vertebrates alike.
Although the mechanism of light disruption has not been fully elucidated for all animals, it is well established in birds. Melatonin rhythms allow many bird species to regulate day/night behaviors and detect seasonal variation in day length. Exposure to light at night suppresses melatonin rhythms and can therefore disrupt normal daily or photoperiodic activities (de Jong et al., 2015). The detection of the onset of day and night can therefore be altered (Dominoni & Partecke, 2015). Additionally, ALAN disrupts the daily corticosterone spike at the onset of activity, which alters waking cues (Russ et al., 2015) and disrupts the timing of morning arousal. Birds arise earlier with ALAN, and songbirds sing earlier in the morning, which can affect predator–prey interactions, as well as mating (Kempenaers, Borgström, Loës, Schlicht, & Valcu, 2010; Miller, 2006).
Many studies suggest a similar effect from ALAN in mammals (Le Tallec, Théry, & Perret, 2016; Robert, Lesku, Partecke, & Chambers, 2015). However, laboratory rodents, which typically lack a strong melatonin rhythm, also undergo behavioral and physiological alterations from exposure to ALAN, suggesting these effects may be independent of melatonin in some species. Instead, circadian dysregulation from ALAN likely alters these activities independent of melatonin.
Activity patterns can change under ALAN by altering circadian timing or as a direct response to light, independent of circadian rhythms (Spoelstra, Verhagen, Meijer, & Visser, 2018). Light drives individuals to balance the ability to obtain resources (Foraging Efficiency Hypothesis; Imber, 1975) with the risk of predation (Predation Risk Hypothesis; Mougeot & Bretagnolle, 2000) in order to maximize their energy expenditure and overall fitness. Daily activity patterns are driven by many factors, including resource availability, population structure, social interaction, mate selection, and predator-prey interactions (reviewed in Lima, 2002; Mougeot & Bretagnolle, 2000). Laboratory studies suggest activity patterns are altered when nocturnal rodents are exposed to ALAN. Hamsters (Phodopus sungorus) exposed to dim levels of ALAN reduced their locomotor activity during the dark phase (Bedrosian et al., 2013a). Although locomotor activity does not necessarily increase during the light phase compared with rodents exposed to dark nights, behaviors such as food intake increased during the daytime (Fonken et al., 2010, 2013), suggesting increased arousal during the daytime. Studies also indicate activity patterns are altered in wild populations as well. Common spiny mice (Acomys cahirinus), which are nocturnal, decreased activity during nights with ALAN (Rotics, Dayan, & Kronfeld-Schor, 2011). Decreased nocturnal activity increased intraspecific competition in this population by reducing the amount of time individuals were actively foraging. In this same study, golden spiny mice (Acomys russatus), which are diurnal, did not expand their activity into the night with exposure to ALAN (Rotics et al., 2011). In another study, wood mice (Apodemus sylvaticus) also decreased their activity during nights with ALAN (Spoelstra et al., 2015). Additionally, ALAN also affects communication and activity in amphibians. Frogs decrease the frequency of calling and also have less complex calls when exposed to ALAN (Hall, 2016; Tuttle & Ryan, 1982). Calling is a main behavioral tactic for attracting mates by male frogs, and therefore ALAN could interfere with mate selection. Although these studies strongly suggest that ALAN alters activity patterns and intraspecific interactions, very few studies address whether these changes affect biological fitness. Indeed, one study that tracked survival following exposure to ALAN indicates that even with changes in daily activity patterns and spatial usage, survival was not altered (Hoffmann, Palme, & Eccard, 2018). More studies that address the effect of ALAN on fitness are required to determine whether survival is a concern in other species.
Artificial lighting exerts strong effects on foraging behavior as well (Brown, Kotler, Smith, & Wirtz, 1988). In laboratory studies, exposure to dim levels of ALAN shifts the timing of food intake in nocturnal rodents to the daytime (Fonken et al., 2010, 2013). In these studies, the mice also gained additional body fat with the same amount of food intake as mice restricted to nocturnal feeding. Salamanders, a family of amphibians undergoing population decline (Semlitsch, Walls, Barichivich, & O’Donnell, 2017), forage at night. However, salamanders forage less and are less active at night when exposed to ALAN (Wise, unpublished data). Amphibian populations, especially near human development, are declining, and much of the decline has been attributed to chemical pollutants in water bodies. However, light pollution and the resulting effects on behavior could also contribute to the population decline in amphibians and deserves study. Although gaining body fat with equivalent food intake may be beneficial to animals in the wild, there could be significant fitness costs to altered feeding and active timing via resource misalignment, increased exposure to predators, or decreased success in finding a mate.
Seasonal changes in foraging behavior and food intake are under neuroendocrine regulation in mammals through ghrelin and melatonin signaling, respectively (Keen-Rhinehart & Bartness, 2005; Le Tallec et al., 2016; Nelson & Drazen, 1999); peripheral and central ghrelin signaling induces food foraging, hoarding, and intake in Siberian hamsters (Phodopus sungorus) (Keen-Rhinehart & Bartness, 2005; Le Tallec et al., 2016). Melatonin stimulates food intake (Angers, Haddad, Selmaoui, & Thibault, 2003); thus, a reduction in melatonin production at night could reduce food intake at night, and potentially shift a portion of the caloric intake to daytime in nocturnal animals. Although the precise mechanism for the changes in foraging behaviors with exposure to ALAN remains unknown, an interaction between ghrelin and altered melatonin rhythms might contribute.
In addition to altering fitness via altered activity and predator–prey dynamics, exposure to ALAN can also impair learning and memory in many species (Chellappa et al., 2011; Fonken, Kitsmiller, Smale, & Nelson, 2012). Spatial learning is an important skill for migratory and movement patterns toward resources. In a laboratory setting, spatial learning was impaired by exposure to dim levels of ALAN in diurnal Nile grass rats (Arvicanthis niloticus) (Fonken et al., 2012). Nocturnal rodents do not seem to be affected in this way (Aubrecht et al., 2013; Fonken et al., 2012). Although few studies directly examine ALAN and cognitive effects, it is well known that circadian dysregulation from sleep disruption or jet lag affect learning and memory (Gibson, Wang, Tjho, Khattar, & Kriegsfeld, 2010; Karatsoreos, Bhagat, Bloss, Morrison, & McEwen, 2011). Thus, circadian dysregulation by exposure to ALAN likely impairs cognitive functions as well, and further study in this area is required.
Daily patterns in activity and behavior are intimately tied with light–dark patterns. A fine balance between activity patterns and environment influences fitness through access to resources, access to mates, exposure to disease, and predator–prey interactions. Human influence in these dynamics through ALAN have not been well studied, but have the potential to drive large-scale ecosystem changes.
3 | SEASONAL BEHAVIORS ARE ALTERED BY ALAN
Many species are photoperiodic and use day length to assess the time of year. As noted, the length of day is signaled via melatonin; long durations of elevated circulating melatonin signal short-days, and conversely, short durations of elevated circulating melatonin signal long days. Photoperiodic animals must be able to detect seasons for two major reasons: preparation for a resource-poor season and reproductive synchronization with mates and favorable seasonal conditions for production of offspring. In preparation for winter, many animals divert their energy expenditures away from reproduction and territorial defense toward hoarding food and increasing energy stores in fat. These animals undergo physiological changes in response to short-days, such as changes to pelage and increasing body weight. Exposure to nighttime lighting suppresses melatonin rhythms and thus alters the detection of short days. Another strategy is to reduce body mass in response to short days to reduce surface volume in order to conserve heat and reduce caloric requirements during winter (Bartness & Wade, 1985). Exposure to dim ALAN disrupts the effects of short days on decreased body mass in Siberian hamsters (Phodopus sungorus) (Ikeno, Weil, & Nelson, 2014).
Reproduction is one of the most important events in an individual’s life, and is directly related to individual fitness. Successful reproduction requires production of oocytes and sperm, interaction with a mate, successful birth, and, in some cases, maternal/paternal care of offspring. Photoperiodic animals rely on day length cues to discern long days from short days, and detect, well in advance, the arrival or departure of seasons favoring reproductive success. Thus, exposure to ALAN could interfere with individuals’ detection of day length, skewing seasonal timing (Dominoni, Quetting, & Partecke, 2013). Small mammals that specifically mate during the spring become refractory to the inhibitory effects of short days, then undergo various physiological changes to hypothalamic, pituitary, and gonadal function to prepare for mating. However, ALAN during short days can interfere with processing day length information and maintain reproductive function (Ikeno et al., 2014). Presumably, exposure to ALAN in the field would allow reproduction to occur “out-of-season” which likely could compromise survival of both offspring and parents. In animals stimulated to breed by long days, light at night could inaccurately signal long days during winter. In lemurs (Microcebus murinus) exposed to 50 lx of ALAN, melatonin was decreased and sexual recrudescence was premature, indicated by increased testis size and testosterone concentrations (Le Tallec et al., 2016). Many photoperiodic breeders also give birth at a particular time of year, which is genetically fixed (Wolcott, Reitz, & Weckerly, 2015). Light pollution delayed births in tammar wallabies (Macropus eugenii) that typically give birth following a dormancy during short days (Robert et al., 2015). The effects of delayed birth are largely unknown in mammals. However, mismatch of reproductive timing and resources reduced offspring production fourfold in at least one population (Post & Forchhammer, 2008), therefore, altered reproductive timing by ALAN could presumably result in population declines. ALAN can also interfere in reproductive hormone signaling (reviewed in Ouyang, Davies, & Dominoni, 2018), although the effect of these alterations are mostly unkown in wild popluations.
Sky glow can reach far from urban areas, and thus a wide area of the planet today is exposed to altered nighttime lighting. Although large-scale ecosystem-level studies addressing the effects of ALAN are limited, small changes in activity and movement patterns in response to sky glow could potentially produce large-scale ecosystem changes in species distribution and thus interfere in several ecosystem-level processes. ALAN is a threat to individual fitness and might be a hidden detriment to biodiversity. Studies investigating the effects of ALAN in natural habitats and at the population and ecosystem level are required to elucidate the possible outcomes.
4 | SOLUTIONS AND CONCLUSIONS
ALAN is unavoidable in modern times, but there are measures that can limit the amount of sky glow produced from urban areas. In many species, blue light exerts a greater effect on circadian rhythms than light in the red spectrum (Brainard, Richardson, King, & Reiter, 1984, 2008; Figueiro & Rea, 2010). Blue light also produces more sky glow than red (Kyba, Ruhtz, Fischer, & Hölker, 2012). Thus, types of light sources are not all equal. LED-based white street lighting typically emits light from all wavelengths, but peaks in the blue and green wavelengths (Elvidge, Keith, Tuttle, & Baugh, 2010). Cities are considering changing from traditionally used bulbs to LED bulbs, which are more energy efficient, but the impact on wildlife might be greater (Stone, Wakefield, Harris, & Jones, 2015). Thus, balancing the energy-saving benefits of changing street lighting to LED lights with possible costs to ecosystem health must be considered. LED lights can now be tuned to emit light of different wavelengths, which could alleviate these consequences. Additionally, the design of lamps can alter the amount of sky glow as well. Light emitted at an angle slightly above the horizon produces more sky glow than light emitted toward the street (Cinzano, Falchi, Elvidge, & Baugh, 2000). Controlling these factors can limit sky glow as seen in Lombardia, Italy, where sky glow levels have been maintained at a constant level from 1998 to 2010 despite an estimated doubling of street lighting (Falchi, Cinzano, Elvidge, Keith, & Haim, 2011). Thus, selecting lighting sources while also considering the ecosystem could mitigate effects.
The extent to which wildlife species, communities, and ecosystems have responded to ALAN remains unspecified, and it also remains unknown whether species can adapt to additional temporal changes. However, studies on individuals strongly suggest wildlife species could be greatly affected in their daily and seasonal activities. These changes likely effect individual fitness, but additional studies are needed to determine the selective pressure ALAN exerts. An interdisciplinary approach is required to determine broader scale effects of ALAN on wildlife biodiversity and ecosystem health, and to determine strategies to mitigate the effects.
Acknowledgments
Preparation of this review was partially supported by NIH grant NS R01092388.
Funding information
National Institutes of Health, Grant/Award Number: R01092388
Footnotes
ORCID
Kathryn L. G. Russart http://orcid.org/0000-0001-9093-2618
References
- Angers K, Haddad N, Selmaoui B, Thibault L. Effect of melatonin on total food intake and macronutrient choice in rats. Physiology & Behavior. 2003;80:9–18. doi: 10.1016/s0031-9384(03)00215-4. [DOI] [PubMed] [Google Scholar]
- Aubrecht TG, Weil ZM, Magalang UJ, Nelson RJ. Dim light at night interacts with intermittent hypoxia to alter cognitive and affective responses. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2013;305:R78–R86. doi: 10.1152/ajpregu.00100.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN. Photoperiodic control of seasonal body weight cycles in hamsters. Neuroscience & Biobehavioral Reviews. 1985;9:599–612. doi: 10.1016/0149-7634(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Beauchamp G. Visual obstruction and vigilance: A natural experiment. Journal of Avian Biology. 2015;46:476–481. [Google Scholar]
- Beauchamp G, McNeil R. Vigilance in greater flamingos foraging at night. Ethology. 2003;109:511–520. [Google Scholar]
- Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology. 2011;36:1062–1069. doi: 10.1016/j.psyneuen.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Vaughn CA, Galan A, Daye G, Weil ZM, Nelson RJ. Nocturnal light exposure impairs affective responses in a wavelength-dependent manner. Journal of Neuroscience. 2013a;33:13081–13087. doi: 10.1523/JNEUROSCI.5734-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Vaughn CA, Weil ZM, Nelson RJ. Behaviour of laboratory mice is altered by light pollution within the housing environment. Animal Welfare. 2013b;22:483–487. [Google Scholar]
- Benarroch EE. Suprachiasmatic nucleus and melatonin: Reciprocal interactions and clinical correlations. Neurology. 2008;71:594–598. doi: 10.1212/01.wnl.0000324283.57261.37. [DOI] [PubMed] [Google Scholar]
- Bennie JJ, Duffy JP, Inger R, Gaston KJ. Biogeography of time partitioning in mammals. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13727–13732. doi: 10.1073/pnas.1216063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Richardson BA, King TS, Reiter RJ. The influence of different light spectra on the supression of pineal melatonin content in the Syrian hamster. Brain Research. 1984;294:333–339. doi: 10.1016/0006-8993(84)91045-x. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Sliney D, Hanifin JP, Glickman G, Byrne B, Greeson JM, Rollag MD. Sensitivity of the human circadian system to short-wavelength (420-nm) light. Journal of Biological Rhythms. 2008;23:379–386. doi: 10.1177/0748730408323089. [DOI] [PubMed] [Google Scholar]
- Brown JS, Kotler BP, Smith RJ, Wirtz WO. The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia. 1988;76:408–415. doi: 10.1007/BF00377036. [DOI] [PubMed] [Google Scholar]
- Chellappa SL, Steiner R, Blattner P, Oelhafen P, Götz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: Can blue-enriched light keep us alert? PLoS One. 2011;6:e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinzano P, Falchi F, Elvidge CD, Baugh KE. The artificial night sky brightness mapped from DMSP satellite Operational Linescan System measurements. Monthly Notices of the Royal Astronomical Society. 2000;318:641–657. [Google Scholar]
- Clarke JA. Moonlight’s influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus) Behavioral Ecology and Sociobiology. 1983;13:205–209. [Google Scholar]
- Davies TW, Bennie J, Inger R, Gaston KJ. Artificial light alters natural regimes of night-time sky brightness. Scientific Reports. 2013;3:1722. [Google Scholar]
- Dominoni D, Quetting M, Partecke J. Artificial light at night advances avian reproductive physiology. Proceedings Biological Sciences. 2013;280:20123017. doi: 10.1098/rspb.2012.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominoni DM. The effects of light pollution on biological rhythms of birds: An integrated, mechanistic perspective. Journal of Ornithology. 2015;156:409–418. [Google Scholar]
- Dominoni DM, Partecke J. Does light pollution alter daylength? A test using light loggers on free-ranging European blackbirds (Turdus merula) Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2015;370:20140118. doi: 10.1098/rstb.2014.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doren BM, Horton KG, Dokter AM, Klinck H, Elbin SB, Farnsworth A. High-intensity urban light installation dramatically alters nocturnal bird migration. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:11175–11180. doi: 10.1073/pnas.1708574114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvidge CD, Keith DM, Tuttle BT, Baugh KE. Spectral identification of lighting type and character. Sensors. 2010;10:3961–3988. doi: 10.3390/s100403961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, Furgoni R. The new world atlas of artificial night sky brightness. Science Advances. 2016;2:e1600377–e1600377. doi: 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. Limiting the impact of light pollution on human health, environment and stellar visibility. Journal of Environmental Economics and Management. 2011;92:2714–2722. doi: 10.1016/j.jenvman.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Rea MS. The effects of red and blue lights on circadian variations in cortisol, alpha amylase, and melatonin. International Journal of Endocrinology. 2010;2010:1–9. doi: 10.1155/2010/829351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. Journal of Biological Rhythms. 2013;28:262–271. doi: 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. Journal of Biological Rhythms. 2012;27:319–327. doi: 10.1177/0748730412448324. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston KJ, Bennie J, Davies TW, Hopkins J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol Rev. 2013;88:912–927. doi: 10.1111/brv.12036. [DOI] [PubMed] [Google Scholar]
- Gauthreaux SAJ, Belser CG. Effects of artificial night lighting on migrating birds. In: Rich C, Longcore T, editors. Ecological consequences of artificial night lighting. Washington, DC: Island Press; 2006. pp. 67–93. [Google Scholar]
- Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental “jet lag” inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5:e15267. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenzel WP, Salmon TP. Characteristics of American crow urban roosts in California. The Journal of Wildlife Management. 1995;59:638–645. [Google Scholar]
- Griffin PC, Griffin SC, Waroquiers C, Mills LS. Mortality by moonlight: Predation risk and the snowshoe hare. Behavioral Ecology. 2005;16:938–944. [Google Scholar]
- Hall AS. Acute artificial light diminishes central Texas anuran calling behavior. The American Midland Naturalist Journal. 2016;175:183–193. [Google Scholar]
- Hoffmann J, Palme R, Eccard JA. Long-term dim light during nighttime changes activity patterns and space use in experimental small mammal populations. Environmental Pollution. 2018;238:844–851. doi: 10.1016/j.envpol.2018.03.107. [DOI] [PubMed] [Google Scholar]
- Hogan MK, Kovalycsik T, Sun Q, Rajagopalan S, Nelson RJ. Combined effects of exposure to dim light at night and fine particulate matter on C3H/HeNHsd mice. Behavioural Brain Research. 2015;294:81–88. doi: 10.1016/j.bbr.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölker F, Wolter C, Perkin EK, Tockner K. Light pollution as a biodiversity threat. Trends in Ecology & Evolution. 2010;25:681–682. doi: 10.1016/j.tree.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Ikeno T, Weil ZM, Nelson RJ. Dim light at night disrupts the short-day response in Siberian hamsters. General and Comparative Endocrinology. 2014;197:56–64. doi: 10.1016/j.ygcen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Imber MJ. Behaviour of petrels in relation to the moon and artificial lights. Notornis. 1975;22:302–306. [Google Scholar]
- Jones KA, Krebs JR, Whittingham MJ. Vigilance in the third dimension: Head movement not scan duration varies in response to different predator models. Animal Behaviour. 2007;74:1181–1187. [Google Scholar]
- de Jong M, Ouyang JQ, Da Silva A, van Grunsven RHA, Kempenaers B, Visser ME, Spoelstra K. Effects of nocturnal illumination on life-history decisions and fitness in two wild songbird species. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2015;370:20140128–20140128. doi: 10.1098/rstb.2014.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Current Biology. 2010;20:1735–1739. doi: 10.1016/j.cub.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Schor N, Dominoni D, de la Iglesia H, Levy O, Herzog ED, Dayan T, Helfrich-Forster C. Chronobiology by moonlight. Proceedings Biological Sciences. 2013;280:20123088. doi: 10.1098/rspb.2012.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurvers RHJM, Holker F. Bright nights and social interactions: A neglected issue. Behavioral Ecology. 2015;26:334–339. [Google Scholar]
- Kyba CCM, Ruhtz T, Fischer J, Hölker F. Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLoS One. 2011;6:e17307. doi: 10.1371/journal.pone.0017307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba CCM, Ruhtz T, Fischer J, Hölker F. Red is the new black: How the colour of urban skyglow varies with cloud cover. Monthly Notices of the Royal Astronomical Society. 2012;425:701–708. [Google Scholar]
- van Langevelde F, Ettema JA, Donners M, WallisDeVries MF, Groenendijk D. Effect of spectral composition of artificial light on the attraction of moths. Biological Conservation. 2011;144:2274–2281. [Google Scholar]
- Last KS, Hobbs L, Berge J, Brierley AS, Cottier F. Moonlight drives ocean-scale mass vertical migration of zooplankton during the Arctic winter. Current Biology. 2016;26:244–251. doi: 10.1016/j.cub.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Lima SL. Putting predators back into behavioral predator–prey interactions. Trends in Ecology & Evolution. 2002;17:70–75. [Google Scholar]
- Lima SL, Bednekoff PA. Back to the basics of antipredatory vigilance: Can nonvigilant animals detect attack? Animal Behaviour. 1999;58:537–543. doi: 10.1006/anbe.1999.1182. [DOI] [PubMed] [Google Scholar]
- Longcore T, Rich C. Ecological light pollution. Frontiers in Ecology and the Environment. 2004;2:191–198. [Google Scholar]
- Ludvigsen M, Berge J, Geoffroy M, Cohen JH, De La Torre PR, Nornes SM, Singh H, Johnsen G. Use of an autonomous surface vehicle reveals small-scale diel vertical migrations of zooplankton and susceptibility to light pollution under low solar irradiance. Science Advances. 2018;4:eaap9887. doi: 10.1126/sciadv.aap9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung MA, Childress MJ. The influence of conspecifics and predation risk on the vigilance of elk (Cervus elaphus) in Yellowstone National Park. Behavioral Ecology. 2006;18:12–20. [Google Scholar]
- Miller MW. Apparent effects of light pollution on singing behavior of American robins. Condor. 2006;108:130–139. [Google Scholar]
- Montevecchi WA. Influences of artificial light on marine birds. In: Rich C, Longcore T, editors. Ecological consequences of artificial night lighting. Washington, DC: Island Press; 2006. pp. 94–113. [Google Scholar]
- Mougeot F, Bretagnolle V. Predation risk and moonlight avoidance in nocturnal seabirds. Journal of Avian Biology. 2000;31:376–386. [Google Scholar]
- Nelson RJ, Drazen DL. Melatonin mediates seasonal adjustments in immune function. Reproduction Nutrition Development. 1999;39:383–398. doi: 10.1051/rnd:19990310. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Davies S, Dominoni D. Hormonally mediated effects of artificial light at night on behavior and fitness: Linking endocrine mechanisms with function. The Journal of Experimental Biology. 2018;221:jeb156893. doi: 10.1242/jeb.156893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends in Cell Biology. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E, Forchhammer MC. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363:2369–2375. doi: 10.1098/rstb.2007.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert KA, Lesku JA, Partecke J, Chambers B. Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proceedings of the Royal Society of London Series B, Biological Sciences. 2015;282:20151745. doi: 10.1098/rspb.2015.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotics S, Dayan T, Kronfeld-Schor N. Effect of artificial night lighting on temporally partitioned spiny mice. Journal of Mammalogy. 2011;92:159–168. [Google Scholar]
- Russ A, Reitemeier S, Weissmann A, Gottschalk J, Einspanier A, Klenke R. Seasonal and urban effects on the endocrinology of a wild passerine. Ecology and Evolution. 2015;5:5698–5710. doi: 10.1002/ece3.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch RD, Walls SC, Barichivich WJ, O’Donnell KM. Extinction debt as a driver of amphibian declines: An example with imperiled flatwoods salamanders. Journal of Herpetology. 2017;51:12–18. [Google Scholar]
- La Sorte FA, Fink D, Buler JJ, Farnsworth A, Cabrera-Cruz SA. Seasonal associations with urban light pollution for nocturnally migrating bird populations. Global Change Biology. 2017;23:4609–4619. doi: 10.1111/gcb.13792. [DOI] [PubMed] [Google Scholar]
- Spoelstra K, van Grunsven RHA, Donners M, Gienapp P, Huigens ME, Slaterus R, Veenendaal E. Experimental illumination of natural habitat–an experimental set-up to assess the direct and indirect ecological consequences of artificial light of different spectral composition. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2015;370:20140129. doi: 10.1098/rstb.2014.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelstra K, Verhagen I, Meijer D, Visser ME. Artificial light at night shifts daily activity patterns but not the internal clock in the great tit (Parus major) Proceedings of the Royal Society of London Series B, Biological Sciences. 2018;285:20172751. doi: 10.1098/rspb.2017.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EL, Wakefield A, Harris S, Jones G. The impacts of new street light technologies: Experimentally testing the effects on bats of changing from low-pressure sodium to white metal halide. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2015;370:20140127. doi: 10.1098/rstb.2014.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaddle JP, Francis CD, Barber JR, Cooper CB, Kyba CCM, Dominoni DM, Longcore T. A framework to assess evolutionary responses to anthropogenic light and sound. Trends in Ecology & Evolution. 2015;30:550–560. doi: 10.1016/j.tree.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Le Tallec T, Théry M, Perret M. Melatonin concentrations and timing of seasonal reproduction in male mouse lemurs (Microcebus murinus) exposed to light pollution. Journal of Mammalogy. 2016;97:753–760. [Google Scholar]
- Tuttle MD, Ryan MJ. The role of synchronized calling, ambient light, and ambient noise in anti-bat behaviour of a treefrog. Behavioral Ecology and Sociobiology. 1982;154:171–174. [Google Scholar]
- Tuxbury SM, Salmon M. Competitive interactions between artificial lighting and natural cues during seafinding by hatchling marine turtles. Biological Conservation. 2005;121:311–316. [Google Scholar]
- Weaver RE. Effects of simulated moonlight on activity in the desert nightsnake (Hypsiglena chlorophaea) Northwest Science. 2011;85:497–500. [Google Scholar]
- Wiltschko W, Munro U, Ford H, Wiltschko R. Red light disrupts magnetic orientation of migratory birds. Nature. 1993;364:525–527. [Google Scholar]
- Wiltschko W, Wiltschko R. Migratory orientation of European robins is affected by the wavelength of light as well as by a magnetic pulse. Journal of Comparative Physiology A. 1995;177:363–369. [Google Scholar]
- Wolcott DM, Reitz RL, Weckerly FW. Biological and environmental influences on parturition date and birth mass of a seasonal breeder. PLoS One. 2015;10:e0124431. doi: 10.1371/journal.pone.0124431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorzinski JL, Chisholm S, Byerley SD, Coy JR, Aziz A, Wolf JA, Gnerlich AC. Artificial light pollution increases nocturnal vigilance in peahens. Peer J. 2015;3:e1174. doi: 10.7717/peerj.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurk H, Trites AW. Experimental attempts to reduce predation by harbor seals on out-migrating juvenile salmonids. Transactions of the American Fisheries Society. 2000;129:1360–1366. [Google Scholar]


