Abstract
Background:
Phosphodiesterase type-1 (PDE1) hydrolyzes cyclic AMP and cGMP and is constitutively expressed in the heart, though cardiac effects from its acute inhibition in vivo are largely unknown. Existing data are limited to rodents expressing mostly the cGMP-favoring PDE1A isoform. Human heart predominantly express PDE1C with balanced selectivity for cAMP and cGMP. Here, we determined acute effects of PDE1 inhibition in PDE1C-expressing mammals, dogs and rabbits, in normal and failing hearts, and explored its regulatory pathways.
Methods:
Conscious dogs chronically instrumented for pressure-volume relations were studied before and after tachypacing-induced heart failure (HF). A selective PDE1 inhibitor (ITI-214) was administered orally or intravenously ± dobutamine. Pressure-volume analysis in anesthetized rabbits tested the role of beta-adrenergic and adenosine receptor signaling on ITI-214 effects. Sarcomere and calcium dynamics were studied in rabbit left-ventricular myocytes.
Results:
In normal and HF dogs, ITI-214 increased load-independent contractility, improved relaxation, and reduced systemic arterial resistance, raising cardiac output without altering systolic blood pressure. Heart rate increased, but less so in HF dogs. ITI-214 effects were additive to beta-adrenergic receptor (β-AR) agonism (dobutamine). Dobutamine but not ITI-214 increased plasma cAMP. ITI-214 induced similar cardiovascular effects in rabbits, whereas mice displayed only mild vasodilation and no contractility effects. In rabbit, β-AR-blockade (esmolol) prevented ITI-214-mediated chronotropy, but inotropy and vasodilation remained unchanged. By contrast, adenosine A2B-receptor blockade (MRS-1754) suppressed ITI-214 cardiovascular effects. Adding fixed-rate atrial pacing did not alter the findings. ITI-214 alone did not affect sarcomere or whole-cell calcium dynamics, whereas β-AR agonism (isoproterenol) or PDE3 inhibition (cilostamide, CIL) increased both. Unlike CIL, which further enhanced shortening and peak calcium when combined with isoproterenol, ITI-214 had no impact on these responses. Both PDE1 and PDE3 inhibitors increased shortening and accelerated calcium decay when combined with forskolin, yet only CIL increased calcium transients.
Conclusions:
PDE1 inhibition by ITI-214 in vivo confers acute inotropic, lusitropic, and arterial vasodilatory effects in PDE1C-expressing mammals with and without HF. The effects appear related to cAMP signaling that is different from that provided via beta-AR receptors or PDE3 modulation. ITI-214, which has completed Phase I trials, may provide a novel therapy for HF.
Keywords: heart failure, cyclic nucleotide phosphodiesterase, cyclic AMP, beta-adrenergic, adenosine, calcium
INTRODUCTION
Heart failure (HF) affects an estimated 30–50 million patients worldwide. Despite recent therapeutic advances, its prevalence is increasing, partly due to a fall in mortality, but also from higher rates of major co-morbidities such as obesity, diabetes, and age1. A major factor underlying cardiac dysfunction in HF resides in second messenger signaling defects coupled to 3’,5’-cyclic adenosine and guanosine monophosphate (cAMP, cGMP). Cyclic AMP stimulates protein kinase A (PKA) and exchange protein activated by cAMP (EPAC), acutely enhancing excitation-contraction coupling and sarcomere function2. Cyclic GMP acts as a brake on this signaling by activating protein kinase G3, 4 (PKG). Both cyclic-nucleotides have relevant vascular and fibroblast activity, reducing vessel tone, altering permeability and proliferation, and suppressing fibrosis.5–7 They are synthesized by adenylyl or guanylyl cyclases and degraded (hydrolyzed) by phosphodiesterases (PDEs)8, 9, to provide tissue and cell specific intracellular nano-regulation10.
From a therapeutic standpoint, PDEs are of particular interest because their structure is amenable to selective small molecule inhibition, and their cell-specific expression provides better organ targeting11. There are 11 main PDE members expressed as >100 isoforms, with PDE4, PDE7, and PDE8 being highly selective for cAMP; and PDE5, PDE6, and PDE9 for cGMP. The remaining PDEs hydrolyze both with selectivity depending on biological conditions and isoforms11, 12. The heart and/or myocytes express mRNA for all but PDE6, and functional roles have been identified for PDE1–5, PDE8, and PDE9. Inhibitors of several of these PDEs have been translated to humans. PDE3 inhibitors are used as inotrope/vasodilators for acute HF13, and PDE5 inhibitors for pulmonary hypertension and erectile dysfunction14, though cardiac indications have also been explored15–17. PDE4 inhibitors are used for forms of chronic pulmonary disease18, while PDE9 inhibitors have been tested for Alzheimer’s disease19, and recent data suggest they may hold promise for HF as well20.
PDE1 is constitutively and robustly expressed in the heart. It is activated by a Ca2+/calmodulin-binding domain and provides a substantial percent of in vitro cAMP and cGMP hydrolytic activity in mammals, including humans21, 22. Remarkably, however, virtually nothing is known about its role in acute cardiovascular regulation. PDE1 is expressed as three isoforms: PDE1A and PDE1C are in heart and vessels, whereas PDE1B is primarily found in brain. The isoforms are not redundant, as PDE1A is >30 times more selective for cGMP, whereas PDE1C has similar affinities for both cyclic nucleotides9. Humans predominantly express PDE1C in the heart, whereas rodents express mostly PDE1A. Yet, all reported cardiovascular studies are from rodents. In mice, non-isoform selective PDE1 inhibition attenuates cardiac hypertrophy and fibrosis induced by 1–2 weeks of isoproterenol or angiotensin infusion23, 24, and is coupled to enhanced cGMP levels. Mice genetically lacking PDE1C are also protected against pressure-overload25, though here the mechanism relates to cAMP. PDE1 also regulates sino-atrial beat frequency via cAMP-stimulated HCN4 potassium channels26. Cardio-vascular effects of PDE1 inhibition in mammals similar to humans (mostly expressing PDE1C), have not been reported. In addition, whether such effects are altered in HF or by β-adrenergic stimulation/blockade is unknown. These are all essential pre-clinical questions if small molecule PDE1 inhibitors are to find applications in human heart disease.
The recent development of ITI-21427, a potent and highly selective PDE1 inhibitor first studied for treating neurodegenerative and neuropsychiatric disease28, offers a new tool to address these questions. To our knowledge, it is the only PDE1 inhibitor yet studied in humans (NCT01900522 and NCT03257046), so far for neurocognitive diseases, with a Phase Ib/IIa safety/tolerability dosing study underway in humans with HF (NCT03387215). It is also the only compound for which sufficient quantities are available for large animal testing. The current study determined cardiovascular effects of ITI-214 in the dog and rabbit, both of which primarily express PDE1C, and further assessed its impact on failing hearts. We dissected signaling pathways engaged by PDE1 inhibition in the intact rabbit and isolated rabbit myocytes, where comparisons were also made to that of PDE3 inhibition. The data reveal acute positive inotropic, lusitropic, and arterial vasodilator effects in vivo that persist in HF, are not impacted by concomitant β-adrenergic stimulation or blockade, but regulate adenylate cyclase-coupled activity and require adenosine receptor A2BR signaling in vivo. They differ from PDE3 with respect to calcium handling and interaction with β-adrenergic stimulation.
METHODS
The authors declare that all supporting data are available within the article [and its online supplementary files].
Pharmaceuticals
ITI-214 (full molecular weight (phosphate salt) = 606, 6aR,9aS)-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-3-(phenyl-amino)-5,6a,7,8,9,9a-hexahydrocyclopenta-[4,5]imidazo[1,2-a]pyrazolo[4,3-e]pyrimidin-4-(2H)-one phosphate), was synthesized by Intra-Cellular Therapies, Inc. (New York, NY). Its Ki for full-length recombinant r-hPDE1A, 1B, and 1C is 34, 380, and 37 pM, respectively, with 900-fold greater activity toward PDE1C isoforms compared with the next nearest PDE family enzyme, PDE4D (Ki = 33 nM), and 104-3×105 -fold selectivity toward all other PDE enzyme families28. Additional pharmaceuticals were dobutamine (Hospira Inc, Lake Forest, IL), esmolol HCl (Mylan, Rockford, IL), cilostamide, MRS1754 (Tocris Bioscience, Bristol, UK), Hespan (6% Hetastarch in 0.9% NaCl, B. Braun Medical Inc., Bethlehem, PA), forskolin, isoproterenol HCl, and 3-Isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, St. Louis, MO).
Animal Studies
All animal study procedures were performed in accordance with the Guide to the Care and Use of Laboratory Animals, and approved by the Johns Hopkins University Institutional Animal Care and Use Committee (IACUC).
In Vivo Canine Studies
Adult mongrel dogs (25–30 kg; n=6) were chronically instrumented with left ventricular sonomicrometers, micromanometer, inferior vena caval cuff occluder, and indwelling arterial and venous catheters for conscious pressure-volume (PV) hemodynamic analysis. Dogs were studied in the conscious state before and after inducing dilated cardiomyopathy by tachypacing. Details of this preparation have been reported29. On separate days, dogs were administered oral (0.1–10 mg/kg) or intravenous (0.01 and 0.1 mg/kg) ITI-214 with and without concomitant beta-adrenergic receptor stimulation by dobutamine (10 μg/kg/min). Intravenous infusion data were measured over 15 minutes post bolus injection or after steady-state infusion was achieved. Oral ITI-214 was provided in gelatin capsules encased in peanut butter, and hemodynamic data recorded over the ensuing two hours. Based on plasma levels and responses in normal and HF dogs, an optimal dose (10 mg/kg) was identified and is reported. This dose was repeated on a separate day, with dogs first receiving dobutamine, then after re-baseline, oral ITI-214, and after 2 hours, repeat dobutamine testing. Intravenous ITI-214 was dissolved in 0.05 M citrate-phosphate buffer (pH 3.2), and administered via a central venous catheter.
In Vivo Rabbit Studies
New Zealand White rabbits (male, 2–3 kg, n=25) were sedated with 35 mg/kg ketamine and 5 mg/kg xylazine, intubated and ventilated (Model 683, Harvard Apparatus, Holliston, MA), and anesthesia maintained by isoflurane inhalation (1–2%). A pressure-volume catheter (SPR-894, Millar, Inc., Houston, TX) was inserted via the common carotid artery and advanced to the LV apex, and a 2-French pacing catheter (Arrow Intern. Inc., Cleveland, OH) was positioned in the right atrium via the right jugular vein to provide atrial pacing. A 4-Fr Berman balloon catheter (AI-07134, Teleflex, Wayne, PA) was positioned in the inferior vena cava (IVC) via a femoral vein and used to transiently reduce venous return to assess PV relations. Volumes were calibrated by the hypertonic saline method30, with an assumed gain of 1.0 Rabbits were infused with 6% hetastarch in saline during the procedure to stabilize arterial pressure. All pharmaceuticals were administered intravenously in two protocols: ITI-214 (bolus 0.1 mg/kg administered over 1 minute) with or without 15 minute pre-treatment with 1) esmolol (0.5 mg/kg bolus injection followed by 0.05 mg/kg/min continuous infusion) or 2) MRS1754 (1 mg/kg intravenous bolus).
In vivo Mouse Studies
Adult C57BL/6J mice (3 months, n=5–7) were anesthetized, instrumented with a miniature pressure-volume catheter and studied as described30. ITI-214 was delivered at 0.1 and 0.5 mg/kg iv over a 30-second period, and cardiovascular function recorded for 10 minutes.
Myocardial Tissue Analysis
Heart tissue was rapidly excised from euthanized adult C57BL/6J mice, Sprague Dawley rats, mongrel dogs, and New Zealand White Rabbits, washed and then frozen in liquid nitrogen. Human myocardial tissue was obtained from donor control hearts (n=14) and end-stage cardiomyopathic hearts (n=14) from a tissue bank at the University of Pennsylvania. Both types of heart tissue were harvested under controlled surgical procedures using ice-cold cardioplegia, transported on ice, and snap-frozen in liquid nitrogen shortly thereafter.
Isolated Myocyte Studies
New Zealand White rabbits (male, 2–3 kg, n=13) were sedated as above, and the heart perfused retrograde using a Langendorff preparation to isolate left ventricular myocytes. Details of the procedure are provided in Supplemental Methods.
Real-time Quantitative PCR Analysis
RNA was extracted and analyzed using standard protocols (see Supplemental Methods).
Protein Immunoblot Analysis
Details of protein immunoblot analysis are provided in Supplemental Methods.
LC/MS-MS Analysis of ITI-214 in Plasma
Blood was collected in K2EDTA tubes, centrifuged at 2,000 × g for 15 min, and the plasma fraction frozen. ITI-214 levels were assessed by a rapid, sensitive liquid chromatography–tandem mass spectrometric (LC/MS–MS) method (details in Supplemental Methods) In an initial dose titration study, normal conscious dogs were exposed to oral and IV doses of ITI-214 ranging 0.1–10 mg/kg, and pharmacodynamics assays were performed. With 10 mg/kg p.o., plasma ITI-214 free base concentrations were 103.9±8.5 ng/mL at 120 min post dose in normal dogs, and 178.4 ± 100.5 ng/mL in HF dogs. At 0.1 mg/kg i.v., in normal dogs, plasma ITI-214 concentrations peaked within minutes, falling to 211.2±60.7 at 10 min, and 94.5±21.2 ng/mL at 30 min. These levels were somewhat higher in failing dogs (441.4±92.5 and 184.9±28.9 ng/mL at 10 and 30 min, respectively (Supplemental Figure S1)). Rabbit dosing at 0.1 mg/kg yielded 213±88 ng/mL at 15 min. Mouse plasma levels 15 min after intravenous dosing was 334.1±74 ng/mL. Plasma levels of 100–200 ng/mL correspond to free plasma concentrations of 1–2 nM ITI-214 (which is >99% plasma protein bound), above the Ki for recombinant PDE1, but far below the Ki of other PDEs28.
Cyclic Nucleotide Assay
Details for cAMP and cGMP plasma analysis are provided in Supplemental Methods.
Data Analysis and Statistics
Hemodynamic recordings were obtained with a custom AD acquisition system or Powerlab (AD Systems) and analyzed with custom software (WinPVAN, JHU Baltimore) as described30. Results are presented as means ± SEM. For steady state analysis, multiple cycles (10–20) were time-averaged to generate a single beat that was analyzed. For multiple-beat pressure-volume analysis, data during inferior vena cava (IVC) occlusion were used to derive load-independent measures of contractility (end-systolic elastance (Ees), preload-recruitable stroke work (PRSW), maximal rate of pressure rise normalized to instantaneous pressure (dP/dtmax/IP), rate constant of relaxation, and various other hemodynamic indexes as previously described29. Myocyte shortening and calcium data were analyzed using IonOptix software (MyoCam). Data were statistically analyzed using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA). Western blot and qPCR data were analyzed using two-way ANOVA to assess interaction of isoform and disease. In vivo time-series data were examined using two-way repeated measures ANOVA with post-hoc Sidak’s multiple comparison test, to assess interaction of time and drug intervention. One-way ANOVA (or Kruskal Wallis test, if D’Agostino & Pearson normality test failed) with post-hoc multiple comparison testing, and unpaired t-test (or Mann Whitney test) were also used where appropriate and are indicated in figure legends.
RESULTS
PDE1A vs PDE1C isoform expression in dog and rabbit versus human
In order to establish an experimental model in a mammal expressing primarily PDE1C in the myocardium, protein and gene expression were examined in human, dog, rabbit, rat, and mouse. Dog and rabbit express primarily PDE1C (Fig. 1A, 1B), with a smaller amount of PDE1A and opposite the profile in rat or mouse (Supplemental Figure S2). The expression profile is not significantly altered by HF in dogs. Human left ventricle (LV) overwhelmingly expresses PDE1C at the transcript level, though some PDE1A is present. At the protein level, both are present, with PDE1C dominating in normal animals, and PDE1A increasing with end-stage HF (Fig. 1C). PDE1B is undetected in heart but present in the brain for all the species.
Figure 1. PDE1 isoform protein and gene expression in canine and rabbit.
(A) PDE1 isoform expression for protein (left and center) and mRNA (right) in both non-failing and failing canine left ventricle (n=5). (B) PDE1 isoform expression for protein (left and center) and mRNA (right) from normal rabbit left ventricle (n=5). (C) PDE1 isoform expression for protein (left and center) and mRNA (right) in the hearts of normal and DCM human patients (Western blot n=14, qPCR n=10). Brain lysate is provided as a positive control, and same amount of protein loaded as for the heart extract. For A and C: *p<0.0001 vs PDE1A or 1B (normal), †p<0.0001 vs PDE1A or 1B (HF/DCM), ‡p<0.0001 vs PDE1C (normal), §p<0.0001 vs PDE1A, #p<0.01, **p<0.001 vs PDE1A (Normal); ††p<0.01 vs PDE1A (DCM) by two-way ANOVA with Sidak’s post-hoc test. For B: §p<0.0001, ||p<0.01 vs PDE1A or 1B by one-way ANOVA with Tukey’s post-hoc test. HF = heart failure, LV = left ventricle, DCM = dilated cardiomyopathy, a.u. = arbitrary units.
Acute PDE1 inhibition alters cardiovascular function in normal and failing canine hearts
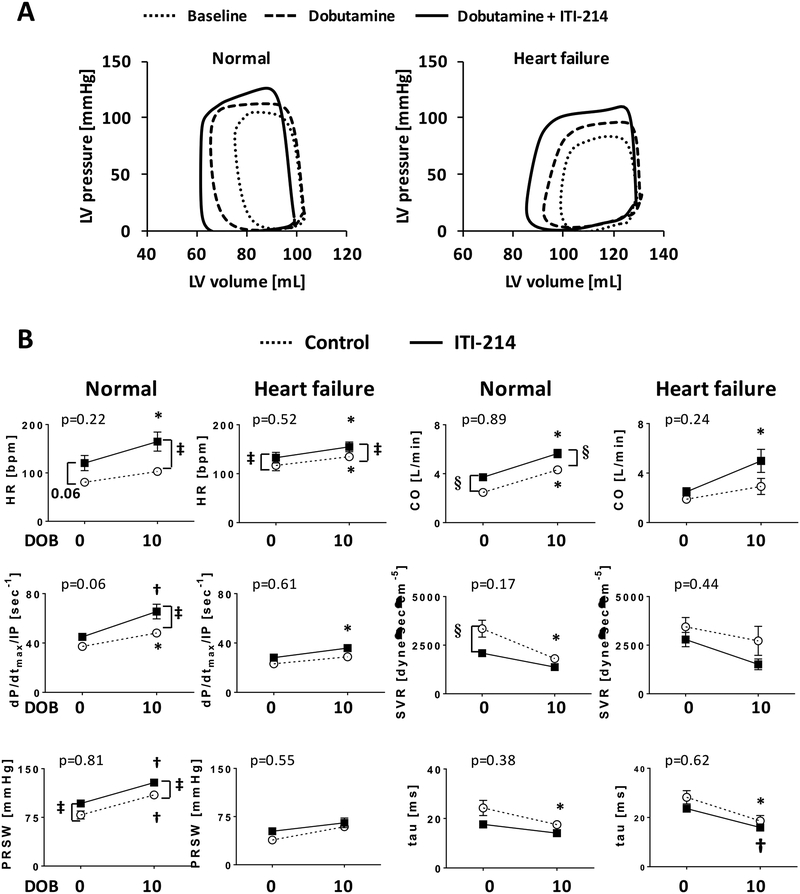
To assess the cardiac effects of acute PDE1 inhibition, we obtained pressure-volume relationships in intact dogs with healthy or failing hearts and analyzed hemodynamic and cardiac mechanics at baseline and 120 minutes after a single oral ITI-214 dose (10 mg/kg). This time point was based on pharmacokinetic data described in Methods. Figure 2A shows representative PV loops and relationships for a control and HF dog at the two time points. There was an increase in contractility (higher end-systolic elastance) with little change in LV preload (end-diastolic volume) or systolic pressure. Summary data for both conditions are provided in Figure 2B and Supplemental Table S1. In normal dogs, ITI-214 increased heart rate (HR), reduced systemic vascular resistance, and increased cardiac output, without altering systolic blood pressure. Load insensitive indexes of contractility (dP/dtmax/IP and PRSW) increased. Relaxation reflected by peak rate of pressure decline (dP/dtmin) and relaxation time constant (tau) also improved. With HF, basal contractility was depressed and ITI-214 increased inotropy and induced vasodilation, similar to controls. The rise in HR was blunted in HF animals (p=0.07 for interaction by 2-way ANOVA). Thus, ITI-214 produced a net rise in cardiac output (+50% in controls, +32% in HF) without altering systemic pressure.
Figure 2. Hemodynamic effect of acute PDE1 inhibition in dogs with normal and failing hearts.
A) Representative LV pressure-volume loops and relations defined by transient preload reduction. The end-systolic PV relationship is depicted by the line connecting the upper left-corner of the loops. Data are shown at baseline and 120 min post oral 10 mg/kg ITI-214 (n=5). B) Summary results for both non-failing and HF dogs. *p<0.05, #p<0.01 vs baseline, paired t-test. HR – heart rate, SBP – systolic blood pressure, SVR – systemic vascular resistance, dP/dtmax/IP – peak rate of pressure rise normalized to instantaneous pressure, PRSW – preload-recruitable stroke work, CO – cardiac output, Tau – relaxation time constant, dP/dtmin – peak rate of pressure decline, LVEDV – left ventricular end-diastolic volume.
Intravenous ITI-214 produced similar effects in both normal and HF conditions, but the response was more rapid, peaking after 5–10 minutes (Supplemental Figure S3). At 0.01 mg/kg (23.6±8.6 ng/mL plasma ITI-214 concentrations at 10 min), responses in all but HR were negligible at this dose. However, at 0.1 mg/kg (211.2±60.7 ng/mL plasma ITI-214 concentrations at 10 min), positive chronotropic, inotropic, lusitropic, and vasodilator responses were observed, with a net rise in cardiac output similar to that with 10 mg/kg oral dose. These effects were again slightly abated with HF, but inotropy, lusitropy, and vasodilation remained significantly improved.
Hemodynamic effects of PDE1 inhibition in dog is additive to β-adrenergic stimulation
The acute cardiovascular responses to ITI-214 suggested cAMP signaling over cGMP. This raised the question of whether its net impact was redundant to or amplified by co-activation of the β-AR pathway. This was tested by administering 10 μg/kg/min dobutamine with or without concurrent 10 mg/kg ITI-214. Dobutamine increased PV loop area (stroke work) and shifted the upper corner of each loop to the left (increased contractility), and adding ITI-214 enhanced both of these effects (Fig. 3A). These effects were additive, not synergistic. This was determined from the drug interaction term by 2-way ANOVA (Fig. 3B, p-values indicate interaction, none of which were significant (i.e. lines were generally parallel)).
Figure 3. ITI-214 does not alter acute cardiovascular effects of dobutamine in dogs.
A) Example PV loops and B) summary data of hemodynamics at baseline, post 10 μg/kg/min dobutamine, and dobutamine + 10 mg/kg ITI-214. The effects of ITI-214 or dobutamine alone were generally matched, and their combination was additive (e.g. no interaction effect between drugs; lines as shown were effectively parallel). P values for each graph are for this interaction term from 2-way repeated measures ANOVA. *p<0.05, †p<0.01 vs baseline (i.e. dobutamine = 0), ‡p<0.05, §p<0.01 vs control (n=4–5). DOB = dobutamine.
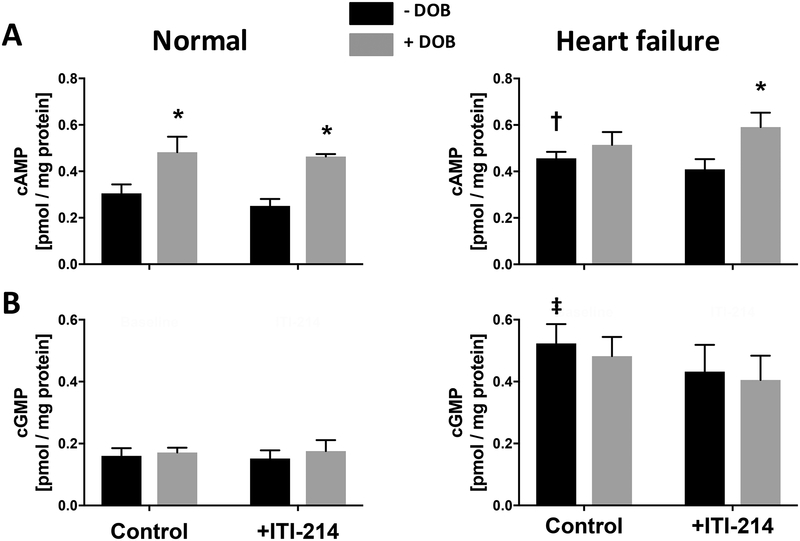
In normal dogs, plasma cAMP increased with dobutamine infusion but not with ITI-214. When combined, the same increase in cAMP was observed as with dobutamine alone (Fig. 4). ITI-214 alone also did not elevate plasma cAMP in HF dogs, whereas dobutamine+ITI-214 did. Plasma cGMP was unchanged at all conditions in normal and HF dogs. Both baseline cAMP and cGMP were significantly greater in HF versus control dogs.
Figure 4. ITI-214 does not increase plasma cAMP or cGMP, whereas dobutamine increases cAMP.
A) Blood plasma cAMP and B) cGMP levels measured in normal and HF dogs after exposure to 10 μg/kg/min dobutamine (intravenous × 15 min), 10 mg/kg ITI-214 (2-hours after oral consumption), or their combination. Only dobutamine increased plasma cAMP, and this was similar with or without ITI-214 co-treatment. Neither altered cGMP levels. 2-way ANOVA, with Sidak’s multiple comparisons test. *p<0.05 vs corresponding -DOB; † p=0.02, ‡ p=0.002 compared to Control (minus DOB) levels measured in normal dogs. n=4–5 for each assay. DOB=dobutamine.
Cardiovascular effects of PDE1 inhibition in rabbit are similar to dog, and unaltered by β-AR blockade but suppressed by adenosine A2B receptor inhibition.
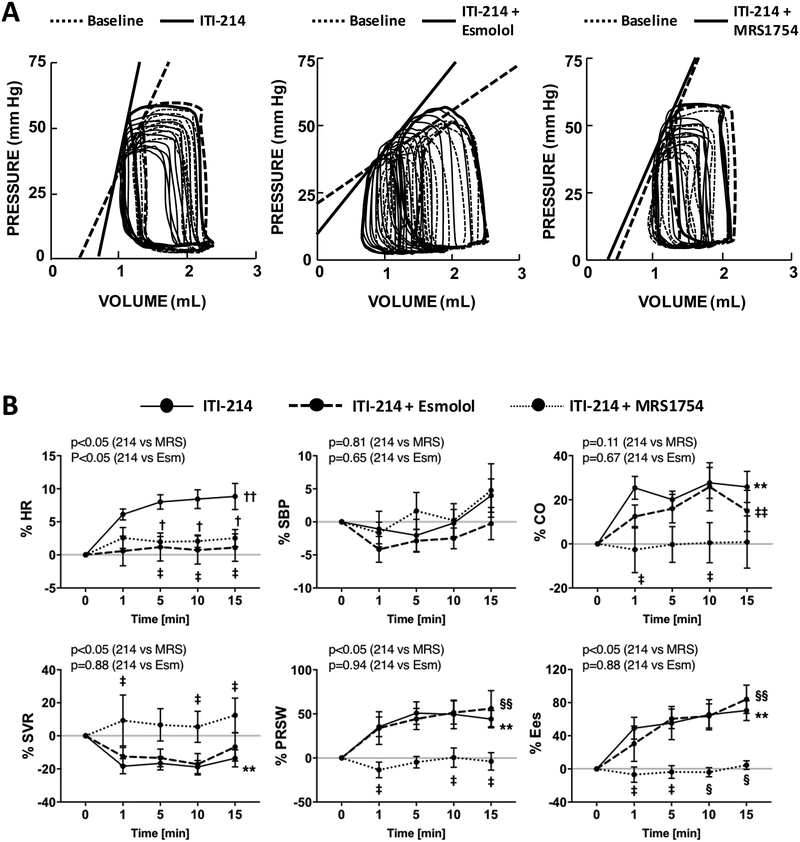
Cyclic AMP is principally generated by transmembrane adenylyl cyclase activated via stimulatory G protein (Gs), with β-adrenergic (β-AR), adenosine (notably A2B), glucagon, prostanoids, histamine, and serotonin receptors all coupling to Gs in cardiomyocytes2. Here, we focused on β-AR and adenosine receptors, examining the A2BR in particular as it is reported to directly stimulate cAMP-dependent contractility2, 31. Anesthetized rabbits received intravenous ITI-214 at 0.1 mg/kg (dose based on dog results) with or without prior treatment with the β-AR blocker esmolol or adenosine A2BR blocker MRS-1754. Figure 5A displays representative PV loops, and summary data are shown in Figure 5B, Supplemental Figure S4, and Supplemental Table S2. As in the dog, acute PDE1 inhibition alone increased cardiac output due to a rise in contractility (Ees and PRSW rose by 50%), modestly elevated HR, and lowered systemic resistance, and did not alter systolic pressure. Relaxation rate was faster (Supplemental Table S2). Mechanical efficiency as reflected by ventricular/arterial coupling ratio (Ees/Ea), increased (Supplemental Figure S4).
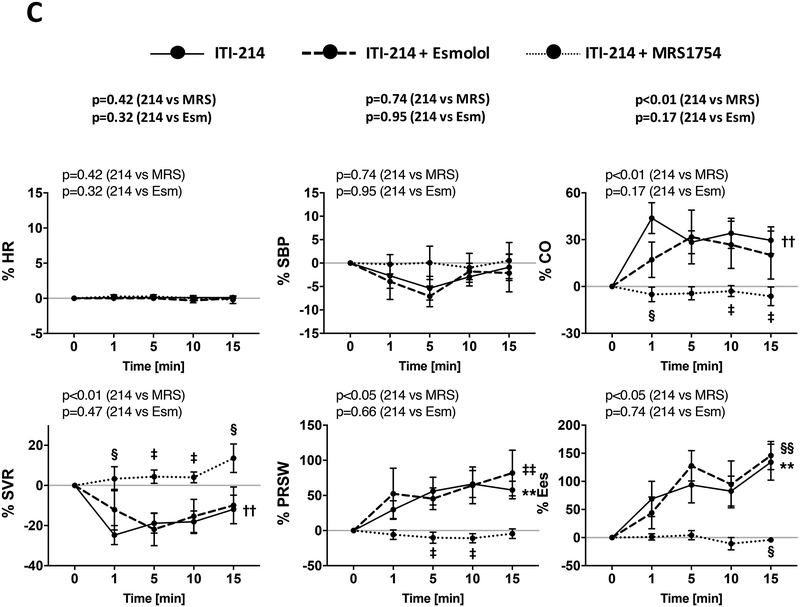
Figure 5. Influence of β-adrenergic or adenosine A2B receptor blockade on ITI-214 response.
A) Example pressure-volume relations before and after ITI-214 infusion with vehicle, esmolol (β-blocker), or MRS1754 (A2BR blocker). B) Summary time course plots of the 15-minute response to ITI-214 under the three co-treatment conditions. Data from rabbits (n=19 vehicle + ITI-214 0.1 mg/kg i.v.; n=11 for esmolol + ITI-214; and n=7 for MRS1754 + ITI-214) are shown. C) Summary data for same three conditions with constant atrial pacing (n=13 vehicle+ITI-214 0.1 mg/kg i.v.; n=8 for esmolol+ITI-214; and n=6 for MRS1754+ITI-214). †p<0.01 for ITI-214 vs ITI-214+Esmolol; ‡p<0.05, §p<0.01 for ITI-214 vs ITI-214+MRS1754 (two-way ANOVA with post hoc Sidak’s test); **p<0.01, ††p<0.001 (one-way ANOVA for ITI-214); ‡‡p<0.05, §§p<0.01 (one-way ANOVA for ITI-214+Esmolol). P values above each graph are for interaction of time and drug intervention from 2-way repeated measures ANOVA. 214 = ITI-214, MRS = MRS1754, Esm = esmolol.
To test the role of different cAMP-stimulation pathways on ITI-214 effects, we repeated these experiments with pre-treatment with esmolol to block β-AR receptors or MRS-1754 to block A2B receptors (Figure 5A, 5B, Supplemental Table S2). Each blocker was first infused and data obtained after 10–15 minutes prior to the addition of ITI-214. Neither blocker altered resting cardiac function or hemodynamics alone (Supplemental Table S2). When ITI-214 was infused in rabbits following β-AR blockade, HR did not increase yet contractile and vasodilation effects remained intact. Importantly, this esmolol dose virtually prevented cardiac responses to 10μg/kg/min dobutamine (Supplemental Fig. S5). By contrast, when ITI-214 was administered after A2B-R blockade, all of its cardiovascular responses including HR, contractility, and vasodilation were blocked. Thus, A2BR but not β-AR signaling is required for the acute in vivo contractility and vasodilator effects of PDE1 inhibition by ITI-214. Chronotropic effects are blocked by antagonism of either receptor.
HR itself influences cardiac function and hemodynamics, and as this differed between experimental animal groups, we controlled for HR using atrial pacing set at ~20% above the sinus rate. ITI-214 induced nearly identical responses under these conditions as when HR was allowed to increase; these effects were again unaltered by esmolol but suppressed by MRS-1754 (Fig. 5C, Supplemental Figure S6).
ITI-214 has mild vasodilator but no inotropic effects in intact mice
The isoform disparity among dog, rabbit and mice predicted that the mouse might respond differently to acute ITI-214 since PDE1A favors cGMP over cAMP hydrolysis. To performed studies in anesthetized mice, administering the same concentration (0.1 mg/kg, i.v.) as in dogs and rabbits while measuring PV relations (Supplemental Table S3). At this dose, there was only a small rise in cardiac output consistent with transient arterial vasodilation, no other parameters displayed significant differences. At a much higher dose (0.5 mg/kg), we observed chronotropy (+4%) and reduced arterial resistance (−15%) associated with a rise in cardiac output, but no increases in contractility. Thus, the mouse hemodynamic response had similarities with dog and rabbit with respect to systemic vasodilation and heart rate increase, but not inotropy.
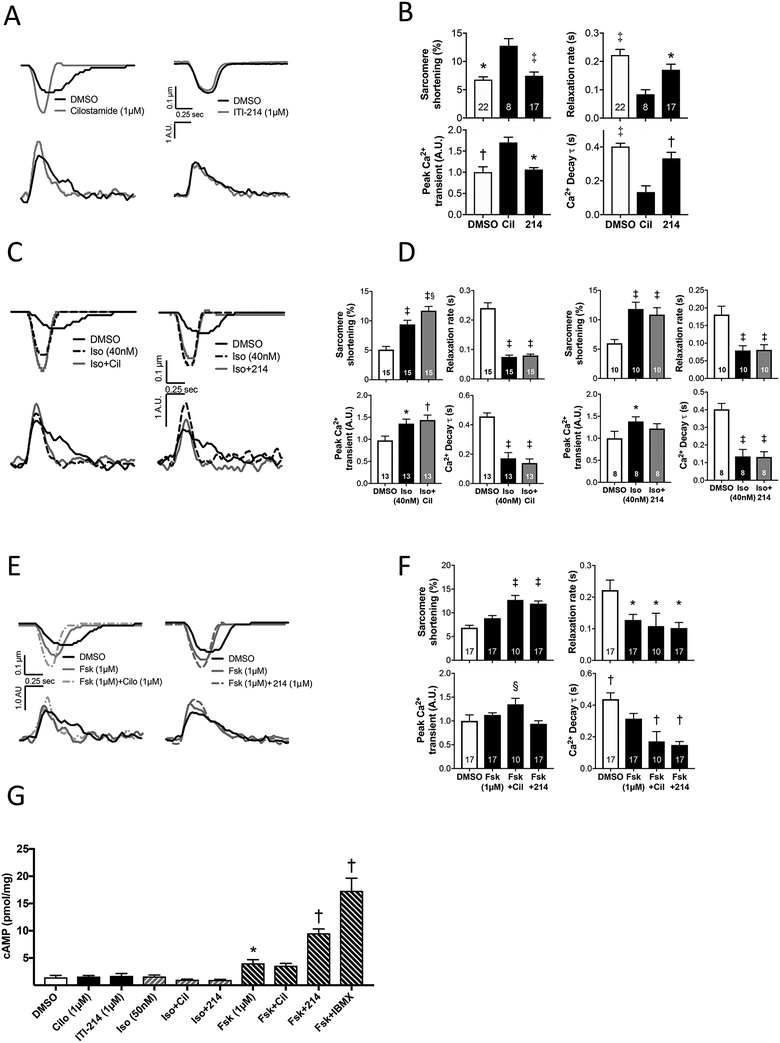
Effects of ITI-214 on myocyte contractility and calcium in comparison to PDE3 inhibition in isolated rabbit myocytes.
We next turned to isolated rabbit myocytes to determine the effect of PDE1 inhibition on cardiomyocyte contraction and whole cell Ca2+ transients, and compared the results to those with a PDE3 inhibitor, cilostamide (Cil, 1 μM). Figure 6A shows example time-tracings. Percent sarcomere shortening (%SS) and peak-Ca2+ transients rose and their decay kinetics accelerated in cells treated with Cil; however, this was not observed with ITI-214 (1 μM).
Figure 6. ITI-214 increases cAMP-mediated contractility in isolated rabbit cardiomyocytes.
A) Example traces of sarcomere shortening and Ca2+ transients in response cilostamide or ITI-214. B) Mean change of sarcomere shortening and relaxation rate, and normalized peak Ca2+ transient and exponential time-constant of Ca2+ decline (*p<0.05, †p<0.01, ‡ p<0.001 vs cilostamide; C) Example traces in response to isoproterenol ± cilostamide or ITI-214, and D) corresponding grouped data (*p<0.05, † p<0.01, ‡ p<0.001 vs DMSO, §p<0.001 vs Iso by two-way RM-ANOVA with Sidak’s post-hoc test). E) Example traces in response to forskolin ± cilostamide or ITI-214, and F) corresponding grouped data (*p<0.05 vs DMSO, † p≤0.05 vs Fsk, ‡ p<0.005 vs Fsk and DMSO, § p<0.03 vs DMSO and Fsk+ITI-214, Kruskal-Wallis test with Dunn’s post-hoc test). The number of myocyte for each group is shown. G) Intracellular cAMP in response to various activators/PDE inhibitors. Doses are shown and are the same as used in the functional assays (e.g. panels A, C, and E). *p<0.05 vs DMSO, †p<0.0005 vs Fsk. All analysis is performed by One-way ANOVA with Sidak’s multiple comparison test unless individual noted above. Cil = cilostamide, 214 = ITI-214, Iso = isoproterenol, Fsk = forskolin.
To compare the influence of PDE1 and PDE3 in modulating β-AR signaling, cells were first exposed to a non-saturating dose of isoproterenol (Iso, 40 nM near EC50 based on dose-response curve, Supplemental Figure S7), then Iso combined with either Cil or ITI-214. As expected, Iso increased %SS and peak-Ca2+, and quickened relaxation times. Addition of Cil further increased %SS (p=0.0002) and peak-Ca2+ remained elevated over baseline (p=0.001). By contrast, when ITI-214 was added to Iso, %SS did not change further, and peak-Ca2+ was no longer significantly different from pre-Iso baseline (Figure 6C, D). We conclude that PDE1 does not interact with β-AR signaling, but PDE3 does.
An alternative approach to augmenting cAMP independent of β-AR is to directly stimulate adenylate cyclase using forskolin (Fsk). We again performed a dose response study, identifying a non-saturating Fsk dose that still generated significant inotropic effects (1 μM, Supplemental Figure S8). When Cil was added to Fsk, we observed an increase in %SS, peak-Ca2+ transient, and faster decay of the Ca2+ transient. When ITI-214 was added to FSK, %SS rose as with Cil, but there was no corresponding increase in peak Ca2+ transient which was significantly less that with Cil. Interestingly, FSK+ITI-214 enhanced the Ca2+ decay rate similarly as did Cil (Figure 6E, 6F).
Lastly, we compared the effect of Iso, Cil, ITI-214, and their combination on myocyte cAMP. Despite increases in %SS and Ca2+ transients, Iso, Cil, or their combination did not measurably increase whole cell cAMP. Neither did ITI-214 and ISO+ITI-214. This is consistent with prior studies showing locally generated cAMP from β-AR and its modulation by PDE3 occur in local subcellular domains that are not easily detected in whole cell lysates32. Fsk resulted in a rise in cAMP which was further increased by the addition of ITI-214 but not Cil. The latter reached about half the maximal change as assessed by adding the broad PDE inhibitor IBMX to Fsk. Taken together, these data identify a different pool of cAMP under PDE1 regulation that is not modulated by β-AR but can be revealed with direct adenylate cyclase stimulation.
DISCUSSION
This study reveals potent acute cardiovascular effects from a highly selective pan-isoform PDE1 inhibitor (ITI-214) tested in mammalian hearts that like humans principally express PDE1C. The positive inotropy, chronotropy, and vasodilation effects are most consistent with modulation of cAMP, and were not seen in mice where the dominant isoform is PDE1A that favors cGMP hydrolysis. The combination of effects in both dog and rabbit results in a rise in cardiac output with negligible changes in cardiac preload and arterial systolic pressure. Unlike in vivo β-AR stimulation, PDE1 inhibition does not elevate plasma cAMP, nor are its cardiovascular effects blunted by β-AR blockade or constant-rate pacing. By contrast, these effects were markedly suppressed in the intact rabbit by concurrent A2BR inhibition, supporting engagement of an alternative cAMP pathway. Lastly, in studies performed in rabbit myocytes we reveal disparities between PDE1 and PDE3 inhibitory effects. PDE3 inhibition enhances contraction and peak-Ca2+ transients and amplifies β-AR stimulation, whereas PDE1 inhibition does not. PDE1 inhibition augments Fsk-stimulated cAMP and cell shortening without increasing Ca2+, whereas PDE3 inhibition increases both shortening and Ca2+ yet does not increase whole-cell measurable cAMP. Collectively, these in vivo and in vitro results define a pharmacological profile of ITI-214 that is different from β-AR agonism and PDE3 inhibition, and suggests potential utility as a clinical HF therapeutic engaging novel mechanisms. Indeed, we confirm increases of PDE1 isoform expression occur in human heart failure patients.
Given the array of cyclic nucleotide-signaling effects, the multiple cyclases involved in their synthesis, and >100 PDE isoforms engaged in their hydrolysis, it is not surprising that this regulation is highly compartmentalized within the cell10. In the case of cAMP regulating proteins, this transpires in conjunction with the A-kinase anchoring proteins (AKAPs) that coalesce these regulating proteins within nanodomains.33 PDE3, the focus of much prior cardiac research and the only species currently used for clinical HF, exists in different isoforms: PDE3A1 localizes to the SR-ATPase-phospholamban complex34, and its inhibition induces positive inotropic effects in conjunction with increasing whole-Ca2+transients32. Different PDE isoforms also couple with β-AR signaling, with PDE4 playing a prominent role in modulating inotropic responses in rodents35 whereas in larger mammals such as the dog, PDE3 plays a greater role36. The localization of PDE1 isoforms is less well studied, with one study showing PDE1C primarily in the soluble fraction of human myocytes, displaying a striated distribution that may reflect T-tubule mitochondria junctions22.
In the current study, evidence for microdomain regulation by PDE1 was provided by the whole cell cAMP measurements that showed little change despite Iso, Cil, or Iso+Cil stimulation, all of which (at the same concentrations) at doses we showed stimulated myocyte function and calcium handling. Fsk stimulates cAMP synthesis in multiple compartments as it targets adenylate cyclase directly, and here we detected a rise in cAMP that further increased with ITI-214; Iso+ITI-214 did not alter cAMP. This is consistent with a prior study employing FRET biosensors in adult mouse myocytes where a rise in cAMP with PDE1 inhibition was impacted by Fsk and not β-AR co-stimulation37. That cAMP appeared similar with Fsk and Fsk+Cil further supports different compartments being engaged.
While we did not directly probe for the sub-cellular cAMP signaling compartment(s) controlled by PDE1, we obtained substantial evidence supporting differences to cAMP regulation by β-AR activation or PDE3 inhibition. PDE1 inhibition failed to potentiate β-AR stimulation (in vivo and in vitro), unlike what occurs with PDE3 inhibition. In addition, ITI-214 did not augment whole-Ca2+ transients whereas this is observed with Iso stimulation +/− PDE3 inhibition38. The lack of Ca2+ increase was still seen even when ITI-214 did enhance cell function, as when combined with Fsk. By contrast, PDE3 inhibition increased both function and Ca2+. This is notable as prior safety concerns regarding PDE3 inhibitors often noted their effects on increasing myocyte Ca2+, including arrhythmia13. The lack of intracellular Ca2+ increase despite functional improvement suggest PDE1 inhibition likely enhances phosphorylation of sarcomere proteins to improve myofilament calcium sensitivity. Furthermore, the ability of ITI-214 to accelerate the rate of Ca2+ decline even as peak levels were slightly reduced, suggests it also modulates internal Ca2+ recycling and less so intracellular Ca2+ entry. The precise targets remain to be determined.
Another major new finding was the capacity of an A2B-R antagonist to block ITI-214 cardiovascular effects in vivo. The heart expresses multiple adenosine receptors, including A1, A2A, A2B, and A3. A1R and A3R couple to inhibitory G-proteins (Gi,0) and Gq/11 signaling, and blunt β-AR stimulation39. By contrast, A2A and A2B couple with stimulatory Gs-cAMP, though regulation of contraction by the former may relate to blunting A1R-anti-adrenergic effects, while A2BR reportedly has more direct effects31. Work from the Kitakaze40, Strasser41, and Downey42 laboratories showed A2BR as an important mediator of ischemic protection43, 44, with benefits in human HF. Immuno-histochemistry localizes A2BR at mitochondria45 and it is also expressed in vascular tissue and fibroblasts where it regulates proliferation, vascular tone, and anti-fibrotic signaling46. PDE1 inhibition is also antifibrotic47, regulates smooth muscle proliferation48, and as shown by Zhang et al49, also protects against doxorubicin-induced myocyte apoptosis in vitro and in vivo. In this study, they found PDE1 co-localizes with A2R in a membrane macromolecular complex overlapping t-tubules, and that both A2AR and A2BR blockade prevents the anti-apoptotic efficacy from PDE1 inhibition. While we found A2BR blockade sufficient to prevent acute inotropic and vasodilator responses to ITI-214 in vivo, the A2AR may also play a role and this will require further investigation.
For the in vivo studies, we left reflexes intact so responses potentially relevant to human could be observed. While positive chronotropic and inotropic effects of ITI-214 could conceivably result from reflex activation, systolic pressure actually rose which would be an over-compensation and unusual for a reflex mechanism. Its persistence with β-AR blockade and fixed rate pacing further counters the likelihood that ITI-214 inotropy was due to a baroreflex. Based on recent data, the HR changes are more likely related to PDE1 modulation of sinoatrial nodal cells26, though involvement of central or peripheral nervous signaling cannot be ruled out. We were surprised to find A2B-R blockade prevented HR rate increases, and cannot find studies linking this pathway to rate control, so other factors may apply. From a translational perspective, our data predict that in HF patients, most receiving β-AR blockade, ITI-214 should change HR little while still potentially conferring inotropic, lusitropic, and systemic vascular effects. The effect of ITI-214 on myocardial oxygen consumption remains to be determined, though if A2BR signaling dominates, we would predict little rise in contrast to β-AR stimulants.
ITI-214 is the only potent and selective PDE1 inhibitor yet developed and tested in humans, as it has successfully completed several Phase I clinical trials. These studies have not focused on the cardiovascular system at all, but on important CNS functions that are impacted in neurodegenerative and neuropsychiatric disease. These earlier studies provided the safety data in support of a current Phase Ib/IIA dosing, safety, and tolerance study of ITI-214 in stable, Class II-III HF patients (www.clinicaltrials.gov: NCT03387215). This study will assess the impact of ITI-214 on cardiovascular function in humans for the first time, and help determine if the observations we made here in the dog and rabbit models are indeed translational.
Supplementary Material
What Is New.
Selective inhibition of phosphodiesterase type 1, a dual (cAMP and cGMP) phosphodiesterase, with ITI-214 induces positive inotropic, lusitropic, chronotropic, and arterial vasodilatory effects in mammals expressing the dominant human isoform – PDE1C.
The effects occur via cAMP modulation and are observed in failing hearts.
ITI-214 contractile increase is insensitive to β-adrenergic blockade or heart rate increase, but inhibited in vivo by adenosine receptor (A2B) blockade.
Isolated myocytes reveal differences between PDE1 and PDE3 inhibition.
PDE3 inhibition augments β-receptor agonism, and when combined with adenylate cyclase activation, augments cell shortening and calcium transients, whereas PDE1 inhibition enhances function without calcium increase.
Clinical Perspective.
ITI-214 has been previously completed four Phase I studies, and is currently under study for Parkinson’s disease (NCT03257046).
A Phase Ib/IIa safety and tolerability study with non-invasive hemodynamic data in heart failure patients is also underway (NCT03387215).
Adenosine signaling is cardioprotective against ischemic and heart failure stress, so the finding that PDE1 couples to A2B receptor signaling rather than β-adrenergic signaling suggests the potential for providing cardioprotection.
In contrast to PDE3 inhibition, myocyte results show negligible change in calcium transients with PDE1 inhibition despite improved cell shortening, suggesting different cAMP regulation and potentially a safer clinical profile than PDE3.
Acknowledgments
FUNDING
Supported by National Institute of Health (HL135827–01, P01HL107153, HL119012) (DAK), the Japanese Circulation Society Overseas Research Fellowship and Uehara Memorial Foundation Research Fellowship (TH), T32 Training Program (GEK, SH, TA), American Heart Association Fellowship Grant (RN). ITI-214 described in this study was provided by Intra-Cellular Therapies, Inc., which also provided funding for the research.
Footnotes
DISCLOSURES
Dr. Kass is a paid consultant for Intra-Cellular Therapies, Inc. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. JJO’B, JPH, RED, WY, DB, HRH, and LPW are full-time employees of Intra-Cellular Therapies, Inc.
REFERENCES
- 1.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN and Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 2.Boularan C and Gales C. Cardiac cAMP: production, hydrolysis, modulation and detection. Front Pharmacol. 2015;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, Dostmann WR, Smith CJ and Kass DA. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–109. [DOI] [PubMed] [Google Scholar]
- 4.Mehel H, Emons J, Vettel C, Wittkopper K, Seppelt D, Dewenter M, Lutz S, Sossalla S, Maier LS, Lechene P, Leroy J, Lefebvre F, Varin A, Eschenhagen T, Nattel S, Dobrev D, Zimmermann WH, Nikolaev VO, Vandecasteele G, Fischmeister R and El-Armouche A. Phosphodiesterase-2 is up-regulated in human failing hearts and blunts beta-adrenergic responses in cardiomyocytes. J Am Coll Cardiol. 2013;62:1596–1606. [DOI] [PubMed] [Google Scholar]
- 5.Insel PA, Murray F, Yokoyama U, Romano S, Yun H, Brown L, Snead A, Lu D and Aroonsakool N. cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol. 2012;166:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgado M, Cairrao E, Santos-Silva AJ and Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci. 2012;69:247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lezoualc’h F, Fazal L, Laudette M and Conte C. Cyclic AMP Sensor EPAC Proteins and Their Role in Cardiovascular Function and Disease. Circ Res. 2016;118:881–897. [DOI] [PubMed] [Google Scholar]
- 8.Bobin P, Belacel-Ouari M, Bedioune I, Zhang L, Leroy J, Leblais V, Fischmeister R and Vandecasteele G. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Arch Cardiovasc Dis. 2016;109:431–443. [DOI] [PubMed] [Google Scholar]
- 9.Bender AT and Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol.Rev 2006;58:488–520. [DOI] [PubMed] [Google Scholar]
- 10.Kokkonen K and Kass DA. Nanodomain Regulation of Cardiac Cyclic Nucleotide Signaling by Phosphodiesterases. Annu Rev Pharmacol Toxicol. 2017;57:455–479. [DOI] [PubMed] [Google Scholar]
- 11.Maurice DH, Ke H, Ahmad F, Wang Y, Chung J and Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov. 2014;13:290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keravis T and Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol. 2012;165:1288–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Movsesian M Novel approaches to targeting PDE3 in cardiovascular disease. Pharmacol Ther. 2016;163:74–81. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad F, Murata T, Shimizu K, Degerman E, Maurice D and Manganiello V. Cyclic nucleotide phosphodiesterases: important signaling modulators and therapeutic targets. Oral Dis. 2015;21:e25–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E and Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y and Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat.Med 2005;11:214–222. [DOI] [PubMed] [Google Scholar]
- 17.Das A, Durrant D, Salloum FN, Xi L and Kukreja RC. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther. 2015;147:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garnock-Jones KP. Roflumilast: A Review in COPD. Drugs. 2015;75:1645–1656. [DOI] [PubMed] [Google Scholar]
- 19.Schwam EM, Nicholas T, Chew R, Billing CB, Davidson W, Ambrose D and Altstiel LD. A multicenter, double-blind, placebo-controlled trial of the PDE9A inhibitor, PF-04447943, in Alzheimer’s disease. Curr Alzheimer Res. 2014;11:413–421. [DOI] [PubMed] [Google Scholar]
- 20.Lee DI, Zhu G, Sasaki T, Cho GS, Hamdani N, Holewinski R, Jo SH, Danner T, Zhang M, Rainer PP, Bedja D, Kirk JA, Ranek MJ, Dostmann WR, Kwon C, Margulies KB, Van Eyk JE, Paulus WJ, Takimoto E and Kass DA. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan C, Zhao AZ, Bentley JK and Beavo JA. The calmodulin-dependent phosphodiesterase gene PDE1C encodes several functionally different splice variants in a tissue-specific manner. J Biol Chem. 1996;271:25699–25706. [DOI] [PubMed] [Google Scholar]
- 22.Vandeput F, Wolda SL, Krall J, Hambleton R, Uher L, McCaw KN, Radwanski PB, Florio V and Movsesian MA. Cyclic nucleotide phosphodiesterase PDE1C1 in human cardiac myocytes. J Biol.Chem 2007;282:32749–32757. [DOI] [PubMed] [Google Scholar]
- 23.Wu MP, Zhang YS, Xu X, Zhou Q, Li JD and Yan C. Vinpocetine Attenuates Pathological Cardiac Remodeling by Inhibiting Cardiac Hypertrophy and Fibrosis. Cardiovasc Drugs Ther. 2017;31:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, Xu H, Florio V, Rybalkin SD, Beavo JA, Chen YF, Li JD, Blaxall BC, Abe J and Yan C. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ.Res 2009;105:956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight WE, Chen S, Zhang Y, Oikawa M, Wu M, Zhou Q, Miller CL, Cai Y, Mickelsen DM, Moravec C, Small EM, Abe J and Yan C. PDE1C deficiency antagonizes pathological cardiac remodeling and dysfunction. Proc Natl Acad Sci U S A. 2016;113:E7116–E7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukyanenko YO, Younes A, Lyashkov AE, Tarasov KV, Riordon DR, Lee J, Sirenko SG, Kobrinsky E, Ziman B, Tarasova YS, Juhaszova M, Sollott SJ, Graham DR and Lakatta EG. Ca(2+)/calmodulin-activated phosphodiesterase 1A is highly expressed in rabbit cardiac sinoatrial nodal cells and regulates pacemaker function. J Mol Cell Cardiol. 2016;98:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P, Zheng H, Zhao J, Zhang L, Yao W, Zhu H, Beard JD, Ida K, Lane W, Snell G, Sogabe S, Heyser CJ, Snyder GL, Hendrick JP, Vanover KE, Davis RE and Wennogle LP. Discovery of Potent and Selective Inhibitors of Phosphodiesterase 1 for the Treatment of Cognitive Impairment Associated with Neurodegenerative and Neuropsychiatric Diseases. J Med Chem. 2016;59:1149–1164. [DOI] [PubMed] [Google Scholar]
- 28.Snyder GL, Prickaerts J, Wadenberg ML, Zhang L, Zheng H, Yao W, Akkerman S, Zhu H, Hendrick JP, Vanover KE, Davis R, Li P, Mates S and Wennogle LP. Preclinical profile of ITI-214, an inhibitor of phosphodiesterase 1, for enhancement of memory performance in rats. Psychopharmacology (Berl). 2016;233:3113–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbah HN, Tocchetti CG, Wang M, Daya S, Gupta RC, Tunin RS, Mazhari R, Takimoto E, Paolocci N, Cowart D, Colucci WS and Kass DA. Nitroxyl (HNO): A novel approach for the acute treatment of heart failure. Circ Heart Fail. 2013;6:1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cingolani OH and Kass DA. Pressure-volume relation analysis of mouse ventricular function. Am J Physiol Heart Circ Physiol. 2011;301:H2198–206. [DOI] [PubMed] [Google Scholar]
- 31.Chandrasekera PC, McIntosh VJ, Cao FX and Lasley RD. Differential effects of adenosine A2a and A2b receptors on cardiac contractility. Am J Physiol Heart Circ Physiol. 2010;299:H2082–H2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beca S, Ahmad F, Shen W, Liu J, Makary S, Polidovitch N, Sun J, Hockman S, Chung YW, Movsesian M, Murphy E, Manganiello V and Backx PH. Phosphodiesterase type 3A regulates basal myocardial contractility through interacting with sarcoplasmic reticulum calcium ATPase type 2a signaling complexes in mouse heart. Circ Res. 2013;112:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres-Quesada O, Mayrhofer JE and Stefan E. The many faces of compartmentalized PKA signalosomes. Cell Signal. 2017;37:1–11. [DOI] [PubMed] [Google Scholar]
- 34.Movsesian M, Ahmad F and Hirsch E. Functions of PDE3 Isoforms in Cardiac Muscle. J Cardiovasc Dev Dis. 2018;5: pii: E10. doi: 10.3390/jcdd5010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ and Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–1091. [DOI] [PubMed] [Google Scholar]
- 36.Molina CE, Johnson DM, Mehel H, Spatjens RL, Mika D, Algalarrondo V, Slimane ZH, Lechene P, Abi-Gerges N, van der Linde HJ, Leroy J, Volders PG, Fischmeister R and Vandecasteele G. Interventricular differences in beta-adrenergic responses in the canine heart: role of phosphodiesterases. J Am Heart Assoc. 2014;3:e000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprenger JU, Bork NI, Herting J, Fischer TH and Nikolaev VO. Interactions of Calcium Fluctuations during Cardiomyocyte Contraction with Real-Time cAMP Dynamics Detected by FRET. PLoS One. 2016;11:e0167974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, Fischmeister R and Vandecasteele G. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res 2006;98:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Headrick JP, Ashton KJ, Rose’meyer RB and Peart JN. Cardiovascular adenosine receptors: expression, actions and interactions. Pharmacol Ther 2013;140:92–111. [DOI] [PubMed] [Google Scholar]
- 40.Wakeno M, Minamino T, Seguchi O, Okazaki H, Tsukamoto O, Okada K, Hirata A, Fujita M, Asanuma H, Kim J, Komamura K, Takashima S, Mochizuki N and Kitakaze M. Long-term stimulation of adenosine A2b receptors begun after myocardial infarction prevents cardiac remodeling in rats. Circulation. 2006;114:1923–1932. [DOI] [PubMed] [Google Scholar]
- 41.Simonis G, Wiedemann S, Joachim D, Weinbrenner C, Marquetant R and Strasser RH. Stimulation of adenosine A2b receptors blocks apoptosis in the non-infarcted myocardium even when administered after the onset of infarction. Mol Cell Biochem. 2009;328:119–126. [DOI] [PubMed] [Google Scholar]
- 42.Methner C, Schmidt K, Cohen MV, Downey JM and Krieg T. Both A2a and A2b adenosine receptors at reperfusion are necessary to reduce infarct size in mouse hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1262–H1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, Zvara DA and Xu Z. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol. 2009;47:684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo SW, Koeppen M, Bonney S, Gobel M, Thayer M, Harter PN, Ravid K, Eltzschig HK, Mittelbronn M, Walker L and Eckle T. Differential Tissue-Specific Function of Adora2b in Cardioprotection. J Immunol. 2015;195:1732–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grube K, Rudebusch J, Xu Z, Bockenholt T, Methner C, Muller T, Cuello F, Zimmermann K, Yang X, Felix SB, Cohen MV, Downey JM and Krieg T. Evidence for an intracellular localization of the adenosine A2B receptor in rat cardiomyocytes. Basic Res Cardiol. 2011;106:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vecchio EA, White PJ and May LT. Targeting Adenosine Receptors for the Treatment of Cardiac Fibrosis. Front Pharmacol. 2017;8:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller CL, Cai Y, Oikawa M, Thomas T, Dostmann WR, Zaccolo M, Fujiwara K and Yan C. Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart. Basic Res Cardiol. 2011;106:1023–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai Y, Nagel DJ, Zhou Q, Cygnar KD, Zhao H, Li F, Pi X, Knight PA and Yan C. Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ Res. 2015;116:1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Knight W, Chen S, Mohan A and Yan C. A Multi-Protein Complex with TRPC, PDE1C, and A2R Plays a Critical Role in Regulating Cardiomyocyte cAMP and Survival. Circulation. 2018. (DOI: 10.1161/circulationaha.118.034189). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.