Abstract
Tryptic peptide imaging is a primary workflow for matrix-assisted laser desorption/ ionization imaging mass spectrometry (MALDI IMS) and has led to new information reporting highly multiplexed protein localization. Technological advances within the last few years have produced robust tools for automated spraying of both matrix and enzymes. When combined with high mass resolution and high mass accuracy instrumentation studies now generally result in two dimensional mapping of well over 1,000 peptide peaks. This protocol describes sample preparation, spraying and application of enzymes and matrices, and MALDI FT-ICR instrumental considerations for two dimensional mapping of tryptic peptides from fresh frozen (FF) or formalin-fixed, paraffin-embedded (FFPE) tissue sections. Procedures for extraction of tryptic peptides from tissue sections for LC-MS/MS identification are also described.
Keywords: imaging mass spectrometry, proteomics, tryptic peptide imaging, peptide identification for imaging mass spectrometry, formalin-fixed, paraffin-embedded tissue imaging, tissue imaging, MALDI imaging mass spectrometry
Introduction
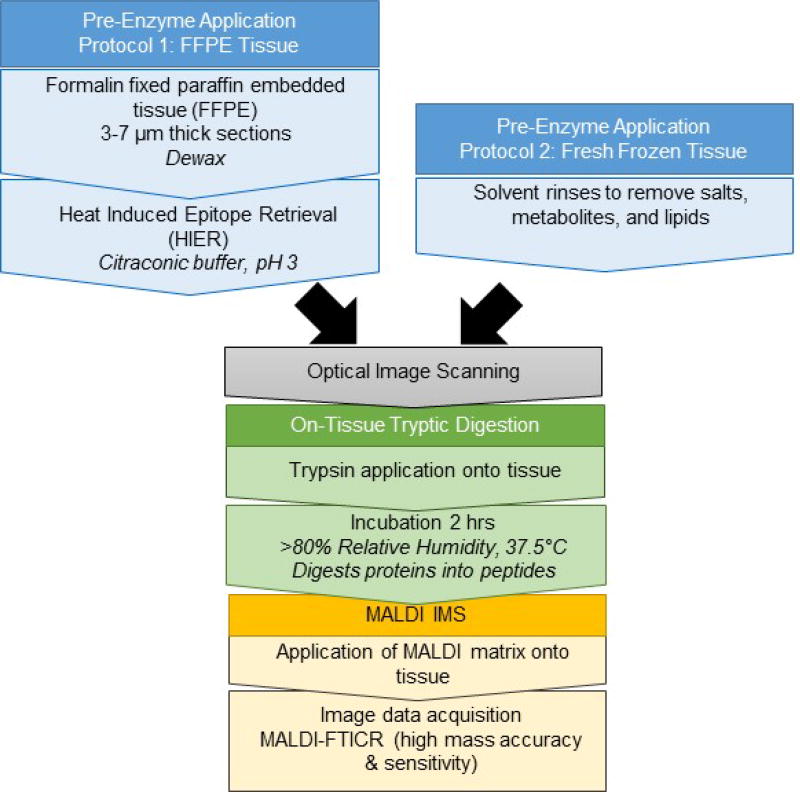
Technological advances within the last few years allow robust application of both enzyme and matrices to tissue sections, with current work focused on increasing confidence in peptide identifications from tissue using high mass accuracy imaging instrumentation.(Cillero-Pastor & Heeren, 2013; Schober, Schramm, Spengler, & Römpp, 2011) This unit first describes pre-enzyme sample preparation that is unique to the type of tissue section, that is either fresh frozen (FF) or formalin-fixed, paraffin-embedded (FFPE). The protocol then details experimental strategies for generating robust 2D maps of tryptic digestion on FF or FFPE tissues using an automated sprayer found in many MALDI IMS labs, the TM-Sprayer™ (HTXImaging), for application of enzymes and MALDI matrix. Considerations to retain high mass accuracy (≤2 ppm) while performing the imaging experiment using a MALDI Fourier Transform Ion Cyclotron Resonance (FT-ICR) instrument are detailed. Strategies for peptide identification by either MALDI MS/MS or liquid chromatography coupled to high mass accuracy tandem mass spectrometry (LC-MS/MS) are discussed.
Abbreviations: MALDI, matrix-assisted laser desorption/ionization; FT-ICR, Fourier Transform Ion Cyclotron Resonance; MS- mass spectrometry; FFPE, formalin-fixed, paraffin-embedded; FF, fresh frozen; HR/AM- high resolution/accurate mass; AB, ammonium bicarbonate; ACN, acetonitrile; methanol, MeOH; ethanol-EtOH; TFA, trifluoroacetic acid; FA- formic acid, CHCA, alpha-cyano-4-hydroxycinnamic acid.
Materials
Note that some brands are specified due to a desired quality different from generic brands
Coplin jars capable of holding 8–10 slides minimum (8 plastic, 2 glass; glass is for FF tissues)
5 slide mailer with side opening
Antigen retrieval device (vegetable steamer used here e.g., Rival Model CKRVSTLM21)
Cell culture dish, plastic 100 × 15 mm
Kimwipes (Kimberly-Clark)
Wypall X60 paper towel
Desiccator hooked to laboratory vacuum
High Resolution Document Scanner
Syringe pump capable of 30 µL/min within 0.05% accuracy
Pump capable of 100 µL/min within 0.05% accuracy
TM-Sprayer™ (HTXImaging)
A 1-mL syringe with Luer lock and PTFE coated plunger
A 5-mL glass airtight Luer lock syringe with a PTFE coated plunger
A 0.2 µm PTFE filter suitable for HPLC grade solvents with Luer lock, 13 mm
Oven operating at 37.5 ± 1.5°C
Oven operating at 60 ± 1.5°C
Chemicals & Enzymes
Note that solvents must be used in the specified grade for high quality images
Xylenes, histology grade
200 proof ethanol, USP grade
Water, HPLC grade
Chloroform
Glacial acetic acid
Ammonium bicarbonate (purchase in small amounts (e.g., <25 grams) to minimize keratin contamination)
Sequencing grade modified trypsin
Trifluoroacetic acid
Alpha-cyano-4-hydroxycinnamic acid (CHCA)
Acetonitrile, HPLC grade
Methanol, HPLC grade
Ultra pure, nitrogen gas, 300 L cylinder
Strategic Planning
A. Slide Considerations
MALDI IMS instruments from different vendors support the use of standard microscope slides, including the MALDI FT-ICRs from Bruker Daltonics and the MALDI ion mobility instruments from Waters. However, almost all MALDI time-of-flight instruments require a conductively charged slide to aid in production of ions, typically created by coating a glass slide with indium tin oxide to obtain a specified resistivity.
For most imaging mass spectrometers, slide holders are engineered to fit the standard 75 mm × 25 mm × 1 mm microscope slide. Slides for fresh frozen tissue sections should be purchased at the correct size prior to mounting the tissue section on the slide. Slides with FFPE tissue sections may arrive in the MALDI IMS lab with the incorrect size. If a slide with tissue on it does not fit the sample holder for the instrument, it may be sized to fit while the tissue is still covered with wax, as this prevents contamination and limits damage to the tissue. A small hand held rotary tool may be used to grind down slide edges to fit into the instrument sample holder. Afterwards, slides may be lightly rinsed in tap water and allowed to air dry. For frozen tissues, ensure that the slides fit the holder before mounting the tissue to the slides.
B. Sample Considerations
Evaluate the sample prior to starting the MALDI IMS experimental workflow to limit work and instrument time on tissues that may produce poor quality image data. Fresh frozen sections are cryosectioned at 8–10 µm for MALDI IMS. FFPE sections should be 3–7 µm thick to obtain high quality images. At this thickness, surrounding wax should be slightly transparent. Too thick of a section, detected as opaque wax surrounding the FFPE tissue, will result in poor quality images because under these conditions the wax will not be removed completely and the enzyme will not penetrate uniformly. Scratches in the tissue will produce gaps in the image data and limit detection of molecules from that area. Tissue that has folds and wrinkles will produce poor quality images. In general, the tissue section should be smooth with no significant distortions of the section. If the tissue is either too thick or wrinkled, use a different tissue section, if available.
FFPE tissue must be dewaxed to remove paraffin that would otherwise obscure peptide signal from tissue. In our experience, other crosslinking preservation methods paired with paraffin embedding should be treated the same way as FFPE using heating and dewaxing (e.g., paraformaldehyde fixation). FF tissue must be rinsed in organic solvents capable of removing salts, lipids and metabolites for greatly improved detection of peptides from proteins.(Seeley, Oppenheimer, Mi, Chaurand, & Caprioli, 2008; Yang & Caprioli, 2011)
For FF tissue, it is preferable to cryosection and image on the same day. If long term storage is unavoidable, store at −80°C. FFPE sections should be stored in a cool dark place (Economou et al., 2014) prior to the sample preparation described in this protocol.
C. Number of Samples to Prepare
This protocol describes the use of a TM-Sprayer™ (HTXImaging) to apply enzyme and matrix. This robotic sprayer is used in many MALDI IMS labs both commercial and academic and is able to process up to four standard microscope slides (each 75 mm × 25 mm) at a time. It is possible to go through the described protocol for enzyme and matrix application twice in a single 8 hour workday using the TM-Sprayer™, producing 8 processed slides for MALDIIMS. Other automated sprayers include the ImagePrep™ (Bruker Daltonics) that processes one slide at a time, and the SunCollect (SunChrom) that processes up to eight slides, although four is the typical number prepared at any one time. Any number of tissue sections may be placed onto any one slide with a 5 mm allowance at the edges for the slide holder.
D. Solvents
Ensure that all solvents are of the specified grade, including those for dewaxing and washing. Using low quality solvents greatly reduces analyte detection by MALDI IMS.
E. Humidity Chamber Preparation
Have specified materials ready for preheating the incubation chamber. This allows the humidity chamber to start producing moisture and is essential to complete the digest in two hours. The humidity chamber is prepared by cutting a Wypall*X60 paper towel to line the bottom of the 100×15 mm culture dish. Two 4×6 Kimwipes are folded in half, rolled to form a cylinder, and placed on opposite sides of the culture dish (Figure 1). The slide will eventually be placed on top of these two Kimwipes, so they provide not only a reservoir for extra moisture, but also a support holder for the slide. A squirt bottle of distilled water is used to wet the Kimwipes and paper towel until saturated. Water should be seen barely leaking out from under the towel when the dish is tilted. This is generally about 5 mL of water. Use of different types of Kimwipes and paper towels affects amount of water needed and evaporation time and will have to be adjusted experimentally to obtain >80% relative humidity. Placing the incubation chamber in an oven set at 37°C should produce condensation within 15 minutes (Figure 1B). If this does not happen, the actual oven temperature may be set wrong (versus the digital display) or not enough water was placed in the dish.
Figure 1.
Example of humidity chamber setup in culture dish. A) Paper towel (Wypall X60) is placed on the bottom of the dish, cut to fit. Two Kimwipes are rolled into bundles and placed either end of the dish. Distilled water is used to saturate the paper towel and both Kimwipes. B) Example of condensation that should be seen on the cell culture dish prior to placing samples into the humidity chamber to digest.
F. Experimental Workflow
Figure 1 shows the experimental workflow, taking into consideration preparation for either FFPE or FF tissue. For FFPE tissue, the citraconic buffer for antigen retrieval may be prepared while dewaxing. The antigen retrieval device may be preheated, if necessary (e.g. when using a vegetable steamer), while dewaxing/washing. During antigen retrieval, the humidity chamber is set up and placed in the oven to begin warming and producing humidity. The MALDI matrix may be prepared in the last half hour of enzyme incubation. The TM-Sprayer™ takes around 10 minutes to set up and reach set temperatures for either enzyme or matrix application. TM-Sprayer™ set up for enzyme spraying may be performed during the antigen retrieval step. TM-Sprayer™ set up for matrix spraying and MALDI matrix preparation may be performed in the last half hour of enzyme digestion.
Basic Protocol 1
In situ Imaging of Tryptic Peptides by MALDI Imaging Mass Spectrometry FFPE tissue should be prepared as described below. FF tissue is prepared as described in Alternate Protocol 1.
FFPE Tissue Section Dewaxing and Antigen Retrieval
FFPE tissue is embedded in paraffin and highly crosslinked. Dewaxing removes paraffin which can interfere with peptide detection by MALDI IMS. Antigen retrieval breaks protein crosslinks formed by formalin and increases access of enzymes to the protein structure.
Materials
Oven operating at 60 ± 1.5°C
8 Coplin jars capable of holding 8–10 slides minimum
5 slide mailer with side opening
Antigen retrieval device (vegetable steamer used here e.g., Rival Model CKRVSTLM21)
Cell culture dish, plastic 100 × 15 mm
Kimwipes (Kimberly-Clark)
Wypall X60 paper towel
Desiccator hooked to laboratory vacuum
High Resolution Document Scanner
Oven operating at 37 ± 1.5°C
-
1Pour the following solvents into Coplin jars for tissue dewaxing:
- Xylenes, two Coplin jars;
- 200 proof ethanol, USP grade, two Coplin jars;
- 95% ethanol, one Coplin jar;
- 70% ethanol, one Coplin jar;
- double distilled water, two Coplin jars.
- Solvents are used for dewaxing up to eight slides before changing to fresh solvents.
-
2
Dewax the slides. These steps remove the paraffin wax using xylenes and ethanol and then rehydrates the tissue in preparation for antigen retrieval. It is important for the repeated washes to use fresh solvent each time. For each step, immerse the slides completely in the solution for the stated length of time.
-
3
Xylenes, histology grade, repeat twice, 3 minutes each
-
4
100% ethanol, repeat twice, 1 minute each
-
5
95% ethanol 1 minute
-
6
70% ethanol 1 minute
-
7
HPLC grade water, repeat twice, 3 minutes each
-
8
Inspect the slides after dewaxing. Incomplete paraffin removal leaves behind waxes that look like opaque white residue and will result in poor quality images. Infrequently, additional xylene washes followed by ethanol washes and rehydration are needed to remove wax.
-
9
Slides may be stored overnight in desiccator prior to completion. If continuing with the workflow, dry the slides in a vacuum-desiccator 5 minutes.
Antigen Retrieval
This step is the same as heat-induced epitope retrieval used for immunohistochemistry and breaks methylene bridges formed by formalin fixation. This allows enzyme access to more protein content. We use a kitchen vegetable steamer to perform antigen retrieval steps. It is preferable to complete antigen retrieval and trypsin digestion on the same day.
-
10
Fill the vegetable steamer to appropriate water levels and preheat for 5 minutes prior to antigen retrieval.
-
11
Add around ~10 mL of the Citraconic acid buffer to a 5 slide mailer with side opening.
-
12
Place no more than 3 slides into each 5 slide mailer with side opening. Slides should be placed with tissue facing outward to the solution in positions 1 and 5, and NOT facing the slide mailer walls. Position 3 may face either way. This allows good solvent access to the tissue.
-
13
Completely fill the slide mailer the rest of the way with the buffer so that all tissue is completely recovered. This ensures that adequate antigen retrieval is accomplished and prevents drying artifacts.
-
14
If the mailer has no holes punched in the lid, only snap close one corner of the mailer. This allows steam to exit.
-
15
Place the mailer in the center of the vegetable steamer.
-
16
Set the heating for 30 minutes.
Note: For antigen retrieval, some fragile tissues require shorter heating times to limit loss of tissue; For instance, we have found that antigen retrieving breast tissue for 20 minutes instead of 30 minutes limits tissue loss.
-
17
Cool the slides after antigen retrieval. Cooling too fast may cause tissue damage.
-
18
Remove mailer and place in a container holding cool water from the faucet. Water should come midway up the side of the mailer.
-
19
Cool for 5 minutes in the cool water bath.
-
20
Remove half the buffer from the mailer and replace with distilled water.
-
21
Place the mailer on the countertop to cool for 5 minutes.
-
22
Repeat removal of half the buffer two more times, each with 5 minutes of cooling.
-
23
Complete by rinsing in 100% distilled water.
-
24
Dry the slides 5 minutes in a desiccator.
Note that all steps throughout the remainder of this protocol are applicable to either FFPE tissue types prepared as described above or FF tissue prepared as described in Alternate Protocol 1
Slide Scanning
A scanned image of the slide with the tissue on it produces an optical image that is used when programming tissue regions to map by MALDI IMS. Scanning collects a grayscale image that demonstrates tissue densities due to tissue histology and is the minimal amount of information needed to understand how maps of IMS data correlate to tissue features. Performing the scanning after application of matrix obscures tissue density features that would otherwise be useful in interpreting the image. A high resolution document scanner may be used to produce the scanned optical image.
-
25
Mark fiducials at the four corners of each slide. Fiducials are points of reference used when “teaching” the instrument software the coordinates of the slide. Fiducials allow accurate targeting of tissue regions for imaging. Use a reflective metallic marker to make a small circle at each corner. Use a black marker to draw a cross or hash mark on top of each silver circle. The reflective marker provides a contrasting background for easy visualization of the black fiducial mark by the instrument camera.
-
26
For images that will be acquired by mass spectrometry at ≥100 µm spatial resolution, scan the whole slide at a minimum of 1200 dpi resolution. Samples requiring higher spatial resolution will require a higher resolution scanned image. For example, images that will be acquired with a ≤50 µm step size require a 2400 dpi scanned image. This is because each image data pixel requires 4 optical pixels for image interpolation.
-
27
Save the images to a project specific folder.
Trypsin Application by TM-Sprayer™
-
28
Have on hand a syringe pump with 0.05% accuracy when pumping 30 µL/min, which is used for enzyme application and a glass or plastic 1-mL syringe with Luer lock for loading enzyme into the sprayer.
-
29
Rinse the syringe with water, fill with three volumes of water, and aspirate into waste.
-
30
Fill the syringe with the prepared trypsin solution, ensuring that there are no bubbles in the syringe. Tip: After loading all the trypsin solution required, pull a small volume of air into the syringe. Gently dispense the syringe until the air bubble is gone.
Note: The trypsin solution usually has a small bubble or two in the solution that can be difficult to remove. Pulling a large volume of air into the syringe causes the bubble(s) to merge with the air, which makes it easier to eliminate all air from the syringe.
-
31
Using the Luer lock mechanism, fasten the syringe to the TM-Sprayer™ line used for enzyme spraying (this is the shorter line).
-
32
Place the syringe onto the syringe pump. Check that the syringe head is seated correctly into the syringe pump so that pumping is accurate. The syringe should not move freely when correctly locked into place.
-
33
Ensure that the diameter on the syringe pump is set appropriate to the syringe and that the rate is set at 30 µL/min. Do not start the pump at this time.
-
34
Place the samples on the TM-Sprayer™ platform, fastening them with tape. For this protocol, there is a 40 mm distance from the tip of the sprayhead to the surface of the slide; some TM-Sprayer™ systems may have lower platforms.
-
35
Turn on the TM-Sprayer™ and controlling computer.
-
36
Open the nitrogen gas tank, setting the regulator to 10 psi.
-
37
In the TM-Sprayer™ software, set the temperature to 45°C. Temperature will not adjust without the nitrogen gas flowing.
-
38
Program the TM-Sprayer™ to cover the appropriate number of slides, allowing a 5 mm additional edge distance for sprayhead turn round.
Note: Effort should be made to program the TM-Sprayer™ turn around pattern to be completely off tissue to prevent delocalization and excess matrix application.
-
39
Program the TM-Sprayer™ method for trypsin to use 8 passes, crisscross pattern, velocity of 1200, and 3.0 mm track spacing. The dry time should remain as zero for four slides. The concentration and type of enzyme, the flow rate set on the syringe pump, and the sprayhead distance should be recorded in the method. This method may be used repeatedly for any sample preparation requiring trypsin spraying.
-
40
Ensure that the nitrogen gas pressure reading on the front of the TM-Sprayer™ is at 10 psi.
-
41
Start the syringe pump.
-
42
Place a blank microscope slide under the nozzle of the spray head to check the TM-Sprayer™ to monitor the start of enzyme solution spraying. It generally takes about 1–3 minutes to start emitting solution.
-
43
Once moisture is detected on the blank slide, press “Start” in the TM-Sprayer™ software.
-
44
Trypsin solution will be applied in a thin layer onto target tissues.
-
45
While the trypsin is being applied, set up the incubation chambers as detailed in strategic planning.
Trypsin Digestion
-
46
The incubation chamber should be already preheated in an oven at 37.5°C and a thin layer of condensation should have formed on the top of the incubation dish as detailed in strategic planning.
-
47
After application of trypsin onto the slide, place the slide with the tissue facing upwards into the incubation chamber using the Kimwipes as supports. Gently push the slide down slightly so that when the cover is placed on, the tissue does not touch the incubation chamber cover.
-
48
Incubate 2 hours in the oven set at 37.5 ±1.5°C. Ensure that the internal oven temperature is at the correct temperature using a secondary thermometer.
-
49
Be aware that condensation will have developed underneath the slide. Remove the slide slowly while holding it parallel with the countertop. Wipe off the condensation before rotating the slide to prevent liquid rolling onto the tissue surface and delocalizing peptides.
-
50
Store the slide in a 5 slide mailer to protect the released peptides. If matrix cannot be sprayed the same day, store in a desiccator (6–12 hours) or at −20°C for long term (2–3 days).
-
51
It is recommended to immediately spray matrix onto the slide.
MALDI Matrix Application by TM-Sprayer™
-
52
Have on hand an isocratic pump with 0.05% accuracy when pumping 100 µL/min, which is used for enzyme application, and a glass 5-mL syringe with Luer lock for loading matrix into the sprayer.
-
53
Ensure that the isocratic pump is set to pump 100 µL/min of 50% methanol/water. Solvent may be degassed to limit flow variation.
-
54
The TM-Sprayer™ has a 5 mL loop that comes standard. Move the flow line that goes to the sprayhead from the lower syringe port to the upper port that includes the 5-mL loop in the flow path. This forms a flowpath from the higher flowing pump to the sprayhead.
-
55
Turn on the pump.
-
56
Fill a glass 5-mL syringe with 50% methanol/water, ensuring that there are no bubbles in syringe.
-
57
Connect the syringe to the loading line using the Luer lock.
-
58
Turn the 6-port valve on the front of the TM-Sprayer™ to “Load” and inject all of the 50% methanol/water.
-
59
Turn the 6-port valve on the front of the TM-Sprayer™ to “Spray” and allow to flow for 5 minutes at 100 µL/min.
-
60
Repeat filling the syringe, loading into the loop, and flowing for 5 minutes a total three times. This cleans the loop and associated lines. The TM-Sprayer™ does not need to be powered on to perform cleaning.
Note: Cleaning the TM-Sprayer® before and after spraying matrix contamination and prevents recrystallization and clogging of the lines.
-
61
Turn on the TM-Sprayer™ and controlling computer.
-
62
Open the nitrogen gas tank, setting the regulator to 10 psi.
-
63
In the TM-Sprayer™ software, set the temperature to 80°C. Temperature will not adjust without the nitrogen gas flowing.
-
64
Program the TM-Sprayer™ method for CHCA matrix application to use 8 passes, crisscross pattern, velocity of 1300, and 2.5 mm track spacing. The dry time should remain as zero for four slides. The concentration and type of matrix, the flow rate set on the syringe pump, and the spray head distance should be recorded in the method. This method may be used repeatedly for any sample preparation requiring CHCA spraying after trypsin digestion.
-
65
Program the TM-Sprayer™ pattern to cover the appropriate number of slides, allowing a 5 mm additional edge distance for sprayhead turn around.
Note: Effort should be made to program the TM-Sprayer™ turn around pattern to be completely off tissue to prevent delocalization and excess matrix application on top of the tissue.
-
66
Ensure that the nitrogen gas pressure reading on the front of the TM-Sprayer™ is at 10 psi.
-
67
Place the samples on the TM-Sprayer™ platform, fastening them with tape. For this protocol, there is a 40 mm distance from the tip of the sprayhead to the surface of the slide; some TM-Sprayer™ systems may have lower platforms.
-
68
Fill a glass 5-mL syringe with the filtered CHCA solution, ensuring that there are no bubbles in syringe.
-
69
Using the Luer lock mechanism, fasten the syringe to the TM-Sprayer™ line going to the 6-port valve.
-
70
Move the switch to “LOAD” and steadily depress the syringe plunger until all the sample is loaded.
-
71
Ensure that the pump is flowing at 100 µL//minute and that appropriate pump pressure readouts are stable.
-
72
Move the 6-port valve switch to “Spray”.
-
73
Use a blank microscope slide to check the TM-Sprayer™ nozzle for spraying of solution. It generally takes about two minutes to start spraying matrix.
-
74
Once matrix is detected as a light film on the dummy slide, press “Start” in the TM-Sprayer™ software.
-
75
CHCA solution will be applied in a thin layer onto target tissues.
-
76
When finished, matrix coated slides may be imaged immediately or stored in a desiccator.
Image Acquisition: Basic Considerations for MALDI IMS of Peptides by FT-ICR
The discussed parameters are used on a 7.0 Tesla solariX Legacy™ MALDI FT-ICR (Bruker Daltonics).
-
77
Select a lockmass. To retain high mass accuracy in the image data, use a trypsin autolysis product as a lockmass. The tryptic autolysis peptide (R)VATVSLPR(S) at 842.50943 or (K)LSSPATLNSR(V) at m/z 1045.56365 are useful lockmasses that should be detectable across the entire tissue section.
-
78
Set resolving power. Resolving power determines attainable mass resolution on tissue and with transient length is a tradeoff in mass resolution and time spent analyzing each pixel. A general peptide imaging method uses a time domain dataset size of 512K word in broadband mode over m/z range 495–5,000, resulting in a transient length of 1.2059 seconds and an estimated resolving power of 160,000 calculated by instrument software at m/z 400. Higher mass resolutions require increased computational resources for studies and increased data storage space. General methods may be used in triage with higher mass resolution methods to reduce data storage size and allow computational handling of large collections of tissue. A higher mass resolution method may then be useful to further define target peptides found by the general imaging experiment.
-
79
Set laser power. Optimize the laser power on-tissue. High laser power results in significant increases in matrix peaks. Lower laser power improves signal-to-noise. A general peptide imaging method uses a 16% laser power, which increases slightly as the laser ages.
-
80
Set laser shots. Optimize the number of laser shots per spot on-tissue.
-
81
Set the laser to 25 shots and shoot in sequence at the same spot without moving the laser. Stop collecting when peptide signal diminishes. A general peptide imaging method typically uses 200 shots per pixel without rastering, where rastering means random movements of the sample plate to increase sampling area per pixel. Rastering with an increased number of laser shots may be used to increase sensitivity, but may limit attainable spatial resolution as the area sampled by the laser is significantly larger.
-
82
Collect a single scan per pixel during the imaging run by setting Number of Scans” to a value of 1.
-
83
Collect data. Bruker MALDI FT-ICRs allow accumulating ions from one pixel while detecting ions from another pixel in the ICR cell. This saves a significant amount of time performing the imaging experiment. Check the box for the function “Accumulate During Detect” (under the Mode Tab). Checking the “Save FID file” saves the time domain data and allows image data to be recalibrated at a later date. It is highly recommended to check this box for imaging experiments.
-
84
Data storage – Bruker MALDI FT-ICRs allow reduction of data to save space and help with downstream computational resources. In the Mode Tab, ensure that “Save Reduced Profile Spectrum” and “Save Reduced Profile Spectrum Peaklist” are checked. This is necessary for both data storage and for downstream exporting into imaging mass spectrometry statistical software (e.g., SCiLS, Bruker Daltonics). A general peptide imaging method reduces the data 95%, minimizing needed data storage space.
-
85
Example data size with the above settings is 2.198 Mb per pixel. An image with 10,000 pixels would result in a data size of 22 Gb.
Alternate Protocol 1: Preparation of Fresh Frozen Tissue for MALDI Imaging
Fresh frozen tissue is not cross-linked, but retains many salts, metabolites, and lipids that suppress signal detected by MALDI IMS. FF tissue sections are washed in solutions that remove these species. Antigen retrieval is not required for FF tissue sections. Washing should be done immediately before slide scanning and application of protease.
Materials (also see Basic Protocol 1)
Coplin jars capable of holding 8–10 slides minimum (8 plastic, 2 glass).
Note: the glass is for Carnoy’s solution, which can extract from polymers from some plastics. Extracted polymer signatures cause ion suppression in the image data.
5 slide mailer with side opening
Cell culture dish, plastic 100 × 15 mm
Kimwipes (Kimberly-Clark)
Wypall X60 paper towel
Desiccator hooked to laboratory vacuum
High Resolution Document Scanner
Oven operating at 37 ± 1.5°C
- Pour the following solvents into Coplin jars for tissue rinsing:
- Xylenes, two Coplin jars;
- 200 proof ethanol, USP grade two Coplin jars;
- Carnoy’s solution, two glass Coplin jars
- 95% ethanol, one Coplin jar;
- 70% ethanol, one Coplin jar;
- double distilled water, two Coplin jars.
Rinse the slides. For each step, immerse the slides completely in the solution for the stated length of time. For repeated rinses, use fresh solvent each time to increase removal of unwanted species.
Xylenes, histology grade, repeat twice, 3 minutes each
100% ethanol, 1 minute
Carnoy’s solution, repeat twice, 2 minutes each
100% ethanol, 1 minute
95% ethanol 1 minute
70% ethanol 1 minute
HPLC grade water, repeat twice, 3 minutes each
Dry the slides in a vacuum-desiccator 5 minutes.
Continue with Basic Protocol 1 starting with slide scanning (steps 40–84).
Support Protocol 1: Extraction of Peptides from Tissue for LC-MS/MS Identification: Whole Tissue
This protocol is applicable to either FF or FFPE tissue types
The goal is to collect peptides from the on-tissue tryptic digestion so they may be analyzed by routine LC-MS/MS proteomics analysis using high mass resolution, high mass accuracy instrumentation (e.g., an Orbitrap or FT-ICR instrument). A serial section is used to collect tryptic peptides produced by the imaging workflow for LC-MS/MS analysis. Figure 2 illustrates the workflow for matching MALDI IMS data to HR/AM LC-MS/MS data. This protocol does not cover HPLC, MS/MS and data search procedures, which are reviewed elsewhere (Gundry et al., 2009).
-
On a serial section, perform Basic Protocol steps 1–51 for on-tissue tryptic digestion for MALDI IMS. Do not apply MALDI matrix.
Note: Use trypsin from the same vendor for both imaging and peptide identification to ensure consistency in production of tryptic peptides.
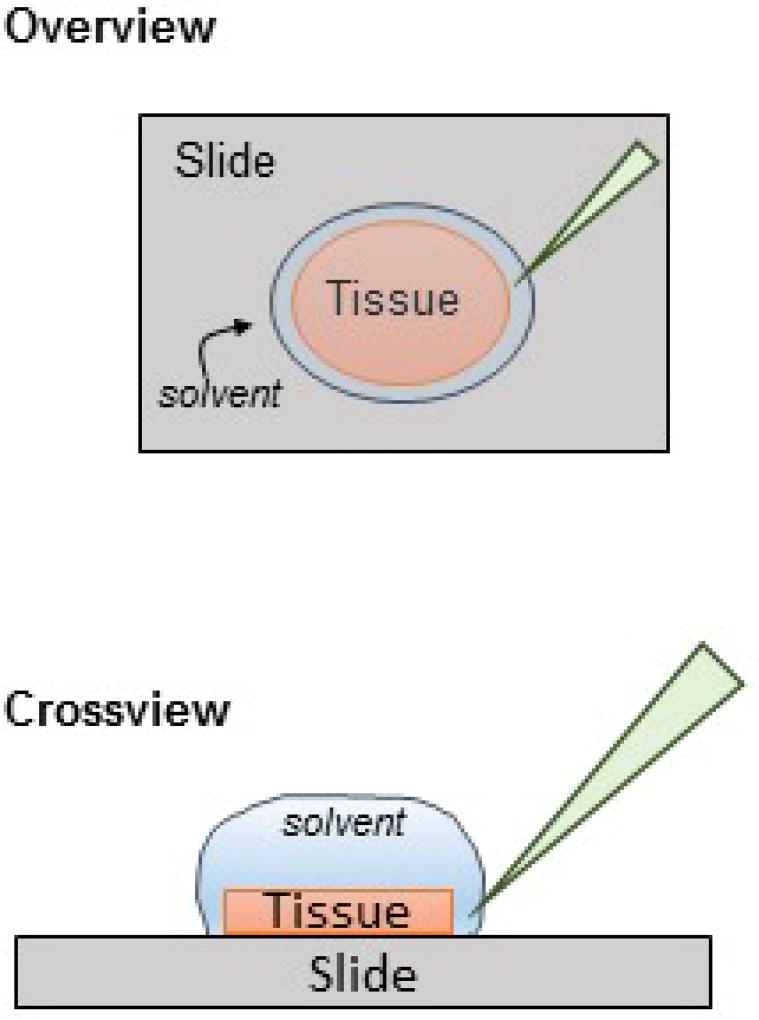
Using 50–100 µl of 15% acetonitrile, 0.1% formic acid, slowly pipette solvent onto the tissue until it is completely covered. Liquid should bead up to the edges of the tissue.
Cover the slide to prevent drying, allowing 3 minutes of incubation
Gently tilt the slide so that solvent bead swells on the lower edge.
Holding the pipette at an angle near the edge of the tissue, place the pipette tip into the bead. Do not touch the tissue with the pipette tip (Figure 4).
Gently suction up the volume and place into a low protein binding 1.5 mL microcentrifuge tube.
Repeat the process with 25% acetonitrile, 0.1% formic acid, placing the collected volume into the same microcentrifuge tube.
Repeat the process with 45% acetonitrile, 0.1% formic acid, placing the collected volume into the same microcentrifuge tube.
If LC-MS/MS will be performed immediately, dry down to ~ 1–5 µl volume and then add solvent recommended by your proteomics group (usually 2–5% acetonitrile, 0.1% formic acid).
If the sample requires long term storage, dry down completely and store at −20°C in a sealed container up to 3 months.(Mateos et al., 2014)
Figure 2.
Experimental workflow for generating image data by on-tissue tryptic digestion.
Figure 4.
Extraction of peptides from an entire tissue slice for identification by LC-MS/MS proteomics. Solvent is placed on the tissue forming a bead and allowed to incubate for 3 min, covered. A pipette tip is inserted at a 45° angle on the edge of the tissue and bead of solvent. The solvent is aspirated into the pipette tip and placed in a microcentrifuge tube.
Support Protocol 2: Extraction of Peptides for LC-MS/MS Identification from Target Regions
The following steps are applicable to either FF or FFPE tissue types
The goal is to collect peptides from a specific target region using on-tissue tryptic digestion so they may be analyzed by routine LC-MS/MS proteomics using high mass resolution, high mass accuracy instrumentation (e.g., an Orbitrap or FT-ICR). Either the same section or a serial section may be used to collect peptides from a target region. Figure 2 illustrates the workflow for matching MALDI IMS data to HR/AM LC-MS/MS data. This protocol does not cover HPLC, MS/MS and data search procedures, which are reviewed elsewhere (Gundry et al., 2009).
Materials
500 microliter low protein binding microcentrifuge tubes (e.g., Axygen MCT-060-L-C).
2 microliter pipette (for regions less than approximately 5×5 mm) capable of delivering volumes down to 200 nanoliters with at minimum 1.5% relative standard deviation.
10 microliter pipette (for tissues greater than 5×5 mm).
On a serial section, perform the protocol steps 1–46 for on-tissue tryptic digestion for MALDI IMS. Do not apply MALDI matrix.
Note: Use trypsin from the same vendor for both imaging and peptide identification to ensure consistency in production of tryptic peptides.
Fill a low protein binding microcentrifuge tube with 10 µl of 10% ACN/0.1% FA. This is used for dispelling and rinsing low volumes of peptide solution.
Flip the slide over, and using a black marker, mark the target region on the microscope glass from where the peptides will be extracted. Consult the image data if needed.
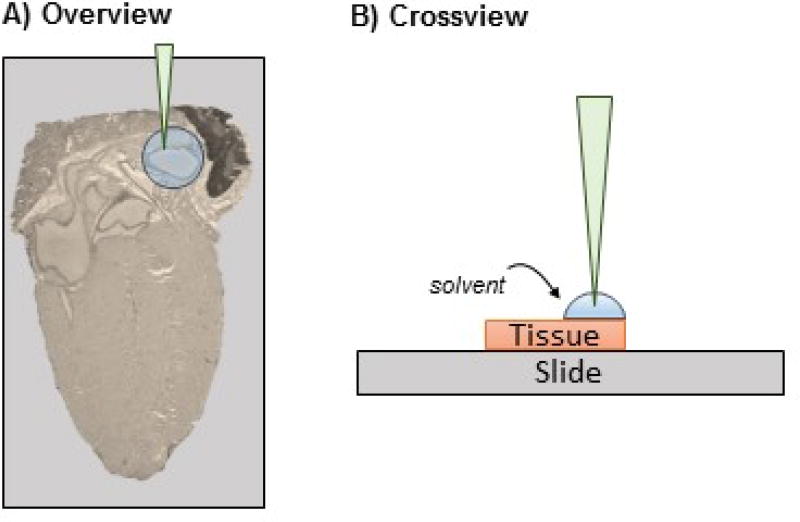
Using the appropriate pipette, pull up a volume of fluid into the pipette. For large regions (e.g., a tumor that occupies half of the tissue section), use enough solvent to cover the target region only. Generally, 2–5 µl of solution covers 2–5 mm of area for 45% acetonitrile/water. For small regions, (e.g., vessels), use 200–500 nl of solution.
Place the pipette with solvent vertical onto the target spot with the pipette tip barely touching the tissue.
Slowly eject liquid onto the target region, then immediately draw the liquid back into the pipette. The liquid may extrude outside of the pipette tip; this is expected (Figure 5).
Pump the pipette 5–10 times, finishing with the solvent drawn into the pipette tip.
Place the extracted solvent into the low protein binding microcentrifuge tube containing 10 µl of 10% ACN, 0.1% formic acid.
Use the solvent in the microcentrifuge tube to flush the pipette tip.
Repeat steps 5–9 with 25% acetonitrile, 0.1% formic acid, placing the collected volume into the same microcentrifuge tube.
Repeat steps 5–9 with 45% acetonitrile, 0.1% formic acid, placing the collected volume into the same microcentrifuge tube.
If LC-MS/MS will be performed immediately, dry down to ~ 1–5 µl volume and then add solvent recommended by your proteomics group (usually 2–5% acetonitrile, 0.1% formic acid).
If the sample requires long term storage, dry down completely and store at −20°C in a sealed container up to 3 months.(Mateos et al., 2014).
Figure 5.
Extraction of peptides from a target area on the tissue for identification by LC-MS/MS proteomics. A) A small amount of solvent (200–500 nl) is placed by pipette on the target region, forming a bead, shown here on mouse heart. B) The solvent is ejected and withdrawn from the target region 5–10 times for extraction without moving the pipette tip from a position perpendicular to the tissue. The solvent is then placed in a low protein binding microcentrifuge tube until LC-MS/MS.
Support Protocol 3: On-tissue MS/MS analysis by MALDI FT-ICR using a solariX Legacy™ Instrument (Bruker Daltonics)
This protocol describes simple MS/MS by collision-induced dissociation on the solariX Legacy™ mass spectrometer (Bruker Daltonics). The following steps are applicable to either FF or FFPE tissue types. The goal is to collect enough MS/MS data from a target region on-tissue to interpret MS/MS from a target peptide. Typically, the candidate peptide is a peptide that has unique localization to a tissue feature or has been calculated to be altered in a biological state. All peptides may not be examined by on-tissue MS/MS as the process of MALDI depletes the matrix and analytes, preventing further analyses. Either the same section or a serial section may be used to collect MS/MS on target peptides from a region. If a serial section is used, it should be coated with MALDI matrix.
Materials
Have on hand the image pattern reported by MALDI IMS of the target peptides of interest from steps 1–78.
-
Insert the sample into the instrument and navigate to the target region.
Note: The same sample that was imaged previously should be used for MS/MS unless the matrix has been depleted by the imaging experiment. If this is impossible due to matrix depletion by the image experiment, use a serial section prepared by steps 1–78 and image the tissue at a low spatial resolution to obtain information on molecular localization. Performing MS/MS on tissue sections that are far removed from the section used for the image data may result in inaccurate reporting of peptide identity.
Ensure the instrument is calibrated to subppm mass accuracy.
Open the same method as used for image data collection.
Set the “Number of scans to average” as 10 and collect 10 scans in broadband mode.
Identify that the peak of interest is observed in the MS scan. Record the reported mass as the target m/z.
-
Open the Source MS/MS tab and select “Isolate”. Input the target m/z as reported in the broadband MS scan. Collect 10 scans, averaging into one MS spectrum. This reports the precursor mass.
Note: The trypsin autolysis products may be used to evaluate methods for accuracy and fragmentation before attempting to fragment a target peptide. However, amino acid combinations affect fragmentation and more or less collision energy may be needed to fragment the target peptide. This is determined on a per-peptide basis.
In the MS/MS tab, deselect “Isolate” and select CID. Increase the collision energy to 35 V. Set the isolation window at 2 Dalton and collect 10 scans from the target region. Increasing the isolation window may improve signal, but may also fragment near-isobaric peaks which can complicate interpretation of the MS/MS data.
Input main fragments into a database search engine such as Mascot (matrixscience.com) or a utility program such as Protein Prospector (Baker, P.R. and Clauser, K.R. http://prospector.ucsf.edu). Ensure the correct protein database is used, allow for variable oxidative modification of methionine, and up to three missed cleavages by trypsin. Precursor mass accuracy should be set to 2 ppm, fragmentation mass accuracy 10 ppm.
Ideal fragmentation will produce overlapping ladders of a-, b- and y-ion fragments spaced by amino acid residue masses as well as neutral losses of water from these fragments. Increases in collision energy may be used to improve fragmentation. Large increases in the number of scans will accumulate more MS/MS data and may improve data interpretation. There are many instances where on-tissue MS/MS is unable to clearly define the peptide sequence; thus it is helpful to have available the whole tissue or regional extraction method followed by LC-MS/MS for further validation of identification.
Reagents and Solutions
Solvents used in Tissue Preparation
A. citraconic buffer, pH 3.0 ± 0.5
Pour 25 mL double distilled water into a clean 50 to 100 mL bottle.
Add 25 µL of Citraconic buffer (Thermo Scientific 20907) to the water.
Add 2 µL of 12 M HCl.
Cap the bottle and shake to mix.
Add an additional 25 mL of double distilled water to the bottle to bring final volume to 50 mL.
Cap the bottle and shake to mix.
Check that pH is around 3.0 ±0.5 by spotting 1–2 µL of the prepared buffer onto a pH strip. If it is not within the desired pH range, adjust with solutions of HCl or NaOH until a pH 3 is reached. A potential reason why pH will not be 3 are that the citraconic buffer is too old and has crystallized. In this case, the citraconic buffer should be discarded and re-purchased.
Use the same day as prepared.
B. 25 mM ammonium bicarbonate, pH 7.5 ± 0.5
Pour 50 mL double distilled water into a clean 100 mL bottle.
Weigh out 1.97 ± 0.05 g of ammonium bicarbonate
Pour the ammonium bicarbonate into the 50 ml water and mix.
Add an additional 50 mL of double distilled water to the bottle for a total of 100 mL and mix.
Check the pH with a pH paper strip. The pH should be 7.5 ± 0.5.
Store at 4°C for up to 2 weeks.
C. 0.1 µg/µL trypsin
To 100 µg of trypsin add 1000 µl of prepared 25 mM ammonium bicarbonate and mix.
Use the same day as prepared.
Notes: 1) We use sequencing grade modified trypsin. 2) One batch allows spraying of 4 slides on the TM-Sprayer™.
D. (For Fresh Frozen tissue) Preparation of 1 liter of Carnoy’s solution
To a clean glass bottle, add 600 mL of 200 proof ethanol, 300 mL of chloroform, and 100 mL of glacial acetic acid.
Mix well.
Store for 3 months tightly sealed in a well ventilated location.
E. 50% Acetonitrile (ACN), 0.1% TFA
To a clean 1000-mL bottle, add 250 mL of double distilled water.
Carefully add 1 mL of trifluoroacetic acid to the water
Add 500 mL of acetonitrile and mix.
Add 249 mL of double distilled water and mix.
Store for up to 2 months in a tightly sealed container.
F. MALDI matrix for peptide imaging: Alpha-cyano-4-hydroxycinnamic acid (CHCA), 7 mg/mL in 50% acetonitrile/0.1% TFA
Prepare fresh each time.
Weigh out 42 ± 1 mg CHCA
Add the solid CHCA to a 50 mL Falcon tube.
Add 6 ml of prepared 50% acetonitrile/0.1% TFA.
Vortex briefly and sonicate 5 minutes using a benchtop sonicator.
Filter CHCA solution using a 13 mm 0.2 µm PTFE hydrophilic syringe filter graded for use with HPLC solvents. This prevents clogging the solvent lines of the TM-Sprayer™.
G. Preparation of 5 mL solution for extraction of peptides 10% ACN, 0.1% formic acid
Add 4,495 µL of HPLC grade water to a clean container.
Add 5 µL of formic acid, 99% purity to the water
Add 500 µL of acetonitrile to the clean container.
Vortex.
Store at room temperature for up to 2 weeks.
H. Preparation of 5 mL solution for extraction of peptides 25% ACN, 0.1% formic acid
Add 3,745 µL of HPLC grade water to a clean container.
Add 5 µL of formic acid, 99% purity to the water
Add 1,250 µL of acetonitrile to the clean container.
Vortex.
Store at room temperature for up to 2 weeks.
I. Preparation of 5 mL solution for extraction of peptides 45% ACN, 0.1% formic acid
Add 2,745 µL of HPLC grade water to a clean container.
Add 5 µL of formic acid, 99% purity to the water
Add 2,250 µL of acetonitrile to the clean container.
Vortex.
Store at room temperature for up to 2 weeks.
Commentary
Background information
Tryptic peptide imaging was one of the first workflows developed for matrix-assisted laser desorption/ ionization imaging mass spectrometry (MALDI IMS)(Casadonte & Caprioli, 2011; Groseclose, Andersson, Hardesty, & Caprioli, 2007), allowing access to proteomic-level information from the same type of thin tissue sections as used for histology studies. The technique leverages aspects of classical immunohistochemistry combined with the tryptic digestion used for mass spectrometry-based proteomics. by Multidimensional Protein Identification Technology (MudPIT). Trypsin is especially useful for proteomic 2D mapping as it cleaves proteins at the C-terminal side of arginine or lysine residues.(Olsen, Ong, & Mann, 2004) This results in several advantages for MALDI IMS including that 1) the basic C-terminal lysine or arginine residue results in intense ion reporting of peptide peaks from complex tissue matrices; 2) protease digestion increases access to both high molecular weight proteins and cross-linked proteins; 3) cleaving proteins into peptides increases the total number of proteins detected; and 4) it is highly compatible with LC-MS/MS-based proteomic approaches. The output reports 2D mapping of 300 or more peptides (instrument dependent) across tissue sections, filling a technological niche between MUDPIT proteomics and laser capture microdissection. Studies of tryptic peptide localization on tissue allow basic research on disease mechanisms as well as clinical research on prognostic and diagnostic markers of disease.(Kriegsmann, Kriegsmann, & Casadonte, 2015; McDonnell et al., 2010; Norris & Caprioli, 2013; Schwamborn & Caprioli, 2010)
There are now several ways to homogenously apply enzymes and matrix to tissues for high quality, high resolution images. Initial studies reporting tryptic peptide imaging utilized automated acoustic spotting of both enzyme and matrix. This allowed efficient production of tryptic peptides and extraction into the matrix, but commercial acoustic spotters limited the spatial resolution to a 200–250 µm step size.(Aerni, Cornett, & Caprioli, 2006; Groseclose et al., 2007; Groseclose, Massion, Chaurand, & Caprioli, 2008) The Imageprep device developed by Bruker Daltonics uses a piezo-electric sprayhead to disperse solutions by vibrational vaporization for higher spatial resolution application of enzymes and matrices (25 – 50 µm stepsize).(Heeren, Kükrer-Kaletaş, Taban, MacAleese, & McDonnell, 2008). The pneumatic sprayhead, SunCollect (SunChrome) achieves homogenous application of solutions in low volumes, allowing valuable enzyme to be conserved, with spatial resolutions of less than 10 µm step size achievable. The TM-Sprayer™ (HTXImaging) is a pneumatic sprayer that applies low volumes of solutions using a 20 µm inner diameter stainless steel, heated sprayer. This device is able to achieve matrix crystals of less than 3 µm and allows imaging data to be collected down to a 1 µm stepsize (Zavalin, Yang, Hayden, Vestal, & Caprioli, 2015). Many MALDI IMS labs have more than one automated sprayer for matrix application, including the TM-Sprayer™. These developments in enzyme and matrix application occurred parallel with decreases in laser spot size and commercial instrumentation, which now allows routine achievement of 5–10 µm spatial resolution.
Critical parameters
An important aspect to consider is that appropriate dewaxing or washing of tissues is rigorously followed, as these steps expose the tissue protein for enzymatic access. For fresh frozen tissues, washing in solutions with high concentrations of organic solvents (e.g., over 50% such as the Carnoy’s solution which is 10% glacial acetic acid, 30% chloroform, 60% ethanol) are critical for eliminating salts, lipids and metabolites that can reduce signal due to ion suppression.
Having an appropriate high humidity chamber setup is critical for successful digestion. The humidity chamber should produce a thin layer of condensation prior to placing the sample inside. Low humidity results in poor digestion and limited signal.
The oven temperature must be correct to achieve consistent digestion. Frequently, digital readouts on the front of ovens are inaccurate and can lead to poor digestion within the two-hour digestion time. A secondary thermometer placed in the oven is recommended for more accurate temperature readings.
Troubleshooting
Matrix peaks can contribute significantly to total ion current in an image, especially in early steps of method evaluation when peptide signal may be low. It is recommended that the protocol be evaluated using two tissue sections, one that is not sprayed with trypsin, and a control tissue that is covered with a cover slip or aluminum foil so that trypsin is not applied to the tissue, (not sprayed with trypsin) but proceeds through the rest of the steps.
Table 1 summarizes common problems encountered in the procedure along with suggested remedies.
Table 1.
Troubleshooting guide.
| Problem | Possible Cause | Solution |
|---|---|---|
| Detection of polymers on FFPE tissue | Wax remaining on tissue |
|
| Poor detection of peptides from fresh frozen tissue vs FFPE tissue | Salts, lipids and metabolites remain on the tissue |
|
| Tissue loss during sample preparation | Antigen retrieval conditions too harsh for tissue type |
|
| Slide not adhesive |
|
|
| Poor detection of peptides (from either FFPE or FF) | Low humidity during digestion |
|
| Incorrect temperature during digestion |
|
|
| Instrument dirty |
|
|
| Laser degradation |
|
|
| Poor detection of peptides from whole tissue extraction | Low or small amount of tissue |
|
| Poor detection of peptides from regional locations on tissue | Low amount of peptide due to small region |
|
| Poor fragmentation of peptides by MALDI – MS/MS | Poor instrument settings |
|
Anticipated Results
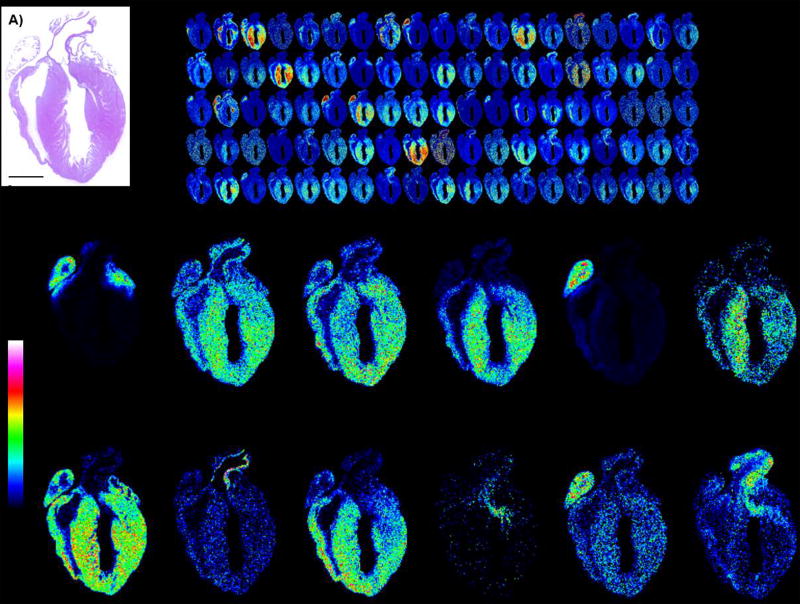
Figure 6 shows anticipated results from a peptide imaging experiment. Typically, the MALDI FT-ICR instrument as described in this protocol reports over 4,500 peaks from the protocol, resulting in over 1,500 peptides (accounting for second and third peaks in peptide isotopic envelopes). MALDI TOF instruments yield around 100–300 two-dimensional peptide maps of tissues; this is because MALDI TOF instruments have a much lower sensitivity. On-tissue extraction from the whole tissue is highly dependent on sample size. Generally, over 200 peptides with high confidence (less than 1% false discovery rate) may be detected from tissue 2 mm × 2 mm by ESI-LC-MS/MS (using an Orbitrap Elite, Thermo Scientific). However, this is highly dependent on the fibrous or fatty content of the tissue. Homogenizing the remaining tissue section and analyzing by ESI-LC-MS/MS can increase protein coverage of imaged proteins. Table 2 summarizes the expected results of peptide identification strategies.
Figure 6.
Example images of expected output shown as MALDI IMS of tryptic peptides from mouse heart. A) H&E stain of heart. B) Example images of tryptic peptide expression showing 110 images out of 1,469 monoisotopic peaks (excluding isotopes 2 and 3 of isotopic envelopes). C) Example single images. Mouse heart tissue section donated by Dr. Christine Kern, Medical University of South Carolina. Mouse heart was fixed in 4% paraformaldehyde, paraffin embedded, and sectioned at 5 µm thickness.
Table 2.
Summary of anticipated results from peptide identification strategies.
| Method | Result | Advantage | Disadvantage |
|---|---|---|---|
| Extraction from whole tissue section | Database of all peptides generated by on-tissue digestion | Database can be used to search peptides extracted from small target regions | Ambiguity from homologous or isobaric peptides |
| Ability to calculate bioinformatic processes | |||
|
| |||
| Extraction from target region | Target peptides from region | Enrichment of distinct peptides from a region | Poor sequence coverage; missing peptide information due to sensitivity issues |
|
| |||
| On-tissue MS/MS | On-tissue peptide sequence information | Direct link to sequence information from tissue | May require a high number of averaged scans to produce an interpretable MS/MS |
Time Considerations
The entire protocol on four slides can be easily completed in one 8-hour day. It is possible to dewax FFPE tissue, scan and store the slide overnight in a desiccator. Peptides produced by tryptic digestion on FFPE may be stored overnight in a desiccator before matrix application. For fresh frozen tissue, it is recommended to perform the entire protocol in one day since this tissue has not gone through the lengthy fixative processes of FFPE tissue.
Figure 3.
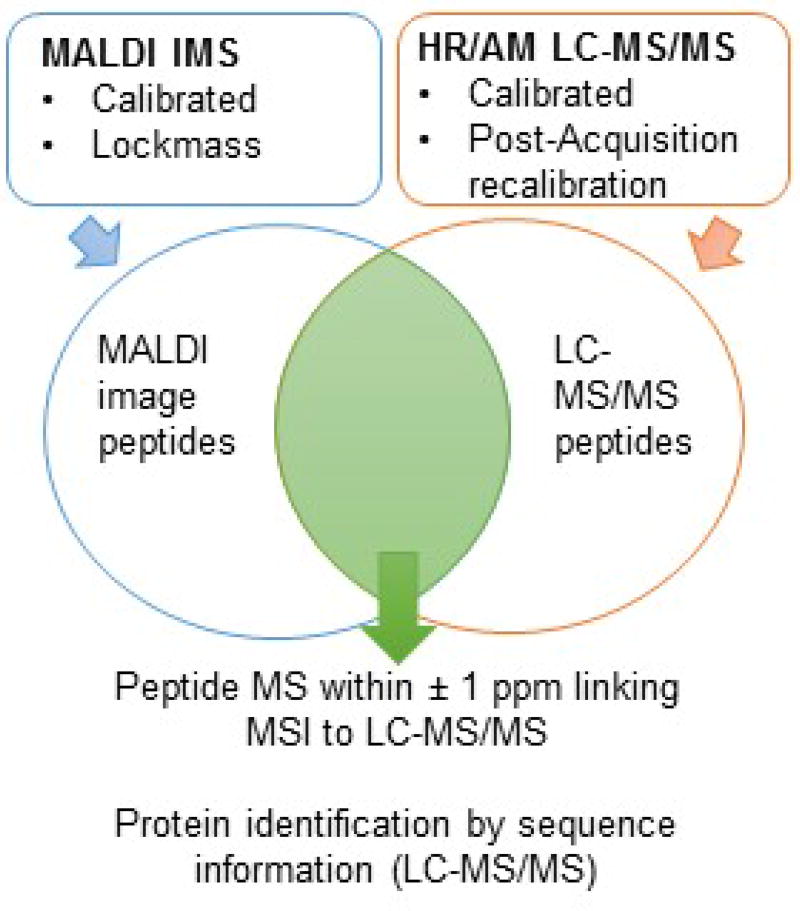
Correlation of MALDI IMS data to LC-MS/MS data by high mass accuracy. MALDI IMS uses calibration of the instrument before each imaging run, combined with the lockmass function to stabilize calibration during imaging. LC-MS/MS uses daily calibration to maintain high resolution, high accurate mass (HR/AM). Data is also recalibrated post acquisition to ensure the highest mass accuracy. MALDI IMS and LC-MS/MS are matched by precursor mass accuracy within ± 1 ppm. Protein identification is made using sequence information obtained by LC-MS/MS or by MALDI MS/MS.
Significance Statement.
Protein imaging mass spectrometry through release of tryptic peptides allows researcher to obtain two dimensional maps on protein localization from thin tissue sections and is compatible with routine proteomic approaches. Approaches to achieving tryptic peptide imaging have improved due to advances in automated spraying and instrumentation. This protocol details the use of automated equipment for spraying enzymes and matrix that is now available in many MALDI imaging mass spectrometry (IMS) labs. This information facilitates reproducible production of tryptic peptide images by IMS for biomedical research studies.
Acknowledgments
This work was supported by the National Institute of Health/National Cancer Institute R21 CA185799 to RRD. PMA appreciates support from the National Institutions of Health through the National Institute of General Medical Sciences P20GM103542.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- Aerni H-R, Cornett DS, Caprioli RM. Automated acoustic matrix deposition for MALDI sample preparation. Analytical chemistry. 2006;78(3):827–834. doi: 10.1021/ac051534r. [DOI] [PubMed] [Google Scholar]
- Casadonte R, Caprioli RM. Proteomic analysis of formalin-fixed paraffin-embedded tissue by MALDI imaging mass spectrometry. Nature protocols. 2011;6(11):1695–1709. doi: 10.1038/nprot.2011.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillero-Pastor B, Heeren RMA. Matrix-assisted laser desorption ionization mass spectrometry imaging for peptide and protein analyses: a critical review of on-tissue digestion. Journal of proteome research. 2013;13(2):325–335. doi: 10.1021/pr400743a. [DOI] [PubMed] [Google Scholar]
- Economou M, Schöni L, Hammer C, Galván JA, Mueller D-E, Zlobec I. Proper paraffin slide storage is crucial for translational research projects involving immunohistochemistry stains. Clinical and translational medicine. 2014;3(1):1–3. doi: 10.1186/2001-1326-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseclose MR, Andersson M, Hardesty WM, Caprioli RM. Identification of proteins directly from tissue: in situ tryptic digestions coupled with imaging mass spectrometry. Journal of Mass Spectrometry. 2007;42(2):254–262. doi: 10.1002/jms.1177. [DOI] [PubMed] [Google Scholar]
- Groseclose MR, Massion PP, Chaurand P, Caprioli RM. High-throughput proteomic analysis of formalin-fixed paraffin-embedded tissue microarrays using MALDI imaging mass spectrometry. Proteomics. 2008;8(18):3715–3724. doi: 10.1002/pmic.200800495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundry RL, White MY, Murray CI, Kane LA, Fu Q, Stanley BA, Van Eyk JE. Preparation of Proteins and Peptides for Mass Spectrometry Analysis in a Bottom-Up Proteomics Workflow. Current protocols in molecular biology. 2009:10–25. doi: 10.1002/0471142727.mb1025s88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren RMA, Kükrer-Kaletaş B, Taban IM, MacAleese L, McDonnell LA. Quality of surface: the influence of sample preparation on MS-based biomolecular tissue imaging with MALDI-MS and (ME-) SIMS. Applied Surface Science. 2008;255(4):1289–1297. [Google Scholar]
- Kriegsmann J, Kriegsmann M, Casadonte R. MALDI TOF imaging mass spectrometry in clinical pathology: a valuable tool for cancer diagnostics (review) International journal of oncology. 2015;46(3):893–906. doi: 10.3892/ijo.2014.2788. [DOI] [PubMed] [Google Scholar]
- Mateos Js, Pintor-Iglesias A, Fernández-Puente P, García-Camba M, Ruiz-Romero C, Doménech N, Blanco FJ. Cryoconservation of peptide extracts from trypsin digestion of proteins for proteomic analysis in a hospital biobank facility. Journal of proteome research. 2014;13(4):1930–1937. doi: 10.1021/pr401046u. [DOI] [PubMed] [Google Scholar]
- McDonnell LA, Corthals GL, Willems SM, van Remoortere A, van Zeijl RJM, Deelder AM. Peptide and protein imaging mass spectrometry in cancer research. Journal of proteomics. 2010;73(10):1921–1944. doi: 10.1016/j.jprot.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Norris JL, Caprioli RM. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chemical reviews. 2013;113(4):2309–2342. doi: 10.1021/cr3004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Ong S-E, Mann M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Molecular & Cellular Proteomics. 2004;3(6):608–614. doi: 10.1074/mcp.T400003-MCP200. [DOI] [PubMed] [Google Scholar]
- Schober Y, Schramm T, Spengler B, Römpp A. Protein identification by accurate mass matrix-assisted laser desorption/ionization imaging of tryptic peptides. Rapid communications in mass spectrometry. 2011;25(17):2475–2483. doi: 10.1002/rcm.5135. [DOI] [PubMed] [Google Scholar]
- Schwamborn K, Caprioli RM. Molecular imaging by mass spectrometry - looking beyond classical histology. Nature Reviews Cancer. 2010;10(9):639–646. doi: 10.1038/nrc2917. [DOI] [PubMed] [Google Scholar]
- Seeley EH, Oppenheimer SR, Mi D, Chaurand P, Caprioli RM. Enhancement of protein sensitivity for MALDI imaging mass spectrometry after chemical treatment of tissue sections. Journal of the American Society for Mass Spectrometry. 2008;19(8):1069–1077. doi: 10.1016/j.jasms.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Caprioli RM. Matrix sublimation/recrystallization for imaging proteins by mass spectrometry at high spatial resolution. Analytical chemistry. 2011;83(14):5728–5734. doi: 10.1021/ac200998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavalin A, Yang J, Hayden K, Vestal M, Caprioli RM. Tissue protein imaging at 1 µm laser spot diameter for high spatial resolution and high imaging speed using transmission geometry MALDI TOF MS. Analytical and bioanalytical chemistry. 2015;407(8):2337–2342. doi: 10.1007/s00216-015-8532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]