Abstract
Because of its unique properties, plasma technology has gained much prominence in the microelectronics industry. Recently, environmental and energy applications of plasmas have gained a lot of attention. In this area, the focus is on converting CO2 and reforming hydrocarbons, with the goal of developing an efficient single-step ‘gas-to-liquid’ (GTL) process. Here we show that applying tri-reforming principles to plasma—further called ‘plasma-based multi-reforming’—allows us to better control the plasma chemistry and thus the formed products. To demonstrate this, we used chemical kinetics calculations supported by experiments and reveal that better control of the plasma chemistry can be achieved by adding O2 or H2O to a mixture containing CH4 and CO2 (diluted in N2). Moreover, by adding O2 and H2O simultaneously, we can tune the plasma chemistry even further, improving the conversions, thermal efficiency and methanol yield. Unlike thermocatalytic reforming, plasma-based reforming is capable of producing methanol in a single step; and compared with traditional plasma-based dry reforming, plasma-based multi-reforming increases the methanol yield by more than seven times and the thermal efficiency by 49%, as revealed by our model calculations. Thus, we believe that by using plasma-based multi-reforming, ‘gas-to-liquid’ conversion may be made efficient and scalable.
Introduction
The world needs an easily scalable gas reforming technology more than ever. From an environmental perspective, we need a technology that can efficiently convert CO2—together with a co-reactant—into value-added chemicals and fuels1–6. From an energetic and economic perspective, we want a technology that can transform gaseous hydrocarbons (mainly methane) into liquids; this is known as the ‘gas-to-liquid’ (GTL) process7. Interest in GTL began in the early 20th century; yet, despite some first commercial GTL plants, we still do not have an efficient scalable GTL process to convert gaseous hydrocarbons, that are currently flared in the (petro)chemical industry. An efficient GTL process would make it possible to liquefy both natural gas and methanated CO2, and would enable us to efficiently utilize the growing feedstocks of biogas and landfill gas. In contrast to GTL, interest in converting CO2 is more recent, still both GTL and CO2 conversion technologies share a common goal; both technologies aim to convert gases into value-added chemicals and fuels.
In 2004 Song et al.8 introduced a novel concept called tri-reforming of methane. The proposed single reactor process was a combination of dry reforming of methane (DRM), steam methane reforming (SMR) and partial oxidation of methane (POX). By this process, syngas with industrially desired H2/CO ratios (1.5–2.0) can be produced and the DRM coking issue can be eliminated8,9. Nevertheless, this innovative approach still relies on several energy intensive post-processing steps in order to transform the syngas into desired liquid products10. This issue makes a single-step GTL process that we present here more beneficial. It is exactly in this area that non-thermal plasma technology could excel1,11,12. A key feature of plasma technology is that it can activate gas molecules at room temperature through electron impact reactions, rather than through heat. Several plasma reactors have already been studied for a variety of plasma-based reforming processes, including pure CO2 splitting, CO2 hydrogenation, artificial photosynthesis, methane reforming, DRM, SMR and POX1,11–13. However, obtaining a high selectivity and yield of the desired chemicals and fuels proves to be challenging.
Recently, adding O2 to the mixture has gained some attention14–23, but adding H2O to plasma-based DRM has not, given that, to our knowledge, only two papers on this topic exist in the literature24,25. Here we show that introducing the principles of tri-reforming in a plasma reactor significantly enhances the conversions, thermal efficiency and methanol yield. This is the first fundamental investigation on the benefits of—what we will further call—‘plasma-based multi-reforming’.
Combining Experiments and Chemical Kinetics Calculations
To investigate the possibilities of plasma-based multi-reforming, we performed chemical kinetics calculations supported by experiments. The reactor used for the experiments26–28 and the chemistry set29 (including its experimental validation) used for the calculations were presented previously. A detailed sensitivity analysis on this chemistry set was presented by Wang et al.30 (detailed information on both the model and experiments can be found in the respective references as well as the Supplementary Information).
The experimental setup consists of a temperature-controlled coaxial DBD reactor with a plasma reactor volume of 3.31 cm3, a reactant feeding system, a high-voltage power supply, and an analytical system. The experiments were performed for a fixed temperature of 673 K (to prevent condensation of the liquid products) and a fixed pressure of 1 atm. The Specific Energy Input (SEI) is defined as the plasma power divided by the total gas flow rate, while the energy for heating the gas is taken into account separately when calculating the thermal efficiency (see equation S22 in the Supplementary Information).
The model used in this work to predict the plasma chemistry is a zero-dimensional (0D) chemical kinetics model, called ZDPlaskin31. In this model, the time evolution of the species densities is calculated by balance equations, taking into account the various production and loss terms by chemical reactions. The temperature-dependent rate coefficients of the heavy particle reactions (i.e., atoms, molecules, radicals, ions, and excited species) are assumed to be constant, in accordance to the findings reported in the literature and were adopted for the temperature of 673 K, used in the experiments. The rate coefficients for the electron impact reactions are calculated with a Boltzmann solver, BOLSIG+32, which is integrated into ZDPlaskin. A more detailed description of ZDPlaskin is provided by Pancheshnyi et al.31.
Here, we first validated the general model in more detail, based on a series of specific experiments with the addition of 2 up to 8% O2 or H2O, of which the results can be found in the Supplementary Information. We then further explore the plasma-based multi-reforming process relying on extensive model calculations only, in which we extrapolate the validated range up to 32% O2 or H2O added, as well as for the combined addition of O2 and H2O. Hence, these results should be qualitatively evaluated (rather than quantitatively) as an indication of the possibilities the addition of specific compounds has on tuning the chemistry. Especially, to our knowledge this is the first study that focuses not just on using a model for analysing the plasma reforming chemistry, but actually using the plasma chemistry to specifically tailor the production of chemicals based on the insights obtained from such previous combined modelling and experimental studies. As a result it is a—much needed—way more fundamental change to looking at plasma reforming processes.
In the Supplementary Information, we report very detailed information about the experiments and the model used, including detailed experimental and modelling results, and equations to calculate conversions, yields, selectivities and thermal efficiency. Also in the Supplementary Information, we report the comparison between the experimental and modelling results. We will limit the discussion (sections 3, 4 and 5 below) to modelling results only to highlight the potential impact of plasma-based multi-reforming in the field of gas reforming, thus stressing the importance of further research in this field.
Controlling the Chemistry by Moving to Plasma-Based Multi-Reforming
A central issue in plasma-based reforming processes is to enhance the selectivity to liquid products over the selectivity to gaseous products (mainly syngas)1,12,33. As a baseline case, we modelled a DBD operating at an SEI of 3 kJ/L, with a DRM mixture of 10% CH4 and 10% CO2 diluted in 80% N2. This yielded a H-based selectivity towards methanol of only 3.29%, while the main products were H2 (40.2%), C2H6 (30.2%) and H2O (9.65%), as shown in Figs 1 and 2. (More details are provided in the Supplementary Information). We focus on the H-based selectivity, not the C-based selectivity (which can be found in the Supplementary Information), because the hydrogen atoms are the most valuable species for this application. These selectivities resulted in a syngas ratio of 1.34 for the standard plasma-based DRM baseline case. To explore the specific effects of O2 and H2O through plasma-based multi-reforming, we added either O2 or H2O to the mixture. In both cases, we kept the CH4 and CO2 concentrations fixed at 10%, with the remainder being N2.
Figure 1.
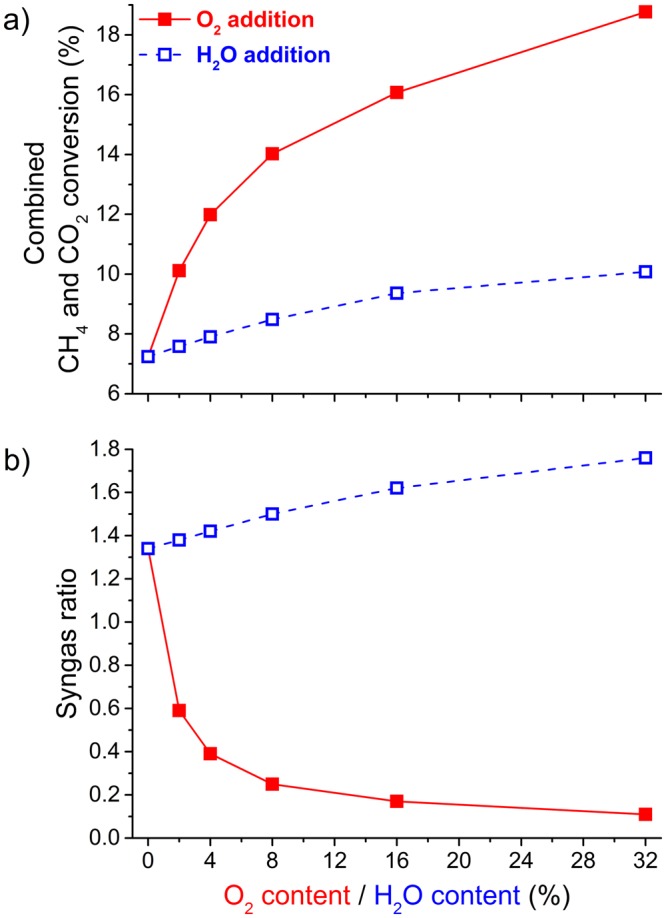
Calculated values of the combined CH4 and CO2 conversion (a); and the syngas ratio (H2/CO) (b) as a function of O2 or H2O content, for a modelled DBD operating at an SEI of 3 kJ/L, with a mixture of 10% CH4 and 10% CO2 diluted in N2.
Figure 2.
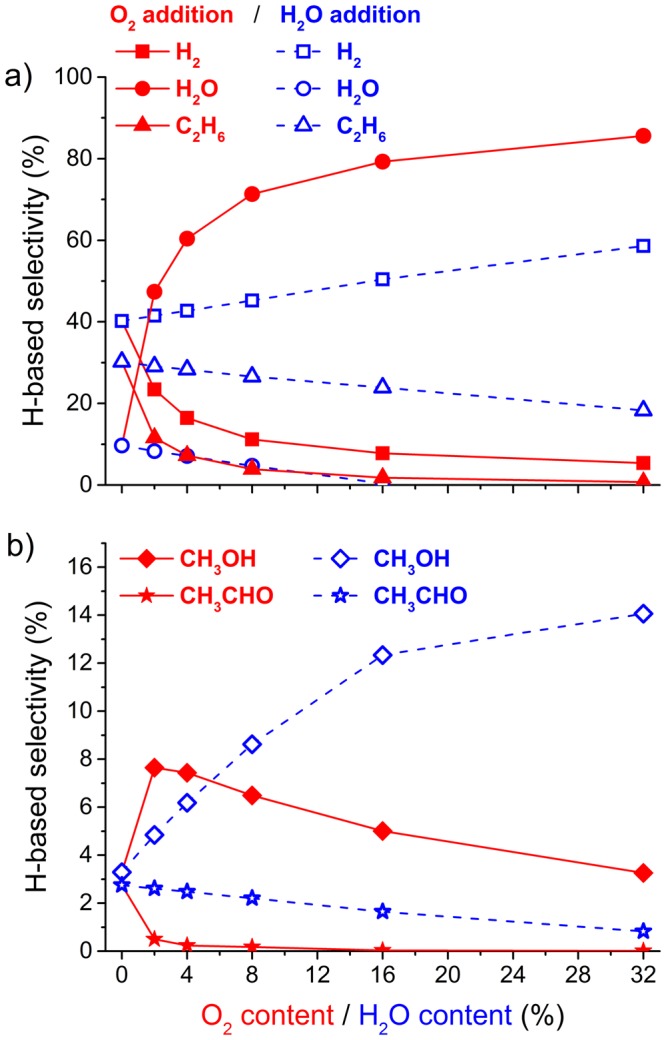
Calculated values of the H-based selectivity towards H2, H2O, C2H6 (a), and CH3OH and CH3CHO (b) as a function of O2 or H2O content, for a modelled DBD operating at an SEI of 3 kJ/L, with a mixture of 10% CH4 and 10% CO2 diluted in N2.
At first, adding O2 seemed to be more beneficial than adding H2O, as 2% of O2 was sufficient to markedly increase, the combined conversion of CH4 and CO2 (Fig. 1a) and methanol selectivity (Fig. 2b). However, we observed that the syngas ratio decreased (Fig. 1b), and the H2O selectivity increased (Fig. 2a) as soon as O2 was added, which leads us to conclude the opposite. O2—and especially its electron impact dissociation product O—is a strong oxidant and thus very effective at converting CH4. This explains why the combined conversion rapidly increased. Unfortunately, O2 mainly converted CH4 into H2O and CO/CO2. When 2 to 8% of O2 was added, 50 to 70% of the hydrogen atoms from CH4 were lost to H2O. This is a huge loss because the hydrogen atoms are the most valuable species. Preferably we aspire them to form methanol (CH3OH) or hydrogen gas (H2), definitely not water (H2O).
When H2O was added to the mixture, instead of O2, the combined conversion of CH4 and CO2 increased slowly but steadily (Fig. 1a), and the syngas ratio also increased slowly but steadily (Fig. 1b). These results are contrary to the fact that adding H2O is detrimental to the conversion process in a DBD for a mixture containing only CO234. The most interesting result came from the selectivity. Contrary to O2, when H2O was added, the selectivity of H2O decreased whereas the selectivity of H2 and that of CH3OH increased (Fig. 2a,b). The selectivity of the other main products, such as CH3CHO and C2H6, decreased as the H2O content increased. When the H2O fraction reached 32%, a selectivity of 14.1% was obtained for methanol, 58.6% for H2, 18.3% for C2H6 and there was no more selectivity to H2O (Fig. 2); in fact, 0.21% of the initial H2O was effectively converted. This change in H-based selectivity from H2O to more valuable products also led to a higher thermal efficiency when H2O was added compared to when O2 was added (Fig. 3).
Figure 3.
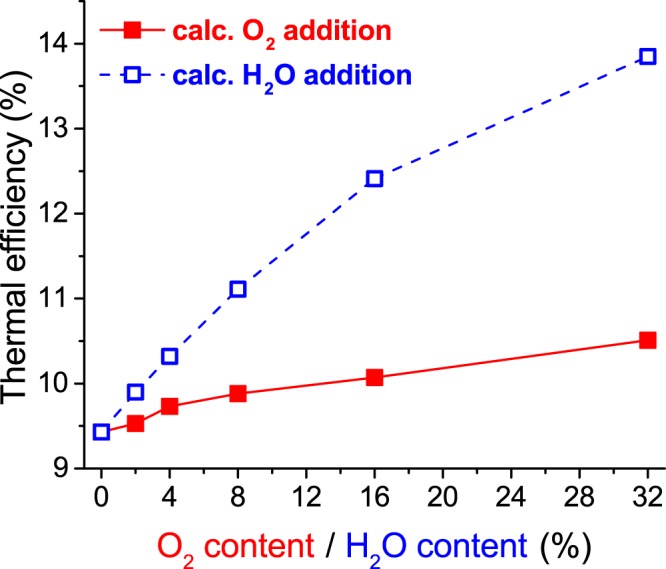
Calculated values of the thermal efficiency as a function of O2 or H2O content, for a modelled DBD operating at an SEI of 3 kJ/L, with a mixture of 10% CH4 and 10% CO2 diluted in N2.
The methanol selectivity appears to saturate when the H2O content reaches 32% (Fig. 2b). This indicates that we are near a point of chemical equilibrium for these specific conditions of the plasma. By adding H2O to the mixture, we shifted the chemistry from water production to methanol production. Details are provided in Fig. 2 (for selectivities) and in Supplementary Fig. S3 (for yields). At one point, between 16 and 32% water, so much water is added that the chemistry undergoes another change and effectively starts to convert part of the water present in the mixture (see details in the Supplementary Information). Despite the thermodynamic non-equilibrium character of the plasma and its influence on reactive species generation, it appears that the overall reaction chemistry is still following Le Chateliers’ principle. From a chemist’s perspective, it is reasonable to believe that this is partially the case, together with the physical changes that influence the chemistry.
When 32% of water was added, the effective conversion of CH4 increased by 44% (from 1.01 to 1.45%), that of CO2 increased by 27% (from 0.44 to 0.56%), and the syngas ratio increased by 31% (from 1.34 to 1.76). At the same time, the (H-based) methanol selectivity increased by more than a factor 4 (from 3.29 to 14.1%), and the yield increased by more than a factor 6 (from 0.033 to 0.219%). Finally, this led to a 47% increase in the thermal efficiency (from 9.43 to 13.85%).
Thus, when H2O (or O2) is added to a CH4:CO2:N2 mixture, plasma-based multi-reforming not only allows us to control the chemistry to increase the yield(s) of the desired products(s) (in this case, methanol), but it also allows us to achieve higher conversion rates and thermal efficiencies than standard plasma-based DRM does.
Enhanced Methanol Yield by Further Tuning Plasma-Based Multi-Reforming
When we added up to 32% H2O, the chemistry reached a point of equilibrium, where the methanol selectivity starts to saturate and the initial H2O in the mixture begins converting. Therefore, we assumed that adding O2 at this point might help to further tune the changes in the plasma chemistry following the tri-reforming principles. So we added several combinations of H2O and O2. (See the Supplementary Information for complete details). It appeared that the oxidising character of O2 remains so dominant that the further enhancement of the plasma chemistry occurs only when a very small amount of O2 is added (Table 1).
Table 1.
Calculated values of effective CH4, CO2 and H2O conversion, methanol (CH3OH) selectivity and yield, and thermal efficiency for plasma-based multi-reforming conditions containing varying O2 and H2O concentrations, for a modelled DBD operating at an SEI of 3 kJ/L, with a mixture of 10% CH4 and 10% CO2 diluted in N2.
| Content | Conversion | Selectivity | Yield | Thermal efficiency | ||
|---|---|---|---|---|---|---|
| H2O O2 | CH4 (%) | CO2 (%) | H2O (%) | CH3OH (%) | CH3OH (mol%) | ηThermal, HHV (%) |
| 0% H2O 0% O2 | 1.01 | 0.44 | n.a. | 3.29 | 0.033 | 9.43 |
| 32% H2O 0% O2 | 1.45 | 0.56 | 0.21 | 14.1 | 0.219 | 13.85 |
| 32% H2O 0.5% O2 | 1.76 | 0.54 | 0.00 | 13.7 | 0.241 | 14.01 |
| 32% H2O 1.0% O2 | 2.00 | 0.52 | 0.00 | 12.3 | 0.246 | 13.81 |
When 0.5% O2 was added to the mixture containing 32% H2O, the effective CH4 conversion increased by 21% (from 1.45 to 1.76%), and the effective CO2 conversion decreased slightly by 4% (from 0.56 to 0.54%). More importantly, despite a slight decrease in the methanol selectivity from 14.1 to 13.7%, the methanol yield further increased by 10% (from 0.219 to 0.241%). Finally, the thermal efficiency increased by 1.2% (from 13.85 to 14.01%). When we added 1% O2, the CH4 conversion and methanol yield continued to increase, but the thermal efficiency decreased. Hence, we expect the highest thermal efficiency to occur when O2 is added between 0 and 0.5%.
Thus, these results indicate that devising the proper mixture makes it possible to tune the chemical equilibrium of the plasma chemistry in such a way that the reaction pathways, hence the selectivity and yield, towards liquid products can be significantly altered in our favour.
Role of the Diluting Agent N2 and the CH4/CO2 Ratio
To determine whether the diluting agent N2 also affects the chemistry, we changed the N2 concentration for a fixed 1:1:3 ratio of CH4:CO2:H2O. For a N2 content of 95%, we added 1% CH4, 1% CO2 and 3% H2O; for a N2 content of 80%, we added 4% CH4, 4% CO2 and 12% H2O; and for a N2 content of 50%, we added 10% CH4, 10% CO2 and 30% H2O. As soon as the N2 content was lowered, the thermal efficiency increased drastically, which was not unexpected. Indeed, earlier experiments and model calculations revealed that for high N2 contents, a lot of the supplied energy goes into exciting the N2 molecules at the expense of dissociating CH4 and CO21,35,36. Interestingly, however, is the increase in methanol selectivity by more than a factor 2 (from 5.46 to 14.2%), and the increase in methanol yield by more than a factor 6 (from 0.035 to 0.219%), as the N2 content decreased from 95 to 50% (Table 2).
Table 2.
Calculated values of effective CH4, CO2 and H2O conversion, methanol (CH3OH) selectivity and yield, and thermal efficiency for plasma-based multi-reforming conditions, varying the concentration of the diluting agent N2, for a modelled DBD operating at an SEI of 3 kJ/L, with a fixed 1:1 mixture of CH4 and CO2.
| Content | Conversion | Selectivity | Yield | Thermal efficiency | ||
|---|---|---|---|---|---|---|
| N2 (%) | CH4 (%) | CO2 (%) | H2O (%) | CH3OH (%) | CH3OH (mol%) | ηThermal, HHV (%) |
| 95 | 0.62 | 0.09 | 0.03 | 5.46 | 0.035 | 5.45 |
| 80 | 0.97 | 0.27 | 0.06 | 10.3 | 0.103 | 8.98 |
| 50 | 1.45 | 0.56 | 0.18 | 14.2 | 0.219 | 13.73 |
To explore the effect of the CH4/CO2 ratio, we varied its value from 0.333 to 3 while keeping the concentration of N2 fixed at 80% and that of H2O fixed at 10%. We found the highest methanol selectivity for a ratio of 1, while the highest methanol yield was found for a ratio of 3 (Table 3). Additionally, the thermal efficiency increased by 27% (from 7.98 to 10.13%) as the CH4/CO2 ratio increased from 0.333 to 3. Our investigations lead us to attribute this increase in thermal efficiency to a shift from CO production to C2H6 production, with the higher methanol yield playing a negligible role.
Table 3.
Calculated values of effective CH4, CO2 and H2O conversions, methanol (CH3OH) selectivity and yield, and thermal efficiency for plasma-based multi-reforming conditions, varying the CH4/CO2 ratio, for a modelled DBD operating at an SEI of 3 kJ/L, diluted in a fixed N2 content of 80%.
| Ratio | Conversion | Selectivity | Yield | Thermal efficiency | ||
|---|---|---|---|---|---|---|
| CH4/CO2 | CH4 (%) | CO2 (%) | H2O (%) | CH3OH (%) | CH3OH (mol%) | ηThermal, HHV (%) |
| 0.333 | 0.82 | 0.48 | 0.00 | 8.29 | 0.068 | 7.98 |
| 1 | 1.01 | 0.32 | 0.01 | 9.63 | 0.098 | 9.26 |
| 3 | 1.12 | 0.16 | 0.05 | 9.19 | 0.107 | 10.13 |
To summarize, in spite of its inert character, the diluting agent N2 significantly influences the plasma chemistry. Additionally, the CH4/CO2 ratio also influences the plasma chemistry and thermal efficiency, but its effect is more nuanced. Depending on the objective a different ratio is needed. (For example to obtain the highest methanol selectivity a different ratio is required than that needed to obtain the highest methanol yield). Thus, these results indicate that many factors can affect the plasma chemistry, and this is why controlling these different parameters is crucial.
Conclusion and Outlook
We have demonstrated that ‘plasma-based multi-reforming’ is a novel concept that can be successfully applied to improve the gas-to-liquid process. We showed that adding H2O and O2 to a plasma-based DRM baseline case significantly increases the CH4 and CO2 conversions, methanol production, and thermal efficiency. These results highlight the benefits of plasma-based multi-reforming over regular plasma-based reforming processes such as DRM and POX.
Our findings suggest that the plasma chemistry can be controlled by adding O2 or H2O to a CH4:CO2:N2 mixture, and that adding H2O yields more promising results. Additionally, we showed that the plasma chemistry can be tuned further if the right combination of initial reactants are added. When we added 32% H2O and 0.5% O2, the methanol yield increased up to 645%, and the thermal efficiency increased up to 49%, compared to the plasma-based DRM baseline case. Thus, plasma-based multi-reforming appears to have greater significant benefits than those the regular individual reforming processes can offer, similarly to tri-reforming with classic thermocatalysis. Moreover, plasma-based multi-reforming allows to produce methanol through a single-step process; this gives it a huge competitive edge and makes it far more attractive for GTL than the thermocatalytic multi-step process. This shows that in the short term it might be more worthwhile to play with the pure plasma chemistry by working with well thought through additives (O2 and H2O in this case), without immediately turning to catalyst materials for selectivity enhancements. In the same sense at a later stage the combination of plasma and catalysis will most probably benefit from this approach as well.
These results are promising; but more research is needed. For example, the calculated product yields need further experimental validation and the underlying chemical pathways accessible through the model need to be thoroughly analysed. This data is needed to further unravel the underlying chemical kinetic reaction pathways for us to figure out how we can intervene even more in the chemistry, and eventually to be able to determine the most optimum conditions. Additionally, in this study, we observed that N2 significantly alters the chemistry as a diluting agent. Raising the question what is the influence of other diluting agents or mixtures? We also found that the CH4/CO2 ratio plays a dual role in this story. Which role do we prefer? Temperature dependent studies should be performed as well because product selectivities in plasma-based reforming heavily depend on temperature26. Finally, and most importantly, the concept of plasma-based multi-reforming should be explored with more energy efficient and higher throughput reactors, such as gliding arc, microwave and ns-pulsed discharges, as well as their combinations with catalysts1. We strongly believe that the effects observed here will also be present in those reactors. Hence, although the current study is limited in scope and we have mountains of work ahead of us, the results presented here are significant in that they give us a sneak peek at the potential of plasma-based multi-reforming and its potentially bright future as GTL technology.
Electronic supplementary material
Acknowledgements
The authors acknowledge financial support from the Competitive Research Funding from King Abdullah University of Science and Technology (KAUST), the European Marie Skłodowska-Curie Individual Fellowship “GlidArc” within Horizon2020 (Grant No. 657304), the Fund for Scientific Research Flanders (FWO) (grant nos G.0217.14 N, G.0254.14 N and G.0383.16 N) and the IAP/7 (Inter-university Attraction Pole) program ‘PSI-Physical Chemistry of Plasma-Surface Interactions’, financially supported by the Belgian Federal Office for Science Policy (BELSPO). This work was carried out, in part, using the Turing HPC infrastructure at the CalcUA core facility of the Universiteit Antwerpen, a division of the Flemish Supercomputer Center VSC, funded by the Hercules Foundation, the Flemish Government (department EWI) and the University of Antwerp.
Author Contributions
R. Snoeckx and W. Wang contributed equally to the model development, modelling results, data analysis, data interpretation and manuscript writing; X. Zhang contributed the experimental set-up development and experimental results; M. S. Cha contributed the experimental results and supervision of the experiments; A. Bogaerts contributed the modelling results and supervision of the modelling. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ramses Snoeckx and Weizong Wang contributed equally.
Contributor Information
Ramses Snoeckx, Email: ramses.snoeckx@kaust.edu.sa.
Weizong Wang, Email: weizong.wang@uantwerpen.be.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34359-x.
References
- 1.Snoeckx R, Bogaerts A. Plasma technology – a novel solution for CO2 conversion? Chem. Soc. Rev. 2017;46:5805–5863. doi: 10.1039/C6CS00066E. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Z, Xiao T, Kuznetsov VL, Edwards PP. Turning carbon dioxide into fuel. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010;368:3343–3364. doi: 10.1098/rsta.2010.0119. [DOI] [PubMed] [Google Scholar]
- 3.Aresta M, Dibenedetto A, Angelini A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 2014;114:1709–1742. doi: 10.1021/cr4002758. [DOI] [PubMed] [Google Scholar]
- 4.Centi G, Quadrelli EA, Perathoner S. Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013;6:1711. doi: 10.1039/c3ee00056g. [DOI] [Google Scholar]
- 5.Goeppert A, Czaun M, Jones J-P, Surya Prakash GK, Olah GA. Recycling of carbon dioxide to methanol and derived products – closing the loop. Chem. Soc. Rev. 2014;43:7995–8048. doi: 10.1039/C4CS00122B. [DOI] [PubMed] [Google Scholar]
- 6.Mikkelsen M, Jørgensen M, Krebs FC. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010;3:43–81. doi: 10.1039/B912904A. [DOI] [Google Scholar]
- 7.Wood DA, Nwaoha C, Towler BF. Gas-to-liquids (GTL): A review of an industry offering several routes for monetizing natural gas. J. Nat. Gas Sci. Eng. 2012;9:196–208. doi: 10.1016/j.jngse.2012.07.001. [DOI] [Google Scholar]
- 8.Song C, Pan W. Tri-reforming of methane: a novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios. Catal. Today. 2004;98:463–484. doi: 10.1016/j.cattod.2004.09.054. [DOI] [Google Scholar]
- 9.Pakhare D, Spivey J. A review of dry (CO 2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014;43:7813–7837. doi: 10.1039/C3CS60395D. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm DJ, Simbeck DR, Karp AD, Dickenson RL. Syngas production for gas-to-liquids applications: Technologies, issues and outlook. Fuel Process. Technol. 2001;71:139–148. doi: 10.1016/S0378-3820(01)00140-0. [DOI] [Google Scholar]
- 11.Adamovich I, et al. The2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D. Appl. Phys. 2017;50:323001. doi: 10.1088/1361-6463/aa76f5. [DOI] [Google Scholar]
- 12.Snoeckx R, Rabinovich A, Dobrynin D, Bogaerts A, Fridman A. Plasma-based liquefaction of methane: The road from hydrogen production to direct methane liquefaction. Plasma Process. Polym. 2017;14:1600115. doi: 10.1002/ppap.201600115. [DOI] [Google Scholar]
- 13.Fridman, A. Plasma Chemistry, 10.1017/CBO9780511546075 (Cambridge University Press, 2008).
- 14.Hwang N, Song Y, Cha MS. Efficient Use of CO2 Reforming of Methane With an Arc-Jet Plasma. IEEE Trans. Plasma Sci. 2010;38:3291–3299. doi: 10.1109/TPS.2010.2064179. [DOI] [Google Scholar]
- 15.Kolb T, Voigt JH, Gericke K-H. Conversion of Methane and Carbon Dioxide in a DBD Reactor: Influence of Oxygen. Plasma Chem. Plasma Process. 2013;33:631–646. doi: 10.1007/s11090-013-9448-6. [DOI] [Google Scholar]
- 16.Zhu B, Li XS, Liu JL, Zhu AM. Optimized mixed reforming of biogas with O2 addition in spark-discharge plasma. Int. J. Hydrogen Energy. 2012;37:16916–16924. doi: 10.1016/j.ijhydene.2012.08.091. [DOI] [Google Scholar]
- 17.Rueangjitt N, Sreethawong T, Chavadej S. Reforming of CO 2-containing natural gas using an AC gliding Arc system: Effects of operational parameters and oxygen addition in feed. Plasma Chem. Plasma Process. 2008;28:49–67. doi: 10.1007/s11090-007-9119-6. [DOI] [Google Scholar]
- 18.Liu JL, Park HW, Chung WJ, Ahn WS, Park DW. Simulated biogas oxidative reforming in AC-pulsed gliding arc discharge. Chem. Eng. J. 2016;285:243–251. doi: 10.1016/j.cej.2015.09.100. [DOI] [Google Scholar]
- 19.Li K, Liu JL, Li XS, Zhu X, Zhu AM. Warm plasma catalytic reforming of biogas in a heat-insulated reactor: Dramatic energy efficiency and catalyst auto-reduction. Chem. Eng. J. 2016;288:671–679. doi: 10.1016/j.cej.2015.12.036. [DOI] [Google Scholar]
- 20.Zhu X, Li K, Liu JL, Li XS, Zhu AM. Effect of CO2/CH4 ratio on biogas reforming with added O2 through an unique spark-shade plasma. Int. J. Hydrogen Energy. 2014;39:13902–13908. doi: 10.1016/j.ijhydene.2014.01.040. [DOI] [Google Scholar]
- 21.Liu JL, et al. Renewable and high-concentration syngas production from oxidative reforming of simulated biogas with low energy cost in a plasma shade. Chem. Eng. J. 2013;234:240–246. doi: 10.1016/j.cej.2013.08.056. [DOI] [Google Scholar]
- 22.Li K, Liu JL, Li XS, Zhu XB, Zhu AM. Post-plasma catalytic oxidative CO2 reforming of methane over Ni-based catalysts. Catal. Today. 2015;256:96–101. doi: 10.1016/j.cattod.2015.03.013. [DOI] [Google Scholar]
- 23.Li X-S, Zhu B, Shi C, Xu Y, Zhu A. Carbon Dioxide Reforming of Methane in Kilohertz Spark-Discharge Plasma at Atmospheric Pressure. AIChE J. 2011;57:2854–2860. doi: 10.1002/aic.12472. [DOI] [Google Scholar]
- 24.Kolb T, Kroker T, Voigt JH, Gericke KH. Wet conversion of methane and carbon dioxide in a dbd reactor. Plasma Chem. Plasma Process. 2012;32:1139–1155. doi: 10.1007/s11090-012-9411-y. [DOI] [Google Scholar]
- 25.Chun YN, Yang YC, Yoshikawa K. Hydrogen generation from biogas reforming using a gliding arc plasma-catalyst reformer. Catal. Today. 2009;148:283–289. doi: 10.1016/j.cattod.2009.09.019. [DOI] [Google Scholar]
- 26.Zhang X, Cha MS. Electron-induced dry reforming of methane in a temperature-controlled dielectric barrier discharge reactor. J. Phys. D. Appl. Phys. 2013;46:415205. doi: 10.1088/0022-3727/46/41/415205. [DOI] [Google Scholar]
- 27.Zhang X, Cha MS. Partial oxidation of methane in a temperature-controlled dielectric barrier discharge reactor. Proc. Combust. Inst. 2015;35:3447–3454. doi: 10.1016/j.proci.2014.05.089. [DOI] [Google Scholar]
- 28.Liu J-L, Snoeckx R, Cha MS. Steam reforming of methane in a temperature-controlled dielectric barrier discharge reactor: the role of electron-induced chemistry versus thermochemistry. J. Phys. D. Appl. Phys. 2018;51:385201. doi: 10.1088/1361-6463/aad7e7. [DOI] [Google Scholar]
- 29.Wang W, Snoeckx R, Zhang X, Cha MS, Bogaerts A. Modeling Plasma-based CO2 and CH4 Conversion in Mixtures with N2, O2, and H2O: The Bigger Plasma Chemistry Picture. J. Phys. Chem. C. 2018;122:8704–8723. doi: 10.1021/acs.jpcc.7b10619. [DOI] [Google Scholar]
- 30.Wang Weizong, Berthelot Antonin, Zhang Quanzhi, Bogaerts Annemie. Modelling of plasma-based dry reforming: how do uncertainties in the input data affect the calculation results? Journal of Physics D: Applied Physics. 2018;51(20):204003. doi: 10.1088/1361-6463/aab97a. [DOI] [Google Scholar]
- 31.Pancheshnyi, S., Eismann, B., Hagelaar, G. J. M. & Pitchford, L. C. Computer code ZDPlasKin (2008).
- 32.Hagelaar GJM, Pitchford LC. Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci. Technol. 2005;14:722–733. doi: 10.1088/0963-0252/14/4/011. [DOI] [Google Scholar]
- 33.Tao X, et al. CH4-CO2 reforming by plasma - Challenges and opportunities. Prog. Energy Combust. Sci. 2011;37:113–124. doi: 10.1016/j.pecs.2010.05.001. [DOI] [Google Scholar]
- 34.Snoeckx R, Ozkan A, Reniers F, Bogaerts A. The Quest for Value-Added Products from Carbon Dioxide and Water in a Dielectric Barrier Discharge: A Chemical Kinetics Study. ChemSusChem. 2017;10:409–424. doi: 10.1002/cssc.201601234. [DOI] [PubMed] [Google Scholar]
- 35.Snoeckx R, Heijkers S, Van Wesenbeeck K, Lenaerts S, Bogaerts A. CO2 conversion in a dielectric barrier discharge plasma: N2 in the mix as a helping hand or problematic impurity? Energy Environ. Sci. 2016;9:999–1011. doi: 10.1039/C5EE03304G. [DOI] [Google Scholar]
- 36.Snoeckx R, et al. Influence of N2 concentration in a CH4/N2 dielectric barrier discharge used for CH4 conversion into H2. Int. J. Hydrogen Energy. 2013;38:16098–16120. doi: 10.1016/j.ijhydene.2013.09.136. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


