Our study demonstrated real-world, direct effectiveness of 13-valent pneumococcal conjugate vaccine against vaccine-type community-acquired pneumonia following introduction into a routine immunization program among adults aged ≥65 years, many of whom had immunocompromising and chronic medical conditions.
Keywords: PCV13, vaccine effectiveness, community-acquired pneumonia, test-negative, adult
Abstract
Background
Following universal recommendation for use of 13-valent pneumococcal conjugate vaccine (PCV13) in US adults aged ≥65 years in September 2014, we conducted the first real-world evaluation of PCV13 vaccine effectiveness (VE) against hospitalized vaccine-type community-acquired pneumonia (CAP) in this population.
Methods
Using a test-negative design, we identified cases and controls from a population-based surveillance study of adults in Louisville, Kentucky, who were hospitalized with CAP. We analyzed a subset of CAP patients enrolled 1 April 2015 through 30 April 2016 who were aged ≥65 years and consented to have their pneumococcal vaccination history confirmed by health insurance records. Cases were defined as hospitalized CAP patients with PCV13 serotypes identified via culture or serotype-specific urinary antigen detection assay. Remaining CAP patients served as test-negative controls.
Results
Of 2034 CAP hospitalizations, we identified PCV13 serotypes in 68 (3.3%) participants (ie, cases), of whom 6 of 68 (8.8%) had a positive blood culture. Cases were less likely to be immunocompromised (29.4% vs 46.4%, P = .02) and overweight or obese (41.2% vs 58.6%, P = .01) compared to controls, but were otherwise similar. Cases were less likely to have received PCV13 than controls (3/68 [4.4%] vs 285/1966 [14.5%]; unadjusted VE, 72.8% [95% confidence interval, 12.8%−91.5%]). No confounding was observed during adjustment for patient characteristics, including immunocompromised status, body mass index, and history of influenza and pneumococcal polysaccharide vaccination (adjusted VE range, 71.1%−73.3%).
Conclusions
Our study is the first to demonstrate real-world effectiveness of PCV13 against vaccine-type CAP in adults aged ≥65 years following introduction into a national immunization program.
Invasive pneumococcal disease (IPD) represents only a fraction of the adult pneumococcal disease burden [1, 2]. By comparison, nonbacteremic community-acquired pneumonia (CAP) makes up the vast majority of pneumococcal disease in adults [2]. In 2014, the Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA), a double-blind, placebo-controlled randomized clinical trial (RCT) conducted in the Netherlands, demonstrated efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) against both overall and nonbacteremic vaccine-type CAP (VT-CAP) in adults aged ≥65 years [3]. As a result of this trial [4] and evidence that, despite herd protection induced by the PCV13 infant vaccination program [5, 6], roughly 10% of all adult CAP was still caused by PCV13 serotypes [7], in September 2014 the US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) revised their 17-year-old pneumococcal vaccination recommendation for older adults to include PCV13 use for all adults aged ≥65 years [7, 8]. This update augmented long-standing use of 23-valent pneumococcal polysaccharide vaccine (PPSV23) [8] with PCV13 in this population [7, 8].
Since that time, >30% of US adults aged ≥65 years have received PCV13 [9]. As with any vaccine, it is important to assess not only the clinical efficacy and safety, but also the real-world effectiveness of vaccination following routine introduction into a broader population. Namely, for PCV13, it is not currently known whether the efficacy observed in the RCT setting of the Netherlands [3] would be reflective of the real-life experience of an adult population as clinically and demographically diverse as the United States. We addressed this important question by evaluating PCV13 effectiveness against hospitalized VT-CAP in adults aged ≥65 years following the age-based ACIP recommendation for use of PCV13 in this population [7] using an observational study design.
METHODS
Design and Setting
We estimated PCV13 VE against hospitalized VT-CAP using a test-negative design (TND). The TND is considered a robust type of observational study for evaluating VE against infectious respiratory diseases [10–13]. This TND was conducted as a nested case-control substudy within a large, population-based CAP surveillance study. The surveillance study prospectively enrolled adults living in Louisville, Kentucky, who were hospitalized with CAP in 1 of 9 adult acute-care hospitals from 7 October 2013 through 30 September 2016 [14].
Participants
We obtained institutional review board approval (IRB) from the University of Louisville Human Subjects Research Protection Program Office (IRB number 13.0408) and from the research offices at each participating study hospital. All participants provided written, informed consent prior to the conduct of any study-related procedures. Clinical and radiological criteria were used to define CAP [14] based on prior, similar studies [15, 16]. CAP was defined based on (i) clinical evidence of ≥2 of the following: fever, hypothermia, chills or rigors, pleuritic chest pain, cough, sputum production, dyspnea, tachypnea, malaise, or abnormal auscultatory findings suggestive of pneumonia; and (ii) radiographic evidence, which included a chest radiograph and/or a computed tomographic image with an infiltrate consistent with pneumonia as determined by the treating healthcare provider or radiologist at the time of presentation. Patients with hospital-acquired pneumonia or who did not provide a urine sample or did not have a final diagnosis of pneumonia at discharge were excluded. Patients could contribute >1 CAP hospitalization event to the study if a subsequent CAP hospitalization for the same patient occurred >30 days after the previous hospitalization.
Clinical and demographic characteristics were obtained for all patients. Clinical characteristics were based on medical record review. Risk for pneumococcal disease was defined by the presence or absence of underlying immunocompromising and chronic medical conditions with each patient categorized as high-risk (immunocompromised), at-risk (immunocompetent, but chronic disease present), or healthy [8, 17, 18]. Categories were based on CDC designations, which are applied to current ACIP recommendations for pneumococcal vaccination [8, 17, 18].
Analysis Population
We limited our analysis to persons aged ≥65 years because current vaccination guidelines recommended universal PCV13 vaccination for this age group [7]. In addition, only patients enrolled between 1 April 2015 and 30 April 2016 were included because (i) it occurred after universal recommendation for adult PCV13 use (September 2014) [7], and (ii) detailed pneumococcal vaccination history was obtained from health insurers during this time period. Participants had to consent to have their pneumococcal vaccination history confirmed by health insurance records. Patients were excluded if, after consent, their health insurer could not be reached. Finally, we excluded patients who received pneumococcal vaccination ≤30 days prior to urine sample collection because a previous study suggested that recent pneumococcal vaccination could impact detection of pneumococcal serotypes in urine samples [19].
Defining Cases and Test-Negative Controls
We performed blood culture, BinaxNOW (Alere, Walthman, Massachusetts), and a PCV13 serotype-specific urinary antigen detection (UAD) assay (Pfizer Inc) on all enrolled patients as a study-related procedure. The UAD assay is ≥95% sensitive and specific for detecting PCV13-type pneumococcal serotypes in patients with bacteremic or nonbacteremic radiographically confirmed CAP when validated against a gold standard of bacteremic pneumonia [20–22]. We collected sputum/respiratory and pleural fluid cultures (when appropriate) as a part of standard medical care.
We defined cases as patients hospitalized for CAP in whom PCV13 serotypes were identified by any method, including from UAD or routine culture from blood, respiratory tract, or pleural fluid. All other patients who met study inclusion criteria but for whom PCV13 serotypes were not identified from any source served as test-negative controls. This approach mimics the definition of test-negative controls commonly used in TND studies of influenza VE [13].
In sensitivity analyses, we estimated PCV13 VE using 2 different control groups. In the first sensitivity analysis, controls were defined as non-PCV13-type pneumococcal CAP (Broome method) [23]. That is, controls were patients in whom pneumococcus was detected by routine culture or BinaxNOW, but PCV13 serotypes were not identified from culture or UAD. This method has been most commonly used to estimate VE against IPD [23]; however, this approach was limited for identifying controls in our study given that UAD only detects PCV13 serotypes. In the second sensitivity analysis, we defined controls as CAP patients in whom pneumococcus was not detected by any method (culture, UAD, or BinaxNOW), similar to a recently published TND study [24].
Vaccine Exposure
Pneumococcal vaccination status was based on whether receipt of ≥1 dose of PCV13 or PPSV23 could be confirmed by health insurance records. We contacted all health insurers that provided coverage for each patient included in the analysis and requested a record of any pneumococcal vaccine(s) received in the last 5 years, including which pneumococcal vaccine(s) were received and the date of administration. Patients were considered vaccinated if they received pneumococcal vaccination >30 days before hospitalization for CAP. We obtained previous influenza vaccination status (within the last year) via patient self-report.
Statistical Analysis
Odds of having received PCV13 for cases and controls were constructed and compared using odds ratios (ORs) and 95% confidence intervals (CIs). We calculated VE as 1 minus the OR multiplied by 100%. We also estimated PCV13 VE against nonbacteremic (only) VT-CAP by restricting the primary analysis to patients in whom blood culture results were negative. In addition to constructing crude OR and VE estimates, we performed logistic regression modeling to assess PCV13 VE after adjustment for potentially confounding factors. Any variable(s) that changed the estimated OR for PCV13 by ≥10% (ie, confounder) [25] remained in the final model. We used a 2-sided α of .05 for all analyses.
RESULTS
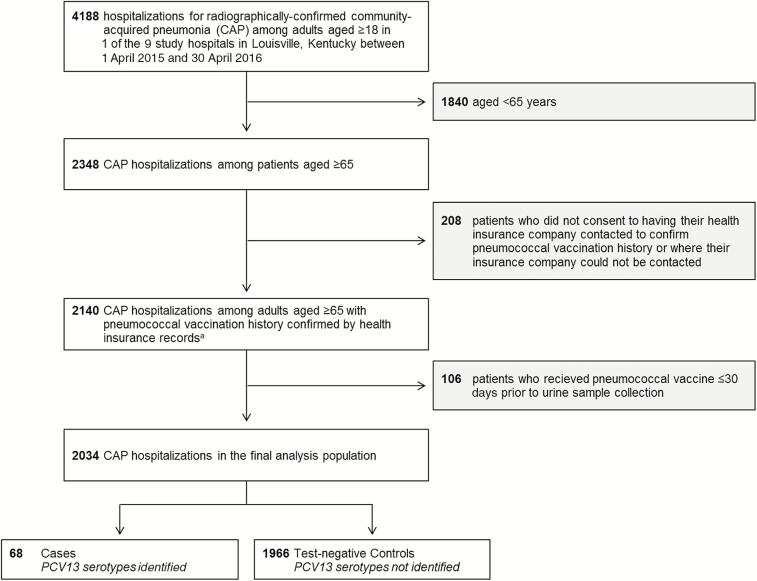
Of 4188 adult hospitalizations for CAP, 2348 occurred among patients aged ≥65 years. Of these, 314 (13.4%) participants for whom pneumococcal vaccination status could not be obtained from health insurance records (n = 208) or pneumococcal vaccination occurred ≤30 days before urine sample collection (n = 106) were excluded, leaving a final analysis population of 2034 CAP hospitalizations (Figure 1). Only 111 of 2034 (5.5%) were repeat CAP hospitalizations from a previously enrolled study patient.
Figure 1.
Selection criteria for test-negative design analysis. aData describing pneumococcal vaccination history were obtained from health insurers for all eligible and consented adult patients in the community-acquired pneumonia (CAP) surveillance study (n = 4813); however, only patients who ultimately met all final criteria for the CAP surveillance study are presented here. Abbreviations: CAP, community-acquired pneumonia; PCV13, 13-valent pneumococcal conjugate vaccine.
Median participant age was 76 years (range, 65−102 years); 35.4% were aged ≥80 years. Most participants were white (88.5%), non-Hispanic (>99%), and had ≥1 at-risk or high-risk condition (87.9%). The most prevalent comorbid conditions were chronic obstructive pulmonary disease (52.6%), coronary artery disease (35.4%), congestive heart failure (31.9%), and diabetes mellitus (32.2%). Almost half (45.8%) were high risk/immunocompromised, with chronic renal disease (22.8%) and malignancy (19.1%) being the most common high-risk/immunocompromising conditions. Smoking prevalence was 19.0%. Median body mass index (BMI [kg/m2]) was 26.2; 30.2% of participants were obese. Median Pneumonia Severity Index (PSI) [26] was 106, with 27.0% of participants having PSI = 5. Median hospital stay for CAP was 6 days (range, 1−49 days); 132 (6.5%) patients died during the initial hospitalization for CAP and 258 (12.7%) patients died within 30 days of hospitalization.
Self-reported receipt of influenza vaccination within the previous year was 68.0%. Based on health insurance records, in the last 5 years, 21.2% received PPSV23, 14.2% received PCV13, and 3.0% received PCV13 and PPSV23. Median time since last dose of PPSV23 and PCV13 was 553 and 157 days, respectively. Participants who received PCV13 were more likely to be enrolled during the winter (38.2 vs 28.0, P < .001), to be white (92.0% vs 88.0%, P = .05), to live at home (95.5% vs 89.5%, P < .01), and to have had an influenza vaccination within the past year (87.2% vs 64.8%, P < .001), but were similar across other patient characteristics (Table 1).
Table 1.
Participant Demographic and Clinical Characteristics by Health Insurance-Validated 13-Valent Pneumococcal Conjugate Vaccination Status (n = 2034)
| Participant Characteristics | PCV13 (n = 288) | No PCV13 (n = 1746) | P Value |
|---|---|---|---|
| Season/time period | <.001 | ||
| Spring | 53 (18.4) | 471 (27.0) | |
| Summer | 48 (16.7) | 385 (22.1) | |
| Fall | 77 (26.7) | 401 (23.0) | |
| Winter | 110 (38.2) | 489 (28.0) | |
| Age group, y | .59 | ||
| 65–79 | 182 (63.2) | 1132 (64.8) | |
| ≥80 | 106 (36.8) | 614 (35.2) | |
| Gender | .10 | ||
| Female | 133 (46.2) | 899 (51.5) | |
| Male | 155 (53.8) | 847 (48.5) | |
| Race | .05 | ||
| White | 265 (92.0) | 1536 (88.0) | |
| Other | 23 (8.0) | 210 (12.0) | |
| Ethnicity | .89 | ||
| Not Hispanic/Latino | 287 (99.7) | 1739 (99.6) | |
| Hispanic/Latino | 1 (0.3) | 7 (0.4) | |
| Place of residence | <.01 | ||
| Family home | 275 (95.5) | 1563 (89.5) | |
| Other | 13 (4.5) | 183 (10.5) | |
| Risk levela | .44 | ||
| High risk/immunocompromised | 128 (44.4) | 804 (46.0) | |
| At risk | 130 (45.1) | 726 (41.6) | |
| Healthy | 30 (10.4) | 216 (12.4) | |
| BMI categoryb | .43 | ||
| Underweight (<18.5) | 22 (7.6) | 135 (7.7) | |
| Normal/healthy weight (18.5−24.9) | 89 (30.9) | 606 (34.7) | |
| Overweight (25.0−29.9) | 91 (31.6) | 475 (27.2) | |
| Obese (≥30.0) | 86 (29.9) | 528 (30.2) | |
| PSI risk classc | .85 | ||
| I | 0 (0.0) | 2 (0.1) | |
| II | 25 (8.7) | 148 (8.5) | |
| III | 62 (21.5) | 386 (22.1) | |
| IV | 129 (44.8) | 732 (41.9) | |
| V | 72 (25.0) | 477 (27.3) | |
| Healthcare facility exposure in past 3 mo | 82 (28.5) | 597 (34.2) | .06 |
| Weekly exposure to children aged <5 yd | 21 (7.3) | 179 (10.3) | .12 |
| Current drug abusee | 1 (0.3) | 8 (0.5) | .79 |
| Antibiotic use within 14 d | 237 (82.3) | 1468 (84.1) | .45 |
| Influenza vaccination within previous y | 251 (87.2) | 1132 (64.8) | <.001 |
| PPSV23 receipt in past 5 y | 62 (21.5) | 370 (21.2) | .90 |
Data are presented as No. (%).
Abbreviations: BMI, body mass index; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine; PSI, Pneumonia Severity Index.
aRisk level was based on Centers for Disease Control and Prevention classifications of risk for pneumococcal disease using information about chronic medical conditions collected from the medical record. “High-risk” patients were defined as having certain immunocompromising conditions including immunodeficiency, human immunodeficiency virus/AIDS, generalized malignancy (excluding skin cancer), hematologic malignancy, diseases requiring treatment with immunosuppressive drugs including long-term corticosteroids or radiation therapy, nephrotic syndrome, chronic renal failure (including end-stage renal disease), or organ transplantation. “At-risk” patients were defined as the absence of immunocompromising conditions but the presence of ≥1 chronic medical condition including congestive heart failure, diabetes mellitus, chronic obstructive pulmonary disease, asthma, liver disease, or current alcoholism or smoking. “Healthy patients” were defined as participants without any immunocompromising or chronic medical conditions listed above [8, 17, 18].
bBMI was measured as weight in kilograms divided by height in meters squared (kg/m2). Two participants were missing information about BMI.
cOne participant was missing information about PSI.
dOne person was missing information about whether or not they had weekly exposure to a child aged <5 years.
eFour participants were missing information about current drug abuse.
UAD and BinaxNOW results were available for all participants. Culture results (predominately from blood) were available for 93.7% (1905/2034) of participants. Of 2034 CAP hospitalizations, we identified PCV13 serotypes in 68 (3.3%) who served as cases. The remainder (1966/2034 [96.7%]) served as test-negative controls. Nearly all of the cases (66/68 [97.1%]) were identified by UAD, 8 of 66 (12.1%) of whom also had positive cultures (5 blood, 2 sputum, 1 bronchoalveolar lavage). Serotyping results were not available for 2 of the 5 blood cultures. Two additional cases, for whom UAD was negative, were identified by culture alone (1 blood, 1 sputum). Thus, most cases 62 of 68 (91.2%) were nonbacteremic.
Cases were less likely to be high risk/immunocompromised (29.4% vs 46.4%, P = .02) and overweight or obese (BMI ≥25.0) (41.2% vs 58.6%, P = .01) compared to controls, but were otherwise similar (Table 2). Cases were less likely to have received PCV13 than controls (3/68 [4.4%] vs 285/1966 [14.5%]; unadjusted VE, 72.8% [95% CI, 12.8%−91.5%]; Tables 2 and 3). Effectiveness against nonbacteremic VT-CAP was similar, with an unadjusted VE of 70.1% (95% CI, 4.1%−90.7%). No confounding was observed during univariate and multivariable adjustment for clinical and demographic characteristics, including immunocompromised status, BMI, and history of influenza and PPSV vaccination for VT-CAP (adjusted VE range, 71.1%−73.3%) or for nonbacteremic VT-CAP (adjusted VE range, 67.5%−70.7%; Table 3). Thus, our crude model served as the final model for both outcomes. We also compared patients who received PCV13 (n = 288) to patients who did not (n = 1746) to compare the proportion of hospitalized CAP caused by PCV13 serotypes by vaccination status. CAP patients who received PCV13 were less likely to have PCV13 serotypes detected (3/288 [1.0%] vs 65/1746 [3.7%], P = .02).
Table 2.
Participant Demographic and Clinical Characteristics for Cases and Test-Negative Controls (n = 2034)
| Participant Characteristics | Cases (n = 68): PCV13-Type Hospitalized CAP | Controls (n = 1966): Non-PCV13-Type Hospitalized CAP | P Value |
|---|---|---|---|
| Primary exposure: received PCV13 in past 5 y | .02 | ||
| Yes | 3 (4.4) | 285 (14.5) | |
| No | 65 (95.6) | 1681 (85.5) | |
| Season/time period | .15 | ||
| Spring | 24 (35.3) | 500 (25.4) | |
| Summer | 9 (13.2) | 424 (21.6) | |
| Fall | 18 (26.5) | 460 (23.4) | |
| Winter | 17 (25.0) | 582 (29.6) | |
| Age group, y | .62 | ||
| 65–79 | 42 (61.8) | 1272 (64.7) | |
| ≥80 | 26 (38.2) | 694 (35.3) | |
| Gender | .17 | ||
| Female | 40 (58.8) | 992 (50.5) | |
| Male | 28 (41.2) | 974 (49.5) | |
| Race | .76 | ||
| White | 61 (89.7) | 1740 (88.5) | |
| Other | 7 (10.3) | 226 (11.5) | |
| Ethnicity | .60 | ||
| Not Hispanic/Latino | 68 (100.0) | 1958 (99.6) | |
| Hispanic/Latino | 0 (0.0) | 8 (0.4) | |
| Place of residence | .52 | ||
| Family home | 63 (92.6) | 1775 (90.3) | |
| Other | 5 (7.4) | 191 (9.7) | |
| Risk levela | .02 | ||
| High risk/immunocompromised | 20 (29.4) | 912 (46.4) | |
| At risk | 38 (55.9) | 818 (41.6) | |
| Healthy | 10 (14.7) | 236 (12.0) | |
| BMI categoryb | .01 | ||
| Underweight (<18.5) | 4 (5.9) | 153 (7.8) | |
| Normal/healthy weight (18.5−24.9) | 36 (52.9) | 659 (33.5) | |
| Overweight (25.0−29.9) | 14 (20.6) | 552 (28.1) | |
| Obese (≥30.0) | 14 (20.6) | 600 (30.5) | |
| PSI risk classc | .47 | ||
| I | 0 (0.0) | 2 (0.1) | |
| II | 3 (4.4) | 170 (8.6) | |
| III | 13 (19.1) | 435 (22.1) | |
| IV | 28 (41.2) | 833 (42.4) | |
| V | 24 (35.3) | 525 (26.7) | |
| Healthcare facility exposure in past 3 mo | 25 (36.8) | 654 (33.3) | .55 |
| Weekly exposure to children aged <5 yd | 7 (10.3) | 193 (9.8) | .90 |
| Current drug abusee | 0 (0.0) | 9 (0.5) | .58 |
| Antibiotic use within 14 d | 58 (85.3) | 1647 (83.8) | .74 |
| Influenza vaccination within previous y | 41 (60.3) | 1342 (68.3) | .17 |
| PPSV23 receipt in past 5 y | 12 (17.6) | 420 (21.4) | .46 |
Data are presented as No. (%).
Abbreviations: BMI, body mass index; CAP, community acquired pneumonia; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine; PSI, Pneumonia Severity Index.
aRisk level was based on Centers for Disease Control and Prevention classifications of risk for pneumococcal disease using information about chronic medical conditions collected from the medical record. “High-risk” patients were defined as having certain immunocompromising conditions including immunodeficiency, human immunodeficiency virus/AIDS, generalized malignancy (excluding skin cancer), hematologic malignancy, diseases requiring treatment with immunosuppressive drugs including long-term corticosteroids or radiation therapy, nephrotic syndrome, chronic renal failure (including end-stage renal disease), or organ transplantation. “At-risk” patients were defined as the absence of immunocompromising conditions but the presence of ≥1 chronic medical condition including congestive heart failure, diabetes mellitus, chronic obstructive pulmonary disease, asthma, liver disease, or current alcoholism or smoking. “Healthy patients” were defined as participants without any immunocompromising or chronic medical conditions listed above [8, 17, 18].
bBMI was measured as weight in kilograms divided by height in meters squared (kg/m2). Two participants were missing information about BMI.
cOne participant was missing information about PSI.
dOne person was missing information about whether or not they had weekly exposure to a child aged <5 years.
eFour participants were missing information about current drug abuse.
Table 3.
Vaccine Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Hospitalized Vaccine-Type Community-Acquired Pneumonia
| Logistic Regression Modela | All VT-CAP (n = 2034) | Nonbacteremic VT-CAP (n = 2014) |
|---|---|---|
| Cases, No. | 68 | 62 |
| Controls, No. | 1966 | 1952 |
| VE, % (95% CI) | ||
| Crude modelb | 72.8 (12.8−91.5) | 70.1 (4.1−90.7) |
| Univariate adjustment | ||
| Seasonality/time period | 72.4 (11.4−91.4) | 69.2 (.8−90.4) |
| Age group | 72.8 (13.0−91.5) | 70.2 (4.4−90.7) |
| Gender | 72.3 (11.3−91.4) | 69.8 (2.9−90.7) |
| Race | 72.9 (13.3−91.6) | 70.4 (4.9−90.8) |
| Ethnicity | 72.8 (12.9−91.5) | 70.2 (4.1−90.7) |
| Place of residence | 73.3 (14.4−91.7) | 70.5 (5.3−90.8) |
| Risk level | 73.3 (14.2−91.7) | 70.7 (5.9−90.9) |
| BMI category | 72.1 (10.4−91.3) | 69.3 (1.3−90.5) |
| PSI | 72.3 (11.3−91.4) | 69.8 (2.9−90.6) |
| Healthcare facility exposure in last 3 mo | 72.6 (12.1−91.4) | 69.9 (3.3−90.6) |
| Weekly exposure to children aged <5 y | 72.8 (12.7−91.5) | 70.4 (4.8−90.8) |
| Influenza vaccination within previous y | 71.1 (6.9−91.0) | 67.5 (–5.2 to 90.0) |
| History of PPSV23 in last 5 y | 72.8 (12.7−91.5) | 70.1 (4.1−90.7) |
| Fully adjustedc | 71.2 (6.1−91.2) | 67.6 (–6.2 to 90.1) |
Abbreviations: BMI, body mass index; CAP, community-acquired pneumonia; CI, confidence interval; PPSV23, 23-valent pneumococcal polysaccharide vaccine; PSI, pneumonia severity index; VE, vaccine effectiveness; VT, vaccine-type (ie, 13-valent pneumococcal conjugate vaccine [PCV13]–type).
aVE was calculated as 1 minus the odds ratio from the logistic regression model of PCV13 vs no PCV13 multiplied by 100%. Season of enrollment was modeled as spring vs summer vs fall vs winter. Age group was modeled as age 65–79 vs ≥80 years. Gender was modeled as male vs female. Race was modeled as white vs non-white. Ethnicity was modeled as Hispanic vs non-Hispanic. Place of residence was modeled as home vs other. Risk level was based on Centers for Disease Control and Prevention classifications of risk for pneumococcal disease and modeled as healthy vs at-risk vs high-risk [8, 17, 18]. BMI (kg/m2) was modeled as obese vs overweight vs normal weight vs underweight; two patients were missing information about BMI and were excluded from the univariate and fully-adjusted models. PSI was modeled as a continuous variable; one patient was missing information about PSI and was excluded from the univariate and fully-adjusted models. Healthcare exposure in last 3 months, weekly exposure to children aged <5 years, influenza vaccine receipt within previous year, and history of PPSV23 in last 5 years were modeled as yes vs no. One patient was missing information about weekly exposure to children aged <5 years and was excluded from the univariate and fully-adjusted models.
bThe crude model served as the final model because no evidence of confounding was observed in univariate or multivariable modeling [25].
cThe fully adjusted model was simultaneously adjusted for all covariates listed in the table.
The 3 VT-CAP cases among PCV13-vaccinated persons were serotypes 3 (n = 2) and 19A (n = 1). All 3 of these cases occurred among at-risk female patients; 2 occurred among patients who were aged ≥80 years. Among the 65 patients who did not receive PCV13, serotype 3 was most commonly detected (n = 25 [38.5%]), followed by serotypes 19A (n = 12 [18.5%]) and 6A (n = 9 [13.8%]). Among the 6 cases that were positive for PCV13 serotypes in UAD and culture, serotyping results disagreed for 2. For 1 patient, UAD identified serotype 19A while blood culture identified serotype 3. For the other patient, UAD identified serotype 3 whereas sputum culture identified a non-PCV13 serotype. Removing these 2 patients from the analysis or defaulting to culture results in these 2 instances, however, had no appreciable effect on our results.
In the first sensitivity analysis where controls were defined as non-PCV13-type pneumococcal CAP, 164 (8.1%) participants aged ≥65 years had pneumococcus detected by any method (culture, UAD, or BinaxNOW). A lower proportion of cases received PCV13 than controls (3/68 [4.4%] vs 11/96 [11.5%], P = .16); however, this difference was no longer statistically significant after the majority of nonpneumococcal controls from the primary analysis were excluded. This corresponded to an unadjusted VE of 64.3% (95% CI, –33.1% to 90.4%). Evidence of confounding in univariate and multivariable models was limited (adjusted VE range, 60.3%−69.2%) (Table 4). Results from the second sensitivity analysis, where controls were defined as nonpneumococcal CAP, were nearly identical to the primary analysis with VE estimates ranging from 71.5% to 73.8% in univariate and multivariable models (Table 4).
Table 4.
Sensitivity Analyses of Vaccine Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Hospitalized Vaccine-Type Community-Acquired Pneumonia Based on Alternative Definitions of Test-Negative Controls
| Logistic Regression Modela | Sensitivity Analysis 1 (n = 164): Controls Defined as Non-PCV13-Type Pneumococcal CAP | Sensitivity Analysis 2 (n = 1938): Controls Defined as Nonpneumococcal CAP |
|---|---|---|
| Cases, No. | 68 | 68 |
| Controls, No. | 96 | 1870 |
| VE, % (95% CI) | ||
| Crude modelb | 63.8 (–37.7 to 90.5) | 73.1 (13.9−91.6) |
| Univariate adjustment | ||
| Seasonality/time period | 60.3 (–50.6.4 to 89.5) | 72.9 (12.8−91.6) |
| Age group | 64.7 (–31.8 to 90.6) | 73.2 (14.1−91.6) |
| Gender | 64.3 (–33.1 to 90.4) | 72.7 (12.5−91.5) |
| Race | 64.3 (–33.4 to 90.4) | 73.3 (14.4−91.7) |
| Ethnicity | 64.3 (–33.1 to 90.4) | 73.1 (13.9−91.6) |
| Place of residence | 65.9 (–27.7 to 90.9) | 73.6 (15.4−91.8) |
| Risk level | 60.3 (–50.6 to 89.5) | 73.8 (16.1−91.8) |
| BMI category | 65.9 (–30.2 to 91.0) | 72.4 (11.3−91.4) |
| PSI | 62.4 (–41.2 to 90.0) | 72.7 (12.5−91.5) |
| Healthcare facility exposure in last 3 mo | 61.2 (–46.2 to 89.7) | 73.0 (13.3−91.6) |
| Weekly exposure to children aged <5 y | 64.8 (–31.6 to 90.6) | 73.1 (13.8−91.6) |
| Influenza vaccination within previous y | 64.8 (–35.0 to 90.8) | 71.5 (8.0−91.1) |
| History of PPSV23 in last 5 y | 67.7 (–21.8 to 91.4) | 73.1 (13.7−91.6) |
| Fully adjustedc | 69.2 (–47.0 to 93.5) | 72.0 (8.7−91.4) |
Abbreviations: BMI, body mass index; CAP, community-acquired pneumonia; CI, confidence interval; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine; PSI, Pneumonia Severity Index; VE, vaccine effectiveness.
aVE was calculated as 1 minus the odds ratio from the logistic regression model of PCV13 vs no PCV13 multiplied by 100%. Season of enrollment was modeled as spring vs summer vs fall vs winter. Age group was modeled as age 65–79 vs ≥80 years. Gender was modeled as male vs female. Race was modeled as white vs nonwhite. Ethnicity was modeled as Hispanic vs non-Hispanic. Place of residence was modeled as home vs other. Risk level was based on Centers for Disease Control and Prevention classifications of risk for pneumococcal disease and modeled as healthy vs at-risk vs high-risk [8, 17, 18]. BMI (kg/m2) was modeled as obese vs overweight vs normal weight vs underweight; two patients were missing information about BMI and were excluded from the univariate and fully-adjusted models. PSI was modeled as a continuous variable; one patient was missing information about PSI and was excluded from the univariate and fully-adjusted models. Healthcare exposure in last 3 months, weekly exposure to children aged <5 years, influenza vaccine receipt within previous year, and history of PPSV23 in last 5 years were modeled as yes vs no. One patient was missing information about weekly exposure to children aged <5 years and was excluded from the univariate and fully-adjusted models.
bThe crude model served as the final model because no evidence of confounding was observed in univariate or multivariable modeling [25].
cThe fully adjusted model was simultaneously adjusted for all covariates listed in the table.
DISCUSSION
Following universal recommendation for PCV13 use in older adults in the United States, PCV13 VE against hospitalized VT-CAP among a clinically and demographically diverse population of adults aged ≥65 years was 73%. VE against nonbacteremic (only) VT-CAP was similar (70%), given that the large majority (91%) of cases were nonbacteremic. These VE estimates were robust against adjustment for potentially confounding participant demographic and clinical characteristics and history of influenza vaccine and PPSV23 use. Furthermore, it is both reassuring and noteworthy that PCV13 VE against VT-CAP was confirmed in a US population with a high prevalence of immunocompromising conditions (46%) and other chronic medical conditions including chronic obstructive pulmonary disease (53%), congestive heart failure (32%), and diabetes mellitus (32%), and where PPSV23 was used (21% in the last 5 years).
PCV13 VE against VT-CAP seen in our observational study (73%) was higher than what was previously observed in the CAPiTA RCT (46%) and was comparable to the 75% efficacy seen against IPD in the same study [3]. Our study differs from the RCT in several ways. First, high-risk/immunocompromised patients were included in our study and made up nearly half of the study population, but were excluded in the RCT. Second, our study was conducted in Louisville, Kentucky, where 21% of patients aged ≥65 years received PPSV23 in the past 5 years. In contrast, in the Netherlands, where the RCT was conducted, PPSV23 was not recommended for use in adults. However, both high-risk/immunocompromised adults and those who have previously received PPSV23 elicit lower immunologic responses to PCV13 compared to adults without high-risk/immunocompromising conditions and adults naive to PPSV23 [27, 28]. Thus, based on these 2 differences alone, a higher VE was unexpected in our study. Our study, however, was observational and, unlike an RCT, is potentially subject to unmeasured confounding. Whether unmeasured confounding alone explains a higher observed point estimate in our study, however, is unclear. Moreover, CIs around our VE estimates were wide and overlapped the range of VT-CAP VE estimates observed in the RCT [3].
Sensitivity analyses that restricted test-negative controls to non-PCV13-type pneumococcal CAP [23] and to nonpneumococcal CAP [24] yielded point estimates that were consistent with those from the primary analysis. This finding is not surprising given that previous studies of influenza VE have also suggested that variations in the definition of test-negative controls have little impact on overall point estimates [29]. Results from sensitivity analyses that restricted controls to non-PCV13-type pneumococcal CAP, however, were not statistically significant at P < .05, likely due to a large reduction in sample size when restricting the analysis population to pneumococcal CAP patients.
Our study has limitations. Although TND studies have been shown to be valid for determining VE, the TND is an observational study and is not immune to selection bias or confounding [10–12, 30]. Our VE results were, however, highly robust to adjustment for many potentially confounding factors (eg, age, risk status, and previous history of PPSV23 and influenza vaccine) and sensitivity analyses of how test-negative controls were defined. In observational settings, the TND is less susceptible to bias caused by differences in healthcare-seeking behavior among cases and controls [10–12, 30]. In traditional case-control studies, it is often difficult to ensure that controls are representative of the source population from which cases were identified [31]. However, test-negative controls are, by definition, patients presenting with similar clinical syndromes as cases (hospitalized CAP in our study).
UAD (alone) detected the large majority of PCV13-type CAP cases in our study. The sensitivity and specificity of UAD were shown to both be nearly 100% based on blood culture, both in the original UAD validation studies [20, 21] and in a recent multicenter study conducted by CDC [22]. It should be noted, however, that no gold standard for nonbacteremic CAP exists. If nonbacteremic pneumonia leads to less antigenuria than bacteremic pneumonia, sensitivity of the UAD may be lower for detecting nonbacteremic cases. There is no reason, however, to assume this would occur differently between vaccinated and unvaccinated persons. Thus, any reduced sensitivity of the UAD for nonbacteremic disease (that occurred nondifferentially) would only drive our VE estimates toward the null (ie, our estimates would underestimate the true VE). Moreover, VE estimates are primarily dependent on test specificity. UAD agreed with the gold standard of blood culture in our study in 4 of 5 instances. In the 1 instance of discordance with blood culture, both UAD and culture both identified a PCV13 serotype, thus this discordance (ie, defaulting to culture) did not impact our results.
Another limitation is that PCV13 uptake during our study period was relatively low (roughly 14%). Nevertheless, pneumococcal vaccination history was collected in a stringent manner that required health insurance company verification for each patient. We excluded patients who received pneumococcal vaccination within 30 days of urine sample collection to prevent false positives by UAD [19]. PCV13 coverage was slightly higher (18%) if these patients were included in population uptake estimates. PCV13 uptake based on health-insurer records in our study (14%−18% with study midpoint between September and October of 2015) was comparable to national estimates of PCV13 coverage based on Medicare claims for the same age group and time period recently published by the CDC (15%−20% between September and October of 2015) [9]. It is possible that some patients received PCV13, but no record of PCV13 receipt could be found in health insurance claims [9]. This potential misclassification bias, however, would drive results toward the null (ie, lower VE estimate), and would only overestimate VE if it occurred differentially between cases and test-negative controls. Finally, the overall number of cases was relatively small (n = 68), and because only 3 patients who received PCV13 developed VT-CAP, meaningful evaluations of serotype-specific PCV13 VE and stratified analyses (eg, VE among different risk groups) were not possible.
The estimated annual incidence of hospitalized CAP in the Louisville surveillance study for adults aged ≥65 years was approximately 2300 per 100000 person-years [32], and the United States has roughly 49 million adults in that age group. Thus, the 73% (95% CI, 13%−92%) effectiveness against 3.7% of CAP observed in our study translates to an estimated rate reduction of hospitalized CAP of approximately 62 (11−78) per 100000 person-years. Over 5 years, for which PCV13 has shown a minimum duration of protection [33, 34], this represents 137000 (24000−173000) cumulative cases of hospitalized CAP that are potentially preventable with PCV13 in the United States. This is comparable to the estimated number of hospitalizations averted from the US seasonal influenza vaccination program in the same age group (13000−166000) based on CDC estimates of annual influenza-related hospitalization in nonpandemic years [35, 36] and assuming a range of influenza VE of 20%−60% in older adults [37]. Further, based on the median hospitalization length of 6 days and the 6.5%−12.7% risk of dying as a result of the CAP hospitalization observed in our study, this could equate to as many as 824000 (145000−1035000) hospital days and 17440 (1570−21920) deaths averted over 5 years with adult PCV13 use in the United States. To date, PCV13 uptake in older US adults has been modest [9]. Given the effectiveness of PCV13 and the remaining VT-CAP disease burden in adults aged ≥65 years observed in our study, the potential public health benefit of continued PCV13 vaccination in this US population, and in other adult populations globally, remains substantial.
Notes
Author contributions. All authors participated in the study design, data interpretation, and the writing of the manuscript. Q. J. and J. M. M. performed data analyses. R. M. C., P. P., T. L. W., W. A. M., and J. A. R. collected the data.
Acknowledgments. Editorial support was provided by Susan DeRocco-Keller at Complete Healthcare Communications, LLC, and was funded by Pfizer.
Financial support. This study was sponsored by Pfizer Inc.
Potential conflicts of interest. J. M. M., R. E. I., H. L. S., D. L. S., B. D. G., L. J., and Q. J. are employees and shareholders of Pfizer Inc. R. M. C., P. P., T. L. W., W. A. M., and J. A. R. received funding support from Pfizer for the conduct of this study. The sponsor was involved with study concept and design, conduct, analysis, and interpretation of the data; drafting of the manuscript; and the decision to submit the manuscript for publication. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014; 20:45–51. [DOI] [PubMed] [Google Scholar]
- 2. Said MA, Johnson HL, Nonyane BA et al. AGEDD Adult Pneumococcal Burden Study Team Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One 2013; 8:e60273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonten MJ, Huijts SM, Bolkenbaas M et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 4. Pfizer Inc. Pfizer announces positive top-line results of landmark Community-Acquired Pneumonia Immunization Trial In Adults (CAPiTA) evaluating efficacy of Prevenar 13. Available at: http://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_positive_top_line_results_of_landmark_community_acquired_pneumonia_immunization_trial_in_adults_capita_evaluating_efficacy_of_prevenar_13. Accessed 27 April 2018. [Google Scholar]
- 5. Moore MR, Link-Gelles R, Schaffner W et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomczyk S, Bennett NM, Stoecker C et al. Centers for Disease Control and Prevention (CDC) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24. [PubMed] [Google Scholar]
- 9. Black CL, Williams WW, Warnock R, Pilishvili T, Kim D, Kelman JA. Pneumococcal vaccination among medicare beneficiaries occurring after the Advisory Committee on Immunization Practices recommendation for routine use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults aged ≥65 years. MMWR Morb Mortal Wkly Rep 2017; 66:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill 2013; 18. pii:20585. [DOI] [PubMed] [Google Scholar]
- 11. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 12. Lipsitch M, Jha A, Simonsen L. Observational studies and the difficult quest for causality: lessons from vaccine effectiveness and impact studies. Int J Epidemiol 2016; 45:2060–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sullivan SG, Feng S, Cowling BJ. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines 2014; 13:1571–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramirez J, Alexander R, Carrico R et al. Distribution of PCV13 pneumococcal serotypes in patients with community-acquired pneumonia presenting at 20 United States hospitals. Open Forum Infect Dis 2015; 2:S379. [Google Scholar]
- 15. Jain S, Self WH, Wunderink RG et al. CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marston BJ, Plouffe JF, File TM Jr et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med 1997; 157:1709–18. [PubMed] [Google Scholar]
- 17. Kyaw MH, Rose CE Jr, Fry AM et al. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005; 192:377–86. [DOI] [PubMed] [Google Scholar]
- 18. Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine 2010; 28:4955–60. [DOI] [PubMed] [Google Scholar]
- 19. Grijalva CG, Wunderink RG, Zhu Y et al. In-hospital pneumococcal polysaccharide vaccination is associated with detection of pneumococcal vaccine serotypes in adults hospitalized for community-acquired pneumonia. Open Forum Infect Dis 2015; 2:ofv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pride MW, Huijts SM, Wu K et al. Validation of an immunodiagnostic assay for detection of 13 Streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Vaccine Immunol 2012; 19:1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pride MW, Jansen KU. Reevaluation of positivity cutoff values for the pneumococcal urinary antigen detection assay. Clin Vaccine Immunol 2017; 24. doi:10.1128/CVI.00239-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wunderink RG, Self WH, Anderson EJ et al. Pneumococcal community-acquired pneumonia detected by serotype-specific urinary antigen detection assays. Clin Infect Dis 2018; 66:1504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Broome CV, Facklam RR, Fraser DW. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine. N Engl J Med 1980; 303:549–52. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki M, Dhoubhadel BG, Ishifuji T et al. Adult Pneumonia Study Group-Japan (APSG-J) Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis 2017; 17:313–21. [DOI] [PubMed] [Google Scholar]
- 25. Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989; 129:125–37. [DOI] [PubMed] [Google Scholar]
- 26. Fine MJ, Auble TE, Yealy DM et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–50. [DOI] [PubMed] [Google Scholar]
- 27. Bhorat AE, Madhi SA, Laudat F et al. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in HIV-infected individuals naive to pneumococcal vaccination. AIDS 2015; 29:1345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenberg RN, Gurtman A, Frenck RW et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine 2014; 32:2364–74. [DOI] [PubMed] [Google Scholar]
- 29. Feng S, Cowling BJ, Kelly H, Sullivan SG. Estimating influenza vaccine effectiveness in the test-negative design using alternative control groups—a systematic review and meta-analysis. Am J Epidemiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haber M, An Q, Foppa IM, Shay DK, Ferdinands JM, Orenstein WA. A probability model for evaluating the bias and precision of influenza vaccine effectiveness estimates from case-control studies. Epidemiol Infect 2015; 143:1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rothman KJ. Invited commentary: when case-control studies came of age. Am J Epidemiol 2017; 185:1012–4. [DOI] [PubMed] [Google Scholar]
- 32. Ramirez JA, Wiemken TL, Peyrani P et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017; 65:1806–12. [DOI] [PubMed] [Google Scholar]
- 33. McLaughlin JM, Swerdlow DL, Isturiz RE, Jodar L. Rethinking number-needed-to-vaccinate for pneumococcal conjugate vaccines in older adults: current and future implications. Vaccine 2017; 35:5360–5. [DOI] [PubMed] [Google Scholar]
- 34. Patterson S, Webber C, Patton M et al. A post hoc assessment of duration of protection in CAPiTA (Community Acquired Pneumonia Immunization Trial in Adults). Trials in Vaccinology 2016; 5:92–6. [Google Scholar]
- 35. Thompson WW, Shay DK, Weintraub E et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–40. [DOI] [PubMed] [Google Scholar]
- 36. Zhou H, Thompson WW, Viboud CG et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 2012; 54:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kostova D, Reed C, Finelli L et al. Influenza illness and hospitalizations averted by influenza vaccination in the United States, 2005–2011. PLoS One 2013; 8:e66312. [DOI] [PMC free article] [PubMed] [Google Scholar]