Abstract
AIM
To investigate changes in spleen stiffness measurements (SSMs) and other non-invasive tests (NITs) after treatment with direct-acting antivirals (DAAs) and identify predictors of SSM change after sustained virological response (SVR).
METHODS
We retrospectively analysed 146 advanced-chronic liver disease (ACLD) patients treated with DAA with available paired SSM at baseline and SVR24. Liver stiffness (LSM), spleen diameter (SD), platelet count (PLT) and liver stiffness-spleen diameter to platelet ratio score(LSPS) were also investigated. LSM ≥ 21 kPa was used as a cut-off to rule-in clinically significant portal hypertension (CSPH). SSM reduction > 20% from baseline was defined as significant.
RESULTS
SSM significantly decreased at SVR24, in both patients with and without CSPH; in 44.8% of cases, SSM reduction was > 20%. LSPS significantly improved in the entire cohort at SVR24; SD and PLT changed significantly only in patients without CSPH. LSM significantly decreased in 65.7% of patients and also in 2/3 patients in whom SSM did not decrease. The independent predictor of decreased SSM was median relative change of LSM. CSPH persisted in 54.4% patients after SVR. Delta LSM and baseline SSM were independent factors associated with CSPH persistence.
CONCLUSION
SSM and other NITs significantly decrease after SVR, although differently according to the patient’s clinical condition. SSM faithfully reflects changes in portal hypertension and could represent a useful NIT for the follow-up of these patients.
Keywords: Clinically significant portal hypertension, Spleen stiffness measurement, Advanced chronic liver disease, Direct-acting antivirals, Portal hypertension, Hepatitis C, Non-invasive test
Core tip: Liver stiffness measurement (LSM) and spleen stiffness measurement (SSM) are widely validated surrogates of portal hypertension (PH) and its complications. Their role in the assessment of therapy response, such as treatment with direct-acting antivirals (DAAs) of hepatitis C virus patients, is still under investigation. We demonstrated in a large cohort that not only LSM, but also SSM, is reduced six months after successful DAA therapy. As opposed to LSM, SSM directly reflects PH and is less influenced by the immediate reduction of liver necro-inflammation. We believe that SSM could represent a helpful tool for the clinician in the follow-up of these patients.
INTRODUCTION
Chronic hepatitis C virus (HCV) infection represents one of the major causes of liver disease and is a leading cause of liver transplantation[1,2]. Recently, the introduction of the highly effective interferon-free direct-acting antivirals (DAAs) has enormously increased the number of patients who have achieved sustained virological response (SVR), even in patients with liver cirrhosis[3-5].
Studies, mostly from the interferon era, have shown that achieving SVR improves liver function[6,7], liver histology[8] and overall clinical outcomes[9]. However, the real impact of SVR in the DAA era, in terms of changes in portal hypertension (PH) and risk of decompensation on immediate follow-up, is not completely known, especially in patients with advanced chronic liver diseases (ACLD). PH is a progressive condition that represents a key point in the natural history of liver diseases[10]; therefore, its assessment by the hepatic venous pressure gradient (HVPG) measurement is fundamental in ACLD patients[11-14]. Indeed, the development of clinically significant portal hypertension (CSPH) in patients with compensated ACLD (cACLD)[11] is highly associated with the risk of clinical decompensation events (ascites, variceal bleeding, jaundice and hepatic encephalopathy)[10].
To date, several studies have demonstrated a significant reduction in HVPG (> 10%-20%) after achieving SVR, both after interferon-based[15-17] and DAA-based regimens[18-21]. Although the HVPG measurement is the gold standard to assess PH[11], it remains an invasive method[22] and its use is still limited only to highly specialized centres[12]; thus, its repeated measurements during the follow-up would hardly be applicable.
Consequently, many non-invasive tests (NITs) in the last decade, including liver and spleen stiffness measurements (LSM and SSM) as well as liver stiffness- spleen diameter to platelet ratio score (LSPS), have been developed and validated to accurately assess PH degree and its complications[11,22-29]. In fact, the Baveno VI Consensus recently recommended that LSM values of 10 kPa should rule out cACLD patients, and values of 20-25 kPa should accurately identify CSPH in patients with viral hepatitis[11]. However, to date, few studies have evaluated the role of NITs in the PH assessment of SVR patients after DAA treatment, and their role in the follow-up. Even if most studies agree on the fact that LSM rapidly decreases after virus eradication[18,19,30-32], controversial data have emerged regarding the changes of SSM after SVR[30-32].
MATERIALS AND METHODS
Aims of the study
We aimed to: (1) investigate the possible effect of HCV-DAA treatment on PH, evaluated by spleen stiffness changes as a mirror of PH; (2) as well as those of other NITs, after HCV-DAA treatment; moreover, we aimed to (3) identify the presence of predictors of the SSM changes in SVR patients after DAA therapy.
Study design and population
This is a retrospective analysis of prospectively collected data of HCV-related cACLD patients treated with DAAs between January 2015 and September 2017 at our department, with valid measurements of LSM and SSM by transient elastography (TE) at baseline (BL) and at six mo after the end of DAA treatment (SVR24).
According to the Baveno VI Criteria[11], values of LSM > 10 kPa at TE were considered suggestive of having cACLD; LSM cut-off ≥ 21 kPa was used to rule-in CSPH, as previously described[33,34]. At baseline, laboratory values, Model for end-stage liver disease (MELD) and Child-Turcotte Pugh (CTP) scores were also reported for each patient.
We excluded patients who (1) had incomplete response to surgical resection or loco-regional ablation of previous HCC; (2) developed HCC during antiviral treatment; (3) developed variceal bleeding and/or endoscopic banding legation (EBL) during the study period; and (4) initiated or changed the dosage of non-selective beta-blockers (NSBB) or had portal vein thrombosis, transjugular intrahepatic portosystemic shunt (TIPS) and non-cirrhotic PH. A subgroup of the patients who did not achieve SVR were separately investigated.
Antiviral treatment
Eligibility for treatment of HCV patients with DAAs was assessed following the priority criteria established in the protocol approved by the Italian Medicines Agency committee. The choice of DAA and treatment duration (12 or 24 wk) was based on viral genotype and stage of disease, according to the guidelines available at the time of enrollment[35]. SVR was defined as undetectable HCV-RNA using real-time PCR, with a detection limit of 15 IU/mL at the 12-wk post-treatment follow-up visit.
NITs for PH assessments
LSM values were assessed by expert operators using the FibroScan® apparatus with “M” probe (Echosens®, Paris, France) after overnight fasting and after a complete abdominal US examination. LSM values were obtained as previously reported[16], and the reliability criteria considered were according to the last EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography[36]. SSM was assessed on the same day as LSM assessment, with the same probe utilized to perform LSM using the FibroScan® apparatus, as previously described[24]. Since no specific literature is present, we translated data from HVPG experience[11] and defined significant SSM as a reduction > 20% from BL. LSPS was calculated as liver stiffness × (spleen diameter (SD)/platelet count)[37]. SD was considered to be the bipolar diameter of the spleen as assessed by ultrasound.
Statistical analysis
Categorical data are expressed as numbers (percentages) and continuous variables as medians (IQR or range). For group comparison, the Mann-Whitney U test was used for continuous variables and the chi2 test for categorical variables. Group comparisons among NITs at BL and SVR24 were evaluated with Friedman’s non-parametric test, and Bonferroni-corrected alphas were used for post hoc pairwise comparison. Demographic, clinical, functional and elastometric variables were evaluated with univariate and multivariate Logistic Regression models in order to assess the predictive factors associated with PH improvement as assessed by SSM. After evaluation of multicollinearity, variables with a P-value < 0.10 upon univariate analysis were included in several multivariate Logistic Regression models with stepwise backward procedures. Prevalence of esophageal varices (EV) was not included in the multivariate analysis due to the limited number of patients with available EGD data (within 6 mo from TE assessment). The estimated odds ratios with their 95% confidence intervals, LR chi2 and Area under ROC Curve were presented. For each multivariable logistic regression, the model discrimination and calibration were reported together with Akaike information criterion and Bayesian information criterion measures for comparing maximum likelihood models. Only P-values < 0.05 were considered statistically significant. The statistical analysis was conducted using Stata/SE (Version 14.0; Stata Corp, Texas, United States).
Ethics
The DAAs treatment protocol was approved by the National Institutional Review Board (IRB) of the Italian Medicines Agency committee. Local IRB [Institutional Ethics Committee of Sant’Orsola-Malpighi University Hospital (Bologna, Italy)] approval was authorized.
RESULTS
Patients characteristics
One hundred-ninety-seven cACLD patients treated with DAAs and with available valid baseline LS and SS measurements were evaluated. The following patients were excluded: two (1%) had HCC occurrence and three (1.5%) presented with active HCC, one (0.5%) underwent EBL during the study period, four (2%) had previous EBL, two (1%) patients presented with complete portal vein thrombosis, one (0.5%) required an increase in NSBB dosage and one (0.5%) had previous TIPS placement. An additional 37 (18.8%) patients were excluded: 22 (out of 197, 11.2%) due to lack of follow-up and 15 (out of 197, 7.6%) due to unfeasible SSM at follow-up. Accordingly, a total of 134 patients with paired LSM and SSM at BL and SVR24 were included in the final analysis; 12 (6%) patients who did not achieve SVR were analysed separately (Figure 1).
Figure 1.
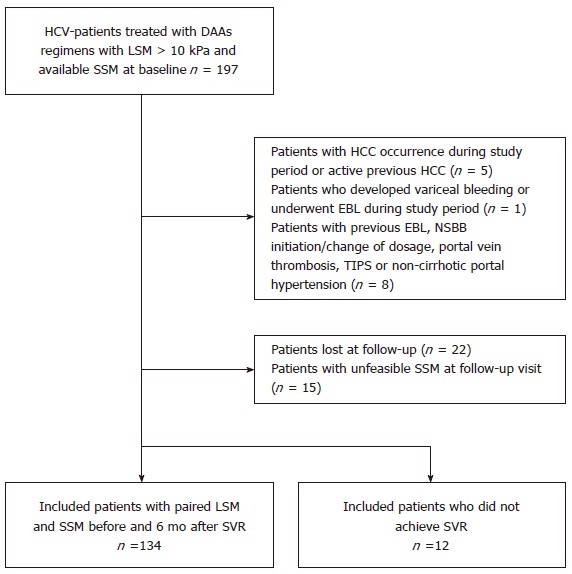
Flowchart of study design. DAA: Direct-acting antiviral; EBL: Endoscopic band ligation; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; LSM: Liver stiffness measurement; NSBB: Non-selective beta-blocker; SSM: Spleen stiffness measurement; SVR: Sustained virological response; TIPS: Transjugular intrahepatic portosystemic shunt.
Table 1 depicts the baseline characteristics of the study cohort. Regarding main NITs, the median values at BL were LSM 19.3 kPa (14.1-27 kPa) and SSM 58.8 kPa (42.2-75 kPa). In a sub-analysis, patients with CSPH (LSM ≥ 21 kPa) differed significantly for MELD score, platelet count, total serum bilirubin, INR, SSM, LSM, SSM and LSPS.
Table 1.
Baseline characteristics of included patients
| Variable | Overall (n = 134) | CSPH (LSM ≥ 21 kPa) (n = 60) | No CSPH (LSM < 21 kPa) (n = 74) |
| Age (yr) | 60 (51-69) | 57 (50.5-65) | 61.5 (51-70) |
| Male | 92 (68.7) | 42 (70) | 50 (67.6) |
| HCV-genotype | |||
| 1 | 95 (70.9) | 41 (68.3) | 54 (72.5) |
| 2 | 12 (8.9) | 4 (6.7) | 8 (10.8) |
| 3 | 20 (14.9) | 11 (18.3) | 9 (12.2) |
| 4 | 7 (5.3) | 4 (6.7) | 3 (4.5) |
| Treatment regimen | |||
| SOF/RBV | 33 (24.6) | 10 (16.7) | 23 (31.1) |
| SOF/SMV | 29 (21.6) | 15 (25) | 14 (18.9) |
| SOF/DCV | 38 (28.4) | 19 (31.6) | 19 (25.6) |
| SOF/LDV | 16 (12) | 7 (11.7) | 9 (12.2) |
| Other | 18 (13.4) | 9 (15) | 9 (12.2) |
| Child Pugh Score | |||
| A | 115 (85.8) | 52 (86.7) | 63 (85.1) |
| B | 19 (14.2) | 8 (13.3) | 11 (14.9) |
| MELD Score | 8 (7-10) | 9 (8-10) | 8 (7-10) |
| Spleen Diameter (cm) | 14 (12.3-15.5) | 14.7 (12.8-15.8) | 13.9 (12.1-15) |
| Laboratory results | |||
| Platelets (cells × 109/L) | 110 (79-150) | 102 (74-132) | 134 (92-159) |
| ALT (U/L) | 58 (39-95) | 55 (39-84) | 60 (38-105) |
| Bilirubin (mg/dL) | 0.9 (0.67-1.29) | 1 (0.84-1.52) | 0.8 (0.6-1.1) |
| Albumin (g/dL) | 3.8 (3.6-4.1) | 3.8 (3.5-4.1) | 3.8 (3.6-4.1) |
| Creatinine (mg/dL) | 0.8 (0.7-0.98) | 0.8 (0.70-0.96) | 0.85 (0.71-1.08) |
| INR | 1.1 (1.06-1.2) | 1.17 (1.1-1.21) | 1.08 (1.04-1.13) |
| NITs | |||
| SSM (kPa) | 58.8 (42.2-75) | 69.9 (55.7-75) | 46.2 (31.6-63.9) |
| LSM (kPa) | 19.3 (14.1-27) | 29.1 (23.9-39.7) | 14.6 (12-17) |
| LSPS | 2.78 (1.4-4.94) | 5.1 (3.05-7.48) | 1.58 (1.09-2.79) |
Qualitative data were expressed as number and percentage (%); quantitative data were expressed as median (25%-75% quantiles). ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CSPH: Clinically significant portal hypertension; DCV: Daclatasvir; HRV: High risk varices; INR: International normalized ratio; LDV: Ledipasvir; LSM: Liver stiffness measurement; LSPS: Liver stiffness to spleen/platelet score; MELD: Model for end-stage liver disease; NITs: Non-invasive tests; RBV: Ribavirin; SMV: Simeprevir; SOF: Sofosbuvir; SVR: Sustained virological response; SSM: Spleen stiffness measurement.
Changes in SSM and LSM after SVR
In the patients who achieved SVR, the median SSM significantly decreased from 58.8 kPa to 38.2 kPa (P = 0.001), with a median delta change in SSM of – 12.3%. The decrease in SSM was statistically significant in both groups, CSPH and not (Figure 2A); the median delta SSM was higher in patients without CSPH at baseline when compared to patients with CSPH (-20.4% vs -4.7%), although this difference did not reach statistical significance. A decrease in SSM values was found in 92 (68.7%) patients, of whom 73 (54.5%) had a decrease > 10% and 60 (44.8%) > 20% (Table 2 and Figure 3A). LSM values also decreased after SVR, with respective median values of 19.3 kPa and 13.8 kPa at baseline and SVR24 (P < 0.0001). The median delta LSM was -30% with similar changes in both groups; LSM decreased in 114 (85.1%) patients, of whom 88 (65.7%) had a decrease of > 20% (Table 2 and Figure 3A).
Figure 2.
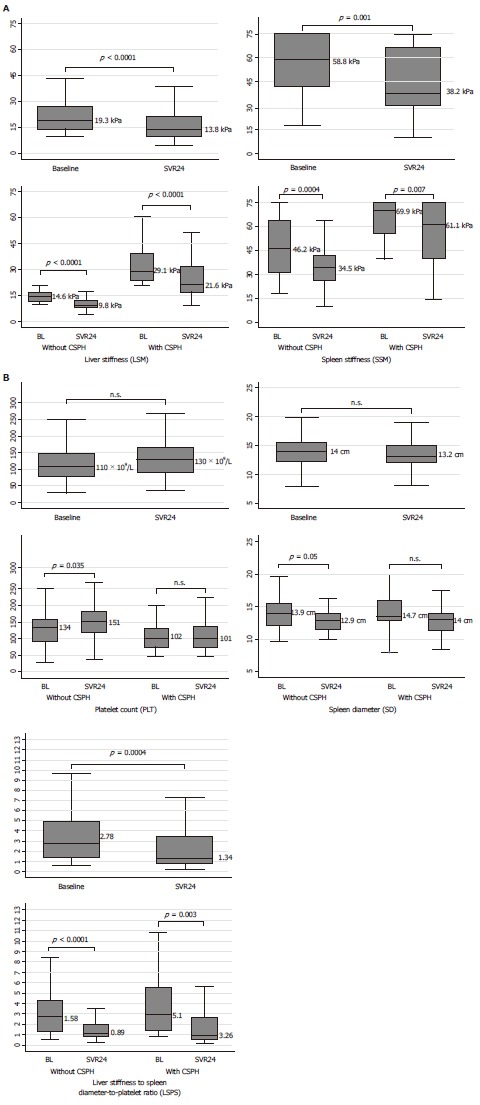
Non-invasive tests changes after sustained viral response by clinically significant portal hypertension presence. A: LSM and SSM changes; B: PLT, SD, LSPS changes. CSPH: Clinically significant portal hypertension; LSM: Liver stiffness measurement; SSM: Spleen stiffness measurement; PLT: Platelet count; SD: Spleen diameter; LSPS: Liver stiffness-to-spleen diameter-to-platelet count ratio score.
Table 2.
Liver and Spleen stiffness measurement decreases after sustained viral response
| Variable | Overall (n = 134) | CSPH (LSM ≥ 21 kPa) (n = 60) | No CSPH (LSM < 21 kPa) (n = 74) |
| Relative SSM decrease (%) | 12.3 (0-36.3) | 4.7 (0-32.5) | 20.4 (0-39.7) |
| Overall SSM decrease | 92 (68.7) | 40 (66.7) | 52 (70.3) |
| > 10% | 73 (54.5) | 31 (51.7) | 42 (56.8) |
| > 20% | 60 (44.8) | 23 (38.3) | 37 (50) |
| Relative LSM decrease (%) | 30 (13.5-42.4) | 28.3 (11.4-41.9) | 30.8 (13.9-42.4) |
| Overall LSM decrease | 114 (85.1) | 51 (85) | 63 (85.1) |
| > 10% | 108 (80.6) | 48 (80) | 60 (81.1) |
| > 20% | 88 (65.7) | 40 (66.7) | 48 (64.9) |
| PLT Increase (%) | 12.4 (-10.1 to 29.6) | 5.5 (-15.6 to 25.9) | 17.4 (-0.67 to 35.6) |
CSPH: Clinically significant portal hypertension; LSM: Liver stiffness measurement; PLT: Platelet count; SSM: Spleen stiffness measurement.
Figure 3.
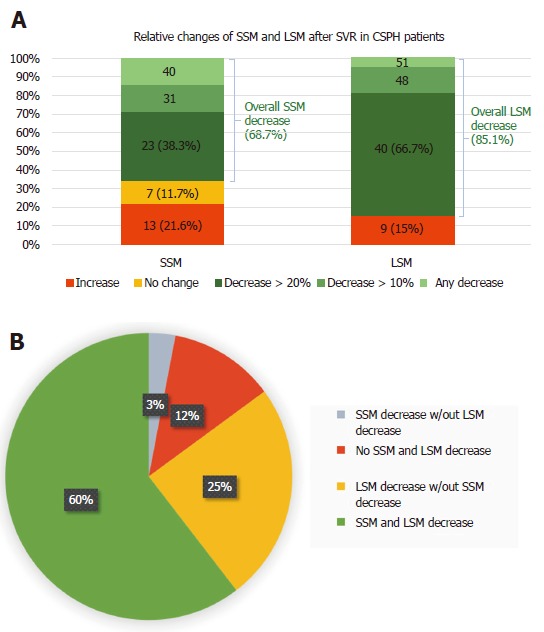
Spleen and liver stiffness measurement decreases after sustained viral response (A), and liver stiffness measurement decreases in patients without spleen stiffness measurement improvements (B). BL: Baseline; CSPH: Clinically significant portal hypertension; LSM: Liver stiffness measurement; SSM: Spleen stiffness measurement; SVR: Sustained virological response.
A LSM decrease was found in almost all patients in whom SSM decreased (95.3%). On the other hand, LSM significantly decreased (P = 0.022) in 2/3 of the patients in whom SSM did not decrease, with a median delta LSM of -28.3%. (Figure 3B).
Changes in other NITs after SVR
The median spleen diameter (SD) at baseline and SVR24 were 14 cm and 13.2 cm, respectively. Although the reduction was not statistically significant in the overall population, it reached significance in the subgroup of patients without CSPH. The increase of PLT (from 110 109/L to 130 109/L) did not reach statistical significance in the entire cohort either, but only in patients without CSPH (Figure 2B). Moreover, median LSPS differed significantly between baseline (2.78) and SVR (1.34), and in both subgroups as well.
Non-SVR patients
Twelve patients did not achieve SVR in our cohort. Baseline characteristics did not statistically differ from the patients included in the final analysis. In particular, in non-SVR patients, an LSM decrease (23.2 kPa at BL vs 21.6 kPa at FU24), an SSM increase (45.6 kPa at BL vs 57.8 kPa at FU24) and a PLT decrease (128 × 109/L at BL vs 100 × 109/L at FU24) were observed; none of these changes reached statistical significance (Supplementary Table 1).
Predictors of significant SSM Decrease (> 20%)
Table 3 shows the differences observed between patients who had an SSM decrease > 20% and those who did not. In the entire cohort, patients with significant SSM reduction differed in the prevalence of EV, MELD score, albumin levels, as well as baseline SSM, LSPS values and LSM-related variables. In multivariate analysis, relative LSM changes remained as the only independent predictor of an SSM decrease > 20%. Furthermore, predictors of an SSM decrease > 20% (Supplementary Table 2) were investigated among patients with CSPH at baseline. Once again, a higher prevalence of EV, higher creatinine levels, lower LSM values at SVR24 and higher delta LSM were observed among patients with an SSM decrease > 20%. In multivariate analysis, higher serum creatinine levels and delta LSM > 20% were the predictors of a significant SSM decrease.
Table 3.
Univariate and multivariate analysis of factors associated with a SSM decrease > 20%
| Entire Population (n = 134) | ||||||
| Variable |
Univariate analysis |
Multivariate analysis |
||||
| LR Chi-2 = 16.48 | AIC = 171.8 | |||||
| AUROC = 0.6821 | BIC = 177.6 | |||||
| SSM Decrease > 20% (n = 60) | No SSM Decrease > 20% (n = 74) | OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | 62 (52-69) | 56 (50-68) | 1.005 (0.975-1.037) | 0.727 | ||
| Sex (male) | 21 (28.4) | 21 (35) | 1.359 (0.653-2.828) | 0.412 | ||
| Presence of varices (n = 67) (yes) | 9 (34.2) | 24 (82.8) | 0.110 (0.031-0.388) | 0.001 | ||
| Spleen diameter (cm) | 13.6 (11.65-15.15) | 14.5 (13-16) | 0.800 (0.660-0.970) | 0.023 | ||
| Child Pugh Score | 5 (5-6) | 5 (5-6) | 0.885 (0.601-1.303) | 0.535 | ||
| Child Pugh Score B (yes) | 8 (13.3) | 11 (14.9) | 0.881 (0.330-2.352) | 0.801 | ||
| MELD score | 8 (7-10) | 9 (8-10) | 0.786 (0.648-0.954) | 0.015 | ||
| MELD > 10 | 12 (20) | 30 (40.5) | 0.367 (0.167-0.804) | 0.012 | ||
| AST (U/L) | 54.5 (38-85) | 56 (35.5-87) | 0.996 (0.987-1.004) | 0.322 | ||
| ALT (U/L) | 62 (37-105) | 53 (40-90) | 1.002 (0.995-1.008) | 0.620 | ||
| ALT ≥ 2 × ULN at BL | 59.5 (37.5-101) | 54 (40-91.5) | 1.326 (0.597-2.944) | 0.489 | ||
| INR | 1.09 (1.05-1.17) | 1.12 (1.09-1.21) | 0.127 (0.001-0.551) | 0.023 | ||
| Bilirubin (mg/dl) | 0.85 (0.65-1.16) | 1.02 (0.71-1.52) | 0.903 (0.535-1.525) | 0.703 | ||
| Albumin (g/dl) | 3.8 (3.52-4.12) | 3.78 (3.52-4.12) | 2.096 (0.959-4.581) | 0.063 | ||
| Creatinine (mg/d) | 0.8 (0.7-1) | 0.81 (0.69-0.93) | 0.327 (0.674-1.585) | 0.165 | ||
| Platelet count (10^9/L) | 118 (92-154) | 91 (74-137) | 1.002 (0.996-1.007) | 0.579 | ||
| LSM BL (kPa) | 18 (14.6-25.7) | 21.1 (14-38.5) | 0.988 (0.962-1.015) | 0.391 | ||
| LSM SVR24 (kPa) | 12.4 (9.4-18) | 17.5 (10.4-32.4) | 0.944 (0.908-0.981) | 0.004 | ||
| SSM BL (kPa) | 60.4 (45.7-70.7) | 53.2 (37.4-75) | 1.012 (0.992-1.032) | 0.225 | ||
| LSPS BL | 2.17 (1.33-3.77) | 4.15 (1.65-6.26) | 0.817 (0.684-0.975) | 0.025 | ||
| LSM decrease (Delta, %) | 33 (18.1–44.6) | 19.4 (0–31.3) | 0.0332 (0.005-0.225) | < 0.0001 | 0.0332 (0.005-0.225) | < 0.0001 |
| LSM decrease > 10% (yes) | 54 (90) | 54 (73) | 3.333 (1.242-8.946) | 0.017 | ||
| LSM decrease > 20% (yes) | 47 (78.3) | 41 (55.4) | 2.910 (1.352-6.262) | 0.006 | ||
Qualitative data were expressed as number and percentage (%); quantitative data were expressed as median (25%-75% quantiles). AIC: Akaike information criterion; ALT: Alanine aminotransferase; AUROC: Area under curve ROC; AST: Aspartate aminotransferase; BIC: Bayesian information criterion; CSPH: Clinically significant portal hypertension; DCV: Daclatasvir; HRV: High risk varices; INR: International normalized ratio; LDV: Ledipasvir; LR: Like-hood ratio; LSM: Liver stiffness measurement; LSPS: Liver stiffness to spleen/platelet score; MELD: Model for end-stage liver disease; NITs: Non-invasive tests; RBV: Ribavirin; SMV: Simeprevir; SOF: Sofosbuvir; SVR: Sustained virological response; SSM: Spleen stiffness measurement.
Changes of CSPH state after SVR
Figure 4 shows that 60 (44.8%) patients presented with CSPH at baseline, defined as an LSM ≥ 21 kPa. After a 6 mo follow-up, none of the 74 patients without CSPH at baseline progressed to CSPH. In patients with CSPH, 46.7% of them had reduced LSM under the CSPH threshold after treatment. Supplementary Table 3 shows the predictors of CSPH persistence after DAA treatment.
Figure 4.
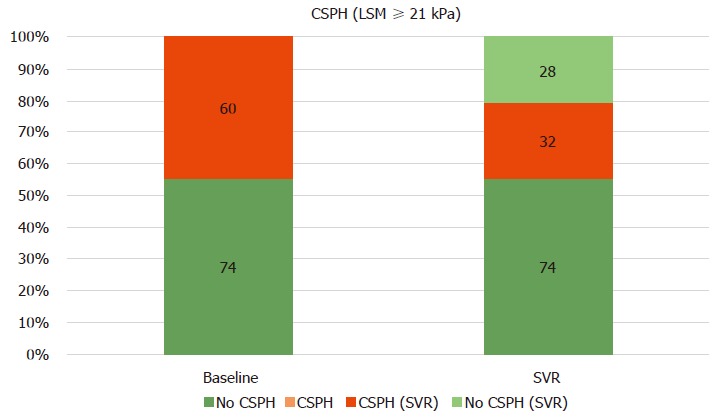
Clinically significant portal hypertension presence, according to Baveno VI (liver stiffness measurement ≥ 21 kPa) at baseline and after SVR24. CSPH: Clinically significant portal hypertension; LSM: Liver stiffness measurement; SSM: Spleen stiffness measurement; SVR: Sustained virological response.
DISCUSSION
The main aim of our study was to evaluate PH changes assessed by non-invasive methods after successful viral eradication in patients treated with DAAs. Our data show that SSM and LSM significantly decrease after SVR, according to the baseline clinical patient condition.
The IFN-free regimens are highly effective, allowing to treat and achieve SVR in patients who also have ACLD[4,38]. However, the individual clinical benefit in these patients is still under debate, especially in terms of changes in PH and CSPH-driven complications[39-41]. While results from the interferon era might not necessarily be translatable to DAA regimens[21], recent studies have also unanimously demonstrated that HVPG significantly decreases after SVR[18-21]. Although many studies have shown that LSM rapidly decreases after DAA treatment[42,43], not much is known about the changes of PH surrogate NITs, such as SSM and LSPS, after viral eradication. In fact, NITs have yet to be validated in SVR patients, and their role in the clinical follow-up is still to be determined.
The main finding of this study is that SSM significantly changes after 24 wk of SVR in patients with cACLD, with a median relative change of -12.3% (Table 2). To our knowledge, only two complete papers[30,32] and one letter to the editor[39] have investigated the changes in SSM after SVR, with opposing results. In fact, only in the study by Pons et al[32] SSM was found to rapidly decrease at only 4 wk after therapy initiation in 41 patients, with no ulterior significant changes until 48 wk of follow-up; the other studies concluded that SSM did not significantly decrease at SVR24[30,32].
In our study that analyzed a large cohort of cACLD patients, we demonstrated that SSM significantly decreased after DAA treatment. These results confirm previous studies in which PH was assessed by paired HVPG measurements[18-21]. Moreover, our study is the first to assess and demonstrate the improvement of LSPS, another accurate surrogate of PH, after SVR24. Moreover, in the eight patients who did not achieve SVR, SSM and other NITs did not significantly differ during follow-up measurements (Supplementary Table 1).
We classified patients with and without CSPH according to a LSM cut-off of 21 kPa[33,34]. Interestingly, the relative changes in SSM and LSM performed differently in patients with and without CSPH. In fact, while the median delta LSM in patients with and without CSPH was very similar (-28.3% vs -30.8%), the reduction of SSM was much more evident in patients without CSPH (-20.4% vs -4.7%). This last result is consistent with the relative HVPG changes described by Mandorfer et al[18]. Moreover, the other surrogates of PH, including the platelet and spleen diameter, significantly changed only when split by CSPH presence. Regarding the different changes of NITs in patients with and without CSPH, we could speculate that this behaviour can reflect the different stages of underlying PH pathogenic mechanisms. Indeed, determinants of portal pressure affecting SSM, such as intrahepatic resistance and liver necro-inflammation[44], improve in both subgroups. However, in CSPH, other major actors of PH, such as extra-hepatic hemodynamic factors[34] and spleen structural changes[45], might not ameliorate in the short-term follow-up (6 mo after SVR). This hypothesis could explain why we found a less prominent SSM decrease (-4.7% vs -20.4%), even when liver necro-inflammation reduction as assessed by delta LSM (-28.3% vs -30.8%) was the same.
SSM reduction was present in 68.7% of patients after 6 mo of follow-up. We found that the only independent predictor of a significant PH improvement, as reflected by a SSM decrease > 20%, was the relative change in LSM (Table 3), confirming previous studies with HVPG[18,21]. However, when we assessed PH improvement as reflected by SSM in our study, as PH surrogate, and by HVPG in the study by Lens et al[21], we noticed similar proportions of patients with a significant response (> 20%) when comparing SSM and HVPG (38.3% vs 39.8%, respectively), but not LSM and HVPG (66.7% vs 39.8%, respectively) (Figure 3A). Even if a correlation between HVPG and SSM changes after DAA treatment has not been demonstrated to date, our data may suggest that an SSM reduction > 20% could be a more accurate non-invasive predictor of a significant HVPG reduction[11].
A statement in the Baveno VI consensus was that the main therapeutic goal in patients with mild PH (6-9 mmHg) is to prevent CSPH development[11]. In our cohort, none of the patients who achieved SVR progressed to CSPH. More challenging, however, is the concept of assessment of CSPH presence/absence after SVR due to its clinical implications, since there is not sufficient evidence showing that the cut-offs after DAAs are the same as the ones used in the pre-treatment phase[23,46]. However, promising data documented that a LSM of 20-25 kPa could be an accurate cut-off to rule-in CSPH after DAA therapy[21]. Accordingly, we also investigated CSPH persistence after SVR (Figure 4). Using these cut-offs, we found that 53% of the patients with CSPH at baseline presented CSPH at SVR24. In multivariate analysis, higher baseline values of SSM (indicating a more severe PH) and lower LSM relative changes were found to be predictors of CSPH persistence (Supplementary Table 3). These results are in line with another study[21] in which higher BL HVPG and relative LSM changes were predictors of CSPH persistence after DAA treatment.
All of the above results seem to reflect the different dynamics in LSM and SSM changes after achieving SVR. LSM consensually decreased in almost all patients with SSM reduction (95.2%), while the opposite was not found to be true. In fact, LSM significantly decreased, with a median delta -28.3%, in 2/3 of the patients in whom no SSM reduction was found. This result emphasizes the fact that LSM is heavily influenced by the reduction of liver necro-inflammation[44] after SVR, and that changes in LSM might not be the most adequate predictors of PH changes in this context. On the other hand, a SSM decrease > 20% could identify patients who significantly clinically benefit from viral eradication. When looking at the bigger picture, SSM could represent a feasible tool to monitor therapy response and assess its benefit. This is also supported by a recent study by Buechter et al[47] that investigated LSM and SSM changes after TIPS placement.
The present study has some limitations: (1) its retrospective nature, even though SSM and LSM were prospectively collected according to the Italian Medicines Agency committee eligibility criteria for the treatment of HCV patients with DAAs, and (2) the absence of a gold-standard reference for PH assessment. However, according to the Baveno VI consensus[11], we could consider NITs, in addition to LSM, to be good surrogates of invasive methods, such as liver biopsy and HVPG. The time of follow-up was too short to fully correlate SSM changes with clinical outcomes after viral eradication, such as events of decompensation after SVR[48]. As in previous studies that include SSM, the upper limit of 75 kPa for SSM affects the possibility to detect changes in patients with severe PH[49,50]; in fact, both BL and SVR24 values were 75 kPa in seven (5.2%) patients.
In conclusion, SSM could be an accurate and useful NIT for the follow-up of patients after SVR, as it faithfully reflects changes in PH better than other NITs, including LSM. Further prospective studies are required in order to confirm the accuracy and usefulness of SSM and other NITs in the follow-up of patients with ACLD and its correlation with clinical outcomes.
ARTICLE HIGHLIGHTS
Research background
The long-term benefits of achieving sustained virological response (SVR) in cirrhotic patients are still to be established. Non-invasive tests (NITs), such as liver (LSM) and especially spleen stiffness (SSM), are widely validated in hepatology as portal hypertension (PH) surrogates. However, their use in SVR patients and their changes after virus eradication is still under discussion.
Research motivation
Many studies have reported rapid LSM decrease after achieving SVR. However, only a few have investigated changes in SSM in such patients, with contrasting results. Given that there is a decrease in SSM after therapy, it means that SSM could be exploited to assess changes in PH and PH-driven complication after achieving SVR.
Research objectives
The main objective of the study was to investigate changes in PH after successful eradication of HCV infection, as reflected by its non-invasive assessment by SSM and other NITs.
Research methods
This is a retrospective study of prospectively collected data. Patients with available paired SSM assessment at baseline and 6 mo after end-of-therapy (SVR24) were included in the study.
Research results
Our main result is that a significant SSM decrease at SVR24 was demonstrated in a large cohort of 134 patients. This is the first study that also reveals a decrease in LSPS after SVR. SSM reduction differed according to the patient’s clinical condition, especially when divided by the presence of clinically significant PH. An LSM decrease of > 20% was evident in the majority of patients, and also in patients in whom no SSM reduction was present. This finding likely reflects the reduction in liver necro-inflammation rather than PH improvement.
Research conclusions
PH, reflected by NITs, improves after achieving SVR in cirrhotic patients. SSM is a direct surrogate of PH and less influenced by liver necro-inflammation, as opposed to LSM. Its decrease (> 20%) could help the clinician to stratify the risk for PH-related complication after DAA therapy.
Research perspectives
Future prospective studies should investigate whether changes in SSM are predictive of clinical decompensation or other complications of cirrhosis after viral eradication. SSM could become a helpful and accurate method to assess therapy response and the risk of complications.
Footnotes
Institutional review board statement: This study was approved by the National Institutional Review Board of the Italian Medicines Agency Committee. Local IRB [Institutional Ethics Committee of Sant’Orsola-Malpighi University Hospital (Bologna, Italy)] approval was authorized.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors disclose no conflicts.
STROBE statement: The guidelines of the STROBE statement have been adopted and a fulfilled version of the checklist has been attached with the submission of the manuscript.
Manuscript source: Invited manuscript
Peer-review started: July 3, 2018
First decision: July 24, 2018
Article in press: August 13, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Ferraioli G, Furuichi Y, Kahraman A S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Song H
Contributor Information
Federico Ravaioli, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy.
Antonio Colecchia, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy; Unit of Gastroenterology, Borgo Trento University Hospital, Verona 37100, Italy. antonio.colecchia@aovr.veneto.it.
Elton Dajti, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy.
Giovanni Marasco, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy.
Luigina Vanessa Alemanni, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy.
Mariarosa Tamè, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy.
Francesco Azzaroli, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy.
Stefano Brillanti, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy.
Giuseppe Mazzella, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy.
Davide Festi, Gastroenterology Unit, Sant’Orsola-Malpighi University Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna 40138, Italy.
References
- 1.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 2.Millman AJ, Nelson NP, Vellozzi C. Hepatitis C: Review of the Epidemiology, Clinical Care, and Continued Challenges in the Direct Acting Antiviral Era. Curr Epidemiol Rep. 2017;4:174–185. doi: 10.1007/s40471-017-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 4.Ferenci P, Kozbial K, Mandorfer M, Hofer H. HCV targeting of patients with cirrhosis. J Hepatol. 2015;63:1015–1022. doi: 10.1016/j.jhep.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Babatin MA, Alghamdi AS, Albenmousa A, Alaseeri A, Aljarodi M, Albiladi H, Alsahafi A, Almugharbal M, Alothmani HS, Sanai FM, et al. Efficacy and Safety of Simeprevir or Daclatasvir in Combination With Sofosbuvir for the Treatment of Hepatitis C Genotype 4 Infection. J Clin Gastroenterol. 2018;52:452–457. doi: 10.1097/MCG.0000000000000896. [DOI] [PubMed] [Google Scholar]
- 6.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, Fried MW, Terrault NA, O’Leary JG, Vargas HE, Kuo A, Schiff E, Sulkowski MS, Gilroy R, Watt KD, Brown K, Kwo P, Pungpapong S, Korenblat KM, Muir AJ, Teperman L, Fontana RJ, Denning J, Arterburn S, Dvory-Sobol H, Brandt-Sarif T, Pang PS, McHutchison JG, Reddy KR, Afdhal N; SOLAR-1 Investigators. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Deterding K, Höner Zu Siederdissen C, Port K, Solbach P, Sollik L, Kirschner J, Mix C, Cornberg J, Worzala D, Mix H, et al. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment Pharmacol Ther. 2015;42:889–901. doi: 10.1111/apt.13343. [DOI] [PubMed] [Google Scholar]
- 8.Cammà C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, Marcellin P, Balart L, Alberti A, Craxì A. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–342. doi: 10.1002/hep.20073. [DOI] [PubMed] [Google Scholar]
- 9.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 11.de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280–282. doi: 10.1002/hep.20062. [DOI] [PubMed] [Google Scholar]
- 13.Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902–908. doi: 10.1053/jhep.2003.50133. [DOI] [PubMed] [Google Scholar]
- 14.Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–582. doi: 10.1038/nrgastro.2009.149. [DOI] [PubMed] [Google Scholar]
- 15.Rincon D, Ripoll C, Lo Iacono O, Salcedo M, Catalina MV, Alvarez E, Nuñez O, Matilla AM, Clemente G, Bañares R. Antiviral therapy decreases hepatic venous pressure gradient in patients with chronic hepatitis C and advanced fibrosis. Am J Gastroenterol. 2006;101:2269–2274. doi: 10.1111/j.1572-0241.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 16.Roberts S, Gordon A, McLean C, Pedersen J, Bowden S, Thomson K, Angus P. Effect of sustained viral response on hepatic venous pressure gradient in hepatitis C-related cirrhosis. Clin Gastroenterol Hepatol. 2007;5:932–937. doi: 10.1016/j.cgh.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Reiberger T, Payer BA, Ferlitsch A, Sieghart W, Breitenecker F, Aichelburg MC, Schmied B, Rieger A, Trauner M, Peck-Radosavljevic M; Vienna Hepatic Hemodynamic Lab and Vienna HIV & Liver Study Group. A prospective evaluation of pulmonary, systemic and hepatic haemodynamics in HIV-HCV-coinfected patients before and after antiviral therapy with pegylated interferon and ribavirin. Antivir Ther. 2012;17:1327–1334. doi: 10.3851/IMP2349. [DOI] [PubMed] [Google Scholar]
- 18.Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, Chromy D, Stättermayer AF, Reiberger T, Beinhardt S, et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692–699. doi: 10.1016/j.jhep.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Mauro E, Crespo G, Montironi C, Londoño MC, Hernández-Gea V, Ruiz P, Sastre L, Lombardo J, Mariño Z, Díaz A, et al. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology. 2018;67:1683–1694. doi: 10.1002/hep.29557. [DOI] [PubMed] [Google Scholar]
- 20.Afdhal N, Everson GT, Calleja JL, McCaughan GW, Bosch J, Brainard DM, McHutchison JG, De-Oertel S, An D, Charlton M, et al. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepat. 2017;24:823–831. doi: 10.1111/jvh.12706. [DOI] [PubMed] [Google Scholar]
- 21.Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, Fortea JI, Ibañez L, Ariza X, Baiges A, et al. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology. 2017;153:1273–1283.e1. doi: 10.1053/j.gastro.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399–411. doi: 10.1016/j.jhep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 23.European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646–654. doi: 10.1053/j.gastro.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Ma X, Wang L, Wu H, Feng Y, Han X, Bu H, Zhu Q. Spleen Stiffness Is Superior to Liver Stiffness for Predicting Esophageal Varices in Chronic Liver Disease: A Meta-Analysis. PLoS One. 2016;11:e0165786. doi: 10.1371/journal.pone.0165786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraldes JG, Bureau C, Stefanescu H, Augustin S, Ney M, Blasco H, Procopet B, Bosch J, Genesca J, Berzigotti A; Anticipate Investigators. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The “Anticipate” study. Hepatology. 2016;64:2173–2184. doi: 10.1002/hep.28824. [DOI] [PubMed] [Google Scholar]
- 27.Voutilainen S, Kivisaari R, Lohi J, Jalanko H, Pakarinen MP. A Prospective Comparison of Noninvasive Methods in the Assessment of Liver Fibrosis and Esophageal Varices in Pediatric Chronic Liver Diseases. J Clin Gastroenterol. 2016;50:658–663. doi: 10.1097/MCG.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 28.Chon YE, Jung ES, Park JY, Kim DY, Ahn SH, Han KH, Chon CY, Jung KS, Kim SU. The accuracy of noninvasive methods in predicting the development of hepatocellular carcinoma and hepatic decompensation in patients with chronic hepatitis B. J Clin Gastroenterol. 2012;46:518–525. doi: 10.1097/MCG.0b013e31825079f1. [DOI] [PubMed] [Google Scholar]
- 29.Colecchia A, Ravaioli F, Marasco G, Colli A, Dajti E, Di Biase AR, Bacchi Reggiani ML, Berzigotti A, Pinzani M, Festi D. A combined model based on spleen stiffness measurement and Baveno VI criteria to rule out high-risk varices in advanced chronic liver disease. J Hepatol. 2018;69:308–317. doi: 10.1016/j.jhep.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Knop V, Hoppe D, Welzel T, Vermehren J, Herrmann E, Vermehren A, Friedrich-Rust M, Sarrazin C, Zeuzem S, Welker MW. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J Viral Hepat. 2016;23:994–1002. doi: 10.1111/jvh.12578. [DOI] [PubMed] [Google Scholar]
- 31.Verlinden W, Francque S, Michielsen P, Vanwolleghem T. Successful antiviral treatment of chronic hepatitis C leads to a rapid decline of liver stiffness without an early effect on spleen stiffness. Hepatology. 2016;64:1809–1810. doi: 10.1002/hep.28610. [DOI] [PubMed] [Google Scholar]
- 32.Pons M, Santos B, Simón-Talero M, Ventura-Cots M, Riveiro-Barciela M, Esteban R, Augustin S, Genescà J. Rapid liver and spleen stiffness improvement in compensated advanced chronic liver disease patients treated with oral antivirals. Therap Adv Gastroenterol. 2017;10:619–629. doi: 10.1177/1756283X17715198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llop E, Berzigotti A, Reig M, Erice E, Reverter E, Seijo S, Abraldes JG, Bruix J, Bosch J, García-Pagan JC. Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. J Hepatol. 2012;56:103–108. doi: 10.1016/j.jhep.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 34.Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 35.European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version) Ultraschall Med. 2017;38:e16–e47. doi: 10.1055/s-0043-103952. [DOI] [PubMed] [Google Scholar]
- 37.Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Kim DY. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol. 2010;105:1382–1390. doi: 10.1038/ajg.2009.750. [DOI] [PubMed] [Google Scholar]
- 38.Chayama K, Suzuki F, Karino Y, Kawakami Y, Sato K, Atarashi T, Naganuma A, Watanabe T, Eguchi Y, Yoshiji H, et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 1 hepatitis C virus infection with and without cirrhosis. J Gastroenterol. 2018;53:557–565. doi: 10.1007/s00535-017-1391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sack J, Garcia-Tsao G. Variceal Hemorrhage in a Patient With Hepatitis C Virus Cirrhosis in Whom Liver Synthetic Function had Normalized After Viral Elimination. Hepatology. 2016;63:1733–1735. doi: 10.1002/hep.28470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dailey F, Ayoub WS. Hepatitis C Virus Therapy for Decompensated and Posttransplant Patients. J Clin Gastroenterol. 2017;51:215–222. doi: 10.1097/MCG.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 41.Saadi T, Khoury J. Is There a Relationship Between Treatment With Direct Antiviral Agents for HCV Infection and the Development of Malignancies? J Clin Gastroenterol. 2018;52:353–359. doi: 10.1097/MCG.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:27–38.e4. doi: 10.1016/j.cgh.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Facciorusso A, Del Prete V, Turco A, Buccino RV, Nacchiero MC, Muscatiello N. Long-term liver stiffness assessment in hepatitis C virus patients undergoing antiviral therapy: Results from a 5-year cohort study. J Gastroenterol Hepatol. 2018;33:942–949. doi: 10.1111/jgh.14008. [DOI] [PubMed] [Google Scholar]
- 44.Pinzani M. Liver Fibrosis in the Post-HCV Era. Semin Liver Dis. 2015;35:157–165. doi: 10.1055/s-0035-1550056. [DOI] [PubMed] [Google Scholar]
- 45.Mejias M, Garcia-Pras E, Gallego J, Mendez R, Bosch J, Fernandez M. Relevance of the mTOR signaling pathway in the pathophysiology of splenomegaly in rats with chronic portal hypertension. J Hepatol. 2010;52:529–539. doi: 10.1016/j.jhep.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Castera L. Non-invasive tests for liver fibrosis progression and regression. J Hepatol. 2016;64:232–233. doi: 10.1016/j.jhep.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Buechter M, Manka P, Theysohn JM, Reinboldt M, Canbay A, Kahraman A. Spleen stiffness is positively correlated with HVPG and decreases significantly after TIPS implantation. Dig Liver Dis. 2018;50:54–60. doi: 10.1016/j.dld.2017.09.138. [DOI] [PubMed] [Google Scholar]
- 48.Colecchia A, Colli A, Casazza G, Mandolesi D, Schiumerini R, Reggiani LB, Marasco G, Taddia M, Lisotti A, Mazzella G, et al. Spleen stiffness measurement can predict clinical complications in compensated HCV-related cirrhosis: a prospective study. J Hepatol. 2014;60:1158–1164. doi: 10.1016/j.jhep.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Ravaioli F, Montagnani M, Lisotti A, Festi D, Mazzella G, Azzaroli F. Noninvasive Assessment of Portal Hypertension in Advanced Chronic Liver Disease: An Update. Gastroenterol Res Pract. 2018;2018:4202091. doi: 10.1155/2018/4202091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colecchia A, Ravaioli F, Marasco G, Festi D. Cham: Springer International Publishing; 2018. Spleen Stiffness by Ultrasound Elastography. In: Diagnostic Methods for Cirrhosis and Portal Hypertension; pp. 113–137. [Google Scholar]


