Abstract
We previously showed that PAUF is involved in tumor development and metastases in cervical cancer. This study was conducted to discover novel molecular markers linked with PAUF in cervical cancer using genomic network analysis and to assess their prognostic value in cervical cancer. Three PAUF-related genes were identified using in-silico network-based analysis of the open genome datasets. To assess the expression of these genes and their relationship to the outcome of cervical cancer, immunohistochemical analysis was performed using cervical cancer TMA. The associations of the identified proteins with clinicopathologic characteristics and prognosis were examined. AGR2, BRD7, and POM121 were identified as interconnected with PAUF through in-silico network-based analysis. AGR2 (r = 0.213, p < 0.001) and POM121 (r = 0.135, p = 0.013) protein expression were positively correlated with PAUF. BRD7High and AGR2Low were significantly associated with favorable disease-free survival (DFS) (p = 0.009 and p < 0.001, respectively), and in combination with PAUFHigh, even more significantly favorable DFS observed (p < 0.001 for both). In multivariate analysis, AGR2High (HR = 3.16, p = 0.01) and BRD7High (HR = 0.5, p = 0.025) showed independent prognostic value for DFS. In a random survival forest (RSF) model, the combined clinical and molecular variable model predicted DFS with significantly improved power compared with that of the clinical variable model (C-index of 0.79 vs. 0.75, p < 0.001). In conclusion, AGR2 and BRD7 expression have prognostic significance in cervical cancer and provide opportunities for improved treatment options. Genomic network-based approaches using the cBioPortal may facilitate the discovery of additional biomarkers for the prognosis of cervical cancer and may provide new insights into the biology of cervical carcinogenesis.
Keywords: PAUF, AGR2, BRD7, POM121, prognosis, uterine cervical neoplasms
Introduction
Cervical cancer is the second most commonly diagnosed cancer and is the third leading cause of cancer death among females in less-developed countries (1). Although screening for precancerous lesions and human papilloma virus (HPV) infection and preventive HPV vaccinations are good options to prevent cervical cancer, once invasive cancer occurs, recurrence remains a major problem despite improvements in treatment (2).
To improve survival in cervical cancer patient, proper treatment should be adopted. If tumor behavior could be reliably predicted at the initial diagnosis, tailored treatments could be implemented to improve survival. Although clinical factors such as International Federation of Gynecology and Obstetrics (FIGO) stage, lymph node metastasis, and tumor size may serve as markers for overall prognosis, they have limitations in accurately predicting survival (3). Thus, novel biomarkers including molecular markers are needed for accurate survival estimates. Recent advances in molecular biology and well-curated, publicly available data, such as The Cancer Genome Atlas (TCGA), have made it possible to identify multiple valuable prognostic biomarkers for cancer studies.
We previously reported that pancreatic adenocarcinoma up-regulated factor (PAUF) can be used as a prognostic molecular marker in patients with cervical cancer (4). PAUF expression is upregulated in cancers, particularly in glandular cells or adenocarcinoma, and cytoplasmic expression is independently associated with poor survival. However, the specific receptor involved, its downstream signaling pathways, and the molecular mechanisms of PAUF-dependent gene regulation need to be elucidated. Recently, there have been several network-based studies utilizing interaction information between genes in cancer (5, 6). Considering the complex nature of the interaction between genes, a single genetic abnormality can spread along the links of the complex intracellular network to alter the functions of related genes (7–9). In addition, we hypothesized that multi-gene markers could have more accuracy in prognostication than a single molecular marker (10). To this end, the markers interconnected with PAUF could be identified with in silico analysis using publicly available datasets. The purpose of this study was to find novel proteins connected with PAUF in cervical cancer using in silico analysis and to investigate the clinical significance of those proteins in a well-defined cohort of cervical cancers using immunohistochemistry.
Materials and methods
in silico analysis to select candidate genes connected with PAUF
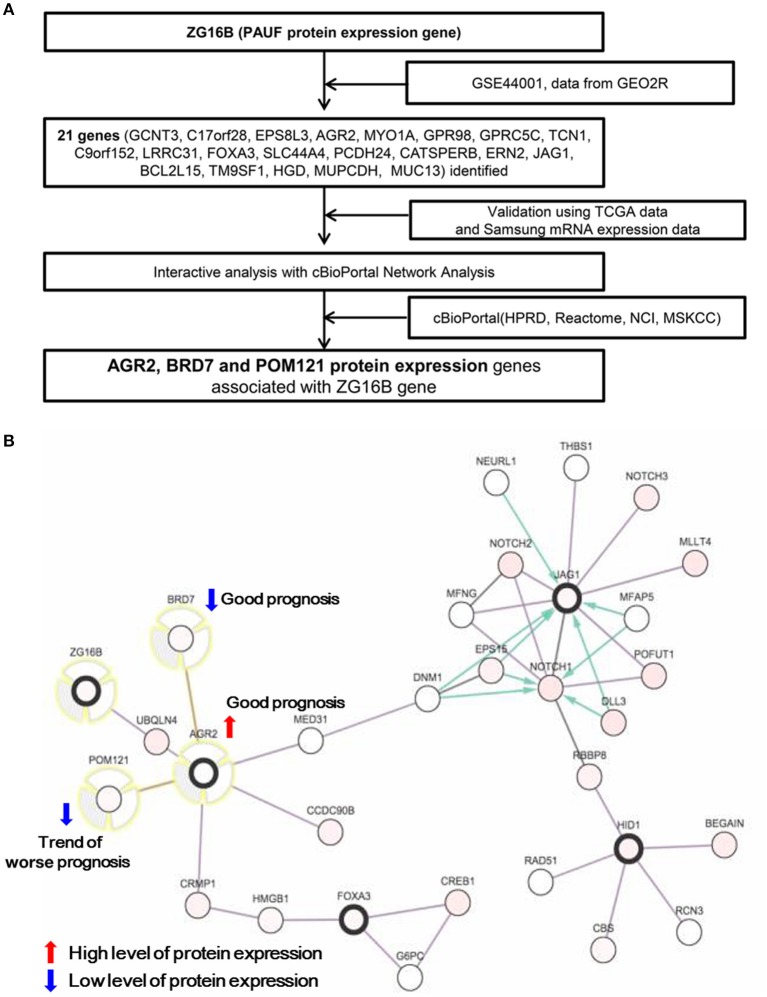
To identify the novel prognostic molecular markers related to PAUF protein expression, data from the Gene Expression Omnibus (GSE44001) and TCGA datasets were analyzed, and a detailed schematic is depicted in Figure 1A. For the details, we downloaded the GES44001 dataset from GEO website (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44001). The obtained data were dichotomized by the median expression of ZG16b (Entrez Gene ID: 124220, ID: ILMN_1753139), and each 150 samples were allocated as high or low PAUF expression (File S1). Subsequently, the differential expression analysis was performed with the “Analyze with GEO2R” in the GEO website with options of Benjamini & Hochberg (False discovery rate), Auto-detect, and Submitter supplied (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44001). The detailed instruction of GEO2R is available in https://www.ncbi.nlm.nih.gov/geo/info/geo2r.html#how_to_use. The genes selected after the above steps are described in Table S1. An interactive analysis was conducted to explore genes related to ZG16B, the PAUF protein expression gene, using mutual exclusivity and network analysis with cBioPortal network analysis. cBioPortal for Cancer Genomics (http://cbioportal.org) is a web resource for exploring, visualizing, and analyzing multi-dimensional cancer genomics data (11). The detailed queries for the analysis are as follows; samples were “cervical squamous cell carcinoma and endocervical adenocarcinoma,” and genomic profile was “mRNA Expression z-Scores (RNA Seq V2 RSEM)”. The access date was November 2014.
Figure 1.
(A) Flow chart identifying the candidate PAUF-associated prognostic markers. (B) Network view of 22 genes using interactive analysis with cBioPortal network analysis (http://cbioportal.org). Among candidate genes, high expression of AGR2 protein and low expression of BRD7 protein are significantly associated with favorable disease-free survival while the low expression of POM121 protein shows a trend of worse prognosis in patients with cervical cancer.
Patients and tumor samples
As previously described, tissue microarrays (TMAs) were constructed using samples from 336 early-stage cervical cancer patients who were primarily treated with radical hysterectomy at the Department of Gynecologic Oncology, Samsung Medical Center, between 2002 and 2009 (12, 13). Tissue samples were collected from patients who had signed an informed consent form and the study was approved by the Institutional Review Board at Samsung Medical Center, Seoul, Korea (IRB No: 2015-07-122). No patients had undergone previous radiation or chemotherapy. All patients underwent radical hysterectomy with pelvic lymphadenectomy. Para aortic lymph node sampling was performed on patients who were diagnosed with pelvic lymph node metastasis in frozen biopsy during operation, or patients with para aortic lymph node metastasis in pre-operative radiologic findings. Patients with rare histology or limited availability of tissue block specimens were excluded from the TMA cohort. Following radical hysterectomy, adjuvant radiotherapy with or without concurrent chemotherapy was given according to the following risk factors: lymph node metastasis, parametrial involvement, positive resection margins, stromal invasion of more than half of the cervix, lymphovascular space invasion (LVSI), or a tumor larger than 4 cm in diameter. During follow-ups conducted every 3 months for 2 years then every 6 months for the next 3 years, Pap smears, tumor markers, and CT scans were performed every 3–12 months. Patients who relapsed within 3 years after adjuvant chemoradiation were classified as resistant to chemoradiation. Disease-free survival (DFS) was assessed from the date of surgery to the date of recurrence or the date of the last follow-up visit. Overall survival (OS) was measured from the date of surgery to the time of death or the date of last contact (for living patients). The data for patients who had not experienced an event as of the date of the final observation were censored.
Immunohistochemistry
Immunohistochemistry was performed using cervical cancer TMAs as previously described (12, 13). TMA sections were cut at 4 μm thickness followed by deparaffinization in xylene and dehydration with graded ethanols. The deparaffinized and rehydrated slides were treated with heat-induced antigen retrieval buffer (Dako, Carpinteria, CA) at pH 6.0 (for BRD7 and POM121) or pH 9.0 (for AGR2) for 20 min. The endogenous peroxidase activity was blocked with 3% H2O2 for 10 min. The sections were incubated with anti-AGR2 rabbit polyclonal antibody (Novus Biologicals, NBP2-27393, CO, USA) at a 1:250 dilution, anti-BRD7 rabbit polyclonal antibody (Novus Biologicals, NBP1-28727, CO, USA) at a 1:500 dilution, or anti-POM121 rabbit polyclonal antibody (Novus Biologicals) at a 1:500 dilution for 120 min at room temperature. The antigen-antibody reaction was detected with EnVision+ Dual Link System-HRP (Dako) and DAB+ (3,3′-Diaminobenzidine; Dako). Negative controls were performed by omitting the primary antibody and rabbit immunoglobulin (IgG).
Quantitative evaluation of immunohistochemical staining
Immunohistochemical staining was quantitatively evaluated using computer-assisted image analysis software (Visiopharm, Hoersholm, Denmark) as described previously (14). In brief, the slides were scanned using a whole-slide scanner (NanoZoomer 2.0, Hamamatsu Photonics, Hamamatsu City, Japan) and imported into Visiopharm software (for Windows 7, version 4.5.1.324) using the TMA workflow. Staining intensity was categorized as 0, 1+, 2+, or 3+ according to the distribution pattern across cores. A brown staining intensity (0-negative, 1-weak, 2-moderate, or 3-strong) was obtained using the predefined algorithm and optimized settings. The overall immunohistochemical scores (histoscore) for AGR2, BRD7, and POM121 were expressed as the percentage of positive cells multiplied by their staining intensity (possible range, 0–300). For the survival analysis and hierarchical clustering, the expression values were dichotomized (High vs. Low, described in superscript) using cut-off values affording the most discriminative power (histoscore of 39 for AGR2, 46 for BRD7, and 204 for POM121). PAUF protein expression was evaluated as previously described (4) to compare with that of the three proteins identified.
Statistical analysis
Statistical analysis was performed using R software, version 3.1.3 (R Foundation, Vienna, Austria; http://www.R-project.org). The expression levels of the proteins according to the clinicopathological characteristics were analyzed using Student's t-test or Mann–Whitney U-test. Analysis using Spearman's rho coefficient was used to assess the correlations between the expressions of the proteins. Survival distributions were estimated using the Kaplan–Meier method, and the relationship between survival and each parameter was analyzed with the log-rank test. A Cox proportional hazards model was created to identify independent predictors of survival. Statistical significance was defined as p < 0.05. Hierarchical clustering was performed to determine the clustering of samples according to PAUF and PAUF-related protein expression. In addition, the random survival forest (RSF) method was modified to include both clinical and molecular features to assess the predictive power of integrating the molecular data with clinical variables (15). A detailed description of the analysis has been previously described (12).
Results
Candidate genes connected with PAUF (ZG16B)
Analysis of the GSE44001 dataset showed that 21 genes were differentially expressed according to ZG16B gene expression (dichotomized using the median cutoff). These identified genes were validated in the TCGA cervix dataset (Table S1). The cBioPortal network view of these genes showed that anterior gradient protein 2 (AGR2), bromodomain-containing protein 7 (BRD7), and nuclear envelope pore membrane protein (POM121) are interconnected with the ZG16B gene (Figure 1B). These three proteins were selected to evaluate their prognostic value in cervical carcinoma.
Clinicopathological characteristics of patients
The clinicopathological characteristics are summarized in Table 1. The mean age of the patients was 48.9 ± 11.2 years. Of the 336 patients with cervical cancer, 291 (86.6%) were stage IIA or less, and 45 (13.4%) were stage IB2 or IIB. In Korea, many surgeons perform radical hysterectomy on patients who were suspected of having a stage IIB diagnosis in pre-operative radiologic findings or physical examination. In this study, we intended to include possible samples which were obtained from radical hysterectomy even if the sample sizes were small. Of the patients, 256 (76.2%) had squamous cell carcinoma and 80 (23.8%) had adenocarcinoma. Lymph node metastasis was found in 80 (23.8%) patients and parametrial involvement in 31 (9.2%) patients. One hundred and sixty (47.6%) patients were treated with adjuvant radiation with or without concurrent chemotherapy due to the presence of risk factors. Among them, 20 patients were radiation resistant.
Table 1.
The correlation between the PAUF associated proteins expressions with clinicopathologic characteristics of cervical cancer.
| No. | AGR2 | BRD7 | POM121 | ||||
|---|---|---|---|---|---|---|---|
| Mean Histoscore [95% CI] | p-value | Mean Histoscore [95% CI] | p-value | Mean Histoscore [95% CI] | p-value | ||
| GROUP | |||||||
| Normal | 81 | 92 [85–99] | 0.724 | 266 [256–275] | <0.001* | 228 [209–247] | <0.001* |
| Cancer | 336 | 90 [81–99] | 63 [59–68] | 144 [134–153] | |||
| AGE | |||||||
| <50 years | 203 | 94 [83–105] | 0.274 | 61 [55–67] | 0.227 | 143 [131–155] | 0.902 |
| >50 years | 133 | 84 [70–98] | 67 [59–74] | 145 [128–161] | |||
| FIGO STAGE | |||||||
| ≤IIA | 291 | 88 [79–98] | 0.346 | 64 [59–68] | 0.749 | 141 [131–152] | 0.208 |
| IB2 or IIB | 45 | 101 [76–126] | 61 [50–73] | 158 [134–183] | |||
| CELL TYPE | |||||||
| SCC | 256 | 70 [62–79] | <0.001* | 67 [62–72] | 0.004* | 148 [137–159] | 0.123 |
| AC/ASC | 80 | 154 [136–172] | 52 [44–60] | 130 [111–150] | |||
| TUMOR SIZE | |||||||
| <4 cm | 256 | 93 [83–103] | 0.267 | 64 [59–69] | 0.64 | 143 [132–154] | 0.764 |
| >4 cm | 80 | 81 [64–99] | 61 [52–71] | 146 [127–165] | |||
| LVSI | |||||||
| Negative | 202 | 99 [88–111] | 0.007* | 63 [57–69] | 0.792 | 149 [136–162] | 0.192 |
| Positive | 133 | 76 [63–89] | 64 [57–71] | 136 [122–150] | |||
| DEPTH OF INVASION | |||||||
| <50% | 108 | 103 [87–119] | 0.042* | 57 [48–65] | 0.047* | 140 [123–158] | 0.645 |
| >50% | 228 | 84 [73–94] | 66 [61–72] | 145 [134–157] | |||
| LN METASTASIS | |||||||
| Negative | 256 | 92 [82–102] | 0.438 | 63 [57–68] | 0.664 | 144 [133–155] | 0.901 |
| Positive | 80 | 84 [67–101] | 65 [56–73] | 143 [123–162] | |||
| PM INVOLVEMENT | |||||||
| Negative | 305 | 92 [82–101] | 0.218 | 62 [57–67] | 0.082 | 145 [135–155] | 0.291 |
| Positive | 31 | 74 [47–101] | 75 [61–89] | 130 [102–157] | |||
| RESECTION MARGIN | |||||||
| Negative | 323 | 91 [82–100] | 0.446 | 63 [59–68] | 0.765 | 144 [134–154] | 0.957 |
| Positive | 13 | 72 [19–124] | 67 [41–92] | 142 [82–203] | |||
| RT RESISTANCE | |||||||
| Resistant | 20 | 102 [71–134] | 0.212 | 45 [28–62] | 0.053 | 89 [62–116] | 0.001* |
| Sensitive | 113 | 81 [66–95] | 63 [56–70] | 147 [130–164] | |||
| PRIMARY TREATMENT | |||||||
| OP only | 171 | 97 [84–110] | 0.266 | 65 [59–72] | 0.704 | 143 [129–157] | 0.989 |
| OP+ RT | 70 | 75 [58–92] | 63 [52–74] | 145 [123–167] | |||
| OP + CCRT | 90 | 88 [72–104] | 60 [52–67] | 144 [127–162] | |||
| Neoadjuvant | 5 | 87 [35–139] | 53 [-9–114] | 131 [-7–268] | |||
FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous cell carcinoma; LVSI, lymphovascular space invasion; LN, lymph node; PM, parametrium; OP, operation; RT, radiotherapy; CCRT, concurrent chemoradiation.
Significant at the level of p < 0.05.
AGR2, BRD7, and POM121 protein expression
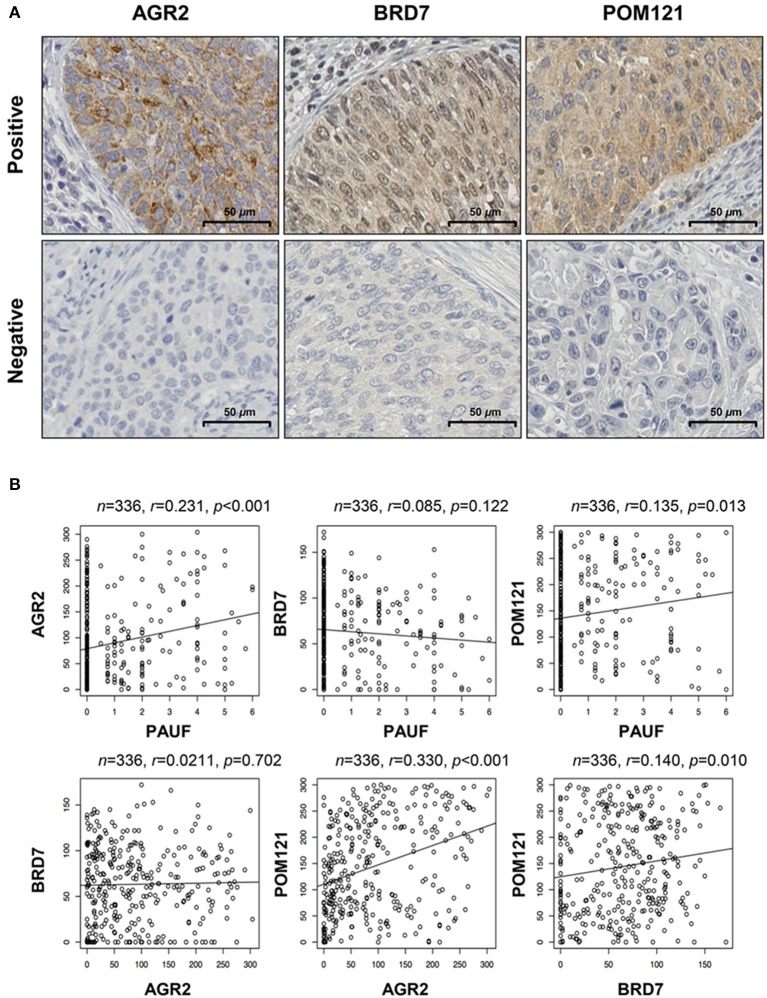
AGR2 and POM121 protein expression was mainly observed in the cytoplasm, and BRD7 expression was observed in both the cytoplasm and nucleus. Representative examples of positive and negative staining are shown in Figure 2A. Subsequently, we examined the correlations between PAUF protein expression and these three proteins using subgroup analysis. Although there were no statistically significant differences, AGR2 and POM121 protein expression showed the tendency of a positive association with PAUF protein expression (r = 0.213; p < 0.001 and r = 0.135; p = 0.013, respectively; Figure 2B). Moreover, AGR2 protein expression was positively associated with POM121 protein expression as shown in Figure 2B (r = 0.330; p < 0.001). In the subgroup analysis, PAUF and POM121 protein expression was positively correlated with AGR2 protein expression (r = 0.305; p = 0.001 and r = 0.456; p < 0.001, respectively) in the radiation-sensitive group (n = 113; Figure S1A). Furthermore, in the radiation-resistant group (n = 20), BRD7 protein expression was positively associated with POM121 protein expression (r = 0.55; p = 0.012) (Figure S1B). These findings are consistent with the TCGA dataset and suggest that these proteins are correlated in cervical cancer, especially within subgroups according to radiation therapy.
Figure 2.
Expression of the PAUF-associated proteins AGR2, BRD7, and POM121 in patients with cervical cancer. (A) Representative examples of positive and negative expression of the AGR2, BRD7, and POM121 proteins. The scale bar represents 50 μm. (B) Correlations between expression of PAUF and PAUF-related proteins in cervical cancer tissues. AGR2 protein expression was positively correlated with POM121 expression (r = 0.33, p < 0.001).
When compared with normal cervix tissues, a significant decrease in BRD7 (mean histoscore 63 vs. 266, p < 0.001) and POM121 (mean histoscore 144 vs. 228, p < 0.001) expression was detected in cancer tissues, but the expression of AGR2 showed no significant difference (Table 1). The expression of AGR2 and BRD7 was cell type-dependent; AGR2 was more highly expressed in adeno/adenosquamous carcinoma, and BRD 7 expression was more prominent in squamous cell carcinoma (p < 0.001 and p = 0.004, respectively; Table 1). These results suggest that AGR2 and BRD7 potentially have different roles in cervical cancer according to cell type. In addition, the higher expression of AGR2 was negatively correlated with LVSI and the depth of invasion (p = 0.007 and p = 0.042, respectively), and POM121 expression was decreased (p = 0.001) in radiation-resistant cervical cancer.
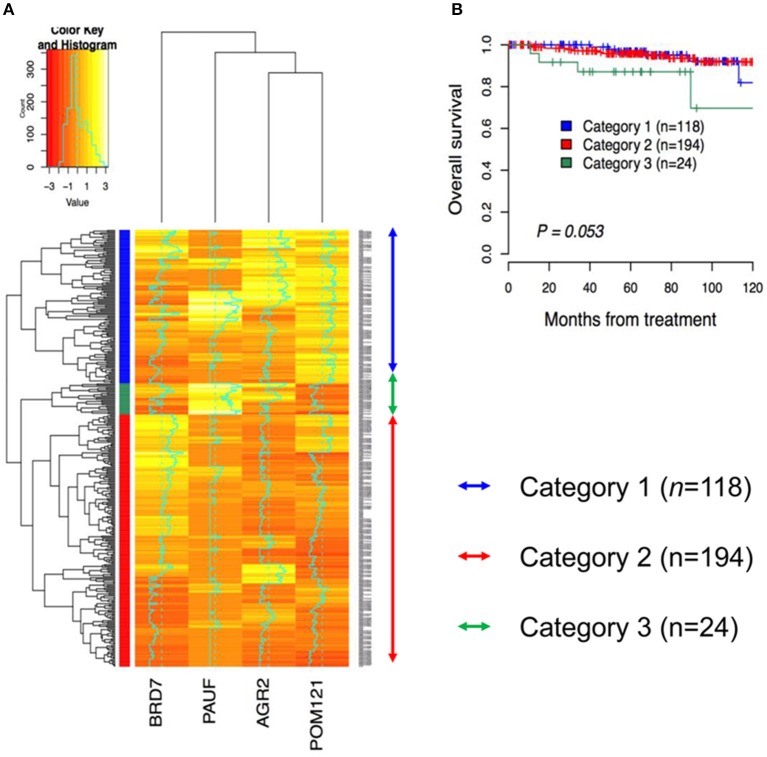
Unsupervised hierarchical clustering of PAUF-associated markers
To examine whether patients can be grouped according to protein expression, a total of 336 cervical cancer cases were analyzed using hierarchical clustering with histoscore. As shown in Figure 3A, the patients were divided to three groups. Category 1 (n = 118) consisted exclusively of AGR2High and POM 121High expression. Category 2 (n = 194) consisted exclusively of AGR2Low and PAUFLow expression. Category 3 (n = 24) consisted of PAUFHigh and POM 121Low expression. There were significant differences between categories 1, 2, and 3 in terms of cell type, depth of invasion, and radiation therapy resistance (p < 0.001, p = 0.008, and p < 0.001, respectively; Table 2). Notably, category 3 was associated with adenocarcinoma cell type and the radiation-resistant phenotype and showed a poor overall survival (OS) tendency (p = 0.053; Figure 3B).
Figure 3.
Hierarchical clustering analysis for immunohistochemical expression of PAUF-associated proteins in cervical cancer patients. (A) Three cluster groups were identified in the present study. Category 1 (n = 118, blue arrow) consists of relatively high AGR2 and POM121 expression. Category 2 (n = 194, red arrow) consists of relatively low AGR2 and PAUF expression. Category 3 (n = 24, green arrow) consists of relatively high PAUF and low POM121 expression. (B) Kaplan–Meier plot for overall survival according to group by hierarchical clustering. A tendency toward decreased overall survival was observed in Category 3 than in the other groups (log-rank test, p = 0.053).
Table 2.
Clinicopathological characteristics between three groups defined with hierarchical cluster analysis.
| Category 1 n (%) | Category 2 n (%) | Category 3 n (%) | p-value | |
|---|---|---|---|---|
| AGE | ||||
| <50 years | 71 (35) | 118 (58) | 14 (7) | 0.970 |
| >50 years | 47 (35) | 76 (57) | 10 (8) | |
| FIGO STAGE | ||||
| ≤ IIA | 101 (35) | 167 (57) | 23 (8) | 0.380 |
| IB2 or IIB | 17 (38) | 27 (60) | 1 (2) | |
| CELL TYPE | ||||
| SCC | 83 (32) | 162 (63) | 11 (5) | <0.001* |
| AD/ASC | 35 (44) | 32 (40) | 13 (16) | |
| TUMOR SIZE | ||||
| ≤ 4 cm | 88 (34) | 147 (57) | 21 (8) | 0.390 |
| >4 cm | 30 (37) | 47 (59) | 3 (4) | |
| LVSI | ||||
| Negative | 78 (39) | 109 (54) | 15 (7) | 0.240 |
| Positive | 40 (30) | 84 (63) | 9 (7) | |
| DEPTH OF INVASION | ||||
| <1 cm | 61 (39) | 79 (50) | 17 (11) | 0.008* |
| >1 cm | 57 (32) | 115 (64) | 7 (4) | |
| LN METASTASIS | ||||
| Negative | 91 (35) | 145 (57) | 20 (8) | 0.620 |
| Positive | 27 (34) | 49 (61) | 4 (5) | |
| PM INVOLVEMENT | ||||
| Negative | 111 (36) | 171 (56) | 23 (8) | 0.140 |
| Positive | 7 (23) | 23 (74) | 1 (3) | |
| RT RESISTANT | ||||
| Resistant | 2 (10) | 13 (65) | 5 (25) | <0.001* |
| Sensitive | 41 (36) | 69 (61) | 3 (3) | |
| PRIMARY TREATMENT | ||||
| OP only | 60 (35) | 96 (56) | 15 (9) | 0.510 |
| OP + RT | 24 (34) | 45 (64) | 1 (2) | |
| OP + CCRT | 32 (36) | 50 (55) | 8 (9) | |
| Neoadjuvant | 2 (40) | 3 (60) | 0 | |
FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous cell carcinoma; LVSI, lymphovascular space invasion; LN, lymph node; PM, parametrium; OP, operation; RT, radiotherapy; CCRT, concurrent chemoradiation.
Significant at the level of p < 0.05.
Prognostic significance of AGR2, BRD7, and POM121
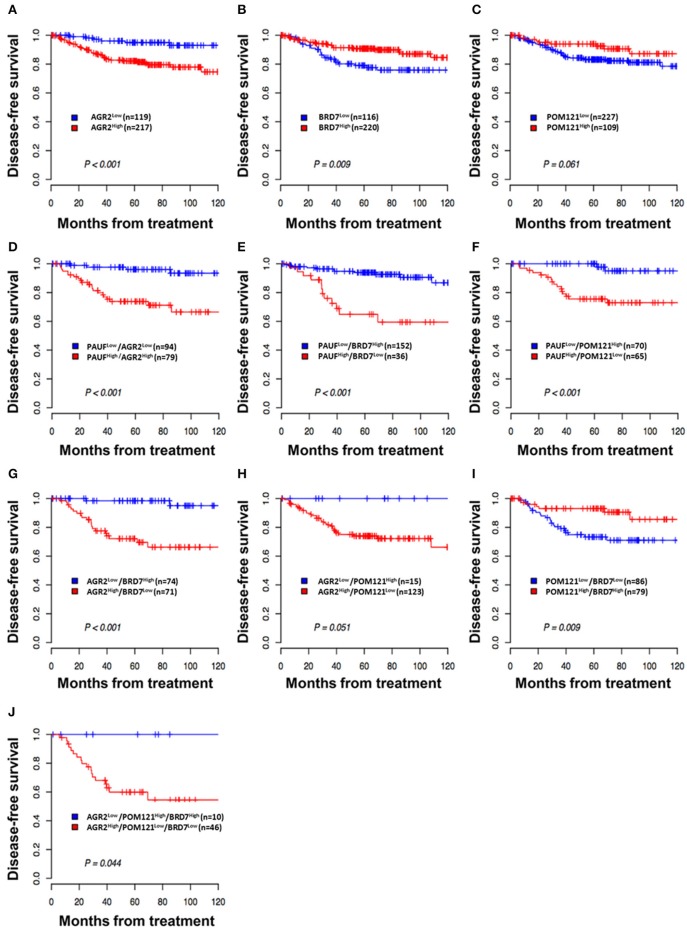
With a median follow-up period of 66 months (range 1–143), the 5-year disease-free survival (DFS) and OS rates for the whole group were 87% (95% CI 83–91) and 96% (95% CI 93–98), respectively. BRD7High and AGR2Low were significantly associated with favorable DFS (p = 0.009 and p < 0.001, respectively; Figures 4A,B). The 5-year DFS was 94.9% (95% CI, 90.7–99.4) for patients with AGR2Low and 90.2% (95% CI, 86.2–94.4) and 94.0% (95% CI, 89.4–98.8) for patients with BRD7High and POM121High (Figure 4C), respectively, compared with 82.1% (95% CI, 76.9–87.7), 78.9 (95% CI, 71.1–87.7), and 83.2% (95% CI, 78.1–88.5) for patients with the opposite protein-expression pattern. Furthermore, we compared DFS using the combined expression of all three markers and PAUF according to a previous study (4). The combination of PAUFLow with AGR2Low, BRD7High, and POM121High had a significantly more favorable DFS than the opposite protein-expression profile (p < 0.001 for all; Figures 4D–F).
Figure 4.
Kaplan–Meier plot of disease-free survival according to expression of PAUF and PAUF-associated proteins. (A,B) Patients with high AGR2 expression (AGR2High) and low BRD7 expression (BRD7Low) showed worse disease-free survival (log-rank test, p < 0.001 and p = 0.009, respectively) than patients with low AGR2 expression (AGR2Low) and high BRD7 expression (BRD7High). (C) Patients with low POM121 expression (POM121Low) showed a trend of worse disease-free survival. (D–F) Patients with combined PAUFHigh/AGR2High, PAUFHigh/BRD7Low, or PAUFHigh/POM121Low expression showed shorter disease-free survival (log-rank test, p < 0.001, p < 0.001, and p < 0.001, respectively) than patients with the opposite combined expression profile. (G–J) Patients with combined AGR2High/BRD7Low, BRD7Low/POM121Low, or AGR2High/BRD7Low/POM121Low expression showed shorter disease-free survival (log-rank test, p < 0.001, p = 0.009, and p = 0.044, respectively) than patients with combined AGR2Low/BRD7High, BRD7High/POM121High, or AGR2Low/BRD7High/POM121High expression.
In addition, the combination of AGR2, BRD7, and POM121 expression had also significant prognostic significance. Patients with combined AGR2High/BRD7Low, BRD7Low/POM121Low, or AGR2High/BRD7Low/POM121Low expression showed shorter disease-free survival (log-rank test, p < 0.001, p = 0.009, and p = 0.044, respectively) than patients with combined AGR2Low/BRD7High, BRD7High/POM121High, or AGR2Low/BRD7High/POM121High expression (Figures 4G–J).
Similarly, the 5-year OS was 98% (95% CI, 95.2–100) for patients with AGR2Low and 96.4% (95% CI, 93.9–99.1) and 97.0% (95% CI, 92.8–100) for patients with BRD7High and POM121High, respectively, compared with 94% (95% CI, 90.7–97.6), 93.3% (95% CI, 88.3–98.7), and 93.9% (95% CI, 90.5–97.3) for patients with the opposite protein-expression profile (Figure S2). BRD7High and AGR2Low were significantly associated with favorable OS (p = 0.047 and p = 0.008, respectively; Figures S2A,B).
Using the Cox proportional hazards model, the expression of AGR2 and BRD7 remained independent prognostic factors for DFS (HR = 3.16; 95% CI, 1.32–7.58; p = 0.01 and HR = 0.50; 95% CI, 0.28–0.92; p = 0.025, respectively) in the multivariate analysis (Table 3). Furthermore, combined PAUFHigh and AGR2High showed independent prognostic value for both DFS and OS in the multivariate analysis (HR13.2; 95% CI, 3.72–46.89; p < 0.001 and HR43.07; 95% CI, 3.72–499.18; p = 0.003, respectively; Table S2).
Table 3.
Univariate and multivariate analyses of disease free survival according to prognostic variables in cervical cancer patients (n = 336).
| Risk factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard ratio [95%CI] | p-value | Hazard ratio [95%CI] | p-value | |
| FIGO stage (>IIB) | 2.44 [1.24–4.82] | 0.010* | 2.29 [0.72–7.25] | 0.160 |
| Cell type (Adeno vs. SCC) | 2.88 [1.62–5.14] | <0.001* | 2.18 [0.75–6.28] | 0.151 |
| LN metastasis | 4.13 [2.31–7.38] | <0.001* | 4.52 [1.64–12.46] | 0.004* |
| Tumor size (>4 cm) | 1.7 [0.92–3.15] | 0.092 | 0.65 [0.18–2.32] | 0.508 |
| PM involvement | 2.24 [1.05–4.81] | 0.038* | 2.87 [0.85–9.66] | 0.088 |
| AGR2High | 4.02 [1.7–9.49] | 0.002* | 3.16 [1.32–7.58] | 0.010* |
| BRD7High | 0.47 [0.26–0.84] | 0.010* | 0.5 [0.28–0.92] | 0.025* |
| POM121High | 0.5 [0.24–1.05] | 0.066 | 0.52 [0.25–1.08] | 0.078 |
| PAUFHigh/AGR2High | 7.17 [2.45–20.94] | <0.001* | 13.2 [3.72–46.89] | <0.001* |
| PAUFHigh/BRD7Low | 5.47 [2.4–12.48] | <0.001* | 4.16 [1.57–11.02] | 0.004* |
| PAUFHigh/POM121Low | 9.21 [2.12–40.09] | 0.003* | 15.46 [2.68–89.08] | 0.002* |
| AGR2High/BRD7Low | 11.88 [2.77–50.95] | 0.001* | 8.15 [1.79–37.16] | 0.007* |
| AGR2High/POM121Low | 79227176.83 [0–Inf] | 0.997 | 47108676.12 [0–Inf] | 0.996 |
| POM121High/BRD7High | 0.34 [0.15–0.8] | 0.013* | 0.38 [0.16–0.91] | 0.030* |
| AGR2High/POM121Low/BRD7Low | 251011694.96 [0–Inf] | 0.998 | 349083474.2 [0–Inf] | 0.998 |
FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; AC, adenocarcinoma; LN, lymph node; PM, parametrium.
Significant at the level of p < 0.05.
Improved prognostic power of combined molecular and clinical model
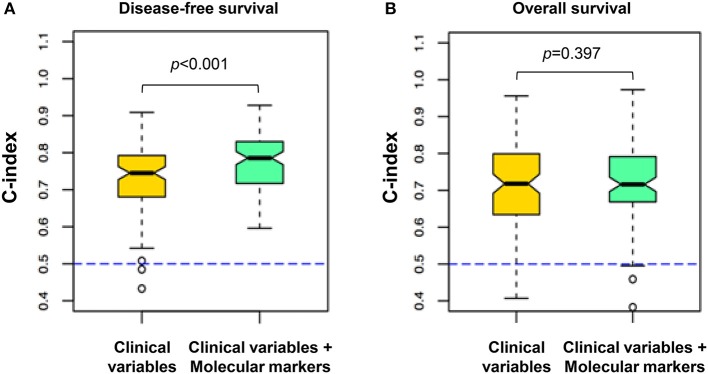
To examine whether a network-based model using expression of PAUF and related genes provided enhanced prognostic power compared to clinical prognostic factors (i.e., FIGO stage, lymph node metastasis, lymphovascular invasion, stromal depth of invasion, parametrial involvement, and resection margin), we compared the C-index for predicting recurrence of the clinical model and a combined clinical/network-based molecular model. In molecular model, PAUF and the related genes (AGR2, BRD7, and POM121) were included. Notably, the combined clinical/molecular model predicted recurrence (median C-index, 0.79; range, 0.55–0.93) with significantly higher power compared to the clinical variable-only model (median C-index, 0.74; range, 0.35–0.90; p < 0.001; Figure 5A), whereas, there was no difference in OS [C index of 0.71 (0.31–0.96) for combined model vs. 0.71 (0.33–0.94) for clinical model, p = 0.397; Figure 5B).
Figure 5.
Comparison of survival predictive power between the clinical variables and the combined clinical and molecular data. The plots indicate the distribution of C-indexes from 100 rounds of cross-validation. The yellow box highlights the model built from the clinical variables, and the green box highlights the models integrating the molecular variables of PAUF-related proteins and the clinical variables. (A) Using the combined clinical and molecular markers (median C-index 0.79) to predict recurrence yielded improved predictive power compared with the clinical variable model (median C-index 0.74; p < 0.001). (B) The combined clinical/molecular-variable model showed similar performance compared with the clinical variable model in predicting death (p = 0.397). The dashed line indicates the C-index equivalent to a random guess (C-index = 0.5). A C-index of 1 indicates perfect prediction.
Discussion
In the present study, we identified novel molecular markers interconnected with PAUF using genomic network-based analysis and evaluated their relationships and prognostic potential in a large cohort of cervical cancer patients. The identified proteins (AGR2, BRD7, and POM121) were differentially expressed according to cell type, clinical prognostic factors, and resistance to radiation therapy in cervical cancer patients. Furthermore, we demonstrated for the first time that low expression of AGR2, high expression of BRD7 or POM121 combined with low expression of PAUF predict delayed recurrence in cervical cancer patients. Based on these results, we suggest that a combined clinical/molecular variable model may have more prognostic potential than a model using clinical variables alone to predict disease recurrence. Based on the above findings, results from in silico analysis of publicly available genomic data were confirmed to show clinical significance with regard to protein levels of the new patient cohort in this study (11, 16).
The pro-oncogenic anterior gradient 2 (AGR2) protein stimulates cancer cell initiation, proliferation, invasion, and metastasis (17), and previous studies have shown that AGR2 is involved in p53 regulation and cell survival control (18). Additionally, elevated AGR2 protein expression is observed in numerous cancer types including gastric adenocarcinoma (19), breast (20), non-small cell lung (21), ovarian (22, 23), esophageal, prostate, and pancreatic cancer (24), and overexpression of the AGR2 protein is correlated with poor prognosis in these carcinomas. Monoclonal antibodies against AGR2 markedly reduce tumor growth and metastasis in pancreatic ductal adenocarcinoma, but this reduction has only been observed in preclinical mouse models (25). In gynecological cancer, overexpression of the AGR2 protein was reported in high-grade ovarian carcinoma, which is related with metastasis and poor prognosis (22, 23). Furthermore, AGR2 was associated with chemotherapy resistance in an analysis of survival in high-grade serous ovarian carcinoma (22). Consistent with previous studies, AGR2 expression was positively correlated with adenocarcinoma and poor prognosis in cervical cancer in this study.
It is well known that cervical cancers result from a persistent infection with high-risk types of HPV (26). HPV infects the basal cells of the squamous epithelium, causing entry of its DNA into the nucleus of the host cells. The HPV DNA replicates as the basal cells differentiate, and induce a dramatic increase in the expression of the two HPV oncoproteins (E6 and E7). Herfs et al. have suggested that cuboidal cells at the cervical squamocolumnar junction are the origin of cervical cancer and its precursors (27). They also identified the squamocolumnar junction cell-specific biomarkers keratin 7, AGR2, CD63, matrix metalloproteinase 7, and guanine deaminase and documented their expression in HPV-infected cell lines, high-grade cervical intraepithelial neoplasms, and cervical carcinoma (27). Recently, Morbini et al. showed that only AGR2 and keratin 7 were associated with active HPV infection in oropharyngeal carcinomas (28). Taken together, these data support AGR2 involvement in cervical cancer tumorigenesis by promoting the proliferation of human cervical cancer cells.
Bromodomain-containing protein 7 (BRD7), also known as celtix-1, is a subunit of the SWI/SNF complex specific for polybromo-associated BRG1-associated factor (PBAF), which was first identified as a tumor suppressor in nasopharyngeal carcinoma cells in 2000 (29). Published studies show that BRD7 plays an important role as a transcription co-factor in the expression of the TP53 gene (30) and interferes with the PI3K signaling pathways (31). Down-regulation of BRD7 expression has been observed in several malignancies and is correlated with poor prognosis of various cancer types, including breast (32), colorectal (33), gastric (29), and prostate cancer (34), as well as hepatocellular carcinoma (35), and osteosarcoma (36). Endometrial cancer (37) and ovarian cancer exhibit low expression of BRD7, which is correlated with down-regulated beta-catenin accumulation (38). In ovarian cancer, BRD7 expression is decreased in high-grade serous carcinoma relative to normal or low-grade carcinoma (38). In our study, high expression of BRD7 was associated with improved DFS in cervical cancer patients. Cox regression analysis confirmed that BRD7 and its combination with PAUF and other PAUF-related proteins is an important prognostic indicator in cervical cancer.
The nuclear envelope pore membrane protein (POM121) gene is one of the PAX5 fusion genes and is related to the development of B-ALL leukemia through gene rearrangement (39). POM121 is one of the integral membrane components of the nuclear pore complex (NPC) in vertebrate cells, which maintains NPC structure (40). Decreased POM121 expression significantly reduces assembled NPCs on the nuclear envelope and induces clustering of NPCs, which regulate molecular transport through the nuclear envelope (often referred to as the nuclear permeability barrier) (41). In our cervical cancer samples, low expression of POM121 alone was not correlated with decreased DFS. However, in combination with high expression of PAUF or low expression of BRD7, low expression of POM121 predicted significantly reduced DFS when compared with BRD7 or PAUF alone. BRD7 was found predominantly in the nucleus and the distribution ratio of BRD7 in nucleus/cytoplasm has effect on its function of transcriptional regulation (42). Also, in our previous study, PAUF had prognostic value when it was located in cytoplasm (4). Accordingly, we deduced that POM121 may have function of transporting BRD7 and PAUF proteins between nucleus and cytoplasm. However, further research is needed to identify the accurate mechanism of POM121 in cervix carcinoma tumorigenesis.
In this study, we performed integrative analysis using the cBioPortal to identify molecules in the PAUF pathway and found that AGR2, BRD7, and POM121 are possible important molecules of the oncogenic function of PAUF. Furthermore, we demonstrated that AGR2, BRD7, and POM121 protein expression had prognostic value when combined with PAUF expression using an analysis of a large cohort of cervical cancer patients. These findings suggest the usefulness of network-based prognostic markers because the prognostic accuracy of a clinical variable model was enhanced by the addition of molecular markers (i.e., AGR2, BRD7, and POM121). With these efforts to find novel molecular makers in cervical carcinoma prognostication, patients with high risk of recurrence might have opportunities to improve their treatment outcomes with closer follow up.
Author contributions
JK, CC, J-YC, and SH conceived of the study and devised the experimental design. CC, J-YC, and JK performed the experiments. CC, J-YC, T-JK, J-WL, B-GK, D-SB, and SH performed data analysis for the experiments and the clinical records. CC, JK, and J-YC drafted the final version of the manuscript and figure legends. SH revised the figures, added critical content to the discussion, and was responsible for revising all submitted portions of the manuscript. All of the authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This research was supported by grants from the Intramural Research Program of the National Institutes of Health (NIH), the National Cancer Institute (NCI), the Center for Cancer Research, and the Laboratory of Pathology at Samsung Medical Center (OTC1180321).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00465/full#supplementary-material
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Park JY, Ngan HY, Park W, Cao Z, Wu X, Ju W, et al. Asian Society of Gynecologic Oncology International Workshop 2014. J Gynecol Oncol. (2015) 26:68–74. 10.3802/jgo.2015.26.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. Cervical cancer. J Natl Compr Canc Netw. (2013) 11:320–43. 10.6004/jnccn.2013.0043 [DOI] [PubMed] [Google Scholar]
- 4.Choi CH, Chung JY, Park HS, Jun M, Lee YY, Kim BG, et al. Pancreatic adenocarcinoma up-regulated factor expression is associated with disease-specific survival in cervical cancer patients. Hum Pathol. (2015) 46:884–93. 10.1016/j.humpath.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofree M, Shen JP, Carter H, Gross A, Ideker T. Network-based stratification of tumor mutations. Nat Methods (2013) 10:1108–15. 10.1038/nmeth.2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ideker T, Krogan NJ. Differential network biology. Mol Syst Biol. (2012) 8:565. 10.1038/msb.2011.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. (2012) 2:881–98. 10.1158/2159-8290.CD-12-0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 9.Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. (2011) 12:56–68. 10.1038/nrg2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Lenferink AE, Deng Y, Collins C, Cui Q, Purisima EO, et al. Identification of high-quality cancer prognostic markers and metastasis network modules. Nat Commun. (2010) 1:34. 10.1038/ncomms1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal (2013) 6:l1. 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi CH, Chung JY, Chung EJ, Sears JD, Lee JW, Bae DS, et al. Prognostic significance of annexin A2 and annexin A4 expression in patients with cervical cancer. BMC Cancer (2016) 16:448. 10.1186/s12885-016-2459-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi CH, Chung JY, Kim JH, Kim BG, Hewitt SM. Expression of fibroblast growth factor receptor family members is associated with prognosis in early stage cervical cancer patients. J Transl Med. (2016) 14:124. 10.1186/s12967-016-0874-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi CH, Chung JY, Cho H, Kitano H, Chang E, Ylaya K, et al. Prognostic significance of AMP-dependent kinase alpha expression in cervical cancer. Pathobiology (2015) 82:203–11. 10.1159/000434726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishwaran H, Kogalur UB. Consistency of random survival forests. Stat Probab Lett. (2010) 80:1056–64. 10.1016/j.spl.2010.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. (2012) 2:401–4. 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brychtova V, Vojtesek B, Hrstka R. Anterior gradient 2: a novel player in tumor cell biology. Cancer Lett. (2011) 304:1–7. 10.1016/j.canlet.2010.12.023 [DOI] [PubMed] [Google Scholar]
- 18.Pohler E, Craig AL, Cotton J, Lawrie L, Dillon JF, Ross P, et al. The Barrett's antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol Cell Proteomics (2004) 3:534–47. 10.1074/mcp.M300089-MCP200 [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Jin Y, Xu S, Zheng J, Zhang QI, Wang Y, et al. AGR2 is associated with gastric cancer progression and poor survival. Oncol Lett. (2016) 11:2075–83. 10.3892/ol.2016.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barraclough DL, Platt-Higgins A, de Silva Rudland S, Barraclough R, Winstanley J, West CR, et al. The metastasis-associated anterior gradient 2 protein is correlated with poor survival of breast cancer patients. Am J Pathol. (2009) 175:1848–57. 10.2353/ajpath.2009.090246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alavi M, Mah V, Maresh EL, Bagryanova L, Horvath S, Chia D, et al. High expression of AGR2 in lung cancer is predictive of poor survival. BMC Cancer (2015) 15:655. 10.1186/s12885-015-1658-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darb-Esfahani S, Fritzsche F, Kristiansen G, Weichert W, Sehouli J, Braicu I, et al. Anterior gradient protein 2 (AGR2) is an independent prognostic factor in ovarian high-grade serous carcinoma. Virchows Arch. (2012) 461:109–16. 10.1007/s00428-012-1273-4 [DOI] [PubMed] [Google Scholar]
- 23.Sung HY, Choi EN, Lyu D, Park AK, Ju W, Ahn JH. Aberrant hypomethylation-mediated AGR2 overexpression induces an aggressive phenotype in ovarian cancer cells. Oncol Rep. (2014) 32:815–20. 10.3892/or.2014.3243 [DOI] [PubMed] [Google Scholar]
- 24.Dumartin L, Whiteman HJ, Weeks ME, Hariharan D, Dmitrovic B, Iacobuzio-Donahue CA, et al. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. (2011) 71:7091–102. 10.1158/0008-5472.CAN-11-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arumugam T, Deng D, Bover L, Wang H, Logsdon CD, Ramachandran V. New blocking antibodies against novel AGR2-C4.4A pathway reduce growth and metastasis of pancreatic tumors and increase survival in mice. Mol Cancer Ther. (2015) 14:941–51. 10.1158/1535-7163.MCT-14-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol (1999) 189:12–9. [DOI] [PubMed] [Google Scholar]
- 27.Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci USA. (2012) 109:10516–21. 10.1073/pnas.1202684109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morbini P, Capello GL, Alberizzi P, Benazzo M, Paglino C, Comoli P, et al. Markers of squamocolumnar junction cells in normal tonsils and oropharyngeal cancer with and without HPV infection. Histol Histopathol. (2015) 30:833–9. 10.14670/HH-11-590 [DOI] [PubMed] [Google Scholar]
- 29.Yu X, Li Z, Shen J. BRD7: a novel tumor suppressor gene in different cancers. Am J Transl Res. (2016) 8:742–8. Available online at: http://europepmc.org/abstract/MED/27158366 [PMC free article] [PubMed] [Google Scholar]
- 30.Drost J, Mantovani F, Tocco F, Elkon R, Comel A, Holstege H, et al. BRD7 is a candidate tumour suppressor gene required for p53 function. Nat Cell Biol. (2010) 12:380–9. 10.1038/ncb2038 [DOI] [PubMed] [Google Scholar]
- 31.Chiu YH, Lee JY, Cantley LC. BRD7, a tumor suppressor, interacts with p85alpha and regulates PI3K activity. Mol Cell (2014) 54:193–202. 10.1016/j.molcel.2014.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harte MT, O'Brien GJ, Ryan NM, Gorski JJ, Savage KI, Crawford NT, et al. BRD7, a subunit of SWI/SNF complexes, binds directly to BRCA1 and regulates BRCA1-dependent transcription. Cancer Res. (2010) 70:2538–47. 10.1158/0008-5472.CAN-09-2089 [DOI] [PubMed] [Google Scholar]
- 33.Wu WJ, Hu KS, Chen DL, Zeng ZL, Luo HY, Wang F, et al. Prognostic relevance of BRD7 expression in colorectal carcinoma. Eur J Clin Invest. (2013) 43:131–40. 10.1111/eci.12024 [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi M, Okumura F, Tsukiyama T, Watanabe M, Miyajima N, Tanaka J, et al. TRIM24 mediates ligand-dependent activation of androgen receptor and is repressed by a bromodomain-containing protein, BRD7, in prostate cancer cells. Biochim Biophys Acta (2009) 1793:1828–36. 10.1016/j.bbamcr.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 35.Chen CL, Wang Y, Pan QZ, Tang Y, Wang QJ, Pan K, et al. Bromodomain-containing protein 7 (BRD7) as a potential tumor suppressor in hepatocellular carcinoma. Oncotarget (2016) 7:16248–61. 10.18632/oncotarget.7637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu K, Liao D, Wu W, Han AJ, Shi HJ, Wang F, et al. Targeting the anaphase-promoting complex/cyclosome (APC/C)- bromodomain containing 7 (BRD7) pathway for human osteosarcoma. Oncotarget (2014) 5:3088–100. 10.18632/oncotarget.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park YA, Lee JW, Choi JJ, Jeon HK, Cho Y, Choi C, et al. The interactions between MicroRNA-200c and BRD7 in endometrial carcinoma. Gynecol Oncol. (2012) 124:125–33. 10.1016/j.ygyno.2011.09.026 [DOI] [PubMed] [Google Scholar]
- 38.Park YA, Lee JW, Kim HS, Lee YY, Kim TJ, Choi CH, et al. Tumor suppressive effects of bromodomain-containing protein 7 (BRD7) in epithelial ovarian carcinoma. Clin Cancer Res. (2014) 20:565–75. 10.1158/1078-0432.CCR-13-1271 [DOI] [PubMed] [Google Scholar]
- 39.Nebral K, Denk D, Attarbaschi A, Konig M, Mann G, Haas OA, et al. Incidence and diversity of PAX5 fusion genes in childhood acute lymphoblastic leukemia. Leukemia (2009) 23:134–43. 10.1038/leu.2008.306 [DOI] [PubMed] [Google Scholar]
- 40.Funakoshi T, Maeshima K, Yahata K, Sugano S, Imamoto F, Imamoto N. Two distinct human POM121 genes: requirement for the formation of nuclear pore complexes. FEBS Lett. (2007) 581:4910–6. 10.1016/j.febslet.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 41.Talamas JA, Hetzer MW. POM121 and Sun1 play a role in early steps of interphase NPC assembly. J Cell Biol. (2011) 194:27–37. 10.1083/jcb.201012154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou M, Liu H, Xu X, Zhou H, Li X, Peng C, et al. Identification of nuclear localization signal that governs nuclear import of BRD7 and its essential roles in inhibiting cell cycle progression. J Cell Biochem. (2006) 98:920–30. 10.1002/jcb.20788 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.