Abstract
Background
Excess weight, low physical activity, low intakes of dietary fiber, fruits, and vegetables, and high meat and salt intake increase cancer risk.
Methods
Numbers and proportions (population-attributable fractions, PAF) of incident cancer cases in Germany in 2018 attributable to these factors were estimated by sex and age groups for ages 35 to 84 years using population projections, national cancer incidence and exposure data, and published risk estimates.
Results
Estimated numbers (percentages) of attributable cancers were 30 567 (7%) for excess weight, 27 081 (6%) for low physical activity, 14 474 (3%) for low dietary fiber intake, 9447 (2%) for low fruit and vegetable consumption, 9454 (2%) and 1687 (0.4%) for processed meat and high red meat consumption, respectively, and 1204 (0.3%) for high salt intake. Excess weight substantially contributed to endometrial, renal, and liver cancer (PAF = 24 to 35%). Low physical activity contributed to endometrial, renal, and lung cancer (PAF = 15 to 19%), and dietary factors mainly contributed to colorectal, breast, and lung cancer (PAF = 9 to 16%).
Conclusion
A considerable proportion of cancer cases are attributable to excess weight, physical inactivity, and unhealthy dietary habits. Major prevention efforts are needed to reduce the cancer incidence attributable to these avoidable factors.
Excess weight, low physical activity, and unhealthy diet contribute substantially to the development of cancer (1– 3). However, no information on the attributable cancer incidence is available for the general population in Germany. By applying the concept of population-attributable fractions (PAF), we estimated the incidence of cancers attributable to excess weight, low physical activity, and unhealthy diet in people aged 35–84 years in Germany in 2018. Health professionals and politicians need such information to design and implement effective measures to reduce the prevalence of obesity, physical inactivity, and unhealthy diet.
Methods
Lifestyle factors and site-specific cancer risk
Our definitions of normal body weight, recommended level of physical activity and a healthy diet followed the cancer prevention guidelines of the World Cancer Research Fund (WCRF) (eSupplement A) (4). We considered all cancer types that have been shown to be related to those lifestyle factors in published meta-analyses of prospective studies comprising 5000 or more cancer cases (eSupplement B– D, eTables 1– 3).
Statistical methods
In analogy to our alcohol analysis in this issue (5), we used PAFs (for details see the Box in Mons et al. [5], this issue) to estimate the proportion of lifestyle-associated cancers in the population aged 35 to 84 years, assuming a 10-year latency period between exposure and cancer incidence. We used prevalence data of 6962 men and women aged 25 to 74 years from the nationally representative German Health Interview and Examination Survey for Adults for the period 2008 to 2011 (DEGS1) (6) (eSupplement, eTables 4– 8). We estimated the number of cancer cases attributable to each lifestyle factor by multiplying the PAF by the expected cancer incidence for the year 2018 (eSupplement E, eTables 9– 21).
Key Messages.
We estimated the proportions of new cancers among the population aged 35 to 84 years in Germany in 2018 that can be attributed to excess weight, low physical activity, and an unhealthy diet. Our estimates are based on the concept of population-attributable fractions (PAF).
According to our calculations, more than 30 000 cancers (7% of the estimated total of 440 000 cancers in that age range) are attributable to excess weight.
A comparably high number of cancers (>27 000, 6%) are attributable to low physical activity.
Lower but still substantial numbers of cancers are attributable to low dietary fiber intake (>14 000, 3%), low fruit and non-starchy vegetable consumption (>9000, 2%), and high processed meat consumption (>9000, 2%).
Our findings suggest that potentially modifiable lifestyle factors, including excess weight, low physical activity, and an unhealthy diet, contribute substantially to the development of potentially severe cancers in Germany.
Results
Prevalence of lifestyle factors
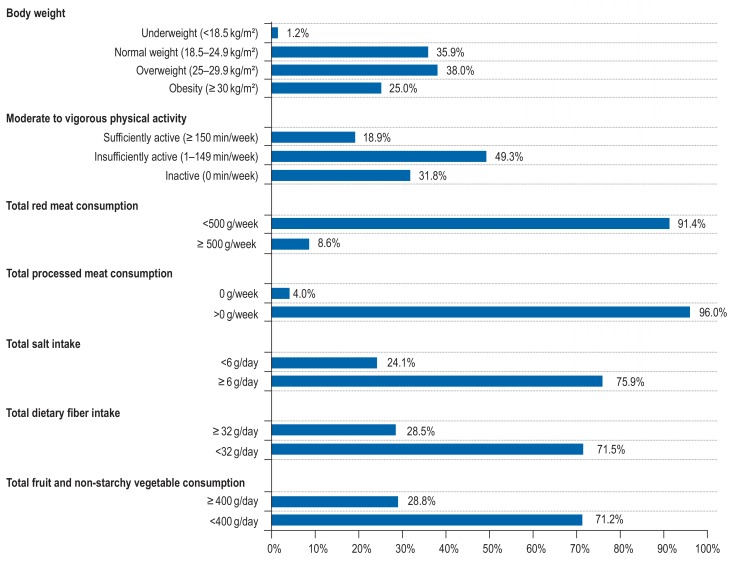
According to DEGS1, 63% of men and women aged 25 to 74 years living in Germany were overweight (38%) or obese (25%) (Figure 1, eTable 4). Furthermore, 81% of the study population were insufficiently physically active (49%; 1–149 min/week of moderate to vigorous physical activity) or physically inactive (32%; 0 min/week of moderate to vigorous physical activity) (eTable 5). In addition, 9% of the study population reported a high red meat consumption of =500 g/week (eTables 6– 8), 96% of them ate processed meat (including hamburger/kebab, bratwurst/currywurst, sausage, and ham), 76% had a high salt intake of =6 g/day, 72% had a low dietary fiber intake (<2 g/day), and 71% did not consume enough fruit and non-starchy vegetables (<400 g/day).
Figure 1.
Prevalence of selected lifestyle factors among men and women aged 25–74 years (N = 6087 for body weight, N = 6696 for physical activity, N = 6129 for dietary factors) from the nationally representative DEGS1 survey, 2008 to 2011, Germany
Site-specific cancer risk
Published meta-analyses revealed that obesity (as compared to normal weight) increases the risk of cancers of the stomach (by 17%), colorectum (by 33%), liver (by 83%), gallbladder (by 67%), pancreas (by 36%), breast (postmenopausal, by 20%), endometrium (by 154%), ovary (by 27%), prostate (advanced, by 14%), kidney (by 77%), bladder (by 10%), thyroid gland (by 29%), and additionally the risks of non-Hodgkin lymphoma (by 19%), multiple myeloma (by 21%), and leukemia (by 26%) (eTable 1, eFigure 1).
Because cohort studies of physical inactivity and cancer risk used heterogeneous physical activity assessments and categories, meta-analyses could only provide general estimates of the cancer risk among physically inactive individuals as compared to the cancer risk among individuals who were sufficiently physically active (eTable 2). In DEGS1, the comparison between sufficient physical activity and physical inactivity corresponded to the comparison of engaging in an average of 248 min/week of moderate to vigorous physical activity versus not engaging in any moderate to vigorous physical activity (0 min/week). According to this interpretation, a 150 min/week decrease in moderate to vigorous physical activity is associated with risk increases of 5% for gastric cancer, 11% for colorectal cancer, 3% for pancreatic cancer, 20% for lung cancer, 7% for breast cancer, 15% for endometrial cancer, 17% for renal cancer, and 9% for bladder cancer (eFigure 2).
Dose–response meta-analyses of dietary factors and cancer risk reported a risk increase per 200 g/week increase in red meat consumption of 3% for colorectal cancer, 3% for pancreatic cancer, 7% for lung cancer, and 3% for breast cancer, and risk increases per 200 g/week increase in processed meat consumption of 9% for colorectal cancer and 5% for breast cancer (eTable 3, eFigure 3). A 2 g/day increase in salt intake increases the risk of gastric cancer by 5%. A 10 g/day decrease in dietary fiber intake is associated with an 11% increased risk of colorectal cancer and a 5% increased risk of developing breast cancer. A 200 g/day decrease in fruit and non-starchy vegetable consumption is associated with a risk increase of 2% for colorectal cancer and a 9% increase in the risk of lung cancer.
Cancers attributable to the selected lifestyle factors
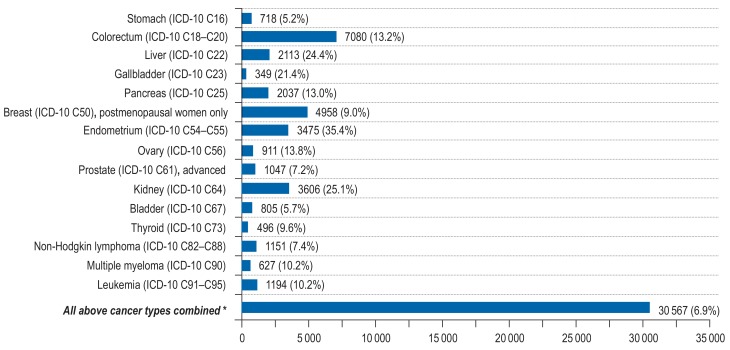
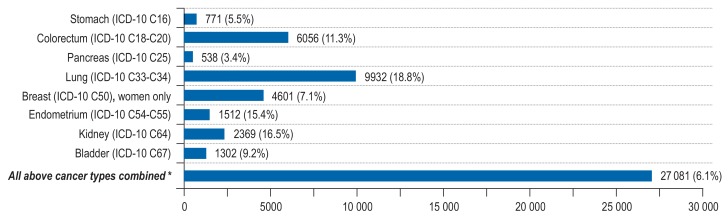
We expected 440 373 incident cancers among adults aged 35 to 84 years in 2018 in Germany. Excess weight and low physical activity increased the cancer incidence substantially (excess weight: N = 30 567 cases, PAF = 7%; low physical activity: N = 27 081 cases, PAF = 6%), exerting a substantial effect on endometrial cancer (PAF for excess weight = 35%; PAF for low physical activity = 15%), renal cancer (PAF for excess weight = 25%; PAF for low physical activity = 17%), liver cancer (PAF for excess weight = 24%), and lung cancer (PAF for low physical activity = 19%) (Figures 2– 3, eTables 12– 17, eFigures 4– 5).
Figure 2.
Estimated number of site-specific incident cancer cases attributable to excess weight (BMI=25 kg/m²) among men and women aged 35 to 84 years in Germany for the year 2018, assuming a 10-year latency period between exposure and cancer incidence. *The PAF for the category “All above cancer types combined” was computed with respect to total cancer incidence (ICD-10 C00-C99 without C44).
ICD, International Classification of Diseases; PAF, population-attributable fraction, BMI, body mass index
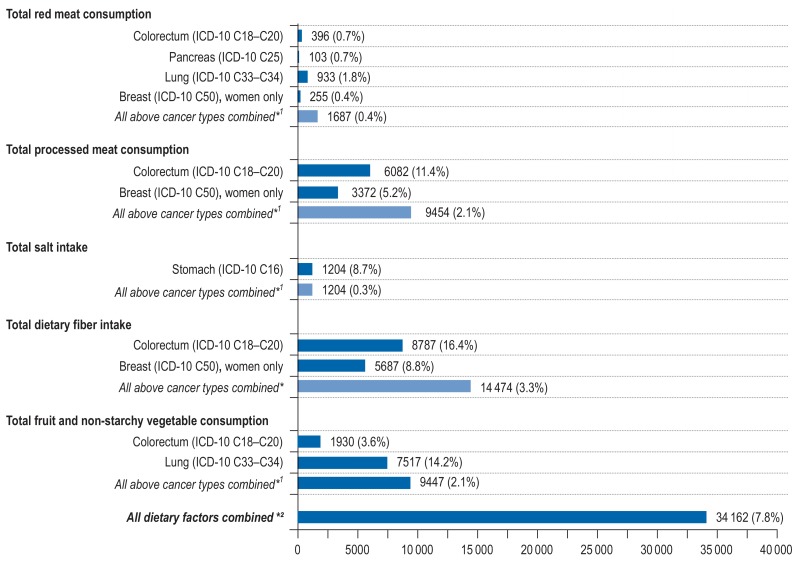
Substantially lower contributions to total cancer risk were observed for intakes of dietary fiber, fruit, non-starchy vegetables, and processed meat (any consumption of processed meat: N = 9454, PAF = 2%; low intake of dietary fiber: N = 14 474, PAF = 3%; low consumption of fruit and non-starchy vegetables: N = 9447, PAF = 2%) (Figure 4, eTables 18– 20, eFigure 6). Low intake of dietary fiber and the consumption of processed meat products favored the development of colorectal cancer and breast cancer (consumption of processed meat: PAF for colorectal cancer = 11 %, PAF for breast cancer = 5 %; low dietary fiber: PAF for colorectal cancer = 16 %, PAF for breast cancer = 9 %). Low consumption of fruit and non-starchy vegetables influenced the development of colorectal cancer (PAF = 4 %) and lung cancer (PAF = 14 %). High intakes of salt and red meat had considerable effects on gastric cancer (PAF for high salt intake = 9%) and lung cancer (PAF for high red meat consumption = 2%), but negligible effects on total cancer (high red meat consumption: N = 1687, PAF = 0.4%; high salt intake: N = 1204, PAF = 0.3%). We estimated that a total of 34 162 cancers (PAF = 8%) were attributable to all dietary factors combined (Figure 4, eTable 21).
Figure 4.
Estimated number of site-specific incident cancer cases attributable to high consumption of red meat (=500 g/week), any consumption of processed meat (>0 g/week), high intake of salt (=6 g/day), low intake of dietary fiber (<32 g/day) and low consumption of fruit and vegetables (<400 g/day) among men and women aged 35 to 84 years in Germany for the year 2018, assuming a 10-year latency period between exposure and cancer incidence.
*1 The PAF for the category “All above cancer types combined” was computed with respect to total cancer incidence (ICD-10 C00-C99 without C44).
*2 The PAF for the category “All dietary factors combined” was computed with the sequential PAF formula separately for each cancer type and each age group. The resulting age- and sex-specific attributable cancer cases were then summated to yield an overall estimate for the whole population and set against the total number of cancer cases (ICD-10 C00-C99 without C44; cf. eSupplement E and eTable 21).
ICD, International Classification of Diseases; PAF, population-attributable fraction
We observed no strong correlations between the individual lifestyle factors in our population, but there were moderate correlations between high consumption of processed meat and high salt intake, and between high intake of dietary fiber and high intakes of salt, fruit and non-starchy vegetables (Spearman correlation coefficients = 0.22–0.45, eTable 22).
Sensitivity analyses using the 95% confidence limits of risk estimates in the PAF formulae indicated an estimated range of 19 513 to 41 723 cancer cases attributable to excess weight, 19 714 to 34 857 to low physical activity, and 16 695 to 52 547 to unhealthy diet (eTables 23– 25).
Discussion
Our study revealed a high prevalence of excess weight, low physical activity, and unhealthy diet among the population in Germany in the period 2008 to 2011. For the population aged 35 to 84 years in 2018 in Germany, we therefore estimated that 30 567 incident cancers will be attributable to excess weight and 27 081 to low physical activity in 2018, corresponding to 7% and 6%, respectively, of the expected total of 440 373 incident cancers in this population. 9000 to 14 000 cancers (2–3%) will be attributable to low intakes of dietary fiber, fruit and non-starchy vegetables and high consumption of processed meat, and some 1000 to 2000 cases (<1%) to high intakes of salt and red meat.
Overweight and obesity
Earlier studies (7– 11) estimated the cancer risk attributable to overweight and obesity under the model assumption that the natural logarithm of the relative risk depends linearly on BMI (log linearity). This assumption is not always true (12), potentially leading to distorted estimates. We therefore dispensed with this model assumption and instead used direct comparisons of the cancer risk for normal weight, overweight, and obesity. Some previous studies also used direct risk comparisons to calculate the cancer incidence attributable to overweight and obesity (13– 16). Due to lower prevalence rates for overweight and obesity, those studies yielded lower attributable cancer incidence estimates for overweight and obesity than our study (13– 16).
It is biologically plausible that overweight and obesity contribute to the development of cancer. Potential biological mechanisms and factors linking excess body fat to cancer incidence include insulin resistance, chronic inflammatory processes, sex hormones, and growth factors (1).
Low physical activity
Previous estimates of the cancer incidence attributable to low physical activity differ from our estimates because previous studies used lower physical activity target levels (13, 14), lower prevalence rates (15, 17), or lower or higher assumed cancer risks for their estimates (9, 11, 16, 18, 19).
A high level of physical activity may prevent cancer through reductions of adipose tissue and insulin resistance, through decreases in chronic inflammation, sex hormones, and growth factors, and through improved resistance to oxidative stress and DNA damage (3).
Unhealthy diet
Previous attributable cancer incidence studies reported a greater or lesser cancer prevention potential of a healthy diet because they applied more or less rigorous target intake levels than in our study (11, 20– 26), because their mean intake was further away from/closer to the target level than in our study (15, 16, 20, 21), and because they used higher or lower cancer risk estimates for an unhealthy diet (11, 14, 27). Our estimate for the combined impact of dietary factors on cancer incidence was comparable to that from previous studies from other countries (28, 29).
Low intakes of red meat, processed meat, and salt and high intakes of dietary fiber, fruit, and non-starchy vegetables may contribute to the prevention of cancer through (2, 30– 32):
Reduced exposure to exogenous and endogenous carcinogens including N-nitroso compounds
Decreased formation of cyto- and genotoxic aldehydes
Lower levels of chronic inflammation
Increased antioxidative capacities
Improved DNA repair
Modulated estrogen metabolism.
In addition, changing from diets with high intakes of energy-dense foods to diets with high intakes of dietary fiber, fruit, and vegetables may decrease cancer risk through reductions in adipose tissue, insulin levels, chronic inflammation, and circulating sex and growth hormones (2).
Strengths and limitations
The present study of attributable cancer incidence followed the methodological recommendations issued by the World Health Organization (WHO, eSupplement F). Our study provides up-to-date estimates of the total number of cancers attributable to excess weight, low physical activity and unhealthy diet in Germany for the year 2018, assuming a 10-year latency period, based on the latest nationally representative prevalence and cancer incidence data. As discussed above, there is sufficient biological evidence in support of a causal relationship between the selected lifestyle factors and the development of cancer.
Our comprehensive systematic literature search yielded the most recent data on the relations of lifestyle factors to site-specific cancer risk. The present study is the first to consider, in estimating the attributable cancer incidence, the relation of obesity to bladder cancer, the relations of low physical activity to cancers of the stomach, lung, and bladder, and the relations of high consumption of red and processed meat and of low intake of dietary fiber to cancers of the pancreas, lung, and breast. All of these relations have been established in published meta-analyses of prospective studies including =5000 incident cancer cases.
As a limitation, we may have underestimated the cancer prevention potential of the selected lifestyle factors because we did not consider any potential relations of these factors to site-specific cancer risk that have not yet been confirmed in meta-analyses of prospective studies including =5000 incident cancer cases (eSupplement F).
We accounted for potential confounding by age and sex, the most important predictors of cancer incidence, by stratifying our analyses by age and sex. However, we were not able to consider additional potential confounding factors, including genetic traits, medical conditions, and sociodemographic factors, because cancer registries do not provide data stratified by such factors. Unfortunately we could not calculate any PAF for combinations of lifestyle factors, because the required risk estimators were insufficiently precise (33, 34). Therefore, we were also unable to take account of the potential biological interactions among the individual lifestyle factors and the resultant potential confounding. Because the concept of PAF does not allow the summation of attributable cancer cases across individual risk factors, we used the sequential PAF formula to assess the combined impact of dietary factors on cancer incidence. That formula requires the assumption of uncorrelated dietary factors, which was approximately met in our population.
For the selected lifestyle factors, the assumed cancer latency period of 10 years is within the realistic range of 5–15 years—a range in which attributable cancer incidence estimates vary little (eSupplement F). In sensitivity analyses, we used the lower and upper bounds of the cancer site–specific relative risk estimates in the PAF formulas and observed substantial numbers of attributable cancer cases across all scenarios. Potential changes in prevalence of lifestyle factors in recent years are probably small and should not affect our estimates substantially.
Conclusion
The present study identified excess weight (>30 000 annual cases, 7%) and low physical activity (>27 000 annual cases, 6%) as major contributors to the current cancer incidence (>440 000 annual cases) among adults aged 35–84 years in Germany. Substantial numbers of incident cancers were also due to dietary factors, including low intake of dietary fiber (>14 000 annual cases, 3%), low consumption of fruit and non-starchy vegetables (>9000 annual cases, 2%), any consumption of processed meat (>9000 annual cases, 2%), high consumption of red meat (>1600 annual cases, 0.4%) and high salt intake (>1200 annual cases, 0.3%). These figures suggest that adherence to a healthy lifestyle is vital in cancer prevention at both the individual level and the population level. In view of the high prevalence of unhealthy lifestyle factors in the population, health professionals and politicians should increase their efforts to encourage people to lead a healthy lifestyle. According to the World Cancer Research Fund (WCRF), a cancer-preventive lifestyle should include adherence to a normal weight (BMI 18.5–24.9 kg/m²), regular physical activity (=150 min/week of moderate to vigorous physical activity), and a healthy diet (=32 g/day of dietary fiber, =400 g/day of fruit and non-starchy vegetables, 0 g/week of processed meat, <500 g/week of red meat, <6 g/day of salt). Encouragement from physicians is an effective means of increasing adherence to a healthy lifestyle due to the trust that patients have in their doctors’ medical advice (35). Adherence to a healthy lifestyle may be effectively supported by the creation of healthy living environments and incentives. Potential strategies to promote physical activity and reduce overweight include physical activity interventions at school and work-places, the creation of sport facilities, parks, and nature recreation areas in neighborhoods, and the development of public transport, safe bike lanes, safe sidewalks, and pleasant walking environments (36). The extension of public transportation represents a health-enhancing option because people often walk or cycle to the next public transport station (36). A healthy diet may be promoted through price policies, advertising restrictions, nutrition labeling, school and workplace interventions, information campaigns, and greater availability of healthy foodstuffs in restaurants, kiosks, and fast-food outlets (37– 39).
Figure 3.
Estimated number of site-specific incident cancer cases attributable to low physical activity (<150 min/week of moderate to vigorous physical activity) among men and women aged 35 to 84 years in Germany for the year 2018, assuming a 10-year latency period between exposure and cancer incidence.
*The PAF for the category “All above cancer types combined” was computed with respect to total cancer incidence (ICD-10 C00-C99 without C44).
ICD, International Classification of Diseases; PAF, population-attributable fraction
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
Funding
The study was funded by German Cancer Aid (“Deutsche Krebshilfe”), grant number 70112097.
References
- 1.Anderson AS, Key TJ, Norat T, et al. European Code Against Cancer 4th Edition: obesity, body fatness and cancer. Cancer Epidemiol. 2015;39(1):S34–S45. doi: 10.1016/j.canep.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Norat T, Scoccianti C, Boutron-Ruault MC, et al. European Code Against Cancer 4th Edition: diet and cancer. Cancer Epidemiol. 2015;39(1):S56–S66. doi: 10.1016/j.canep.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Leitzmann M, Powers H, Anderson AS, et al. European Code Against Cancer 4th edition: physical activity and cancer. Cancer Epidemiol. 2015;39 Suppl 1:S46–S55. doi: 10.1016/j.canep.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: 2007. www.aicr.org/assets/docs/pdf/reports/Second_Expert_Report.pdf (last accessed on 8 August 2017) [Google Scholar]
- 5.Mons U, Gredner T, Behrens G, Stock C, Brenner H. Cancers due to smoking and high alcohol consumption—estimation of the attributable cancer burden in Germany. Dtsch Arztebl Int. 2018;115:571–577. doi: 10.3238/arztebl.2018.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheidt-Nave C, Kamtsiuris P, Gosswald A, et al. German Health Interview and Examination Survey for Adults (DEGS)—design, objectives and implementation of the first data collection wave. BMC Public Health. 2012;12 doi: 10.1186/1471-2458-12-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126:692–702. doi: 10.1002/ijc.24803. [DOI] [PubMed] [Google Scholar]
- 8.Parkin DM, Boyd L. 8 Cancers attributable to overweight and obesity in the UK in 2010. Br J Cancer. 2011;105(2):S34–S37. doi: 10.1038/bjc.2011.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131–139. doi: 10.1016/j.ypmed.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Kendall BJ, Wilson LF, Olsen CM, et al. Cancers in Australia in 2010 attributable to overweight and obesity. Aust N Z J Public Health. 2015;39:452–457. doi: 10.1111/1753-6405.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 12.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 52. 4 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson A, Hayes J, Frampton C, Potter J. Modifiable lifestyle factors that could reduce the incidence of colorectal cancer in New Zealand. N Z Med J. 2016;129:13–20. [PubMed] [Google Scholar]
- 14.Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005—systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23:1362–1369. doi: 10.1093/annonc/mdr437. [DOI] [PubMed] [Google Scholar]
- 15.Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118:1130–1141. doi: 10.1038/s41416-018-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azevedo ESG, de Moura L, Curado MP, et al. The fraction of cancer attributable to ways of life, infections, occupation, and environmental agents in Brazil in 2020. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148761. e0148761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkin DM. 9 Cancers attributable to inadequate physical exercise in the UK in 2010. Br J Cancer. 2011;105 (2):S38–S41. doi: 10.1038/bjc.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Olsen CM, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to insufficient physical activity. Aust N Z J Public Health. 2015;39:458–463. doi: 10.1111/1753-6405.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundy A, Poirier AE, Khandwala F, McFadden A, Friedenreich CM, Brenner DR. Cancer incidence attributable to red and processed meat consumption in Alberta in 2012. CMAJ Open. 2016;4:e768–e775. doi: 10.9778/cmajo.20160036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries E, Quintero DC, Henriquez-Mendoza G, Herran OF. Population attributable fractions for colorectal cancer and red and processed meats in Colombia—a macro-simulation study. Colomb Med (Cali) 2017;48:64–69. [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy A, Poirier AE, Khandwala F, McFadden A, Friedenreich CM, Brenner DR. Cancer incidence attributable to insufficient fruit and vegetable consumption in Alberta in 2012. CMAJ Open. 2016;4:e760–e767. doi: 10.9778/cmajo.20160037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagle CM, Wilson LF, Hughes MC, et al. Cancers in Australia in 2010 attributable to inadequate consumption of fruit, non-starchy vegetables and dietary fibre. Aust N Z J Public Health. 2015;39:422–428. doi: 10.1111/1753-6405.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkin DM, Boyd L. 4 Cancers attributable to dietary factors in the UK in 2010. I. Low consumption of fruit and vegetables. Br J Cancer. 2011;105(2):S19–S23. doi: 10.1038/bjc.2011.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundy A, Poirier AE, Khandwala F, McFadden A, Friedenreich CM, Brenner DR. Cancer incidence attributable to insufficient fibre consumption in Alberta in 2012. CMAJ Open. 2017;5:e7–e13. doi: 10.9778/cmajo.20160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkin DM, Boyd L. 6 Cancers attributable to dietary factors in the UK in 2010. III. Low consumption of fibre. Br J Cancer. 2011;105(2):S27–S30. doi: 10.1038/bjc.2011.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkin DM. 7 Cancers attributable to dietary factors in the UK in 2010. IV. Salt Br J Cancer. 2011;105(2):S31–S33. doi: 10.1038/bjc.2011.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkin DM, Boyd L, Walker LC. 16 The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(2):S77–S81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteman DC, Webb PM, Green AC, et al. Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health. 2015;39:477–484. doi: 10.1111/1753-6405.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steppeler C, Haugen JE, Rodbotten R, Kirkhus B. Formation of nalondialdehyde, 4-hydroxynonenal, and 4-hydroxyhexenal during in vitro digestion of cooked beef, pork, chicken, and salmon. J Agric Food Chem. 2016;64:487–496. doi: 10.1021/acs.jafc.5b04201. [DOI] [PubMed] [Google Scholar]
- 31.Cheng XJ, Lin JC, Tu SP. Etiology and prevention of gastric cancer. Gastrointest Tumors. 2016;3:25–36. doi: 10.1159/000443995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins AR, Azqueta A, Langie SA. Effects of micronutrients on DNA repair. Eur J Nutr. 2012;51:261–279. doi: 10.1007/s00394-012-0318-4. [DOI] [PubMed] [Google Scholar]
- 33.Romaguera D, Vergnaud AC, Peeters PH, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012;96:150–163. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- 34.Jankovic N, Geelen A, Winkels RM, et al. Adherence to the WCRF/AICR dietary recommendations for cancer prevention and risk of cancer in elderly from Europe and the United States: a meta-analysis within the CHANCES project. Cancer Epidemiol Biomarkers Prev. 2017;26:136–144. doi: 10.1158/1055-9965.EPI-16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones DE, Carson KA, Bleich SN, Cooper LA. Patient trust in physicians and adoption of lifestyle behaviors to control high blood pressure. Patient Educ Couns. 2012;89:57–62. doi: 10.1016/j.pec.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Institute of Medicine. Physical activity: moving toward obesity solutions: workshop summary. Washington, DC. The National Academies Press. 2015 [PubMed] [Google Scholar]
- 37.Capacci S, Mazzocchi M, Shankar B, et al. Policies to promote healthy eating in Europe: a structured review of policies and their effectiveness. Nutr Rev. 2012;70:188–200. doi: 10.1111/j.1753-4887.2011.00442.x. [DOI] [PubMed] [Google Scholar]
- 38.Lake AA. Neighbourhood food environments: food choice, foodscapes and planning for health. Proc Nutr Soc. 2018;77:239–246. doi: 10.1017/S0029665118000022. [DOI] [PubMed] [Google Scholar]
- 39.Finkelstein EA, Strombotne KL, Zhen C, Epstein LH. Food prices and obesity: a review. Adv Nutr. 2014;5:818–821. doi: 10.3945/an.114.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.WCRF/AICR. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC2007. www.aicr.org/assets/docs/pdf/reports/Second_Expert_Report.pdf (last accessed on 8 August 2017) [Google Scholar]
- E2.Scheidt-Nave C, Kamtsiuris P, Gosswald A, et al. German Health Interview and Examination Survey for Adults (DEGS)—design, objectives and implementation of the first data collection wave. BMC Public Health. 2012;12 doi: 10.1186/1471-2458-12-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Marshall AL, Smith BJ, Bauman AE, Kaur S. Reliability and validity of a brief physical activity assessment for use by family doctors. Br J Sports Med. 2005;39:294–297. doi: 10.1136/bjsm.2004.013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Washburn RA, Goldfield SR, Smith KW, McKinlay JB. The validity of self-reported exercise-induced sweating as a measure of physical activity. Am J Epidemiol. 1990;132:107–113. doi: 10.1093/oxfordjournals.aje.a115622. [DOI] [PubMed] [Google Scholar]
- E5.Kohl HW, Blair SN, Paffenbarger RS Jr, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–1239. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- E6.Siconolfi SF, Lasater TM, Snow RC, Carleton RA. Self-reported physical activity compared with maximal oxygen uptake. Am J Epidemiol. 1985;122:101–105. doi: 10.1093/oxfordjournals.aje.a114068. [DOI] [PubMed] [Google Scholar]
- E7.Krug S, Jordan S, Mensink GB, Muters S, Finger J, Lampert T. [Physical activity: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:765–771. doi: 10.1007/s00103-012-1661-6. [DOI] [PubMed] [Google Scholar]
- E8.Gruner C, Alig F, Muntwyler J. Validity of self-reported exercise-induced sweating as a measure of physical activity among patients with coronary artery disease. Swiss Med Wkly. 2002;132:629–632. doi: 10.4414/smw.2002.10094. [DOI] [PubMed] [Google Scholar]
- E9.Boyle P, Koechlin A, Autier P. Sweetened carbonated beverage consumption and cancer risk: meta-analysis and review. Eur J Cancer Prev. 2014;23:481–490. doi: 10.1097/CEJ.0000000000000015. [DOI] [PubMed] [Google Scholar]
- E10.Romaguera D, Vergnaud AC, Peeters PH, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012;96:150–163. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- E11.Haftenberger M, Heuer T, Heidemann C, Kube F, Krems C, Mensink GB. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr J. 2010;9 doi: 10.1186/1475-2891-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.U.S. Department of Agriculture ARS. USDA Food and Nutrient Database for Dietary Studies 2013-2014. Food Surveys Research Group Home Page. 2016 [Google Scholar]
- E13.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1–19. [Google Scholar]
- E14.Chen Y, Liu L, Wang X, et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev. 2013;22:1395–1408. doi: 10.1158/1055-9965.EPI-13-0042. [DOI] [PubMed] [Google Scholar]
- E15.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053916. e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–2547. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- E17.Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer. 2012;48:2137–2145. doi: 10.1016/j.ejca.2012.02.063. [DOI] [PubMed] [Google Scholar]
- E18.Li L, Gan Y, Li W, Wu C, Lu Z. Overweight, obesity and the risk of gallbladder and extrahepatic bile duct cancers: a meta-analysis of observational studies. Obesity (Silver Spring) 2016;24:1786–1802. doi: 10.1002/oby.21505. [DOI] [PubMed] [Google Scholar]
- E19.Xue K, Li FF, Chen YW, Zhou YH, He J. Body mass index and the risk of cancer in women compared with men: a meta-analysis of prospective cohort studies. Eur J Cancer Prev. 2017;26:94–105. doi: 10.1097/CEJ.0000000000000231. [DOI] [PubMed] [Google Scholar]
- E20.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–136. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Jenabi E, Poorolajal J. The effect of body mass index on endometrial cancer: a meta-analysis. Public Health. 2015;129:872–880. doi: 10.1016/j.puhe.2015.04.017. [DOI] [PubMed] [Google Scholar]
- E22.Poorolajal J, Jenabi E, Masoumi SZ. Body mass index effects on risk of ovarian cancer: a meta- analysis. Asian Pac J Cancer Prev. 2014;15:7665–7671. doi: 10.7314/apjcp.2014.15.18.7665. [DOI] [PubMed] [Google Scholar]
- E23.Xie B, Zhang G, Wang X, Xu X. Body mass index and incidence of nonaggressive and aggressive prostate cancer: a dose-response meta-analysis of cohort studies. Oncotarget. 2017;8:97584–97592. doi: 10.18632/oncotarget.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2014;135:1673–1686. doi: 10.1002/ijc.28813. [DOI] [PubMed] [Google Scholar]
- E25.Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB. Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119313. e0119313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E26.Ma J, Huang M, Wang L, Ye W, Tong Y, Wang H. Obesity and risk of thyroid cancer: evidence from a meta-analysis of 21 observational studies. Med Sci Monit. 2015;21:283–291. doi: 10.12659/MSM.892035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev. 2015;16:1042–1054. doi: 10.1111/obr.12321. [DOI] [PubMed] [Google Scholar]
- E28.Larsson SC, Wolk A. Obesity and risk of non-Hodgkin‘s lymphoma: a meta-analysis. Int J Cancer. 2007;121:1564–1570. doi: 10.1002/ijc.22762. [DOI] [PubMed] [Google Scholar]
- E29.Wallin A, Larsson SC. Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47:1606–1615. doi: 10.1016/j.ejca.2011.01.020. [DOI] [PubMed] [Google Scholar]
- E30.Castillo JJ, Reagan JL, Ingham RR, et al. Obesity but not overweight increases the incidence and mortality of leukemia in adults: a meta-analysis of prospective cohort studies. Leuk Res. 2012;36:868–875. doi: 10.1016/j.leukres.2011.12.020. [DOI] [PubMed] [Google Scholar]
- E31.Gaudet MM, Kitahara CM, Newton CC, et al. Anthropometry and head and neck cancer: a pooled analysis of cohort data. Int J Epidemiol. 2015;44:673–681. doi: 10.1093/ije/dyv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E32.Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol. 2013;24:609–617. doi: 10.1093/annonc/mds244. [DOI] [PubMed] [Google Scholar]
- E33.Sergentanis TN, Antoniadis AG, Gogas HJ, et al. Obesity and risk of malignant melanoma: a meta-analysis of cohort and case-control studies. Eur J Cancer. 2013;49:642–657. doi: 10.1016/j.ejca.2012.08.028. [DOI] [PubMed] [Google Scholar]
- E34.Niedermaier T, Behrens G, Schmid D, Schlecht I, Fischer B, Leitzmann MF. Body mass index, physical activity, and risk of adult meningioma and glioma: a meta-analysis. Neurology. 2015;85:1342–1350. doi: 10.1212/WNL.0000000000002020. [DOI] [PubMed] [Google Scholar]
- E35.Larsson SC, Wolk A. Body mass index and risk of non-Hodgkin‘s and Hodgkin‘s lymphoma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47:2422–2430. doi: 10.1016/j.ejca.2011.06.029. [DOI] [PubMed] [Google Scholar]
- E36.Duan P, Hu C, Quan C, et al. Body mass index and risk of lung cancer: systematic review and dose-response meta-analysis. Sci Rep. 2015;5 doi: 10.1038/srep16938. 16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E37.Hidayat K, Du X, Chen G, Shi M, Shi B. Abdominal obesity and lung cancer risk: systematic review and meta-analysis of prospective studies. Nutrients. 2016;8 doi: 10.3390/nu8120810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Chen GC, Chen SJ, Zhang R, et al. Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obes Rev. 2016;17:1167–1177. doi: 10.1111/obr.12443. [DOI] [PubMed] [Google Scholar]
- E39.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer—a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–1671. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- E40.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 524 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E41.Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv088. pii:djv088. [DOI] [PubMed] [Google Scholar]
- E42.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E43.Psaltopoulou T, Ntanasis-Stathopoulos I, Tzanninis IG, Kantzanou M, Georgiadou D, Sergentanis TN. Physical activity and gastric cancer risk: a systematic review and meta-analysis. Clin J Sport Med. 2016;26:445–464. doi: 10.1097/JSM.0000000000000316. [DOI] [PubMed] [Google Scholar]
- E44.Liu L, Shi Y, Li T, et al. Leisure time physical activity and cancer risk: evaluation of the WHO‘s recommendation based on 126 high-quality epidemiological studies. Br J Sports Med. 2016;50:372–378. doi: 10.1136/bjsports-2015-094728. [DOI] [PubMed] [Google Scholar]
- E45.Behrens G, Jochem C, Schmid D, Keimling M, Ricci C, Leitzmann MF. Physical activity and risk of pancreatic cancer: a systematic review and meta-analysis. Eur J Epidemiol. 2015;30:279–298. doi: 10.1007/s10654-015-0014-9. [DOI] [PubMed] [Google Scholar]
- E46.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 14. 4 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E47.Neilson HK, Farris MS, Stone CR, Vaska MM, Brenner DR, Friedenreich CM. Moderate-vigorous recreational physical activity and breast cancer risk, stratified by menopause status: a systematic review and meta-analysis. Menopause. 2017;24:322–344. doi: 10.1097/GME.0000000000000745. [DOI] [PubMed] [Google Scholar]
- E48.Behrens G, Leitzmann MF. The association between physical activity and renal cancer: systematic review and meta-analysis. Br J Cancer. 2013;108:798–811. doi: 10.1038/bjc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E49.Behrens G, Jochem C, Keimling M, Ricci C, Schmid D, Leitzmann MF. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. Eur J Epidemiol. 2014;29:151–170. doi: 10.1007/s10654-014-9895-2. [DOI] [PubMed] [Google Scholar]
- E50.Vieira AR, Abar L, Chan D, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017;28:1788–1802. doi: 10.1093/annonc/mdx171. [DOI] [PubMed] [Google Scholar]
- E51.Zhao Z, Yin Z, Pu Z, Zhao Q. Association between consumption of red and processed meat and pancreatic cancer risk: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15:486–493. doi: 10.1016/j.cgh.2016.09.143. [DOI] [PubMed] [Google Scholar]
- E52.Xue XJ, Gao Q, Qiao JH, Zhang J, Xu CP, Liu J. Red and processed meat consumption and the risk of lung cancer: a dose-response meta-analysis of 33 published studies. Int J Clin Exp Med. 2014;7:1542–1553. [PMC free article] [PubMed] [Google Scholar]
- E53.Guo J, Wei W, Zhan L. Red and processed meat intake and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2015;151:191–198. doi: 10.1007/s10549-015-3380-9. [DOI] [PubMed] [Google Scholar]
- E54.Wu J, Zeng R, Huang J, et al. Dietary protein sources and incidence of breast cancer: a dose-response meta-analysis of prospective studies. Nutrients. 2016;8 730. doi: 10.3390/nu8110730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E55.Fang X, Wei J, He X, et al. Landscape of dietary factors associated with risk of gastric cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. 2015;51:2820–2832. doi: 10.1016/j.ejca.2015.09.010. [DOI] [PubMed] [Google Scholar]
- E56.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343 doi: 10.1136/bmj.d6617. d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E57.Aune D, Chan DS, Greenwood DC, et al. Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2012;23:1394–1402. doi: 10.1093/annonc/mdr589. [DOI] [PubMed] [Google Scholar]
- E58.Aune D, Lau R, Chan DS, et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology. 2011;141:106–118. doi: 10.1053/j.gastro.2011.04.013. [DOI] [PubMed] [Google Scholar]
- E59.Vieira AR, Abar L, Vingeliene S, et al. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol. 2016;27:81–96. doi: 10.1093/annonc/mdv381. [DOI] [PubMed] [Google Scholar]
- E60.Luo J, Yang Y, Liu J, et al. Systematic review with meta-analysis: meat consumption and the risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2014;39:913–922. doi: 10.1111/apt.12678. [DOI] [PubMed] [Google Scholar]
- E61.Zhang S, Wang Q, He J. Intake of red and processed meat and risk of renal cell carcinoma: a meta-analysis of observational studies. Oncotarget. 2017;8:77942–77956. doi: 10.18632/oncotarget.18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E62.Kolahdooz F, van der Pols JC, Bain CJ, et al. Meat, fish, and ovarian cancer risk: results from 2 Australian case-control studies, a systematic review, and meta-analysis. Am J Clin Nutr. 2010;91:1752–1763. doi: 10.3945/ajcn.2009.28415. [DOI] [PubMed] [Google Scholar]
- E63.Wang A, Zhu C, Fu L, et al. Citrus fruit intake substantially reduces the risk of esophageal cancer: a meta-analysis of epidemiologic studies. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001390. e1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E64.Lunet N, Lacerda-Vieira A, Barros H. Fruit and vegetables consumption and gastric cancer: a systematic review and meta-analysis of cohort studies. Nutr Cancer. 2005;53:1–10. doi: 10.1207/s15327914nc5301_1. [DOI] [PubMed] [Google Scholar]
- E65.Vieira AR, Vingeliene S, Chan DS, et al. Fruits, vegetables, and bladder cancer risk: a systematic review and meta-analysis. Cancer Med. 2015;4:136–146. doi: 10.1002/cam4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E66.Chen GC, Lv DB, Pang Z, Liu QF. Fruits and vegetables consumption and risk of non-Hodgkin‘s lymphoma: a meta-analysis of observational studies. Int J Cancer. 2013;133:190–200. doi: 10.1002/ijc.27992. [DOI] [PubMed] [Google Scholar]
- E67.Robert Koch-Institut. German Centre for Cancer Registry Data. Database query 2017. www.krebsdaten.de/Krebs/DE/Datenbankabfrage/datenbankabfrage_stufe1_node.html (last accessed on 12 December 2017) [Google Scholar]
- E68.Statistisches Bundesamt. Bevölkerung Deutschlands bis 2060 - Ergebnisse der 13. koordinierten Bevölkerungsvorausberechnung. Wiesbaden, Germany, Statistisches Bundesamt 2015. www.destatis.de/DE/Publikationen/Thematisch/Bevoelkerung/VorausberechnungBevoelkerung/BevoelkerungDeutschland2060_5124202159004.pdf;jsessionid=D06F3A9634A843F70E6D0D907E659818.InternetLive1?__blob=publicationFile (last accessed on 14 December 2017) [Google Scholar]
- E69.Robert Koch-Institut. Krebs in Deutschland. Berlin: Robert Koch-Institute 2017. www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_/krebs_in_deutschland_.pdf?__blob=publicationFile (last accessed on 19 December 2017) [Google Scholar]
- E70.Robert Koch-Institut. Zentrum für Krebsregisterdaten. Methoden. Vollzähligkeitsschätzung. 2017. www.krebsdaten.de/Krebs/DE/Content/Methoden/Vollzaehligkeitsschaetzung/vollzaehligkeitsschaetzung_node.html (last accessed on 4 December 2017) [Google Scholar]
- E71.Parkin DM, Boyd L, Walker LC. 16 The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105)(2):S77–S81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E72.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;99:325–332. doi: 10.1093/oxfordjournals.aje.a121617. [DOI] [PubMed] [Google Scholar]
- E73.James WPT, Jackson-Leach R, Ni Mhurchu C, et al. Overweight and obesity (high body mass index). Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, et al., editors. World Health Organization. Geneva, Switzerland: 2004. pp. p. 497–p. 596. [Google Scholar]
- E74.Bull FC, Armstrong TP, Dixon T, Ham S, Neiman A, Pratt M. Physical inactivity Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. World Health Organization. Geneva, Switzerland: 2004. pp. p. 729–p. 882. [Google Scholar]
- E75.Lock K, Pomerleau J, Causer L, McKee M. Low fruit and vegetable consumption Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. World Health Organization. Geneva, Switzerland: 2004. pp. p. 597–p. 728. [Google Scholar]
- E76.Murray CJL, Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S. Comparative quantification of health risks: conceptual framework and methodological issues. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. World Health Organization. Geneva, Switzerland: 2004. pp. p. 1–p 38. [Google Scholar]
- E77.Vander Hoorn S, Ezzati M, Rodgers A, Lopez AD, Murray CJL. Estimating attributable burden of disease from exposure and hazard data. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. World Health Organization. Geneva, Switzerland: 2004. pp. p. 2129–p 2140. [Google Scholar]
- E78.Ezzati M, Vander Hoorn S, Rodgers A, Lopez AD, Mathers CD, Murray CJL. Potential health gains from reducing multiple risk factors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. World Health Organization. Geneva, Switzerland: 2004. pp. p. 2167–p 2190. [Google Scholar]
- E79.Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131–139. doi: 10.1016/j.ypmed.2014.06.018. [DOI] [PubMed] [Google Scholar]
- E80.de Vries E, Quintero DC, Henriquez-Mendoza G, Herran OF. Population attributable fractions for colorectal cancer and red and processed meats in Colombia—a macro-simulation study. Colomb Med (Cali) 2017;48:64–69. [PMC free article] [PubMed] [Google Scholar]
- E81.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- E82.Grundy A, Poirier AE, Khandwala F, McFadden A, Friedenreich CM, Brenner DR. Cancer incidence attributable to red and processed meat consumption in Alberta in 2012. CMAJ Open. 2016;4:E768–E775. doi: 10.9778/cmajo.20160036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E83.Grundy A, Poirier AE, Khandwala F, McFadden A, Friedenreich CM, Brenner DR. Cancer incidence attributable to insufficient fibre consumption in Alberta in 2012. CMAJ Open. 2017;5:E7–E13. doi: 10.9778/cmajo.20160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E84.Grundy A, Poirier AE, Khandwala F, McFadden A, Friedenreich CM, Brenner DR. Cancer incidence attributable to insufficient fruit and vegetable consumption in Alberta in 2012. CMAJ Open. 2016;4:E760–E767. doi: 10.9778/cmajo.20160037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E85.Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005–systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23:1362–1369. doi: 10.1093/annonc/mdr437. [DOI] [PubMed] [Google Scholar]
- E86.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- E87.Kendall BJ, Wilson LF, Olsen CM, et al. Cancers in Australia in 2010 attributable to overweight and obesity. Aust N Z J Public Health. 2015;39:452–457. doi: 10.1111/1753-6405.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E88.Nagle CM, Wilson LF, Hughes MC, et al. Cancers in Australia in 2010 attributable to inadequate consumption of fruit, non-starchy vegetables and dietary fibre. Aust N Z J Public Health. 2015;39:422–428. doi: 10.1111/1753-6405.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E89.Nagle CM, Wilson LF, Hughes MC, et al. Cancers in Australia in 2010 attributable to the consumption of red and processed meat. Aust N Z J Public Health. 2015;39:429–433. doi: 10.1111/1753-6405.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E90.Olsen CM, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to insufficient physical activity. Aust N Z J Public Health. 2015;39:458–463. doi: 10.1111/1753-6405.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E91.Parkin DM, Boyd L. 4 Cancers attributable to dietary factors in the UK in 2010. I. Low consumption of fruit and vegetables. Br J Cancer. 2011;105(2):S19–S23. doi: 10.1038/bjc.2011.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E92.Parkin DM. 5Cancers attributable to dietary factors in the UK in 2010. II. Meat consumption. Br J Cancer. 2011;105(2):S24–S26. doi: 10.1038/bjc.2011.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E93.Parkin DM, Boyd L. 6 Cancers attributable to dietary factors in the UK in 2010. III. Low consumption of fibre. Br J Cancer. 2011;105(2):S27–S30. doi: 10.1038/bjc.2011.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E94.Parkin DM. 7 Cancers attributable to dietary factors in the UK in 2010. IV. Salt. Br J Cancer. 2011;105(2):S31–S33. doi: 10.1038/bjc.2011.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E95.Parkin DM, Boyd L. 8 Cancers attributable to overweight and obesity in the UK in 2010. Br J Cancer. 2011;105(2):S34–S37. doi: 10.1038/bjc.2011.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E96.Parkin DM. 9 Cancers attributable to inadequate physical exercise in the UK in 2010. Br J Cancer. 2011;105(2):S38–S41. doi: 10.1038/bjc.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E97.Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126:692–702. doi: 10.1002/ijc.24803. [DOI] [PubMed] [Google Scholar]
- E98.Richardson A, Hayes J, Frampton C, Potter J. Modifiable lifestyle factors that could reduce the incidence of colorectal cancer in New Zealand. N Z Med J. 2016;129:13–20. [PubMed] [Google Scholar]
- E99.Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118:1130–1141. doi: 10.1038/s41416-018-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E100.Azevedo ESG, de Moura L, Curado MP, et al. The fraction of cancer attributable to ways of life, infections, occupation, and environmental agents in brazil in 2020. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148761. e0148761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E101.Vingeliene S, Chan DS, Aune D, et al. An update of the WCRF/AICR systematic literature review on esophageal and gastric cancers and citrus fruits intake. Cancer Causes Control. 2016;27:837–851. doi: 10.1007/s10552-016-0755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E102.WCRF/AICR. www.aicr.org/assets/docs/pdf/reports/Second_Expert_Report.pdf (last accessed on 8 August 2017) Washington DC: Food, nutrition, physical activity, and the prevention of cancer: a global perspective. [Google Scholar]
- E103.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women‘s Health Study. Int J Obes Relat Metab Disord. 2003;27:1447–1452. doi: 10.1038/sj.ijo.0802437. [DOI] [PubMed] [Google Scholar]
- E104.Mensink GB, Schienkiewitz A, Haftenberger M, Lampert T, Ziese T, Scheidt-Nave C. [Overweight and obesity in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:786–794. doi: 10.1007/s00103-012-1656-3. [DOI] [PubMed] [Google Scholar]
- E105.Völzke H, Ittermann T, Schmidt CO, et al. Prevalence trends in lifestyle-related risk factors—two cross-sectional analyses with a total of 8728 participants from the Study of Health in Pomerania from 1997 to 2001 and 2008 to 2012. Dtsch Arztebl Int. 2015;112:185–192. doi: 10.3238/arztebl.2015.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E106.Gose M, Krems C, Heuer T, Hoffmann I. Trends in food consumption and nutrient intake in Germany between 2006 and 2012: results of the German National Nutrition Monitoring (NEMONIT) Br J Nutr. 2016;115:1498–1507. doi: 10.1017/S0007114516000544. [DOI] [PubMed] [Google Scholar]
- E107.Jankovic N, Geelen A, Winkels RM, et al. Adherence to the WCRF/AICR dietary recommendations for cancer prevention and risk of cancer in elderly from Europe and the United States: a meta-analysis within the CHANCES project. Cancer Epidemiol Biomarkers Prev. 2017;26:136–144. doi: 10.1158/1055-9965.EPI-16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E108.Lin XJ, Wang CP, Liu XD, et al. Body mass index and risk of gastric cancer: a meta-analysis. Jpn J Clin Oncol. 2014;44:783–791. doi: 10.1093/jjco/hyu082. [DOI] [PubMed] [Google Scholar]
- E109.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- E110.Yang P, Zhou Y, Chen B, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867–2873. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- E111.Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: A quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34:521–543. doi: 10.1080/07315724.2014.992553. [DOI] [PMC free article] [PubMed] [Google Scholar]