Abstract
Background
Causal relationships with the occurrence of cancer have been established for a number of infections and environmental risk factors.
Methods
Numbers and proportions (population-attributable fractions, PAF) of cancer cases attributable to these factors in Germany were calculated by sex and age groups for ages 35 to 84 years based on population projections, national cancer incidence, exposure data, and published risk estimates.
Results
For 2018, more than 17 600 cancer cases (4.0% of all incident cancers) were estimated to be attributable to infections. The largest contributions come from Helicobacter pylori (n = 8764) and human papillomavirus (n = 7669) infections. Infection with hepatitis B and C, human immunodeficiency virus, and human herpesvirus 8 were estimated to cause 983 cases, 144 cases, and 116 cases, respectively. More than 5400 cancer cases (1.2% of all incident cancers) were estimated to be attributable to selected environmental factors, of which the largest contributor is indoor radon (n = 3185), followed by particulate matter (n = 1049), sunbed use (n = 892), and secondhand smoke (n = 309).
Conclusion
Of all cancers expected in 2018 in Germany, at least 5% are attributable to potentially avoidable infections and environmental factors. Further research should be directed towards more comprehensive identification and quantification of environmental risks as a basis for targeted cancer prevention.
In a broad sense, people are exposed to a wide range of carcinogenic agents from different sources in their environment. A causal relationship with the occurrence of cancer has been established between exposure to numerous infections and environmental factors (1, 2). This study provides estimates of the burden of incident cancer cases attributable to infections (Helicobacter pylori, human papillomavirus, hepatitis B, hepatitis C, human immunodeficiency virus, human herpesvirus 8) and major environmental factors (secondhand smoke, indoor radon, particulate matter, sunbed use) in Germany in 2018.
Methods
To provide estimates of cancer cases attributable to infectious and environmental agents, we calculated population-attributable fractions (PAF; for details see the Box in Mons et al., this issue). In this study, we considered six infectious and four environmental agents that fulfilled the following criteria (table):
Key Messages.
We used population-attributable fractions (PAF) to estimate the proportion of cancer incidence attributable to exposure to infectious agents and selected environmental factors in 2018.
Of all incident cancer cases expected to occur at ages 35 to 84 years in Germany in 2018 (ca. 440 000 cases), an estimated 4% (ca. 17 600 cases) are caused by infections and at least 1.2% (5400 cases) are caused by excess levels of selected environmental factors.
About 90% of cancer cases estimated to be attributable to infections are caused by infection with Helicobacter pylori and human papillomavirus.
Both national vaccination programs and individual vaccination advice delivered by health professionals could be effective in reducing the incidence of infection-related cancer cases.
Given our exclusive focus on environmental factors for which both reliable exposure data and reliable risk estimates for specific cancers are available and the lack of such data for many other environmental factors, the total numbers and proportions of cancers attributable to environmental factors are likely to be much higher.
Table. Infectious agents and environmental factors with corresponding cancer sites, reference exposure level, and prevalence.
| Exposure | Cancer site (ICD-10) | Reference exposure level | Prevalence (%) | ||
| of exposure in population | of DNA in tumor cells | Reference | |||
| Infections | |||||
| Helicobacter pylori (H. pylori) | Stomach (C16), non-cardia | No infection | 40.0 | (26) | |
| Gastric MALT lymphoma (C88.4) | |||||
| Human papillomavirus (HPV) | Oral cavity (C02–C04) | No infection | 3.7 | (6) | |
| Oropharynx (C01, C05, C09, C10) | 19.9 | (6) | |||
| Anus and anal canal (C21) | 87.6 | (7) | |||
| Vulva (C51) | 18.3 | (8) | |||
| Vagina (C52) | 71.0 | (9) | |||
| Cervix (C53) | 100.0 | (11) | |||
| Penis (C60) | 32.2 | (10) | |||
| Hepatitis B virus (HBV) | Liver (C22), hepatocellular carcinoma | No infection | 0.3 | (27) | |
| Hepatitis C virus (HCV) | Liver (C22), hepatocellular carcinoma | No infection | 0.3 | (27) | |
| Non-Hodgkin lymphoma (C82–C88) | |||||
| Human immunodeficiency virus (HIV) | Non-Hodgkin lymphoma (C82–C88) | No infection | 0.1 | (15) | |
| Human herpesvirus 8 (HHV-8) | Kaposi sarcoma (C46) | No infection | 100.0 | (5) | |
| En | |||||
| Environmental factors | |||||
| Secondhand smoke (SHS) | Lung (C33–C34) | No exposure to SHS | 25.9* | (12) | |
| Particulate matter (PM10) | Lung (C33–C34) | Mean annual ‧exposure to PM10 below the WHO ‧guideline value | 23.0 | (22) | |
| Indoor radon | Lung (C33–C34) | Natural outdoor radon ‧concentration | 100.0 | (19) | |
| Sunbed use | Skin melanoma (C43) | Never use of sunbeds | 28.0 | (24) | |
* For secondhand smoke, the population refers to all never smokers
MALT, mucosa-associated lymphoid tissue
The risk factor has been classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC);
The exposure is potentially modifiable, i.e., a decrease of risk factor exposure can be deemed effective in reducing the risk of cancer; and
Suitable data on the distribution of risk factors could be obtained for Germany.
Statistical methods
As described in Mons et al. in this issue, we calculated PAF by sex and age groups for ages 35 to 84 years combining the prevalence of exposure and the cancer site–specific relative risk (RR). For infection with human papillomavirus, PAF estimates are based on the proportion of tumors in which viral DNA was prevalent. To estimate the number of cancer cases attributable to each risk factor, we multiplied the PAFs by the projected cancer incidence for the year 2018 (eTables 1– 3). The methods are reported in detail in the supplement to this article (eSupplement A and B).
Data sources and assumptions—infectious agents
Helicobacter pylori
Helicobacter pylori infection is typically acquired in childhood; in the absence of specific treatment, it typically persists throughout adulthood. National prevalence data on H. pylori infection were available from the German National Health Interview and Examination Survey 1998 (GNHIES98; eSupplement C, eTable 4). For the association between H. pylori infection and risk of non-cardia stomach cancer and low-malignant mucosa-associated lymphoid tissue (MALT) gastric lymphoma, we used pooled risk estimates from prospective studies (3, 4) (eFigure 1).
Human papillomavirus (HPV)
In order to estimate the PAF for infection with HPV, the prevalence of viral DNA in tumor material is sufficient to infer that HPV caused the cancer (5). The PAF for malignancies of the oral cavity or oropharynx were estimated using the combination of positivity for HPV-DNA and for either E6*I mRNA expression or p16INK4a (6). For cancers of the anus (7), vulva (8), vagina (9), and penis (10), the PAF were derived from HPV-DNA prevalence in invasive cancers. For cancer of the cervix uteri all cases were assumed to be attributable to HPV (11) (table).
Hepatitis B (HBV) and hepatitis C (HCV)
Assuming that combined infection by both HBV and HCV is very rare, and assuming a latency period of 10 years between ascertainment of infection status and cancer diagnosis, we used seroprevalence from the nationally representative German Health Interview and Examination Survey for Adults for the period 2008 to 2011 (DEGS1) (12). In the process, seropositivity for HBsAG and the prevalence of HCV antibodies were used as indicators for chronic infection with HBV or HCV (eTable 4). We used the relative risks for hepatocellular carcinoma reported in a meta-analysis of HBV and HCV mono-infection comprising studies from low-endemic countries (13). Summary relative risks for non-Hodgkin lymphoma associated with HCV were taken from a meta-analysis of epidemiological studies (14) (eFigure 1).
Human immunodeficiency virus (HIV) and human herpesvirus 8 (HHV-8)
Based on the absolute number of people living with HIV in Germany in 2016, the relative prevalence in Germany in 2018 was calculated (15) (eSupplement C, eTable 4).
For uterine cervix and Kaposi sarcoma the PAF were not calculated separately, as both HPV and HHV-8 are considered necessary causal factors (5, 11). The PAF were also not estimated for anal cancer and Hodgkin lymphoma, because it is assumed that these cancers occur due to co-infection with HPV and Epstein–Barr virus. Due to the absence of information on incidence, conjunctival cancer was not considered in the analysis. To estimate the number of cases of HIV-attributable non-Hogkin lymphoma, relative risk estimates were taken from a population-based registry-linkage study (16) (eFigure 1).
Data sources and assumptions—environmental factors
Secondhand smoke (SHS)
Assuming a latency period of 10 years between SHS exposure and cancer incidence, we used prevalence of self-reported exposure to SHS at home, at the workplace, and in leisure time from DEGS1 2008–2011 (12) (eTable 5). A meta-analysis of the relative risk of lung cancer due to SHS among never smokers provided a lung cancer risk associated with workplace exposure (17), which we assumed to apply to home and leisure time exposure as well (eFigure 2). The estimated numbers of lung cancers occurring among never smokers were taken from the study by Mons et al. in this issue.
Indoor radon
For exposure to indoor radon, the mean annual indoor radon concentration for Germany was used (18, 19). Concentrations below the estimated outdoor air concentration were not considered for the calculation of attributable cases (eSupplement C). The results from a collaborative analysis of individual data from 13 European studies (20) showed a linear dose–response association between radon exposure and lung cancer risk without a threshold below which an exposure would carry no risk (eFigure 2). As most of the radon-induced cancer cases arise from a synergistic effect of radon and smoking (21), we also estimated the attributable cancer cases caused by indoor radon exposure combined with smoking (eSupplement A).
Particulate matter (PM10)
The proportions of the population at different PM exposure levels for the years 2007–2011 were obtained from the Federal Environmental Agency (22). Based on these, we calculated the mean prevalence of the population exposed to PM10 concentrations (particles with aerodynamic diameter = 10 µm) exceeding the WHO mean annual guideline value (>20 µg/m3) and the corresponding population-weighted mean PM10-concentrations. We defined the excess exposure level by the difference between the mean exposure in those exceeding the guideline value and those who are within the limit (eSupplement A, eTable 6). Risk estimates were taken from a meta-analysis of nine studies examining the risk for lung cancer associated with particulate matter (23) (eFigure 2).
Sunbed use
Sunbeds are a source of artificial ultraviolet (UV) radiation. Assuming a latency period of 10 years between sunbed use and cancer incidence, we used lifetime prevalence from a representative population-based telephone survey from 2007 (24) (eTables 7, 8). We used risk estimates from a meta-analysis of sunbed use and risk of developing malignant melanoma (25) (eFigure 2).
Sensitivity analyses
The methods used for sensitivity analyses are described in eSupplement D.
Results
Infections
Helicobacter pylori
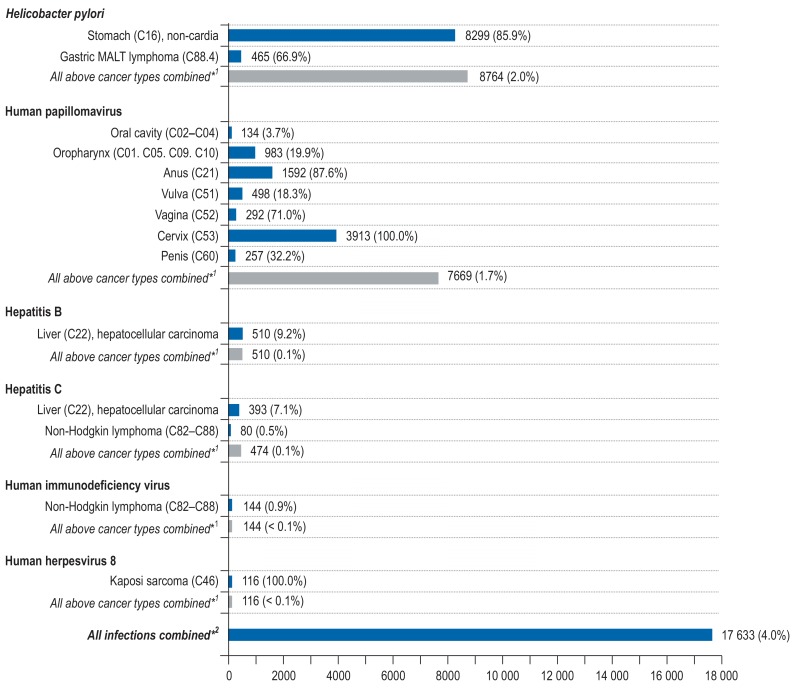
According to GNHIES98, the overall seroprevalence of H. pylori antibodies in the German population in 1998 was 40% (26). We estimated that a total of 8299 (86%) non-cardia stomach cancers (men 4833, women 3466) and 465 (67%) gastric MALT lymphomas (men 208, women 257) are attributable to H. pylori infection (Figure 1, eTables 9– 11).
Figure 1.
Estimated number and proportion of site-specific incident cancer cases attributable to infections among men and women aged 35 to 84 years in Germany for the year 2018.
*1 The population-attributable fraction (PAF) for the category “All above cancer types combined” was computed with respect to total cancer incidence (ICD-10 C00–C99 without C44).
*2 A joint PAF of all infections was calculated assuming independence of infections (28)
Human papillomavirus (HPV)
A total of 7669 cancers of the oral cavity (n=134), oropharynx (n=983), anus (n=1592), vulva (n=498), vagina (n=292), cervix (n=3913), and penis (n=257) were estimated to be attributable to HPV (men 1691, women 5978) (Figure 1, eTables 9– 11).
Hepatitis B (HBV) and hepatitis C (HCV)
The overall prevalence of both HBV and HCV infection in Germany in the period 2008 to 2011 was about 0.3% (27). A total of 903 cases (16%) of hepatocellular carcinoma were estimated to be attributable to HBV and HCV infection (men 732, women 171), and a total of 80 non-Hodgkin lymphomas (<1%) to HCV infection (men 37, women 43) (Figure 1, eTables 9– 11).
Human immunodeficiency virus (HIV) and human herpesvirus 8 (HHV-8)
We estimated that 0.1% of the German population aged 35 to 84 years is infected with HIV in 2018. A total of 144 non-Hodgkin lymphomas (<1%) and 116 cases (100%) of Kaposi sarcoma were estimated to be attributable to HIV and HHV-8 infection (men 224, women 36) (Figure 1, eTables 9– 11).
Sensitivity analyses
Sensitivity analyses using the 95% confidence limits of risk estimates and of HPV prevalences respectively indicated a potential range of 16 000 to 18 800 cancer cases attributable to infections (eTables 12– 13).
Environmental factors
Secondhand smoke
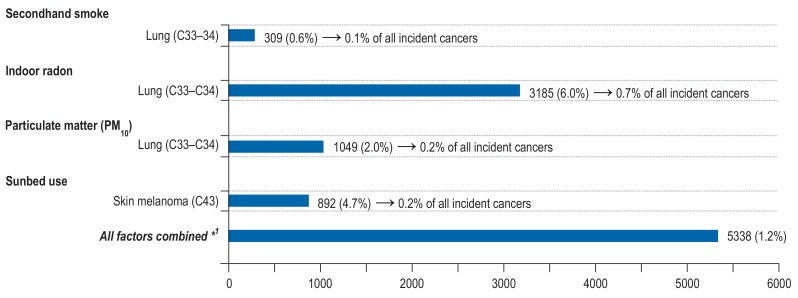
In the period 2008 to 2011, 26% of never smokers aged 25 to 74 years living in Germany were exposed to secondhand smoke. Overall, 309 lung cancer cases were estimated to be attributable to SHS exposure, 212 among men and 97 among women. This corresponds to a PAF of 5% among never-smoking men aged 35 to 84, and a PAF of 3% among never-smoking women (Figure 2, eTable 14).
Figure 2.
Estimated number and proportion of site-specific incident cancer cases attributable to selected environmental factors among men and women aged 35 to 84 years in Germany for the year 2018.
*1A joint PAF of all selected environmental factors was calculated assuming independence of the selected risk factors (28)
Indoor radon
After subtraction of the mean radon outdoor air concentration, the mean annual excess indoor radon concentration was 40 Bq/m³ for Germany. A total of 3185 (6%) lung cancer cases were estimated to be attributable to residential radon (men 2071, women 1114), of which 425 cases (1%) were estimated to be caused by radon alone and 2760 cases (5%) by the combination of smoking and radon (Figure 2, eTable 15).
Particulate matter (PM10)
According to the data of the Federal Environmental Agency, 23% of the German population were exposed to PM10 concentrations exceeding the WHO mean annual guideline value for PM10 in the period 2007 to 2011. Based on this, a total of 1049 (2%) lung cancer cases were estimated to be attributable to PM10 exposure at concentrations above the WHO guideline value (men 682, women 367) (figure 2).
Sunbed use
According to a national representative population-based survey on sunbed use in Germany in 2007, 28% of the German population have used sunbeds at some time in their life (24). We estimated that a total of 892 (5%) malignant melanomas are attributable to ever use of sunbeds (men 360, women 532) (Figure 2, eTable 16). Additional analyses indicated that the vast majority of these cases could be due to highly frequent sunbed use (eTable 17).
Sensitivity analyses
In sensitivity analyses using the 95% confidence limits of risk estimates, estimated numbers of cancer cases attributable to the selected environmental factors ranged from 1500 to 9500 (eTable 18).
Summary of results
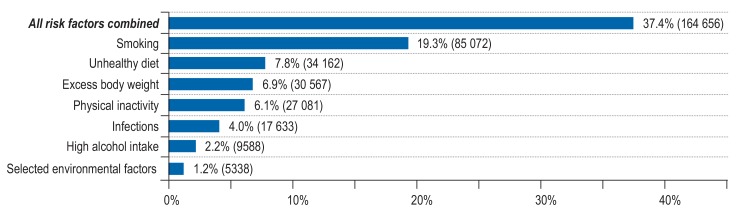
To estimate the total proportion of cancer cases in Germany in 2018 that could be attributed to all modifiable cancer risk factors considered in this article and in the articles by Mons et al. and Behrens et al. in this issue, we combined the single PAF to estimate a joint PAF assuming independence of risk factors (28). We estimated that of all cancer cases to be expected at ages 35 to 84 years in Germany in 2018 (about 440 000 cases), 37.4% of incident cancer cases are attributable to the considered modifiable risk factors and thus are potentially avoidable (figure 3). The lifestyle-related factors, in particular smoking, but also dietary factors, overweight, and physical inactivity, contribute most to this cancer burden. More than 17 600 cases (4.0%) are attributable to infections and more than 5400 cases (1.2%) are attributable to selected environmental factors. Reducing the prevalence of these risk factors in the German population has the potential to substantially reduce the cancer burden.
Figure 3.
Estimated number and proportion of all incident cancer cases (ICD-10 C00-C99 without C44) attributable to the reported lifestyle-related factors, environmental factors and infections among men and women aged 35 to 84 years in Germany for the year 2018
Discussion
Cancer burden attributable to infections
Our analyses indicate that about 4.9% (ca. 9900) of all cancer cases in women and about 3.2% (ca. 7700) of cases in men are due to infections. The cancer burden attributable to infections was estimated to be greater in women than in men, largely because all cervical cancer cases (ca. 3700 cases) are counted as due to HPV infection, as it is considered a necessary causal factor. Among infections, the largest contributors to the cancer burden in the German population were estimated to be H. pylori and HPV infection, with about 8700 and 7600 attributable cancer cases, respectively. In total, they account for about 90% of all cancer cases estimated to be attributable to infectious agents. Overall, our cancer burden estimates for infections were slightly greater than those from previous studies in other countries with comparable income levels, which found proportions of cancer cases attributable to infections in the range of 3.3% to 3.6%. The differences can be explained by a higher prevalence of H. pylori infection in Germany and the use of more recent risk estimates for the association between H. pylori infection and gastric MALT lymphoma.
Effective strategies to reduce the cancer burden attributable to infectious agents include infection control and vaccination promotion. While prevalence of H. pylori infection seems to decline substantially in younger birth cohorts even without specific intervention, enhanced promotion of and adherence to HPV vaccination could reduce the burden of HPV-related cancers. For example, Australia has achieved a considerable fall in the prevalence of the most common high-risk HPV types among 18- to 35-year old women in recent years through the implementation of a comprehensive government-funded national school-based vaccination program (29).
Cancer burden attributable to selected environmental factors
About 1.4% (ca. 3300) of all cancer cases in men and 1.0% (ca. 2000) of cases in women were estimated to be attributable to selected environmental risk factors. Of all these factors, indoor radon accounted for the highest proportion and number of cancer cases (ca. 3100). Overall, the estimated PAF are roughly in line with previous studies (30– 32). With respect to air pollution, a recent study (32) estimated the number of lung cancer cases attributable to fine particles (PM2.5) in the UK to be considerably greater (1.0% of all incident cancer cases) than those in this study, which likely reflects the differences in particle size and reference exposure levels. Generally, it is important to keep in mind that the assumptions that were made to estimate the number of PM10-attributable cancers are quite conservative, as a guideline-based reference level was chosen, even though an increased risk for lung cancer has also been reported for exposure levels below current guidelines (1, 31). All of the considered environmental factors are potentially avoidable and there is evidence that exposure could be reduced by policy measures, such as comprehensive smoke-free legislation (34), building regulations for radon prevention and mitigation (35), road traffic-related emission control interventions to improve air quality (36), and restriction of access to sunbeds (37).
Strengths and limitations
This is the first study to provide estimates of the cancer burden attributable to infections and selected environmental factors in Germany in 2018. Our estimates are based on the latest population projections and cancer registry information and—for most of the considered risk factors—nationally representative survey data on seroprevalence of infections and prevalence of exposure to environmental factors. In agreement with previous studies from other countries, we assumed latency periods for most risk factors, taking into account that current cancer cases were caused by past exposure (38). We were not able to quantify the combined impact of different risk factors due to lack of appropriate data. Generally, we may have underestimated the cancer burden attributable to environmental factors because reliable population-based prevalence estimates were not available for some established risk factors, including exposure to natural solar radiation, which is considered the most important environmental cause of skin cancer (25). In addition, we could not consider numerous potentially carcinogenic environmental factors because they have not yet been sufficiently examined in epidemiological studies.
Conclusion
Our findings suggest that of all cancers expected in 2018 in Germany, at least 5% are attributable to infections and selected environmental factors. Although the estimates rely on several assumptions and not all risk factors could be considered, the results indicate that many thousand cancer cases could be avoided if exposure to these risk factors were reduced or eliminated through effective prevention measures. Further research should be directed towards more comprehensive identification and quantification of environmental risks as a basis for targeted cancer prevention.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
Funding
The study was funded by German Cancer Aid (“Deutsche Krebshilfe”), grant number 70112097.
References
- 1.Espina C, Straif K, Friis S, et al. European Code against Cancer 4th edition: environment, occupation and cancer. Cancer Epidemiol. 2015;39(1):84–92. doi: 10.1016/j.canep.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 3.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 4.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 5.De Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 6.Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv403. djv403. [DOI] [PubMed] [Google Scholar]
- 7.Alemany L, Saunier M, Alvarado-Cabrero I, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136:98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Sanjosé S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49:3450–3461. doi: 10.1016/j.ejca.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Alemany L, Saunier M, Tinoco L, et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer. 2014;50:2846–2854. doi: 10.1016/j.ejca.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Alemany L, Cubilla A, Halec G, et al. Role of human papillomavirus in penile carcinomas worldwide. Eur Urol. 2016;69:953–961. doi: 10.1016/j.eururo.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Scheidt-Nave C, Kamtsiuris P, Gößwald A, et al. German health interview and examination survey for adults (DEGS)-design, objectives and implementation of the first data collection wave. BMC Public Health. 2012;12 doi: 10.1186/1471-2458-12-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho LY, Yang JJ, Ko KP, et al. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Cancer. 2011;128:176–184. doi: 10.1002/ijc.25321. [DOI] [PubMed] [Google Scholar]
- 14.Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15:2078–2085. doi: 10.1158/1055-9965.EPI-06-0308. [DOI] [PubMed] [Google Scholar]
- 15.an der Heiden M, Marcus U, Kollan C, et al. Schätzung der Zahl der HIV-Neuinfektionen und der Gesamtzahl von Menschen mit HIV in Deutschland, Stand Ende 2016. Epid Bull. 2017;47:531–545. [Google Scholar]
- 16.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4:e495–e504. doi: 10.1016/S2352-3018(17)30125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Health Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta 2006. www.surgeongeneral.gov/library/reports/secondhandsmoke/fullreport.pdf (last accessed on 22 July 2018) [Google Scholar]
- 18.Menzler S, Piller G, Gruson M, Rosario AS, Wichmann H-E, Kreienbrock L. Population attributable fraction for lung cancer due to residential radon in Switzerland and Germany. Health Phys. 2008;95:179–189. doi: 10.1097/01.HP.0000309769.55126.03. [DOI] [PubMed] [Google Scholar]
- 19.Schmid K, Kuwert T, Drexler H. Radon in indoor spaces: an underestimated risk factor for lung cancer in environmental medicine. Dtsch Arztebl Int. 2010;107 doi: 10.3238/arztebl.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330 doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McColl N, Auvinen A, Kesminiene A, et al. European Code against Cancer 4th edition: ionising and non-ionising radiation and cancer. Cancer Epidemiol. 2015;39:93–100. doi: 10.1016/j.canep.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Kallweit D, Wintermeyer D. Berechnung der gesundheitlichen Belastung der Bevölkerung in Deutschland durch Feinstaub (PM10) UMID. Umwelt und Mensch-Informationsdienst. 2013;4:18–24. [Google Scholar]
- 23.Hamra GB, Guha N, Cohen A, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect. 2014;122 doi: 10.1289/ehp/1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borner FU, Schutz H, Wiedemann P. A population-based survey on tanning bed use in Germany. BMC Dermatol. 2009;9 doi: 10.1186/1471-5945-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345 doi: 10.1136/bmj.e4757. e4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bornemann R, Gaber E. Themenheft 55 „Gastritis, Magen-und Zwölffingerdarmgeschwüre“ Berlin: Robert Koch-Institut, 2013. www.rki.de/DE/Content/Gesundheitsmonitoring/Gesundheitsberichterstattung/GBEDownloadsT/gastritis.pdf?__blob=publicationFile (last accessed on 30 July 2018) [Google Scholar]
- 27.Poethko-Muller C, Zimmermann R, Hamouda O, et al. [Epidemiology of hepatitis A, B, and C among adults in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:707–715. doi: 10.1007/s00103-013-1673-x. [DOI] [PubMed] [Google Scholar]
- 28.Ezzati M, Vander Hoorn S, Rodgers A, et al. Estimates of global and regional potentil health gains from reducing muliple major risk factors. Lancet. 2003;362:271–280. doi: 10.1016/s0140-6736(03)13968-2. [DOI] [PubMed] [Google Scholar]
- 29.Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus (HPV) types among 18 to 35 year old Australian women, nine years following implementation of vaccination. J Infect Dis. 2018;217:1590–1600. doi: 10.1093/infdis/jiy075. [DOI] [PubMed] [Google Scholar]
- 30.Wilson LF, Antonsson A, Green AC, et al. How many cancer cases and deaths are potentially preventable? Estimates for Australia in 2013. Int J Cancer. 2018;142:691–701. doi: 10.1002/ijc.31088. [DOI] [PubMed] [Google Scholar]
- 31.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 32.Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. 2018;118:1130–1141. doi: 10.1038/s41416-018-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 34.Frazer K, Callinan JE, McHugh J, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst Rev. 2016;2 doi: 10.1002/14651858.CD005992.pub3. CD005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coskeran T, Denman A, Phillips P, Tornberg R. A critical evaluation of the cost-effectiveness of radon protection methods in new homes in a radon affected area of England. Environ Int. 2009;35:943–951. doi: 10.1016/j.envint.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Zhong B, Vardoulakis S, et al. Air quality strategies on public health and health equity in Europe-a systematic review. Int J Environ Res Public Health. 20161;3 doi: 10.3390/ijerph13121196. pii: E1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson M, Holman DM, Fox KA, et al. Preventing skin cancer through reduction of indoor tanning: current evidence. Am J Prev Med. 2013;44:682–689. doi: 10.1016/j.amepre.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkin D. 1 The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105 doi: 10.1038/bjc.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–541. [PubMed] [Google Scholar]
- E2.US Department of Health Human Services. Atlanta: 2006. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, [Google Scholar]
- E3.Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330 doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Hamra GB, Guha N, Cohen A, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect. 2014;122 doi: 10.1289/ehp/1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345 doi: 10.1136/bmj.e4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Cho LY, Yang JJ, Ko KP, et al. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Cancer. 2011;128:176–184. doi: 10.1002/ijc.25321. [DOI] [PubMed] [Google Scholar]
- E7.Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15:2078–2085. doi: 10.1158/1055-9965.EPI-06-0308. [DOI] [PubMed] [Google Scholar]
- E8.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- E9.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pyloriinfection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- E10.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4:495–504. doi: 10.1016/S2352-3018(17)30125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Schmid K, Kuwert T, Drexler H. Radon in indoor spaces: an underestimated risk factor for lung cancer in environmental medicine. Dtsch Arztebl Int. 2010;107 doi: 10.3238/arztebl.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Ezzati M, Vander Hoorn S, Rodgers A, et al. Estimates of global and regional potentil health gains from reducing muliple major risk factors. Lancet. 2003;362:271–280. doi: 10.1016/s0140-6736(03)13968-2. [DOI] [PubMed] [Google Scholar]
- E13.Statistisches Bundesamt. Bevölkerung Deutschlands bis 2060 - Ergebnisse der 13 koordinierten Bevölkerungsvorausberechnung. Wiesbaden, Germany. www.destatis.de/DE/Publikationen/Thematisch/Bevoelkerung/VorausberechnungBevoelkerung/BevoelkerungDeutschland2060_5124202159004.pdf;jsessionid=D06F3A9634A843F70E6D0D907E659818.InternetLive1?__ blob=publicationFile: Statistisches Bundesamt 2015 (last accessed on14 December 2017) [Google Scholar]
- E14.Robert Koch Institute. German Centre for Cancer Registry Data. Database Query 2017. www.krebsdaten.de/Krebs/DE/Datenbankabfrage/datenbankabfrage_stufe1_node.html(last accessed 12 December 2017) [Google Scholar]
- E15.Robert Koch Institute. Bericht zum Krebsgeschehen. Berlin: Robert Koch Institute 2016. www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebsgeschehen/Krebsgeschehen_download.pdf?__ blob=publicationFile (last accessed 14 February 2018) [Google Scholar]
- E16.Robert Koch Institute. Krebs in Deutschland für 2013/2014. Berlin: Robert Koch Institute 2017; www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_7/krebs_in_ deutschland_7.pdf?__blob=publicationFile (last accessed on 14 February 2018) [Google Scholar]
- E17.Wu X-C, Andrews P, Chen VW, Groves FD. Incidence of extranodal non-Hodgkin lymphomas among whites, blacks, and Asians/Pacific Islanders in the United States: anatomic site and histology differences. Cancer Epidemiol. 2009;33:337–346. doi: 10.1016/j.canep.2009.09.006. [DOI] [PubMed] [Google Scholar]
- E18.Feller A. Springer. Berlin Heidelberg: 2006. Klassifikation der Non-Hodgkin-Lymphome Kompendium Internistische Onkologie; pp. 2829–2844. [Google Scholar]
- E19.Scheidt-Nave C, Kamtsiuris P, Gößwald A, et al. German health interview and examination survey for adults (DEGS)-design, objectives and implementation of the first data collection wave. BMC public health. 2012;12 doi: 10.1186/1471-2458-12-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.Poethko-Muller C, Zimmermann R, Hamouda O, et al. [Epidemiology of hepatitis A, B, and C among adults in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:707–715. doi: 10.1007/s00103-013-1673-x. [DOI] [PubMed] [Google Scholar]
- E21.Bornemann R, Gaber E. Themenheft 55“ Gastritis, Magen-und Zwölffingerdarmgeschwüre“. 2013 [Google Scholar]
- E22.Statistisches Bundesamt. Bevölkerung und Erwerbstätigkeit - Rückgerechnete und fortgeschriebene Bevölkerung auf Grundlage des Zensus 2011 Wiesbaden, Germany. www.destatis.de/DE/Publikationen/Thematisch/Bevoelkerung/Bevoelkerungsstand/RueckgerechneteBevoelkerung5124105119004.pdf?__blob=publicationFile: Statistisches Bundesamt 2016 (last accessed 22 February 2018) [Google Scholar]
- E23.an der Heiden M, Marcus U, Kollan C, et al. Schätzung der Zahl der HIV-Neuinfektionen und der Gesamtzahl von Menschen mit HIV in Deutschland, Stand Ende 2016. Epid Bull. 2017;47:531–545. [Google Scholar]
- E24.McColl N, Auvinen A, Kesminiene A, et al. European Code against Cancer 4th Edition: ionising and non-ionising radiation and cancer. Cancer Epidemiol. 2015;39:93–100. doi: 10.1016/j.canep.2015.03.016. [DOI] [PubMed] [Google Scholar]
- E25.Kummel M, Dushe C, Muller S, Gehrcke K. Outdoor (222)Rn-concentrations in Germany - part 1 - natural background. J Environ Radioact. 2014;132:123–130. doi: 10.1016/j.jenvrad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- E26.Borner FU, Schutz H, Wiedemann P. A population-based survey on tanning bed use in Germany. BMC Dermatol. 2009;9 doi: 10.1186/1471-5945-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- E28.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- E29.Umweltbundesamt. Gesundheitsrisiken der Bevölkerung durch Feinstaub. Berlin: Umweltbundesamt 2017. www.umweltbundesamt.de/daten/umwelt-gesundheit/gesundheitsrisiken-der-bevoelkerung-durch-feinstaub (last accessed on 14 December 2017) [Google Scholar]