Abstract
Background: Epidemiological evidences regarding the association between the use of non-steroidal anti-inflammatory drugs (NSAIDs) and the risk of prostate cancer (PC) is still controversial. Therefore, we conducted a meta-analysis to explore the controversy that exists.
Methods: Electronic databases including Medline, EMBASE, Web of Science, Cochrane Library, BIOSIS, Scopus, CBM, CNKI, WANFANG, and CQVIP were used to search for and identify eligible studies published until December 31, 2017. Pooled effect estimates for the relative risk (RR) were computed through fixed-effects or random-effects models as appropriate. Publication bias was evaluated by Egger's and Begg's tests and potential sources of heterogeneity were investigated in subgroup analyses.
Results: A total of 43 observational studies were eligible for this meta-analysis. A protective effect was identified for the intake of any NSAIDs on the risk of PC (pooled RR = 0.89, 95% CI = 0.81–0.98). Moreover, the long-term intake of NSAIDs (≥5 years rather than ≥4 years) was associated with reduced PC incidence (pooled RR = 0.882, 95% CI = 0.785–0.991). Aspirin intake was also associated with a 7.0% risk reduction of PC (pooled RR = 0.93, 95% CI = 0.89–0.96). The inverse association became stronger for advanced PC and PC with a Gleason score ≥7 compared to the association with total PC. Interestingly, it was the daily dose (≥1 pill/day) rather than, long-term aspirin intake (≥4 or ≥5 years) that was associated with reduced PC incidence (pooled RR = 0.875, 95% CI = 0.792–0.967). The pooled effects for non-aspirin NSAIDs demonstrated no significantly adverse or beneficial effects on total PC, advanced PC, or PC with Gleason score ≥7, though all pooled RRs were >1.
Conclusions: Our findings suggested a protective effect of the intake of any NSAIDs on the risk of PC, especially in those who took the NSAIDs for a long period. Moreover, aspirin intake was also associated with a decreased risk of PC, and there was a dose related association between aspirin intake and the risk of PC, while no significant effects of long-term aspirin intake were found on the PC incidence.
Keywords: non-steroidal anti-inflammatory drugs, aspirin, prostate cancer, risk, meta-analysis
Introduction
Rationale
PC is the most prevalent cancer in male and the third leading cause of cancer-related death worldwide (1). It has been estimated that 26,730 American men died of PC in 2017 (2). Except for the three already well-established non-modifiable risk factors, age, race, and family history, the etiology of PC remains largely unknown (3, 4). Therefore, it is important to identify effective methods of preventing PC, which may subsequently reduce the substantial burden placed on society by this significant health issue.
Experimental studies suggested that chronic inflammation is involved in the carcinogenesis of PC, especially high-grade PC (5–8). It was demonstrated that tumor cell proliferation and resistance to apoptosis were enhanced through the synthesis of pro-tumor and immunosuppressive cytokines that are present in a chronically inflamed environment. Given the anti-inflammatory and antithrombotic properties of non-steroidal anti-inflammatory drugs (NSAIDs), it is very important to discern their potential role in the development of PC. NSAIDs suppress inflammation and the synthesis of prostaglandin by inhibiting the cyclooxygenase enzyme (COX). Mechanistically, studies verified that NSAIDs, like aspirin, exhibit their chemopreventive effects through both isoforms of the COX enzyme pathway (COX-1 and COX-2). The micrometastasis of PC cells was impaired by the antithrombotic effect of COX-1 inhibition in platelets, which could release pro-angiogenic factors to facilitate the escape of cancer cells from immune surveillance (9, 10). Meanwhile, COX-2 is significantly over-expressed in human PC tumor tissues (2, 11), and the blockage of COX-2 could prevent the production of downstream prostanoids, which contribute to tumorigenesis by promoting cell proliferation, induction, angiogenesis, invasion, and metastasis (12).
Epidemiological studies reported an inverse association between the intake of NSAIDs and the risk of colorectal cancer, gastric cancer, and breast cancer (13–15). Nevertheless, studies on the use of NSAIDs and the risk of PC produced conflicting results (16–19). Although many observational studies revealed a modest inverse association between the use of NSAIDs and PC occurrence, at the same time, other investigations, including several recent meta-analyses, conversely reported no association or even a positive association (18, 20–23). A meta-analysis (from articles up to October 2013) revealed a positive relationship between any type of the use of NSAIDs use and the incidence of PC (20), while another meta-analysis (without language restrictions) demonstrated that NSAIDs did not have either adverse or beneficial effects on the risk of developing PC (22). These two studies were both published around the same time. Interestingly, results regarding the association of the use of NSAIDs with the prevalence of localized PC, advanced PC, and overall PC were also inconsistent. Currently, several large-scale studies performed after the meta-analysis mentioned above was conducted may provide more reliable statistical evidence to help us better understand this issue.
Given the widespread use of NSAIDs, more information is needed to carefully weigh their role in the incidence of PC. Therefore, we performed this meta-analysis to clarify the potential association between the use of NSAIDs and the risk of PC to investigate the sources of variability between studies, which may highlight the importance of considering methods of preventing PC.
Objectives
This meta-analysis aimed to explore the association between the use of NSAIDs and the risk of total PC, advanced PC and PC with Gleason score ≥7.
Research question
Does the intake of NSAIDs reduce the risk of total PC, advanced PC and PC with Gleason score ≥7?
Methods
Study design
This study was conducted in accordance with the 2015 Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines (24, 25).
Participants, interventions, comparators
We included retrieved articles whose design was a case-control, cohort or cross-sectional study evaluating the association between the use of NSAIDs and the incidence of PC. No restrictions were imposed regarding language.
We included the participants who were exposed to any single NSAID or a mixture of NSAIDs.
We included studies whose results included odds ratios (ORs), relative risks (RRs), hazard ratios (HRs), standardized incidence ratios (SIRs), or incidence rate ratios (IRRs) and 95% confidence intervals (95% CIs), or provided the available raw data needed to calculate the RRs, wherever possible.
Systematic review protocol
This meta-analysis is registered with the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42018090475).
Search strategy
Ten computerized literature databases were searched systematically by a professional librarian for relevant studies up to December 31, 2017. Medical subject headings (MeSH) in combination with free text searches were used. The full search strategy is presented in Table 1. In particular, those negative studies published in “gray literature,” such as theses, book chapters, and meeting abstracts, were also searched manually. The bibliographies of retrieved articles and previous meta-analyses were also screened to identify additional citations. No exclusion criteria were imposed.
Table 1.
Search strategy.
| DATABASES USED IN THE SEARCH | |
|---|---|
| Medline, EMBASE, Web of science, Cochrane Library, BIOSIS, Scopus, CBM (Chinese Biomedical Literature Database), CNKI (China National Knowledge Infrastructure), WANFANG (Wanfang Database), and CQVIP (Chongqing VIP Database) | |
| SEARCH ALGORITHM | |
| Search terms #1 | “Prostatic Neoplasms”[Mesh] (OR) prostate neoplasm* (OR) prostatic neoplasm* (OR) prostate cancer* (OR) prostatic cancer* (OR) prostate carcinoma* (OR) prostatic carcinoma* (OR) prostate adenocarcinoma* (OR) prostatic adenocarcinoma* |
| Search terms #2 | “Anti-Inflammatory Agents, Non-Steroidal”[Mesh] (OR) NSAID* (OR) non-steroidal anti-inflammatory drugs (OR) non-steroidal anti-inflammatory drugs (OR) non-steroidal anti-inflammatory drugs (OR) non-steroidal anti-inflammatory drugs (OR) non-steroidal anti-inflammatory drugs (OR) non-steroidal anti-inflammatory drugs (OR) non-steroidal anti-inflammatory drugs (OR) non-steroidal anti-inflammatory drugs (OR) non-steroidal anti-inflammatory drugs (OR) non-steroidal anti-inflammatory agents (OR) non-steroidal anti-inflammatory agents (OR) non-steroidal anti-inflammatory agents (OR) non-steroidal anti-inflammatory agents (OR) non-steroidal anti-inflammatory agents (OR) non-steroidal anti-inflammatory agents (OR) non-steroidal anti-inflammatory agents (OR) non-steroidal anti-inflammatory agents (OR) non-steroidal anti-inflammatory agents (OR) “Cyclooxygenase 2 Inhibitors”[Mesh] (OR) Cyclooxygenase-2 inhibitors (OR) Cyclooxygenase 2 inhibitors (OR) Cyclooxygenase2 inhibitors (OR) COX-2 inhibitors (OR) COX 2 inhibitors (OR) COX2 inhibitors (OR) “Aspirin”[Mesh] (OR) aspirin (OR) acetylsalicylic acid (OR) “Celecoxib”[Mesh] (OR) celecoxib (OR) “Diclofenac”[Mesh] (OR) diclofenac (OR) “Diflunisal”[Mesh] (OR) diflunisal (OR) “Etodolac”[Mesh] (OR) etodolac (OR) “Fenoprofen”[Mesh] (OR) fenoprofen (OR) “Flurbiprofen”[Mesh] (OR) flurbiprofen (OR) “Ibuprofen”[Mesh] (OR) ibuprofen (OR) “Indomethacin”[Mesh] (OR) indomethacin (OR) “Ketoprofen”[Mesh] (OR) ketoprofen (OR) “Mefenamic Acid”[Mesh] (OR) mefenamic acid (OR) meloxicam (OR) nabumetone (OR) “Naproxen”[Mesh] (OR) naproxen (OR) “Phenylbutazone”[Mesh] (OR) phenylbutazone (OR) “Piroxicam”[Mesh] (OR) piroxicam (OR) rofecoxib (OR) “Sulindac”[Mesh] (OR) sulindac (OR) tiaprofenic acid (OR) “Tolmetin”[Mesh] (OR) tolmetin (OR) zomepirac (OR) “Acetaminophen”[Mesh] (OR) acetaminophen (OR) paracetamol |
| Search terms #3 | Search terms #1 AND search terms #2 |
Data sources, studies sections, and data extraction
Reviews, case reports, letters, commentaries, and animal experimental studies were all excluded. If overlapping study populations were identified, the study with the larger population or greater amount of information was selected for inclusion, but relevant articles with required information were also included. The identification of relevant studies was performed independently by two different authors (JW and XW), and disagreements were resolved through consultation with a third reviewer (HY).
The methodological quality of the included articles was assessed by two independent authors (QW and XW) according to the Newcastle–Ottawa scale (NOS) (for case-control and cohort study) and a modified version of the NOS (for cross-sectional study, Supplementary File S1 in Supplementary Material). When study comparability was evaluated by the NOS, one of the three well-established risk factors (age, race, and family history) was selected as the most important adjusted covariate. Similarly, any of the comorbidities or drugs used simultaneously was chosen as the second most important adjusted factors. “High-quality studies” were defined as having a total NOS score of ≥7, while the others were considered “poor-quality studies.” For each article included, the following information was extracted: the first author's name, year of publication, country, study design, type of controls, numbers of cases and controls (exposure and non-exposure for cohort studies), study period, information source, types of NSAIDs used, definition of NSAIDs uses, adjusted factors, effects estimates as reported or associated raw data and corresponding 95% CIs. Estimates of the association between the intake of NSAIDs and the risk of advanced PC were also extracted. Data were obtained and reviewed independently by two reviewers (ZS and HY), and discrepancies were resolved by group consensus.
Data analysis
The effect estimates, such as ORs, HRs, SIRs and IRRs, were extracted. Since the absolute risk of PC is low, the above-mentioned measures of association are mathematically approximately equal to the estimates of RRs. Consequently, the pooled RR and its 95% CIs were used to assess the association between the intake of NSAIDs and the risk of PC, making it possible to conduct a comprehensive analysis and to maximize the statistical power (26). If data from different durations of NSAIDs use or different NSAIDs intake levels were available, we selected the data from the longest duration or highest level of intake. Considering that ≥4 or ≥5 years were the most common definition of long-term drugs intake period in the original studies, both were adopted to investigate the pooled effect estimates of long-term drugs intake. Besides, advanced PC was defined as prostatic specific antigen (PSA) ≥20 ng/mL, tumor stage ≥T2cN0M0, or Gleason score ≥7.
Statistical analyses were performed using STATA Statistical Software version 11.0 (STATA Corp, College Station, Texas, USA). The Cochrane Q-test and the Higgins I2-test were used to explore the extent of heterogeneity across the included articles (27, 28). When the I2-value exceeded 50%, a random-effects model was employed; otherwise, a fixed-effect model was adopted. A χ2-based Q test was also performed to check between-study heterogeneity, with P < 0.1 indicating statistical significance. Potential sources of heterogeneity were investigated in subgroup analyses, which were based on study design, study quality (total NOS score ≥7), participants, geographic location, dose or duration of drug intake, the sources of drugs, adjusted confounders (numbers of the three main factors and whether they were adjusted for comorbidity or the simultaneous use of other medications), types of effect measures (ORs, RRs, or HRs), information source, and study period. Given that PSA-based screening for PC may be more popular after 2000 than that before 2000. Thus, studies were stratified by study period after 2000 or that before 2000. Publication bias was evaluated by Egger's and Begg's tests. A sensitivity analysis was subsequently conducted to explore whether the pooled result was influenced by individual studies (29, 30).
Results
Flow diagram
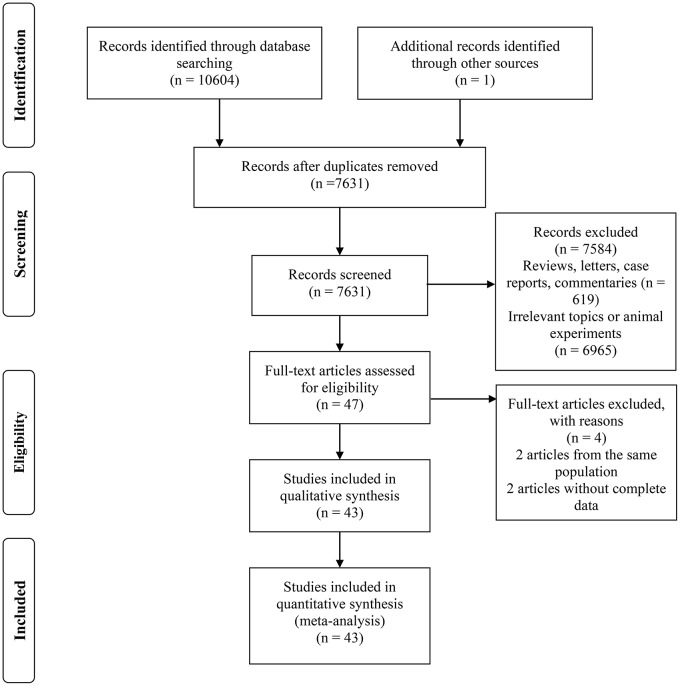
Figure 1 illustrates the PRISMA trial flow diagram for identifying and selecting articles.
Figure 1.
The PRISMA trial flow diagram for identifying and selecting articles.
Study selection and characteristics
A total of 10,604 articles were identified according to the keywords. One article was identified through references and included. After screening titles or abstracts, we identified 47 articles for full-text review. Two articles were excluded due to the lack of complete data needed to evaluate the estimates of the effect of the intake of NSAIDs on PC incidence, and 2 articles were excluded because they had less data than that of another 2 articles from the same populations. Finally, a total of 43 articles were included.
Among the identified articles, there were 19 case-control studies (17, 21, 31–47), 22 cohort studies (18, 19, 23, 48–66), and 2 cross-sectional studies (67, 68). Specifically, most studies (79.07%) were population-based, and more than half of the studies (51.16%) were performed in the USA (23, 31, 35–38, 43, 46, 51–54, 56–58, 61–67). Thirty-two (74.42%) studies also attempted to explore the effect of aspirin intake on the incidence of total PC (17–19, 21, 31–35, 37–42, 45–47, 49, 50, 52–57, 60, 62–65, 67). For advanced PC, 19 studies were included for analysis, which were composed of 8 case-control studies and 11 cohort studies (19, 21, 23, 31–33, 35, 38, 45, 48, 51–54, 58, 62, 65, 66, 68). Moreover, the association between the use of NSAIDs and highly aggressive tumors with Gleason scores ≥7 was investigated in eight studies (19, 21, 32, 48, 51, 53, 54, 68). Information was collected from either databases or questionnaires. Regarding the quality of all the eligible studies, 29 studies (67.44%) were classified as “high-quality studies,” and the others were classified as “poor-quality studies.” Detailed characteristics of the included studies are presented in Table 2 and their NOS score are showed in Supplementary File S2 in Supplementary Material.
Table 2.
Characteristics of studies included in the meta-analysis.
| References | Study design | Country | Participant | Age | Drugs | Definition of drugs use | Cases/exposure | Controls/non-exposuree | Effect estimate | 95%CI | Outcomes | Adjusted factors | Study period | Information sourceurce | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (48) | Cohort | Finland | Population-based | 55–63 | NSAIDs | 57,531 | 21,083 | RR | 0.75 (0.74–0.75) | Total PC, advanced PC | Age | 1995–2009 | Database | 7 | |
| (31) | Case-Control | USA | Population-based | 40–90 | Aspirin | At least 1 tablet daily in the past 5 years | 811 | 1,023 | OR | 0.86 (0.71–1.06) | Total PC, advanced PC | Age, BMI, diabetes, education, family history of prostate cancer, race, smoking history, and Tylenol and pain relievers not containing Tylenol or aspirin-containing compounds | 2005–2015 | Questionnaire | 7 |
| (32) | Case-Control | France | Population-based | ≤ 75 | NSAIDs, Aspirin, non-aspirin NSAIDs | At least once a month | 819 | 879 | OR | 0.77 (0.61–0.98) | Total PC, advanced PC | Age, family history of cancer at first degree, race, educational level, history of prostatitis, waist to hip ratio | 2012–2013 | Questionnaire | 7 |
| (49) | Cohort | Sweden | Population-based | ≥18 | Aspirin, non-aspirin NSAIDs | SIRs | 0.87 (0.85–0.88) | Total PC | Age | 2005–2012 | Questionnaire | 7 | |||
| (18) | Cohort | Korea | Population-based | ≥40 | NSAIDs, Aspirin | 1,305 | 142,565 | HR | 1.35 (1.14–1.58) | Total PC | Age distribution, sex, insurance eligibility status, Charlson Comorbidity Index, participant's income level and whether or not drug usage and drug types | 2004–2013 | Database | 8 | |
| (66) | Cohort | USA | Health professionals | 40–84 | Aspirin | >3 tablets/week for at least 1 year | 12,454 | 9,496 | HR | 0.68 (0.52–0.89) | Advanced PC | Age, race, BMI, height, smoking, hypertension, and type 2 diabetes | 1981/82–2009 | Questionnaire | 7 |
| (21) | Case-Control | Denmark | Population-based | 70 | Aspirin, non-aspirin NSAIDs | ≥ 2 redeemed prescriptions on separate days, more than 1 year prior to the index date | 35,600 | 177,992 | OR | 0.94 (0.91–0.97) | Total PC, advanced PC | Age and residence, use of high-dose aspirin, 5-alpha reductase inhibitors, statins, selected cardiovascular drugs, and antidepressants or neuroleptics, history of diabetes mellitus; educational level; income; and mutual adjustment for use of low-dose aspirin or non-aspirin NSAIDs | 2000–2012 | Database | 7 |
| (67) | Cross-sectional | USA | Population-based | ≥20 | Aspirin | At least 3 times a week | 2,457,316 | 104,026,095 | OR | 0.60 (0.38–0.94) | Total PC | Age, race/ethnicity, education, US citizen, and cancer-related health beliefs, insurance, family income, region of residence, regular finasteride use, regular use of non-aspirin NSAIDs or COX-2 inhibitors, and antidiabetic drug use, family history of prostate cancer, smoking status, alcohol drinking status, frequency of vigorous physical activity, nutritional status, health status, numbers of PSA tests performed during the past 5 years, BMI, and selfreported diabetes mellitus | 1987–2010 | Questionnaire | 7 |
| (50) | Cohort | Italy | Population-based | ≥18 | Aspirin | 7,747 | 5,706 | HR | 0.64 (0.48–0.86) | Total PC | Age, presence of obesity, smoking, alcohol abuse or related diseases, Charlson Comorbidity Index, benign prostatic hypertrophy, numbers of PSA requests, use of ACE inhibitors, NSAIDs, statins, alpha-adrenoreceptor antagonists, testosterone 5-alpha reductase inhibitors, immunosuppressive drugs | 2002–2013 | Database | 7 | |
| (23) | Cohort | USA | Population-based | 40–75 | Aspirin | 3,748 | 3,823 | RR | 0.97 (0.85–1.1) | Advanced PC | Race, height, BMI, family history of cancer, physical examination in the past 2 years, history of colonoscopy or sigmoidoscopy, smoking, physical activity, alcohol intake, current multivitamin use, total energy intake, red and processed meat intake, folate intake, calcium intake, and Alternate Healthy Eating Index 2010 prostate-specific antigen test in the past 2 years | 1986–2012 | Questionnaire | 8 | |
| (51) | Cohort | USA | Population-based | 50–75 | NSAIDs | 3,221 | 3,169 | OR | 0.92 (0.78–1.08) | Total PC, advanced PC | Age, race, baseline PSA, prostate volume, DRE findings, BMI, treatment arm, geographic region, smoking, cardiovascular disease, diabetes, alcohol use, statin medication, and hypertension hypertension | 2003–2007 | Database | 6 | |
| (19) | Cohort | Sweden | Population-based | 71.6 | Aspirin | 26,409 | 177,822 | OR | 1.115 (0.995–1.25) | Total PC, advanced PC | Age, natural log-transformed PSA concentration, PSA quotient, Charlson Comorbidity Index, educational level, use of aspirin, use of statin and use of antidiabetic medication | 2003–2012 | Database | 7 | |
| (33) | Case-Control | Finland | Population-based | 20–96 | Aspirin, NSAIDs | 24,657 | 24,657 | OR | 0.95 (0.88–1.0) | Total PC, advanced PC | Age and simultaneous use of other medications (cholesterol lowering drugs, anti-diabetic drugs, antihypertensive drugs and benign prostatic hyperplasia medication) | 1995–2002 | Database | 8 | |
| (52) | Cohort | USA | Population-based | 62.8 | Aspirin, non-aspirin NSAIDs | 15,893 | 13,539 | RR | 0.92 (0.85–0.99) | Total PC, advanced PC | Race, study center, family history of prostate cancer, the number of screening exams, aspirin use (for non-aspirin NSAIDs only), and non-aspirin NSAIDs use (for aspirin only) | 1993–2009 | Questionnaire | 7 | |
| (53) | Cohort | USA | Health professionals | 40–75 | Aspirin | ≥ 2 days/week | 18,570 | 24,494 | HR | 0.94 (0.87–1.02) | Total PC, advanced PC | Age, period, family history, ethnic, height, BMI, tomato sauce, vigorous physical activity, smoking, vitamin D, fish, red meat, cholesterol-lowering drugs and total kcal | 1988–2006 | Questionnaire | 7 |
| (17) | Case-Control | UK | Population-based | 50–69 | NSAIDs, Aspirin, non-aspirin NSAIDs | 1,016 | 5,043 | OR | 1.24 (1.06–1.46) | Total PC | Age, family history of prostate cancer, BMI and self-reported diabetes status | 2001–2008 | Questionnaire | 7 | |
| (34) | Case-Control | Canada | Population-based | ≥40 | NSAIDs, Aspirin, non-aspirin NSAIDs | 9,007 | 35,891 | OR | 0.87 (0.80–0.94) | Total PC | Ever visited a urologist 1–11 years prior, volume of family physician visits in the 5 years prior to the index date and, when appropriate, for use of other NSAIDs classes, Binary variable with 1 indicating whether at any point prior to the index date a subject had a physician visit for BPH, prostatitis, other disorders of prostate or any point during the 11 years prior to the index date, a subject received at least one prescription for finasteride or an a-blocker or had prostatic ablation or resection, or testing of prostatic secretions | 1985–2000 | Database | 7 | |
| (54) | Cohort | USA | Population-based | 50–76 | Aspirin, non-aspirin NSAIDs | 10,767 | 23,265 | HR | 0.98 (0.87–1.09) | Total PC, advanced PC | Age, race, education, BMI, multivitamin use, PSA test in the past 2 years, benign prostate biopsy, enlarged prostate, family history of prostate cancer, diabetes, coronary artery disease, osteoarthritis, rheumatoid arthritis, chronic joint pain, chronic headaches, and migraines | 2000–2007 | Questionnaire | 7 | |
| (35) | Case-Control | USA | Population-based | 35–74 | Aspirin, non-aspirin NSAIDs | At least once per week for a period of 3 months or longer | 1,000 | 942 | OR | 0.82 (0.68–0.99) | Total PC, advanced PC | Age at reference date, race, prostate cancer screening within 5 years before reference date | 2002–2005 | Questionnaire | 7 |
| (36) | Case-Control | USA | Hospital-based | 40–79 | NSAIDs | 1,367 | 2,007 | OR | 0.9 (0.4–2.2) | Total PC | Age, study center, interview year and BMI, alcohol use, pack-years of smoking, race, family history of PC, number of doctor visits made 2 years before hospital admission and education | 1992–2008 | Questionnaire | 6 | |
| (55) | Cohort | Netherlands | Population-based | ≥ 55 | NSAIDs, Aspirin, non-aspirin NSAIDs | HR | 1.02 (0.76–1.37) | Total PC | Age, BMI, C-reactive protein level and pack years of smoking | 1989–1993 | Questionnaire | 7 | |||
| (56) | Cohort | USA | Population-based | ≥50 | Aspirin | 53,573 | 16,237 | RR | 0.81 (0.7–0.94) | Total PC | Age, race, education, smoking, BMI, physical activity level, history of PSA testing, history of colorectal endoscopy, use of non-aspirin NSAIDs, and history of heart attack, diabetes and hypertension etes, and hypertension | 1992–2001 | Questionnaire | 8 | |
| (37) | Case-Control | USA | Population-based | Aspirin | More than 1 pill per week for more than 1 year | 229 | 285 | OR | 0.52 (0.29–0.93) | Total PC | Age, body mass, family history, smoking, alcohol intake | 2002–2004 | Database | 6 | |
| (68) | Cross-sectional | Canada | Population-based | 59–71 | NSAIDs | Daily | 494 | 805 | OR | 0.71 (0.48–1.03) | Total PC, advanced PC | age, family history of prostate cancer, history of ischaemic heart disease, intake of acetaminophen, reasons for referral and prostate volume | 1999–2003 | Questionnaire | 7 |
| (38) | Case-Control | USA | Hospital-based | 67.1 | Aspirin | At least once a week for at least 6 months | 1,029 | 1,029 | OR | 1.05 (0.89–1.25) | Total PC, advanced PC | Age, education, family history of prostate cancer, cigarette smoking, race, and BMI | 1982–1998 | Questionnaire | 6 |
| (39) | Case-Control | Italy | Hospital-based | 46–74 | Aspirin | At least once a week for more than 6 months | 1,261 | 1,131 | OR | 1.10 (0.81–1.50) | Total PC | Age, study center, education and family history of prostate cancer | 1991–2002 | Questionnaire | 6 |
| (47) | Case-Control | Canada | Hospital-based | ≥65 | NSAIDs, Aspirin | ≥ 1 prescription more than 4 months | 2,025 | 2,150 | OR | 0.71 (0.58–0.86) | Total PC | Age and finasteride use | 1999–2002 | Questionnaire | 6 |
| (58) | Cohort | USA | Population-based | ≥ 50 | NSAIDs | 41,094 | 29,050 | RR | 0.95 (0.86–1.05) | Total PC, advanced PC | Age, race, diabetes, history of heart attack, history of PSA testing, education, and family history of prostate cancer in a brother or father | 1992–2001 | Questionnaire | 8 | |
| (57) | Cohort | USA | Population-based | 70 | NSAIDs, Aspirin, non-aspirin NSAIDs | RR | 0.71 (0.49–1.02) | Total PC | Age and analgesic drugs | 1980–2004 | Questionnaire | 8 | |||
| (40) | Case-Control | UK | Hospital-based | 50–79 | Aspirin, non-aspirin NSAIDs | Current use | 2,183 | 10,000 | OR | 0.70 (0.61–0.79) | Total PC | Age, calendar year, prior BPH history, number of visits to general practitioners, referrals, hospitalizations | 1995–2001 | Database | 6 |
| (41) | Case-Control | Canada | Population-based | 73–79 | NSAIDs, Aspirin | At least 325 mg daily | 2,221 | 11,105 | OR | 1.14 (0.85–1.54) | Total PC | Age and recent medical contacts | 1993–1995 | Database | 6 |
| (60) | Cohort | Denmark | Population-based | 70 | Aspirin | 15,058 | SIR | 1.1 (1.0–1.3) | Total PC | Age | 1989–1995 | Questionnaire | 7 | ||
| (59) | Cohort | Denmark | Population-based | 47.2 | Non-aspirin NSAIDs | 78,562 | SIR | 1.3 (1.2–1.5) | Total PC | 1989–1995 | Questionnaire | 6 | |||
| (65) | Cohort | USA | Health professionals | 40–75 | Aspirin | RR | 1.10 (1.01–1.19) | Total PC, advanced PC | Age, time period, BMI at age 21, height, pack-years of smoking in the previous decade, family history of prostate cancer, vigorous physical activity, intake of total energy, calcium, fructose, tomato-based foods, red meat, fish, supplemental vitamin E, linoleic acid, and α-linolenic acid | 1986–1996 | Questionnaire | 6 | |||
| (62) | Cohort | USA | Population-based | 18–84 | Aspirin | More than 6 aspirin almost every day | 2,466 | 87,634 | RR | 0.76 (0.60–0.98) | Total PC, advanced PC | Birth year, education, race, and number of health checkups | 1964–1973 | Questionnaire | 7 |
| (61) | Cohort | USA | Community-based | 56.7–70.9 | NSAIDs | Daily | 592 | 861 | OR | 0.45 (0.28–0.73) | Total PC | 1990–1996 | Questionnaire | 5 | |
| (42) | Case-Control | France | Population-based | 66.8 | NSAIDs, Aspirin, non-aspirin NSAIDs | Any of these medications some time during the 5 years before the interview | 639 | 659 | RR | 0.90 (0.86–0.93) | Total PC | Age, farming, ethnic origin, frequency of red meat and red wine consumption, aspirin and non-aspirin NSAIDs, finasteride and urological center | 1999–2000 | Questionnaire | 8 |
| (44) | Case-Control | UK | Population-based | NSAIDs | Prescribed NSAIDs in 13–36 months before diagnosis of case | 1,813 | 5,354 | OR | 1.33 (1.07–1.64) | Total PC | Age and smoking | 1990–1993 | Database | 7 | |
| (43) | Case-Control | USA | Population-based | 64 | NSAIDs | 417 | 420 | OR | 0.34 (0.2–0.58) | Total PC | Age, race, and other factors | 1992–1995 | Questionnaire | 6 | |
| (45) | Case-Control | New Zealand | Population-based | 40–80 | NSAIDs, Aspirin, non-aspirin NSAIDs | 317 | 480 | OR | 0.88 (0.64–1.20) | Total PC, advanced PC | Age, socio-economic status, total polyunsaturated fat consumption, α-linolenic acid and ratio of dietary n-6: long-chain n-3 polyunsaturated fatty acids | 1996–1997 | Questionnaire | 6 | |
| (46) | Case-Control | USA | Hospital-based | 69.6 | Aspirin | 319 | 189 | OR | 1.6 (0.82–3.11) | Total PC | Age, race, and history of coronary heart disease, diabetes | 1984–1986 | Questionnaire | 7 | |
| (63) | Cohort | USA | Population-based | 25–74 | Aspirin | IRR | 0.95 (0.66–1.35) | Total PC | Age, race, education, socioeconomic status, BMI, alcohol consumption, and arthritis | 1971–1987 | Questionnaire | 7 | |||
| (64) | Cohort | USA | Population-based | 73 | Aspirin | 1,561 | 3,490 | RR | 0.95 (0.84–0.97) | Total PC | Age | 1981–1988 | Questionnaire | 6 |
Synthesized findings
Intake of any NSAIDs and total or advanced PC risk
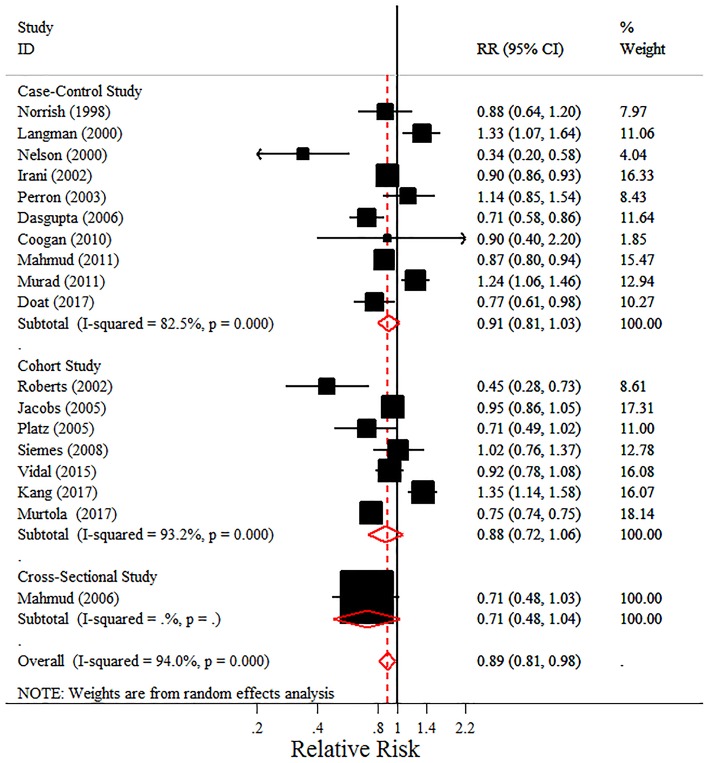
Unlike previously conducted meta-analyses, this meta-analysis found a protective effect of the intake of any NSAIDs on PC risk due to the inclusion of recent research (pooled RR = 0.89, 95% CI = 0.81–0.98) (Figure 2). However, there was some evidence of heterogeneity (I2 = 94.00%, P < 0.001). Possible reasons for that heterogeneity were explored in the subgroup analyses. In addition, a non-significant decreased risk was detected for advanced PC or PC with Gleason score ≥7, though the pooled RRs were <1.
Figure 2.
Forest plot and meta-analysis of the association between the intake of any NSAIDs and the risk of prostate cancer.
In the subgroup analyses, we observed that the intake of NSAIDs was associated with a decreased PC risk in hospital-based studies and studies from North America (pooled RR 0.719, 95% CI = 0.593–0.871, I2 = 0.00%; pooled RR 0.797, 95% CI = 0.698–0.910, I2 = 72.10%, respectively). Interestingly, long-term intake of NSAIDs (≥5 years rather than ≥4 years) was associated with an 11.8% reduction in PC incidence, with little evidence of heterogeneity (pooled RR = 0.882, 95% CI = 0.785–0.991, P = 0.035; I2 = 27.40%, P = 0.248). Notably, no significantly beneficial effects were found in the “High-quality studies” on total PC. However, when we restricted our analysis to studies adjusting for comorbidities, the association did not remain and there was no significance when the studies were stratified by the numbers of the three main factors, though the pooled RR was also <1 (Table 3).
Table 3.
Stratified pooled effects and 95% confidence intervals of NSAIDs intake and prostate cancer risk.
| Study characteristics | Number of studies | Effect estimates (95% CI) | P value | Effect model | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 (%) | P value | ||||||
| STUDIES OF TOTAL PROSTATE CANCER | |||||||
| NSAIDs | |||||||
| Study design | Case–control studies | 10 | 0.913 (0.807, 1.032) | 0.147 | Random | 82.50 | <0.001 |
| Cohort studies | 7 | 0.877 (0.722, 1.065) | 0.185 | Random | 93.20 | <0.001 | |
| Study quality | High quality studies | 11 | 0.950 (0.847, 1.066) | 0.381 | random | 96.10 | <0.001 |
| Poor quality studies | 7 | 0.742 (0.580, 0.950) | 0.018 | random | 76.40 | <0.001 | |
| Participant | Population-based studies | 15 | 0.927 (0.836, 1.028) | 0.15 | Random | 95.00 | <0.001 |
| Hospital-based studies | 2 | 0.719 (0.593, 0.871) | 0.001 | Fixed | 0.00 | 0.595 | |
| Country | Studies from North America | 10 | 0.797 (0.698, 0.910) | <0.001 | Random | 72.10 | <0.001 |
| Studies from Europe | 6 | 0.960 (0.829, 1.111) | 0.582 | Random | 97.00 | <0.001 | |
| Studies from other countries | 2 | 1.114 (0.734, 1.691) | 0.612 | Random | 82.20 | 0.018 | |
| Duration | Long-time NSAIDs use (≥4 years) | 6 | 1.023 (0.833, 1.255) | 0.83 | Random | 72.40 | 0.003 |
| Long-time NSAIDs use (≥5 years) | 4 | 0.882 (0.785, 0.991) | 0.035 | Fixed | 27.40 | 0.248 | |
| Effect estimates | Effect estimate OR | 13 | 0.878 (0.786, 0.980) | 0.021 | Random | 80.30 | <0.001 |
| Effect estimate RR | 2 | 0.868 (0.667, 1.130) | 0.294 | Random | 55.70 | 0.133 | |
| Effect estimate HR | 2 | 1.207 (0.922, 1.579) | 0.171 | Random | 62.40 | 0.103 | |
| NSAIDs source | Prescription database | 7 | 0.865 (0.663, 1.129) | 0.286 | Random | 93.70 | 0.109 |
| Adjusted factors | Less than 2 of three main adjusted factors | 10 | 0.905 (0.775, 1.056) | 0.205 | Random | 91.90 | <0.001 |
| Equal or more than 2 of three main adjusted factors | 8 | 0.887 (0.782, 1.006) | 0.062 | Random | 78.30 | <0.001 | |
| Comorbidity | Did not adjust for comorbidity | 13 | 0.892 (0.799, 0.995) | 0.04 | Random | 94.30 | <0.001 |
| Adjusted for comorbidity | 4 | 0.976 (0.824, 1.157) | 0.781 | Random | 74.90 | 0.008 | |
| Concomitant use of medication | Did not adjust for concomitant use of other medications | 9 | 0.851 (0.700, 1.034) | 0.105 | Random | 92.50 | <0.001 |
| Adjusted for concomitant use of other medications | 9 | 0.911 (0.820, 1.013) | 0.085 | Random | 77.50 | <0.001 | |
| Information source | Questionnaires | 12 | 0.826 (0.732, 0.933) | 0.002 | Random | 77.30 | <0.001 |
| Database | 6 | 1.016 (0.838, 1.233) | 0.871 | Random | 95.30 | <0.001 | |
| Study period | Study period before 2000 | 8 | 0.895 (0.780, 1.027) | 0.114 | Random | 81.40 | <0.001 |
| Study period after 2000 | 4 | 1.054 (0.831, 1.335) | 0.666 | Random | 86.10 | <0.001 | |
| Aspirin | |||||||
| Study design | Case–control studies | 16 | 0.914 (0.868, 0.961) | 0.001 | Random | 71.70 | <0.001 |
| Cohort studies | 17 | 0.940 (0.887, 0.996) | 0.037 | Random | 81.70 | <0.001 | |
| Study quality | High quality studies | 24 | 0.942 (0.906, 0.979) | 0.002 | Random | 78.40 | <0.001 |
| Poor quality studies | 10 | 0.870 (0.771, 0.981) | 0.024 | Random | 83.40 | <0.001 | |
| Participant | Population-based studies | 28 | 0.934 (0.899, 0.971) | 0.001 | Random | 80.20 | <0.001 |
| Hospital-based studies | 5 | 0.927 (0.756, 1.137) | 0.469 | Random | 80.70 | <0.001 | |
| Country | Studies from North America | 20 | 0.906 (0.857, 0.958) | 0.001 | Random | 66.50 | <0.001 |
| Studies from Europe | 12 | 0.938 (0.888, 0.991) | 0.024 | Random | 87.40 | <0.001 | |
| Studies from other countries | 2 | 1.017 (0.935, 1.107) | 0.691 | Fixed | 15.80 | 0.276 | |
| Dose | Daily aspirin use (≥ 1/day) | 7 | 0.875 (0.792, 0.967) | 0.009 | Random | 64.30 | 0.01 |
| Duration | Long-time aspirin use (≥ 4 years) | 15 | 0.823 (0.571, 1.186) | 0.295 | Random | 99.10 | <0.001 |
| Long-time aspirin use (≥ 5 years) | 11 | 0.792 (0.514, 1.219) | 0.288 | Random | 99.20 | <0.001 | |
| Effect estimates | Effect estimate OR | 19 | 0.916 (0.870, 0.963) | 0.001 | Random | 73.60 | <0.001 |
| Effect estimate RR | 7 | 0.921 (0.843, 1.007) | 0.069 | Random | 73.00 | 0.001 | |
| Effect estimate HR | 5 | 0.950 (0.862, 1.047) | 0.301 | Random | 61.50 | 0.034 | |
| Aspirin source | Prescription database | 13 | 0.936 (0.878, 0.996) | 0.0038 | Random | 90.20 | 0.009 |
| Adjusted factors | Less than 2 of three main adjusted factors | 15 | 0.919 (0.870, 0.971) | 0.003 | Random | 86.20 | <0.001 |
| Equal or more than 2 of three main adjusted factors | 19 | 0.934 (0.888, 0.983) | 0.009 | Random | 59.30 | 0.001 | |
| comorbidity | Did not adjust for comorbidity | 23 | 0.933 (0.892, 0.976) | 0.003 | Random | 83.60 | <0.001 |
| Adjusted for comorbidity | 11 | 0.903 (0.835, 0.976) | 0.01 | Random | 59.40 | 0.006 | |
| Concomitant use of medication | Did not adjust for concomitant use of other medications | 18 | 0.941 (0.876, 1.011) | 0.095 | Random | 79.70 | <0.001 |
| Adjusted for concomitant use of other medications | 16 | 0.925 (0.888, 0.963) | <0.001 | Random | 69.20 | <0.001 | |
| Information source | Questionnaires | 24 | 0.937 (0.898, 0.978) | 0.003 | Random | 74.50 | <0.001 |
| Database | 10 | 0.892 (0.824, 0.965) | 0.005 | Random | 84.80 | <0.001 | |
| Study period | Study period before 2000 | 12 | 0.978 (0.920, 1.040) | 0.479 | Random | 67.80 | <0.001 |
| Study period after 2000 | 12 | 0.926 (0.871, 0.986) | 0.016 | Random | 82.50 | <0.001 | |
| NA-NSAID | |||||||
| Study design | Case–control studies | 8 | 1.002 (0.881, 1.140) | 0.978 | Random | 88.60 | <0.001 |
| Cohort studies | 7 | 1.001 (0.866, 1.157) | 0.992 | Random | 89.10 | <0.001 | |
| Study quality | High quality studies | 12 | 0.966 (0.867, 1.076) | 0.524 | Random | 95.2 | <0.001 |
| Poor quality studies | 3 | 1.219 (1.122, 1.325) | 0.001 | Fixed | 45.30 | 0.16 | |
| Participant | Population-based studies | 15 | 1.001 (0.908, 1.103) | 0.987 | Random | 94.50 | <0.001 |
| Country | Studies from North America | 6 | 0.932 (0.886, 0.981) | 0.007 | Fixed | 36.30 | 0.165 |
| Studies from Europe | 8 | 1.036 (0.900, 1.192) | 0.624 | Random | 97.00 | <0.001 | |
| Dose | Daily NA-NSAIDS use (≥ 1/day) | 2 | 0.975 (0.790, 1.203) | 0.813 | Fixed | 0.00 | 0.773 |
| Duration | Long-time NA-NSAIDS use (≥ 4 years) | 6 | 1.080 (1.079, 1.080) | <0.001 | Fixed | 0.00 | 0.451 |
| Long-time NA-NSAIDS use (≥ 5 years) | 3 | 1.080 (1.079, 1.080) | <0.001 | Fixed | 30.90 | 0.235 | |
| Effect estimates | Effect estimate OR | 8 | 1.002 (0.881, 1.140) | 0.978 | Random | 88.60 | <0.001 |
| Effect estimate RR | 3 | 0.985 (0.889, 1.092) | 0.776 | Fixed | 0.00 | 0.487 | |
| Effect estimate HR | 2 | 1.010 (0.897, 1.138) | 0.87 | Fixed | 0.00 | 1 | |
| NA-NSAIDs source | Prescription database | 5 | 1.046 (0.895, 1.223) | 0.574 | Random | 98.30 | 0.030 |
| Adjusted factors | Less than 2 of three main adjusted factors | 9 | 0.995 (0.873, 1.134) | 0.934 | Random | 96.70 | <0.001 |
| Equal or more than 2 of three main adjusted factors | 6 | 1.015 (0.945, 1.090) | 0.68 | Fixed | 43.90 | 0.113 | |
| Comorbidity | Did not adjust for comorbidity | 12 | 0.981 (0.878, 1.097) | 0.741 | Random | 95.60 | <0.001 |
| Adjusted for comorbidity | 3 | 1.048 (0.948, 1.158) | 0.358 | Fixed | 47.10 | 0.151 | |
| Concomitant use of medication | Did not adjust for concomitant use of other medications | 10 | 1.029 (0.899, 1.178) | 0.681 | Random | 88.20 | <0.001 |
| Adjusted for concomitant use of other medications | 5 | 0.951 (0.812, 1.113) | 0.531 | Random | 92.90 | <0.001 | |
| Information source | Questionnaires | 12 | 0.987 (0.880, 1.106) | 0.819 | Random | 83.80 | <0.001 |
| Database | 3 | 1.041 (0.869, 1.245) | 0.664 | Random | 95.70 | <0.001 | |
| Study period | Study period before 2000 | 5 | 0.985 (0.782, 1.239) | 0.895 | Random | 88.80 | <0.001 |
| Study period after 2000 | 6 | 1.005 (0.857, 1.177) | 0.955 | Random | 97.60 | <0.001 | |
| STUDIES OF ADVANCED PROSTATE CANCER | |||||||
| Drugs | NSAIDs intake | 7 | 0.906 (0.702, 1.168) | 0.445 | Random | 94.50 | <0.001 |
| Asprin intake | 17 | 0.909 (0.875, 0.945) | <0.001 | Fixed | 16.20 | 0.264 | |
| NA-NSAIDs intake | 7 | 1.030 (0.988, 1.074) | 0.161 | Fixed | 0.00 | 0.803 | |
| STUDIES OF PROSTATE CANCER WITH GLEASON SCORE ≥7 | |||||||
| Drugs | NSAIDs intake | 4 | 0.868 (0.715, 1.053) | 0.152 | Random | 50.40 | 0.109 |
| Aspirin intake | 6 | 0.918 (0.875, 0.964) | 0.001 | Fixed | 28.40 | 0.222 | |
| NA-NSAIDs intake | 3 | 1.032 (0.988, 1.077) | 0.156 | Fixed | 0.00 | 0.543 | |
Intake of aspirin and total or advanced PC risk
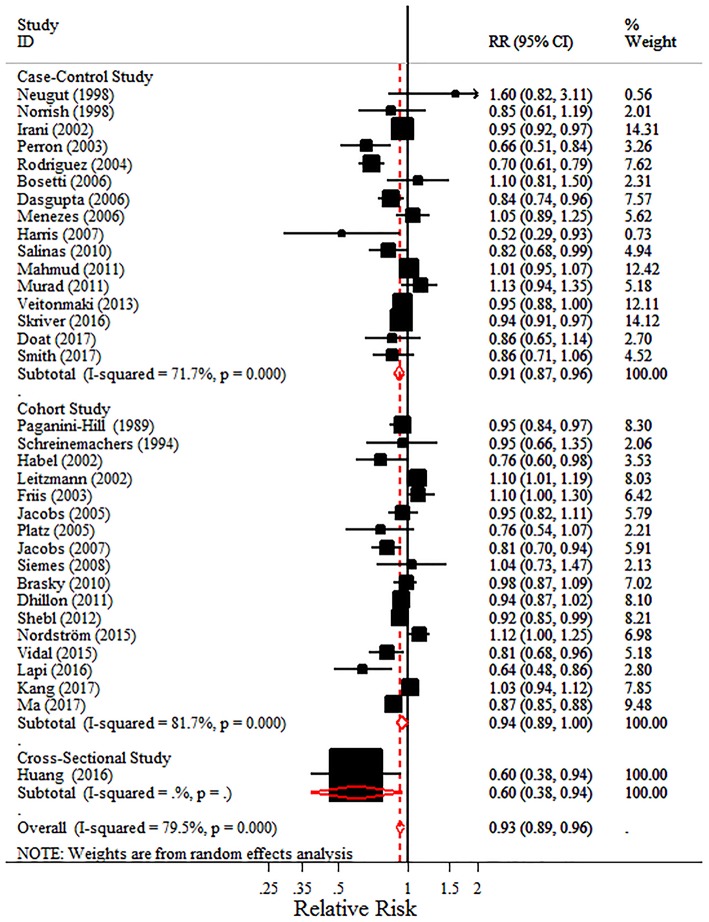
Considering the pharmacology of aspirin differ from that of other NSAIDs, the meta-analyses for aspirin and non-aspirin NSAIDs (NA-NSAIDs) were also conducted, respectively. The 34 studies that evaluated aspirin intake and total PC risk showed a 7.0% risk reduction of PC with aspirin intake (pooled RR = 0.93, 95% CI = 0.89–0.96) and displayed considerable heterogeneity (I2 = 79.5%, P < 0.001) (Figure 3). Especially, Table 3 shows that with aspirin intake, the risks of advanced PC and PC with Gleason score ≥7 were lower than that of total PC (pooled RR = 0.909, 95% CI = 0.875–0.945; pooled RR = 0.918, 95% CI = 0.875–0.964, respectively) with little heterogeneity (I2 = 16.20 and 28.40%, respectively).
Figure 3.
Forest plot and meta-analysis of the association between the intake of aspirin and the risk of prostate cancer.
In the subgroup analyses, a similar negative trend of PC risk with aspirin intake was detected regardless of study design (for case-control studies, pooled RR = 0.914, 95% CI = 0.868 −0.961; for cohort studies, pooled RR = 0.940, 95% CI = 0.887–0.996), quality of studies (for high quality studies, pooled RR = 0.942, 95% CI = 0.906–0.979; for poor quality studies, pooled RR = 0.870, 95% CI = 0.771–0.981), geographic region (for studies from North America, pooled RR = 0.906, 95% CI = 0.857–0.958; for studies from Europe, pooled RR = 0.938, 95% CI = 0.888–0.991), information source (information collected from questionnaires, pooled RR = 0.937, 95% CI = 0.898–0.978; information collected from databases, pooled RR = 0.892, 95% CI = 0.824–0.965), comorbidities, the source of aspirin, and the number of the three main adjusted factors. Contrary to a recent study, it was the daily dose (≥1 pill/day) not the long-term intake of aspirin (≥4 years or ≥5 years) that was associated with reduced PC incidence (pooled RR = 0.875, 95% CI = 0.792–0.967). In addition, we summarized the final pooled effects of aspirin intake from 16 studies which have adjusted for the concomitant use of other medications and found a lower risk of PC (pooled RR = 0.925, 95% CI = 0.888–0.963), compared with the 7.0% risk reduction of PC among all aspirin users. Interestingly, studies performed after the year 2000 showed a negative association unlike those performed before 2000 (pooled RR = 0.926, 95% CI = 0.871–0.986), and the subgroup analysis on ORs also demonstrated a similar negative trend (pooled RR = 0.916, 95% CI = 0.870–0.963) (Table 3).
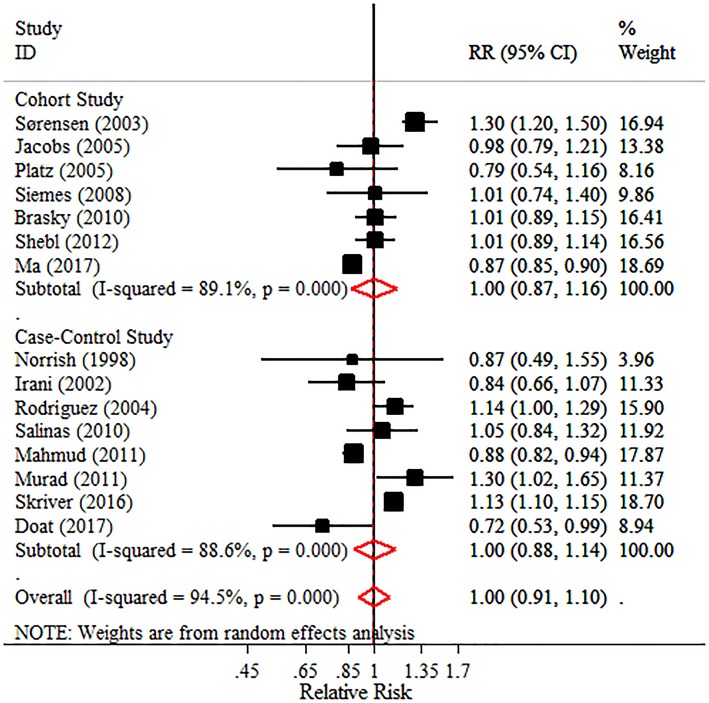
Intake of NA-NSAIDs and total or advanced PC risk
The pooled effects for non-aspirin NSAIDs demonstrated no significantly adverse or beneficial effects on total PC, advanced PC, or PC with Gleason score ≥7. However, all pooled RRs were >1 (Figure 4 and Table 3). Notably, results in the subgroup analyses were not consistent. A decreased PC risk was observed in studies from North America (pooled RR = 0.932, 95% CI = 0.886–0.981), while the long-term intake of non-aspirin NSAIDs (≥4 years or ≥5 years) may be a potential risk factor in PC incidence, with little heterogeneity (I2 = 0.00 and 30.90%, respectively). Moreover, a non-significant decreased risk was detected in “high quality studies,” though an adverse effect of non-aspirin NSAIDs on total PC was observed in “poor quality studies.” The detailed data are shown in Table 3.
Figure 4.
Forest plot and meta-analysis of the association between the intake of NA-NSAIDs and the risk of prostate cancer.
Risk of bias
No such publication bias was detected by either Begg's or Egger's test (for the intake of any NSAIDs, p = 0.185; for the intake of aspirin, p = 0.537; for the intake of non-aspirin NSAIDs, p = 0.953, respectively) (Figure 5). In sensitivity analyses, none of the individual studies substantially altered the pooled effect estimates for drugs intake on PC incidence (Figure 6). Age, race, and family history of participants were well-established risk factors for PC. In the individual studies, analyses should be systematically adjusted for the three risk factors. In this meta-analysis, most of the included individual studies (93.62%) take at least one of the three risk factors into account. In addition, based on the number of the three main adjusted factors included, subgroup analyses were performed. The NOS and a modified version of the NOS were used to assess the quality of case-control or cohort study and cross-sectional study respectively. More than half of the studies (67.44%) were considered as high-quality studies. Meta-analysis of only these high-quality studies revealed similar risk reduction of PC with aspirin intake, while no significant effects of NSAIDs or non-aspirin NSAIDs intake were found on the PC incidence. Differences in the definitions of drug intake, ages of participants, sample sizes of studies, comorbidities, simultaneous use of other medications and information source of the included individual studies could result in some selection bias, confounding bias and information bias, which may be interpreted by subgroup analyses to some extent.
Figure 5.
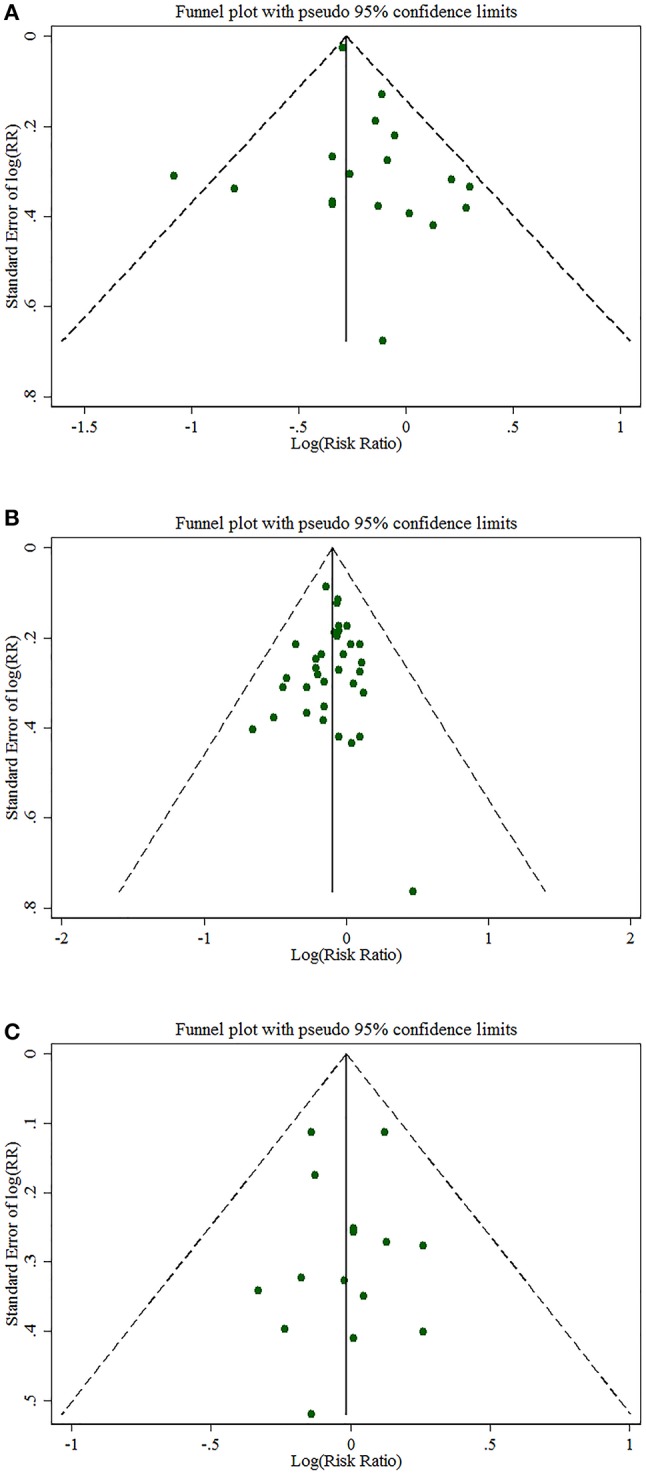
Funnel plots of Begg's test (A) for the intake of any NSAIDs, (B) for the intake of aspirin, (C) for the intake of NA-NSAIDs.
Figure 6.
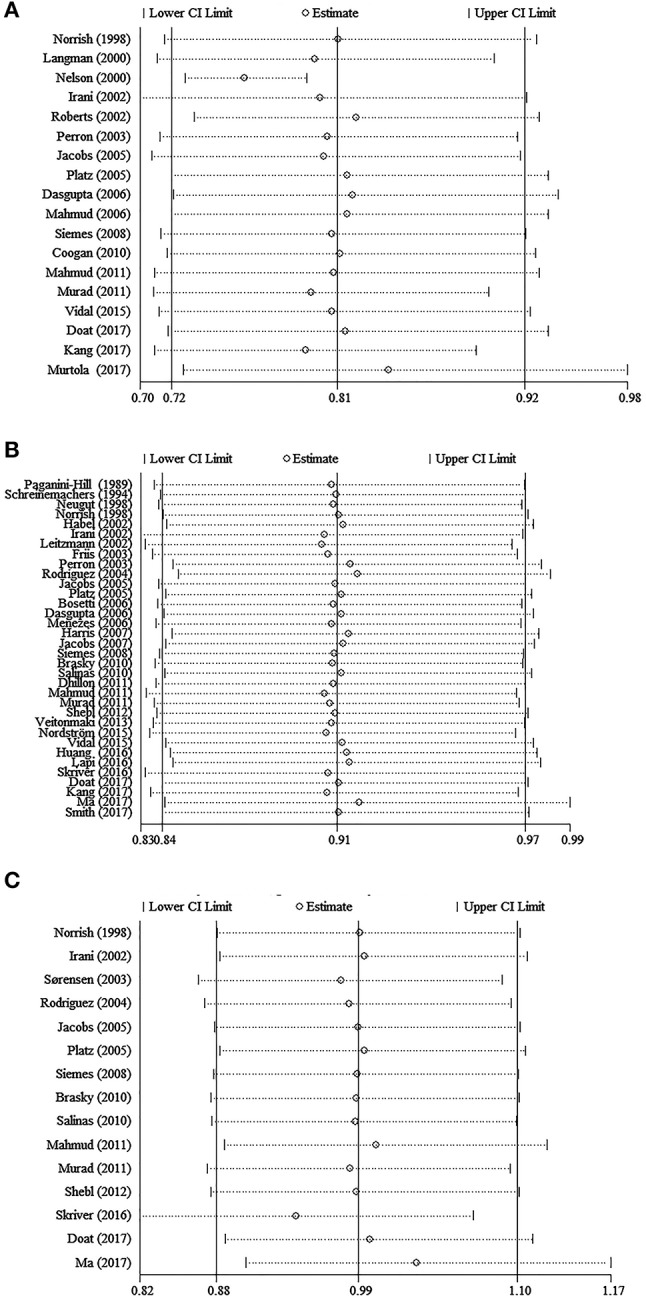
Sensitivity analyses for the pooled risk of prostate cancer (A) for the intake of any NSAIDs, (B) for the intake of aspirin, (C) for the intake of NA-NSAIDs.
Discussion
Summary of main findings
To the best of our knowledge, this is the first meta-analysis that attempted to explore the association between PC incidence and the dose and duration of the intake of NSAIDs, which could be the most important factors affecting the effect of NSAIDs on PC and which have not been addressed by previous meta-analyses. In our meta-analysis of 43 studies, we observed that the intake of any NSAIDs and the intake of aspirin were inversely related to PC incidence. Aspirin use was also associated with a 9.1 and 8.2% reduction in advanced PC risk and the risk of PC with Gleason score ≥7, respectively. Particularly, long-term intake of NSAIDs (≥5 years rather than ≥4 years) was associated with an 11.8% reduction in total PC incidence, while it was the daily dose of aspirin (≥1 pill/day) not the long-term aspirin intake (≥4 years or ≥5 years) that was associated with reduced total PC incidence.
Inconsistent with previous meta-analyses, this meta-analysis found a protective effect of any NSAIDs intake on total PC risk. A recent meta-analysis suggested a positive relation between the intake of any NSAIDs and the risk of PC (20), while neither adverse nor beneficial effects were found by another meta-analysis around the same time (22). To explore the reasons, we made an intensive study of the newly included studies. Although one study illustrated a significantly elevated risk of total PC among any NSAIDs users (18), other studies seem to carry more weight by avoiding detection bias to some extent, due to participants undergoing biopsies independent of PSA levels (51). In addition, similar inconsistency was found between long-term aspirin intake (≥4 or ≥5 years) and the risk of total PC. Based on eight included studies, Liu et al. recently found a significantly reduced PC incidence among those who took aspirin for at least 4 years (22). However, after including 15 studies with more information about the dose and frequency of aspirin intake, this meta-analysis did not find significantly beneficial effects of long-term aspirin intake on the risk of total PC. When it comes to the pooled effects of non-aspirin NSAIDs on the risk of PC, our findings were not completely in accordance with that of previous studies. In this meta-analysis, a non-significant decreased PC risk was detected in “high quality studies,” while an adverse effect of non-aspirin NSAIDs on total PC was observed in “poor quality studies.” It seems that the possibility of a non-significant decreased PC risk for non-aspirin NSAIDs is higher.
Compared to that in all aspirin users (7.0%), our finding of a larger (12.5%) PC risk reduction among those who took at least 1 aspirin tablet daily may indicate a dose related association between the intake of aspirin and the risk of total PC, although we could not pool the data to investigate the association between aspirin intake of less than 1 pill/day and the risk of PC. The above dose related association was reported in several studies (21, 31, 33, 35, 53). However, pooled effect estimates have never generated before. The 12.5% risk reduction observed in this study is in line with the results of recent epidemiological studies. For example, one study conducted by Skriver reported that aspirin intake (≥1 pill/day) was associated with an 11% reduction in PC risk (21). Similarly, Smith et al. suggested that daily aspirin use (≥1 pill/day) also decreased the risk of advanced PC in the NCI-Maryland Prostate Cancer Case-Control Study (31). Experimental studies have also clearly demonstrated that aspirin can inhibit the growth of prostate epithelial cells at the concentration of 0.5 mmol/L, which is the therapeutically relevant concentration (69). In particular, the dose related association between the intake of aspirin and the risk of total PC may result from the fact that lower doses of aspirin may only inhibit the COX-1 isoform, whereas at higher doses, aspirin also inhibits COX-2. Therefore, the dose effect may be explained by the pharmacology of aspirin, which is different from that of other NSAIDs, where there are non-specific inhibition of both COX-1 and COX-2 at all doses. However, with respect to the correlation between the dose of NSAID and PC risk, the pooled effect estimates were not generated due to the limited number of studies; one study suggested that systematic differences may result in a non-dose-dependent association (33). In our opinion, further study is required to obtain convincing evidence.
Regarding long-term intake of NSAIDs or aspirin, the association became less consistent. We found that long-term intake of NSAIDs (≥5 years rather than ≥4 years) and non-aspirin NSAIDs (≥4 or ≥5 years) was associated with reduced PC incidence. The results for NSAIDs or non-aspirin NSAIDs may be valid due to little evidence of heterogeneity. However, no significantly beneficial effects were found between long-term intake of aspirin (≥4 or ≥5 years) and the risk of total PC, though the pooled RRs were <1. Previously, a modest 12–18% reduction in total PC risk was reported among long-term aspirin users (≥4 years) (22, 70). Moreover, Ma et al. recently found that long-term use of aspirin (≥5 years) or non-aspirin NSAIDs (≥3 years) decreased the risk of PC (49). It is interesting to consider what accounts for the final pooled effects of long-term aspirin use. First, there is a higher likelihood that those who take aspirin for a long time simultaneously take other medications for a long time, which may greatly impact the perceived effects of long-term aspirin use. Second, considering the substantial heterogeneity among the included studies, the crudely estimated duration of aspirin intake in some studies may have led to over- or underestimation of the real effects of long-term aspirin use on PC incidence.
As for the geographic difference, we observed that the intake of NSAIDs or aspirin was associated with a decreased PC risk in both Europe and North America, though there was no statistical difference in PC risk due to the intake of NSAIDs in Europe. Additionally, a negative association was also observed between PC incidence and the intake of non-aspirin NSAIDs in North America, while there was an insignificant positive relation between PC incidence and the intake of non-aspirin NSAIDs in Europe. Some previous studies indicated that the effect of the intake of NSAIDs on PC incidence seemed to vary by geographic region, which may result from a potential bias. For example, studies of European men reported that the use of NSAIDs was associated with an increased risk of total PC (33, 17, 44), while a reduced risk of total PC was found among NSAIDs users from North America (40, 43, 47, 71). Given that previously, PSA testing was practiced less frequently in Europe than in North America, PC in European men taking NSAIDs were likely to be missed and to be detected at a later stage, which may account for the positive associations to some degree. However, recent studies from Europe showed an overall modest protective effect of the intake of NSAIDs or non-aspirin NSAIDs on the incidence of PC, which could be attributed to the increasing popularity of PSA screening (32, 49, 50).
Furthermore, we detected that there was no remaining association between the intake of NSAIDs and PC incidence when we restricted our analysis to studies adjusting for comorbidities. The final pooled effects of aspirin intake seemed to be influenced by the concomitant use of other medications. Comorbidity and the concomitant use of other medications could influence the risk of PC and introduce an indication bias due to the fact that several comorbidities (cardiovascular and rheumatologic diseases) were the main reasons for their intake of NSAIDs. In addition, cardiovascular events and PC shared several common risk factors, such as smoking, alcohol, obesity, and low levels of physical activity. Thus, PC may be more prevalent in those with certain risk factors than in the general population (50). It should also be noted that other medications used simultaneously, such as statins and metformin, were commonly prescribed to NSAIDs users and their combined effects on the risk of PC should not be neglected. Interestingly, statins also showed some promising chemopreventive effects against PC, although insufficient evidence from multiple reports was merely suggestive rather than conclusive. For example, Ma et al. found that the protective effect of aspirin was less pronounced among those who took aspirin and statins simultaneously (49).
Meanwhile, we found that the intake of aspirin, rather than NSAIDs or non-aspirin NSAIDs, was associated with a greater decrease in the risk of advanced PC and PC with Gleason score ≥7, with little heterogeneity (I2 = 16.20 and 28.40%, respectively). Although the final pooled effect of aspirin intake on the incidence of advanced PC was consistent with the results of previous studies, the degree of risk reduction in those studies varied from 11 to 30% (20, 22, 70, 72). For fear of misclassification of the stage due to the lack of complete information based on TNM classifications, a pooled analysis was further performed to investigate the association between drug use and PC with Gleason score ≥7. However, this association was not consistent either (21, 53, 54, 66).
Considering the different effect estimates included, subgroup analyses were conducted based on different effect estimates. Interestingly, the subgroup analysis on ORs, instead of RRs or HRs, demonstrated a negative trend of PC risk in NSAIDs or aspirin users. There seems to be further room for methodological improvement in these studies.
Limitations
The limitations of this study should also be acknowledged. Firstly, heterogeneity was an inevitable problem, and it was also a very significant problem in the previous meta-analyses. Though sensitivity analyses indicated that none of the individual datasets substantially altered the pooled effect estimates of drugs intake on PC incidence, the summarized estimates in this meta-analysis may be ambiguous to the public and should be treated with caution due to a considerable heterogeneity. In our meta-analysis, methodological heterogeneities across included studies were observed and could not be reduced and interpreted in some subgroup analyses. Specifically, the definitions of drug intake, ages of participants, and sample sizes of studies were rather different among included studies, for example, the definitions of drug intake vary from daily to once a month, making it difficult to determine an optimum dividing-class value to conduct corresponding stratified analyses and resulting in a certain level of heterogeneity undoubtedly. Accordingly, the reference groups were defined differently, which may also bias the pooled effect estimates. Moreover, it may be inappropriate to choose a single effect estimate to pool the data, though the heterogeneity across included studies could not explained completely by stratified analyses based on study design or types of effect measures.
Secondly, possible publication bias remains another potential impact, although we included as many English and Chinese databases as possible, and no publication bias was detected according to the Begg's or Egger's tests. However, studies with negative results are less likely to be published in indexed journals. Thus, those unpublished negative studies may result in possible publication bias, though those published in “gray literature,” such as theses, book chapters, and meeting abstracts, were also searched particularly in this meta-analysis. Furthermore, another possible publication bias could attribute to the exclusion of studies without available information, which may also lead to the downgrading of evidence.
Thirdly, the inherent limitations resulting from the design of included studies might involve a certain level of recall bias and selection bias, which may contribute to potential misclassification of exposure and outcome. Evaluations of drug use based on interviews or questionnaires are likely to be prone to recall related measurement errors, particularly regarding the dose and duration of drug intake (49). Although the assessment of the intake of NSAIDs obtained by complete prescription histories minimize the risk of misclassification as much as possible, some NSAIDs may be bought over-the-counter, and therefore, complete and accurate information was not available. In addition, it also should be mentioned that the over-the-counter use of NSAIDs was not included in some studies. Furthermore, there was likely drop-in and drop-out of NSAIDs users if the updated data was insufficient (51).
Moreover, the underestimation of PC incidence resulting from the potential ability of NSAIDs to alter PSA levels may lead to another type of selection bias. PSA levels were reported to be lowered by a modest 6% in individuals who used NSAIDs for more than 5 years (73). As is well known, PSA screening plays a leading role in PC detection. Therefore, some studies may have suffered from a selection bias, in which only men with abnormal PSA levels were referred for biopsy. In other words, men with lower PSA levels due to the use of NSAIDs may have received fewer biopsies, and therefore, their PC was detected at a later stage (31, 51).
Finally, studies of men with advanced PC whose PSA levels were much higher than those of men with early-stage disease would not suffer from such a detection bias because their PSA levels at diagnosis would still meet the biopsy criteria even though their use of NSAIDs may result in a modest reduction in PSA levels (31). Thus, the relationship between the intake of NSAIDs and advanced PC is unlikely to be influenced by disease detection bias caused by the use of NSAIDs. However, it was also a concern that the protective effect of the intake of NSAIDs on advanced PC may be over-estimated due to the more frequent PSA screening of patients with advanced PC, thereby leading to earlier cancer detection (19), which could be a source of screening bias.
Conclusions
The results of this meta-analysis provided quantitative evidence regarding the protective effect of the intake of any type of NSAIDs on the risk of PC, especially among those with long-term NSAID use (≥5 years). Moreover, aspirin intake was also associated with a decreased risk of PC, and it was the daily dose (≥1 pill/day) not the long-term intake of aspirin (≥4 or ≥5 years) that was associated with the reduced incidence of PC. No significantly adverse or beneficial effects were found between the intake of non-aspirin NSAIDs and the risk of total PC, advanced PC, or PC with Gleason score ≥7. It is necessary to perform further well-designed large-scale randomized controlled trials to draw a definitive conclusion, which could adjust for the potential known and unknown confounders.
Author contributions
ZS and TO conceived and designed the study. ZS, XW, KY, JW, HY, and QW contributed to the extraction of data and analysis of the results. All the authors contributed in writing and editing the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
ZS thanks his girlfriend for her greatest support and assistance.
Footnotes
Funding. This work was supported by the National Nature Science Foundation of China (grant number 81500578) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (grant number ZYLX201801).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00437/full#supplementary-material
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Zhou CK, Daugherty SE, Liao LM, Freedman ND, Abnet CC, Pfeiffer R, et al. Do aspirin and other NSAIDs confer a survival benefit in men diagnosed with prostate cancer? a pooled analysis of NIH-AARP and PLCO cohorts. Cancer Prev Res. (2017) 10:410–20. 10.1158/1940-6207.CAPR-17-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel AR, Klein EA. Risk factors for prostate cancer. Nat Clin Pract Urol. (2009) 6:87–95. 10.1038/ncpuro1290 [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, Culig Z, et al. Prevention and early detection of prostate cancer. Lancet Oncol. (2014) 15:e484–92. 10.1016/S1470-2045(14)70211-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thapa D, Ghosh R. Chronic inflammatory mediators enhance prostate cancer development and progression. Biochem Pharmacol. (2015) 94:53–62. 10.1016/j.bcp.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454:436–44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. (2009) 10:501–7. 10.1016/S1470-2045(09)70035-X [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. (2015) 26:47–57. 10.1093/annonc/mdu225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helley D, Banu E, Bouziane A, Banu A, Scotte F, Fischer AM, et al. Platelet microparticles: a potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur Urol. (2009) 56:479–84. 10.1016/j.eururo.2008.06.038 [DOI] [PubMed] [Google Scholar]
- 10.Usman MW, Luo F, Cheng H, Zhao JJ, Liu P. Chemopreventive effects of aspirin at a glance. Biochim Biophys Acta (2015) 1855:254–63. 10.1016/j.bbcan.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 11.Kirschenbaum A, Klausner AP, Lee R, Unger P, Yao S, Liu XH, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology (2000) 56:671–6. 10.1016/S0090-4295(00)00674-9 [DOI] [PubMed] [Google Scholar]
- 12.Bilani N, Bahmad H, Abou-Kheir W. Prostate cancer and aspirin use: synopsis of the proposed molecular mechanisms. Front Pharmacol. (2017) 8:145. 10.3389/fphar.2017.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strum WB. Colorectal adenomas. N Engl J Med. (2016) 374:1065–75. 10.1056/NEJMra1513581 [DOI] [PubMed] [Google Scholar]
- 14.Coyle C, Cafferty FH, Langley RE. Aspirin and colorectal cancer prevention and treatment: is it for everyone? Curr Colorectal Cancer Rep. (2016) 12:27–34. 10.1007/s11888-016-0306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patrignani P, Patrono C. Aspirin and cancer. J Am Coll Cardiol. (2016) 68:967–76. 10.1016/j.jacc.2016.05.083 [DOI] [PubMed] [Google Scholar]
- 16.Veitonmaki T, Murtola TJ, Maattanen L, Taari K, Stenman UH, Tammela TL, et al. Prostate cancer risk and nonsteroidal antiinflammatory drug use in the Finnish prostate cancer screening trial. Br J Cancer (2014) 111:1421–31. 10.1038/bjc.2014.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murad AS, Down L, Davey Smith G, Donovan JL, Athene Lane J, Hamdy FC, et al. Associations of aspirin, nonsteroidal anti-inflammatory drug and paracetamol use with PSA-detected prostate cancer: findings from a large, population-based, case-control study (the ProtecT study). Int J Cancer (2011) 128:1442–8. 10.1002/ijc.25465 [DOI] [PubMed] [Google Scholar]
- 18.Kang M, Ku JH, Kwak C, Kim HH, Jeong CW. Effects of aspirin, nonsteroidal anti-inflammatory drugs, statin and COX2 inhibitor on the developments of urological malignancies: a population-based study with 10-year follow-up data in Korea. Cancer Res Treat (2017). 50:984–91. 10.4143/crt.2017.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordstrom T, Clements M, Karlsson R, Adolfsson J, Gronberg H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer (2015) 51:725–33. 10.1016/j.ejca.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Lin YW, Wu J, Zhu Y, Xu XL, Xu X, et al. Meta-analysis of nonsteroidal anti-inflammatory drug intake and prostate cancer risk. World J Surg Oncol. (2014) 12:304. 10.1186/1477-7819-12-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skriver C, Dehlendorff C, Borre M, Brasso K, Sørensen HT, Hallas J, et al. Low-dose aspirin or other nonsteroidal anti-inflammatory drug use and prostate cancer risk: a nationwide study. Cancer Causes Control (2016) 27:1067–79. 10.1007/s10552-016-0785-7 [DOI] [PubMed] [Google Scholar]
- 22.Liu YQ, Chen JQ, Xie L, Wang J, Li TJ, He Y, et al. Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. BMC Med. (2014) 12:15. 10.1186/1741-7015-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y, Nishihara R, Wu K, Wang M, Ogino S, Willett WC, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. (2016) 2:762–9. 10.1001/jamaoncol.2015.6396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA (2000) 283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 26.Siristatidis C, Sergentanis TN, Kanavidis P, Trivella M, Sotiraki M, Mavromatis I, et al. Controlled ovarian hyperstimulation for IVF: impact on ovarian, endometrial and cervical cancer–a systematic review and meta-analysis. Hum Reprod Update (2013) 19:105–23. 10.1093/humupd/dms051 [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials (1986) 7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315:629. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 31.Smith CJ, Dorsey TH, Tang W, Jordan SV, Loffredo CA, Ambs S. Aspirin use reduces the risk of aggressive prostate cancer and disease recurrence in African-American men. Cancer Epidemiol Biomarkers Prev. (2017) 26:845–53. 10.1158/1055-9965.EPI-16-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doat S, Cenee S, Tretarre B, Rebillard X, Lamy P-J, Bringer J-P, et al. Nonsteroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk: results from the EPICAP study. Cancer Med. (2017) 6:2461–70. 10.1002/cam4.1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veitonmaki T, Tammela TLJ, Auvinen A, Murtola TJ. Use of aspirin, but not other non-steroidal anti-inflammatory drugs is associated with decreased prostate cancer risk at the population level. Eur J Cancer (2013) 49:938–45. 10.1016/j.ejca.2012.09.030 [DOI] [PubMed] [Google Scholar]
- 34.Mahmud SM, Franco EL, Turner D, Platt RW, Beck P, Skarsgard D, et al. Use of non-steroidal anti-inflammatory drugs and prostate cancer risk: a population-based nested case-control study. PLoS ONE (2011) 6:e16412. 10.1371/journal.pone.0016412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salinas CA, Kwon EM, FitzGerald LM, Feng Z, Nelson PS, Ostrander EA, et al. Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol. (2010) 172:578–90. 10.1093/aje/kwq175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coogan PF, Kelly JP, Strom BL, Rosenberg L. Statin and NSAID use and prostate cancer risk. Pharmacoepidemiol Drug Saf. (2010) 19:752–5. 10.1002/pds.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris RE, Beebe-Donk J, Alshafie GA. Cancer chemoprevention by cyclooxygenase 2 (COX-2) blockade: results of case control studies. Sub Cell Biochem. (2007) 42:193–212. 10.1007/1-4020-5688-5_9 [DOI] [PubMed] [Google Scholar]
- 38.Menezes RJ, Swede H, Niles R, Moysich KB. Regular use of aspirin and prostate cancer risk (United States). Cancer Causes Control (2006) 17:251–6. 10.1007/s10552-005-0450-z [DOI] [PubMed] [Google Scholar]
- 39.Bosetti C, Talamini R, Negri E, Franceschi S, Montella M, La Vecchia C. Aspirin and the risk of prostate cancer. Eur J Cancer Prev. (2006) 15:43–5. 10.1097/01.cej.0000180665.04335.de [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez LAG, Gonzalez-Perez A. Inverse association between nonsteroidal anti-inflammatory drugs and prostate cancer. Cancer Epidemiol Biomark Prevent. (2004) 13:649–53. [PubMed] [Google Scholar]
- 41.Perron L, Bairati I, Moore L, Meyer F. Dosage, duration and timing of nonsteroidal antiinflammatory drug use and risk of prostate cancer. Int J Cancer (2003) 106:409–15. 10.1002/ijc.11250 [DOI] [PubMed] [Google Scholar]
- 42.Irani J, Ravery V, Pariente JL, Chartier-Kastler E, Lechevallier E, Soulie M, et al. Effect of nonsteroidal anti-inflammatory agents and finasteride on prostate cancer risk. J Urol. (2002) 168:1985–8. 10.1016/S0022-5347(05)64277-2 [DOI] [PubMed] [Google Scholar]
- 43.Nelson JE, Harris RE. Inverse association of prostate cancer and non-steroidal anti-inflammatory drugs (NSAIDs): results of a case-control study. Oncol Rep. (2000) 7:169–70. 10.3892/or.7.1.169 [DOI] [PubMed] [Google Scholar]
- 44.Langman MJS, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. Br Med J. (2000) 320:1642–6. 10.1136/bmj.320.7250.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norrish AE, Jackson RT, McRae CU. Non-steroidal anti-inflammatory drugs and prostate cancer progression. Int J Cancer (1998) 77:511–5. [DOI] [PubMed] [Google Scholar]
- 46.Neugut AI, Rosenberg DJ, Ahsan H, Jacobson JS, Wahid N, Hagan M, et al. Association between coronary heart disease and cancers of the breast, prostate, and colon. Cancer Epidemiol Biomark Prevent. (1998) 7:869–73. [PubMed] [Google Scholar]
- 47.Dasgupta K, di Cesar D, Ghosn J, Rajan R, Mahmud S, Rahme E. Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J. (2006) 12:130–5. [PubMed] [Google Scholar]
- 48.Murtola TJ, Vettenranta AM, Talala K, Taari K, Stenman UH, Tammela TLJ, et al. Outcomes of prostate-specific antigen-based prostate cancer screening among men using nonsteroidal anti-inflammatory drugs. Eur Urol Focus (2017). 10.1016/j.euf.2017.03.005. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 49.Ma Y, Brusselaers N. Maintenance use of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk. Prostate Cancer Prostatic Dis. (2017). 21, 147–152. 10.1038/s41391-017-0021-x [DOI] [PubMed] [Google Scholar]
- 50.Lapi F, Levi M, Simonetti M, Cancian M, Parretti D, Cricelli I, et al. Risk of prostate cancer in low-dose aspirin users: A retrospective cohort study. Int J Cancer (2016) 139:205–11. 10.1002/ijc.30061 [DOI] [PubMed] [Google Scholar]
- 51.Vidal AC, Howard LE, Moreira DM, Castro-Santamaria R, Andriole GL, Freedland SJ. Aspirin, NSAIDs, and risk of prostate cancer: results from the REDUCE study. Clin Cancer Res (2015) 21:756–62. 10.1158/1078-0432.CCR-14-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shebl FM, Sakoda LC, Black A, Koshiol J, Andriole GL, Grubb R, et al. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. Br J Cancer (2012) 107:207–14. 10.1038/bjc.2012.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL. Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988-2006. Int J Cancer (2011) 128:2444–52. 10.1002/ijc.25811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brasky TM, Velicer CM, Kristal AR, Peters U, Potter JD, White E. Nonsteroidal anti-inflammatory drugs and prostate cancer risk in the VITamins And Lifestyle (VITAL) cohort. Cancer Epidemiol Biomark Prevent. (2010) 19:3185–8. 10.1158/1055-9965.EPI-10-0942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siemes C, Visser LE, Coebergh JWW, Hofman A, Uitterfnden AG, Stricker BHC. Protective effect of NSAIDs on cancer and influence of COX-2 C(-765)G genotype. Curr Cancer Drug Targets (2008) 8:753–64. 10.2174/156800908786733414 [DOI] [PubMed] [Google Scholar]
- 56.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. (2007) 99:608–15. 10.1093/jnci/djk132 [DOI] [PubMed] [Google Scholar]
- 57.Platz EA, Rohrmann S, Pearson JD, Corrada MM, Watson DJ, De Marzo AM, et al. Nonsteroidal anti-inflammatory drugs and risk of prostate cancer in the baltimore longitudinal study of aging. Cancer Epidemiol Biomark Prevent. (2005) 14:390–6. 10.1158/1055-9965.EPI-04-0532 [DOI] [PubMed] [Google Scholar]
- 58.Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, et al. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. (2005) 97:975–80. 10.1093/jnci/dji173 [DOI] [PubMed] [Google Scholar]
- 59.Sørensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, McLaughlin JK, et al. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer (2003) 88:1687–92. 10.1038/sj.bjc.6600945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friis S, Sorensen HT, McLaughlin JK, Johnsen SP, Blot WJ, Olsen JH. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br J Cancer (2003) 88:684–8. 10.1038/sj.bjc.6600760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts RO, Jacobson DJ, Girman CJ, Rhodes T, Lieber MM, Jacobsen SJ. A population-based study of daily nonsteroidal anti-inflammatory drug use and prostate cancer. Mayo Clin Proc. (2002) 77:219–25. 10.4065/77.3.219 [DOI] [PubMed] [Google Scholar]
- 62.Habel LA, Zhao W, Stanford JL. Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Causes Control (2002) 13:427–34. 10.1023/A:1015788502099 [DOI] [PubMed] [Google Scholar]
- 63.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast-cancer incidence in a prospective-study. Epidemiology (1994) 5:138–46. 10.1097/00001648-199403000-00003 [DOI] [PubMed] [Google Scholar]
- 64.Paganinihill A, Chao A, Ross RK, Henderson BE. Aspirin use and chronic diseases - a cohort study of the elderly. Br Med J. (1989) 299(6710):1247–50. 10.1136/bmj.299.6710.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leitzmann MF, Stampfer MJ, Ma J, Chan JM, Colditz GA, Willetts WC, et al. Aspirin use in relation to risk of prostate cancer. Cancer Epidemiol Biomark Prevent. (2002) 11:1108–11. [PubMed] [Google Scholar]
- 66.Downer MK, Allard CB, Preston MA, Gaziano JM, Stampfer MJ, Mucci LA, et al. Regular aspirin use and the risk of lethal prostate cancer in the physicians' health study. Eur Urol (2017) 72:821–7. 10.1016/j.eururo.2017.01.044 [DOI] [PubMed] [Google Scholar]
- 67.Huang WT, Erickson SR, Hansen RA, Wu CH. The association between regular use of aspirin and the prevalence of prostate cancer: results from the National Health Interview Survey. Medicine (2016) 95:e3909. 10.1097/MD.0000000000003909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahmud SM, Tanguay S, Begin LR, Franco EL, Aprikian AG. Non-steroidal anti-inflammatory drug use and prostate cancer in a high-risk population. EurJ Cancer Prev. (2006) 15:158–64. 10.1097/01.cej.0000197451.02604.25 [DOI] [PubMed] [Google Scholar]
- 69.Murtola TJ, Pennanen P, Syvala H, Blauer M, Ylikomi T, Tammela TL. Effects of simvastatin, acetylsalicylic acid, and rosiglitazone on proliferation of normal and cancerous prostate epithelial cells at therapeutic concentrations. Prostate (2009) 69:1017–23. 10.1002/pros.20951 [DOI] [PubMed] [Google Scholar]
- 70.Huang T, Yan Y, Guo Z, Zhang X, Liu H, Geng J, et al. Aspirin use and the risk of prostate cancer: a meta-analysis of 24 epidemiologic studies. Int Urol Nephrol. (2014) 46:1715–28. 10.1007/s11255-014-0703-4 [DOI] [PubMed] [Google Scholar]
- 71.Liu X, Plummer SJ, Nock NL, Casey G, Witte JS. Nonsteroidal antiinflammatory drugs and decreased risk of advanced prostate cancer: modification by lymphotoxin alpha. Am J Epidemiol. (2006) 164:984–9. 10.1093/aje/kwj294 [DOI] [PubMed] [Google Scholar]
- 72.Mahmud SM, Franco EL, Aprikian AG. Use of nonsteroidal anti-inflammatory drugs and prostate cancer risk: a meta-analysis. Int J Cancer (2010) 127:1680–91. 10.1002/ijc.25186 [DOI] [PubMed] [Google Scholar]
- 73.Chang SL, Harshman LC, Presti JC Jr. Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol (2010) 28:3951–7. 10.1200/JCO.2009.27.9406 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.