Abstract
The most important insect pests causing severe economic damages to soybean (Glycine max L.) production worldwide are Chrysodeixis includens (Walker, Noctuidae), Anticarsia gemmatalis (Hübner, Erebidae), Helicoverpa gelotopoeon (Dyar, Noctuidae), Crocidosema aporema (Walsingham; Tortricidae), Spodoptera albula (Walker, Noctuidae), S. cosmiodes (Walker, Noctuidae), S. eridania (Stoll, Noctuidae), S. frugiperda (Smith; Noctuidae), Helicoverpa armigera (Hübner, Noctuidae), H. zea (Boddie; Noctuidae) and Telenomus podisi (Hymenoptera,Platygastidae). Despite the success of biotech Bacillus thuringiensis (Bt)/herbicide tolerance (HT)-soybean in the past decade in terms of output, unforeseen mitigated performances have been observed due to changes in climatic events that favors the emergence of insect resistance. Thus, there is a need to develop hybrids with elaborated gene stacking to avert the upsurge in insect field tolerance to crystal (Cry) toxins in Bt-soybean. This study covers the performance of important commercial transgenic soybean developed to outwit destructive insects. New gene stacking soybean events such as Cry1Ac-, Cry1AF- and PAT-soybean (DAS-81419-2®, Conkesta™ technology), and MON-87751-7 × MON-87701–2 × MON 87708 × MON 89788 (bearing Cry1A.105 [Cry1Ab, Cry1F, Cry1Ac], Cry2Ab, Cry1Ac) are being approved and deployed in fields. Following this deployment trend, we recommend herein that plant-mediated RNA interference into Bt-soybean, and the application of RNA-based pesticides that is complemented by other best agricultural practices such as refuge compliance, and periodic application of low-level insecticides could maximize trait durability in Bt-soybean production in the twenty-first century.
Keywords: Armyworm, RNA inference, Resistance, Gene pyramiding, RNAi-based pesticides, Refuge strategy
Introduction
Soybean (Glycine max L.; Merrill; Fabaceae: Phaseoleae; 2n = 40) is a native crop of East Asia which gained importance for its nutritional attributes and was introduced in Africa by the Chinese in the late nineteenth century and is now cultivated worldwide (Shurtleff and Aoyagi 2013). Commercial soybean expressing crystal (Cry) proteins derived from Bacillus thuringiensis (Bt) is thereafter referred to as Bt-soybean. Prior to global commercialization of Bt-soybean, harmful insects caused the destruction of 20–30% of the world’s crops annually (Estruch et al. 1996). Today, farmers have steadily gained interest in growing genetically modified (GM) soybean either because of their herbicide-tolerant potential or insecticidal activities. For instance, biotech soybean occupies 83% (i.e., 92.1 million hectares) of the total 111 million hectares of soybean farmed globally (James 2015; ISAAA 2016; Marques et al. 2017), of which majority (> 80%) is the Bt-soybean, such as the MON 87701 × MON 87788 hybrid (Monsanto 2012). Soybean is endowed with physiologically active metabolites such as tocopherols, lecithins, isoflavones, saponins, and proteins (Sugan 2005; Yamada et al. 2012). It is a cheap source of nutrition and plays an active role in disease prevention in animals (Sugan 2005; Yamada et al. 2012). Daily multipurpose usage of soybean and the increase in oil demand compelled an increase in soybean farming (Hartman et al. 2011).
The steady increase in human population obliges the agricultural sector to diversify the methods of crop production to match global food demand. Given that the world’s population is predicted at 9.2 billion by 2050, farmers are further compelled to produce an extra 200,000 billion calories per annum (Evans 1998; Bebber and Sarah 2015). This means increasing global acceptance of GM-crops is imminent. Intriguingly, farming indigenous varieties via traditional methods failed to match population demand for soybean and profits for farmers due to damages caused by phytopathogens and harmful insects. Efforts to improve indigenous varieties of soybean began mainly via marker-assisted breeding (MAB) and transgenic approaches (Parrot et al. 1994; Dufourmantel et al. 2005; Cregan et al. 1999; Fast et al. 2015; Ortega et al. 2016; Marques et al. 2017). Nevertheless, the latter approach gained prominence over the former because of the ease and short span of time required to release new hybrids with desired agronomic traits. Despite the preference for transgenic approach to improve plant qualities and agronomic characteristics over classical breeding, the recalcitrance of many countries to accept transgenic food due to perceived safety issues limited the frequency at which biotech soybeans enter the market. Key techniques for development of biotech soybean has been the cotyledonary node Agrobacterium-mediated transformation and somatic embryo particle bombardment-mediated transformation (Yamda et al. 2012).
Globally farmed Bt-soybean such as “Roundup-Ready” cultivar (MON 87701—expressing Cry1Ac protein for insect resistance × MON 89788—glyphosate tolerance for herbicide tolerance) has helped solve some specific problems but exposes the crop to phytopathogens and new emerging destructive insects. This is a major unforeseen problem for the global production of Bt-soybean prompting rethinking the strategies to better enhance the resistance of Bt-soybean to destructive insects. Currently, the most cultivated Bt-soybean expresses Cry1Ac, Cry1Ab, Cry1AF, and phosphinothricin acetyltransferase (PAT) proteins. In this study, we search the literature for recent performances of Bt-soybean vis-à-vis targeted harmful insects, evaluate the impact of emerging insect resistance to Cry-protein, and provide a blueprint for gene pyramiding aimed at enhancing Bt-soybean production in the twenty-first century.
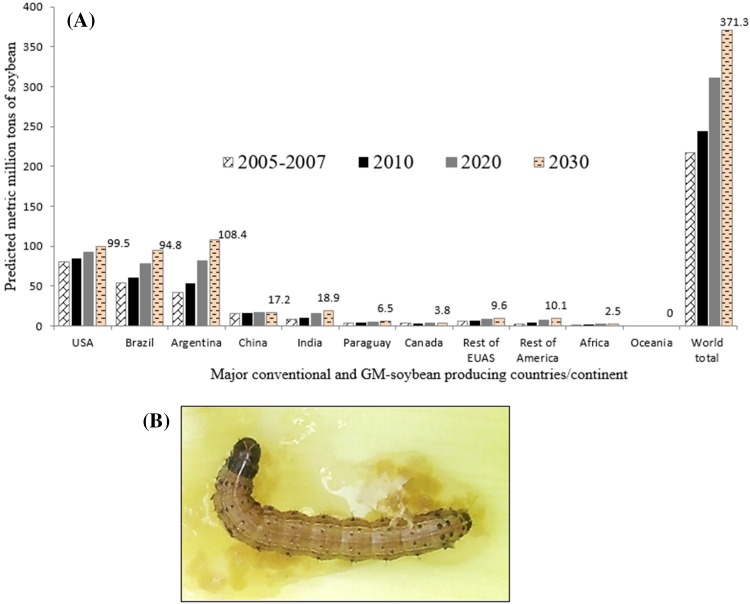
Global predictions of commercialized soybean by 2030 reveal upsurge in output
In USA alone, studies in Arkansas, Mississippi, North Carolina, and Tennessee showed that losses caused by bean leaf beetle Cerotoma trifurcata (Forster; Coleoptera: Chrysomelidae) ranges from 3 to 20% (Musser et al. 2014, 2015, 2016, 2017). Despite the deployment of glyphosate-resistant soybean in the field to improve weed management, yield losses associated with early-season weed competition was estimated at 9.3 and 3.1% across 30 fields in 2008 and 40 fields in 2009 in Wisconsin, USA, respectively (Fickett et al. 2009) and a net loss in Bt-soybean worth $16 billion between 2007 and 2013 (Dillie et al. 2016). While current statistics on soybean losses is absent, the United States of America (USA) Department of Agriculture estimated that global soybean production in 2017/2018 will be 351 million metric tons (USDA 2017). As of 2017, we found that there are 37 soybean genetically modified events approved by the International Service for Acquisition of Agri-Biotech Applications (ISAAA; http://www.isaaa.org/gmapprovaldatabase/) indicating the resolve to maintain the current production trends of soybean. We found that Bt-soybean will be predominantly produced by 2050, and that global production is anticipated at 311.10 and 371.30 million metric tons in 2020 and 2030, respectively (FAOSTAT2012). Based on predictions, the rate of soybean production in Argentina is likely to surge by 4.5% from 2010 to 2020 and by 2.8% from 2020 to 2030 (Fig. 1a). It is envisaged that Argentina would have an overall output of 108.4 million metric tons by 2030 (Masuda and Goldsmith 2009; FAOSTAT 2012). Thus, Argentina is expected to become the top soybean grower, contributing 29.2% of the world’s soybean output. Based on this prediction, USA would become the second largest producer (with 99.50 million metric tons) and Brazil the third (with 94.80 million metric tons) of soybean by 2030. Consequently, about 90% of the world’s soybean output by 2030 mostly GM-soybean will be supplied by Argentina, USA, Brazil, China, while India will be supplying high-yielding conventional soybean (Fig. 1a). The world’s total farmed land occupied by soybean would increase to 140.90 million hectares by 2030, which is 1.5 times larger than the area harvested between 2005 and 2007. This could trigger a serious unforeseen global challenge to the production of other crops due to insufficient acreage for farming.
Fig. 1.
a World soybean production forecast summary. The production growth rate is disaggregated into area harvested and yield growth rates. We generated the graph based on prediction data generated in Masuda and Goldsmith (2009) and FAOSTAT (2012). At present, India is only producing high-yielding conventional soybean. b Emergence of destructive fall armyworm (Spodoptera frugiperda) observed in Bt-soybean farms in Vanderbijlpark, Gauteng, South Africa in 2017
According to Food and Agricultural Organization (FAOSTAT2012), Argentina and Paraguay by 2030 would have increased GM-soybean cultivation areas by 31.4 million hectares and 5.0 million hectares, respectively. Regardless of the rising output of GM-soybean, global acceptance still tends to be low, although Bt-soybean does not show any substantial damage to animals, environment, and human health (James 2010, 2015). The major obstacle in acceptance of transgenic soybean is the lack of awareness and the fact that government regulations in some African, European and Asian countries do not permit the commercialization of transgenic plants.
Global profile of destructive insect pest hampering soybean production
We found that fall armyworm (Spodoptera frugiperda, Lepidoptera: Noctuidae J.E. Smith) which was mainly restricted to the South American continent has invaded Africa, especially Bt-soybean farms in South Africa (Fig. 1b). Over the years, some insect species belonging to the order Lepidoptera, Coleoptera, Hemiptera and Orthoptera have been discovered to be the major soybean pests (Grossi-de-Sá et al. 2011). Soybean farming suffers from caterpillars and stink bugs contributing to severe yield losses worldwide (Gamundi and Sosa 2008). Even so, the most important stink bug is Euschistus heros (Hemiptera, Pentatomidae) endemic to South American countries causing severe damage to soybean pods, and it is difficult to control because of its resistance to different insecticides (Sosa-Gomez and Silva 2 010). The most common insects affecting soybean production includes Chrysodeixis includens (Lepidoptera, Noctuidae), Anticarsia gemmatalis (Lepidoptera, Erebidae) (Bernardi et al. 2012), Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) (Azambuja et al. 2015) and the black armyworm, Spodoptera cosmioides (Lepidoptera: Noctuidae) (Silva et al. 2016). Ecological concerns, health hazards and increase in the insect’s population have led to the progressive preference of biological control methods such as the farming of Bt-soybean.
Types of δ -endotoxins engineered in commercialized soybean
It is observed that since the first isolation of Bacillus thuringiensis (Bt) in Japan by Ishiwata (1901) from silkworm larvae and the subsequent discovery of insecticidal δ-endotoxins crystal proteins (Cry), Bt has been applied as oil-based formulations, granules, and powders to curb insect grazing. This is because Cry-protein kills insect species at the larval stages such as those of the order Lepidoptera (specifically targeted by Cry1Aa, Cry1Ab, Cry1Ac, Cry2Ab), Diptera (specifically targeted by Cry4Aa, Cry4Ba), and Coleoptera (specifically targeted by Cry3A, Cry4b and Cry8Ea) (Ferre and van-Rie 2002). This eco-friendly strategy offers effectiveness in selective elimination of insect chewing pest and acts within a narrow range, but rapidly breakdowns in nature and thus, reduces dependence on chemical insecticides. Advances in genetic engineering led to the development of Bt-soybean to overcome the problem of conventional Bt-insecticide, which is exposed to biodegradation and wash-off. Moreover, specificity of Cry-protein in Bt-soybean greatly reduces insecticide use (Palma et al. 2014), lower production cost, minimizes exposure of lower hazardous chemicals into the environment as well as to humans and animals (Loguercio et al. 2001). The Bt Cry-proteins specifically bind to receptors of the gut epithelial cells by inserting into the target membrane and forming pores (Raymond et al. 2010) and this triggers rapid fluid loss from insect gut, prolongs developmental time, decreases growth rate, reduces the body mass and size of insects, and causes mortality (Lang and Otto 2010). To date, Cry1Ac, Cry1AF, Cry1F, Cry2Ab2, Cry1A.105, and Cry1Ab (Tables 1, 2; Fig. 2) are the most used Cry proteins in Bt-soybean.
Table 1.
List of GM-soybean engineered with Cry-protoxin either by particle bombardment (PB) or Agrobacterium (AG)-mediated transformation
| Introgressed gene | Target tissue | Promotors | Transformation method | Effect | References |
|---|---|---|---|---|---|
| Insecticidal crystal protein (Cry1Ab) | Whole plant | CaMV35S with an alfalfa mosaic virus leader sequence | PB | Resistance to velvetbean caterpillar (Anticarsia gemmatalis) | Parrot et al. (1994) |
| Insecticidal crystal protein (Cry1Ac) | Whole plant | CaMV35S | PB | Resistance to A. gemmatalis, corn earworm (Helicoverpa zea), soybean looper (Pseudoplusia includens) | Stewart et al. (1996) |
| Insecticidal crystal protein (Cry1Ab) | Plastid | 16SrRNA promoter, Nicotiana tabacum Prrn (np) | PB | Resistance to A. gemmatalis | Dufourmantel et al. (2005) |
| Insecticidal crystal protein (Cry1Ab) | Whole plant | Nicotiana tabacum Prrn (np) + bacteriophage T7 of gene 10L | PB | Resistance to A. gemmatalis | Dufourmantel et al. (2007) |
| Insecticidal crystal protein gene (Cry1Ac), Agglutinins gene from Pinellia ternata (PTA) | Whole plant | CaMV35S | PB | Resistance to cotton bollworm (Helicoverpa armigera) | Dang and Wei (2007) |
| Insecticidal crystal protein (Cry1Ac; MON 87701), glyphosate (MON 89788) | Whole plant | 35S RNA from figwort mosaic virus (FMV) for Cry1Ac, CaMV35S for EPSP | AG, then traditional breeding of two genetically modified parental lines | Chrysoperla spp., H. zea, tobacco burworm (Heliothis virescens), Hypena scabra | (Monsanto, 2012) |
| Insecticidal crystal protein gene Cry1AC, Cry1F and PAT proteins, and PAT confers tolerance to the herbicide glufosinate | Whole plant | DAS-81419-2 (Conkesta™ technology) powered by polyubiquitin 10 (UBQ10) gene promotor | AG | Target Elasmopalpus lignosellus, Agrotis ipsilon and Helicoverpa armigera and specific for lepidopteran pests | Fast et al. (2015) |
Table 2.
List of Bt-soybean engineered by stacking Cry genes to counter insect resistance and approved in different countries as from November 2017
| Event code and developer | Stacked genes introduced | Product of engineered genes | Function-engineered genes | Method of trait introduction | Country approved as from 2017 | Targeted insects |
|---|---|---|---|---|---|---|
| MON 87701 × MON 89788 Monsanto Company |
Cry1Ac | Cry1Ac delta-endotoxin | Confers resistance to lepidopteran insects by selectively damaging their midgut lining | Conventional breeding—cross hybridization and selection involving transgenic donor(s) | Argentina, Brazil, China, Colombia, European Union, India, Japan, Mexico, Paraguay, Philippines, South Africa, South Korea, Taiwan, Turkey, Uruguay | Lepidopteran insect resistance |
| Cp4 EPSPS (aroA: CP4) | Herbicide-tolerant form of 5-enolpyruvulshikimate-3-phosphate synthase (EPSPS) enzyme | Confer increase tolerance to glyphosate herbicide | ||||
|
DAS81419 Dow AgroSciences LLC |
Cry1Ac | Cry1Ac delta-endotoxin | Confers resistance to lepidopteran insects by selectively damaging their midgut lining | Agrobacterium tumefaciens-mediated plant transformation | Argentina, Australia, Brazil, Canada, Japan, Mexico, New Zealand, South Korea, Taiwan, USA | Lepidopteran insect resistance |
| Cry1F | Cry1F delta-endotoxin | Confers resistance to lepidopteran insects by selectively damaging their midgut lining | ||||
| PAT | Phosphinothricin N-acetyltransferase (PAT) enzyme | Eliminates herbicidal activity of glufosinate (phosphinothricin) herbicides by acetylation | ||||
| MON 87751 Monsanto Company |
Cry1A.105 | Cry1A.105 protein which comprises the Cry1Ab, Cry1F and Cry1Ac proteins | Confers resistance to lepidopteran insects by selectively damaging their midgut lining | Agrobacterium tumefaciens-mediated plant transformation | Australia, Canada, Colombia, Japan, Mexico, New Zealand, South Korea, USA, Taiwan | Lepidopteran insect resistance |
| Cry2Ab2 | Cry2Ab delta-endotoxin | Confers resistance to lepidopteran insects by selectively damaging their midgut lining | ||||
| MON 87751 × MON 87701 × MON 87708 × MON 89788 Monsanto Company |
Cry1A.105 | Cry1A.105 protein which comprises the Cry1Ab, Cry1F and Cry1Ac proteins | Confers resistance to lepidopteran insects by selectively damaging their midgut lining | Conventional breeding—cross hybridization and selection involving transgenic donor(s) | Taiwan, South Korea | Lepidopteran insect resistance |
| Cry2Ab2 | Cry2Ab delta-endotoxin | Confers resistance to lepidopteran insects by selectively damaging their midgut lining | ||||
| Cry1Ac | Cry1Ac delta-endotoxin | Confers resistance to lepidopteran insects by selectively damaging their midgut lining | ||||
| Dmo | Dicamba monooxygenase (Dmo) enzyme | Confers tolerance to the herbicide dicamba (2-methoxy-3,6-dichlorobenzoic acid) by using dicamba as substrate in an enzymatic reaction | ||||
| Cp4 EPSPS (aroA:CP4) | Herbicide tolerant form of 5-enolpyruvulshikimate-3-phosphate synthase (EPSPS) enzyme | Confer increase tolerance to glyphosate herbicide |
Fig. 2.
Molecular analysis of two Cry-protoxins (Cry1Ac and Cry1Ab) commonly introgressed in Bt-soybean. A Molecular phylogenetic analysis by maximum likelihood (ML) method and bootstrap values ≥ 50% from 1000 iterations are shown and the ML tree was rooted as previously described (Louis et al. 2014). b Three-dimensional structure of Cry1Ac (GenBank accession KKB28329.1) and CryAb (GenBank accession AEV45790.1) protoxins modeled in Phyre2 server at 90% accuracy at intensive mode. c Overall view of the functional domain of Cry1Ac or Cry1Ab
Current field performance of Cry genes in Bt-soybean and insect tolerance
We found reports that when truncated Cry1A(b) was introduced into a soybean line that is partially resistant to defoliating insects, the T1 plants deterred feeding, development, and survival of velvetbean caterpillar Anticarsia gemmatalis Hübner (Parrott et al. 1994), but unfortunately it never entered the regulatory and commercial pipeline. Developed in China, the first Bt-soybean expressing Cry-protein with resistant insect trait was introduced into the commercial and regulatory pipeline in January 2009 (Stein and Rodríguezo 2009). By 2013, Monsanto stacked MON 87701 (expressing Cry1Ac for insect resistance) and MON 89788 [glyphosate tolerance for herbicide tolerance (HT)] in the same soybean (codename: MON 89788 × MON 87701) which were then entered in phase III trials in Brazil (Stein and Rodríguezo 2009) and eventually became the first commercial Bt-soybean in Brazil in 2010 and Argentina in 2012 (Bortolotto et al. 2014). Recently, the deployment of Bt-soybean DAS-81419-2 (Conkesta™ technology) expressing Cry1Ac, Cry1F, and phosphinothricin acetyltransferase (PAT) proteins in Brazil have shown high efficiency in resistance against Lepidoptera insects (Fast et al. 2015; Marques et al. 2017).
Prior to commercialization of MON 8788 × MON 87701, fertile Bt-soybean engineered with synthetic Cry1Ac was developed (Cregan et al. 1999). Here, Cregan et al. (1999) identified two quantitative trait loci (QTLs; 229-H and 229-M) from native Japanese soybean lines conferring antibiosis and antixenosis resistance against Lepidopteran insects. By pyramiding these QLTs (229-H and 229-M) with synthetic Cry1Ac enhanced the plant defense against destructive insects (Cregan et al. 1999). Antibiosis is a resistance phenomenon whereby a plant has a detrimental effect on insect growth, development, and reproduction (Painter 2005). On the other hand, antixenosis is a resistance phenomenon whereby plant affects insect behavior by discouraging oviposition, colonization or feeding (Kogan and Ortman 1978). It was observed that Bt-soybean that constitutively expresses stacked genes viz., Cry1Ac and 229-M generates strong insecticidal activities against larvae of Lepidopteran’s—Heliothis virescens, Helicoverpa zea, and Pseudoplusia includens (Walker et al. 2004). Interestingly, Bt-soybean over-expressing synthetic Cry1A significantly reduced Lepidopteran population densities notably, A.gemmatalis, P. includens, and Hypena scabra under field conditions (Mcpherson and Macrae 2009). Using Bt-soybean (MON 87701 × MON 87788 expressing Cry1Ac protein), Bernardi et al. (2012) found high effectiveness against C. includens (Lepidoptera, Noctuidae) and A. gemmatalis (Lepidoptera, Erebidae), yet, this Bt-soybean failed to show activity against stink bugs E. heros or its egg parasitoid Telenomus podisi (Hymenoptera, Platygastidae) under laboratory conditions (Silva et al. 2014). We equally observed that during the outbreak of fall armyworm (S. frugiperda) in South Africa Bt-soybean (MON 87701 × MON 87788; Fig. 1b) was ravaged. Given the polyphagous nature of S. frugiperda and E. heros, this calls for the development of other Bt-soybean with other Cry genes.
In vivo climatic condition alters the performances of Cry genes in Bt-soybean
From the literature search, we identified that a major problem associated with Cry in Planta is the low expression levels that can go below 3 ng/mg of soluble protein in plant generations succeeding T0 plants. This is because native Bt-proteins contain regulatory sequences and require tRNAs not found in plants, or Cry mRNA form secondary structures that hinder proper translation (Perlak et al. 1990). Also, the expression of transgene and the overall phenotype of the plants are not stable (Parrott et al. 1994). Another key issue affecting the performances of Bt-crops is the variations of Cry-proteins as a function of plant variety, plant age, environmental temperature, relative humidity, light and soil properties (Kranthi et al. 2005; Rochester 2006; Addison and Rogers 2010; Chen et al. 2011; Ranjith-kumar et al. 2010; Hagenbucher et al. 2013). Additionally, it was noted under field conditions that the concentration of Cry1Ac protein was higher in Bt-soybean leaves than in any other tissues but drops during anthesis and gradually rebounds (Yu et al. 2014). This indicated that changes in climatic events can alter Bt-soybean’s global defense against destructive insect and thus, affect farmers’ output.
Though the majority of biotech soybean farmed globally is MON 87701 × MON 87788 expressing Cry1Ac, it failed to affect the development and reproduction of black armyworm S. cosmioides (Silva et al. 2016) as well as fall armyworm (S. frugiperda) (Fig. 1b). Intriguingly, Silva et al. (2016) suggested that MON 87701 × MON 87788 is a good host for the development of S. cosmioides and allows for more than 80% of larvae-to-adult survival. Thus, black armyworm S. cosmioides is a major unforeseen menace for global Bt-soybean industries should there be an outbreak. The control of destructive insects by planting Bt-soybean (MON 87701 × MON 87788) could trigger interspecific competition and prompt an upsurge in the population density of polyphagous insects such as black armyworm and fall armyworm.
Divergence in the interaction dynamics of Cry proteins reduces insecticidal potential
Due to the functional complementarities between Cry1Ab and Cry1Ac and their common use for controlling polyphagous insects, we retrieved their sequences in NCBI database, and check for their phylogenetic and structural differences. The sequence set was filtered at default mode for redundancy using ElimDupes (available at http://hcv.lanl.gov/content/sequence/ELIMDUPES/elimdupes.html). From the unique sequence set, the Akaike Information Criterion, corrected (AICc) and Bayesian Information Criterion (BIC) were determined. A maximum likelihood phylogenetic analysis was performed using MEGA 6.1 software (Tamura et al. 2013). The structure of Cry1Ab and Cry1Ac were model using The Phyre2 web portal at default mode (Kelley et al. 2015).
Based on the comparative phylogenetic placement of the most used Cry proteins (Cry1Ac and Cry1Ab) in soybean, the best substitution method was JJT, AICc was 9252.90, BIC was 9541.13 and the highest log likelihood was− 4587.32. The maximum likelihood tree revolved the independent evolution of Cry1Ac and Cry1Ab (Fig. 2a), yet they function by damaging the midgut lining of targeted insects. Although Cry1Ac and Cry1Ab evolved independently, Cry1Ac (in group “a”) showed more diversity hallmarked by singleton AANO7788.1, AHK23266.1 and ADH94040.1. It is worth noting that independent evolution of three domains (I, II and III) and swapping of domain III between different Cry proteins is responsible for the varieties of specificities (De-Maagd et al. 2001; Kumar and Kumari 2015). Structural analysis revealed strong resemblance at main functional domains (I, II and III; Fig. 2b) for Cry1Ac (Genbank: KKB28329.1) and CryAb (Genbank: AEV45790.1).
It was shown that Cry1Ac protein in cotton do not have an effect on Spodoptera insects (Greenberg et al. 2010) and could be due to natural high tolerance of these insects to Cry1 protein (de Maagd et al. 2000). Interestingly, Cry-protein domain I creates pore in epithelium midgut, while domains II and III are involved in receptor recognition and binding (de Maagd et al. 2000, 2003) and contribute to osmotic cell death (Zhuang et al. 2002; Pardo-Lopez et al. 2006). Additionally, domain III structurally resembles the carbohydrate-binding domain 6 (CBM6; Fig. 2b). However, the interaction dynamics of Cry-protein with Spodoptera differs. It was shown that domain III of Cry1C can function as a specific determinant for Spodoptera exigua, but not in all pyramid Cry1-Cry1C combinations (de Maagd et al. 2000). Based on this, it would not be unreasonable to hypothesize that an unexpected mutation in domain III of Cry1Ac could be fatal for Bt-soybean susceptibility to destructive insects. Incontrovertibly, the midgut of Cry-resistant mutant insect larvae are able to tolerate very high doses of protein because of the following: (1) down-regulation in the expression of Cry-protein receptors such as aminopeptidases, alkaline phosphatase and cadherin-like proteins, (2) decrease in the quantity of proteases as well as increase in the amount of esterases and glycolipids such that the level of lethal free and active toxin is non-optimal, and (3) suppression in the expression of ATP-binding cassette transporter C2 (Griffitts and Aroian 2005; Kumar and Kumari 2015). Interestingly, instead of mutations in the Cry, four Cry1Ac-resistant mutations were discovered from Pectinophora gossypiella and found to be recessive and were later mapped onto the cadherin locus (Morin et al. 2003; Fabrick and Tabashnik 2012). This indicates that in an event of concomitant mutations in Cry genes and mutations in insect gut epithelial cell receptors (such as aminopeptidase, alkaline phosphatase and cadherin-like proteins) could compromise the effectiveness of Cry-protein even if the Cry-protein is highly expressed in the Bt-crop.
Cohort of factors that favors the emergence of insect tolerance to Bt-soybean
A fundamental problem affecting the performance of Cry1Ac-crops against key destructive insects such as S. frugiperda and S. exiguaare (1) poor binding to the midgut (Aranda et al. 1996), and (2) endogenous secretion of insect proteases to deactivate Cry1Ac (Miranda et al. 2001; Rahman et al. 2012). Often intercropping of Bt-crops such as soybean and maize could exert selective pressure on polyphagous feeders such as H. armigera that is distributed in Asia, Africa, Europe, and Australia (Tay et al. 2013) and accelerates the emergence of resistant insects. This is supported by a report of H. armigera resistance to Bt-cotton expressing Cry1Ac protein in Australia (Gunning et al. 2005).
Critical for Cry-protein optimal insecticidal activity, it should be overexpressed at all stages of plant growth that coincide with the occurrence of insect pests at the vulnerable stage. Equally, abiotic factors may cause a decrease or fluctuation in the total soluble proteins and negatively influences insecticidal activity. For instance, Luo et al. (2008) showed that the combination of waterlogging and salinity stress on Cry1Ac-cotton inhibited nitrogen metabolism which reduces the production of total soluble protein and reduces insecticidal potential. Also, higher temperature is known to degrade total soluble protein by 47–55%, thus, decreasing the insecticidal activity of Cry1Ac expressed in cotton (Chen et al. 2005). This may explain in part why Cry1Ac does not sufficiently protect Bt-soybean from fall armyworm (S. frugiperda), Southern armyworm (S. eridania), and the velvet armyworm (S. latifascia) (Bernardi et al. 2014). Bt-soybean the MON 87701 × MON 87788 bearing the Cry1Ac is the most farmed, indicating a wide range of the target and non-target insects that are continuously exposed to this protein. Thus, there is a high likelihood of insects evolving tolerance to Cry1Ac. This could be the case for H. armigera resistance to Cry1Ac-cotton in Australia (Gunning et al. 2005).
Higher temperatures in growing area at high latitudes lead to a longer growing season and this subsequently leads to an increase in pest and disease pressure (Haverkort and Verhagen 2008). Climatic changes could also trigger unfavorable epigenetic DNA changes. For instance, DNA methylation of promotor led to reduce late-season expression of Cry-protein (Müller-Cohn et al. 1996; Dong and Li 2007) and this phenomenon could increase the likelihood of broad insect resistance in regions prone to heavy and unseasonal rains, increased humidity, drought, cyclones and hurricanes, and warmer monsoon temperatures. At present, Bt-soybean based on Cry1Ac, and Cry1Ab are widely cultivated and hypothetically, extensive farming of these hybrids could accelerate the development of resistance since Cry1Ab and Cry1Ac show high-level structural and functional similarities (Fig. 2).
Blueprint in integrated gene pyramiding strategy to counter insect resistance challenges
Current trends in soybean protection revealed that resistance to destructive insects hugely relies on coupling multiple endogenous quantitative resistance traits in succession to achieve the desired trait as a core part of integrated pest management strategy (Fig. 3; Table 2). To avoid insect evolving resistance to Cry1Ac-soybean commonly farmed, gene pyramiding consisting of coupling more than one Cry in a transgenic plant is encouraged (Perlak et al. 1990; Monsanto 2012; Ortega et al. 2016). For instance, pyramiding in Bt-soybean (MON 89788 × MON 87701) solves at least two problems (Monsanto 2012), but reliance on only one Cry-protein cannot guarantee complete elimination of all soybean destructive insects.
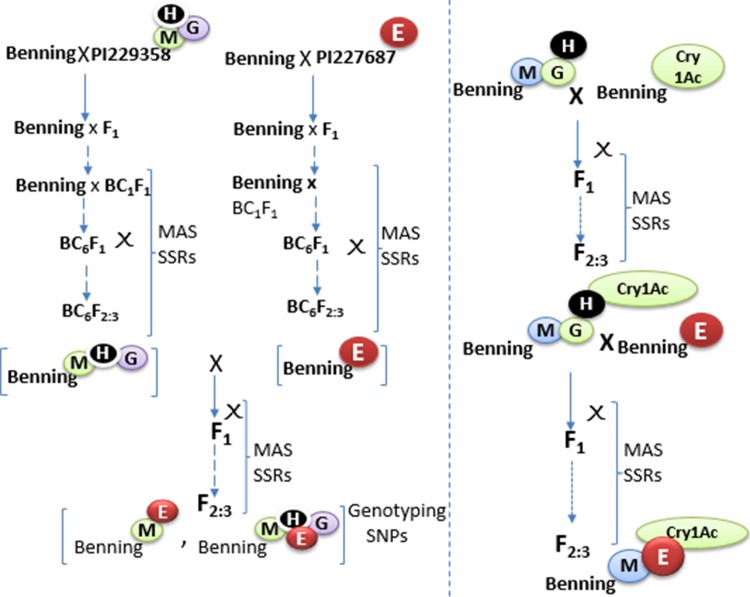
Fig. 3.
a Modification of Ortega et al. (2016) depicting the strategy of pyramiding QTLs resistance with Cry1Ac-protoxin to control various soybean chewing insects. Here, BenningM−E−Cry1Ac was found to be more resistant than BenningME and BenningCry1Acagainst soybean looper Chrysodeixis includens (Walker) and Southern armyworm Spodoptera eridania (Cramer). b Cry was coupled with artificial microRNAs to specifically target pests
Gene pyramiding in plants such as soybean that are recalcitrant to transform is challenging (Joshi and Nayak 2010; Ortega et al. 2016), although marker-assisted selection (MAS) and single nucleotide polymorphism (SNP) has eased the process of selecting the desire trait (Fig. 3; Table 2). Combining dominant resistance traits offers broad spectrum resistance to multiple destructive insects of soybean. For example, Ortega et al. (2016) used soybean line PI 229358 and PI 227687 that harbors broad range resistance to chewing insects. PI 229358’s resistance is conferred by QTLs: M, G, and H. PI 227687’s resistance is conferred by QTL-E, where the letters indicate linkage groups on which the QTL are located, plus the incorporated Cry1Ac-protein to soybean-BenningM−E−Cry1AC (Ortega et al. 2016; Fig. 3a). It was found that BenningM−E−Cry1Acwas more resistant than BenningME and BenningCry1Acagainst soybean looper C. includens and Southern armyworm (Spodoptera eridania). In fact, the rationale behind the success of gene pyramiding depends on the genetic background of the host lines to ensure inheritance of the desired traits. For instance, PI-227687 is resistant to defoliating insects and PI-229358 exhibits antibiosis and antixenosis (Rector et al. 2000a, b) and thus, stacking more traits enhances defense against destructive insects. It would be interesting to observe the field performance of Cry1Ac-, Cry1AF- and PAT-soybean (DAS-81419-2®, Conkesta™ technology; Fast et al. 2015) bearing multiple stack genes. Furthermore, we proposed that engineering a soybean with inherent multiple resistant QTLs with multiple Cry-proteins whose product bind to different receptors in the same insect is an important prospective to effectively counter insect resistance challenges. While Cry is widely accepted in soybean, we proposed that incorporating RNA interference (RNAi) technology into transgenic soybeans could complement the Bt-soybean hybrids already compromised by insect-evolved field tolerance. In this vein, the use of artificial microRNAs designed to be constitutively expressed and target midgut proteases of insects could ensure durability of already developed Bt-soybean (Fig. 4).
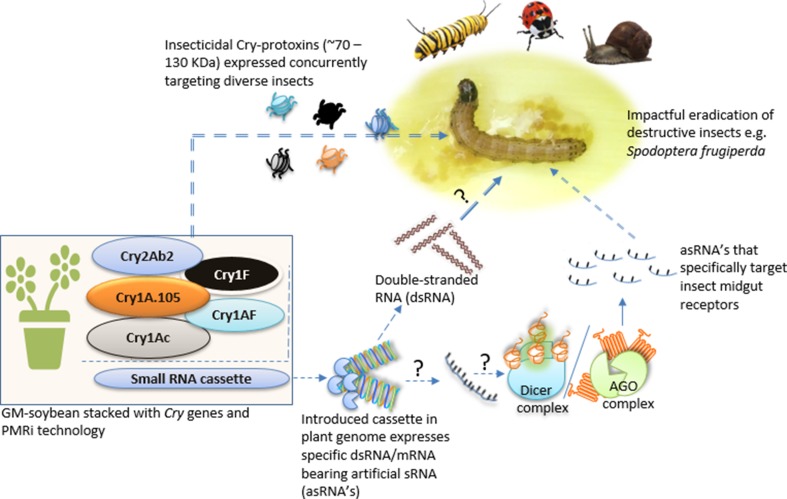
Fig. 4.
Important δ-endotoxins crystal proteins such as Cry1 (~ 130 kDa specifically target butterflies and beetles) Cry2 (~ 70 kDa specifically target butterflies and Diptera), Cry3 (~ 70 kDa specifically target beetles), and Cry4 (~ 130 kDa specifically target Diptera) (Palma et al. 2014; Ibrahim and Shawer 2014). Coupling PMRi with stacked Cry genes could better protect Bt-soybean against destructive insects
Currently, QTLs/Cry-protein applied in soybean is expressed in the whole plant (Cregan et al. 1999; Ortega et al. 2016), whereas insects feed mostly on the soybean leaf and pods. It was suggested that Cry-protein should be expressed in the chloroplast genome since it offers the possibility of gene transfer to pollen (Daniell 2000) and high-level toxicity in the leaves. Therefore, transforming chloroplast genome provides high dosage of Cry-protein/mg leaf consumed and offers a more effective tool of controlling the destructive insects. With advances in DNA delivery method, Dufourmantel et al. (2005) transformed soybean plastid with Cry1Ab (Table 1) and found a strong resistance against velvetbean caterpillar (A. gemmatalis) a difficult insect to control. Plastid transformation offers multiple advantages such as (1) specific placement of the transgene, (2) sub-lethal dosage to leaf-chewing insect are avoided, (3) since plastids genes are inherited via the mother ovary only, the likely risk for out-crossing transgenes to other species in the same biota is greatly reduced (Mikkelsen et al. 1996; Ibrahim and Shawer 2014), and (4) high copy number of transgene is achieved since DNA placed in the chloroplast will be copied 5000–10,000 times in a cell, while a typical genomic DNA will produce 1–4 copies per cell. Thus, plastid transformation offers a direct and rapid approach for controlling destructive insects of soybean. In this light, Bt-soybean developers should consider chloroplast transformation for the stacked Cry genes constructs (Table 2). To preserve the effectiveness of the current Bt-technology, a refuge block (or strips) of non-Bt-crops could be included in the field harboring the same Bt-crop as buffer zones. Furthermore, combining the refuge technique with other traditional techniques such as (1) crop rotation, (2) rotating Bt traits, (3) use of pyramided traits, and (4) scout fields for pest can effectively slow insect resistance to Bt-crop.
Enhancing plant defense by coupling Cry traits with plant-mediated RNAi (PMRi) silencing of insect’s genes
Mobile small RNA (sRNA)-mediated RNA interference (RNAi) is a highly conserved regulatory mechanism that governed cellular processes in eukaryotes and ensure communication between kingdoms (Weiberg et al. 2013). Despite the diversity of sRNA, the most used of this non-coding regulatory molecules in transgenics are small interfering RNAs (siRNAs), piwi-associated RNAs (piRNAs) and microRNAs (miRNAs) generated via the Dicer and Argonaute (AGO) protein complexes (Fig. 4). MicroRNAs designed from acetylcholinesterase 2 gene (MpCHE2) to target two different sites on MpCHE2 of aphid Myzus persicae (family Aphidoidea, order Hemiptera) and introgressed in tobacco plants (Nicotiana tabacum cv. Xanti) exhibited resistance against aphid (Guo et al. 2014). This study demonstrated that plants expressing artificial sRNA (asRNA) can effectively control destructive insects. Other genes used successfully in plant-mediated RNAi (PMRi) silencing of insect’s genes where transgenic plants expressed insect double-stranded RNA (dsRNA) include cytochrome P450 genes CYP6AE14 and CYP6B6, arginine kinase (AK) gene, and HMG-CoA reductase (HMG) gene of cotton bollworm Helicoverpa zea (Mao et al. 2007, 2011; Tian et al. 2015), hormone receptor 3 (HR3) molt-regulating transcription factor involved in metamorphosis of Helicoverpa armigera (Han et al. 2017), and induced cytochrome P450 monooxygenase CYP6B46 and β-glucosidase genes of Manduca sexta and M. quinquemaculata (Lepidoptera, Sphingidae) (Poreddy et al. 2017), all leading resistance against the targeted insects. Taking into consideration that insects bites triggers host-induced gene silencing (HIGS) via transgenes in plants and that HIGS sRNAs are effective against destructive insects by translocating into insect cells (Weiberg et al. 2013), associating PMRi with Cry genes would better protect Bt-soybeans than overdependence on Cry genes only (Fig. 4).
The quiet approval and commercialization of Monsanto non-specific DvSnf7 dsRNA expressed in MON 89034 × TC1507 × MON 87411 × DAS-59122-7 cassette in corn (Bolognesi et al. 2012; Vélez et al. 2016; EPA 2016) to counter the Western corn rootworm (Diabrotica virgifera virgifera LeConte) in USA, marks the beginning of RNAi biospesticide into agriculture. Reasonably, it might not long to see DvSnf7 dsRNA incorporated in different soybean hybrids. Regardless of RNAi approach, it could be less effective because of low uptake in the insect, high alkaline gut environment (pH > 9.0) and a strong intestinal nucleolytic activity which degrade dsRNA. To counter alkaline hydrolysis in insect gut, Christiaens et al. (2018) developed a guanidine-rich polymer capable of protecting dsRNA up to 30 h at pH 11. By feeding beet armyworm S. exigua larvae with cabbage leaf disc coated with protected dsRNA guanidine-rich polymer nanoparticle in vivo, 53% and 16% mortality was observed with protected and naked dsRNA (Christiaens et al. 2018), respectively. This approach of protecting dsRNA with guanidine-rich polymer nanoparticle paved the way for RNAi-based pesticides as sprays for the control of invasive insect pests.
Conclusion
In the twenty-first century, Bt-soybean like other GM crops provide useful agronomic traits for crop protection and production, but not a panacea, yet, Bt-soybean helped to curb the problems caused by specific pests, lower farming input and increase the yield. It could be an oversight not to think of new strategies to control destructive insect and ensure the achievement of 2050 forecast of 371.3 metric million tons. We proposed that combining two Cry-protein that binds to different receptors in the same insect and associated RNAi technology could enhance insecticidal potential of Bt-soybean. While gene pyramiding appeared the way forward to counter destructive insects, stringent caution must be taken not to compromise human health, animal health and abrupt collapse of biota due to the elimination of non-target pests.
Acknowledgements
This research was supported by The World Academy of Sciences (TWAS), Trieste, Italy and the Department of Biotechnology, Government of India (DBT/TWAS PG fellowship no. 3240223450) and Alexander von Humbolt (AvH) foundation.
Abbreviations
- PMRi
Plant-mediated RNA interference
- Bt
Bacillus thuringiensis
- HT
Herbicide tolerance
- GM
Genetically modified
- MAB
Marker-assisted breeding
- PAT
Phosphinothricin acetyltransferase
- AICc
Akaike Information Criterion, corrected
- BIC
Bayesian Information Criterion
- Cry
Crystal
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported by the authors.
References
- Addison SJ, Rogers DJ. Potential impact of differential production of the Cry2Ab and Cry1Ac proteins in transgenic cotton in response to cold stress. J Econ Entomol. 2010;103:1206–1215. doi: 10.1603/ec09369. [DOI] [PubMed] [Google Scholar]
- Aranda E, Sanchez J, Peferoen M, Guereca L, Bravo A. Interactions ofBacillus thuringiensis crystal proteins with the midgut epithelial cells of Spodoptera frugiperda (Lepidoptera: Noctuidae) J Invertebr Pathol. 1996;68:203–212. doi: 10.1006/jipa.1996.0087. [DOI] [PubMed] [Google Scholar]
- Azambuja R, Degrande PE, Dos-Santos RO, De-Souza EP, Gomes CEC. Effect of Bt-soybean on larvae of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) J Agric Sci. 2015;7(8):90–94. [Google Scholar]
- Bebber DP, Sarah JG. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet Biol. 2015;74:62–64. doi: 10.1016/j.fgb.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Bernardi O, Malvestiti GS, Dourado PM, Oliveira WS, Martinelli S, Berger GU, Head GP, Omoto C. Assessment of the high-dose concept and level of control provided by MON 87701 × MON 87788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag Sci. 2012;68:1083–1091. doi: 10.1002/ps.3271. [DOI] [PubMed] [Google Scholar]
- Bernardi O, Sorgatto RJ, Barbosa AD, Domingues FA, Dourado PM, Carvalho RA, Martinelli S, Head GP, Omoto C. Low susceptibility of Spodoptera cosmioides, Spodoptera eridania and Spodoptera frugiperda (Lepidoptera: Noctuidae) to genetically-modifed soybean expressing Cry1Ac protein. Crop Prot. 2014;58:33–40. [Google Scholar]
- Bolognesi R, Ramaseshadri P, Anderson J, Bachman P, Clinton W, Flannagan R, Ilagan O, Lawrence C, Levine S, Moar W, Mueller G, Tan J, Uffman J, Wiggins E, Heck G, Segers G. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte) PLoS One. 2012;7(10):e47534. doi: 10.1371/journal.pone.0047534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotto OC, Silva GV, Bueno AF, Pomari AF, Martinelli S, Head GP, Carvalho RA, Barbosa GC. Development and reproduction of Spodoptera eridania (Lepidoptera: Noctuidae) and its egg parasitoid Telenomus remus (Hymenoptera: Platygastridae) on the genetically modified soybean (Bt) MON 87701 × MON 89788. Bull Entomol Res. 2014;104:724–730. doi: 10.1017/S0007485314000546. [DOI] [PubMed] [Google Scholar]
- Chen D, Ye G, Yang C, Chen Y, Wu Y. The effect of high temperature on the insecticidal properties of Bt cotton. Environ Exp Bot. 2005;53:333e342. [Google Scholar]
- Chen FJ, Wu G, Ge F, Parajulee MN. Relationships between exogenoustoxin quantity and increased biomass of transgenic Bt-crops under elevated carbondioxide. Ecotoxicol Environ Saf. 2011;74:1074–1080. doi: 10.1016/j.ecoenv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Christiaens O, Tardajos MG, Martinez-Reyna ZL, Dash M, Dubruel P, Smagghe G. Increased RNAi efficacy in Spodoptera exigua via the formulation of dsRNA with guanylated polymers. Front Physiol. 2018;9:316. doi: 10.3389/fphys.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregan PB, Jarvik T, Bush AL, Shoemaker RC, Lark KG, Kahler AL, Kaya N, Vantoai TT, Lohnes DG, Chung J, Specht JE. An integrated genetic linkage map of the soybean genome. Crop Sci. 1999;39:1464–1490. [Google Scholar]
- Daniell H. Genetically modified food crops: current concerns and solutions for next generation food crops. Biotechnol Genet Eng Rev. 2000;17:327–352. doi: 10.1080/02648725.2000.10647997. [DOI] [PubMed] [Google Scholar]
- Dang W, Wei Z-M. An optimized Agrobacterium-mediated transformation for soybean for expression of binary insect resistance genes. Plant Sci. 2007;173:381–389. [Google Scholar]
- de Maagd RA, Weemen-Hendriks M, Stiekema W, Bosch D. Bacillus thuringiensis delta-endotoxin Cry1C domain III can function as a specificity determinant for Spodoptera exigua in different, but not all, Cry1-Cry1C hybrids. Appl Environ Microbiol. 2000;66(4):1559–1563. doi: 10.1128/aem.66.4.1559-1563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Maagd RA, Bravo A, Crickmore N. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 2001;17:193–199. doi: 10.1016/s0168-9525(01)02237-5. [DOI] [PubMed] [Google Scholar]
- De-Maagd RA, Bravo A, Berry C, Crickmore N, Schnepf HE. Structure, diversity and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu Rev Genet. 2003;37:409–433. doi: 10.1146/annurev.genet.37.110801.143042. [DOI] [PubMed] [Google Scholar]
- Dillie JA, Sikkema PH, Everman WJ, Davis VM, Burke IC (2016) Perspectives on soybean yield losses due to weeds in North America. http://wssa.net/wp-content/uploads/WSSA-2016-Soybean-Yield-Loss-poster.pdf. Accessed Jan 2016
- Dong HZ, Li WJ. Variability of endotoxin expression in Bt transgenic cotton. J Agron Crop Sci. 2007;193:21–29. [Google Scholar]
- Dufourmantel N, Tissot G, Goutorbe F, Garςon F, Muhr C, Jansens B, Pelissier B. Generation and analysis of soybean plastid transformants expressing Bacillus thuringiensis Cry1Ab protoxin. Plant Mol Biol. 2005;58:659–668. doi: 10.1007/s11103-005-7405-3. [DOI] [PubMed] [Google Scholar]
- Dufourmantel N, Dubald M, Matringe M, Canard H, Garcon F, Job C, Kay E. Generation and characterization of soybean and marker-free tobacco plastid transformants over-expressing a bacterial 4-hydroxyphenylpyruvate dioxygenase which provides strong herbicide tolerance. J Plant Biotechnol. 2007;5:118–133. doi: 10.1111/j.1467-7652.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- EPA (2016) RNAi technology: human health and ecological risk assessments fr SmartStax PRO. 42706: Federal Register. 81(126). https://www.gpo.gov/fdsys/pkg/FR-2016-06-30/pdf/2016-15589.pdf. Accessed 30 June 2016
- Estruch JJ, Carozzi NB, Desai N, Duck NB, Warren GW, Koziel MG. Transgenic plants: an emerging approach to pest control. Nat Biotechnol. 1996;15:137. doi: 10.1038/nbt0297-137. [DOI] [PubMed] [Google Scholar]
- Evans LT. Feeding the ten billion. Plants and population growth. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Fabrick JA, Tabashnik BE. Similar genetic basis of resistance to Bt toxin Cry1Ac in boll-selected and diet-selected strains of pink bollworm. PLoS One. 2012;7:e35658. doi: 10.1371/journal.pone.0035658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT (2012) Rome, IT, Statistics Division of FAO. http://faostat.fao.org/site/339/default.aspx. Accessed 10 Dec 2011
- Fast BJ, Schafer AC, Jonson TY, Potts BL, Herman RA. Insect-protected event DAS-81419-2 soybean (Glycine max L.) grown in the United States and Brazil is compositionally equivalent to non-transgenic soybean. J Agr Food Chem. 2015;63:2063e2073. doi: 10.1021/jf505015y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre J, van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu Rev Entomol. 2002;47:501–533. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- Fickett ND, Stoltenberg DE, Boerboom CM, Hammond CM. Estimated economic losses from early weed competition in Wisconsin corn and soybean fields. N Cent Weed Sci Soc Proc. 2009;64:93. [Google Scholar]
- Gamundi JC, Sosa MA. Caracterización de daňos de chinches en soja y criterios para la tomada de decisions de manejo, pp29–148. In: Trumper EV, Edelstein JD, editors. Chindes fitófages en soja. Revisión y avances en el studio de su ecología y manejo. Buenos Aires: Ediciones INTA, Manfredi; 2008. p. 190. [Google Scholar]
- Greenberg SM, Li YX, Liu TX. Effect of age of transgenic cotton on mortality of lepidopteran larvae. Southwest Entomol. 2010;35:261–268. [Google Scholar]
- Griffitts JS, Aroian RV. Many roads to resistance: how invertebrates adapt to Bt toxins. BioEssays. 2005;27(6):614–624. doi: 10.1002/bies.20239. [DOI] [PubMed] [Google Scholar]
- Grossi-de-Sá MF, Pelegrini PB, Fragoso RR (2011) Genetically modified soybean for insect-pest and disease control, pp 429–452. http://ainfo.cnptia.embrapa.br/digital/bitstream/item/42556/1/FRAGOSO-S1424.pdf. Accessed 11 Apr 2011
- Gunning RV, Dang HT, Kemp FC, Nicholson IC, Moores GD. New resistance mechanism in Helicoverpa armigera threatens transgenic crops expressing Bacillus thuringiensis Cry1Ac toxin. Appl Environ Microbiol. 2005;71:558–2563. doi: 10.1128/AEM.71.5.2558-2563.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Song X, Wang G, Yang K, Wang Y, et al. Plant-generated artificial small RNAs mediated aphid resistance. PLoS One. 2014;9(5):e97410. doi: 10.1371/journal.pone.0097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbucher S, Olson DM, Ruberson JR, Wäckers FL, Romeis J. Resistance mechanisms against arthropod herbivores in cotton and their interactions with natural enemies. Crit Rev Plant Sci. 2013;32:458–482. [Google Scholar]
- Han Q, Wang Z, He Y, Xiong Y, Lv S, Li S, Zhang Z, Qiu D, Zeng H. Transgenic cotton plants expressing the HaHR3 gene conferred enhanced resistance to Helicoverpa armigera and improved cotton yield. Int J Mol Sci. 2017;18:1274. doi: 10.3390/ijms18091874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman GL, West ED, Herman TK. Crops that feed the world 2. Soybean—worldwide production, use, and constraints caused by pathogens and pest. Food Secur. 2011;3:5–17. [Google Scholar]
- Haverkort A, Verhagen A. Climate change and its repercussions for the potato supply chain. Potato Res. 2008;51:223–237. [Google Scholar]
- Ibrahim RA, Shawer DM. Transgenic Bt-plants and the future of crop protection (an overview) Int J Agric Food Res. 2014;3(1):14–40. [Google Scholar]
- ISAAA (2016) International Service for the Acquisition of Agribiotech Applications (ISAAA). http://www.isaaa.org/resources/publications/pocketk/16/. Accessed June 2016
- Ishiwata S. On a kind of severe flasherie (sotto disease) Danihan Sanbshi Kaiho. 1901;9:1–5. [Google Scholar]
- James C. Global status of commercialized biotech GM Crops: 2010 International Service for the Acquisition of Agri-biotech Applications (ISAAA) Ithaca, NY: ISAAA Briefs brief 42; 2010. [Google Scholar]
- James C. 20th Anniversary (1996 to 2015) of the global commercialization of biotech crops and biotech crop highlights in 2015. ISAAA brief No. 51. Ithaca: ISAAA; 2015. [Google Scholar]
- Joshi RK, Nayak S. Gene pyramiding-A broad spectrum technique for developing durable stress resistance in crops. Biotechnol Mol Biol. 2010;5(3):51–60. [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modelling, prediction and analysis. Nation Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan M, Ortman EF. Antixenosis: a new term proposed to define Painter’s non-preference modality of resistance. Bull Entomol Soc Am. 1978;24:175–176. [Google Scholar]
- Kranthi KR, Naidu S, Dhawad CS, Tatwawadi A, Mate K. Temporal and intra-plant variability of Cry1Ac expression in Bt-cotton and it influence on the survival of the cotton bollworm, Helicoverpa armigera (Hubner) (Noctuidae: Lepidoptera) Curr Sci. 2005;89:291–298. [Google Scholar]
- Kumar S, Kumari R. Occurrence of molecularly diverse Bt Cry toxin-resistant mutations in insect pests of Bt + corn and cotton crops and remedial approaches. Curr Sci. 2015;108(8):1483–1450. [Google Scholar]
- Lang A, Otto M. A synthesis of laboratory and field studies on the effects of transgenic Bacillus thurengiensis (Bt) maize on non-target Lepidoptera. Entomol Exp Appl. 2010;135:121–134. [Google Scholar]
- Loguercio LL, Santos CG, Barreto MR, Guimarẩes CT, Paaiva E. Association of PCR and feeding bioassays as a large-scale method to screen tropical Bacillus thuringiensis isolates for a Cry constitution with higher insecticidal effect against Spodoptera frugiperda (Lepidoptera: Nuctuidae) larvae. Lett Appl Microbiol. 2001;32:362–367. doi: 10.1046/j.1472-765x.2001.00920.x. [DOI] [PubMed] [Google Scholar]
- Louis B, Sayanika DW, Pranab R, Pardeep KB, Wakambam MS, Talukdar NC. Host shifting dynamics of Cochliobolus lunatus: from a biocontrol agent to a severe environmental threat. Biomed Res Int. 2014;2014:9. doi: 10.1155/2014/378372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Dong H, Li W, Zhao M, Zhu Y. Individual and combined effects of salinity and waterlogging on Cry1Ac expression and insecticidal efficacy of Bt-cotton. Crop Prot. 2008;27:1485e1490. [Google Scholar]
- Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol. 2007;25:1307–1313. doi: 10.1038/nbt1352. [DOI] [PubMed] [Google Scholar]
- Mao Y-B, Tao X-Y, Xue X-Y, Wang L-J, Chen X-Y. Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Res. 2011;20:665–673. doi: 10.1007/s11248-010-9450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques LH, Santos AC, Castro BA, Moscardini VF, Silva RJ, Zobiole OAN, et al. Field evaluation of soybean transgenic event DAS-81419-2 expressing Cry1F and Cry1Ac proteins for the control of secondary lepidopteranpests in Brazil. Crop Prot. 2017;96:109–115. [Google Scholar]
- Masuda T, Goldsmith PD. World soybean production: area harvested, yield, and long-term projections. Int Food Agribus Manag Assoc. 2009;12(4):143–162. [Google Scholar]
- Mcpherson RM, Macrae TC. Evaluation of transgenic soybean exhibiting high expression of a synthetic Bacillus thuringiensis Cry1A transgene for suppressing lepidopteran population densities and crop injury. J Econ Entomol. 2009;102(4):1640–1648. doi: 10.1603/029.102.0431. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TR, Andersen B, Jørgensen RB. The risk of crop transgene spread. Nature. 1996;380:31. [Google Scholar]
- Miranda R, Zamudio F, Bravo A. Processing of Cry1Ab d-endotoxin from Bacillus thuringiensis by Manduca sexta and Spodoptera frugiperda midgut proteases: role in protoxin activation and toxin inactivation. Insect Biochem Mol Biol. 2001;31:1155–1163. doi: 10.1016/s0965-1748(01)00061-3. [DOI] [PubMed] [Google Scholar]
- Monsanto (2012) IRM grower guide: insect resistance management for US corn- and cotton-growing areas. http://www.monsanto.com/SiteCollectionDocuments/IRM-Grower-Guide.pdf. Accessed 29 Apr 2012
- Morin S, Biggs RW, Sisterson MS, Shriver L, Ellers-Kirk C, Higginson D, Holley D. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. PNAS. 2003;100:5004–5009. doi: 10.1073/pnas.0831036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Cohn J, Chaufaux J, Buisson C, Gilois N, Sanchis V, Lereclus D. Spodoptera littoralis (Lepidoptera: Noctuidae) resistance to Cry1C and cross resistance to other Bacillus thuringiensis crystal toxins. J Econ Entomol. 1996;89:791–797. [Google Scholar]
- Musser FR, Catchot AL, Jr, Davis JA, Herbert DA, Jr, Lorenz GM, Reed T, Reisig DD, Stewart SD. 2013 soybean insect losses in the southern US. Midsouth Entomol. 2014;7:15–28. [Google Scholar]
- Musser FR, Catchot AL, Jr, Davis JA, Herbert DA, Jr, Lorenz GM, Reed T, Reisig DD, Stewart SD. 2014 soybean insect losses in the southern US. Midsouth Entomol. 2015;8:35–48. [Google Scholar]
- Musser FR, Catchot AL, Jr, Davis JA, Herbert DA, Jr, Lorenz GM, Reed T, Reisig DD, Stewart SD. 2015 soybean insect losses in the southern US. Midsouth Entomol. 2016;9:5–17. [Google Scholar]
- Musser FR, Catchot AL, Jr, Davis JA, Lorenz GM, Reed T, Reisig DD, Stewart SD, Taylor S. 2016 soybean insect losses in the southern US. Midsouth Entomol. 2017;10:1–13. [Google Scholar]
- Ortega MA, All JN, Boerma HR, Parrott WA. Pyramids of QTLs enhance host–plant resistance and Bt mediated resistance to leaf chewing insects in soybean. Theor Appl Genet. 2016;129:703–715. doi: 10.1007/s00122-015-2658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RH. Insect resistance in crop plants. Soil Sci. 2005;72:481. [Google Scholar]
- Palma L, Muñoz D, Berry C, Murillo J, Caballero P. Bacillus thuringiensis toxins: an overview of their bioacidal activity. Toxins. 2014;6:3296–3325. doi: 10.3390/toxins6123296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Lopez L, Gomez I, Rausell C, Sanchez J, Soberon M. Structural changes of the Cry1Ac oligomeric pre-pore from Bacillus thuringiensis induced by N-acetylgalactosamine facilitates toxin membrane insertion. Biochemistry. 2006;45:10329–10336. doi: 10.1021/bi060297z. [DOI] [PubMed] [Google Scholar]
- Parrott WA, All JN, Adang MJ, Bailey MA, Boerma HR, Stewart Jr CN. Recovery and evaluation of soybean plants transgenic for a Bacillus thuringiensis var. Kurstaki Insecticidal gene. In Vitro Cell Dev Biol. 1994;30P:144–149. [Google Scholar]
- Perlak FJ, Deaton RW, Armstrong TA. Insect resistant cotton plants. Bio-Technololgy. 1990;8:939–943. doi: 10.1038/nbt1090-939. [DOI] [PubMed] [Google Scholar]
- Poreddy S, Li J, Baldwin IT. Plant-mediated RNAi silences midgut expressed genes in congeneric lepidopteran insects in nature. BMC Plant Biol. 2017;17:199. doi: 10.1186/s12870-017-1149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K, Abdullah M, Ambati S, Taylor MD, Adang MJ. Differential protection of Cry1Fa toxin against Spodoptera frugiperda larval gut proteases by cadherin orthologs correlates with increased synergism. Appl Environ Micro. 2012;78:354–362. doi: 10.1128/AEM.06212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjith-Kumar CT, Lai Y, Sarisky RT, Cheng Kao C. Green tea catechin, epigallocatechin gallate, suppresses signaling by the dsRNA innate immune receptor RIG-I. PLoS One. 2010;5:e12878. doi: 10.1371/journal.pone.0012878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond B, Johnston PR, Nielsen-leroux C, Lereclus D, Crickmore N. Bacillus thurengiensis: an impotent pathogen? Trends Microbiol. 2010;18:189–194. doi: 10.1016/j.tim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Rector BG, All JN, Parrott WA, Boerma HR. Quantitative trait loci for antibiosis resistance to corn earworm in soybean. Crop Sci. 2000;40:233–238. [Google Scholar]
- Rector BG, All JN, Parrott WA, Boerma HR. Quantitative trait loci for antixenosis resistance to corn earworm in soybean. Crop Sci. 2000;40:531–538. [Google Scholar]
- Rochester IJ. Effect of genotype, edaphic, environmental conditions and agronomic practices on Cry1Ac protein expression in transgenic cotton. J Cotton Sci. 2006;10:252–262. [Google Scholar]
- Shurtleff W, Aoyagi A (2013) History of whole dry soybeans, used as beans, or ground, mashed or flaked (240 BCE to 2013). Lafayette, California, p 950
- Silva GV, Pasini A, Bueno A-de-F, Bortolotto OC, Barbosa GC, Cruz YKS. No impact of Bt soybean that express Cry1Ac protein on biological traits of Euschistus heros (Hemiptera, Pentatomidae) and its egg parasitoid Telenomus podisi (Hymenoptera, Platygastridae) Rev Bras Entomol. 2014;58(3):285–290. [Google Scholar]
- Silva GV, de Bueno AF, Bortolotto OC, Dos-Santos A, Pomari-Fernandes A. Biological characteristics of black armyworm Spodoptera cosmioides on genetically modified soybean and corn crops that express insecticide Cry proteins. Rev Bras Entomol. 2016;60:255–259. [Google Scholar]
- Sosa-Gómes DR, Silva JJ. Neotropical brown stink bug (Euschitus heros) resistance to methamidophos in Paraná, Brazil. Pesqui Agropecu Bras. 2010;45:767–811. [Google Scholar]
- Stein AJ, Rodríguez-Cerezo E (2009) The global pipeline of new GM crops: implications of asynchronous approval for international trade. JRC Technical Report EUR 23486 EN. Office for Official Publications of the European Communities, Luxembourg. http://ipts.jrc.ec.europa.eu/publications/pub.cfm?id=2420. Accessed 03 Sept 2009
- Stewart CN, Jr, Adang MJ, All JN, Boerma HR, Cardineau G, Tucker D, Parrott WA. Genetic transformation, recovery, and characterization of fertile soybean transgenic for a synthetic Bacillus thuringiensis CryIAc gene. Plant Physiol. 1996;112(1):121–130. doi: 10.1104/pp.112.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugan M. Soy in health and disease prevention. New York: CRC Press; 2005. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay WT, Soria MF, Walsh T, Thomazoni D, Silvie P, Gajanan TB, Sharon D. A brave new world for an old-world Pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS One. 2013;8(11):80134. doi: 10.1371/journal.pone.0080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Cheng L, Qi X, Ge Z, Niu C, Zhang X, Jin S. Transgenic cotton plants expressing double-stranded RNAs target HMG-CoA reductase (HMGR) gene inhibits the growth, development and survival of cotton bollworms. Int J Biol Sci. 2015;11(11):1296–1305. doi: 10.7150/ijbs.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA (2017) World agricultural supply and demand estimates, economic research service and foreign agricultural service. https://www.usda.gov/oce/commodity/wasde/latest.pdf(ISSN:1554-9089)
- Vélez AM, Jurzenski J, Matz N, Zhou X, Wang H, Ellis M, Siegfried BD. Developing an in vivo toxicity assay for RNAi risk assessment in honey bees Apis mellifera L. Chemosphere. 2016;144:1083–1090. doi: 10.1016/j.chemosphere.2015.09.068. [DOI] [PubMed] [Google Scholar]
- Walker DR, Narvel JM, Boerma HR, All JN, Parrott WA. A QTL that enhances and broadens Bt insect resistance in soybean. Theor Appl Genet. 2004;109(5):1051–1057. doi: 10.1007/s00122-004-1714-9. [DOI] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, Huang HD, Jin H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 2013;342:118–123. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Takagi K, Ishimoto M. Recent advances in soybean transformation and their application to molecular breeding and genomic analysis. Breed Sci. 2012;61:480–494. doi: 10.1270/jsbbs.61.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Romeis J, Li Y, Li X, Wu K. Acquisition of Cry1Ac protein by non-target arthropods in Bt-soybean fields. PLoS One. 2014;9(8):e103973. doi: 10.1371/journal.pone.0103973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang M, Oltean DI, Gomez I, Pullikuth AK, Soberon M. Heliothis virescens and Manduca sexta lipid rafts are involved in Cry1A toxin binding to the midgut epithelium and subsequent pore formation. J Biol Chem. 2002;277:13863–13872. doi: 10.1074/jbc.M110057200. [DOI] [PubMed] [Google Scholar]