Abstract
Background
Data on primary nonadherence remains sparse, due to a lack of data resources that combine information on medication prescribing and dispensing. In addition, previous work on primary nonadherence has used follow-up periods ranging from 30 days up to 18 months, making results difficult to compare.
Objective
To evaluate the prevalence and predictors of primary nonadherence by measuring time until filling in a cohort of elderly patients.
Design
Retrospective cohort study of new prescription episodes.
Patients
Data comes from a linked database of electronic health records and claims for patients aged ≥ 65 years enrolled in Medicare Parts A, B, and D during 2007–2014. We identified patients receiving a new prescription for a chronic disease medication with continuous Medicare enrollment for 180 days prior to the index prescription order and no fills or orders for the medication during this period.
Main Measures
Time until filling of the index prescription for up to 1 year.
Key Results
In 32,586 new medication orders, the majority (75%; 95% confidence interval [CI] 74–75%) of new prescriptions were filled within 7 days, 81% (81–82%) were filled within 30 days, and 91% (91–92%) were filled within 1 year. The rate and timing of dispensing were similar across therapeutic areas. Timing of initial filling within 7 days or within 30 days could be predicted with moderate accuracy (C-statistics = 0.70–0.74). Patients with > 5 current medications on hand at the time of the index prescription and average out-of-pocket medication costs < $5 filled 89% of prescriptions within 7 days. Patients with no current medications and out-of-pocket costs > $50 filled only 25% of prescriptions within 7 days.
Conclusions
Nearly 20% of patients do not fill a new chronic disease prescription within 30 days. Patients with fewer recent fills and higher out-of-pocket costs are at higher risk of primary nonadherence.
KEY WORDS: electronic health records, health insurance claims, primary nonadherence, prediction, lasso
BACKGROUND
Almost 50 years of research has documented that adherence to prescription medications, regardless of diagnosis, is poor.1, 2 Among patients who initiate a new chronic disease medication, half discontinue within the first 6 months.3–5 Moreover, many patients do not even initiate the newly prescribed medication. This failure to initiate a new therapy is referred to as primary nonadherence (PNA).6 In contrast, secondary nonadherence is the failure to maintain a medication regimen after initiation. In either case, clinicians may not identify the underlying cause of continued adverse health outcomes and prescribe additional medications unnecessarily, resulting in adverse health outcomes and higher healthcare costs.7–11
There is now a robust literature documenting the prevalence, predictors, and consequences of secondary nonadherence.2, 12, 13 However, data on PNA remains sparse, as the study of PNA requires linking data on prescriptions written with information on dispensing. Studies of PNA have found rates of non-filling ranging from 10 to 30%, but these studies have used widely varying follow-up periods, ranging from 30 days up to 18 months.6, 14–19 Identification of the patterns and predictors of the timing of initial medication dispensing may improve both the ability to target PNA interventions and the ability to detect meaningful effects of intervention efforts.
We utilize a unique, linked database of electronic health records (EHR) and complete Medicare claims to study the timing of medication filling among the elderly. We focus on patients newly prescribed maintenance therapy for five important chronic diseases to assess patterns of initial filling and develop parsimonious models for simple predictions of timely filling that could be used to target interventions to patients at greatest risk of nonadherence.
METHODS
Data Source
The data for this analysis come from a linked database of electronic health records (EHR) and medical and pharmacy claims for approximately 500,000 patients aged 65+ with Medicare fee-for-service and Part D plan enrollment during 2007–2014 and receiving care within Partners Healthcare, a system of seven hospitals in the Boston area and their affiliated primary care and specialty practices. The EHR data contain flags for each prescription order indicating whether it represents a new prescription, discontinuation, reconciliation, or entry into the EHR for record-keeping purposes. Linked Part D pharmacy claims provide data on subsequent medication filling.
This study, including linkage between the EHR and Medicare claims data, is governed under an IRB review by Partners Healthcare.
Study Design
In this retrospective cohort study, we identified patients receiving a new prescription for (1) an oral anti-diabetic, (2) antihypertensive, (3) statin, (4) asthma/chronic obstructive pulmonary disease (COPD) maintenance medication, or (5) anti-osteoporosis medication, where a new prescription was considered to be any prescription for which a patient had no fills or prescriptions from the same medication class in the preceding 180 days. For example, a patient receiving metformin prior to a new prescription for a sulfonylurea was considered new to the sulfonylurea medication class (see Appendix 1 for a list of specific drugs included in each therapeutic area and medication class).
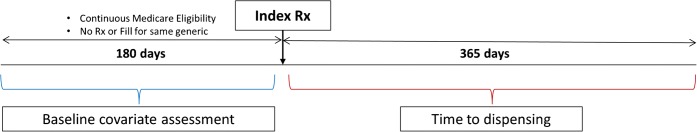
The index date for each prescription episode was defined as the first new prescription date in the medication class where the patient had continuous Medicare fee-for-service and Part D enrollment for at least 180 days prior (Fig. 1). Patients were also required to have had at least one outpatient visit within the year prior to and including the index date to ensure that patients were likely to have sufficient baseline information within the Partners EHR system. Patients could contribute up to one prescription episode per medication class, as long as each prescription episode met the criteria above.
Figure 1.
Study design.
Assessment of Medication Filling
The primary outcome of interest was the time from prescribing to the first pharmacy dispensing of a molecularly-identical medication. Patients who received a prescription for a branded product, but subsequently filled the generic version, and vice versa, were considered adherent. Patients were followed for the outcome beginning on the index date for up to 1 year. Follow-up was censored at the earliest of a discontinuation order for the same generic as the index medication, a new prescription order for any medication in the same class as the index medication, disenrollment from Medicare Part D, an inpatient hospitalization or rehabilitation stay lasting 1 week or more, entry into a nursing home or hospice care, death, or end of available data (December 31, 2014).
Based on analysis of the primary outcome, we focused prediction efforts on filling during two critical periods during follow-up: (1) within 7 days after the index prescription (“fast filling”) and (2) within 30 days after the index prescription (“timely filling”). We chose these windows because they contained the vast majority of filling within the first year and because filling more than 30 days after the index prescription is likely to be influenced by subsequent contact with the healthcare system and therefore may be less predictable from baseline covariates.
Predictors
Patient and prescription-level characteristics were defined to explore potential predictors of initial filling or time until initial filling. The initial selection of covariates was intended to be as broad as possible, since automated variable selection was used to subsequently create more parsimonious sets of predictors. We identified a total of 88 covariates based on information recorded in Medicare claims, EHR, or 2010 Census data linked to patients through their zip code of residence. Details of covariate definitions are provided in Appendix 2.
Statistical Analysis
We calculated the cumulative proportion of patients who filled the index medication over time using a Kaplan-Meier (KM) approach that accounts for patient dropout and other censoring events. We compared time until filling across therapeutic areas using a Cox proportional hazards model with robust standard errors to account for the fact that patients could contribute multiple prescription episodes to the cohort.
We then fit a sequence of logistic regression models predicting each of the two binary outcomes of fast or timely filling. All patients, regardless of whether they filled during follow-up, were used to predict timely filling. In contrast, only patients who filled within the first 30 days were used to predict fast filling. In order to estimate predictive accuracy for each model, we used 10-fold cross-validation to calculate the C-statistic that would be expected if the model were applied to data not used for model estimation.20 The C-statistic ranges from 0.5 (no information) to 1.0 (perfect prediction).21 In addition, we used bootstrapping to judge the statistical significance of differences in prediction accuracy across models.22 In each model, patients whose follow-up was censored during the follow-up period of interest (7 days or 30 days) were dropped.
For each of the two outcomes, logistic regression analysis based on all 88 covariates was used to estimate associations between predictors and filling. We also applied lasso regression to identify a smaller set of useful predictors for each outcome, and we re-estimated the logistic models using only these predictors.23 Finally, for each outcome, we fit boosted logistic regression models, which estimate the association between predictors and outcome without making assumptions about the functional form of the relationships.24 We fit boosted models first using all 88 predictors in order to identify which variables contributed the most to prediction of the outcomes. This was quantified as the percent reduction in prediction accuracy resulting from leaving the variable out of the model. Based on the variable importance summaries from the initial model fit, we selected variables that contributed at least 1% and a smaller set that contributed at least 5% to model fit. We then refit the boosted models using these predictor sets to identify predictive accuracy.
RESULTS
Cohort Characteristics
We identified 32,586 prescriptions for a new medication that met inclusion criteria, written for 20,838 unique patients (Appendix 3). Table 1 provides the complete list of covariates considered, overall and across three discrete filling time intervals: 0–7 days, 8–30 days, and greater than 30 days or no filling. Patients who filled within 0–7 days are fast fillers, while combining patients filling in 0–7 days or 8–30 days provides the group of timely fillers. The average age of patients at the time of receiving the new prescription was 75 years, and almost 63% of the patients were female. Approximately 75% of patients were living in areas of upper-middle income (> $54,400 median annual income).
Table 1.
Characteristics of Patients Initiating a Chronic Disease Medication During 2007–2014 Stratified by Time to Prescription Fill
| Covariate | Overall (N = 32,586) | Fill in 0–7 days | Fill in 8–30 days | Late filling or no fills |
|---|---|---|---|---|
| (N = 24,305) | (N = 2014) | (N = 6267) | ||
| Demographics | ||||
| Age, mean (SD) | 74.7 (7.4) | 74.9 (7.4) | 74.1 (7.4) | 74.4 (7.4) |
| Female | 62.6% | 63.1% | 63.8% | 60.3% |
| Race | ||||
| Asian | 2.1% | 2.2% | 2.0% | 1.8% |
| Black | 8.1% | 8.3% | 8.7% | 7.1% |
| Hispanic | 4.9% | 5.5% | 4.1% | 3.1% |
| Other | 3.0% | 3.2% | 2.9% | 2.7% |
| Unknown | 0.9% | 0.9% | 0.5% | 0.7% |
| White | 81.0% | 80.0% | 81.9% | 84.7% |
| Veteran status | ||||
| Yes | 11.3% | 11.0% | 11.5% | 12.4% |
| No | 69.4% | 70.0% | 67.0% | 67.7% |
| Unknown/not determined | 19.3% | 19.0% | 21.5% | 19.9% |
| Percent high school education in zip code | 89.2 (8.4) | 89.0 (8.5) | 89.6 (8.1) | 89.7 (8.2) |
| Percent black in zip code | 8.4 (13.9) | 8.6 (14.1) | 8.4 (14.1) | 7.6 (13.2) |
| Median household income in zip code | $77,487 ($31,414) | $77,082 ($31,345) | $79,281 ($32,673) | $78,483 ($31,232) |
| Primary language | ||||
| English | 83.8% | 2.6% | 86.6% | 87.6% |
| Non-English | 15.3% | 16.6% | 12.4% | 11.4% |
| Unavailable | 0.9% | 0.8% | 0.9% | 1.1% |
| Relationship status* | ||||
| In a relationship | 46.3% | 45.0% | 47.4% | 50.9% |
| Not in a relationship | 47.9% | 49.0% | 47.0% | 43.8% |
| Unknown | 5.8% | 5.9% | 5.6% | 5.3% |
| Comorbid conditions | ||||
| Atrial fibrillation | 5.1% | 5.2% | 5.1% | 4.8% |
| Alcohol abuse | 0.9% | 0.8% | 1.2% | 0.9% |
| Allergic reaction | 2.4% | 2.4% | 2.3% | 2.5% |
| Coronary artery disease | 7.7% | 7.9% | 6.1% | 7.6% |
| Congestive heart failure | 5.3% | 5.4% | 4.1% | 5.4% |
| Cancer | 36.8% | 36.3% | 36.5% | 38.8% |
| Dementia | 3.3% | 3.3% | 2.7% | 3.1% |
| Depression | 16.1% | 16.6% | 15.2% | 14.5% |
| Diabetes | 29.2% | 29.6% | 28.3% | 28.1% |
| Asthma | 26.2% | 25.6% | 26.6% | 28.5% |
| Hyperlipidemia | 63.2% | 63.0% | 65.4% | 63.4% |
| Hypertension | 79.3% | 79.8% | 78.5% | 77.5% |
| Osteoporosis | 20.9% | 20.8% | 20.8% | 21.3% |
| Prior myocardial infarction | 0.8% | 0.9% | 0.5% | 0.7% |
| Revascularization | 2.5% | 2.6% | 1.6% | 2.4% |
| Stroke | 1.0% | 1.0% | 0.9% | 1.0% |
| Acute renal failure or end stage renal disease | 1.6% | 1.7% | 1.1% | 1.6% |
| Combined comorbidity score | 2.7 | 2.7 | 2.6 | 2.7 |
| Laboratory values and vitals | ||||
| Body mass index | ||||
| < 18.5 | 0.9% | 0.9% | 0.8% | 1.0% |
| ≥ 18.5 and < 24.9 | 15.9% | 15.8% | 16.1% | 16.2% |
| ≥ 25 and < 29.9 | 23.5% | 23.4% | 24.1% | 23.6% |
| ≥ 30 | 24.0% | 24.0% | 24.8% | 23.6% |
| No measurements in data | 35.7% | 35.9% | 34.1% | 35.6% |
| Average HbA1c level, mean (SD)† | 6.8 (1.4) | 6.8 (1.4) | 6.9 (1.6) | 6.7 (1.3) |
| Average LDL level, mean (SD)‡ | 100.4 (38.5) | 100.9 (38.9) | 102.4 (38.2) | 97.7 (36.6) |
| Average systolic blood pressure, mean (SD)§ | 134.1 (21.2) | 134.7 (22.0) | 131.7 (18.4) | 132.1 (18.0) |
| Resource utilization | ||||
| Emergency department visits, mean (SD) | 0.7 (2.6) | 0.7 (2.5) | 0.7 (2.6) | 0.8 (2.8) |
| Hospitalizations, mean (SD) | 0.3 (0.8) | 0.3 (0.8) | 0.3 (0.8) | 0.3 (0.8) |
| Unique physicians, mean (SD) | 3.6 (2.5) | 3.6 (2.5) | 3.5 (2.3) | 3.6 (2.5) |
| Physician office visits, mean (SD) | 5.9 (4.7) | 6.0 (4.7) | 5.4 (4.2) | 5.8 (4.7) |
| Outpatient encounter 7 days before or after index order | 63.2% | 62.3% | 67.8% | 65.3% |
| Benefit characteristics | ||||
| At least 1 month receiving Medicare Part D low-income subsidy | 34.1% | 36.9% | 28.9% | 24.9% |
| Switch in Part D plan | 7.9% | 7.9% | 9.6% | 7.3% |
| Copayment amount corresponding to outpatient encounter 7 days before or after index order (missing if no visit), median (IQR) | $0 ($0) | $0 ($0) | $0 ($0) | $0 ($0) |
| Index order characteristics | ||||
| Quantity ≤ 30 | 56.2% | 58.1% | 47.0% | 52.1% |
| Medication frequency | ||||
| < Once daily | 4.3% | 4.1% | 5.2% | 4.7% |
| Once daily | 70.3% | 71.5% | 70.2% | 65.5% |
| > Once daily | 23.8% | 22.7% | 22.8% | 28.5% |
| Other | 1.6% | 1.6% | 1.7% | 1.3% |
| Number of refills, mean (SD) | 4.2 (3.1) | 4.3 (3.2) | 3.8 (2.7) | 4.1 (3.1) |
| Number of refills | ||||
| 0 | 5.9% | 5.9% | 5.1% | 6.4% |
| 1 | 8.2% | 8.6% | 6.4% | 7.2% |
| 2 | 4.1% | 4.4% | 2.4% | 3.5% |
| 3 | 44.5% | 42.4% | 57.9% | 48.4% |
| 4 | 4.9% | 4.9% | 4.9% | 4.9% |
| 5 | 11.9% | 12.4% | 9.1% | 10.7% |
| 6 | 6.6% | 6.9% | 4.9% | 5.8% |
| 7 | 0.0% | 0.0% | 0.0% | 0.0% |
| 8 | 0.1% | 0.1% | 0.1% | 0.1% |
| 9 | 0.1% | 0.1% | 0.2% | 0.1% |
| 10 | 0.1% | 0.1% | 0.0% | 0.1% |
| 11 | 11.4% | 12.0% | 7.7% | 10.5% |
| 12 | 2.1% | 2.1% | 1.5% | 2.4% |
| > 12 | 0.1% | 0.1% | 0.1% | 0.1% |
| Discontinuation order for same generic as index during baseline | 0.0% | 0.0% | 0.0% | 0.0% |
| Reconciliation order for same generic as index during baseline | 3.0% | 2.7% | 2.6% | 3.8% |
| Dispense as written on index order | 0.9% | 0.5% | 1.4% | 2.0% |
| Prescriber is a primary care physician | 55.5% | 56.3% | 55.4% | 52.8% |
| Prescriber is a specialist physician | 42.2% | 42.5% | 42.0% | 41.2% |
| Adherence predictors | ||||
| Current medications, mean (SD) | 4.9 (3.2) | 5.4 (3.1) | 3.6 (2.9) | 3.3 (3.0) |
| Unique medications during baseline, mean (SD) | 8.1 (4.8) | 8.6 (4.7) | 6.7 (4.4) | 6.3 (4.8) |
| Fills in baseline period, mean (SD) | 18.3 (14.8) | 19.6 (15.0) | 14.3 (12.4) | 14.3 (13.9) |
| Out-of-pocket amount, median (IQR) | $122 ($304) | $118 ($302) | $118 ($290) | $142 ($313) |
| Average out-of-pocket amount per fill, median (IQR) | $11 ($21) | $10 ($20) | $12 ($24) | $14 ($24) |
| New to therapeutic area | 96.7% | 97.0% | 97.0% | 95.7% |
| Day of the week of index order date | ||||
| Sunday | 0.5% | 0.5% | 0.4% | 0.6% |
| Monday | 22.4% | 22.6% | 22.3% | 21.8% |
| Tuesday | 21.5% | 21.2% | 21.9% | 22.4% |
| Wednesday | 19.0% | 18.8% | 20.3% | 19.7% |
| Thursday | 18.9% | 19.1% | 17.8% | 18.1% |
| Friday | 16.8% | 16.9% | 16.7% | 16.4% |
| Saturday | 0.9% | 0.9% | 0.5% | 0.9% |
| Mammogram or PSA test | 27.0% | 26.7% | 29.5% | 27.7% |
*Relationship status is defined as being “in a relationship” if marital status is defined in EHR as either “married” or “partner,” and not as “unknown” or “declined.” Otherwise, relationship status is defined as “not in a relationship”
†Serum glycated hemoglobin (HbA1c) measurements identify average blood plasma glucose concentrations over a period of 2–3 months; in our data, HbA1c test results do not include patients with missing values
‡Low-density lipoprotein (LDL) measurements provide an estimate of the amount of cholesterol in blood which drives progression of atherosclerosis. In our data, LDL test results do not include patients with missing values
§In our data, systolic blood pressure results do not include patients with missing values
Time Until Filling
Across therapeutic areas, the majority (75%; 95% confidence interval [CI] 74–75%) of new prescriptions were filled within 7 days of receiving the prescription, 81% (81–82%) were filled within 30 days, and 91% (91–92%) were filled within 1 year, based on KM estimates. Although all other therapeutic areas had rates of filling that were significantly higher than the rate among anti-osteoporosis prescriptions, the magnitude of the differences was not clinically meaningful (Fig. 2).
Figure 2.
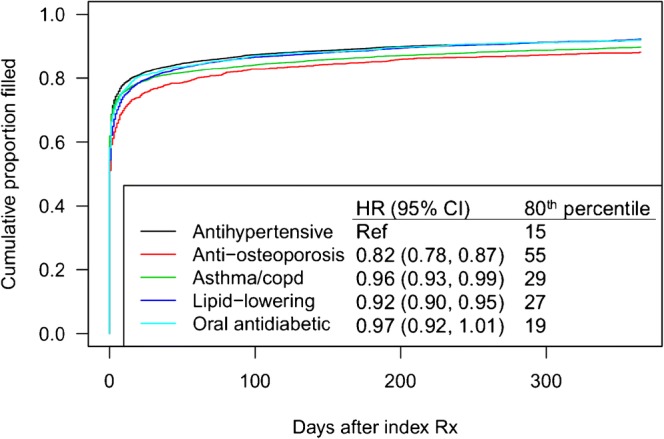
Kaplan-Meier plots showing time-to-fill for newly prescribed medications, by therapeutic area. Relative hazard ratios and the 80th percentile for time until filling in days are also provided.
Predictors Selected by Lasso
Based on logistic models that included predictors selected by lasso regression, we identified factors that were associated with filling (Table 2). Variables associated with a lower likelihood of both fast and timely filling included asthma, diabetes, hyperlipidemia, and higher average out-of-pocket cost per medication during the baseline period, with an additional $10 in copay per medication resulting in a 2–3% decrease in the odds of both outcomes. In addition, each additional filled medication with days supply overlapping the index date was associated with a 30% (27–33%) increase in the odds of fast filling and a 27% (25–29%) increase in the odds of timely filling.
Table 2.
Odds Ratio Estimates and 95% Confidence Intervals from Logistic Regression Models Using Lasso-Selected Variables Only for Fast and Timely Filling
| Variable | Fast filling (≤ 7 days); OR (95% CI) | Timely filling (≤ 30 days); OR (95% CI) |
|---|---|---|
| Therapeutic area (antihypertensive) | 1.20 (1.08, 1.33) | NA |
| Therapeutic area (asthma/COPD) | NA | 0.72 (0.65, 0.79) |
| Asthma | 0.69 (0.62, 0.77) | 0.81 (0.75, 0.88) |
| Diabetes | 0.71 (0.64, 0.79) | 0.66 (0.61, 0.71) |
| Hypertension | NA | 0.83 (0.77, 0.90) |
| Congestive heart failure | NA | 0.80 (0.69, 0.94) |
| Hyperlipidemia | 0.85 (0.77, 0.94) | 0.93 (0.87, 1.00) |
| Number of refills | 1.03 (1.01, 1.05) | NA |
| Number of providers | NA | 0.93 (0.92, 0.95) |
| Number of current medications | 1.30 (1.27, 1.33) | 1.27 (1.25, 1.29) |
| Total number of medications | NA | 1.05 (1.04, 1.07) |
| Average out-of-pocket amount (per $10) | 0.97 (0.96, 0.99) | 0.98 (0.97, 0.99) |
| Age (per 5 years) | NA | 1.04 (1.02, 1.06) |
| Low-income subsidy in at least 1 month | NA | 1.28 (1.17, 1.39) |
| Median household income in zip code (per $10,000) | NA | 1.02 (1.01, 1.03) |
| Quantity (per 10 pills) | 0.97 (0.96, 0.98) | NA |
| New to therapeutic area | NA | 0.80 (0.68, 0.94) |
| Race: White | NA | 0.89 (0.81, 0.98) |
| Primary language: unavailable | NA | 0.69 (0.51, 0.94) |
| Body mass index: > 30 | NA | 0.82 (0.76, 0.88) |
| Prescriber is a primary care physician | NA | 1.08 (1.01, 1.15) |
| Dispense as written on index prescription | NA | 0.34 (0.26, 0.43) |
| Medication frequency: daily | NA | 1.77 (1.56, 2.01) |
| Medication frequency: > daily | NA | 1.09 (0.95, 1.24) |
| Interaction terms | ||
| Female × relationship status: not in a relationship vs. others | NA | 1.05 (0.99, 1.13) |
| Abnormal HbA1c* × therapeutic area: diabetes | NA | 1.65 (1.22, 2.27) |
| Abnormal LDL† × therapeutic area: hyperlipidemia | NA | 1.44 (1.22, 1.71) |
| Abnormal SBP‡ × therapeutic area: hypertension | 1.53 (1.25, 1.88) | 1.54 (1.36, 1.75) |
| Number of refills × quantity (per 100 pills) | NA | 0.97 (0.95, 0.98) |
| Quantity (per 10 pills) × med frequency: > daily | NA | 0.96 (0.95, 0.97) |
NA indicates that the variable was not selected by lasso for that outcome and was therefore not included in the relevant model
*Defined as HbA1c ≥ 8.0
†Defined as LDL ≥ 130
‡Defined as SBP ≥ 150
Among the predictors selected for prediction of timely filling but not selected for prediction of fast filling, having “dispense as written” on the index prescription was associated with a 66% (95% CI 57–74%) reduction in the odds of timely filling. In addition, having fewer providers, a higher number of medications filled during baseline, older age, low-income subsidy for Medicare Part D, living in a higher income zip code, and higher medication frequency were associated with higher rates of timely filling. Predictors associated with fast filling but not selected for prediction of timely filling were an indicator of the antihypertensive therapeutic area, a higher number of refills available, and a lower quantity of pills on the index medication.
Predictors Selected by Boosted Regression
The predictors selected by the boosted models and their variable importance are provided in Appendix 4. The two most important predictors for each outcome based on these models were the number of medications on hand at index and the average out-of-pocket cost for medications filled during baseline. The most parsimonious model for fast filling also included the number of refills, the quantity of pills on the index prescription, and patient age; for timely filling, the total number of medications filled during baseline was also included.
Summary of Relative Model Performance
When predicting filling within 30 days after the index prescription, the full logistic model with 88 predictors resulted in a cross-validated C-statistic of 0.72, indicating moderately good prediction accuracy (Table 3). Reducing the set of predictors to the 27 selected by the lasso model did not impact the predictive performance. When using a nonparametric boosted model, the most parsimonious model that included only three predictors reduced predictive accuracy, but using a slightly richer model that was restricted to the 18 variables contributing at least 1% to the predictive performance improved the C-statistic to 0.74. When predicting filling within 7 days, the full logistic regression model had a C-statistic of 0.70, which was essentially unchanged in the more parsimonious models.
Table 3.
Cross-Validated Accuracy and Number of Predictors Across All Models Considered. P Values Are for the Comparison of Each Parsimonious Model with the Full Model
| Model | Fill within 7 days (N = 26,037) | Fill within 30 days (N = 32,001) | ||||
|---|---|---|---|---|---|---|
| C-statistic | P value | No. predictors | C-statistic | P value | No. predictors | |
| Full model | 0.70 | 88 | 0.72 | 88 | ||
| Reduced model (lasso-selected predictors) | 0.70 | 0.84 | 9 | 0.72 | 0.94 | 27 |
| Boosted model—1% | 0.71 | 0.24 | 16 | 0.74 | < 0.001 | 18 |
| Boosted model—5% | 0.70 | 0.14 | 5 | 0.70 | < 0.001 | 3 |
N provides the number of patients used in model estimation for each outcome
Simple Stratification
Because the number of current medications and the average out-of-pocket cost for medications during baseline were selected by all models for both outcomes, we stratified patients based on these two variables and evaluated the probability of fast filling and timely filling within strata, provided in Table 4. Among patients with more than five medications on hand at index and an average copay on prior medications of $5 or less, 89% and 93% filled within 7 and 30 days, respectively. Among patients with no current medications and an average copay or more than $5 on prior medications, < 30% or < 50% filled within 7 or 30 days.
Table 4.
The Observed Probabilities of Fast and Timely Filling, Stratified by the Two Strongest Predictors of Filling: Number of Medications at Index and Average Out-of-pocket Medication Costs During Baseline
| No. current medications at index date | Average out-of-pocket cost for prior medications | No. (%) patients in stratum | Probability of fast filling | Probability of timely filling |
|---|---|---|---|---|
| 0 | ≤ $5 | 414 (1.3%) | 0.43 | 0.63 |
| $5–50 | 1626 (5.2%) | 0.28 | 0.39 | |
| > $50 | 115 (0.4%) | 0.25 | 0.48 | |
| 1 | ≤ $5 | 607 (1.9%) | 0.63 | 0.75 |
| $5–50 | 1395 (4.4%) | 0.55 | 0.67 | |
| > $50 | 243 (0.8%) | 0.42 | 0.59 | |
| 2–3 | ≤ $5 | 2081 (6.6%) | 0.77 | 0.85 |
| $5–50 | 4540 (14.4%) | 0.70 | 0.78 | |
| > $50 | 624 (2.0%) | 0.64 | 0.74 | |
| 4–5 | ≤ $5 | 2581 (8.2%) | 0.83 | 0.89 |
| $5–50 | 4895 (15.6%) | 0.78 | 0.85 | |
| > $50 | 530 (1.7%) | 0.70 | 0.77 | |
| > 5 | ≤ $5 | 5014 (15.9%) | 0.89 | 0.93 |
| $5–50 | 6119 (19.4%) | 0.85 | 0.90 | |
| > $50 | 692 (2.2%) | 0.79 | 0.85 |
DISCUSSION
In this large study of new prescriptions of chronic disease medications in elderly Medicare beneficiaries, 75% were filled within 7 days and 81% within 30 days. When allowing up to 1 year for filling, this rate increased to 91%. Timing and rates of filling were similar for all five therapeutic areas and could be predicted with moderately good accuracy based on only a few variables. A higher number of dispensed medications with days supply overlapping the index date and lower average out-of-pocket cost for all medications filled in the prior 180 days were both strongly associated with lower rates of PNA.
The prevalence of PNA found in this study is slightly lower than the prevalence reported in previous studies.6, 14, 19 However, these studies included a more diverse set of prescribed medications in their sample, with many non-critical medications or medications that were prescribed to be taken as needed. In other studies that focused on medications for hypertension, diabetes, or hyperlipidemia, 18-month PNA ranged from 5 to 13%17 and 90-day PNA was 15%,16 which aligns well with the current findings. The five chronic conditions we evaluated are some of the most prevalent conditions in the Medicare population. The conditions comprised both symptomatic conditions (e.g., asthma) and silent conditions (e.g., hypertension); however, the nature of the underlying conditions did not translate into meaningful differences in time-to-filling. Our prediction accuracy, as measured by the C-statistic, was higher than that observed in other studies of PNA, where C-statistics ranged from 0.60 to 0.63. This finding may partly be explained by our inclusion of information on prior fills, such as recency and cost, which were generally highly predictive of the outcome.16, 17
Given the strong discrimination in filling observed, the models developed in this study may be useful for targeting interventions designed to encourage initiation of a new medication, thereby improving the cost-effectiveness of interventions. In this study, 68% of patients had a predicted probability of filling within 7 days that was > 0.75 based on the two strongest predictors. Therefore, removing these patients would leave just 32% of the population in need of intervention. Although automated telephone reminders and letters have been shown to improve PNA to statins,25 personal calls from patients’ physicians did not result in further improve PNA.26 Therefore, improvement in PNA may require tailored solutions that would be infeasible for use across all patients.
The two strongest predictors in this study, the number of current medications and average out-of-pocket medication cost, were both derived from pharmacy claims. Therefore, the prediction models presented in this paper would be most useful to providers with real-time access to pharmacy claims data. In addition, only 26% of patients who did not fill within the first 7 days after the prescription date went on to fill within the first 30 days. Thus, if real-time data on patient filling is available, patients who remain nonadherent after 7 days can also be targeted immediately to improve PNA and subsequent clinical outcomes.
In addition to out-of-pocket cost for prior medications, several other variables related to cost and ability to pay were associated with the timely filling of prescriptions, indicating that efforts to reduce patient cost are likely to result in improved adherence. The small subset of prescriptions (0.9%) with “dispense as written” had a much lower rate of filling than that of prescriptions without this designation. This indication prevents pharmacists from substituting cheaper generic versions of branded products and would be likely to lead to higher out-of-pocket costs for the patient.27 Patients receiving the low-income subsidy through Medicare Part D were more likely to fill, as these patients receive help with coinsurance and copayments and are not subject to coverage gaps based on prior spending. Patients living in zip codes with higher incomes were also more likely to fill. In contrast to these predictors of timely filling, a higher number of refills and lower quantity on the index prescription were associated with fast filling but not with timely filling, indicating that modifying these factors could encourage patients to fill quickly, but may not affect whether patients choose to fill at all.
In studying medication adherence, pharmacy claims are generally considered to be a highly accurate source of information.28, 29 However, patients may have obtained prescriptions through mechanisms that do not result in submission of a claim to the insurer, for example low-cost generic medications available from certain chain pharmacies. Given the overall high rates of filling, it is unlikely that a large proportion of patients filled without capture in claims. In addition, the distribution of medication samples was prohibited within Partners Healthcare system throughout the period of data collection, so patients were unlikely to have delayed filling due to use of samples. Although we were able to identify prescriptions that were discontinued in the EHR system, we would not be able to identify such instances that were communicated verbally to the patient.
Despite the unique data resource that allowed us to link claims and EHR data, it was difficult to identify truly new prescriptions for this cohort of elderly patients. Review of patient notes indicated that many of these patients should have been on the drug of interest previously, but were nonadherent, so that the “new” prescriptions were in reality resumptions of prior therapy or renewed attempts to prescribe a medication for which PNA by the patient had occurred in the past. Our study population was also restricted to patients aged 65+ continuously enrolled in a Medicare Part D plan and receiving care from a single health system. The utilization of e-prescribing and other features to encourage medication initiation may be different at other providers, which would impact the rates and possibly the predictors of filling.
This study suggests that a substantial proportion of elderly patients receiving a new prescription for a chronic disease medication do not fill it. However, it is possible to identify patients at the time of the index prescription with low likelihood of timely filling based on a small set of predictors related to recent medication filling and cost derived from claims. Additional work should investigate the effectiveness of interventions for reducing PNA that incorporate these factors.
Contributons
None.
Funders
This work was funded by Merck & Co., Inc., Kenilworth, NJ, USA.
Compliance with Ethical Standards
Prior presentations
Preliminary results from this paper were presented at the 2017 Society of General Internal Medicine Annual Meeting, April 19–22, 2017.
Conflict of Interest
HB and CM were employees of Merck & Co at the time of writing. All other authors declare no conflicts of interest.
References
- 1.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 2.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 3.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 4.Caro JJ, Salas M, Speckman JL, Raggio G, Jackson J. Persistence with treatment for hypertension in actual practice. Can Med Assoc J. 1999;160(1):31–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165(20):2414–2419. doi: 10.1001/archinte.165.20.2414. [DOI] [PubMed] [Google Scholar]
- 6.Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25(4):284–290. doi: 10.1007/s11606-010-1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes a meta-analysis. Med Care. 2002:794–811. [DOI] [PubMed]
- 8.Mahoney JJ. Reducing patient drug acquisition costs can lower diabetes health claims. Am J Manag Care. 2005;11(5 Suppl):S170–6. [PubMed] [Google Scholar]
- 9.Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117(8):1028–1036. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- 10.Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76–87. doi: 10.1111/j.1742-1241.2007.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitton A, Choudhry NK, Matlin OS, Swanton K, Shrank WH. The impact of medication adherence on coronary artery disease costs and outcomes: a systematic review. Am J Med. 2013;126(4):357. e7–357. e27. doi: 10.1016/j.amjmed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Franklin JM, Shrank WH, Lii J, et al. Observing versus Predicting: Initial Patterns of Filling Predict Long-Term Adherence More Accurately Than High-Dimensional Modeling Techniques. Health Serv Res. 2016;51(1):220–239. doi: 10.1111/1475-6773.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan DC, Shrank WH, Cutler D, et al. Patient, physician, and payment predictors of statin adherence. Med Care. 2010;48(3):196–202. doi: 10.1097/MLR.0b013e3181c132ad. [DOI] [PubMed] [Google Scholar]
- 14.Fischer MA, Choudhry NK, Brill G, et al. Trouble getting started: predictors of primary medication nonadherence. Am J Med. 2011;124(11):1081. e9–1081. e22. doi: 10.1016/j.amjmed.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Carroll NM, Bayliss EA, Schroeder EB, Shetterly S, Steiner JF. Characteristics of patients with primary non-adherence to medications for hypertension, diabetes, and lipid disorders. J Gen Intern Med. 2012;27(1):57–64. doi: 10.1007/s11606-011-1829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheetham TC, Niu F, Green K, et al. Primary nonadherence to statin medications in a managed care organization. J Manag Care Pharm. 2013;19(5):367–373. doi: 10.18553/jmcp.2013.19.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raebel MA, Ellis JL, Carroll NM, et al. Characteristics of patients with primary non-adherence to medications for hypertension, diabetes, and lipid disorders. J Gen Intern Med. 2012;27(1):57–64. doi: 10.1007/s11606-011-1829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds K, Muntner P, Cheetham TC, et al. Primary non-adherence to bisphosphonates in an integrated healthcare setting. Osteoporos Int. 2013;24(9):2509–2517. doi: 10.1007/s00198-013-2326-5. [DOI] [PubMed] [Google Scholar]
- 19.Tamblyn R, Eguale T, Huang A, Winslade N, Doran P. The Incidence and Determinants of Primary Nonadherence With Prescribed Medication in Primary Care. Ann Intern Med. 2014;160(7):441–450. doi: 10.7326/M13-1705. [DOI] [PubMed] [Google Scholar]
- 20.Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Stat. 1983;37(1):36–48. [Google Scholar]
- 21.Harrell FE, Lee KL, Mark DB. Tutorial in biostatistics multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 23.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B Methodol. 1996:267–288.
- 24.Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Stat. 2001:1189–1232.
- 25.Derose SF, Green K, Marrett E, et al. Automated outreach to increase primary adherence to cholesterol-lowering medications. Jama Intern Med. 2013;173(1):38–43. doi: 10.1001/2013.jamainternmed.717. [DOI] [PubMed] [Google Scholar]
- 26.Fischer MA, Jones JB, Wright E, et al. A randomized telephone intervention trial to reduce primary medication nonadherence. J Manag Care Spec Pharm. 2015;21(2):124–131. doi: 10.18553/jmcp.2015.21.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrank WH, Liberman JN, Fischer MA, et al. The consequences of requesting “dispense as written”. Am J Med. 2011;124(4):309–317. doi: 10.1016/j.amjmed.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 29.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/S0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]