Abstract
Background:
A growing body of evidence supports a role for immune alterations in Schizophrenia Spectrum Disorders (SSD). A high prevalence (25–40%) of SSD has been found in patients with 22q11.2 deletion syndrome (22q11.2DS), which is known for T-cell deficits due to thymus hypoplasia. This study is the first to explore the association between the T-cell subsets and psychotic symptoms in adults with 22q11.2DS.
Methods:
34 individuals (aged 19–38 yrs.) with 22q11.2DS and 34 healthy age- and gender matched control individuals were included. FACS analysis of the blood samples was performed to define T-cell subsets. Ultra-high risk for psychosis or diagnosis of SSD was determined based on CAARMS interviews and DSM-5 criteria for SSD. Positive psychotic symptom severity was measured based on the PANSS positive symptoms subscale.
Results:
A partial T-cell immune deficiency in 22q11.2DS patients was confirmed by significantly reduced percentages of circulating T and T-helper cells. Significantly higher percentages of inflammatory Th1, Th17, and memory T-helper cells were found in adults with 22q11.2DS. Most importantly an increased Th17 percentage was found in adults with psychotic symptoms as compared to non-psychotic adults with 22q11.2DS, and Th17 percentage were related to the presence of positive psychotic symptoms.
Conclusions:
Given the literature on the role of T cells and in particular of Th17 cells and IL-17 in hippocampus development, cognition and behavior, these results support the hypothesis for a role of Th17 cells in the development and/or regulation of psychotic symptoms in 22q11.2DS. This pilot study underlines the importance to further study the role of T-cell defects and of Th17 cells in the development of psychiatric symptoms. It also supports the possibility to use 22q11.2DS as a model to study T-cell involvement in the development of SSD.
Keywords: 22q11.2 deletion syndrome, Schizophrenia spectrum disorder, Th17 cells, T-cell immune deficiency, Psychosis, Neuro-inflammation
1. Introduction
During the last decade, the field of immuno-psychiatry focused on the role of chronic processes of neuro-inflammation in the development of schizophrenia spectrum disorders (SSD). There are several lines of evidence for the involvement of chronic mild inflammation in the pathogenesis of SSD. Epidemiological studies have shown that maternal infections during pregnancy are associated with an increased risk for schizophrenia in the child (Brown and Derkits, 2010). Meta-analyses have confirmed that serum levels of pro-inflammatory cytokines such as IL-6 and IL1β are increased in SSD (Miller et al., 2011). Psychotic symptoms are a known side effect of immune-modulating treatments and inversely an improvement of psychotic symptoms has been observed after treatment with anti-inflammatory drugs (Keller et al., 2013; Janssen et al., 1992). However, unraveling the mechanisms of pathogenesis behind the supposed chronic inflammatory processes has proven to be a real challenge. Results of various immune studies have often been conflicting. Explanations for these conflicting results are multiple including the heterogeneity of SSD, the complexity of the immune system and the influence of immune-modulating confounding factors, such as the use of anti-psychotics.
Not only chronic inflammation plays a role in SSD. Reports of a reduced T-cell mediated immune response in acute schizophrenia (Steiner et al., 2010; Achiron et al., 1994) and a failure of women with postpartum psychosis to show the normal postpartum surge in T cells (Bergink et al., 2013) suggest an involvement of T-cell deficits. Various review papers have pointed to the role of inflammation and the role of impaired T-cell function in SSDs and psychosis. Overall, monocyte/macrophage/microglial inflammatory activation, raised levels of inflammation regulating cytokines and a decrease in the percentage of T cells are observed (Miller et al., 2013; Leza et al., 2015). This observation is even more pronounced in drug-naïve first episode psychosis, and can be partially reversed after anti-psychotic treatment (10; 11). As important producers of cytokines, T cells and more specifically T-helper cells can influence the aforementioned inflammatory response and play a role in immune-brain communication. Interestingly, a recent study reveals that not only pro- but also anti-inflammatory subsets of T-helper cells may have a role in the pathogenesis of schizophrenia (Drexhage et al., 2011). More specifically, an inflammatory imbalance with changes in cytokine levels and T-cell subsets including Th17 cells, T-regulatory cells and the Th1/Th2 response has been observed in patients with SSD (Fineberg and Ellman, 2013; Ding et al., 2014). The concept is now arising that defects in the T-regulatory system allow for an excessive pro-inflammatory state in patients by insufficiently damping monocyte/macrophage responses to normal environmental cues.
To further unravel the immunological underpinnings of SSD, studying neuro-inflammation in patients with 22q11.2DS might provide us with some insight. Previously diagnosed as distinct clinical syndromes such as DiGeorge syndrome (DGS) and Velocardio-facial syndrome (VCFS), it is now clear that most patients (with either syndrome) share the 22q11.2 deletion. With 1 in 4000 live births, 22q11.2DS is the most frequent microdeletion (Oskarsdottir and Vujic, 2004). The clinical picture is quite variable but frequent somatic symptoms include congenital heart disease, velopharyngeal insufficiency, hypocalcemia and recurrent infections (Ryan et al., 1997). Up to 80% are estimated to have thymus hypoplasia, predisposing to recurrent infections and to autoimmune dysfunction due to the occurring T-cell defect (Lima et al., 2010). The syndrome is not only characterized by a T-cell deficiency, but also by an increased risk for neuropsychiatric disorders and a high prevalence of neurodevelopmental disorders (Murphy et al., 1999; Gennery, 2012). Frequent pathology includes increased levels of anxiety, Autism Spectrum Disorders (ASD) and Attention Deficit Hyperactivity Disorder (ADHD) (Schneider et al., 2014). With a 20–40 fold higher risk of developing schizophrenia-like symptoms compared to the normal population, the 22q11.2 DS is one of the greatest known risk factors for SSD (18; 20). Moreover, studies estimate that more than half of the adolescent patients with 22q11.2 DS may experience prodromal psychotic symptoms (Shapiro et al., 2011).
In general, the 22q11.2 deletion is thought to be the cause of both the immune dysfunction and the brain neuronal dysfunction and behavioral abnormalities. The candidate genes that have been linked to the immune dysfunction (TBX-1, DGCR8 and CRKL) and the candidate genes for psychosis (COMT, PRODH) are all located in the 22q11.2 region (Jerome and Papaioannou, 2001; Giacomelli et al., 2016; Jeker et al., 2013). However, a single causal gene in the 22q11.2 region which could explain the high prevalence of psychosis in patients has not been identified. This supports the hypothesis that the neuropsychiatric phenotypes are a result of multiple gene dosage effects caused by the deletion, and in addition by the presence of additional genetic and environmental modifiers (gene-environment interaction) and possible epigenetic mechanisms (such as imprinting, DNA-methylation etc.). Interestingly, the immune deficiency arising from the aforementioned T-cell lymphopenia may constitute just such a set of pathogenesis factors, combining biological predisposition and facilitating environmental stress by recurrent immune challenges.
In light of the inborn T-cell defects in patients with 22q11.2DS, and their unusually high risk for the development of psychosis, this syndrome offers a unique opportunity to further explore the nature of the immune alterations that may directly contribute to the onset of psychosis. In this study we investigated the phenotypic profile of circulating T cells (percentages of T cells, T-helper cells, cytotoxic T-cells, T-regulatory cells as well as the Th1, Th2, Th17 and the memory T-helper subsets) of adults with 22q11.2DS compared to healthy age- and gender-matched controls. In addition, we explored -within the 22q11.2 DS group- the relationship between the circulating levels of the different T-cell subsets and the presence of psychotic symptoms.
2. Materials and methods
2.1. Sample collection
34 adults with 22q11.2DS (19–38 years old) and 34 healthy age- and gender matched controls (19–35 years old) were included. The patients with 22q11.2DS were recruited at the Center for Human Genetics of the University Hospital Leuven in Belgium. A microdeletion of the 22q11.2 region was confirmed in all 34 patients by FISH or microarray. Healthy controls were recruited as part of the Virtual Reality study by Veling et al. (2016) (25). Exclusion criteria were a recent infection (<1 week) and the use of immunosuppressive medication and clozapine. Healthy controls were excluded in case of any psychotic history. A MINI plus interview was performed in all adults with 22q11.2DS to screen for psychiatric disorders past or present. Adults with 22q11.2DS who had psychotic symptoms were diagnosed based on DSM-5 criteria (SCID) for SSD. Secondly a comprehensive assessment of at risk mental state (CAARMS) was performed to assess prodromal psychotic symptoms. Based on this standardized interview adults with 22q11.2DS were divided into a group of ultra-high risk, meaning having developed psychosis like symptoms with a significant impact on functioning, or a group of low risk individuals, meaning not having developed any psychosis like symptoms. Additional information for every patient included age, current body mass index (BMI), medication and medical history. An overview of demographic features can be found in Table 1. Adults with 22q11.2DS were consecutively divided into 2 subgroups based on the at risk score on the CAARMS and history of SSD. Because of the limited sample size adults with prodromal symptoms (at risk score on the CAARMS) (n = 4) and adults with a diagnosis of a SSDs (n = 10) were designated to the group “with psychotic symptoms” (22q11 with P) (n = 14). Adults with a low score on the CAARMS (meaning not at risk) and without a history of psychosis were labeled as “without psychotic symptoms” (22q11 without P) (n =20). Symptom severity was measured in almost all adults with 22q11.2DS using the Positive and Negative Symptom Scale (PANSS) (n = 11).
Table 1.
Demographic features of 22q11.2 DS adults and healthy controls.
| Gender | Age | BMI | |||||
|---|---|---|---|---|---|---|---|
| F | M | X2 (p-value) | Mean (±SD) | M-W U (2-sided sig.) | Mean (±SD) | M-W U (2-sided sig.) | |
| Controls | 16 | 18 | 2.15 (0.14) | 24,71 (±4,3) | 379.0 (0.03) | 23,31 (±2,6) | 332.0 (0.003) |
| 22q11.2DS | 22 | 12 | 27,18 (±5,1) | 27,18 (±5,9) | |||
| Non-psychotic | 13 | 7 | 0.002 (0.97) | 26,95 (±4,8) | 139.5 (0.99) | 27,30 (±6,6) | 136.5 (0.90) |
| Psychotic | 9 | 5 | 27,50 (±5,6) | 26,99 (±4,8) | |||
After the psychiatric interview a peripheral blood sample was collected. PBMC isolation with Ficoll-Paque Plus (Amersham Biosciences, Piscataway, New Jersey) was performed at the University Hospital Leuven within 3 h after the blood was drawn. After isolation, PBMCs were frozen in 10% dimethylsulfoxide (DMSO) and stored in liquid nitrogen (Pae et al., 2006). After a collection period of 2 months all 36 samples were transported in dry ice to the Erasmus MC in Rotterdam, the Netherlands for analysis. Controls were collected during the same time period and analyzed in the same assay.
2.2. Flow cytometric analysis
Immune cell composition and activation status was assessed using a Fluorescence-activated Cell Sorting (FACS) at the Erasmus MC in Rotterdam, the Netherlands. For this purpose PBMCs were defrosted and washed once with medium. Average recovery of cells after thawing was 83% and viability 97%, as determined by Trypan blue staining. Differences between different groups were not observed.
Staining A: To determine percentages of T helper lymphocytes, T cytotoxic lymphocytes, natural killer cells and monocytes, 50.000 PBMCs were stained for 15 min at room temperature in tube A containing staining buffer (PBS 0.2%, 0.1% sodium-azide, pH 7.8) with CD45-Pacific Orange (Invitrogen, Carlsbad, CA, USA) 1:80, CD3-PercP-Cy5.5 (BD Biosciences, San Jose, CA, USA) 1:16, CD4-Pacific Blue (BD Biosciences) 1:200, CD8-PC7 (Beckman Coulter, Brea, CA, USA) 1:80, CD14-APC-H7 (BD Biosciences) 1:16, CD56-PE (Cytognos, Salamanca, Spain) 1:40 and CD15-FITC (BD Biosciences) 1:400.
Staining B: To determine T cell subsets (Th1, Th2, Th17, T regulatory cells), 1 × 106 PBMCs were cultured for 4 h at 37 °C stimulated in RPMI-1640 containing 25 mM Hepes and UltraGlutamine (Lonza, Verviers, Belgium), 10% fetal calf serum (Lonza) and 1% Penicillin/Streptomycin). Extra additions for cell stimulation were 50 ng/ml phorbol 12-myristrate 13-acetate (PMA; Sigma Aldrich, St. Louis, MO, USA) and 1.0 μg/ml ionomycin (Sigma) in the presence of Golgistop (BD Biosciences). Cells were harvested and stained for membrane markers with CD45-ROFITC (Dako, Glostrup, Denmark) 1:10, CD3-APC-H7 (BD Biosciences) 1:40, and CD25-APC (BD Biosciences) 1:20 antibodies for 20 min at room temperature. After washing with PBS, cells were fixed and permeabilized according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA) for 45 min at 4 °C. Cells were washed twice and stained with CD4-PercP-Cy5.5 (BD Bioscience) 1:30 and intracellular IFN-gamma-Horizon V500 (BD Biosciences) 1:60, IL-4-PE-Cy7 (eBioscience) 1:240, IL-17A-BV421 (BioLegend, San Diego, CA, USA) 1:40 and Fox-P3-PE (BD bioscience) 1:10 in permeabilization buffer (eBio-science) for 45 min at 4 °C.
All specific staining antibodies used are routinely tested for effectiveness by the manufacturer and titrated for optimal concentrations in our laboratory. Specificity of the staining antibodies was controlled using five isotype controls provided by the manufacturer (BD) and background positivity was negligible (between 0.2% and 1% of the specific staining depending on the isotype control, both in patient samples and controls).
Stained cell samples were analyzed using eight-color flow cytometry (LSR II, BD Biosciences, San Jose, California) and further analyzed with FlowJo (Tree Star, Ashland, Oregon) research software. Counted were 20.000 events for staining A and minimal 200.000 events for staining B.
The gating strategies are given in Supplementary Figs. S1 and S2.
When referring in the results to NK-cell% we refer to the frequency of CD3-CD56+ cells as a percentage of live PBMC counts (see S1), when to NKT-cell% to CD3+CD56+ cells, when referring to T-cell% to CD3+CD56- cells, when to T-helper cell% to CD3 +CD4+CD8- cells and when to cytotoxic T-cell% to CD3+CD4-CD8 + cells, both not containing any CD56+ cells. All populations were expressed as percentage of live PBMC (see S1).
The T-helper subsets are all expressed as percentages of total CD3+CD4+ T-cell count of staining B, when referring to Th1 cells to CD3+CD4+IFN-gamma+ cells, to Th2 to CD3+CD4+IL-4+ cells, to Th17 cells to CD3+CD4+IL17+ cells, to T regulatory cells to CD3+CD25highFoxP3+ cells and to memory T-helper cells to CD3 +CD4+CD45RO+ cells.
2.3. Statistical analysis
Statistical analysis was performed using SPSS version 24. A Multivariate Analysis of COVAriates (MANCOVA) was performed to compare percentages of live PBMC’s for T-cell subpopulations and NK-cells between adults with 22q11.2DS and healthy controls from staining A. This included 7 T-cell subsets as dependent variables and the independent variables of group (control versus 22q11.2DS), BMI and age (Supplementary Table S1). A second MANCOVA was performed for the T-helper subsets as percentage of CD3+CD4+ cells (Supplementary Table S2). The same covariates of BMI and age were included. No significant effect of BMI or age was found in either MANCOVA model and results did not differ from a MANOVA model. Several variables were transformed using a logarithmic transformation to achieve a normal distribution. This included the Th1, Th17, Th2, T regulatory and memory T-helper subsets. A Shapiro-Wilk test was performed to check the normality of the residuals. The presence of outliers was excluded based on the outlier labeling rule for every dependent variable. Homogeneity of covariance was deemed acceptable based on Box’s Test of Equality of Covariance Matrices with p = 0.179 for the first model and p = 0.908 for the second model. Th17:Treg ratio and Th1:Th2 ratio was compared between healthy controls and 22q11.2DS using an non-parametric Mann-Whitney-U test because of non-normal distribution in the healthy control sample.
To look at the difference between 22q11.2DS with and without psychotic symptoms one way ANCOVA’s were performed for 8 of the T-cell subpopulations from staining A and B (all except T-cell%) with the independent variable of psychotic status (psychotic (n = 14) versus non psychotic (n = 20)) and the covariate antipsychotic medication (AP) (Supplementary Table S3). The covariate antipsychotic medication was analyzed as a dichotomous categorical variable with 0 meaning taking no antipsychotic medication and 1 taking any antipsychotic medication at the time of the blood sampling. An overview of the antipsychotics can be found in Table 2. The mean difference in T-cell % was analyzed with a Brown-Forsythe test, as Levene’s test showed heterogeneity of variance. However the use of the Brown-Forsythe test did not permit us to add a covariate. As age, BMI and gender did not significantly differ between psychotic and non-psychotic adults with 22q11.2DS, they were left out of the model. A Shapiro-Wilk test was performed to check normality of the residuals. The presence of outliers was excluded based on the outlier labeling rule. Based on the PANSS positive symptoms scale a second analysis was performed in 30 individuals with 22q11.2DS that were scored at the time of the blood sampling. A general linear model was used including the same 8 T-cell populations from staining A and B as the previous analysis. The PANSS score (log-transformed) and AP were added as respectively a continuous and a categorical independent variable (Supplementary Table S4). A Shapiro-Wilk test was performed to check for multivariate normality of the residuals. As the distribution was not normal, a logarithmic transformation was performed on the cytotoxic T-cell % to normalize the data. The presence of outliers was excluded based on the outlier labeling rule for every dependent variable. Homogeneity of variance for each dependent variable was deemed acceptable based on Levene’s test. The scatterplot of the residuals and White’s test did not show heteroskedasticity. A sub-analysis using a non-parametric Mann-Whitney-U test showed no significant difference in any of the 8 subsets between UHR adults with 22q11.2DS (n = 4) and adults with SSD (n = 10).
Table 2.
Psychotropic medication in 22q11DS patients per risk group.
| With psychosis n = 10 | High risk n = 4 | Low risk n = 20 | Dosage (range) | |
|---|---|---|---|---|
| Antidepressant medication | ||||
| patients treated (n = 8) | 1 | 2 | 5 | |
| total | 10% | 50% | 25% | |
| SSRI | 10% | 50% | 20% | 10–150mg |
| SARI | 10% | 25% | 5% | 50 mg |
| Antipsychotic medication | ||||
| patients treated (n = 10) | 7 | 2 | 0 | |
| 1st generation(n) | 2 | 0 | 0 | |
| total | 20% | 0% | 0% | |
| Thioxanthene | 10% | 0% | 0% | 0,5 mg |
| Pipamperone | 10% | 0% | 0% | 120 mg |
| 2nd generation (n) | 4 | 2 | 0 | |
| total | 40% | 50% | 0% | |
| Quetiapine | 0,00% | 25,00% | 0,00% | 100–180 mg |
| Quetiapine(XR) | 20,00% | 0,00% | 0,00% | 600–1000 mg |
| Paliperidone | 0,00% | 25,00% | 0,00% | 9 mg |
| Olanzapine | 10,00% | 0,00% | 0,00% | 30 mg |
| Aripiprazol | 20,00% | 0,00% | 0,00% | 10 mg |
| Other(n) | 2 | 0 | 0 | |
| total | 20% | 0% | 0% | |
| Clotiapine | 10,00% | 0,00% | 0,00% | 40 mg |
| Risperidone | 20,00% | 0,00% | 0,00% | 3–5 mg |
Psychotropic medication per subclass and risk group for psychotic symptoms. Abbreviations: SSRI = Selective serotonin reuptake inhibitors; SARI = Serotonin antagonist and reuptake inhibitors; n = number.
3. Results
3.1. Adults with 22q11.2DS versus healthy controls
A statistical significant difference between adults with 22q11.2DS and controls was found in the T-cell subpopulations and NK cells from staining A when considered jointly (Wilk’s Ʌ = 0.459, F (8,58) = 9.8, p < 0.0001 partial ƞ2 = 0.541).
As expected in a population with thymus dysfunction caused by thymus hypoplasia, the percentage of T cells was slightly but significantly decreased in the adults with 22q11.2DS (Fig. 1a). This decrease was in particular and statistically significant in the CD3 +CD4+CD8- T-helper population, while the CD3+CD8+CD4-T-cytotoxic population was decreased, but not statistically significant (Fig. 1b and c). Interestingly the double negative (CD3 +CD4-CD8-) T-cell population was also decreased (p < 0.01, corrected for age and BMI), while the double positive population (CD3+CD4+CD8+) was increased (p < 0.01, corrected for age and BMI) (Fig. 1f and g). With regard to the CD3- CD56+CD8-NK and CD3+CD56+ NKT cells, both populations were increased but only with a near significance when corrected for age and BMI (Fig. 1d and e).
Fig. 1.
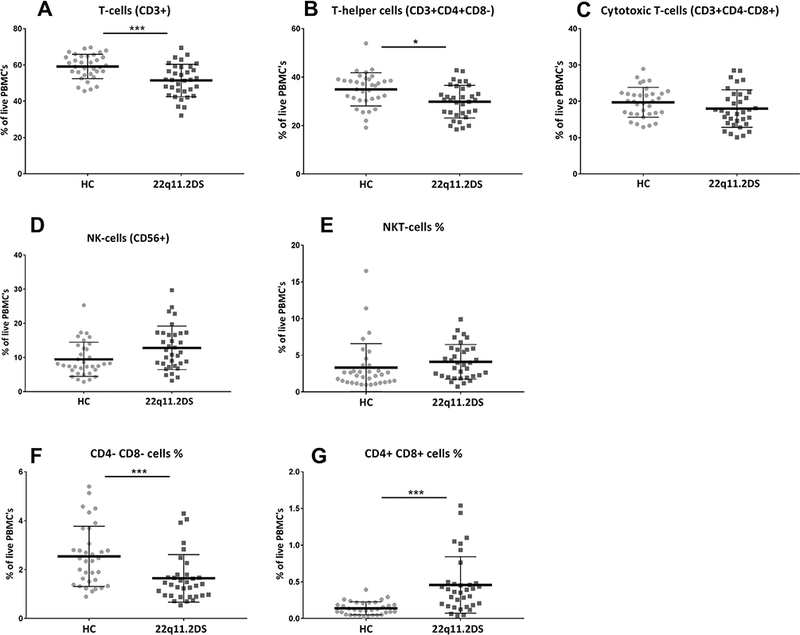
T-cell subset percentages in healthy controls (HC) versus adults with 22q11.2DS. All populations are derived from staining A and are expressed as a % of total live PBMCs. *** < 0.001 (Bonferroni correction applied); * < 0.05.
We also explored whether within the T-helper cell population a different balance was found between the different CD4+ T-cell sub-types that are known to play a role in the pro- and anti-inflammatory immune balance. The changes in differentiation within the T-helper cell compartment was looked at based on staining B, a statistical significant difference between adults with 22q11.2DS and controls was found in the T-cell subpopulations when considered jointly (Wilk’s Ʌ = 0.63, F (5,60) = 7.14, p <0.0001 partial ƞ2 = 0.37). Percentages of the pro-inflammatory Th1 cells and Th17 cells were significantly increased in adults with 22q11.2DS without psychotic symptoms (Fig. 2a & d). The subsets considered as anti-inflammatory (the Th2 and T-regulatory cells) were not significantly different between controls and adults with 22q11.2DS. After Bonferroni correction only the increases of Th17 (F(1,64) = 14.02, partial ƞ2 = 0.18, p = 0.0004) and Th memory cell % (F(1,64) = 22.7, partial ƞ2 = 0.26, p < 0.0001) remained significant. The changes in the various subpopulations resulted in a significant upward change in both the Th1:Th2 (p = 0.03) and the Th17:Treg ratio (p = 0.001) (Fig. 3) with a preponderance of the pro-inflammatory Th1 and Th17cell populations.
Fig. 2.
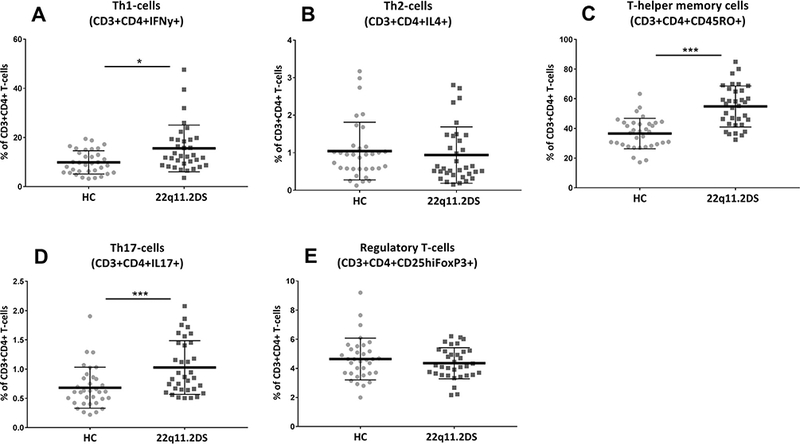
T-helper cell subset percentages in healthy controls versus adults with 22q11.2DS. All populations are derived from staining B and are expressed as a % of total T-helper cell. *** < 0.0001 (Bonferroni corrected); * < 0.05.
Fig. 3.
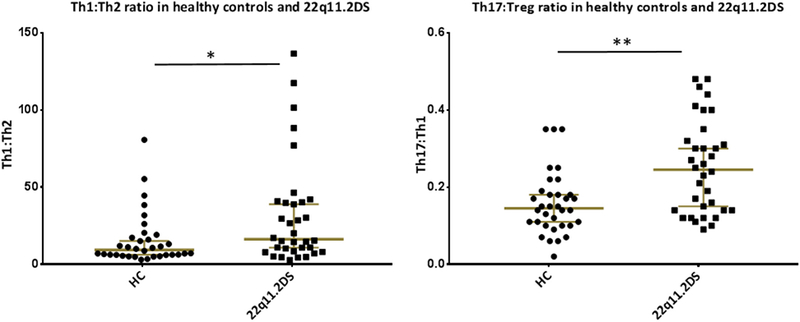
T-cell subset balances in 22q11.2 Adults and in Healthy Controls. ** < 0.01; * < 0.05 Mann-Whitney U test (MVU) with MWU value for Th17:Treg = 316 with median for healthy controls (n = 34) 0.15 and median for 22q11.2DS (n = 34) is 0.25. MWU value for Th1:Th2 ratio = 390 with median for healthy controls 9.32 and median for 22q11.2DS is 16.22.
3.2. The T-cell profile in adults with 22q11.2DS: with versus without psychotic symptoms
We stratified the adults with 22q11.2DS based on the presence of psychotic symptoms. A significant mean difference was present in Th17% (logarithmic transformed) between adults with 22q11.2DS with and without psychotic symptoms based on an ANCOVA model with the covariate antipsychotic medication with (F(1,31) = 4.73, partial ƞ2 = 0.13, p = 0.037). A multivariate general linear model including 8 T-cell populations was found significant for the effect of the PANSS positive subscale when considered jointly (Wilk’s Ʌ = 0.472, F (8,20) = 2.8, p = 0.030, partial ƞ2 = 0.53). Individual ANCOVA’s found an effect of PANSS positive subscale as a predictor of Th17% (F(1,27) = 4.34, p = 0.047, partial ƞ2 = 0.34) and CD8% of live PBMC’s (F(1,27) = 4.33, p = 0.047, partial ƞ2 = 0.34). The PANSS positive symptom subscale was positively related to Th17% (Fig. 4). The effect of antipsychotic medication was not significant.
Fig. 4.
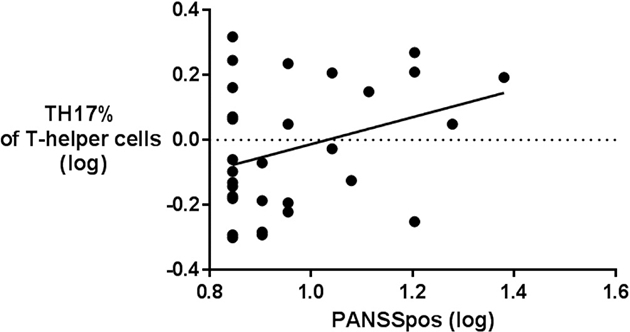
Th17% and positive psychotic symptoms score in psychotic and non-psychotic adults with 22q11.2DS.
4. Discussion
Besides confirming a T-cell defect, our results provide novel insight into the effect of a T-cell imbalance on SSD development in 22q11.2DS. Although both characteristics have been investigated separately (20; Jawad et al., 2001), this study is the first to explore the T-cell subset composition in its relation to the risk state for SSD in 22q11.2DS patients.
Next to the expected T-cell deficit, an increase of memory T-helper cells was found alongside a pro-inflammatory shift within the T-helper population (increased Th1 and Th17) in adults with 22q11.2DS. Both of these shifts can be explained as compensatory mechanisms. Firstly, memory T-cells are primed cells which can efficiently cope with the environmental pressure of foreign and dangerous intruders. It is possible that, against a background of generally reduced T cells and naïve T cells leaving the thymus, the proportion of memory cells needs to increase in order to improve efficient protection from foreign pathogens. Secondly, a similar reasoning may apply to the higher levels of Th1 and Th17 cells, usually needed for combating infection, which outnumbered Th2 and T-regulatory cells respectively. This constant pro-inflammatory state may be needed to cope with the environmental challenges. It is particularly remarkable that the T-cell profile of the current study group with 22q11.2DS resembles the findings described in patients with SSD (Frommberger et al., 1997), more specifically with regard to the decreased T-cell percentages and pro-inflammatory shift. In accordance with our findings, Kanaya et al. (2006) reported increased gene expression levels of the cytokine (TGF-β) responsible for driving the differentiation into Th17 cells, when comparing younger (0–4 years old) to older (7–21 years old) patients with 22q11.2DS. However our findings are not entirely in line with the findings reported by Zemble et al. (2010). These authors did report an increased Th1 percentage in 22q11.2DS only in children but not in adults. In addition they did not find an increase in Th17 cells, but instead increased Th2 cells in adults with 22q11.2DS.
In the current study, a significant increase in particularly the circulating level of Th17 cells related to the presence of psychotic symptoms in patients with 22q11.2DS is found. A pro-inflammatory shift with an increased Th17% seems to be related to positive psychotic symptom levels. This observation supports the previously expressed idea that Th17 cells have a role beyond combating infection and the development of autoimmunity, namely, in contributing to the development of SSD (Debnath and Berk, 2014). In line with our results, increased Th17 proportions have been reported in drug naïve first episode schizophrenia patients and in recent onset schizophrenia patients (12; Zeni-Graiff et al., 2016). Opposite results have also been published, yet not on the cellular level, but on the circulating levels of IL-17, the primary cytokine produced by activated Th17 cells: Zeni-Graiff et al (Noto et al., 2015) found decreased levels of IL-17, in children at ultra-high risk for psychosis. Noto et al (Mekori-Domachevsky et al., 2017) did not find significant changes in IL-17 levels in patients with first episode psychosis. Interestingly a very recent study looking at cytokine profiles and psychotic symptoms in individuals with 22q11.2DS did find higher IL-6 levels and an increased IL-6:IL-10 ratio in psychotic participants (Wolf et al., 2009). Since IL-6 is an important cytokine to drive Th17 differentiation this observation does seem to fit with our findings.
Recent studies shed light on a possible brain function of Th17 cells. In the mouse model of the Kempermann group a deficiency of particular myelin-reactive Th17 cells led to significantly reduced hippocampal neurogenesis and decreased brain-derived neurotrophic factor (BDNF) expression (Niebling and Rünker, 2014). Impaired hippocampal neurogenesis could be restored upon adoptive transfer with homogenous Th17 populations enriched for myelin-reactive T-cell receptors pointing to a brain antigen-specificity of the growth restoring Th17 cell action (Kebir et al., 2007). Th17 and cytokines of the Th17 pathway such as IL-17, IL-22 and IL-23 are also known to play a role in disruption of the blood brain barrier in neuro-immune pathologies such as multiple sclerosis, making the brain more accessible for migration of Th17 and other T cells in pathological conditions (Gyülvészi et al., 2009; Reboldi et al., 2009). However another route to enter the brain and the cerebrospinal fluid, even in healthy conditions, has been identified for Th17 cells (and T-regulatory cells). This route via the choroid plexus, delivers the cells in close vicinity to the limbic structures (Lewitus et al., 2008). In a rodent model, a short exposure to a stressor enhanced anxiety and increased Th17 cell infiltration into the brain via this choroid plexus route. The ability to cope with the induced stress was interrelated with T-cell trafficking to the brain and hippocampal BDNF levels (Moynes et al., 2014).
Whether T cells need to travel to the brain to exert the above described functions is a matter of debate. In the experiments of the Kempermann group the enhanced proliferation was not dependent on direct interactions of infiltrating Th17 cells with cells in the hippocampal neurogenic niche. In other words, brain-infiltration of the Th17 cells was not necessary and the cells might have influenced the brain from a distance by producing IL-17. The IL-17 receptor is expressed in many neural tissues and both neural damaging and beneficial effects of the cytokine have been described (Choi et al., 2016). With regard to behavioral consequences of IL-17 in the brain, Choi et al (41) showed in their mouse model that increased, infection-induced levels of peripheral IL17-A in the mother caused irregularities in the neuron layers of the brain cortex in offspring and these were associated with deficits in social interaction, behavior and communication. Offspring of mice who were lacking an essential regulator of the IL-17 pathway did not show these behavioral deficits (41).
Collectively the above described findings seem to point to a brain-interactive role of particularly Th17-cells in cognition, behavior and psychiatric disorders in 22q11.2DS. This urges for further studies on T-cell function, specificity and character (detrimental, protective) with regard to psychosis liability and cognitive defects. The results of this pilot study suggest that 22q11.2DS could be a usefull human model to further investigate the mechanisms involved in these immune-brain interactions. The high prevalence of SSD and specific cognitive deficits in this group offers the opportunity for longitudinal studies enabling further investigation of the role of immune-brain interactions in important neurodevelopmental windows before onset of the first symptoms.
5. Limitations
As this is the first exploratory study in a cohort of adults with 22q11.2DS, the sample size is too limited to take confounding factors sufficiently into account. We did look at age, gender and BMI. For medication we used immune suppressive medication as one of our exclusion criteria and we added the use of antipsychotics to our statistical models. However, we were not able to control for other psychiatric diagnoses within our population with 22q11.2DS such as major depressive disorders and autism spectrum diagnoses. Most adults with 22q11.2DS have a complex and heterogeneous medical history, which we did not take into account as data were retrospective and accuracy was difficult to determine. This decision was also based on the idea that neuro-immune interactions that are clinically relevant should be apparent in this more naturalistic sample. Future studies should also try to take into account other medication, such as the use of anti-depressants and vitamin D supplements, the time of day of blood sampling, and stress levels.
Furthermore the present study relied on percentages and not absolute values of circulating lymphocyte populations. It is evident from the literature that the absolute and relative counts may not yield equal results: e.g. it has been reported that, although the percentage of T cells was decreased in patients with schizophrenia, there was in contrast an increase in absolute T cell values (8). Future studies should take into account both relative and absolute counts.
6. Conclusion
This study shows that adults with 22q11.2DS are prone to develop a pro-inflammatory imbalance with a significantly increased level of Th17, against the background of a partial T-cell defect. Although limited by the small sample size, our study also reveals that the pro-inflammatory Th17 signal is most pronounced in adults with 22q11.2DS who show symptoms of psychosis when controlling for antipsychotic medication. The resemblance between this aberrant Th17 immune profile of adults with 22q11.2DS and that of schizophrenia patients reported in the literature is remarkable. Given the literature on the role of T cells and in particular of Th17 cells and IL-17 in hippocampus development, cognition and behavior, these results support the hypothesis for a role for T cells and more specifically for Th17 cells in the development and/or regulation of psychotic symptoms in 22q11.2DS.
Supplementary Material
Acknowledgments
We thank all the participants and their families for their willingness to participate in our studies. The authors like to acknowledge the excellent technical assistance of Harm de Wit and Annemarie Wijkhuijs of the immunology lab at the Erasmus MC. They also express their thankfulness to Prof. Adrian Liston, lab of auto-immune genetics , VIB Leuven who kindly enabled us to use his facilities. The study was partly funded by the EU (FP-7 MOODINFLAME and PSYCHAID) and was also supported by the National Institute of Mental Health under Award number U01MH101722. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. The collection of the healthy controls was supported by the Veni scholarship of W. Veling and A. Counotte from the Netherlands Organization for Health Research and Development (916.12.013)). Stephan Claes is a Senior Clinical Investigator of the Fund for Scientific Research Flanders (FWO Vlaanderen).
Footnotes
Financial disclosures
H.A. Drexhage does not have conflicts of interest with commercial firms, except with IgNova which does not have any interest in this study. All other authors have no conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bbi.2018.03.022.
References
- Achiron A, Noy S, Pras E, Lereya J, Hermesh H, Laor N, 1994. T-cell subsets in acute psychotic schizophrenic patients. Biol. Psychiatry 35, 27–31. [DOI] [PubMed] [Google Scholar]
- Bergink V, Burgerhout KM, Weigelt K, Pop VJ, de Wit H, Drexhage RC, et al. , 2013. Immune system dysregulation in first-onset postpartum psychosis. Biol. Psychiatry 73, 1000–1007. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ, 2010. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatry 167, 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. , 2016. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath M, Berk M, 2014. Th17 pathway-mediated immunopathogenesis of schizophrenia: mechanisms and implications. Schizophr. Bull, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Song X, Zhao J, Gao J, Li X, Yang G, et al. , 2014. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 51, 78–82. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJM, Drexhage HA, 2011. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int. J. Neuropsychopharmacol 14, 746–755. [DOI] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM, 2013. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol. Psychiatry 73, 951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M, 1997. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur. Arch. Psychiatry Clin. Neurosci 247, 228–233. [DOI] [PubMed] [Google Scholar]
- Gennery AR, 2012. Immunological aspects of 22q11.2 deletion syndrome. Cell. Mol. Life Sci 69, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli M, Kumar R, Soresina A, Tamassia N, Lorenzini T, Moratto D, et al. , 2016. Reduction of CRKL expression in patients with partial DiGeorge syndrome is associated with impairment of T-cell functions. J. Allergy Clin. Immunol 138 (229–240), e3. [DOI] [PubMed] [Google Scholar]
- Gyülvészi G, Haak S, Becher B, 2009. IL-23-driven encephalo-tropism and Th17 polarization during CNS-inflammation in vivo. Eur. J. Immunol 39, 1864–1869. [DOI] [PubMed] [Google Scholar]
- Janssen HL, Berk L, de Man RA, Heijtink RA, Schalm SW, 1992. Alpha-interferon antiviral treatment in 100 patients with chronic hepatitis B. Ned. Tijdschr. Geneeskd 136, 835–839. [PubMed] [Google Scholar]
- Jawad AF, McDonald-Mcginn DM, Zackai E, Sullivan KE, 2001. Immunologic features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). J. Pediatr 139, 715–723. [DOI] [PubMed] [Google Scholar]
- Jeker LT, Zhou X, Blelloch R, Bluestone JA, 2013. DGCR8-mediated production of canonical microRNAs is critical for regulatory T cell function and stability. PLoS One 8, e66282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome LA, Papaioannou VE, 2001. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet 27, 286–291. [DOI] [PubMed] [Google Scholar]
- Kanaya Y, Ohga S, Ikeda K, Furuno K, Ohno T, Takada H, et al. , 2006. Maturational alterations of peripheral T cell subsets and cytokine gene expression in 22q11.2 deletion syndrome. Clin. Exp. Immunol 144, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. , 2007. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med 13, 1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller WR, Kum LM, Wehring HJ, Koola MM, Buchanan RW, Kelly DL, 2013. A review of anti-inflammatory agents for symptoms of schizophrenia. J. Psychopharmacol 27, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M, 2008. Reducing post-traumatic anxiety by immunization. Brain Behav. Immun 22, 1108–1114. [DOI] [PubMed] [Google Scholar]
- Leza JC, García-Bueno B, Bioque M, Arango C, Parellada M, Do K, et al. , 2015. Inflammation in schizophrenia: a question of balance. Neurosci. Biobehav. Rev 55, 612–626. [DOI] [PubMed] [Google Scholar]
- Lima K, Abrahamsen TG, Foelling I, Natvig S, Ryder LP, Olaussen RW, 2010. Low thymic output in the 22q11.2 deletion syndrome measured by CCR9 +CD45RA+ T cell counts and T cell receptor rearrangement excision circles. Clin. Exp. Immunol 161, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekori-Domachevsky E, Taler M, Shoenfeld Y, Gurevich M, Sonis P, Weisman O, et al. , 2017. Elevated proinflammatory markers in 22q11.2 deletion syndrome are associated with psychosis and cognitive deficits. J. Clin. Psychiatry. OF: 0–0. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B, 2011. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry 70, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A, 2013. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry 73, 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynes DM, Vanner SJ, Lomax AE, 2014. Participation of interleukin 17A in neuroimmune interactions. Brain Behav. Immun 41, 1–9. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ, 1999. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch. Gen. Psychiatry 56, 940. [DOI] [PubMed] [Google Scholar]
- Niebling J, E Rünker A, Schallenberg S, Kretschmer K, Kempermann G, 2014. Myelin-specific T helper 17 cells promote adult hippocampal neurogenesis through indirect mechanisms. F1000Research 3, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto C, Ota VK, Santoro ML, Ortiz BB, Rizzo LB, Higuchi CH, et al. , 2015. Effects of depression on the cytokine profile in drug naïve first-episode psychosis. Schizophr. Res 164, 53–58. [DOI] [PubMed] [Google Scholar]
- Oskarsdottir S, Vujic M, 2004. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Arch. Dis. Child, 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae C-U, Yoon C-H, Kim T-S, Kim J-J, Park S-H, Lee C-U, et al. , 2006. Antipsychotic treatment may alter T-helper (TH) 2 arm cytokines. Int. Immunopharmacol 6, 666–671. [DOI] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, et al. , 2009. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol 10, 514–523. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, et al. , 1997. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J. Med. Genet 34, 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, van den Bree M, et al. , 2014. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am. J. Psychiatry 171, 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro DI, Cubells JF, Ousley OY, Rockers K, Walker EF, 2011. Prodromal symptoms in adolescents with 22q11.2 deletion syndrome and schizotypal personality disorder. Schizophr. Res 129, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Jacobs R, Panteli B, Brauner M, Schiltz K, Bahn S, et al. , 2010. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur. Arch. Psychiatry Clin. Neurosci 260, 509–518. [DOI] [PubMed] [Google Scholar]
- Veling W, Counotte J, Pot-Kolder R, van Os J, van der Gaag M, Andreasen NC, et al. , 2016. Childhood trauma, psychosis liability and social stress reactivity: a virtual reality study. Psychol. Med 49, 1–10. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, et al. , 2009. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J. Immunol 182, 3979–3984. [DOI] [PubMed] [Google Scholar]
- Zemble R, Luning Prak E, McDonald K, McDonald-McGinn D, Zackai E, Sullivan K, 2010. Secondary immunologic consequences in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Clin. Immunol 136, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni-Graiff M, Rizzo LB, Mansur RB, Maurya PK, Sethi S, Cunha GR, et al. , 2016. Peripheral immuno-inflammatory abnormalities in ultra-high risk of developing psychosis. Schizophr. Res 176, 191–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


