Abstract
Background
Although medications play a vital role in the cure, palliation, and inhibition of disease, they also expose patients to drug-related problems (DRPs). DRPs are common in hospitalized patients. Specifically, pediatrics population are easily affected by DRPs, as dynamic and kinetic behaviors of drugs in this population are usually different than in adults.
Objectives
To assess the prevalence of DRPs and associated factors in a pediatric setting in Ethiopia. Setting Pediatric ward of Zewditu Memorial Referral Hospital, Addis Abbeba, Ethiopia.
Methods
A cross-sectional study was conducted on 285 randomly selected patients. Data were obtained through review of physician medication orders and patient files. The prevalence and type of DRPs were studied and documented using the Pharmaceutical Care Network Europe Foundation classification system. The results were summarized using descriptive statistics including frequency, mean, and standard deviation. To identify the independent predicators of DRPs, logistic regression analysis was run and a P value ≤0.05 was considered as statistically significant. Main outcome measure DRPs, types of DRPs, drugs that are frequently involved in DRPs, and factors associated with DRPs. Main outcome measure Number of DRPs.
Results
Of the 1055 medication orders reviewed, a total of 106 DRPs were identified in 90 patients. This gives an overall rate of drug-related problems of 31.57%. The most frequently identified DRPs were dosing problems, with dose too low being 34.9% and dose too high being 7.5%. This was followed by drug–drug interactions(38.67%) and adverse drug reactions (8.49%). The number of prescribed drugs (AOR 2.3, 95% CI 1.3–4.3, P = 0.007) and total number of disease conditions (AOR4.8, 95% CI 1.9, 12.1, P = 0.001) were potential risk factors for occurrence of DRPs.
Conclusion
The present study demonstrated that DRPs were common at the pediatric ward of Zewditu Memorial Referral Hospital and that it needs great attention. The most frequently identified DRPs were dosing problems, followed by drug–drug interactions and adverse drug reaction. Poly-pharmacy and number of disease conditions have been identified as important risk factors for occurrence of DRPs. The investigators recommend establishing a system for reporting DRPs in the pediatric ward of the hospital as it may facilitate appropriate measures for prospective interventions, such as training the healthcare team, as well as detail precautions to be followed by the practitioners. In addition to this, improving communication between the healthcare team members such as physicians, pharmacists, nurses, and other healthcare workers in the hospital is recommended.
Keywords: Drug-related problems, Ethiopia, Pediatrics
Introduction
Although medications play a vital role in the cure, palliation, and inhibition of disease, they also expose patients to drug-related problems (DRPs) [1]. There are a variety of definitions and classification of DRPs. According to the Pharmaceutical Care Network in Europe (PCNE), DRPs have been defined as ‘an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes.’ Accordingly, the PCNE further classified DRPs under one of the following categories: dosage too high, dosage too low, adverse drug reaction (ADR), needs additional drug therapy, drug–drug interactions, unnecessary drug therapy, and non-adherence PCNE V 6.2 [2].
Due to several reasons, hospitalized patients are likely to receive more medications than those treated as outpatients. Joseph et al. compared hospitalized patients to self or family care groups and found a higher number of medications used in the hospitalized group (21 vs. 10%, P = 0.005) [3]. Thus, it is a concern for potential or actual DRPs [4]. Unlike actual DRPs, potential problems do not have manifestations at the time of investigation but if left unresolved may lead to actual drug-related harm to the patient. Therefore, actual problems and their clinical manifestations should be prioritized for intervention. As the number of drugs used in treatment is becoming more complex, this makes appropriate prescribing very challenging [5–7].
Many studies demonstrate the consequences of DRPs, which include additional physician office visits, long-term care admissions, hospitalizations, additional prescriptions, and emergency department visits [1]. In addition to these, substantial costs are also associated with DRPs. For example, the economic burden arising from drug-related morbidity and mortality in ambulatory care environment in the USA in the year 2000 was $177.4 billion [8]. Additionally, the direct costs associated with DRPs identified at two pediatric hospitals in Australia totaled 100,707 lb [9]. Even if less research is done in Africa about costs associated with DRPs, a study done in Lagos State University Teaching Hospital, Nigeria, reported that approximately1.83 million naira (15,466.60 USD) was spent on managing all the patients admitted due to ADRs [10]. Therefore, DRPs are a major area of concern to the patient as well as to the society in terms of psychological, physical, and economic burden [11, 12]. Accordingly, increasing the quality of drug therapy by decreasing the occurrence of drug-related problems may have a substantial impact on the health costs, potentially saving lives and improving patients’ quality of life [13, 14].
The pediatric population is at especially high risk of DRPs, as the pharmacodynamic and pharmacokinetic behavior of drugs in this population is often different than adults [15]. Even if such differences exist, studies that investigate DRPs in children are inadequate and incomplete in resource-limited countries. Such incomplete nature of these studies is unable to represent the wider picture of DRPs in children.
To the best of our knowledge, evidence-based research on DRPs has not been done in Ethiopia. Therefore, this study was designed to determine overall rate of DRPs, common DRPs types, drugs most frequently implicated in DRPs, and factors associated with DRPs at the pediatric ward of Zewditu Memorial Referral Hospital (ZMRH), Addis Ababa, Ethiopia.
Aim of the study
The present study aims to investigate the number of DRPs, drugs that are frequently involved in DRPs, and factors associated with DRPs in hospitalized children identified through PCNE.
Ethics approval
A letter of ethical clearance was obtained from the School of Pharmacy Research Ethics Review Board, Addis Ababa University, Addis Ababa City Health Bureau as well as the pediatric department of ZMRH. Privacy and confidentiality was ensured during review of patients’ chart by data collectors. Name and address of patients were not recorded in the data abstraction. Verbal consent was obtained from each patient or the patient’s caregiver.
Methods and participants
Study area and design
This cross-sectional study was conducted from March 10, 2014, to June 15, 2014, at pediatric ward of ZMRH. The hospital is located in Addis Ababa the capital city of Ethiopia, and it is run by Addis Ababa City Health Bureau. The hospital is a 180-bed referral center and is comprised of 460 health professionals. The pediatric ward has 65 beds, 16 nurses, and a total of 9 doctors of whom 3 are senior pediatricians and 6 are general practitioners.
Study participants
Pediatric patients who were admitted to the pediatric inpatient wards during the study period, who were less than 15 years of age, and whose hospital stay was greater than 48 h were included in the study.
Operational definitions
An ADR is an appreciably harmful or unpleasant reaction resulting from an intervention related to the use of a medicinal product which predicts hazard from future administration and warrants prevention or specific treatment, alteration in the dose regimen, or withdrawal of the product [16].
Data collection procedure
Data were taken from patient medical charts and medical records as well as through patient interviews by two hospitals and one clinical pharmacist. Data collectors were trained by the principal investigators regarding the objective of the study, methods of data collection including data extraction from patient charts as well as techniques of interviewing patients, and DRP identification. The following data were recorded for each patient if available: age of the patient, weight, gender, principal diagnosis, comorbidities, and the length of hospital stay. Specifically regarding medications, the data collected included generic medication name, trade medication name, dosage, dosing frequency, duration of therapy, and start date of the medications. Data collected from the charts and records were supplemented with interviewing the patient to verify ADRs.
DRPs were assessed by reviewing and analyzing all medication orders, administration sheets, laboratory test results, and diagnostic test results. DRPs were identified by evaluating the appropriateness of prescriptions in terms of indication, dosage, and duration of therapy. Appropriateness of drug choice was identified by using the Ethiopia guidelines for the Management of Common Illnesses in Hospitals [17] and the Nelson Textbook of Pediatrics. Potential drug–drug interactions (DDIs) were identified using Medscape (WebMD, LLC) online drug interaction checker, UpToDate®(version 21.2, Wolters Kluwer, The Netherlands), and Micromedex®(Micromedex 2.0).
During data collection, if a DRP was identified, it was recorded and classified using a DRP registration format taken from Cipolle et al. [18] with some modifications. The principal investigators then communicated with the involved physicians to make appropriate interventions.
Data analysis and interpretation
The collected data were entered into Epi Info version 7 and exported to Statistical Package for Social Sciences (SPSS) version 20 for statistical analysis. In order to summarize the results, descriptive statistics including frequency, mean, and standard deviation were used. The incidence of DRPs was determined by dividing the number of people experiencing at least one DRP by the total number of study participants. Both a univariate and multivariate analysis was run. Based on the univariate analysis, the variables that were significant (P ≤ 0.05) were included in the multivariate analysis to control for confounders and to identify factors independently associated with the occurrence of DRPs. The results of univariate and multivariate analysis were reported as crude odds ratio (COR) and adjusted odds ratio (AOR) at 95% confidence intervals (95% CI), respectively. P value ≤0.05 was considered as statistically significant.
Results
Socio-demographic and clinical data of patients
A total of 285 pediatric patients were included in this study of whom 151 (53%) were female. The mean age of the studied population was 2.8 (SD = 1.2) years (range 10 days–14 years). The largest age group was 29 days to ≤1 year which accounted for 31.2%. A majority of the patients had a single disease which accounted for 47.4%. The mean duration of hospital stay was 3.7 (SD = 1.6) days. Most of the patients had ≤3 days of hospital stays which accounted for 57.2%. The details of socio-demographic characteristics and clinical data of the patients are summarized in Table 1.
Table 1.
Socio-demographic and clinical data of the patient in pediatric ward of Zewditu Memorial Referral Hospital
| Patient characteristics | Frequency | Percentage |
|---|---|---|
| Sex | ||
| Male | 134 | 47 |
| Female | 151 | 53 |
| Age | ||
| Neonate (birth to 28 days) | 21 | 7.4 |
| Infant (29 days to ≤1 years) | 89 | 31.2 |
| Toddler (>1 year to ≤3 years) | 69 | 24.2 |
| Preschool (>3 years to ≤5 years) | 16 | 5.6 |
| School age (>5 years to ≤10 years) | 37 | 13.0 |
| Adolescent (>10 years to <15 years) | 53 | 18.6 |
| No of disease conditions | ||
| 1 | 135 | 47.4 |
| 2 | 110 | 38.6 |
| 3 | 30 | 10.5 |
| 4 | 10 | 3.5 |
| No of drugs used | ||
| <5 drugs | 207 | 72.6 |
| ≥5 drugs | 78 | 27.4 |
| Duration of hospital stays | ||
| ≥2 to ≤3 days | 163 | 57.2 |
| 4–6 days | 103 | 36.1 |
| ≥7 days | 19 | 6.7 |
| Type of disease diagnosed | ||
| Infectious and parasitic diseases | 156 | 39.7 |
| Disease of the respiratory system | 117 | 29.8 |
| Nervous system | 45 | 11.4 |
| Disease of genitourinary | 23 | 5.8 |
| Disease of the blood and blood-forming organs | 18 | 4.5 |
| Endocrine and metabolic disease | 17 | 4.3 |
| Cardio vascular system | 5 | 1.3 |
Incidence and nature of DRPs
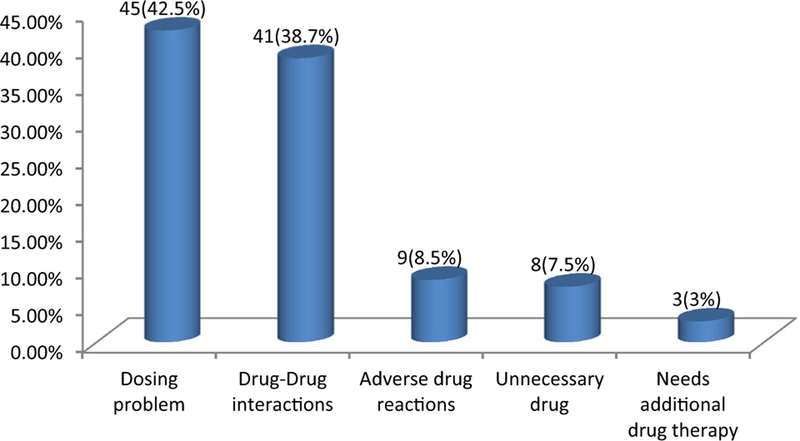
A total of 106 DRPs were identified in 90 patients. The overall incidence was 31.6%. Of all patients having DRPs, 74 (69.8%) of patients had one DRP. Dosing problem was the most frequent DRP, accounting for 45(42.5%): dose too low 34.9% and dose too high 7.5%. Drug–drug interactions (DDIs) accounted for 41 (38.7%) and adverse drug reactions accounted for 9 (8.5%). The details of each type of DRPs identified are summarized in Fig. 1.
Types of DRPs at pediatric ward of Zewditu Memorial Referral Hospital
Drugs and diseases implicated in DRPs
The most prevalent specific diseases implicated in DRPs were pneumonia (41.1%), asthma (20.4%), HIV (15.1%), seizure (10.9%), and TB (9.5%). Drugs involved in DRPs were categorized into different therapeutic classes with antibiotics, analgesics, antipyretics, and anti-epileptics being the most prevalent drug classes. The most frequently implicated drug was ampicillin (14.1%), followed by phenobarbital (12.2%) and diazepam (10.3%). Table 2 summarizes top 10 drugs involved in DRPs.
Table 2.
Top ten specific drugs associated with DRPs in the pediatric ward of Zewditu Memorial Referral Hospital
| Drug name | DDI | ADR | Dosing problems | Untreated conditions | Unnecessary drug | Total % |
|---|---|---|---|---|---|---|
| Ampicillin | 0 | 0 | 15 | 0 | 0 | 14.1 |
| Phenobarbital | 13 | 0 | 0 | 0 | 0 | 12.2 |
| Diazepam | 11 | 0 | 0 | 0 | 0 | 10.3 |
| Diclofenac | 4 | 0 | 0 | 0 | 5 | 8.4 |
| Hydrocortisone | 9 | 0 | 0 | 0 | 0 | 8.4 |
| Salbutamol | 7 | 0 | 0 | 0 | 0 | 6.6 |
| Phenytoin | 6 | 0 | 0 | 0 | 0 | 5.6 |
| Vancomycin | 0 | 0 | 6 | 0 | 0 | 5.6 |
| Gentamycin | 0 | 0 | 6 | 0 | 0 | 5.6 |
| Zidovudine | 0 | 5 | 0 | 0 | 0 | 4.7 |
DDI drug–drug interactions, ADR adverse drug reactions
Factors associated with DRPs
The results of univariate binary logistic regression analysis on the association between different types of independent variables and DRPs showed that patients who had three or more disease conditions had a significant association with DRPs and were around seven (COR 7, 95% CI 2.94–16.22) times more likely to have DRPs compared with patients who had one disease condition. Similarly, patients who took five or more drugs were around four (COR 3.6, 95% CI 2.07–6.18) times more likely to have DRPs compared to those patients who took less than five drugs. The details are summarized in Table 3.
Table 3.
Univariate logistic regression results of factors associated with DRPs in pediatric ward of Zewditu Memorial Referral Hospital
| DRPs | COR | P value at 95% CI | ||
|---|---|---|---|---|
| Yes | No | |||
| Sex | ||||
| Male | 49 (54.4%) | 85 (43.6%) | 1.00 | (Reference) |
| Female | 41 (45.6%) | 110 (56.4%) | 1.5 (0.94,2.6) | 0.089 |
| Age | ||||
| Neonates | 6 (6.7%) | 15 (7.7%) | 1.00 | (Reference) |
| Infant | 21 (23.3%) | 68 (34.9%) | 0.8 (0.2, 2.2) | 0.634 |
| toddler | 20 (22.2%) | 49 (25.1%) | 1.0 (0.3, 3.1) | 0.971 |
| Preschool | 8 (8.9%) | 8 (4.1%) | 2.5 (0.6, 9.8) | 0.188 |
| School age | 16 (17.8%) | 21 (10.8%) | 1.9 (0.6, 6.1) | 0.272 |
| Adolescent | 19 (21.1%) | 34 (17.4%) | 1.4 (0.4, 4.2) | 0.552 |
| Number of diseases | ||||
| 1 | 27 (30.0%) | 108 (55.4%) | 1.00 | (Reference) |
| 2 | 36 (40.0%) | 74 (37.9%) | 1.9 (1.0, 3.5) | 0.024 |
| 3 | 19 (21.1%) | 11 (5.6%) | 7 (2.9, 16.2) | 0.000 |
| 4 | 8 (8.9%) | 2 (1.0%) | 16 (3.2, 79.7) | 0.001 |
| Number of drugs used | ||||
| <5 drugs | 49 (54.4%) | 158 (81.0%) | 1.00 | (Reference) |
| ≥5 drugs | 41 (45.6%) | 37 (19.0%) | 3.6 (2.0, 6.2) | 0.000 |
| Duration of hospital stays | ||||
| ≤3 days | 49 (54.4%) | 114 (58.5%) | 1.00 | (Reference) |
| 4–6 days | 34 (37.8%) | 69 (35.4%) | 1.1 (0.6, 1.9) | 0.613 |
| ≥7 days | 7 (7.8%) | 12 (6.2%) | 1.4 (0.5, 3.7) | 0.546 |
DRPs drug-related problems, COR crude odds ratio
Multivariate logistic regression analysis was run, and the analysis showed that both number of disease conditions and number of drugs taken had a significant association with DRPs. Based on this, patients who had three disease conditions were about five (AOR 4.8, 95% CI 1.9, 12.1) times more likely to have DRPs compared to those patients who had one disease condition. On the other hand, patients who took five or more drugs were about two (AOR 2.3, 95% CI 1.3–4.3) times more likely to have DRPs compared to those patients who took less than five drugs. The details are summarized in Table 4.
Table 4.
Multivariate logistic regression analysis result of factors associated with DRPs in pediatric ward of Zewditu memorial referral hospital
| Variables | DRPs | AOR | P value at 95% CI | |
|---|---|---|---|---|
| Yes | No | |||
| Number of diseases | ||||
| 1 | 27 (30.0%) | 108 (55.4%) | 1.00 | (Reference) |
| 2 | 36 (40.0%) | 74 (37.9%) | 1.7 (0.9, 3.1) | 0.098 |
| 3 | 19 (21.1%) | 11 (5.6%) | 4.8 (1.9, 12.1) | 0.001 |
| 4 | 8 (8.9%) | 2 (1.0%) | 10.7 (1.9, 58.2) | 0.006 |
| Number of drugs used | ||||
| <5 drugs | 49 (54.4%) | 158 (81.0%) | 1.00 | (Reference) |
| ≥5 drugs | 41 (45.6%) | 37 (19.0%) | 2.3 (1.3, 4.3) | 0.007 |
DRPs drug-related problems, AOR adjusted odds ratio
Discussion
The findings of the present study revealed that a significant proportion of hospitalized patients experience DRPs the majority of which arose during hospitalization and this is a major safety issue for them. Therefore, a more comprehensive study of DRPs in hospitalized patients would provide valuable insights for the healthcare professionals trying to reduce the incidence of DRPs.
The incidence of DRPs in this study was 31.57% notably higher than an incidence reported in a Hong Kong study of 21.0% [19]. This difference may be due to differences in hospital settings including composition of healthcare workers, training levels of prescribers, presence or absence of support system, and difference in the definition of DRPs. A higher incidence was reported in UK and the Kingdom of Saudi Arabia study with 45.2% incidence within the same population of pediatric patients [20]. From the total incidence identified in this study, the percentage of male patients with DRPs was higher than of female patients(54.4 vs. 45.6%), although the difference was not significant (COR 1.5, 95% CI 0.9, 2.6, P = 0.089), and this finding is in agreement with the Hong Kong study [19].
In this study, the most frequently identified DRPs were a dosing problem with a percentage of 42.45%: dose too low (34.9%) and dose too high (7.5%). This value is lower than in UK and Kingdom of Saudi Arabia 54% [20]. The most frequently identified drug implicated in dosing problems in this study was ampicillin. This may be due to the higher prevalence of infectious disease and a higher prescription rate of this drug.
Dosing errors in pediatrics might result in ineffective treatment due to subtherapeutic concentration or toxicity due to overdose, both of which may lead to increased mortality. Therefore, a greater focus on improvement in the safety of drugs used in children is necessary. Generally, inappropriate doses are more common in pediatrics than adults because of weight-based dosing calculations, fractional dosing (e.g., mg vs. g), the need for decimal use, and incorrect recording of patients’ weights [21]. The high prevalence of dosing problems in this study would make this an important area requiring further investigation.
The present study analysis on DRPs showed that potential drug–drug interactions were the second most identified DRP 41(38.7%). The drugs most implicated in DDIs for the current study were phenobarbital, diazepam, and hydrocortisone. The high percentage of potential DDIs in this ward probably related to the prevalent of infectious diseases, particularly HIV/AIDS and opportunistic infections, that are managed with complex drug regimens that have a higher potential for interactions. In addition, patients may also have a higher number of comorbid conditions and take more drugs. Patients need to be closely monitored for manifestations such as lack of therapeutic efficacy or toxicity, especially for drugs whose therapeutic effects may be diminished or augmented when used in those combinations. Drug interactions are a major factor that might cause ADR, therapeutic failure, and drug-related harm to patients [22]. These can affect a patient’s clinical outcome, their quality of life, and contribute to unnecessary healthcare cost.
In the present study, 9 (8.5%) DRPs were found to be an ADR most of which were zidovudine-induced anemia and the patients were switched to other drugs. Corticosteroid and griseofulvin drugs were also stopped by the patients following blood sugar elevations and facial swelling, respectively. The high number of ADR occurrence in this ward is probably attributed to the use of multiple medications, which increases the risk of ADRs. Vandenbemt et al. found that patients with five or more drugs prescribed during their hospital stay had the highest risk of developing an ADR three times higher compared to patients receiving between one and four drugs. One possible explanation might be that poly-pharmacy may increase the chances of drug–drug interaction, which leads to increased possibilities for an ADR to occur [23].
In this study, 8 (7.5%) of total DRPs were identified as unnecessary drug use due to duplicate prescriptions. Examples of duplicate prescriptions identified in this study were noted especially with analgesics, antipyretics, and glucocorticosteroids. Concurrent administration of paracetamol and diclofenac as well as dexamethasone and prednisolone was seen in a few cases leading to duplication because both of these drugs belong to the same class. In this era of inflation, drug therapy costs are on the rise. This is a burden for developing nations particularly Ethiopia. Therefore, prevention of duplicate drug therapy will contribute to cost savings among hospitalized patients.
The top three drug classes implicated for DRPs in the present study are anti-infectives, analgesic–antipyretics, and anti-epileptics. Similarly, anti-infectives were the most common classes of drugs involved in DRPs in other studies from Hong Kong [19] and UK [20]. Anti-infectives are used in very high numbers in the pediatric ward of ZMRH as most of the admitted patients were diagnosed with infectious diseases, as presented in Fig. 1. This may be one reason this class of drugs appears as major class responsible for causing DRPs. Melander et al. noted that there is a higher prevalence of infectious diseases in children. As a result, higher numbers of anti-infectives are used in this group than any other class of drugs [24].
The drugs ampicillin, phenobarbital, and diazepam were the three most common drugs involved in DRPs in this study. While phenobarbital and diazepam are implicated in drug–drug interactions, ampicillin was involved in incorrect frequency of administration.
In an attempt to identify risk factors for the occurrence of DRPs in this study, poly-pharmacy and number of disease conditions were significantly associated with DRPs while sex, age, and duration of hospital stays were not significantly associated with DRPs in a univariate analysis. However, in multivariate logistic regression both poly-pharmacy and number of disease conditions were found to be independent predictors of DRPs in the pediatric ward of ZMRH. Other published findings also report on the number of drugs (≥5) taken by a patient as an important risk factor for the occurrence of DRPs [6, 19, 20]. The more complex drug therapy leads to a higher risk of experiencing DRPs such as adverse effects, drug–drug interactions, medication errors, and non-adherence.
Additionally, as the number of disease conditions increases, the number of drugs taken will also increase which may result in ADR, drug–drug interactions, medication errors, and non-adherence [20].
Due to most of the study participants having a short duration of stay in the hospital, patients will on average be given less drugs. Consequently, the occurrence of DRPs in these patients will be decreased because of the decreased occurrence of drug–drug interactions, ADR, and non-adherence.
Limitations of the study
We did not classify the type of DRPs with respect to whether it is potential or actual problem. We also did not classify the DDIs and ADRs based on severity. Adherence was not investigated as part of DRPs, and neither the outcome of DRPs nor the interventions made by the healthcare team were assessed.
Conclusion
The present study demonstrated that a significant proportion of hospitalized patients experience DRPs in the pediatric ward of ZMRH and that it needs great attention. The most frequently identified DRPs were dosing problems, followed by DDIs and ADRs. Poly-pharmacy and number of disease conditions have been identified as important risk factors for the occurrence of DRPs.
The investigators recommend establishing a system for reporting DRPs in the pediatric ward of the hospital as it may facilitate appropriate measures for prospective interventions such as training the healthcare team as well as detail precautions to be followed by the practitioners. In addition, improving communication between the healthcare team members such as physicians, pharmacists, nurses, and other healthcare workers in the hospital is recommended.
Impact on practice.
One out of three pediatric patients in Ethiopia is experiencing drug-related problems. Therefore, healthcare teams working in pediatrics (prescribers, clinical pharmacists, and nurses) in low-income country settings should always exercise maximum precautions while initiating and monitoring treatments.
Since the major drug-related problem identified in the pediatric setting is dose related (especially dose too low), prescribers shall always adhere to the updated guidelines and hospital mangers should ensure the implementation of institutional policies and guidelines.
Acknowledgements
We are very much thankful for the participants in this study as well as the University of Gondar for the financial support.
Funding None.
Footnotes
Conflicts of interest
The authors declare that they have no conflict of interests.
References
- 1.Baena MI, Faus MJ, Fajardo PC, Luque FM, Sierra F, Martinez-Olmos J, et al. Medicine-related problems resulting in emergency department visits. Eur J Clin Pharmacol. 2006;62(5):387–93. [DOI] [PubMed] [Google Scholar]
- 2.Pharmaceutical Care Network Europe. The PCNE classification for drug-related problems V 6.2. [Last accessed on 2014 March 01; Last updated on 2010 Jan 14]. Available from: http://www.pcne.org/sig/drp/documents/PCNE%20classification%20V6-2.pdf.
- 3.Flaherty JH, Perry HM III, Lynchard GS, Morley JE. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55(10):M554–9. [DOI] [PubMed] [Google Scholar]
- 4.Fijn R, Van den Bemt PMLA, Chow M, De Blaey CJ, De Jong-Van den Berg LTW, Brouwers JRBJ. Hospital prescribing errors: epidemiological assessment of predictors. Br J Clin Pharmacol. 2002;53(3):326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blix HS, Viktil KK, Moger TA, Reikvam Å. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm World Sci. 2006;28(3):152. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim N, Wong IC, Patey S, Tomlin S, Sinha MD, Jani Y. Drug-related problem in children with chronic kidney disease. Pediatr Nephrol. 2013;28(1):25–31. [DOI] [PubMed] [Google Scholar]
- 7.Rashed AN, Wong IC, Cranswick N, Hefele B, Tomlin S, Jackman J, et al. Adverse Drug Reactions in Children-International Surveillance and Evaluation (ADVISE): a multicentre cohort study. Drug Saf. 2012;35(6):481–94. [DOI] [PubMed] [Google Scholar]
- 8.Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc. 2001;41(2):192–9. [DOI] [PubMed] [Google Scholar]
- 9.Easton KL, Chapman CB, Brien J-AE. Frequency and characteristics of hospital admissions associated with drug-related problems in paediatrics. Br J Clin Pharmacol. 2004;57(5):611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshikoya KA, Chukwura H, Njokanma OF, Senbanjo IO, Ojo I. Incidence and cost estimate of treating pediatric adverse drug reactions in Lagos, Nigeria. Sao Paulo Med J. 2011;129(3): 153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277(4): 301–6. [PubMed] [Google Scholar]
- 12.Bates DW, Spell N, Cullen DJ, Burdick E, Laird N, Petersen LA, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277(4):307–11. [PubMed] [Google Scholar]
- 13.Winterstein AG, Sauer BC, Hepler CD, Poole C. Preventable drug-related hospital admissions. Ann Pharmacother. 2002;36(7–8): 1238–48. [DOI] [PubMed] [Google Scholar]
- 14.Lagnaoui R, Moore N, Fach J, Longy-Boursier M, Begaud B. Adverse drug reactions in a department of systemic diseases-oriented internal medicine: prevalence, incidence, direct costs and avoidability. Eur J Clin Pharmacol. 2000;56(2):181–6. [DOI] [PubMed] [Google Scholar]
- 15.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. [DOI] [PubMed] [Google Scholar]
- 16.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–9. [DOI] [PubMed] [Google Scholar]
- 17.Pocket book of pediatric hospital care: Ethiopia. Guidelines for the management of common illnesses in hospitals. 2013. [Google Scholar]
- 18.Cipolle RJ, Strand L, Morley P. Pharmaceutical care practice: the clinician’s guide, second edition: the clinician’s guide. New York: McGraw-Hill; 2004. [Google Scholar]
- 19.Rashed AN, Wilton L, Lo CC, Kwong BY, Leung S, Wong IC. Epidemiology and potential risk factors of drug-related problems in Hong Kong paediatric wards. Br J Clin Pharmacol. 2014;77(5):873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashed AN, Neubert A, Tomlin S, Jackman J, Alhamdan H, AlShaikh A, et al. Epidemiology and potential associated risk factors of drug-related problems in hospitalised children in the United Kingdom and Saudi Arabia. Eur J Clin Pharmacol. 2012;68(12):1657–66. [DOI] [PubMed] [Google Scholar]
- 21.Lesar TS, Briceland L, Stein DS. Factors related to errors in medication prescribing. JAMA. 1997;277(4):312–7. [PubMed] [Google Scholar]
- 22.Moura CS, Acurcio FA, Belo NO. Drug–drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci. 2009;12(3):266–72. [DOI] [PubMed] [Google Scholar]
- 23.van den Bemt PM, Egberts AC, Lenderink AW, Verzijl JM, Simons KA, van der Pol WS, et al. Risk factors for the development of adverse drug events in hospitalized patients. Pharm World Sci. 2000;22(2):62–6. [DOI] [PubMed] [Google Scholar]
- 24.Melander E, Nissen A, Henricson K, Merlo J, Mölstad S, Kampmann JP, et al. Utilisation of antibiotics in young children: opposite relationships to adult educational levels in Danish and Swedish counties. Eur J Clin Pharmacol. 2003;59(4):331–5. [DOI] [PubMed] [Google Scholar]