Abstract
Background and Aims:
The factors associated with incident hepatic steatosis are not definitively known. We sought to determine factors associated with incident hepatic steatosis, as measured on computed tomography, in the community.
Methods:
We studied Framingham Heart Study participants without heavy alcohol use or baseline hepatic steatosis who underwent computed tomography scans between 2002–2005 (baseline) and 2008–2011 (follow-up). We performed a stepwise logistic regression procedure to determine the predictors associated with incident hepatic steatosis.
Results:
We included 685 participants (mean age: 45.0 ± 6.2 years, 46.8% women). The incidence of hepatic steatosis in our sample was 17.1% over a mean 6.3 years of follow-up. Participants who developed hepatic steatosis had more adverse cardio-metabolic profiles at baseline compared to those free of hepatic steatosis at follow-up. Multivariable stepwise regression analysis showed that a simple clinical model including age, sex, body mass index, alcohol consumption and triglycerides was predictive of incident hepatic steatosis (C statistic = 0.791, 95% CI: 0.748–0.834). A complex clinical model, which included visceral adipose tissue volume and liver phantom ratio added to the simple clinical model, and had improved discrimination for predicting incident hepatic steatosis (C statistic = 0.826, 95% CI: 0.786–0.866, P < .0001).
Conclusions:
The combination of demographic, clinical and imaging characteristics at baseline was predictive of incident hepatic steatosis. The use of our predictive model may help identify those at increased risk for developing hepatic steatosis who may benefit from risk factor modification although further investigation is warranted.
Keywords: epidemiology, fatty liver, incident disease, risk factors
1 |. INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is now the most common chronic liver disease in the United States.1 NAFLD is a progressive condition that starts with hepatic steatosis and can advance to non-alcoholic steatohepatitis with hepatocyte injury leading to fibrosis and, ultimately, cirrhosis.2 NAFLD is associated with increased overall mortality,3 including liver-related4 and cardiovascular-related death.5,6
Hepatic steatosis, which is diagnosed with liver imaging, may represent an important subclinical condition for which interventions to prevent or treat non-alcoholic steatohepatitis may be employed. Because liver imaging is costly and time-consuming to obtain, few studies have serial abdominal images available to study the incidence and natural history of hepatic steatosis. Prior studies that have attempted to evaluate the incidence of hepatic steatosis have been limited by short duration of follow-up7–11 and small sample sizes.10,12,13 Most prospective studies have been in Asian populations7–11,14–17 and may not be generalizable to the United States. Given the paucity of prospective studies, it is unclear which factors contribute to the pathogenesis of hepatic steatosis in the United States.
We hypothesized that cardiometabolic risk factors contribute to worsened hepatic steatosis. In cross-sectional studies, it is well established that intrahepatic fat is associated with cardiometabolic risk factors above and beyond generalized and visceral adiposity.18–21 Additional evidence to support our hypothesis stems from prior studies which have identified hypertension, hypertriglyceridemia and insulin resistance as predictive of incident diabetes mellitus, which is closely associated with NAFLD.22 These observations suggest that cardiometabolic risk factors may increase the risk of developing NAFLD. Large prospective studies evaluating cardiometabolic risk factors and their associations with the development of hepatic steatosis are lacking. Thus, the goal of our study was to determine the factors associated with incident hepatic steatosis as measured by multi-detector computed tomography (CT) in a community-based, longitudinal cohort study.
2 |. METHODS
2.1 |. Study sample
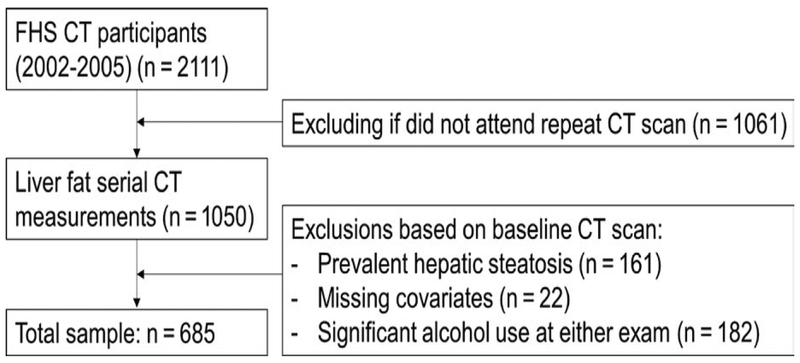
The study sample was derived from the Framingham Heart Study (FHS) Third-Generation cohort.23 Between 2002 and 2005, 2111 participants in the Third-Generation cohort underwent a CT scan as part of a comprehensive assessment of vascular calcification and ectopic fat depots, including liver fat. These participants underwent repeat CT scanning from 2008 to 2011 at the time of the second examination cycle. We included participants in the Third-Generation cohort who had measurements of liver fat at both CT examinations (n = 1050) in the present investigation. Participants were excluded for the following indications: evidence of hepatic steatosis on the baseline examination (n = 161); missing covariate information (n = 22) or significant self-reported alcohol consumption at either exam (as defined as >7 drinks per week for women or >14 drinks per week for men, n = 182, Figure 1). Participants with available liver fat data at both examinations were slightly older (mean age of 45.0 vs 44.2, P value = .02), less likely to be cigarette smokers (9.2% vs 18.7%, P value <.001) and less likely to have diabetes (1.6% vs 3.9%, P value = .03) compared to participants with liver fat data available only at the baseline assessment (Table S1). The Institutional Review Boards of the Boston University Medical Center and Massachusetts General Hospital approved the study protocol. All participants provided written informed consent.
FIGURE 1.
Study sample derivation
2.2 |. Measuring hepatic steatosis and visceral and subcutaneous adipose tissue
The outcome of interest was the development of hepatic steatosis identified on follow-up CT scan. The CT scan protocol for both the baseline CT scans (2002–2005) and follow-up CT scans (2008–2011) have been described previously.21,24 The liver attenuation in Hounsfield Units (HU) was calculated from dividing average HU attenuation measured from three areas in the liver by the HU attenuation of the external phantom to create liver phantom ratios (LPR). The LPR was chosen as the indexed standard since the spleen was not visualized on all scans. A liver spleen ratio of 1.1 corresponds to 30% hepatic steatosis based on studies in liver donors.25 We defined hepatic steatosis as a LPR of ≤0.33 based on the high specificity for detecting a liver spleen ratio of 1.1.21 Participants with prevalent hepatic steatosis at baseline were excluded from analysis. Participants with a LPR >0.33 at the baseline CT examination and a LPR ≤0.33 at the subsequent CT scan were considered to have incident hepatic steatosis.
Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were measured after manually traced the muscular abdominal wall separating the VAT and SAT tissue compartments as previously described.26 The intra-reader and inter-reader reproducibility for LPR and VAT volume were 0.99.26,27
2.3 |. Covariates and baseline measurements
The main exposures of interest were cardiometabolic risk factors given the strong association between cardiometabolic disease and NAFLD. Other exposures included serum aminotransferase levels, serum uric acid and alcohol use. Participants were considered current smokers if they smoked at least one cigarette per day during the previous year. A series of physician-administered questions to assess alcohol use and menopausal status were used. Plasma glucose, triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, total cholesterol, uric acid and liver aminotransferase levels were measured on fasting morning samples. Waist circumference was measured to the nearest 0.25 inch at the level of the umbilicus. Body mass index (BMI) was calculated by dividing the weight (kg) by height in meters squared. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated based on fasting plasma glucose and insulin levels as previously described.28 Diabetes was defined as fasting plasma glucose ≥126 mg/dL or treatment with insulin or hypoglycaemic agent. Impaired fasting glucose was defined by fasting plasma glucose level of 100–125 mg/dL among those not treated for diabetes. Insulin resistance was defined by HOMA-IR ≥75th percentile. High triglycerides were defined by fasting triglycerides ≥150 mg/dL. Low HDL was defined as a HDL <50 mg/dL for women and HDL <40 mg/dL for men. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥ 90 mm Hg or the use of anti-hypertensive medications. Pulse pressure was calculated as the difference in systolic and diastolic blood pressures.
2.4 |. Statistical analysis
The distribution of the demographic and cardiometabolic risk factors between participants who developed and those who remained free of hepatic steatosis during 6.3 years of follow-up was tested using standard descriptive statistics: χ2 test for dichotomous and analysis of variance for continuous variables. We performed a stepwise logistic regression procedure to determine the most parsimonious set of predictors associated with incident hepatic steatosis. Stepwise regression is a variation of forward selection such that after each variable is added, all candidate variables in the model are checked to see whether their significance has been reduced below a tolerated threshold.29 A significance level of 0.10 was used for model entry and a two-sided significance level of 0.05 was used for retention. The rationale for separate models to estimate risk for incident hepatic steatosis was predicated on evaluation of three major levels of health information. The first level includes simple demographic information available without medical consultation. The second level is a simple clinical model that adds to the demographic information typically available at clinic visits including current smoking status, drinks per week, menopausal status, hypertension, pulse pressure, diabetes, fasting glucose, impaired fasting glucose, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, BMI, alanine aminotransferase (ALT) and aspartate aminotransferase levels. The third model, a complex clinical model, adds waist circumference, VAT and SAT volumes, uric acid and the LPR to the simple clinical model. Candidate variables were chosen based on prior studies which have identified cross-sectional associations between prevalent hepatic steatosis and the variables of interest.
Our regression models sequentially included the demographic model, the simple clinical model and the complex clinical model with evaluation of the discriminatory capability of the models using the C statistic, or the area under the receiver operating characteristic curve. The DeLong test was used for the between-model comparisons.30 Participant risk was ranked by decile and we performed a χ2 analysis on the estimates. All analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, NC, USA).
3 |. RESULTS
3.1 |. Study sample characteristics
After exclusions, 685 participants were available for analysis (mean age: 45.0 ± 6.2 years, 46.8% women). The mean follow-up time was 6.3 years (range: 4.0–8.6 years). Baseline characteristics are shown in Table 1. Overall, 17.1% (n = 117) of participants who had an LPR > 0.33 at baseline developed hepatic steatosis over the study period. Baseline comparisons revealed that most clinical variables, including age, sex, smoking status, impaired fasting glucose, metabolic syndrome, systolic blood pressure, diastolic blood pressure, hypertension, HDL cholesterol, triglycerides and ALT were less metabolically favourable in participants who subsequently developed hepatic steatosis. Additionally, participants with higher mean baseline BMI, waist circumference, VAT volume, SAT volume and lower mean LPR were also more likely to develop hepatic steatosis over the study period. Higher mean alcohol use (drinks/d) was associated with lower risk of incident hepatic steatosis. After stratifying by menopausal status, post-menopausal women had a similar incidence of hepatic steatosis compared to men (19% vs 22%) while the incidence of hepatic steatosis was lower for pre-menopausal women (9%).
TABLE 1.
Baseline characteristics stratified by presence of hepatic steatosis on CT scan after a mean 6.3 years of follow-up
| Characteristics | No hepatic steatosis at follow-up (n = 568) | Hepatic steatosis at follow-up (n = 117) |
|---|---|---|
| Age (y) | 44.9 (6.1) | 45.9 (7.0) |
| Women n, (%) | 283 (49.8%) | 38 (32.5%) |
| Smoking n, (%) | 45 (7.9%) | 18 (15.4%) |
| Alcohol (drinks/wk) | 3 (3.2) | 2 (3.2) |
| Diabetes n, (%) | 9 (1.6%) | 2 (1.7%) |
| Impaired fasting glucose n, (%) | 105 (18.8%) | 43 (37.4%) |
| Fasting Glucose (mg/dL) | 95 (18) | 98 (12) |
| Metabolic Syndrome n, (%) | 82 (14.4%) | 47 (40.2%) |
| Systolic blood pressure (mm Hg) | 116 (13) | 121 (13) |
| Diastolic Blood Pressure (mm Hg) | 75(9) | 79(8) |
| Hypertension n, (%) | 74 (13.0%) | 33 (28.2%) |
| Pulse pressure (mm Hg) | 41(9) | 43 (10) |
| Lipid lowering therapy n, (%) | 46 (8.1%) | 19 (16.2%) |
| Total Cholesterol (mg/dL) | 190 (34) | 198 (32) |
| HDL Cholesterol (mg/dL) | 55 (16) | 47 (13) |
| Low HDL Cholesterol n, (%) | 136 (23.9%) | 47 (40.2%) |
| Triglycerides (mg/dL) | 100 (53) | 144 (93) |
| High Triglycerides n, (%) | 120 (21.1%) | 53 (45.3%) |
| LDL Cholesterol (mg/dL) | 115 (31) | 123 (27) |
| Alanine aminotransferase (IU/L) | 24 (14) | 30 (15) |
| Aspartate aminotransferase (IU/L) | 23(8) | 25 (10) |
| BMI (kg/m2) | 25.9 (4.4) | 29.9 (4.8) |
| BMI Category n, (%) | ||
| Normal weight (BMI < 25 kg/m2) | 277 (48.8%) | 13 (11.1%) |
| Overweight (25 < BMI < 30 kg/m2) | 210 (37.0%) | 54 (46.2%) |
| Obese (BMI > 30 kg/m2) | 81 (14.3%) | 50 (42.7%) |
| Visceral adipose tissue (cm3) | 1273 (712) | 2038 (790) |
| Subcutaneous adipose tissue (cm3) | 2444 (1264) | 3368 (1501) |
| Waist circumference (cm) | 91.1 (12.3) | 103.1 (12.9) |
| Liver phantom ratio | 0.38 (0.02) | 0.36 (0.02) |
| Uric acid (mg/dL) | 5.1 (1.3) | 5.9 (1.4) |
BMI, body mass index; HDL, high-density lipoprotein
Continuous variables expressed as mean (SD), categorical variables as n, (%)
3.2 |. Stepwise regression models
Results for the multivariable stepwise regression models predicting incident hepatic steatosis (LPR ≤ 0.33) are shown in Table 2. The demographic model included age and sex only (C statistic = 0.622, 95% CI: 0.567–0.678). For the simple clinical model, after age (OR: 1.04, 95% CI: 1.00–1.08, P = 0.04) and sex (OR: 0.33, 95% CI: 0.20–0.56, P < 0.0001) were forced into the model, BMI (OR: 1.18, 95% CI: 1.12–1.24, P < 0.0001) and triglycerides (OR: 1.01, 95% CI: 1.00–1.01, P = 0.002) were associated with incident hepatic steatosis. Additionally, alcohol use as measured in drinks per week was associated with lower risk of incident hepatic steatosis (OR: 0.90, 95% CI: 0.83–0.96, P = 0.003). The C statistic for the simple clinical model was 0.791 (95% CI: 0.748–0.834). A steatosis risk model calculator, based on the simple clinical model, is included in Appendix S1 and available online at https://www.framinghamheartstudy.org.
TABLE 2.
Stepwise regression procedure for the demographic model, simple clinical model and complex clinical model predicting incident hepatic steatosis
| Predictor | Age and sex only | Simple clinical modela | Complex clinical modelb | |||
|---|---|---|---|---|---|---|
| OR(95%CI) | P value | OR(95%CI) | P value | OR(95%CI) | P value | |
| Age (y) | 1.04 (1.01–1.08) | .01 | 1.04 (1.00–1.08) | .04 | 1.02 (0.98–1.06) | .42 |
| Women | 0.42 (0.27–0.65) | 0.0001 | 0.33 (0.20–0.56) | <.0001 | 0.44 (0.24–0.81) | .008 |
| BMI(kg/m2) | 1.18 (1.12–1.24) | <.0001 | 1.11 (1.04–1.19) | .003 | ||
| Alcoholic drinks/wk | 0.90 (0.83–0.96) | .003 | 0.89 (0.83–0.96) | .003 | ||
| Triglycerides (mg/dL) | 1.01 (1.00–1.01) | .002 | 1.00 (1.00–1.01) | .06 | ||
| LPR | 0.75 (0.67–0.84) | <.0001 | ||||
| VAT (cm3) | 1.34 (1.07–1.69) | .01 | ||||
| AUCc | 0.622 (0.567–0.678) | 0.791 (0748–0.834) | 0.826 (0.786–0.866) | <.0001 | ||
BMI, body mass index; LPR, liver phantom ratio; VAT, visceral adipose tissue; AUC, area under the receiver operating characteristic.
Estimates give OR per 1 unit increase in predictor with the exception of VAT where OR is per 500 cm3 increase and LPR where OR is per 0.01 unit increase.
Stepwise analysis for the simple clinical model included the demographic model with the additional candidate
variables: current smoking status, drinks per week, menopausal status, hypertension, pulse pressure, diabetes, fasting glucose, impaired fasting glucose, total cholesterol levels, HDL cholesterol levels, LDL cholesterol, triglycerides, BMI, alanine aminotransferase levels and aspartate aminotransferase levels.
Stepwise analysis for the complex clinical model included the simple clinical model with the additional candidate variables: waist circumference, VAT and SAT volumes and the LPR.
Between the simple and complex clinical models, the difference in AUC was 0.035 with 95% CI of (0.009–0.061), P < .0001. Between the demographic and complex clinical model, the difference in AUC was 0.204 with 95% CI of (0.147–0.261), P < .0001.
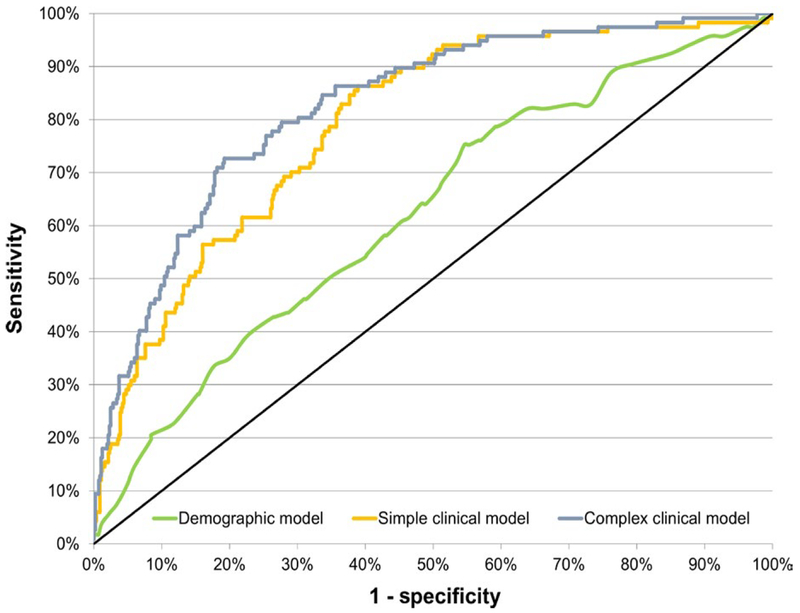
The stepwise regression for the complex clinical model showed that baseline VAT volume (OR: 1.34, 95% CI: 1.07–1.69, P = 0.01) and baseline LPR (OR: 0.75, 95% CI: 0.67–0.84, P < 0.0001) were associated with incident hepatic steatosis after the predictors in the simple clinical model were forced into the model (C statistic 0.826, 95% CI: 0.786–0.866), indicating good capability to discriminate persons who developed hepatic steatosis from those who did not. Figure 2 compares the receiver operating characteristics for the three models, showing graphically how the area under the receiver operating characteristic curve was lower for the demographic and simple clinical model compared to the complex clinical model (P < .0001).
FIGURE 2.
Receiver operating characteristic curves for the demographic, simple clinical and complex clinical models predicting fatty liver. The demographic model includes age and sex only. The simple clinical model adds body mass index, alcoholic drinks per week and triglycerides to the demographic model. The complex clinical model includes the simple clinical model plus the liver phantom ratio and visceral adipose tissue volume
4 |. DISCUSSION
In our community-based cohort study of FHS participants who underwent serial abdominal CT scans, we made several important findings. Firstly, we observed that the incidence of hepatic steatosis defined by CT scan in our cohort of predominately European ancestry, middle-aged, adults was 17.1% over an average of 6.3 years of follow-up. Secondly, we determined that those participants who developed hepatic steatosis had more adverse cardiometabolic risk factor profiles at baseline compared to participants who remained free of hepatic steatosis at the time of follow-up. Finally, we derived a prediction model based on a combination of demographic, clinical variables and more complex measures that had good discriminatory characteristics for determining the risk for incident hepatic steatosis.
Our study supports our prior work where we described the incidence of hepatic steatosis in the Framingham Heart Study. In the present study, we observed a higher incidence of hepatic steatosis (17.1%) compared to our prior work (12%) largely because of differences in the analytical sample and the definition of hepatic steatosis. In the prior study, hepatic steatosis was defined based on sex-and exam-specific 20th percentile cut-offs for the LPR, which sets the prevalence of hepatic steatosis to 20% and does not allow for the expected increase in prevalence between the examination time points. In the present analysis, we chose to define hepatic steatosis based on the previously validated LPR cut-off of 0.33. The incidence of hepatic steatosis of 17.1% in the present analysis is consistent with prior studies that have evaluated the incidence of fatty liver in non-US populations or cohorts with higher disease risk (Table 3). In prior studies, the incidence of NAFLD ranged from 10% to 25.5% depending on the sample characteristics and duration of follow-up. A study from a Japanese cohort of participants in an employee health clinic observed a 10% incidence of NAFLD although the duration of follow-up was only 1.1 years.8 A study in a Korean hospital-based cohort observed the highest incidence of NAFLD, 25.5%, over 5 years of follow-up.14 It is possible that the Korean cohort, being hospital-based, included patients with more NAFLD risk factors compared to community-based cohorts.
TABLE 3.
Literature cited on prior studies on the incidence of NAFLD
| Reference | Study sample | Follow-up, years | NAFLD measurement | Incident NAFLD, n, (%) |
|---|---|---|---|---|
| Maetal.(2017)30 | n: 841 United States Age: 46.2 ± 6.6 y Women: 46% BMI: 30.6 ±5.3 |
6.2 mean | Computed tomography | 101 (12%) |
| Chang etal.(2016)7 | n: 77,425 Chinese Age: 35.7 ± 6.4 y Women: 60.6% BMI: 21.5 ± 2.4 kg/m2 |
4.5 median | Abdominal ultrasonography | 10,340 (13.4%) |
| Hamaguchietal. (2005)8 | n: 3,147 Japanese Age: 47.6 ± 8.8 y Women: 46% BMI: 22.6 kg/m2 |
1.1 mean | Abdominal ultrasonography | 308 (10.0%) |
| Kimetal. (2015)9 | n: 1,375 Korean Age: 51.5 ± 9.1 y Women: 47% BMI: 23.8 kg/m2 |
4.4 mean | Abdominal ultrasonography | 288 (20.9%) |
| Wongetal. (2015)10 | 565 Chinese Age: 48.0 ± 10.0 y Women: 63% BMI: 21.9 ±2.9 kg/m2 |
3.9 median | Proton-magnetic resonance spectroscopy | 78 (13.8%) |
| Zhang etal.(2014)11 | 15,791 Chinese Age: 42.5 ± 15.0 y Women: 50% |
3.3 mean | Abdominal ultrasonography | 3913 (24.8%) |
| Bedognietal. (2007)12 | 144 Italian Age: 47 ± 22 y Women: 43% BMI: 24 kg/m2 |
8.5 mean | Abdominal ultrasonography | 22 (15.3%) |
| Zelber-Sani etal. (2012)13 | 213 Israeli Age: 51.2 ± 9.6 y Women: 46% BMI: 27 kg/m2 |
7 mean | Abdominal ultrasonography | 28 (13.1%) |
| Chung etal.(2017)14 | 2216 Korean Age: 48.1 ± 9.6 y Women: 57% BMI: 21.7 ± 2.4 kg/m2 (women) and 23.6 ± 2.2 kg/m2 (men) |
5 median | Abdominal ultrasonography | 565 (25.5%) |
| Tsunetoetal.(2010)15 | 1,635 Japanese Age: 63.1 ± 8.9 y Women: 63% BMI: 22.5 ±3.0 kg/m2 |
11.6 mean | Abdominal ultrasonography | 323 (19.8%) |
| Wangetal.(2017)16 | 6,948 Chinese Age: 44.5 ± 12.9 y Women: 36.0% BMI: 22.7 ±2.8 kg/m2 |
up to 7 | Abdominal ultrasonography | 1139 (16.4%) |
| Miyakeetal.(2012)17 | 3,215 Japanese Age: 40.2 ± 8.8 y(women) 40.9 ± 9.6 y (men) Women: 72.6% BMI: 20.9 ± 2.6 kg/m2 (women) and 22.5 ± 2.6 kg/m2 (men) |
1.9 mean | Abdominal ultrasonography | 400 (12.4%) |
Few prior studies have developed models to predict incident NAFLD. One study in a large Chinese cohort of ultrasound-defined NAFLD developed and internally validated the NAFL Risk Score which predicted incident NAFLD.31 The predictors of incident NAFLD included BMI, triglycerides x gamma-glutamyl transferase, ALT/aspartate aminotransferase, LDL/HDL, and uric acid and separate models were derived for women and men. In a large Japanese community-based cohort of ultrasound-defined NAFLD, predictors of incident NAFLD over about 2 years of follow-up included BMI, triglycerides, and fasting plasma glucose for women and BMI, ALT, HDL, and uric acid for men.17 However, in our cohort, we did not find that uric acid level was predictive of incident NAFLD. We observed that BMI, alcohol use and serum triglyceride levels were the clinical traits most predictive of incident hepatic steatosis after age and sex in the multivariable stepwise regression. A simple clinical model incorporating these variables had good discriminatory ability to predict incident hepatic steatosis over a mean 6.3 years of follow-up.
In our study, we observed that the odds of incident hepatic steatosis were 18% higher per 1 unit increase in baseline BMI. In multiple prior cross-sectional studies, obesity is associated with prevalent NAFLD.1,21 Our findings support a prior prospective study in the Israeli population which observed a strong association between baseline BMI and incident ultrasound-defined NAFLD after 7 years.13 Additionally, BMI is the strongest predictor of incident NAFLD in the existing incident NAFLD predictive models.17,31
We observed that women had a reduced odds (OR: 0.33) of incident hepatic steatosis compared to men. In the Dallas Heart Study, the prevalence of hepatic steatosis was lower among white women compared to white men.32 The lower prevalence of hepatic steatosis in women may, in part, be owing to lower plasma triglyceride levels in women compared to men.33 Sex differences in how the liver maintains the balance between the synthesis and oxidation of fatty acids may impact triglyceride concentration. Additionally, hormones may influence sex differences. In our study, after stratifying by menopausal status, we observed that post-menopausal women had a similar incidence of hepatic steatosis compared to men, whereas pre-menopausal women had a lower incidence of hepatic steatosis.
After excluding moderate-to-heavy alcohol consumers, we observed that a small-to-moderate amount of alcohol was associated with lower levels of incident hepatic steatosis. This observation is supported by a number of cross-sectional studies, including in a meta-analysis of 8 studies and 43 175 individuals.34–37 There are a number of potential mechanisms by which alcohol may protect against hepatic steatosis. Modest alcohol consumption was associated with reduced cardiovascular mortality,38 which may in part be mediated by a reduction in insulin resistance.39 Alternatively, modest alcohol consumption may be a healthy behaviour that tracks with other healthy behaviours, such as physical activity, which has been shown to improve liver histology.
To test whether more complex variables added to the prediction of incident hepatic steatosis, we derived a complex clinical model. We observed that the addition of baseline VAT volume and baseline LPR improved the models’ assessment of hepatic steatosis risk over and above the simple clinical model. In our study, the odds of hepatic steatosis increased 34% per 500 cm3 increase in baseline VAT volume. Similarly, increasing baseline LPR (less liver fat) was associated with 25% decreased odds of incident hepatic steatosis. In prior cross-sectional studies, VAT volume has been associated with prevalent NAFLD.21 Our findings are supported by a recent Korean study which reported that a higher baseline VAT volume, and not SAT volume, was associated with a dose-dependent, increased risk of incident NAFLD over 4.4 years of median follow-up.9 In the FHS, VAT volume and SAT volume are both correlated with metabolic risk factors; however, VAT is more strongly associated with an adverse metabolic risk profile.26 Future studies aimed at the prevention and treatment of NAFLD should consider interventions aimed at improving visceral adiposity.
The major strength of our investigation includes the use of a community-based cohort that underwent detailed characterization of hepatic steatosis using serial CT scans. In this densely phenotyped sample, we were able to add to the current literature by evaluating the incidence for hepatic steatosis and risk factors for developing incident disease over an average of 6.3 years of follow-up. The major limitation of our study was in the definition of hepatic steatosis by CT imaging, which, like ultrasound, is insensitive to mild steatosis. Participants that developed incident hepatic steatosis tended to have lower baseline LPR values compared to those who did not develop hepatic steatosis (data not shown) which many indicate mild steatosis was present at baseline. Additionally, hepatic steatosis may diminish as NAFLD progresses into steatohepatitis and fibrosis. Steatohepatitis and hepatic fibrosis cannot be measured by CT scan so we are unable to comment on incidence of these conditions. We also lack information about other chronic liver diseases including viral hepatitis status, which can cause the appearance of liver fat on CT scan. This may have led to a misclassification bias. Alcohol use was by self-report, and may also be misclassified. Many participants were on treatment for CVD risk factors which may dampen the effect of CVD risk factors on incident hepatic steatosis. Our study was observational in design and modest in size; we cannot exclude the possibility of residual confounding, and we note the moderate power to identify predictors of NAFLD. Because of our modest sample size, we were not able to internally validate our model. US-based cohorts with serial liver imaging that could be used for external validation are lacking. Finally, FHS participants are mostly of European ancestry, so the generalizability of our findings to other races/ethnicities is not known.
5 |. CONCLUSION
In a community-based cohort of participants, the incidence of hepatic steatosis was 17% over approximately 6 years. The combination of demographic, clinical and imaging characteristics at baseline was predictive of incident hepatic steatosis. Future multi-ethnic studies in US-based cohorts with serial liver imaging are needed to replicate and externally validate our findings. Whether modifying risk factors impacts the development of incident hepatic steatosis or other liver-related endpoints remains unknown and should be explored in future studies.
Supplementary Material
Key points.
Factors associated with incident NAFLD are not definitely known.
The incidence of hepatic steatosis by computed tomography was 17% among community-dwellers over a 6-year period.
Those who developed hepatic steatosis had more adverse cardiometabolic profiles at baseline compared to those free of hepatic steatosis at follow-up.
A simple clinical model including age, sex, body mass index, alcohol consumption and triglycerides was predictive of incident hepatic steatosis.
ACKNOWLEDGEMENTS
This work was supported by the Boston University School of Medicine and the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contract N01-HC-25195 and HHSN268201500001I), and the Division of Intramural Research of the National Heart, Lung, and Blood Institute. Dr. Long is supported by K23DK113252. Dr.Loomba is supported in part by a T. Franklin Williams Scholarship Award, by Atlantic Phianthropies Inc., the John A. Hartford Foundation, OM, the Association of Specialty Professors and by R01 DK106419. Dr. Chung is supported in part by K24 DK078772. Dr. Benjamin is supported in part by 2R01 HL092577 and R01 HL128914.
Funding information
National Heart, Lung, and Blood
Institute, Grant/Award Number: HHSN268201500001, R01 HL092577, R01 HL128914 and R01 DK106419; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: K23 DK113252 and K24 DK078772.
Abbreviations:
- NAFLD
non-alcoholic fatty liver disease
- CT
computed tomography
- FHS
Framingham Heart Study
- HU
Hounsfield units
- LPR
liver phantom ratio
- VAT
visceral adipose tissue
- SAT
subcutaneous adipose tissue
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- BMI
body mass index
- HOMA-IR
homeostatic model assessment of insulin resistance
- ALT
alanine aminotransferase
Footnotes
CONFLICTS OF INTEREST
Alison Pedley is an employee of Merck. The other authors have no conflicts to report.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. [DOI] [PubMed] [Google Scholar]
- 2.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. [DOI] [PubMed] [Google Scholar]
- 3.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. [DOI] [PubMed] [Google Scholar]
- 4.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn W, Xu R, Wingard DL, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Y, Jung HS, Cho J, et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroenterol. 2016;111:1133–1140. [DOI] [PubMed] [Google Scholar]
- 8.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Chung GE, Kwak MS, et al. Body fat distribution and risk of incident and regressed nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2015;14:132–138. [DOI] [PubMed] [Google Scholar]
- 10.Wong VW, Wong GL, Yeung DK, et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol. 2015;62:182–189. [DOI] [PubMed] [Google Scholar]
- 11.Zhang T, Zhang C, Zhang Y, et al. Metabolic syndrome and its components as predictors of nonalcoholic fatty liver disease in a northern urban Han Chinese population: a prospective cohort study. Atherosclerosis. 2015;240:144–148. [DOI] [PubMed] [Google Scholar]
- 12.Bedogni G, Miglioli L, Masutti F, et al. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology. 2007;46:1387–1391. [DOI] [PubMed] [Google Scholar]
- 13.Zelber-Sagi S, Lotan R, Shlomai A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012;56:1145–1151. [DOI] [PubMed] [Google Scholar]
- 14.Chung GE, Yim JY, Kim D, et al. Associations between hemoglobin concentrations and the development of incidental metabolic syndrome or nonalcoholic fatty liver disease. Digestive and Liver Disease. 2017;49:57–62. [DOI] [PubMed] [Google Scholar]
- 15.Tsuneto A, Hida A, Sera N, et al. Fatty liver incidence and predictive variables. Hypertens Res. 2010;33:638–643. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Zhu W, Huang S, et al. Serum apoB levels independently predict the development of non-alcoholic fatty liver disease: a 7-year prospective study. Liver Int. 2017;37:1202–1208. [DOI] [PubMed] [Google Scholar]
- 17.Miyake T, Kumagi T, Hirooka M, et al. Body mass index is the most useful predictive factor for the onset of nonalcoholic fatty liver disease: a community-based retrospective longitudinal cohort study. J Gastroenterol. 2013;48:413–422. [DOI] [PubMed] [Google Scholar]
- 18.Arase Y, Suzuki F, Ikeda K, Kumada H, Tsuji H, Kobayashi T. Multivariate analysis of risk factors for the development of type 2 diabetes in nonalcoholic fatty liver disease. J Gastroenterol. 2009;44:1064–1070. [DOI] [PubMed] [Google Scholar]
- 19.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruberg FL, Chen Z, Hua N, et al. The relationship of ectopic lipid accumulation to cardiac and vascular function in obesity and metabolic syndrome. Obesity. 2010;18:1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167:1068–1074. [DOI] [PubMed] [Google Scholar]
- 23.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 24.Roseman DA, Hwang SJ, Manders ES, et al. Renal artery calcium, cardiovascular risk factors, and indexes of renal function. Am J Cardiol 2014;113:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwasaki M, Takada Y, Hayashi M, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. [DOI] [PubMed] [Google Scholar]
- 26.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 27.Speliotes EK MASSARO JM, HOFFMANN U, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 29.Hocking RR. A Biometrics Invited Paper. The Analysis and Selection of Variables in Linear Regression. Biometrics. 1976;32:1–49. [Google Scholar]
- 30.Delong ER, Delong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 31.Zhou YJ, ZHENG JN, Liu WY, et al. The NAFL Risk Score: a simple scoring model to predict 4-y risk for non-alcoholic fatty liver. Clin Chim Acta. 2017;468:17–24. [DOI] [PubMed] [Google Scholar]
- 32.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab. 2011;96:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriya A, Iwasaki Y, Ohguchi S, et al. Roles of alcohol consumption in fatty liver: a longitudinal study. J Hepatol. 2015;62:921–927. [DOI] [PubMed] [Google Scholar]
- 35.Dunn W, Sanyal AJ, Brunt EM, et al. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD). J Hepatol. 2012;57:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sookoian S, Castano GO, Pirola CJ. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: a meta-analysis of 43175 individuals. Gut. 2014;63:530–532. [DOI] [PubMed] [Google Scholar]
- 37.Liangpunsakul S, Chalasani N. What should we recommend to our patients with NAFLD regarding alcohol use? Am J Gastroenterol. 2012;107:976–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thun MJ, Peto R, Lopez AD, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–1714. [DOI] [PubMed] [Google Scholar]
- 39.Facchini F, Chen YD, Reaven GM. Light-to-moderate alcohol in-take is associated with enhanced insulin sensitivity. Diabetes Care. 1994;17:115–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.